Facile green synthesis of a dahlia-like anatase/TiO2(B) phase junction for boosting the photocatalytic degradation of persistent tetracycline†
Qian
Wang‡
*a,
Xuhua
Wang‡
a,
Pin
Hao
 a,
Xinxin
Fang
a,
Fang
Huang
a,
Xifeng
Shi
a,
Guanwei
Cui
a,
Chengyuan
Song
*b and
Bo
Tang
a,
Xinxin
Fang
a,
Fang
Huang
a,
Xifeng
Shi
a,
Guanwei
Cui
a,
Chengyuan
Song
*b and
Bo
Tang
 *a
*a
aCollege of Chemistry, Chemical Engineering and Materials Science, Institute of Materials and Clean Energy, Collaborative Innovation Center of Functionalized Probes for Chemical Imaging in Universities of Shandong, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Shandong Provincial Key Laboratory of Clean Production of Fine Chemicals, Shandong Normal University, Jinan 250014, P. R. China. E-mail: wangqian@sdnu.edu.cn; tangb@sdnu.edu.cn
bDepartment of Neurology, Qilu Hospital of Shandong University, Jinan 250012, P. R. China. E-mail: songchengyuan@qiluhospital.com
First published on 13th November 2022
Abstract
The simultaneous construction of a phase junction and hierarchical porous architecture can efficiently promote the separation and transfer of photogenerated carriers and then greatly improve the photocatalytic activity of nano-TiO2, while its facile and green preparation is still urgently needed. Herein, a novel dahlia-like TiO2 possessing both an anatase/TiO2(B) phase junction and high specific surface area of 342.7 m2 g−1 was successfully fabricated by a facile and green deep eutectic solvent-tuning strategy. The higher specific surface area results from its 3D hierarchical structure assembled by 2D ultrathin nanosheets with mesopores. Owing to the its structural features, the synthesized TiO2 showed a superior photocatalytic activity and favorable stability for persistent tetracycline degradation, compared with pure anatase TiO2, pure TiO2(B) and commercial P25. Based on the trapping experiments of active radical species, ESR spectra and experimental results, a reasonable photocatalytic mechanism of tetracycline degradation was proposed. These research results will provide an efficient strategy for the green preparation and practical application of multifunctional advanced catalysts.
1. Introduction
Tetracycline (TC), as a typical broad-spectrum antibiotic, has been widely used for preventing and treating bacterial infections in humans and animals.1,2 However, TC residues have been detected in various water bodies due to its heavy usage, low metabolism, poor biodegradation and high chemical stability, which has caused serious harm to human health and the ecological environment.3,4 Various technologies have been developed to eliminate TC. Among them, photocatalytic degradation is regarded as a highly effective and promising technology because of its simple operation, low cost, non-pollution, high efficiency and low energy consumption.5,6 The key to the practical application of this technology is the development of photocatalysts with high efficiency and good stability. Compared to other semiconductors, TiO2 has attracted great attention owing to its intrinsic advantages such as high stability, low cost and nontoxicity.7–9 Nevertheless, fast recombination of photogenerated carriers and low adsorptive capacities for antibiotics greatly limit the enhancement of its photocatalytic performance. Lots of studies have testified that fabricating a phase junction can effectively accelerate the separation of photogenerated carriers and that constructing a 3D hierarchical porous architecture can provide high surface area which not only facilitates antibiotic adsorption and diffusion, but also enables more active sites to participate in the degradation reaction.10,11 Therefore, the design and synthesis of a TiO2 phase junction with a hierarchical porous structure is expected to dramatically improve photocatalytic activity.TiO2 naturally occurs in four polymorphs: anatase, rutile, brookite and TiO2(B). Among these polymorphs, anatase usually possesses higher photocatalytic activity and TiO2(B) more easily forms hierarchical porous architectures originating from its unique layered structure with intrinsic open tunnels,12,13 which provides a good opportunity for the design and construction of an anatase/TiO2(B) phase junction with larger specific surface area. Unfortunately, TiO2(B), as a metastable polymorph, has been rarely studied due to its difficult preparation,14,15 which is not conducive to the development of TiO2(B)-based phase junctions. Besides, the existing synthesis strategies toward the TiO2(B)/anatase phase junction usually depend on high-temperature calcination, strong acids, strong alkalis and complex steps.16,17 As a consequence, it's urgent to explore a green, facile and general preparation strategy for a TiO2(B)/anatase phase junction with a hierarchical porous structure. Deep eutectic solvents (DESs), as a new class of low-cost, easily prepared, versatile and non-toxic solvents, have gotten more and more attention in the nanomaterial synthesis field.18–20 For example, Sandhu et al.21 used a choline chloride/hydroquinone DES as a templating and structure directing agent to synthesize anatase TiO2 with dominant {111} facets, which exhibited higher photocatalytic efficiency in dye degradation. In our previous studies, we found that TiO2 phase junctions can be easily fabricated by a green and mild DES-regulated strategy and the synthesized TiO2 displayed superior activities for photocatalytic water splitting and photocatalytic ofloxacin degradation.22–24 Based on the above advances and understanding, we proposed that both the anatase/TiO2(B) phase junction and hierarchical porous architecture can be simultaneously regulated by tailoring the DES structure to boost TC photocatalytic degradation.
