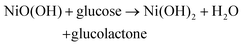A disposable and sensitive non-enzymatic glucose sensor based on a 3D-Mn-doped NiO nanoflower-modified flexible electrode†
Huisi
Yang‡
 a,
Yian
Hu‡
a,
Xinxue
Yin‡
a,
Jiaqing
Huang
a,
Cailin
Qiao
a,
Zhikun
Hu
a,
Congjuan
He
a,
Danqun
Huo
*ac and
Changjun
Hou
a,
Yian
Hu‡
a,
Xinxue
Yin‡
a,
Jiaqing
Huang
a,
Cailin
Qiao
a,
Zhikun
Hu
a,
Congjuan
He
a,
Danqun
Huo
*ac and
Changjun
Hou
 *ab
*ab
aKey Laboratory for Biorheological Science and Technology of Ministry of Education, State and Local Joint Engineering Laboratory for Vascular Implants, Bioengineering College of Chongqing University, Chongqing 400044, China. E-mail: huodq23@163.com; houcj@cqu.edu.cn; Fax: +86 23 6510 2507; Tel: +86 23 6511 2673
bLiquor Making Biology Technology and Application of Key Laboratory of Sichuan Province, College of Bioengineering, Sichuan University of Science and Engineering, Zigong, 643000, PR China
cChongqing Key Laboratory of Bio-perception & Intelligent Information Processing, School of Microelectronics and Communication Engineering, Chongqing University, Chongqing, 400044, PR China
First published on 8th December 2022
Abstract
Herein, nanoflower-shaped Mn-doped NiO nano-enzyme composites with high catalytic performance and excellent conductivity were grown on 3D flexible carbon fiber cloth (CFC) via hydrothermal and calcination methods to construct an efficient flexible glucose-sensitive detection electrode. For electrochemical-based sensors, high conductivity is a prerequisite for reliable data acquisition. To avoid the problems associated with using insulating Nafion or paraffin binders, we adopted a strategy of directly growing Mn-doped NiO onto the electrode surface, thereby avoiding interference due to the oxidization of species present in real samples at higher redox potentials, since Ni2+/Ni3+ has low redox potential. Therefore, the electrode has a linear range of 3–5166 μM for glucose detection, with a detection limit as low as 0.28 μM, showing excellent selectivity and reproducibility. The composite-modified electrode provides accurate detection results with real human serum samples, which are in full agreement with those of commercial blood glucose meters. In addition, we tested the glucose content in tea and sorghum fermentation broth at different stages, further expanding the application range of the Mn-NiO sensors. The nano-enzyme sensor fabricated herein offers a new idea for further integration into wearable flexible electronic devices for accurate glucose detection.
1. Introduction
Glucose occupies an extremely important position in the biomedical field and is an indispensable organic compound in biological metabolism and human activities.1,2 Balance of blood glucose is highly important to maintain the stability of the internal environment in the human body.3,4 Glucose metabolism disorder causes many complications including diabetes, cardiovascular disease and hypoglycemia.5 In addition, research on glucose detection has great application potential in the field of food science, such as in fruits and beverages, and the detection of their glucose content can provide real and effective nutritional data.6 Therefore, it is necessary to monitor glucose concentration in the human body and food continuously and accurately. Nowadays, extensive research has been devoted to developing fast, convenient, low-cost, high-accuracy and non-invasive wearable flexible electrochemical sensors for real-time sensitive detection of glucose and combined with mobile medical technology to achieve scientific and efficient health management for diabetic patients.7,8 Wearable flexible electronic devices have been rapidly developed owing to their real-time, low-cost, easy-to-operate, and portable monitoring of various physiological parameters, and they are expected to gradually replace traditional detection methods that are time-consuming and rely on bulky instruments and trained personnel.9,10,11,12Flexible substrates, such as polydimethylsiloxane (PDMS), polyethylene terephthalate (PET), and polyimide (PI), can be used to support conductive materials or other sensing elements, which greatly contribute to the flexibility, bendability and stretchability of wearable sensors.13,14 Recently, carbon materials (e.g., carbon nanotubes, graphene and other carbon materials) have been found to possess unique advantages of high chemical and thermal stability, low toxicity, and easy functionalization, providing a new option for wearable sensor substrate materials and active materials.15,16 Among them, carbon fiber cloth (CFC) is perfectly suitable as a carbon-based material for wearable or flexible devices owing to its excellent mechanical stability, remarkable flexibility, and low price. However, bare CFC has poor capacitive performance, relatively low hydrophobicity and electrical conductivity, which limit its application in biosensing systems. To this end, researchers modify the surface of CFC by oxidizing, doping, and growing different nanostructures to increase the electroactive surface area necessary for electrochemical interactions.17
The surface modification of CFC with modified functionalized nanostructures further increases the conductivity of electrochemical sensors and provides a large number of electroactive sites for biomolecule adsorption. For example, Horng et al.18 grew polyaniline nanowires (PANI-NWs) with a large specific surface area on 3D flexible CFC by electrochemical polymerization using immobilized glucose oxidase (GOx) on the surface of defect-free nanostructures, which not only effectively accelerated the electron transfer efficiency, but also significantly increased the catalytic sites for glucose capture. Li et al.19 designed a high-performance flexible zinc-ion battery based on flower-like C–MnO2 nanosheets grown on CFC. In situ growth of δ-MnO2 on flexible CFC by using a mussel-derived polydopamine layer as a catalyst significantly improved the specific capacity and effective mass loading of the battery. Zhan et al.20 reported the use of carbon nanotubes (CNTs) as active binders to achieve uniform growth of an organometallic framework (MOF) containing CoP nanoparticles via modification on conductive CFC, thereby effectively increasing the number of its active sites, improving its conductivity, and enhancing the stability of hydrogen release. Although 3D flexible CFC, as a typical super capacitor and flexible electrode, has been fully and widely developed in the field of energy conversion and storage, it still receives inadequate attention, particularly in terms of its integration into the flexible wearable electrochemical sensor and application to the personalized care of human health.
