Stereoselective synthesis of 1,6-diazecanes by a tandem aza-Prins type dimerization and cyclization process†
Gyeong Un
Kim
 ab,
Hyunmi
Cho
ac,
Jae Kyun
Lee
a,
Jae Yeol
Lee
b,
Jinsung
Tae
c,
Sun-Joon
Min
ab,
Hyunmi
Cho
ac,
Jae Kyun
Lee
a,
Jae Yeol
Lee
b,
Jinsung
Tae
c,
Sun-Joon
Min
 *d,
Taek
Kang
*d,
Taek
Kang
 *a and
Yong Seo
Cho
*a
*a and
Yong Seo
Cho
*a
aBrain Science Institute, Korea Institute of Science and Technology (KIST), Seoul 02792, Republic of Korea. E-mail: tkang@kist.re.kr; ys4049@kist.re.kr
bDepartment of Chemistry, Kyung Hee University, Seoul 02447, Republic of Korea
cDepartment of Chemistry, Yonsei University, Seoul 03722, Republic of Korea
dDepartment of Chemical & Molecular Engineering/Applied Chemistry, Center for Bionano Intelligence Education and Research, Hanyang University, Ansan 15588, Republic of Korea. E-mail: sjmin@hanyang.ac.kr
First published on 30th November 2022
Abstract
We report the stereocontrolled synthesis of 1,6-diazecanes via a tandem aza-Prins type reaction of N-acyliminium ions with allylsilanes. It involves an aza-Prins type dimerization and cyclization in a single-step operation. This reaction represents the first example of 10-membered N-heterocycle synthesis using an aza-Prins reaction. Also, the interesting formation of an unusual tetracyclic compound through further cyclization of 1,6-diazecane and bicyclic compounds by the intramolecular cyclization of linear allylsilane are described. This tandem aza-Prins protocol provides a new synthetic strategy for the direct synthesis of medium-sized nitrogen heterocycles.
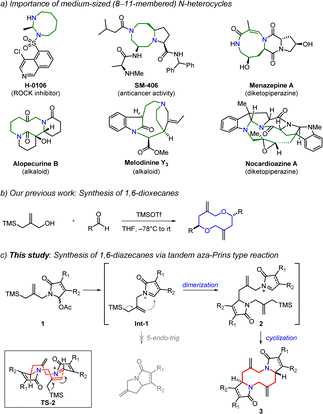
Medium-sized (8–11-membered) heterocycles1 are widely found in medicinally important molecules and natural products (Scheme 1a).2 The unique 3D spatial shapes and conformational constraints of medium-sized rings provide improved biological properties.3 However, medium-sized ring systems are hard to find in drug molecules and compound libraries,4 probably due to their synthetic challenge.5 The synthesis of medium-sized rings via cyclization is difficult because of transannular interactions and entropic factors.6 Although there are diverse approaches for the synthesis of medium-sized rings,7 the development of new strategies is still in high demand to investigate underexplored chemical space.
Aza-Prins cyclization is a powerful strategy to generate a wide range of N-heterocycles through intramolecular trapping of the in situ formed iminium or N-acyliminium ion intermediates.8,9 Aza-Prins cyclization has been broadly utilized to form 5- and 6-membered rings such as pyrrolidines and piperidines.10 A number of complex natural products have also been synthesized using this method.11 Nevertheless, there are very scarce examples to form larger (e.g., 7- and 8-membered) rings through this reaction.12 Herein, we describe the tandem aza-Prins type dimerization and cyclization strategy for the stereoselective synthesis of 1,6-diazecanes (Scheme 1c). Notably, the present protocol is the first example of a 10-membered N-heterocycle synthesis using an aza-Prins reaction.
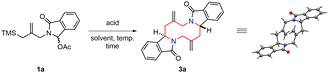
Previously, we reported the synthesis of 1,6-dioxecanes via a Prins type cyclization of allylsilane with an aromatic aldehyde (Scheme 1b).13 It involves an inter- and intramolecular Prins type reaction in a single-step operation. On the basis of this study, we envisioned a new synthetic strategy to form a 1,6-diazecane ring system using an aza-Prins type reaction. An N-acyliminium ion intermediate 2 would be generated by an intermolecular aza-Prins type reaction between an in situ formed N-acyliminium ion and an allylsilane since intramolecular 5-endo-trig cyclization of Int-1 is stereoelectronically unfavourable. Next, the subsequent aza-Prins type intramolecular cyclization of 2 would occur to provide the desired 1,6-diazecane 3. In this process, 2,7-trans stereochemistry would be expected if the cyclization reaction takes place through the transition state TS-2.