In this work, a novel dahlia-like anatase/TiO2(B) phase junction of TiO2 with a large specific surface area (DABTO) was fabricated by a general, facile and green DES-tuning strategy (see Scheme 1). Then, its photocatalytic activity for TC degradation was evaluated. Finally, a possible photocatalytic mechanism was proposed based on the trapping experiments of active radical species, electron spin resonance (ESR) tests and experimental results. Moreover, DABTO also displays better photodegradation efficiencies for two other antibiotics containing chloramphenicol (CAP) and ciprofloxacin (CPFX).
2. Results and discussion
2.1 Synthesis and characterization
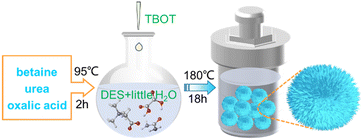
The synthesis strategy of DABTO is shown in Scheme 1. The DES betaine/urea/oxalic acid was firstly synthesized by mixing betaine, urea and oxalic acid in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio at 95 °C for 2 h, and its structure was identified by Fourier transform infrared (FT-IR) spectroscopy and DFT calculations. Then, DABTO was prepared by heating a mixture including tetrabutyl titanate, DES and little distilled water at 180 °C for 18 h, and its morphology and structure were identified by scanning electron microscopy (SEM), transmission electron microscopy (TEM), high resolution TEM (HRTEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), UV-vis diffuse reflectance spectroscopy (UV-DRS) and N2 adsorption–desorption isotherms.
1 molar ratio at 95 °C for 2 h, and its structure was identified by Fourier transform infrared (FT-IR) spectroscopy and DFT calculations. Then, DABTO was prepared by heating a mixture including tetrabutyl titanate, DES and little distilled water at 180 °C for 18 h, and its morphology and structure were identified by scanning electron microscopy (SEM), transmission electron microscopy (TEM), high resolution TEM (HRTEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), UV-vis diffuse reflectance spectroscopy (UV-DRS) and N2 adsorption–desorption isotherms.
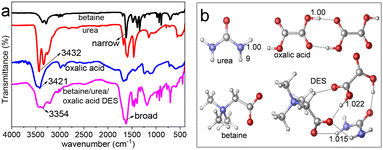
The FT-IR spectra in Fig. 1a not only displayed a clear broadening of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band from betaine in the DES compared to that in single betaine, but also exhibited two obvious red shifts, i.e. –NH2 vibration from 3432 cm−1 of pure urea to 3354 cm−1 of urea in DES, and –OH vibration from 3421 cm−1 of pure oxalic acid to 3354 cm−1 of oxalic acid in DES. These changes are mainly ascribed to the formation of H-bonds between H of –NH2 in urea and O of carboxylate (COO) in betaine, and between H of –OH in oxalic acid and O of COO in betaine.22,25 Moreover, Fig. 1b indicates the changes in bond lengths of major functional groups before and after forming the DES. The bond lengths of –NH of urea and –OH of oxalic acid in the DES both become longer (from 1.009 to 1.015 Å and from 1.000 to 1.022 Å, respectively), compared with those in the two raw materials urea and oxalic acid, which are in concordance with those presented in Fig. 1a. These illustrate that the DES is synthesized by H-bond interaction.