Since the advent of enzymatic glucose biosensors, scientific and technological advances in nanomaterials have driven the development of a new generation of glucose sensors.21 Over the past decade, significant progress has been made in this field, as novel nanomaterials (nanozymes) are able to catalyze glucose efficiently and directly.22,23 Nickel-based nanomaterials and their oxides (Ni and NiO) are gradually becoming strong competitors in transition metal nanozyme materials due to their advantages of low price, low toxicity, excellent stability, and great sensitivity to glucose catalysis.24,25 Under alkaline conditions, NiO can electrocatalyze the oxidation of glucose via a Ni2+/Ni3+ redox reaction, generating current response to detect glucose concentration quantitatively.26 Although nickel-based nanomaterials have good electrocatalytic activity for glucose oxidation, their electron transfer efficiency is weak, and their oxide nanomaterials are easy to accumulate on the electrode in use, which will have adverse effects on the electrocatalytic activity and sensing performance of the sensor.27–29 Therefore, to further eliminate the limitations of nickel-based nanomaterials in this regard, researchers usually design carbon-based nanomaterials as carriers and modulate the morphological structure of the materials by doping and modifying other metal nanomaterials to increase their electrocatalytic active sites, thus improving the conductivity of the electrochemical sensing platform and the accuracy for simultaneous detection of glucose and other small molecules.
In view of this, we synthesized Mn-doped NiO nanospheres and demonstrated their electrooxidative capabilities in terms of sensitivity and selectivity in glucose sensing. Scheme 1A is the schematic diagram of the preparation process of flower-like Mn-doped NiO. The Ni2+ ion is a transition metal coordination center with excellent coordination properties, and Mn2+ coordinates with N in hexamethylene tetramine molecules to form a complex, denoted as Ni(Mn)-HMT. Under high temperature and pressure conditions, this complex will transform into Mn miscellaneous Ni(OH)2 nanoparticle seeds, and then these seeds aggregate to form nanosheets, which further self-assemble to form a flower-shaped Mn-Ni(OH)2 structure. After the high-temperature calcination process, Mn–Ni(OH)2 is finally transformed into Mn-NiO nanoflowers. For comparison, the electrochemical activity of the undoped Mn element was investigated, and the results indicated that both NiO nanospheres can be efficiently used for electrochemical detection of glucose without any enzymes. Among them, Mn-doped NiO nanospheres showed an ultra-wide linear range and low detection limit of non-enzymatic glucose sensors based on nickel-based nanomaterials. Using the results of this study, the practical application value of the sensor can be evaluated, providing new ideas and theoretical basis for the development of wearable flexible electronic devices in diabetes detection and diagnosis in the field of personal “point-of-care” medicine (Scheme 1).
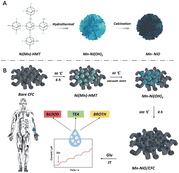 | ||
| Scheme 1 (A) Growth diagram of 3D-Mn doped NiO nano-flower; (B) Schematic of the preparation process of Mn-NiO/CFC electrodes for glucose detection. | ||
2. Experimental section
2.1. Preparation of Mn-NiO/CFC
First, a piece of CFC (3.0 × 3.0 cm2) was immersed in acetone and absolute ethanol in turn, followed by 10 min of ultrasonic treatment to remove organic impurities on the surface. The obtained CFC was washed several times with ultrapure water and then dried for use. In a typical process, 0.713 g NiCl2·6H2O, 0.162 g MnCl2·4H2O and 0.7 g C6H12N4 were dissolved in 30 mL ultrapure water successively, followed by intense mixing for 30 min. The CFC was bombarded with plasma on both sides for 20 s and then put together with the above-mentioned solution into a 100 mL Teflon-lined stainless steel autoclave, reacted at 90 °C for 6 h in an electric thermostatic drying oven. After naturally cooling to room temperature, the obtained CFC was rinsed several times with ultrapure water and absolute ethanol successively. Afterwards, the clean CFC was dried in a vacuum oven at 60 °C overnight to obtain the precursor material. Finally, the above-mentioned material was placed in a single temperature zone open vacuum tube furnace, with the temperature rising to 300 °C at a rate of 10 °C min−1, calcined in air for 4 h, and cooled to room temperature at a rate of 20 °C min−1 to obtain Mn-NiO/CFC with 10.3 wt% doped Mn. In this work, Mn-NiO/CFC was processed into a square with an effective area of 0.7 × 0.7 cm2, and the gap position was the fixed position of the platinum electrode clamp (Fig. S1†). In addition, NiO/CFC without Mn was synthesized by following a similar procedure.2.2. Practical samples analysis
Practical human serum samples containing different concentrations of glucose were prepared. For serum analysis, 20 μL prepared serum samples were dropped into 10 mL 100 mM NaOH solution. At the optimal potential of 0.6 V, the glucose-catalyzed current response heights were carried out by amperometry, and then the measured values were calculated according to the fitted linear equation. The results were compared with those of commercial glucose meter (fish leap glucose meter). To detect the glucose content in tea and sorghum fermentation broth at different stages, the tea was diluted with 0.1 M NaOH solution (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 99
v = 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) before recording the electrochemical signal, and the sorghum fermentation broth was sampled five times every 15 days.
1) before recording the electrochemical signal, and the sorghum fermentation broth was sampled five times every 15 days.
3. Results and discussion
3.1 Morphology and structure characterization of Mn-NiO/CFC
In order to understand the micromorphological structures of the materials, SEM analysis was performed on bare CFC, NiO/CFC, and Mn-NiO/CFC. In Fig. 1A, the low-magnification SEM image shows the typical cross-braided structure of woven fibers of the bare CFC. As shown in Fig. 1B, the single carbon fiber presented a smooth surface and a diameter of about 10 μm under high magnification. Obviously, this structure is favorable for electron transfer in the three-dimensional network and subsequent material growth on the CFC. From the low-magnification SEM image (50 μm) (Fig. 1D) and high-magnification image (10 μm) (Fig. 1E), we could observe dense and uniform thin sheet structures and the interlaced growth of nanosheets on NiO/CFC. The EDS elemental mapping of Fig. 1F indicates that there are three elements, namely, C, O and Ni in NiO/CFC. In Fig. 1G, the low-magnification SEM image shows that tiny granular nanoflowers were clustered or tightly covered on the surface of Mn-NiO/CFC. Furthermore, closer views of Mn-NiO/CFC in Fig. 1H show that the tiny granular nanoflowers were uniform in size and about 2 μm in diameter. Such morphological features increase the specific surface area of the electrode material and provide a large number of adsorption sites for glucose catalysis, which further enhances the electrochemical performance of the sensor. In addition, to obtain elemental compositions of Mn-NiO/CFC, EDS was adopted to identify the elemental mapping of Mn-NiO/CFC. As displayed in Fig. 1I, the C, O, Ni and Mn elements are homogeneously distributed in the entire material, which directly proves the successful preparation of the Mn-NiO/CFC sensor.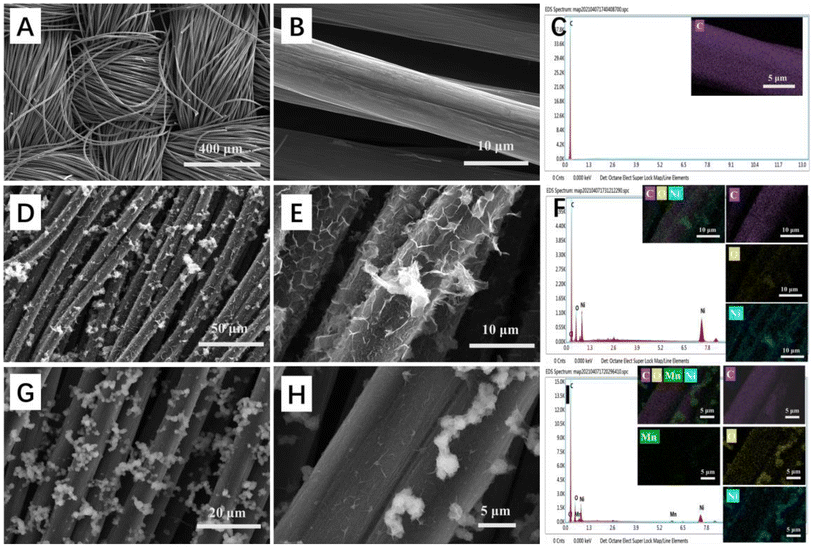 | ||
| Fig. 1 (A and B) SEM images of bare CFC, (D and E) NiO/CFC and (G and H) Mn-NiO/CFC under different magnifications. (C), (F) and (I) EDS elemental mapping of bare CFC, NiO/CFC and Mn-NiO/CFC. | ||
In Fig. 2A and Fig. S2,† the XRD patterns of Mn-NiO/CFC and NiO/CFC were acquired to show their distinct and sharp characteristic peaks at 2θ = 37.2°, 43.3°, 62.9°, 75.4° and 79.4°, corresponding to the (101), (012), (110), (113) and (202) planes of NiO/CFC, respectively, which is highly consistent with the positions of the peaks in the NiO standard card (PDF#44-1159). However, the diffraction peak of Mn-NiO/CFC shows a slight shift, which may be due to the influence of Mn slight doping on the crystal structure of NiO. Moreover, the diffraction peak position of the matrix carbon material appeared on all the three electrodes at 2θ = 27°. The above-mentioned characterization results confirm the successful preparation of the electrode materials.
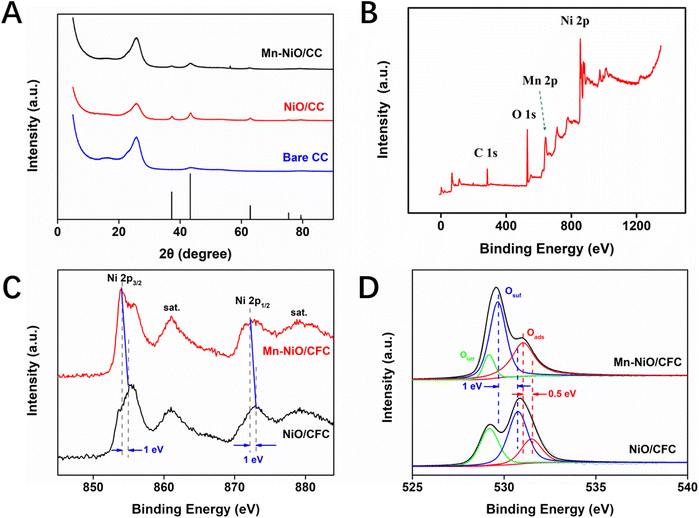 | ||
| Fig. 2 (A) XRD patterns for Mn-NiO/CFC, NiO/CFC and bare CFC. (B) XPS spectrum of Mn-NiO/CFC. (C) High-resolution Ni spectra of Mn-NiO/CFC and NiO/CFC. (D) O 1s spectra of Mn-NiO/CFC and NiO/CFC. | ||
XPS was recorded to investigate the elemental constitution of Mn-NiO/CFC. Fig. 2B exhibits the full spectrum of XPS of Mn-NiO/CFC, which mainly contained four elements, namely, C, O, Mn and Ni. The characteristic peaks of C 1s, O 1s, Mn 2p and Ni 2p located at 284.5 eV, 529.9 eV, 642.2 eV and 855.0 eV, respectively. This result proves that the composite nano-enzyme material doped with Mn and Ni has been successfully modified on the CFC. In addition, from the high-resolution Ni 2p spectra of Mn-NiO/CFC and NiO/CFC in Fig. 2C and D, the characteristic peaks of Ni 2p1/2 and Ni 2p3/2 of Mn-NiO/CFC were found to shift towards the direction of high binding energy compared with NiO/CFC, which indicates that the doping of Mn may change the electronic structure of NiO nanomaterials and increase the valence of Ni element, thus enhancing the catalytic oxidation ability of the electrode modification material on glucose.
3.2. Electrochemical characterizations of Mn-NiO/CFC
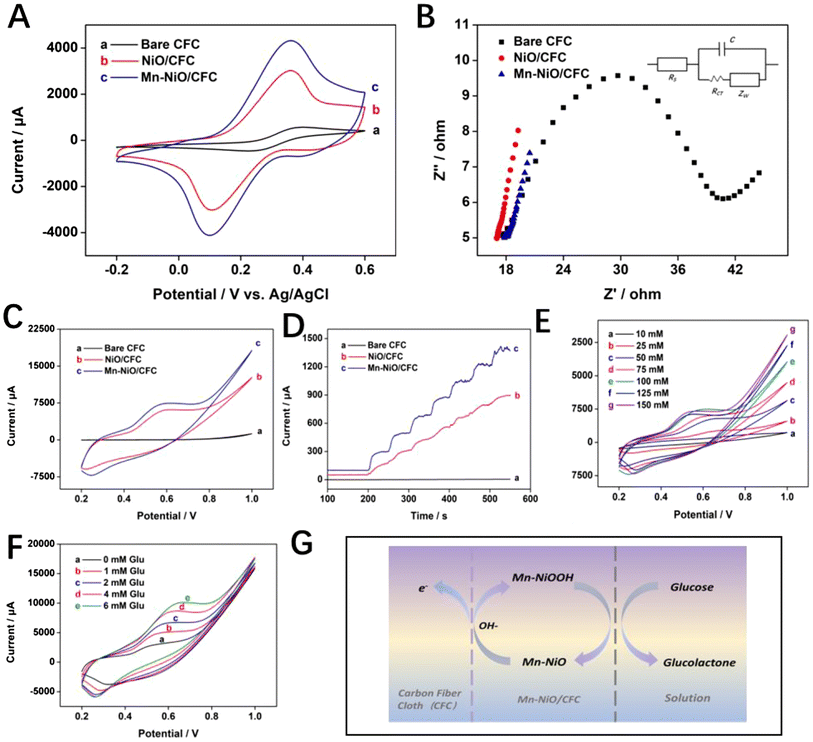
For the purpose of characterizing the conductivity and surface characteristics of Mn-NiO/CFC, the electrochemical performance of the electrode was investigated by CV and EIS in a 10 mL solution system containing 0.1 M KCl and 5 mM K3[Fe(CN)6]3−/4−, and then the results were compared with those of bare CFC and NiO/CFC. The CVs displayed in Fig. 3A show that for all the three electrodes, a typical pair of redox peaks of Fe(III)/Fe(II) appeared. Among them, Mn-NiO/CFC had a higher redox current signal peak than that of NiO/CFC and bare CFC. According to the Randles–Sevcik equation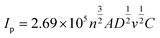 , it can be known that under the same system, the effective active area (A) of the electrode is positively correlated with the peak current (Ip). Based on the CV curves of Fig. 3, the electroactive effective area of the prepared Mn-NiO/CFC electrode is 5.2544 cm2, which is 8.20 times that of the CFC electrode (0.6408 cm2) and 1.37 times that of NiO/CFC (3.8447 cm2). Therefore, Mn-NiO/CFC possesses better electron transfer efficiency than that of the other two electrodes due to its larger electroactive effective area.
, it can be known that under the same system, the effective active area (A) of the electrode is positively correlated with the peak current (Ip). Based on the CV curves of Fig. 3, the electroactive effective area of the prepared Mn-NiO/CFC electrode is 5.2544 cm2, which is 8.20 times that of the CFC electrode (0.6408 cm2) and 1.37 times that of NiO/CFC (3.8447 cm2). Therefore, Mn-NiO/CFC possesses better electron transfer efficiency than that of the other two electrodes due to its larger electroactive effective area.
In addition, the EIS curves of three electrodes with the frequency ranging from 1 Hz to 105 Hz and an AC amplitude of 0.005 V are shown in Fig. 3B. The impedance change at the electrode interface and electron diffusion and transfer process could be observed clearly and intuitively according to the curves. The low-frequency region is the diffusion control process, which is a straight line whose slope represents the Warburg impedance, while the semicircular arc appearing in the high-frequency region is the dynamic process of charge transfer, and its arc diameter represents the resistance of electron transmission (Ret). It could be seen from Fig. 3B that bare CFC has the largest Rct value of about 40 Ω, followed by NiO/CFC and Mn-NiO/CFC in decreasing order, with the high-frequency region of Mn-NiO/CFC approaching a straight line, indicating that Mn-NiO/CFC has extremely superior conductivity. It also illustrates that the interface electron transfer resistance of Mn-NiO/CFC is the smallest, while the electron transfer rate between the electrolyte and the electrode surface is the fastest. This variation trend in EIS plots is in perfect agreement with CV plots shown in Fig. 3A, further verifying the excellent conductivity of Mn-NiO/CFC.
The electrocatalytic oxidation performance of Mn-NiO/CFC on glucose was tested in 10 mL 0.1 mM NaOH solution by CV and amperometry. Fig. 3C displays the CVs of three electrodes with addition of 1 mM glucose in a NaOH solution at a scan rate of 50 mV s−1 and a potential ranging from 0.2 V to 1.0 V. It could be clearly observed from the figure that the naked CFC exhibited no obvious peak current response to glucose, indicating that it has no electrocatalytic ability toward glucose. However, Mn-NiO/CFC and NiO/CFC showed anodizing peaks near 0.6 V, and the current response peak value of the former was significantly higher than that of the latter, which denotes a good catalytic effect on glucose oxidation of the Mn-NiO/CFC. Moreover, amperometry was used to further confirm the above-mentioned results. Specifically, the current–time curve was obtained by continuously dripping 150 μM glucose into a NaOH solution every 50 s. In addition, the curve shows that the electrode of the unmodified material had almost no current response to glucose, showing a straight line, while Mn-NiO/CFC and NiO/CFC produced a rapid current response with the addition of glucose, showing a step growth. Moreover, the growth rate of Mn-NiO/CFC was higher than that of NiO/CFC, thus revealing better electrocatalytic activity. This may be attributed to the fact that doped Mn changes the electronic structure of NiO, exposing more catalytically active sites for glucose oxidation, resulting in the redox reaction of Ni2+/Ni3+ after glucose addition under alkaline conditions, which significantly improves the electrocatalytic performance of Mn-NiO/CFC for glucose oxidation. The speculative reaction mechanism for electrode-catalyzed glucose oxidation is as follows:
| NiO + OH− − e− → NiO(OH) |
The optimal NaOH solution concentration was determined to best catalyze glucose oxidation with the Mn-NiO/CFC electrode. Specifically, Fig. 3E displays the current response height of the electrode to 1 mM glucose at different concentrations of NaOH solutions (10, 25, 50, 75, 100, 125 and 150 mM). The results indicate that as the concentration of NaOH solution increased from 10 mM to 100 mM, the oxidation current signal of the working electrode raised gradually, but when the concentration exceeds 100 mM, the current response decreased. This is because a large amount of OH− in the excessively high-concentration NaOH solution destabilized NiO(OH), the intermediate reaction material on the surface of the electrode material, and limited the adsorption and further reaction of glucose. Therefore, 100 mM NaOH solution was selected as the optimal-concentration electrolyte solution in the subsequent experiments. Fig. 3F shows the CVs of a series of glucose solutions (0, 1, 2, 4, and 6 mM) added into a 10 mL system with the optimal electrolyte solution concentration. From the figure, the oxidation peak signal of the Mn-NiO/CFC increased with the increase in glucose concentration in the potential range of 0.5–0.7 V, which mirrored the excellent performance of Mn-NiO/CFC to glucose electrocatalysis. Therefore, the optimal working potential of the electrode was further explored within the range.
Fig. S3A† presents the electrocatalytic CVs of Mn-NiO/CFC at different scan rates (25–500 mV s−1) to 1 mM glucose in 0.1 M NaOH solution. It could be seen from the figure that the peak current of the oxidation peaks increased progressively with the increase in scanning rate, and the oxidation peaks tended to shift towards high potentials, because of which the increasing scan rate leads to an increase in electrode polarization in NaOH. Otherwise, to further explore the electron transfer mechanism of glucose catalyzed by the electrode, the relationship between the peak current value of the oxidation peak (Ip) and the square root of the scan rate (V1/2) is shown in Fig. S3B,† from which a good linear relationship could be observed. This indicates that the kinetic reaction of Mn-NiO/CFC to glucose catalytic oxidation is a typical diffusion control process.
3.3. Optimization of experimental conditions of Mn-NiO/CFC
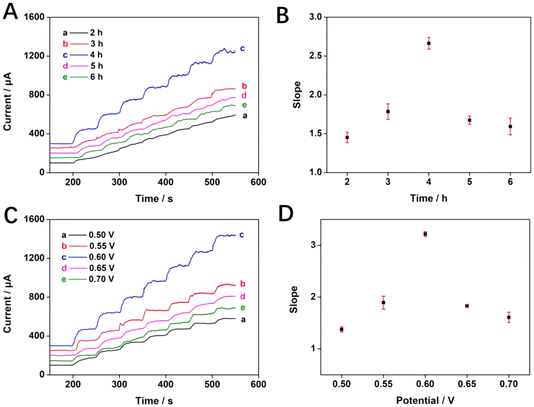
In addition, the calcination time of Mn-NiO/CFC also has a great influence on the electrochemical performance. If the calcination time is too short, the material cannot be fully formed because the particle size is too small, while the calcination time is too long, the particles overgrow and agglomeration occurs. Nonetheless, the optimal calcination time will result in the material with a right particle size and uniform dispersion. Fig. 4A is a graph showing the current–time curve of electrode materials prepared with calcination time periods of 2, 3, 4, 5, and 6 h in 10 mL 100 mM NaOH solution with continuous dripping of glucose. It could be observed that each curve rose step-wise with the continuous dripping of glucose. Especially, the slope of the c curve with a calcination time of 4 h is obviously the largest, and the upward trend is the most significant.In Fig. 4B, a correlation fit of the calcination time scatter for the electrode materials appeared rising and then decreasing for all scatters from 2 h to 6 h of calcination time, with 4 h being the highest value. Based on this, the prepared electrode has the strongest electrocatalytic ability for glucose oxidation under the calcination time of 4 h. In summary, the optimal experimental conditions for the preparation and testing of Mn-NiO/CFC are as follows: 100 mM of NaOH concentration, 0.6 V of working potential, and 4 h of the calcination time for preparing electrodes.
The amperometric method was adopted to investigate the electrocatalytic performance of Mn-NiO/CFC for glucose detection at working potentials of 0.5, 0.55, 0.6, 0.65 and 0.7 V. As shown in Fig. 4C, the current response of the curves increased rapidly with a step-like rise as 150 μM glucose was continuously added to 10 mL 100 mM NaOH solution every 50 s. In addition, the fitting scatter in Fig. 4D of the corresponding different electric potentials shows that the upward trend of the current response gradually increased when the working potential ranged from 0.5 V to 0.6 V. At a potential of 0.6 V, the maximum current response was achieved. Then, the current response height decreased at potentials of 0.65 V and 0.7 V. Therefore, 0.6 V was used as the optimal working potential of the Mn-NiO/CFC electrode to detect glucose.
3.4. Linear detection of Mn-NiO/CFC
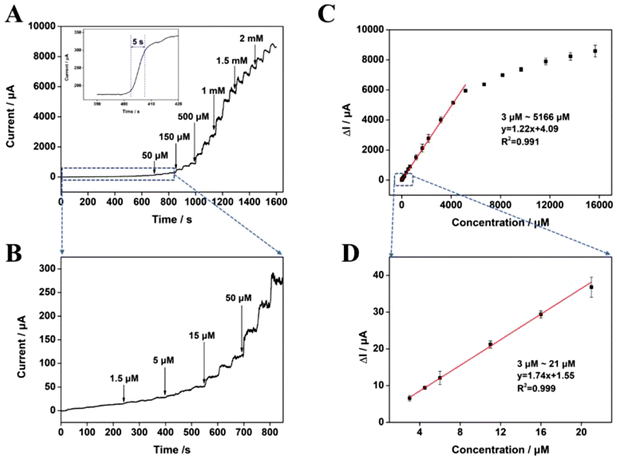
To further explore the electrochemical characteristics of Mn-NiO/CFC with a linear response to glucose, the current–time curves shown in Fig. 5A were obtained by continuously adding a series of glucose solutions with different concentrations to 10 mL 100 mM NaOH solution at an optimal working potential of 0.6 V with Mn-NiO/CFC calcined for 4 h as the working electrode. From the figure, it could be observed that the current response changes rapidly, showing a step-up pattern with the continuous addition of glucose. Additionally, as exhibited in Fig. 5A, the sensing electrode can achieve a stable current response state after the addition of glucose within 5 s, which proves its fast electrochemical response to glucose. Fig. 5B shows the current–time diagram of the electrocatalysis of the electrode at low glucose concentrations, in which the current response signal can be detected as low as 3 μM glucose concentration. Fig. 5C reveals the corresponding calibration curve between glucose concentrations and current density responses from Fig. 5A, which demonstrates a high linear correlation with the electrocatalysis of glucose oxidation of the electrode with the concentration ranging from 3 μM to 5166 μM. The linear equation is described as follows: I (μA) = 1.22CGlu (μM) + 4.09 (R2 = 0.991). A linear equation for low-concentration glucose (3–21 μM) was obtained from Fig. 5D, which could be described as: I (μA) = 1.74CGlu (μM) + 1.55 (R2 = 0.999). According to the equation, the LOD of the Mn-NiO/CFC was calculated to be 0.28 μM (S/N = 3).Furthermore, Table S1† lists the performance parameters of similar types of glucose-sensing electrodes based on nickel-based nanomaterials and conductive CFC that have been reported so far. Compared with other enzyme-free glucose-sensing electrodes constructed using other materials, the prepared Mn-NiO/CFC electrodes indicate better catalytic oxidation performance on glucose to some extent with the simple preparation method, wider detection range or lower detection limit.
3.5. Selectivity and reproducibility study of Mn-NiO/CFC
In the detection of practical samples, the selectivity and reproducibility are also important indicators in the evaluation of glucose electrochemical biosensors. Since many small biological molecules such as urea, AA, and DA coexist with glucose in the actual blood samples of human body (the ratio of glucose to its concentration is about 30/1), which will have a corresponding impact on the sensitive detection of glucose. The current–time curves for the response of the Mn-NiO/CFC electrode shown in Fig. S4A† were plotted after continuous addition of 500 μM glucose and 50 μM other interfering substances, including acetylsalicylic acid (ASA), Acetaminophen (AMP), NaCl and glycine (Gly), AA and DA to 10 mL 100 mM NaOH solution at the optimal working potential of 0.6 V, which was used to further investigate the catalytic oxidation performance of Mn-NiO/CFC toward glucose and its anti-interference ability toward other biological substances. As shown in Fig. S4A,† the response of the interfering substance is negligible and not significant compared to the height of the current response of glucose. Furthermore, a large current change could still occur rapidly with successive additions of glucose after dropping the interfering species into the solution. This indicates that the addition of interfering species had no significant effect on the electrochemical catalytic oxidation of glucose, suggesting the favorable selectivity of the prepared electrode to glucose detection. These analytical features, such as the oxidation of glucose by electrochemically formed Ni3+ in Mn-NiO/CFC, can be attributed to the increased active sites as well as the molecular sieve effect of Mn-NiO/CFC, which prevented the diffusion of interferents into the active sites.The reproducibility of these sensors was confirmed using amperometry with five Mn-NiO/CFC electrodes, which were prepared in the same way. Fig. S4B† shows that the current responses of the five electrodes prepared under the same experimental conditions to the same amount of glucose (150 μM) were highly overlapping, and the relative standard deviation (RSD) of the current response density was calculated, as small as 2.1%. At the same time, the current responses of five Mn-NiO/CFC electrodes were measured in 100 mM NaOH solution with continuous addition of glucose solutions of the same concentration for three times in parallel, respectively. After comparison, a high degree of overlap was found for each electrode in the current response of the three tests under the same conditions, demonstrating the excellent reproducibility of Mn-NiO/CFC.
3.6. Practical sample analysis of Mn-NiO/CFC
The quantitative determination of glucose in human serum, tea and fermentation broth by the amperometric method was used to evaluate the feasibility of applying Mn-NiO/CFC to glucose in actual samples. At a working potential of 0.6 V, the height difference of current response of the sample was measured by adding different concentrations of glucose into 10 mL 100 mM NaOH solution. Then, the corresponding glucose content was calculated according to the linear fitting equation of the current response value measured by the electrode and the glucose concentrations, and the content was compared with the test results of the commercial blood glucose meter, as shown in Table 1. It shows that the recoveries of three human serum samples by the sensor platform were high, in the range of 98.3%–100.5%.| Samples | Reference concentration (mM) | Experimental results (mM) | Blood glucose meter test results (mM) | Recovery (%) |
|---|---|---|---|---|
| 1 | 5.60 | 5.63 | 5.7 | 100.5% |
| 2 | 4.90 | 4.85 | 4.7 | 99.0% |
| 3 | 4.20 | 4.13 | 4.2 | 98.3% |
| Tea1 | 1.00 | 0.99 | 1.13 | 99.7% |
| Tea2 | 2.00 | 2.03 | 2.09 | 102.5% |
| Tea3 | 3.00 | 2.94 | 3.12 | 98.0% |
Moreover, we also tested the glucose content of sorghum fermentation broth with different fermentation cycles using Mn-NiO/CFC sensors. Fig. S5† contains the measured results of samples taken every 15 days, and the measured results are exactly the same as the trend of glucose in conventional sorghum fermentation. This further supports the applicability and detection capability of the sensor. The results indicate that Mn-NiO/CFC has high reliability and accuracy, and has a broad application prospect in glucose detection.
4. Conclusions
We have first grown Mn-NiO nano-bionic enzymes on a piece of 3D flexible CFC and detected glucose with high efficiency. Compared with NiO, Mn-NiO shows a better catalytic oxidation capacity for glucose, which is mainly owing to the combined effect of Mn doping, that changes the electronic structure of NiO, and the sufficient specific surface area provided by the 3D flexible CFC. Furthermore, the biomimetic enzyme sensor constructed from Mn-NiO nanomaterials displays rapid responses toward a series of concentration gradient glucose solutions with a fast response time less than 5 s, wide linear range (3–5166 μM) and a low LOD of 0.28 μM.The Mn-NiO/CFC electrodes display no obvious current response to the interfering substances, AA, UA, Fru, Suc, urea and DA, in the detection of glucose. The small RSD of the current–time curve obtained from the five electrodes in the parallel test (three repetitions per electrode) indicates that the electrodes have great selectivity and reproducibility. In addition, the detection results of glucose in human serum and tea by Mn-NiO/CFC electrodes were consistent with the detection results of commercial glucose meters, indicating that Mn-NiO/CFC has certain practical application potential in glucose detection systems.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81772290), Chongqing science and technology commission (cstc2021jcyj-msxmX0608), Graduate Scientific Research and Innovation Foundation of Chongqing, China (CYS22130), Sichuan Science and Technology Program (21ZYZFZDYF0019), Brew Microorganisms Technology and Application of Key Laboratory Project in Sichuan Province (NJ2020-03), Chongqing Graduate Tutor Team Construction Project and Analytical and Testing Center of Chongqing University for (SEM/XRD/XPS) and the sharing fund of Chongqing University's large equipment.References
- M. Adeel, M. M. Rahman, I. Caligiuri, V. Canzonieri, F. Rizzolio and S. Daniele, Biosens. Bioelectron., 2020, 165, 112331 CrossRef CAS PubMed.
- M. Adeel, K. Asif, M. M. Rahman, S. Daniele, V. Canzonieri and F. Rizzolio, Adv. Funct. Mater., 2021, 31, 2106023 CrossRef CAS.
- E. W. Gregg, N. Sattar and M. K. Ali, Lancet Diabetes Endocrinol., 2016, 4, 537–547 CrossRef PubMed.
- D. Ohayon, G. Nikiforidis, A. Savva, A. Giugni, S. Wustoni, T. Palanisamy, X. X. Chen, I. P. Maria, E. Di Fabrizio, P. M. F. J. Costa, I. McCulloch and S. Inal, Nat. Mater., 2020, 19, 456–463 CrossRef CAS PubMed.
- L. E. Graves and K. C. Donaghue, Ther. Adv. Endocrinol. Metab., 2019, 10, 1–12 Search PubMed.
- L. Shabnam, S. N. Faisal, A. K. Roy, E. Haque, A. I. Minett and V. G. Gomes, Food Chem., 2017, 221, 751–759 CrossRef CAS PubMed.
- E. V. Karpova and A. A. Karyakin, Curr. Opin. Electrochem., 2020, 23, 16–20 CrossRef CAS.
- J. Gao, K. Shang, Y. Ding and Z. Wen, J. Mater. Chem. A, 2021, 9, 8950–8965 RSC.
- B. Peng, F. Zhao, J. Ping and Y. Ying, Small, 2020, 16, 2002681 CrossRef CAS PubMed.
- J. Kim, M. Kim, M. S. Lee, K. Kim, S. Ji, Y. T. Kim, J. Park, K. Na, K. H. Bae, H. K. Kim, F. Bien, C. Y. Lee and J. U. Park, Nat. Commun., 2017, 8, 14997 CrossRef PubMed.
- M. M. Pribil, G. U. Laptev, E. E. Karyakina and A. A. Karyakin, Anal. Chem., 2014, 86, 5215–5219 CrossRef CAS PubMed.
- A. M. Nightingale, C. L. Leong, R. A. Burnish, S. U. Hassan, Y. Zhang, G. F. Clough, M. G. Boutelle, D. Voegeli and X. Niu, Nat. Commun., 2019, 10, 2741 CrossRef PubMed.
- C. Y. Cao, Q. H. Chang, H. J. Qiao, R. Z. Shao, X. Guo, G. N. Xiao, W. Z. Shi and L. Huang, Sens. Actuators, B, 2021, 340, 129943 CrossRef CAS.
- Y. Yao, J. Y. Chen, Y. H. Guo, T. Lv, Z. L. Chen, N. Li, S. K. Cao, B. D. Chen and T. Chen, Biosens. Bioelectron., 2021, 179, 113078 CrossRef CAS PubMed.
- M. Jian, C. Wang, Q. Wang, H. Wang, K. Xia, Z. Yin, M. Zhang, X. Liang and Y. Zhang, Sci. China Mater., 2017, 60, 1026–1062 CrossRef CAS.
- C. Wang, K. Xia, H. Wang, X. Liang, Z. Yin and Y. Zhang, Adv. Mater., 2019, 31, SI Search PubMed.
- A. Mishra, N. P. Shetti, S. Basu, K. R. Reddy and T. M. Aminabhavi, ChemElectroChem, 2019, 6, 5771–5786 CrossRef CAS.
- Y.-Y. Horng, Y.-K. Hsu, A. Ganguly, C.-C. Chen, L.-C. Chen and K.-H. Chen, Electrochem. Commun., 2009, 11, 850–853 CrossRef CAS.
- F. Li, Y.-L. Liu, G.-G. Wang, D. Yan, G.-Z. Li, H.-X. Zhao, H.-Y. Zhang and H.-Y. Yang, J. Mater. Chem. A, 2021, 9, 9675–9684 RSC.
- J. Zhan, X. Cao, J. Zhou, G. Xu, B. Lei and M. Wu, Chem. Eng. J., 2021, 416, 128943 CrossRef CAS.
- M. Adeel, M. M. Rahman, I. Caligiuri, V. Canzonieri, F. Rizzolio and S. Daniele, Biosens. Bioelectron., 2020, 165, 112331 CrossRef CAS PubMed.
- Z. Wang, R. Zhang, X. Yan and K. Fan, Mater. Today, 2020, 41, 81–119 CrossRef CAS.
- P. V. V. Romanholo, C. A. Razzino, P. A. Raymundo-Pereira, T. M. Prado, S. A. S. Machado and L. F. Sgobbi, Biosens. Bioelectron., 2021, 185, 113242 CrossRef CAS PubMed.
- D. K. Pathak, A. Chaudhary, M. Tanwar, U. K. Goutam, P. Mondal and R. Kumar, ACS Appl. Nano Mater., 2021, 4, 2143–2152 CrossRef CAS.
- C. T. Lo, Y. S. Wu, S. M. Huang, P. J. Tsai and C. L. Lee, Food Chem, 2022, 383, 132383 CrossRef CAS PubMed.
- S. Wang, C. Wang, G. Wei, H. Xiao, N. An, Y. Zhou, C. An and J. Zhang, Colloids Surf., A, 2016, 509, 252–258 CrossRef CAS.
- G. Zeng, W. Li, S. Ci, J. Jia and Z. Wen, Sci. Rep., 2016, 6, 36454 CrossRef CAS PubMed.
- H. Yin, J. Zhu, J. Chen, J. Gong and Q. Nie, Mater. Lett., 2018, 221, 267–270 CrossRef CAS.
- Y.-L. T. Ngo, L. T. Hoa, J. S. Chung and S. H. Hur, J. Alloys Compd., 2017, 712, 742–751 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2an01495e |
| ‡ Huisi Yang, Yian Hu and Xinxue Yin contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |