Boosted photothermal hydrogenation of acetylene on an efficient Au–Fe alloy catalyst†
Yuheng
Zhou
 *a,
Xiaohui
Wang
a,
Xubo
Huang
b,
Hui
Deng
a,
Yuntao
Hu
c and
Linfang
Lu
*a,
Xiaohui
Wang
a,
Xubo
Huang
b,
Hui
Deng
a,
Yuntao
Hu
c and
Linfang
Lu
 d
d
aScience and technology research and development center, Sinopec Research Institute of Petroleum Engineering, Beijing, 100000, China. E-mail: tanxishichao@126.com
bKey Lab of Applied Chemistry of Zhejiang Province, Department of Chemistry, Zhejiang University, Hangzhou, 310027, China
cEnvironmental Genomics and Systems Biology, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
dCollege of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou 311121, Zhejiang, China
First published on 24th November 2022
Abstract
A chloroform-assisted method has been developed to prepare Au–Fe alloy catalysts under mild conditions, and they exhibit an excellent activity in the photothermal hydrogenation of acetylene. This research has creative value for the development of efficient heterogeneous catalysts in the photothermal catalysis field, which promotes industrial hydrogenation reactions.
1. Introduction
Ethylene is one of the most important commercial chemicals in the petrochemical industry and is mainly used as a polymer precursor.1 Besides this, it is also widely used as a value-added component in fossil energy and an intermediate in medicine.2 Ethylene is usually obtained by cracking petroleum hydrocarbons, which inevitably produces a small amount of acetylene as a by-product.3 However, residual acetylene in the ethylene gas stream will inactivate the ethylene polymerization catalyst.4 Therefore, the removal of residual acetylene from ethylene is an essential step in the production of polyethylene.5 Unfortunately, most metal catalysts usually have limited selectivity to alkenes and are typically easy to poison in long-term tests at a higher temperature.6 Optimizing the catalytic activity of transition metal catalysts for selective semi-hydrogenation of alkynes is a critical challenge in heterogeneous catalysis.Although the traditional selective hydrogenation of acetylene to ethylene can be realized by thermal catalysis, the secondary waste of energy in the heating process cannot be ignored.7 Using abundant and clean solar energy to drive selective hydrogenation may provide a more cost-effective and sustainable development strategy.8 Therefore, a great deal of research work is now devoted to the development of catalysts that can drive hydrogenation reactions under light.9,10 Despite many reports on photocatalytic acetylene hydrogenation recently, there is still room for improvement in its hydrogenation efficiency.11 A research study shows that photothermal catalysis caused by light irradiation can fully couple the advantages of high selectivity of photocatalysis and high efficiency of thermal catalysis to realize the high-efficiency selective hydrogenation of acetylene.12
As the most used catalysts for acetylene hydrogenation, although Pd catalysts exhibit good activity in the conversion of acetylene to ethylene, they are prone to excessive hydrogenation of ethylene to ethane.13 In contrast, gold catalysts have received new attention in acetylene hydrogenation due to their unique electronic structure and better selectivity. Moreover, the strong plasma effect on nano gold can produce strong photothermal catalytic activity, which has also been widely reported in photo and thermal catalysis.14,15 As active transition metals, iron and nickel have been widely reported to have a good performance in the field of photothermal catalytic selective hydrogenation.16,17 This inspired us to conduct a detailed study on the potential and feasibility of co-using Au and Fe/Ni catalysts to realize the selective hydrogenation of acetylene through photothermal catalysis.18
Based on these, one type of AuFe alloy catalyst loaded on carbon black has been prepared in the chloroform solution to overcome the traditional obstacle of the alloy formation. The research indicates that the Au–Fe/C system has excellent photothermal activity to enhance the semi-hydrogenation of acetylene. The largest conversion can be up to 98.4% with 97.5% selectivity at 403 K, which has never been reported before. In addition, green oil formation in the reaction is suppressed by the addition of Fe, contributing to the improved life of the catalyst. A series of characterization techniques are used to explore the unique structure of Au–Fe catalysts, which may be the origin of their dazzling performance. These results are beneficial for us to understand the reaction mechanism and to create more potential for the industrial application of Au-based catalysts.
2. Results and discussion
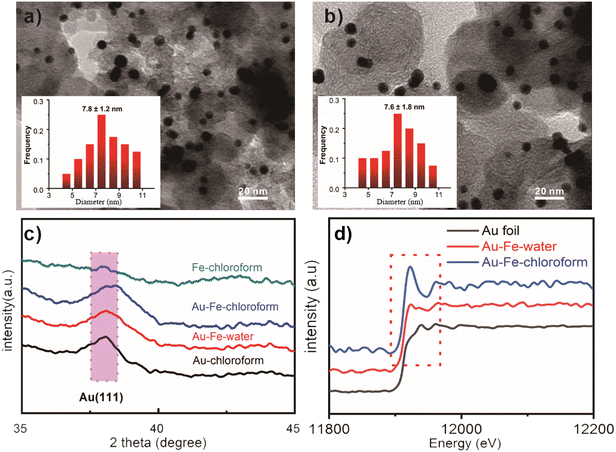
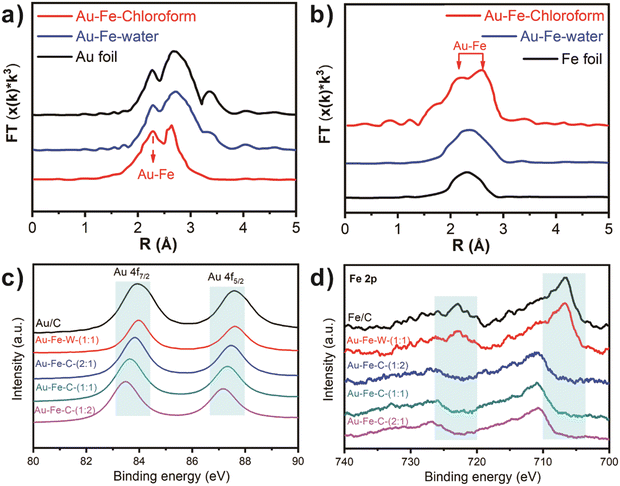
In the synthesis of Au–Fe–chloroform, chloroauric acid is used as the gold source, ferric chloride as the iron source, commercial carbon black as the carrier, and chloroform as the solvent (see the ESI†). For comparison, Au–Fe–water uses water as the solvent and iron nitrate as the iron source and is synthesized by a traditional impregnation method, according to the previous report.19Fig. 1a and b display the TEM photographs of these two catalysts. It can be seen that the nanoparticles are uniformly distributed on the carbon black carrier, and there are no obvious agglomeration and size change due to different synthesis methods (7.8 nm vs. 7.6 nm), which is in good agreement with the XRD patterns (Fig. 1c). Moreover, a typical diffraction peak shift can be found in the catalysts prepared in chloroform (Au–Fe–chloroform), which is obviously different from the main peak of the Au/C catalysts at 38.0°. However, no obvious shift has been observed in Au–Fe–water demonstrating that the gold species in Au–Fe–chloroform are not the same as those in Au–Fe–water. Due to weaker signals for Fe species, we have not found typical peaks for Fe or FeOx in the XRD patterns.The XANES spectra of Au L3-edge are employed to further study the state of the gold species (Fig. 1d). We can observe an obvious normalized white line (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925 eV) in Au–Fe–chloroform compared to other catalysts, indicating two different gold species between Au–Fe–water and Au–Fe–chloroform. Then, we perform EXAFS measurements to explore the detailed structures between Au and Fe, the results of which are shown in Fig. 2a and b. A standard Au foil sample is used as the reference. At 2.72 Å, both Au foil and Au–Fe–water exhibit an apparent peak, which corresponds to the existence of the Au–Au bond.14 Nevertheless, Au–Fe–chloroform has two main peaks at 2.29 Å and 2.68 Å, implying two different bonds in the Au coordination environment. In the EXAFS of Fe K-edge, typical Fe–Fe bonds at 2.33 Å can be discovered in both the Fe foil and Au–Fe–water but not in Au–Fe–chloroform.20 The split peaks at 2.27 Å and 2.60 Å coincide with the result in Au L3-edge, suggesting the formation of Au–Fe bonds.
925 eV) in Au–Fe–chloroform compared to other catalysts, indicating two different gold species between Au–Fe–water and Au–Fe–chloroform. Then, we perform EXAFS measurements to explore the detailed structures between Au and Fe, the results of which are shown in Fig. 2a and b. A standard Au foil sample is used as the reference. At 2.72 Å, both Au foil and Au–Fe–water exhibit an apparent peak, which corresponds to the existence of the Au–Au bond.14 Nevertheless, Au–Fe–chloroform has two main peaks at 2.29 Å and 2.68 Å, implying two different bonds in the Au coordination environment. In the EXAFS of Fe K-edge, typical Fe–Fe bonds at 2.33 Å can be discovered in both the Fe foil and Au–Fe–water but not in Au–Fe–chloroform.20 The split peaks at 2.27 Å and 2.60 Å coincide with the result in Au L3-edge, suggesting the formation of Au–Fe bonds.
Fig. 2c and d reveal the XPS results of both Au 4f and Fe 2p in different catalysts. There are two Au 4f peaks on both Au/C and Au–Fe–water, which correspond to Au 4f7/2 at 84.0 eV and Au 4f5/2 at 87.6 eV. In the Au–Fe–chloroform system, the position of peaks has a distinct change with maximum displacements up to 0.5 eV (Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe = 1
Fe = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). This implies that the existence of Fe has a significant influence on the electronic state of gold. Despite a weaker signal for Fe, it can also be distinguished that in Au–Fe–chloroform catalysts there are two peaks near 710.9 eV and 726.8 eV, corresponding to the signals of Fe 2p3/2 and Fe 2p1/2, which are not in accordance with the Fe0 peaks of Fe/C and Au–Fe–water near 706.7 eV and 722.9 eV. Moreover, according to the results of different mass ratios, similar shifts can also be found at ratios of 1
2). This implies that the existence of Fe has a significant influence on the electronic state of gold. Despite a weaker signal for Fe, it can also be distinguished that in Au–Fe–chloroform catalysts there are two peaks near 710.9 eV and 726.8 eV, corresponding to the signals of Fe 2p3/2 and Fe 2p1/2, which are not in accordance with the Fe0 peaks of Fe/C and Au–Fe–water near 706.7 eV and 722.9 eV. Moreover, according to the results of different mass ratios, similar shifts can also be found at ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, demonstrating strong interaction between Au and Fe in the chloroform system. Thus, it can be inferred that AuFe alloy (Au–Fe alloy) is formed on Au–Fe–chloroform while not in Au–Fe–water.
1, demonstrating strong interaction between Au and Fe in the chloroform system. Thus, it can be inferred that AuFe alloy (Au–Fe alloy) is formed on Au–Fe–chloroform while not in Au–Fe–water.
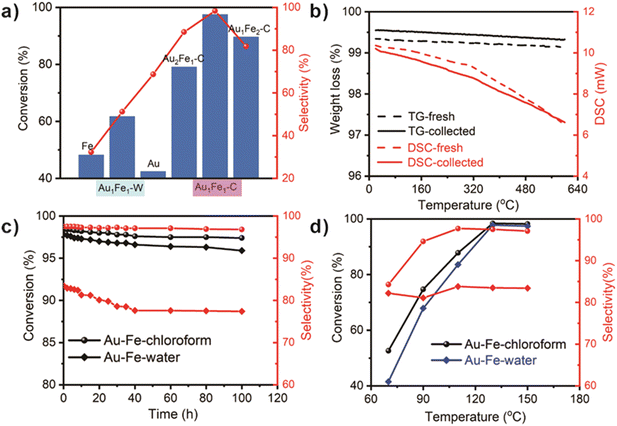
The conversions and selectivities of different catalysts at 220 °C are shown in Fig. 3a, of which the light intensity is at 450 mW cm−2 (see the document in the ESI†). Without the combination, both Au/C and Fe/C have both poor conversions (<50%) and selectivities (<40%). Obvious enhancement has occurred in Au–Fe systems. Compared with the 61.7% conversion and 51.2% selectivity in Au–Fe–water, Au–Fe–chloroform has a preferable activity when the mass ratio of Au and Fe is fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The largest conversion can be up to 98.5% with 97.4% selectivity, which could be attributed to the existence of Au–Fe bonds in Au–Fe–chloroform. Although other ratios in Au–Fe–chloroform also have better activities (>80%) than Au–Fe–water, they are not the optimal solution according to our research. Next, we mechanically mixed Au/C and Fe/C (1
1. The largest conversion can be up to 98.5% with 97.4% selectivity, which could be attributed to the existence of Au–Fe bonds in Au–Fe–chloroform. Although other ratios in Au–Fe–chloroform also have better activities (>80%) than Au–Fe–water, they are not the optimal solution according to our research. Next, we mechanically mixed Au/C and Fe/C (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and tested the performance of the mixed catalyst. After mixing, the effect of the catalyst has not been significantly improved, and the conversion and selectivity are between the two single catalysts (Fig. S1†). This result can roughly infer that the enhancement of activity in Au–Fe–water results from the interaction of gold and iron. However, since there is no formation of the alloy state (Au–Fe bond), its activity does not have a qualitative leap.
1) and tested the performance of the mixed catalyst. After mixing, the effect of the catalyst has not been significantly improved, and the conversion and selectivity are between the two single catalysts (Fig. S1†). This result can roughly infer that the enhancement of activity in Au–Fe–water results from the interaction of gold and iron. However, since there is no formation of the alloy state (Au–Fe bond), its activity does not have a qualitative leap.
Thermal analysis (TG-DSC) is used to detect the formation of multi-carbon products and green oils in the hydrogenation process (Fig. 3b). It turns out that the weight loss is less than 0.4%, and the heating process is a slightly exothermic process without obvious residual by-products, further demonstrating the perfect selectivity of our Au–Fe–chloroform catalyst and guaranteeing the carbon balance in the hydrogenation of acetylene. Then, we perform a 100 h durability test to investigate the stability of Au–Fe–chloroform (Fig. 3c). A 100 h-used catalyst still has an excellent conversion of over 97.5% with a stable selectivity of over 97%, implying the feasibility of Au–Fe–chloroform during a long operation.
Since temperatures have a great influence on the conversions and selectivities in heterogeneous catalysis, we made a serious investigation into the relationship between them. In Fig. 3d, it can be easily found that the conversion of Au–Fe–chloroform is 52.6% at 70 °C, and it can be greatly improved with the increase of temperatures below 130 °C. When the temperature is above 130 °C, the conversion stays at a level of more than 98% without any apparent change. From 70 °C to 110 °C, the selectivity also increases from 84.1% to 97.5% with a typical enhancement, while no further achievements can be obtained above 110 °C. 130 °C is quite an optimal temperature for Au–Fe–chloroform. By comparison, Au–Fe–water has a similar trend for the conversion within this range of temperatures, but the selectivity is still at a low level (82%) which does not have an immense change depending on temperature.
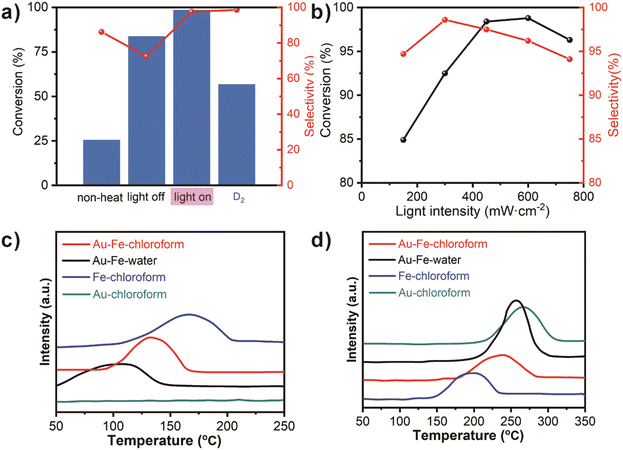
A light switch-controlled experiment is carried out to examine the function of visible light (Fig. 4a). Without heating, the catalyst only has a poor conversion (25.3%) with nearly 90% selectivity. However, when thermal catalysis is performed at 130 °C without any assistance of light, the conversion can be over 75% with a poor selectivity of less than 80%. Thus it can be inferred that the combination with visible light is greatly beneficial to improving the selectivity for achieving the best value of catalysts. When deuterated hydrogen is substituted for hydrogen, the conversion decreases to less than 60% due to a slight isotope effect, further confirming that H2 adsorption and H–H activation have a significant implication in the activity of C2H2 hydrogenation. However, the selectivity does not have any change from the influence of D2, implying that C2H4 adsorption is the key to accelerating selectivity indirectly.
An intensity-dependent experiment was carried out to check the relationship between light intensities and the corresponding catalytic parameters for Au–Fe–chloroform (Fig. 4b). With the increase of light intensity, the conversion has an obvious enhancement from 84.9% to 98.7% before 600 mW cm−2 and decreases subsequently. The selectivity has a similar pattern despite an early peak point at 300 mW cm−2 along with 98.6% selectivity. The experimental condition at 450 mW cm−2 chosen in our research is the optimal solution that considers both the activity and selectivity comprehensively.
With the help of the H2-temperature-programmed-desorption (H2-TPD) and C2H2-TPD profiles, the intensity of H2 and C2H2 desorption can be verified to evidence our deduction (Fig. 4c and d). The typical H2 desorption peak in Au–Fe–chloroform can be observed at 134 °C, which is much higher than 104 °C for Au–Fe–water, but lower than 168 °C for Fe–chloroform. Meanwhile, pure gold does not exhibit an obvious desorption peak when the temperatures are higher than 50 °C. Combined with the experimental results, it can be concluded that only appropriate H2 adsorption within a certain range is beneficial to the activity of catalysts. As for C2H2 adsorption, the typical adsorption peak for Au–Fe–chloroform occurs at 238 °C, which is much lower than 259 °C for Au–Fe–water and Au–chloroform. It further proves the deduction before that the lower adsorption for C2H2 contributes to the selectivity of this reaction, leading to better performance for Au–Fe–chloroform.
We have known that tuning H2 and C2H2 adsorption is important for enhancing both the conversion and selectivity of acetylene hydrogenation. The AuFe alloy can greatly improve these features according to our research, so it is possible to express an excellent activity in other alkynes. A series of alkynes are prepared on Au–Fe catalysts. As shown in Table S1,† despite its weaker conversions and selectivities compared to those of C2H2, Au–Fe–chloroform always exhibits a more excellent performance than Au–Fe–water, indicating that Au–Fe bonds which cause a typical alloy effect in alkyne hydrogenation is universal. Even 2-butylene has the least improvement in selectivity, and 92.1% selectivity can also be achieved using the Au–Fe alloy at 130 °C. We further investigated similar reports of gold-based catalysts in acetylene hydrogenation and listed the relevant parameters in Table S2.† Compared with others, the Au–Fe catalysts that we prepared have excellent performance under the synergistic action of the photothermal effect, with which both high conversion and high selectivity can be realized at a lower temperature.
Conclusions
On the whole, we have successfully synthesized AuFe alloy catalysts with the help of chloroform, following a simple step. The existence of the Au–Fe bond has been confirmed by EXAFS analysis, which exhibits a higher photothermal activity (98.4%) for semi-hydrogenation of acetylene compared to traditional gold catalysts. This unique structure also weakens the adsorption of ethylene and acetylene, which causes an outstanding selectivity (97.5%) to ethylene, as well as super durability during reaction at 130 °C, minimizing the side reaction of deep hydrogenation. This attempt to produce ethylene by photothermal hydrogenation of acetylene has encouraged the development of other photothermal catalyst systems for important industrial chemical conversion, which is an important milestone.Author contributions
Yuheng Zhou: methodology, writing – review & editing, funding acquisition, and supervision. Xiaohui Wang: methodology, data curation, and writing – original draft. Xubo Huang: data curation and writing – original draft. Hui Deng: funding acquisition and conceptualization. Yuntao Hu: characterization support and writing – review & editing. Linfang Lu: characterizations support.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was funded by the Science and Technology Project of China Petrochemical Corporation (P15163, P19029-5 and P19902) and the Zhejiang Provincial Natural Science Foundation of China (LQ20B030011).Notes and references
- Y. Gao, L. Neal, D. Ding, W. Wu, C. Baroi, A. M. Gaffney and F. Li, ACS Catal., 2019, 9, 8592–8621 CrossRef CAS.
- X. Wang, X. Zhang, R. Pandharkar, J. Lyu, D. Ray, Y. Yang, S. Kato, J. Liu, M. C. Wasson and T. Islamoglu, ACS Catal., 2020, 10, 8995–9005 CrossRef CAS.
- K. Bi, S. Zhang, C. Zhang, H. Li, X. Huang, H. Liu and T. Qiu, Chin. J. Chem. Eng., 2021, 38, 1–17 CrossRef.
- Y. Park, S. Lee, K. Hyun, J. Lee, J. Y. Park, R. Ryoo and M. Choi, J. Catal., 2021, 404, 716–725 CrossRef CAS.
- Z. Zhang, S. B. Peh, Y. Wang, C. Kang, W. Fan and D. Zhao, Angew. Chem., 2020, 132, 19089–19094 CrossRef.
- L. Lu, H. Zheng, Y. Li, Y. Zhou and B. Fang, Chem. Eng. J., 2023, 138668 CrossRef CAS.
- H. Jarimi, D. Aydin, Z. Yanan, G. Ozankaya, X. Chen and S. Riffat, Int. J. Low-Carbon Technol., 2019, 14, 44–69 CrossRef CAS.
- F. Arcudi, L. Đorđević, N. Schweitzer, S. I. Stupp and E. A. Weiss, Nat. Chem., 2022, 1–6 Search PubMed.
- J. Wang, M. Wang, X. Li, X. Gu, P. Kong, R. Wang, X. Ke, G. Yu and Z. Zheng, Appl. Catal., B, 2022, 313, 121449 CrossRef CAS.
- Q.-C. Wei, Y. Chen, Z. Wang, D.-Z. Yu, W.-H. Wang, J.-Q. Li, L.-H. Chen, Y. Li and B.-L. Su, Am. Ethnol., 2022, 1–14 Search PubMed.
- Y. Guo, Y. Huang, B. Zeng, B. Han, M. Akri, M. Shi, Y. Zhao, Q. Li, Y. Su and L. Li, Nat. Commun., 2022, 13, 1–12 Search PubMed.
- S. Zhou, L. Shang, Y. Zhao, R. Shi, G. I. Waterhouse, Y. C. Huang, L. Zheng and T. Zhang, Adv. Mater., 2019, 31, 1900509 CrossRef PubMed.
- W. Xie and P. Hu, Catal. Sci. Technol., 2021, 11, 5212–5222 RSC.
- Y. Zhou, Z. Wang, B. Ye, X. Huang and H. Deng, J. Catal., 2021, 400, 274–282 CrossRef CAS.
- H. Zhou, B. Li, Y. Zhang, X. Yan, W. Lv, X. Wang, B. Yuan, Y. Liu, Z. Yang and X. Lou, ACS Appl. Mater. Interfaces, 2021, 13, 40429–40440 CrossRef PubMed.
- M.-J. Cai, C.-R. Li and L. He, Rare Met., 2020, 39, 881–886 CrossRef CAS.
- Z. Li, J. Liu, R. Shi, G. I. Waterhouse, X. D. Wen and T. Zhang, Adv. Energy Mater., 2021, 11, 2002783 CrossRef CAS.
- Y. Wang, X.-H. Liu, R. Wang, B. Cula, Z.-N. Chen, Q. Chen, N. Koch and N. Pinna, J. Am. Chem. Soc., 2021, 143, 9595–9600 CrossRef CAS PubMed.
- M. Okumura and M. Haruta, Chem. Lett., 2000, 29, 396–397 CrossRef.
- Y. Pan, S. Liu, K. Sun, X. Chen, B. Wang, K. Wu, X. Cao, W. C. Cheong, R. Shen and A. Han, Angew. Chem., Int. Ed., 2018, 57, 8614–8618 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cy01500e |
| This journal is © The Royal Society of Chemistry 2023 |