O band from betaine in the DES compared to that in single betaine, but also exhibited two obvious red shifts, i.e. –NH2 vibration from 3432 cm−1 of pure urea to 3354 cm−1 of urea in DES, and –OH vibration from 3421 cm−1 of pure oxalic acid to 3354 cm−1 of oxalic acid in DES. These changes are mainly ascribed to the formation of H-bonds between H of –NH2 in urea and O of carboxylate (COO) in betaine, and between H of –OH in oxalic acid and O of COO in betaine.22,25 Moreover, Fig. 1b indicates the changes in bond lengths of major functional groups before and after forming the DES. The bond lengths of –NH of urea and –OH of oxalic acid in the DES both become longer (from 1.009 to 1.015 Å and from 1.000 to 1.022 Å, respectively), compared with those in the two raw materials urea and oxalic acid, which are in concordance with those presented in Fig. 1a. These illustrate that the DES is synthesized by H-bond interaction.
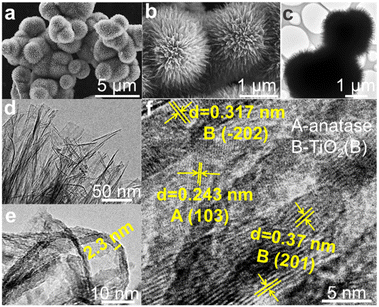
The SEM images in Fig. 2(a and b), TEM images in Fig. 2(c and d) and HRTEM image in Fig. 2e show that the synthesized DABTO has a dahlia-like morphology with a highly open 3D hierarchical structure assembled by about 2.3 nm-thick 2D ultrathin nanosheets. The HRTEM image of PHBATO in Fig. 2f displays that the lattice fringes of 0.317 and 0.37 nm correspond to the (−202) and (201) facets of TiO2(B) respectively, while the lattice fringe of 0.243 nm is indexed to the (103) facet of anatase. These indicate that there are two kinds of polymorphs in the dahlia-like morphology, which shows the successful fabrication of anatase/TiO2(B) phase junctions.
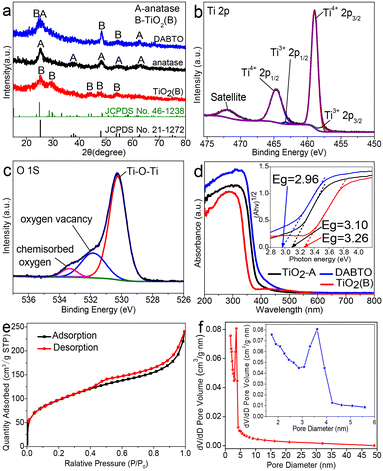
XRD patterns were recorded to identify the phase of the catalysts. As shown in Fig. 3a, the DABTO catalyst prepared by the tailored DES-tuning strategy consists of anatase and TiO2(B), which proves the successful construction of anatase/TiO2(B) phase junctions, together with SEM and HRTEM analysis. Additionally, to explain the role of the phase junction by comparing the photocatalytic activity of DABTO with that of the single phases, pure anatase and sole TiO2(B) were respectively prepared according to the preparation methods reported in studies and their phase structures were also identified by XRD tests.16,24
In their XRD patterns, there are only the diffraction peaks of the pure phase.