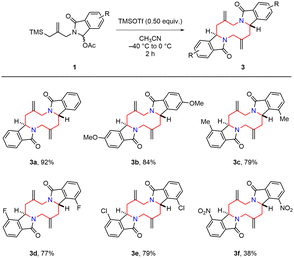
In order to explore the tandem aza-Prins type reaction, the N-acyliminium ion precursor 1a was chosen as a model substrate to perform the reaction under various conditions (Table 1). Among the various acids screened (entries 1–4), only TMSOTf gave the desired 1,6-diazecane product 3a in 67% yield. Other acids such as TfOH, Bi(OTf)3 and TiCl4 failed to give the product. We next examined the effect of solvent on the reaction (entries 4–7). The desired product was obtained in lower yield when we used diethyl ether (entry 5). In the case of THF, the reaction mixture became viscous probably due to cationic polymerization of THF (entry 6).14 We observed the desired product and polyTHF in an aliquot of the reaction mixture by NMR, but we could not isolate the product from the viscous reaction mixture. The yield of 3a was improved when the reaction was carried out in acetonitrile (entry 7). A further increased yield was observed by modifying the reaction time and temperature (entry 8). To our delight, decreasing the amount of TMSOTf to 0.50 equiv. remarkably enhanced the yield to 92% (entry 9). Further decreasing the amount to 0.20 equiv. also led to the desired product albeit in slightly lower yield (entry 10, 83%). Interestingly, TfOH (0.50 equiv.) and Bi(OTf)3 (0.50 equiv.) were also affective when we used acetonitrile as a solvent (entries 11 and 12), although no desired product was observed in dichloromethane. It suggests that the use of acetonitrile as a solvent is crucial for the successful transformation. These results may be explained by the acetonitrile influence on the iminium cation separation from the ion pair15 and stabilization. It was noteworthy that only a single diastereomer of 3a was observed in all cases through the transition state TS-2 in Scheme 1. The structure and stereochemistry of the product (3a) was unambiguously determined by X-ray crystallographic analysis.
| Entry | Acid (equiv.) | Solvent | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reactions performed with substrate (0.60 mmol) and acid in solvent (6.0 mL) at the indicated temperature for the indicated time. the temperature was slowly elevated over the indicated time. b Isolated yield. c The reaction mixture became viscous; ND = not detected. | |||||
| 1 | TfOH (1.1) | CH2Cl2 | −78 to rt | 6 | ND |
| 2 | Bi(OTf)3 (1.1) | CH2Cl2 | −78 to rt | 6 | ND |
| 3 | TiCl4 (1.1) | CH2Cl2 | −78 to rt | 6 | ND |
| 4 | TMSOTf (1.1) | CH2Cl2 | −78 to rt | 6 | 67 |
| 5 | TMSOTf (1.1) | Et2O | −78 to rt | 6 | 47 |
| 6 | TMSOTf (1.1) | THF | −78 to rt | 6 | —c |
| 7 | TMSOTf (1.1) | CH3CN | −40 to rt | 6 | 78 |
| 8 | TMSOTf (1.1) | CH3CN | −40 to 0 | 2 | 81 |
| 9 | TMSOTf (0.50) | CH3CN | −40 to 0 | 2 | 92 |
| 10 | TMSOTf (0.20) | CH3CN | −40 to 0 | 2 | 83 |
| 11 | TfOH (0.50) | CH3CN | −40 to 0 | 2 | 81 |
| 12 | Bi(OTf)3 (0.50) | CH3CN | −40 to 0 | 2 | 42 |
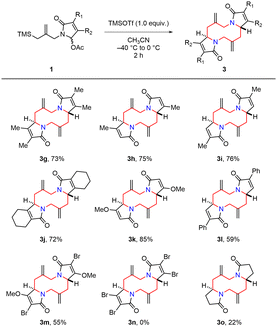
With the optimized conditions in hand (Table 1, entry 9), we first investigated the substrate scope of N-acyliminium ion precursors synthesized from phthalimide derivatives (Scheme 2). Substrates having electron-donating groups, such as methyl and methoxy groups, smoothly reacted under the optimized conditions to afford 1,6-diazecanes 3b (84%) and 3c (79%). Halogen-substituted substrates were also applied to the present procedure to give the products 3d (77%) and 3e (79%). However, a low yield (3f, 38%) was observed when an electron-deficient group substituted substrate was used. These results indicated that electron-donating substituents (3b and 3c) were more suitable for this reaction than electron-withdrawing substituent (3f), probably related to the stability of the highly reactive N-acyliminium ion intermediate.
To expand the scope of the tandem aza-Prins type reaction, we next explored the N-acyliminium ion precursors derived from pyrrole-2,5-dione derivatives (Scheme 3). Unlike substrates 1a–1f derived from phthalimide derivatives, we often encountered low reproducibility with substrates 1g–1o under the optimized reaction conditions. We used 1.0 equiv. of TMSOTf for these substrate 1g–1o because reproducible results were obtained when we simply used an increased amount of acid. Alkyl group substituted substrates 1g–1j produced the corresponding 1,6-diazecanes 3g–3j in good yields (72–76%) regardless of the substitution patterns. Substrate 1k bearing an electron-donating methoxy group at R2 gave 3k in higher yield (85%). When electron-withdrawing phenyl or bromide groups were substituted at R1, the reactions suffered from relatively lower yields (3l and 3m) even in the presence of the methoxy group at R2 (3m). Furthermore, the dihalogen-substituted substrate 1n failed to give the product. The desired 1,6-diazecane 3n was not observed in the complex crude mixture. In the case of substrate 1o prepared from pyrrolidine-2,5-dione, the yield of 1,6-diazecane 3o was only 22%. It implies that the conjugated olefin system stabilizes the highly reactive N-acyliminium ion intermediate during the reaction.
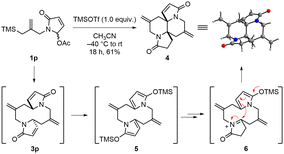
In the course of investigating the substrate scope, we also tested substrate 1p prepared from pyrrole-2,5-dione (Scheme 4). We could not observe the desired 1,6-diazecane 3p under the optimized conditions. However, very interestingly, we obtained an unusual tetracyclic compound 4 in 61% yield instead of 3p under modified reaction conditions. The structure of tetracyclic product 4 was clearly confirmed by X-ray crystallographic analysis. The substrate 1p would be first converted to the corresponding 1,6-diazecane 3p, and then 2-silyloxy dienes 5 could be formed from 3p under the same conditions. It is well known that pyrrole-based 2-silyloxy diene can be generated from a 1,5-dihydropyrrol-2-one moiety in the presence of silyl triflate. The 2-silyloxy diene can act as either a nucleophile or an electrophile to functionalize the 5-position of 1,5-dihydropyrrol-2-one.16 Based on this aspect, one of the 2-silyloxy dienes of 5 would generate the N-acyliminium ion intermediate 6 which could be trapped by the other 2-silyloxy diene intramolecularly to form the tetracyclic compound 4.
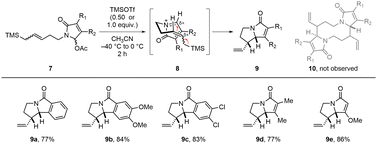
To test the scope of allylsilane, we tried the tandem aza-Prins type reaction with substrates 7a–7e having linear allylsilane (Scheme 5). We obtained intramolecularly cyclized products 9a–9e instead of desired 1,6-diazecanes 10. The stereoselectivity of 9 regardless of double bond configuration can be explained by electrophilic addition mechanism and stereoelectronic effect in transition state 8.17 This results indicated that 5-endo-trig cyclization is faster than dimerization in the case of the linear allylsilane substrate 7.
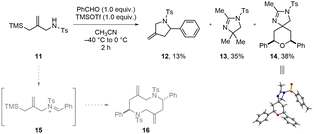
Next, the tandem aza-Prins type reaction of the acyclic iminium ion 15 generated in situ from amine 11 and benzaldehyde was examined (Scheme 6). Unexpected intramolecularly cyclized product (12) and acetonitrile-involved products (13 and 14) were obtained. We also tested the reaction in dichloromethane to avoid a solvent-involved reaction, but we observed an unknown inseparable complex mixture containing the intramolecularly cyclized product 12. It showed that the in situ generated acyclic iminium ion 15 could not provide the desired 1,6-diazecane 16.
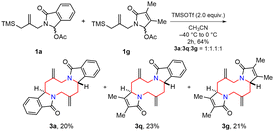
Lastly, a cross-over experiment was performed using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1a and 1g in the presence of 2.0 equiv. TMSOTf to check the mechanistic aspect of the tandem aza-Prins type reaction (Scheme 7). All three possible products (3a, 3q and 3g) were isolated separately in 64% total yield with 1
1 mixture of 1a and 1g in the presence of 2.0 equiv. TMSOTf to check the mechanistic aspect of the tandem aza-Prins type reaction (Scheme 7). All three possible products (3a, 3q and 3g) were isolated separately in 64% total yield with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The cross-over product (3q) was observed as we expected, supporting the intermolecular dimerization process. Also, the product ratio implied that substrates 1a and 1g have similar reactivity. Moreover, this cross-over protocol could be used as an efficient tool for the rapid generation of symmetrical and unsymmetrical 1,6-diazecanes in a single-step process.
1 ratio. The cross-over product (3q) was observed as we expected, supporting the intermolecular dimerization process. Also, the product ratio implied that substrates 1a and 1g have similar reactivity. Moreover, this cross-over protocol could be used as an efficient tool for the rapid generation of symmetrical and unsymmetrical 1,6-diazecanes in a single-step process.
In conclusion, we have described the stereoselective synthesis of 1,6-diazecanes via a tandem aza-Prins type dimerization and cyclization reaction of N-acyliminium ions with allylsilanes. This is the first method to generate 10-membered azacycles using an aza-Prins reaction and can serve as a tool for the synthesis of complex N-heterocycles. In addition, our study showed the formation of an unusual tetracyclic compound through further cyclization of 1,6-diazecane and bicyclic compounds by intramolecular cyclization of a linear allylsilane. Further investigations towards the Prins type cyclization for different kinds of medium-sized heterocycles are currently underway.
This study was supported by the Korea Institute of Science and Technology (KIST) Institutional Program (2E31512) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; NRF-2022M3E5F3085687 and NRF-2022R1A2C1005110).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) A. Hussain, S. K. Yousuf and D. Mukherjee, RSC Adv., 2014, 4, 43241–43257 RSC; (b) P. A. Evans and A. B. Holmes, Tetrahedron, 1991, 47, 9131–9166 CrossRef CAS.
- (a) K. Sumi, Y. Inoue, M. Nishio, Y. Naito, T. Hosoya, M. Suzuki and H. Hidaka, Bioorg. Med. Chem. Lett., 2014, 24, 831–834 CrossRef CAS PubMed; (b) Q. Cai, H. Sun, Y. Peng, J. Lu, Z. Nikolovska-Coleska, D. McEachern, L. Liu, S. Qiu, C.-Y. Yang, R. Miller, H. Yi, T. Zhang, D. Sun, S. Kang, M. Guo, L. Leopold, D. Yang and S. Wang, J. Med. Chem., 2011, 54, 2714–2726 CrossRef CAS PubMed; (c) C. Gao, L. Lin, B. Long, Y. Chen, B. He, H. Sun and R. Huang, Nat. Prod. Res., 2014, 28, 473–476 CrossRef CAS PubMed; (d) Y.-B. Zhang, D. Luo, L. Yang, W. Cheng, L.-J. He, G.-K. Kuang, M.-M. Li, Y.-L. Li and G.-C. Wang, J. Nat. Prod., 2018, 81, 2259–2265 CrossRef CAS PubMed; (e) F.-L. Zhang, J. He, T. Feng and J.-K. Liu, RSC Adv., 2021, 11, 23–29 RSC; (f) R. Raju, A. M. Piggott, X. Huang and R. J. Capon, Org. Lett., 2011, 13, 2770–2773 CrossRef CAS PubMed.
- (a) J. Clayden, W. J. Moran, P. J. Edwards and S. R. LaPlante, Angew. Chem., Int. Ed., 2009, 48, 6398–6401 CrossRef CAS; (b) K. R. Romines, K. D. Watenpaugh, P. K. Tomich, W. J. Howe, J. K. Morris, K. D. Lovasz, A. M. Mulichak, B. C. Finze, J. C. Lynn, M.-M. Horng, F. J. Schwende, M. J. Ruwart, G. L. Zipp, K.-T. Chong, L. A. Dolak, L. N. Toth, G. M. Howard, B. D. Rush, K. F. Wilkinson, P. L. Possert, R. J. Dalga and R. R. Hinshaw, J. Med. Chem., 1995, 38, 1884–1891 CrossRef CAS PubMed; (c) D. F. Veber, S. R. Johnson, H.-Y. Cheng, B. R. Smith, K. W. Ward and K. D. Kopple, J. Med. Chem., 2002, 45, 2615–2623 CrossRef CAS PubMed; (d) T. Rezai, B. Yu, G. L. Millhauser, M. P. Jacobson and R. S. Lokey, J. Am. Chem. Soc., 2006, 128, 2510–2511 CrossRef CAS PubMed.
- (a) J. Shearer, J. L. Castro, A. D. G. Lawson, M. MacCoss and R. D. Taylor, J. Med. Chem., 2022, 65, 8699–8712 CrossRef CAS PubMed; (b) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed; (c) J. Shang, H. Sun, H. Liu, F. Chen, S. Tian, P. Pan, D. Li, D. Kong and T. Hou, J. Cheminf., 2017, 9, 25 CrossRef PubMed.
- (a) G. Illuminati and L. Mandolini, Acc. Chem. Res., 1981, 14, 95–102 CrossRef CAS; (b) H. Kurouchi and T. Ohwada, J. Org. Chem., 2020, 85, 876–901 CrossRef CAS.
- (a) F. C. Lightstone and T. C. Bruice, Bioorg. Chem., 1998, 26, 193–199 CrossRef CAS; (b) C. Galli and L. Mandolini, Eur. J. Org. Chem., 2000, 3117–3125 CrossRef CAS.
- For selected reviews: (a) G. A. Molander, Acc. Chem. Res., 1998, 31, 603–609 CrossRef CAS; (b) L. Yet, Tetrahedron, 1999, 55, 9349–9403 CrossRef CAS; (c) L. Yet, Chem. Rev., 2000, 100, 2963–3008 CrossRef CAS PubMed; (d) M. E. Maier, Angew. Chem., Int. Ed., 2000, 39, 2073–2077 CrossRef CAS; (e) I. Shiina, Chem. Rev., 2007, 107, 239–273 CrossRef CAS PubMed; (f) K. Prantz and J. Mulzer, Chem. Rev., 2019, 110, 3741–3766 CrossRef PubMed; (g) K. C. Majumdar, RSC Adv., 2011, 1, 1152–1170 RSC; (h) M. Choury, A. Basilio Lopes, G. Blond and M. Gulea, Molecules, 2020, 25, 3147–3174 CrossRef CAS PubMed; (i) A. K. Clarke and W. P. Unsworth, Chem. Sci., 2020, 11, 2876–2881 RSC; (j) R. L. Reyes, T. Iwai and M. Sawamura, Chem. Rev., 2021, 121, 8926–8947 CrossRef CAS PubMed.
- For selected reviews: (a) C. Olier, M. Kaafarani, S. Gastaldi and M. P. Bertrand, Tetrahedron, 2010, 66, 413–445 CrossRef CAS; (b) I. M. Pastor and M. Yus, Curr. Org. Chem., 2012, 16, 1277–1312 CrossRef CAS; (c) J. Royer, M. Bonin and L. Micouin, Chem. Rev., 2004, 104, 2311–2352 CrossRef CAS PubMed; (d) P. Wu and T. E. Nielsen, Chem. Rev., 2017, 117, 7811–7856 CrossRef CAS PubMed; (e) S. Abdul-Rashed, C. Holt and A. J. Frontier, Synthesis, 2020, 1991–2007 CAS.
- For selected recent reports: (a) S. Amemiya, S. Okemoto, A. Tsubouchi and A. Saito, Org. Biomol. Chem., 2021, 19, 2959–2967 RSC; (b) S. Biswas, B. Porashar, P. J. Arandhara and A. K. Saikia, Chem. Commun., 2021, 57, 11701–11704 RSC; (c) W. C. Jang, M. Jung and H. M. Ko, Org. Lett., 2021, 23, 1510–1515 CrossRef CAS PubMed; (d) J. J. Hernandez and A. J. Frontier, Org. Lett., 2021, 23, 1782–1786 CrossRef CAS PubMed; (e) Y. Jia, L. Li, L. Duan and Y.-M. Li, Appl. Organomet. Chem., 2020, 34, 5927–5940 Search PubMed; (f) N. Kobayashi, K. Kaneko, S. Amemiya, K. Noguchi, M. Yamanaka and A. Saito, Chem. Commun., 2019, 55, 8619–8622 RSC; (g) R. R. Mittapalli, S. J. J. Guesné, R. J. Parker, W. T. Klooster, S. J. Coles, J. Skidmore and A. P. Dobbs, Org. Lett., 2019, 21, 350–355 CrossRef CAS PubMed; (h) P. Gan, J. Pitzen, P. Qu and S. A. Snyder, J. Am. Chem. Soc., 2018, 140, 919–925 CrossRef CAS PubMed; (i) R. T. Sawant, M. Y. Stevens and L. R. Odell, Chem. Commun., 2017, 53, 2110–2113 RSC; (j) T. Katamura, T. Shimizu, Y. Mutoh and S. Saito, Org. Lett., 2017, 19, 266–269 CrossRef CAS PubMed; (k) A. Mahía, R. Badorrey, J. A. Gálvez and M. D. Díaz-de-Villegas, J. Org. Chem., 2017, 82, 8048–8057 CrossRef PubMed; (l) W. Lin, J. Cheng and S. Ma, Adv. Synth. Catal., 2016, 358, 1989–1999 CrossRef CAS; (m) D. Ma, Z. Zhong, Z. Liu, M. Zhang, S. Xu, D. Xu, D. Song, X. Xie and X. She, Org. Lett., 2016, 18, 4328–4331 CrossRef CAS PubMed; (n) V. Durel, C. Lalli, T. Roisnel and P. van de Weghe, J. Org. Chem., 2016, 81, 849–859 CrossRef CAS PubMed; (o) J. L. Nallasivam and R. A. Fernandes, Eur. J. Org. Chem., 2015, 2012–2022 CrossRef CAS; (p) O. E. Okoromoba, G. B. Hammond and B. Xu, Org. Lett., 2015, 17, 3975–3977 CrossRef CAS PubMed; (q) F. K. I. Chio, S. J. J. Guesné, L. Hassall, T. McGuire and A. P. Dobbs, J. Org. Chem., 2015, 80, 9868–9880 CrossRef CAS PubMed; (r) Y. Sun, P. Chen, D. Zhang, M. Baunach, C. Hertweck and A. Li, Angew. Chem., Int. Ed., 2014, 53, 9012–9016 CrossRef CAS PubMed.
- (a) L. Cuprova and A. P. Dobbs, Adv. Heterocycl. Chem., 2020, vol. 130, pp. 251–278 Search PubMed; (b) C. Díez-Poza, H. Barbero, A. Diez-Varga and A. Barbero, Prog. Heterocycl. Chem., 2018, vol. 30, pp. 13–41 Search PubMed.
- B. V. Subba Reddy, P. N. Nair, A. Antony, C. Lalli and R. Grée, Eur. J. Org. Chem., 2017, 1805–1819 CrossRef CAS.
- (a) A. Barbero, A. Diez-Varga, F. J. Pulido and A. González-Ortega, Org. Lett., 2016, 18, 1972–1975 CrossRef CAS PubMed; (b) V. Sinka, I. Fernández and J. I. Padrón, J. Org. Chem., 2022, 87, 11735–11742 CrossRef CAS PubMed; (c) P. A. Grieco and W. F. Fobare, Tetrahedron Lett., 1986, 27, 5067–5070 CrossRef CAS; (d) B. P. Wijnberg and W. N. Speckamp, Tetrahedron Lett., 1980, 21, 1987–1990 CrossRef CAS.
- P. R. Ullapu, S.-J. Min, S. N. Chavre, H. Choo, J. K. Lee, A. N. Pae, Y. Kim, M. H. Chang and Y. S. Cho, Angew. Chem., Int. Ed., 2009, 48, 2196–2200 CrossRef CAS PubMed.
- J. S. Hrkach and K. Matyjaszewski, J. Polym. Sci., Part A: Polym. Chem., 1995, 33, 285–298 CrossRef CAS.
- H. Mayr, A. R. Ofial, E.-U. Würthwein and N. C. Aust, J. Am. Chem. Soc., 1997, 119, 12727–12733 CrossRef CAS.
- (a) G. Rassu, F. Zanardi, L. Battistini and G. Casiraghi, Chem. Soc. Rev., 2000, 29, 109–118 RSC; (b) N. Langlois and P. K. Choudhury, Tetrahedron Lett., 1999, 40, 2525–2528 CrossRef CAS.
- H. Hiemstra, M. H. A. M. Sno, R. J. Vijn and W. N. Speckamp, J. Org. Chem., 1985, 50, 4014–4020 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2168732, 1847224, and 2220271. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc05133h |
| This journal is © The Royal Society of Chemistry 2023 |