The surface chemical states of Ti and O in DABTO were verified by XPS. The Ti 2p spectrum in Fig. 3b demonstrates that there are two oxidation states of Ti. The two strong peaks at 464.7 eV and 459.0 eV are assigned to the Ti 2p1/2 and Ti 2p3/2 signals of Ti4+, while the other two peaks at 463.3 eV and 457.8 eV are derived from Ti3+.13,26 The O 1s spectrum in Fig. 3c can be deconvoluted into three peaks located at 530.3, 531.9 and 533.4 eV belonging to Ti–O, surface oxygen vacancies and chemisorbed oxygen, respectively.27,28
The light-absorbing capacity of the synthesized DABTO, anatase and TiO2(B) was tested by UV-DRS and their spectra are shown in Fig. 3d. It can be clearly seen that the three catalysts all demonstrate strong absorption in the UV region, and their band gap values were calculated by the Kubelka–Munk function to be 2.96, 3.10 and 3.26 eV, respectively (see inset in Fig. 3d). Obviously, DABTO possesses the best light-absorbing capacity because its band gap energy is the lowest among the three catalysts. This phenomenon can be attributed to the existence of Ti3+ and oxygen vacancies, which have been verified by XPS analysis.29
The Brunauer–Emmett–Teller (BET) surface area and pore size distribution of DABTO were measured using N2 adsorption–desorption isotherms. As shown in Fig. 3e, DABTO displays a characteristic type-IV isotherm with an obvious H3 hysteresis loop between the adsorption and desorption curves, indicating the existence of mesopores.30 BJH analysis as shown in Fig. 3f shows that the pore size in DABTO is mainly distributed in the mesoporous region and the average pore diameter is 3.68 nm (see inset in Fig. 3f), which confirms the mesoporous structure of DABTO. These mesopores mainly exist in the 2D ultrathin nanosheets because lots of small pores can be clearly observed from the HRTEM image in Fig. 2f. Furthermore, DABTO possesses a larger specific surface area of 342.7 m2 g−1, which is higher than those of an overwhelming majority of reported similar catalysts.12,29 It is well-known that the larger specific surface area and mesoporosity are helpful to improve photocatalytic activity.31,32
2.2 Evaluation of photocatalytic performance
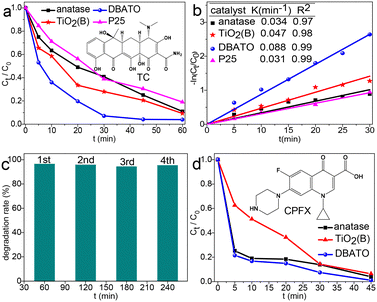
The photocatalytic performance of DABTO was investigated by degrading refractory TC in water. As shown in Fig. 4a, DABTO demonstrates a superior photocatalytic activity to those of anatase, TiO2(B) and commercial P25 under the same degradation conditions. The degradation rate of DABTO reached 92.9% at 30 min, which was almost completely degraded at 45 min. To further evaluate the photocatalytic properties of DABTO, linear fitting of the first-order kinetic equation ln(C0/C) = kt for these catalysts was performed.33Fig. 4b shows that all the linear correlation coefficients R2 were up to 0.97, which illustrated that they well fitted the first-order reaction kinetics. The kinetic constant K values of DABTO, anatase, TiO2(B) and commercial P25 are calculated as 0.088, 0.034, 0.047 and 0.031 min−1, respectively. The K value of DABTO is 2.59, 1.87 and 2.83 times higher than that of anatase, TiO2(B) and commercial P25, respectively. Then, the catalyst stability was examined. Fig. 4c displays that the degradation rate of DABTO for TC does not change obviously after four consecutive cycles. And the XRD patterns in Fig. S1 in the ESI† show that the location and the relative intensity of the characteristic peaks of reused DABTO are consistent with those of the fresh catalyst. These results clearly indicate that the synthesized DABTO catalyst possesses a favorable stability. Besides, in order to expand the application range of DABTO, its catalytic degradation activity for two other common antibiotics was explored. As shown in Fig. 4d and in Fig. S1 in the ESI,† compared with anatase and TiO2(B), DABTO displayed higher photocatalytic activity in the degradation reactions of both CPFX and CAP. These results are further evidence that DABTO prepared by the DES-tuning strategy possesses an excellent photocatalytic performance and shows a promising application prospect in the treatment of several antibiotics in water.2.3 Photoelectrochemical properties
To explore the origins of the improved photocatalytic performance, the photocurrent responses and electrochemical impedance spectroscopy (EIS) spectra were measured and the results are shown in Fig. 5a and b, respectively. It can be seen from Fig. 5a that DABTO displays a higher photocurrent density than pure phase TiO2. This means that DABTO has more efficient carrier separation by introducing the anatase/TiO2(B) phase junction.34–36 It can be seen from Fig. 5b that DABTO displays a smaller arc radius than the pure phase TiO2 samples. This indicates that DABTO has a faster charge transfer ability due to its highly open 3D hierarchical porous architecture assembled by 2D ultrathin nanosheets.37 Overall, just because of the simultaneous construction of a phase junction and hierarchical porous structure which benefitted the separation and migration of photogenerated carriers, DABTO displayed higher photocatalytic activity in the degradation reactions of multiple antibiotics.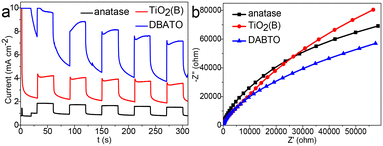 | ||
| Fig. 5 (a) Transient photocurrent responses and (b) EIS Nyquist plots of pure anatase, pure TiO2(B) and DABTO. | ||
2.4 Photocatalytic mechanism for TC degradation in the presence of DABTO
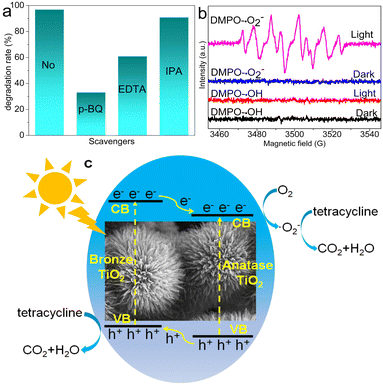
To better understand the photocatalytic mechanism of TC degradation in the presence of DABTO, trapping experiments of the active radical species were performed. p-BQ, EDTA-2Na and IPA were selected as scavengers for ˙O2−, h+ and ˙OH, respectively. As shown in Fig. 6a, the TC degradation rate decreased dramatically from 97% to 33% and 61% when p-BQ and EDTA-2Na were added into the catalytic system, while the degradation rate had no obvious change with IPA added, which illustrated that ˙O2− and h+ were the main active species in the degradation reaction of TC. To verify the generation of the active species in the degradation process, ESR spectra were determined and the results are displayed in Fig. 6b. No signal was detected in the dark. Six peaks for typical DMPO–˙O2− adducts were obviously observed from Fig. 6b under light illumination and the peak intensity is strong, which suggests the formation of lots of ˙O2− radicals.6,38 However, the characteristic peaks of DMPO–˙OH didn't appear, which indicates that ˙OH radicals were not involved in the degradation process. The ESR results are consistent with the trapping experiment results of the active radical species.Based on catalytic activity evaluation, trapping experiments and ESR tests, a possible photocatalytic mechanism was proposed and is shown in Fig. 6c. Under light irradiation, DABTO was excited to generate electron–hole pairs. The excited electrons migrated from the conduction band (CB) of the bronze phase to the CB of the anatase phase in DABTO, while the holes migrated from the valence band (VB) of the anatase phase to the VB of the bronze phase, which could form an internal electric field at the phase interface to promote the separation of electron–hole pairs. Then, the electrons in the CB of the anatase phase in DABTO activated O2 to generate ˙O2− radicals. Under the action of ˙O2− radicals and holes, TC was degraded into small molecules.5
3. Conclusions
In this work, a novel dahlia-like TiO2 photocatalyst possessing both an anatase/TiO2(B) phase junction and high surface area of 342.7 m2 g−1 was successfully fabricated by a general, green and in situ regulation strategy based on a tailored betaine/urea/oxalic acid DES. The 3D hierarchical structure was assembled by 2D ultrathin nanosheets with mesopores. The prepared DABTO showed a superior photocatalytic activity and favorable stability in TC degradation due to its qualities of phase junction structure, high specific surface area and better light-absorbing capacity, compared with the pure anatase phase, pure bronze phase and commercial P25. Based on the ESR spectra and experimental results, a reasonable photocatalytic mechanism of TC degradation using DABTO as the catalyst was proposed. Moreover, DABTO also displayed better photocatalytic activity for the degradation of other antibiotics containing CAP and CPFX. The study provides an efficient strategy for the green preparation and industrial application of multifunctional advanced catalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21927811, 21808129, 22076105, 91753111, 21976110), and the Key Research and Development Program of Shandong Provincial (2018YFJH0502).Notes and references
- H. R. Sun, F. Guo, J. J. Pan, W. Huang, K. Wang and W. L. Shi, Chem. Eng. J., 2021, 406, 126844 CrossRef CAS.
- D. Liu, H. J. Li, R. P. Gao, Q. A. Zhao, Z. Z. Yang, X. Gao, Z. Wang, F. Q. Zhang and W. D. Wu, J. Hazard. Mater., 2021, 406, 124309 CrossRef CAS PubMed.
- C. Hu, Z. T. Liu, K. Y. A. Lin, W. H. Wei and K. H. Wang, J. Ind. Eng. Chem., 2022, 107, 118 CrossRef CAS.
- J. J. Liu, H. He, Z. R. Shen, H. H. Wang and W. Z. Li, J. Hazard. Mater., 2022, 429, 128398 CrossRef CAS.
- S. Q. Wu, H. Y. Hu, Y. Lin, J. L. Zhang and Y. H. Hu, Chem. Eng. J., 2020, 382, 122842 CrossRef CAS.
- X. M. Zhang, H. Wang, M. M. Gao, P. F. Zhao, W. L. Xia, R. L. Yang, Y. C. Huang, L. Wang, M. X. Liu, T. Wei, L. Wang, R. X. Yao, X. Li and Z. J. L. Fan, Chemosphere, 2022, 294, 133782 CrossRef CAS PubMed.
- K. Pandi, M. Preeyanghaa, V. Vinesh, J. Madhavan and B. Neppolian, Environ. Res., 2022, 207, 112188 CrossRef CAS.
- T. H. Zhang, Y. J. Liu, Y. D. Rao, X. P. Li, D. L. Yuan, S. F. Tang and Q. X. Zhao, Chem. Eng. J., 2020, 384, 123350 CrossRef CAS.
- D. M. Ma, W. Y. Liu, Q. Chen, Z. Jin, Y. Zhang, J. Huang, H. Zhang, F. M. Peng and T. Luo, J. Solid State Chem., 2021, 293, 121791 CrossRef CAS.
- Y. Li, M. Q. Zhang, Y. F. Liu, Y. X. Sun, Q. H. Zhao, T. L. Chen, Y. F. Chen and S. F. Wang, Nanomaterials, 2022, 12, 1122 CrossRef CAS.
- S. C. Lu, S. R. Yang, X. L. Hu, Z. Q. Liang, Y. C. Guo, Y. J. Xue, H. Z. Cui and J. Tian, Int. J. Hydrogen Energy, 2019, 44, 24398 CrossRef CAS.
- X. Yan, W. Liu, W. C. Yan, D. Y. Sun, Y. C. Jin, J. Wang, L. Xiang, H. Munakata and K. Kanamura, Electrochim. Acta, 2016, 191, 661 CrossRef CAS.
- D. P. Opra, S. V. Gnedenkov, A. A. Sokolov, A. B. Podgorbunsky, A. Y. Ustinov, V. Y. Mayorov, V. G. Kuryavyi and S. L. Sinebryukhov, J. Mater. Sci. Technol., 2020, 54, 181 CrossRef.
- X. Z. Ye, H. R. Hu, H. Xiong, Y. Wang and J. F. Ye, J. Colloid Interface Sci., 2021, 600, 530 CrossRef CAS.
- Y. Li, Y. F. Liu, M. Q. Zhang, Q. Y. Zhou, X. Li, T. L. Chen and S. F. Wang, Molecules, 2021, 26, 6987 CrossRef CAS PubMed.
- J. He, J. G. Yang, J. Jiang, M. W. Xu and Q. Wang, J. Colloid Interface Sci., 2021, 853, 157330 CAS.
- L. Luo, K. Q. Zhou, R. Q. Lian, Y. Z. Lu, Y. C. Zhen, J. S. Wang, S. Mathur and Z. S. Hong, Nano Energy, 2020, 72, 104716 CrossRef CAS.
- O. Oderinde, I. Hussain, M. M. Kang, Y. F. Wu, K. Mulenga, I. Adebayo, F. Yao and G. D. Fu, J. Ind. Eng. Chem., 2019, 80, 1 CrossRef CAS.
- T. T. Jiao, C. H. Ren, S. J. Lin, L. Z. Zhang, X. Z. Xu, Y. Q. Zhang, W. R. Zhang and P. Liang, J. Mol. Liq., 2022, 347, 118325 CrossRef CAS.
- O. S. Hammond and A. V. Mudring, Chem. Commun., 2022, 58, 3865 RSC.
- S. Sandhu, N. Kumar, V. P. Singh and V. Singh, Vacuum, 2021, 184, 109896 CrossRef CAS.
- Q. Wang, H. Y. Ren, Y. Q. Zhao, X. Y. Xia, F. Huang, G. W. Cui, B. H. Dong and B. Tang, J. Mater. Chem. A, 2019, 7, 14613 RSC.
- Q. Wang, X. X. Fang, P. Hao, H. Y. Ren, Y. Q. Zhao, F. Huang, J. F. Xie, G. W. Cui and B. Tang, Chem. Commun., 2020, 56, 11827 RSC.
- Q. Wang, X. X. Fang, P. Hao, W. W. Chi, F. Huang, X. F. Shi, G. W. Cui, Y. Liu and B. Tang, Chem. Commun., 2021, 57, 13024 RSC.
- M. F. Nava-Ocampo, L. Al Fuhaid, A. Santana, S. S. Bucs, R. Verpoorte, Y. Hae Choi, G. J. Witkamp, J. S. Vrouwenvelder and A. S. F. Farinha, J. Mol. Liq., 2021, 343, 117655 CrossRef CAS.
- R. R. Maça, D. C. Juarez, M. C. Rodríguez and V. Etacheri, Chem. Eng. J., 2020, 391, 123598 CrossRef.
- V. Vinesh, M. Preeyanghaa, T. R. N. Kumar, M. Ashokkumar, C. L. Bianchi and B. Neppolian, Environ. Res., 2022, 207, 112 CrossRef.
- F. C. Lei, Y. F. Sun, K. T. Liu, S. Gao, L. Liang, B. C. Pan and Y. Xie, J. Am. Chem. Soc., 2014, 136, 6826 CrossRef CAS.
- S. Q. Wu, X. Y. Li, Y. Q. Tian, Y. Lin and Y. H. Hu, Chem. Eng. J., 2021, 406, 126747 CrossRef CAS.
- T. Ge, Z. K. Jiang, L. Shen, J. Li, Z. Q. Lu, Y. C. Zhang and F. Wang, Sep. Purif. Technol., 2021, 263, 118401 CrossRef CAS.
- H. Li, B. Chong, B. R. Xu, N. Wells, X. Q. Yan and G. D. Yang, ACS Catal., 2021, 11, 14076 CrossRef CAS.
- X. L. Ren, M. Y. Xia, B. Chong, X. Q. Yan, B. Lin and G. D. Yang, Chem. Eng. Sci., 2022, 257, 117734 CrossRef CAS.
- F. Zhang, Y. C. Zhang, Y. Y. Wang, A. P. Zhu and Y. Zhang, Sep. Purif. Technol., 2022, 283, 120161 CrossRef CAS.
- Y. Zhang, H. H. Zhou, H. G. Wang, Y. C. Zhang and D. D. Dionysiou, Chem. Eng. J., 2021, 418, 129343 CrossRef CAS.
- H. Li, M. Y. Xia, B. Chong, H. Xiao, B. Zhang, B. Lin, B. L. Yang and G. D. Yang, ACS Catal., 2022, 12, 10361 CrossRef CAS.
- X. L. Ren, M. Y. Xia, B. Chong, X. Q. Yan, N. Wells and G. D. Yang, Appl. Catal., B, 2021, 297, 120468 CrossRef CAS.
- Y. Zhang, L. Hu, Y. C. Zhang, X. Z. Wang and H. G. Wang, Appl. Catal., B, 2022, 315, 121540 CrossRef CAS.
- M. Yan, Y. L. Wu and X. L. Liu, J. Alloys Compd., 2021, 855, 157548 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section and catalytic degradation activity for chloramphenicol. See DOI: https://doi.org/10.1039/d2cy01775j |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |