Comment on the “Discovery of high-performance thermoelectric copper chalcogenide using modified diffusion-couple high-throughput synthesis and automated histogram analysis technique” by T. Deng, T. Xing, M. K. Brod, Y. Sheng, P. Qiu, I. Veremchuk, Q. Song, T.-R. Wei, J. Yang, G. J. Snyder, Y. Grin, L. Chen and X. Shi, Energy Environ. Sci., 2020, 13, 3041
P.
Lemoine
 ac,
B.
Raveau
ac,
B.
Raveau
 b,
P.
Boullay
b,
P.
Boullay
 b and
E.
Guilmeau
b and
E.
Guilmeau
 *b
*b
aUniv. Rennes, CNRS, ISCR – UMR 6226, F-35000, Rennes, France
bCRISMAT, CNRS, Normandie Univ., ENSICAEN, UNICAEN, 14000, Caen, France. E-mail: emmanuel.guilmeau@ensicaen.fr
cInstitut Jean Lamour, UMR 7198 CNRS – Université de Lorraine, 2 allée André Guinier-Campus ARTEM, BP 50840, 54011, Nancy Cedex, France
First published on 1st December 2022
Abstract
The recent paper by Deng et al. showed that a copper sulphide with Cu7Sn3S10 composition is a promising thermoelectric material reaching a figure of merit ZT of 0.8 at 750 K via Cl doping. However, structural reinvestigations of the Cu7Sn3S10 sample (i.e., Cu2.10Sn0.90S3) prepared in the reported conditions show that the phase of interest is not tetragonal (space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m, at = 5.4164 Å, ct = 10.832 Å), but crystallizes in the cubic symmetry (space group P
2m, at = 5.4164 Å, ct = 10.832 Å), but crystallizes in the cubic symmetry (space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3n, ac = 10.832 Å) with a composition close to Cu22Sn10S32 similar to the results published by Pavan Kumar et al. These observations indicate that the main phase around the composition Cu7Sn3S10 is cubic, and a revision needs to be imposed for the understanding of the relationships between the structure and thermoelectric properties in this family.
3n, ac = 10.832 Å) with a composition close to Cu22Sn10S32 similar to the results published by Pavan Kumar et al. These observations indicate that the main phase around the composition Cu7Sn3S10 is cubic, and a revision needs to be imposed for the understanding of the relationships between the structure and thermoelectric properties in this family.
Broader contextThe past few years have seen a renewed interest in thermoelectric sulphides as the earth-abundance of sulphur is one critical aspect to encourage the large-scale production of thermoelectric devices with a potentially meaningful impact on mitigating climate change. Following reports of excellent properties in ternary and quaternary sulphides, the performance of cost-efficient sulphides has become closer to that of well-established state-of-the-art materials such as selenides, tellurides, clathrates, skutterudites, Zintl phases, silicides etc.… with ZT values now reaching unity (A. V. Powell, J. Appl. Phys., 2019, 126, 100901). |
In a recent article, Deng et al.1 discovered a high-performance thermoelectric with the composition Cu7Sn3S10 and demonstrated that it is a promising material with ultralow thermal conductivity, moderate band gap, and high electrical conductivity. A figure of merit ZT of 0.8 at 750 K was indeed achieved via Cl doping. In the structural analysis of this material by powder X-ray diffraction, considering stannite-like structural models (i.e., tetragonal superstructure of the sphalerite-type with the space group I
![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m, a = 5.4164 Å, c = 10.832 Å), Deng et al. reported difficulties in the refinement getting either chemical composition Cu39.3Sn10.7S50.0 far from the nominal one Cu35Sn15Sn50, also confirmed by EDXS (model 1), or large spread of the atomic displacement parameters (model 2). A further structural model was mentioned (model 3) assuming chemically improbable mixed cation/anion occupancies of the positions, but yielding expected chemical composition and reasonable values of the atomic displacement parameters. Finally, the authors decided to postpone the clarification of this issue to a separate study, and structural model 2 was considered for the interpretation of the attractive thermoelectric properties of this material.
2m, a = 5.4164 Å, c = 10.832 Å), Deng et al. reported difficulties in the refinement getting either chemical composition Cu39.3Sn10.7S50.0 far from the nominal one Cu35Sn15Sn50, also confirmed by EDXS (model 1), or large spread of the atomic displacement parameters (model 2). A further structural model was mentioned (model 3) assuming chemically improbable mixed cation/anion occupancies of the positions, but yielding expected chemical composition and reasonable values of the atomic displacement parameters. Finally, the authors decided to postpone the clarification of this issue to a separate study, and structural model 2 was considered for the interpretation of the attractive thermoelectric properties of this material.
In a posterior study of the Cu–Sn–S system, Pavan Kumar et al.2 synthesized a low thermal conductivity degenerate semiconductor with a rather close composition, Cu22Sn10S32 (i.e. Cu2.0625Sn0.9375S3), and showed that this sulphide also exhibits attractive thermoelectric properties with a figure of merit ZT of 0.55 at 700 K via Sb for Sn substitution. For the structure solution in the tetragonal symmetry, the authors met with the first step difficulties similar to those encountered by Deng et al. and refined chemical composition far from the nominal one. However, considering the fact that the c parameter is rigorously double the a parameter in the tetragonal structure of the I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m space group, Pavan Kumar et al. reported that it may imply a cubic supercell (ac = 2at = ct).2 Note that refinements reported in Deng et al.1 article lead also to a cell parameters ratio ct/at of 2. Using a combination of synchrotron powder X-ray diffraction, single crystal X-ray diffraction, 119Sn Mössbauer spectroscopy, and transmission electron microscopy, Pavan Kumar et al.2 demonstrated that the phase of interest crystallizes in fact in a cubic superstructure sphalerite-type with the space group P
2m space group, Pavan Kumar et al. reported that it may imply a cubic supercell (ac = 2at = ct).2 Note that refinements reported in Deng et al.1 article lead also to a cell parameters ratio ct/at of 2. Using a combination of synchrotron powder X-ray diffraction, single crystal X-ray diffraction, 119Sn Mössbauer spectroscopy, and transmission electron microscopy, Pavan Kumar et al.2 demonstrated that the phase of interest crystallizes in fact in a cubic superstructure sphalerite-type with the space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3n. Further the group demonstrated that the as-prepared phase is characterized by a semi-ordered cationic distribution: the Cu–Sn disorder being localized on one crystallographic site (see Table 1).
3n. Further the group demonstrated that the as-prepared phase is characterized by a semi-ordered cationic distribution: the Cu–Sn disorder being localized on one crystallographic site (see Table 1).
P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3n, a = 10.8365(1) Å, Cu22.2(1)Sn9.8(1)S32 3n, a = 10.8365(1) Å, Cu22.2(1)Sn9.8(1)S32 |
||||||
|---|---|---|---|---|---|---|
| Atom | Site | x | y | z | B iso (Å2) | SOF |
| Sn1 | 6c | ¼ | ½ | 0 | 1.28(8) | 1.00 |
| Cu2 | 6d | ¼ | 0 | ½ | 1.09(4) | 1.00 |
| Cu3 | 8e | 0.251(1) | x | x | 1.23(5) | 1.00 |
| Cu4/Sn4 | 12f | 0.244(1) | 0 | 0 | 0.95(7) | 0.68(1)/0.32(1) |
| S1 | 8e | 0.127(1) | x | x | 0.78(26) | 1.00 |
| S2 | 24i | 0.378(1) | 0.370(1) | 0.129(1) | 0.72(9) | 1.00 |
Based on the comparison of these results showing strong similitudes between Cu7Sn3S10 and Cu22Sn10S32, we have analysed a sample provided by the group of X. Shi and prepared in the conditions reported in their paper (i.e., heat treatments at 723 K, 1223 K, and 1073 K, followed by ball-milling process, and SPS treatment at 923 K) to revisit the structural analysis of the materials around the composition Cu7Sn3S10 (i.e. Cu2.10Sn0.90S3). The aim of the present comment is to draw attention to the crystal chemistry of the thermoelectric chalcogenides, which requires detailed structural studies to elucidate complex order–disorder phenomena and its impact on the understanding of their transport properties.
Rietveld refinements considering the tetragonal stannite-like (i.e. model 2) and the cubic Cu22Sn10S32 structural models, reported by Deng et al.1 and Pavan Kumar et al.,2 respectively, have been performed on long duration (48 h) powder X-ray diffraction data collected at CRISMAT laboratory using a Bruker D8 Advance Vario A two-circle diffractometer (θ–2θ Bragg–Brentano mode) equipped with a Ge(111) monochromator (Johansson type), a Lynx Eye detector and a Cu X-ray tube (λ = 1.5406 Å). Similar to the description provided by Deng et al.,1 the Rietveld refinement considering the tetragonal stannite-like structural model (I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m) gives at first sight an apparent high-quality refinement and some correct reliability factors (Fig. 1, top panel). However, a careful analysis of the refinement reveals the presence of additional non-indexed diffraction peaks of weak intensity as clearly evidenced in the top panel of Fig. 2 with intensity in the logarithmic scale. These weak intensity diffraction peaks can be indexed as superstructure diffraction reflections in a larger cubic cell similar to that previously observed by Pavan Kumar et al.2 for Cu22Sn10S32. The reinvestigation of the crystal structure of the Cu7Sn3S10 sample was then carried out with the cubic structural model reported for Cu22Sn10S32,2 assuming that a homogeneity range with rather close compositions may exist. Rietveld refinement of the powder X-ray diffraction data considering the cubic structural model (space group P
2m) gives at first sight an apparent high-quality refinement and some correct reliability factors (Fig. 1, top panel). However, a careful analysis of the refinement reveals the presence of additional non-indexed diffraction peaks of weak intensity as clearly evidenced in the top panel of Fig. 2 with intensity in the logarithmic scale. These weak intensity diffraction peaks can be indexed as superstructure diffraction reflections in a larger cubic cell similar to that previously observed by Pavan Kumar et al.2 for Cu22Sn10S32. The reinvestigation of the crystal structure of the Cu7Sn3S10 sample was then carried out with the cubic structural model reported for Cu22Sn10S32,2 assuming that a homogeneity range with rather close compositions may exist. Rietveld refinement of the powder X-ray diffraction data considering the cubic structural model (space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3n) of the Cu22Sn10S32 phase2 also provides very low reliability factors (Fig. 1, bottom panel). Moreover, in contrast to the tetragonal unit cell,1 the cubic structural model allows a perfect fit of all the diffraction peaks, including the weak intensity superstructure reflections (Fig. 2, bottom panel). Importantly, it leads to refined structural parameters and chemical composition Cu22.2(1)Sn9.8(1)S32 (Table 1) in fair agreement with those reported for the cubic phase Cu22Sn10S32.2 The refined composition (i.e., Cu2.08Sn0.92S3), slightly different from the nominal one Cu2.10Sn0.90S3, is supported by the existence of a broad diffraction peak, detected at 2θ = 26.5° (Fig. 2), which may be assigned to a binary copper sulphide present in a minor amount in the sample. Note that the refinement of the atomic parameters (Table 1) results in the composition Cu22.2(1)Sn9.8(1)S32, which – in combination with the refined lattice parameter of 10.8234(2) Å and chemical composition of Cu21.8(1)Sn10.2(1)S32 reported by Pavan Kumar et al.2 from synchrotron PXRD data – may indicate a small homogeneity range of the cubic phase.
3n) of the Cu22Sn10S32 phase2 also provides very low reliability factors (Fig. 1, bottom panel). Moreover, in contrast to the tetragonal unit cell,1 the cubic structural model allows a perfect fit of all the diffraction peaks, including the weak intensity superstructure reflections (Fig. 2, bottom panel). Importantly, it leads to refined structural parameters and chemical composition Cu22.2(1)Sn9.8(1)S32 (Table 1) in fair agreement with those reported for the cubic phase Cu22Sn10S32.2 The refined composition (i.e., Cu2.08Sn0.92S3), slightly different from the nominal one Cu2.10Sn0.90S3, is supported by the existence of a broad diffraction peak, detected at 2θ = 26.5° (Fig. 2), which may be assigned to a binary copper sulphide present in a minor amount in the sample. Note that the refinement of the atomic parameters (Table 1) results in the composition Cu22.2(1)Sn9.8(1)S32, which – in combination with the refined lattice parameter of 10.8234(2) Å and chemical composition of Cu21.8(1)Sn10.2(1)S32 reported by Pavan Kumar et al.2 from synchrotron PXRD data – may indicate a small homogeneity range of the cubic phase.
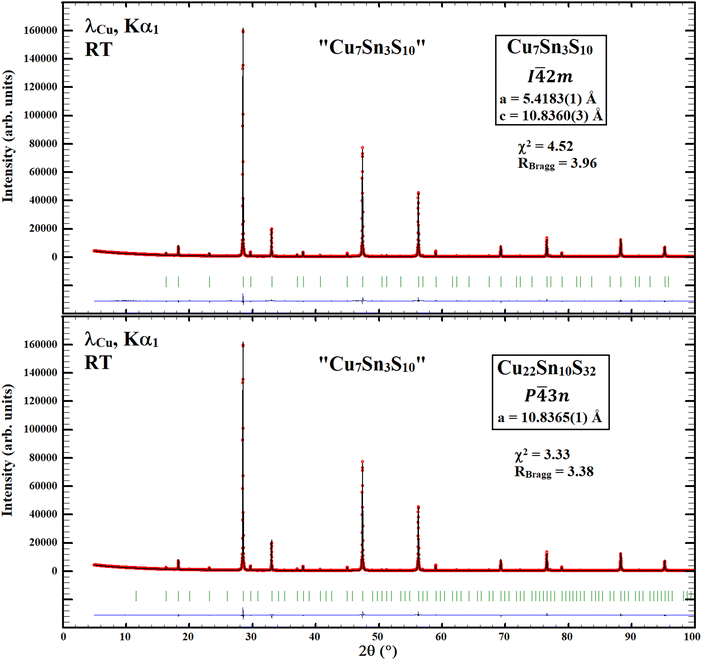 | ||
Fig. 1 Rietveld refinement of the Cu7Sn3S10 sample considering (i) the tetragonal structural model (I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m) proposed by Deng et al.1 (top panel) and (ii) the cubic Cu22Sn10S32 structural model (P 2m) proposed by Deng et al.1 (top panel) and (ii) the cubic Cu22Sn10S32 structural model (P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3n) proposed by Pavan Kumar et al.2 (bottom panel). 3n) proposed by Pavan Kumar et al.2 (bottom panel). | ||
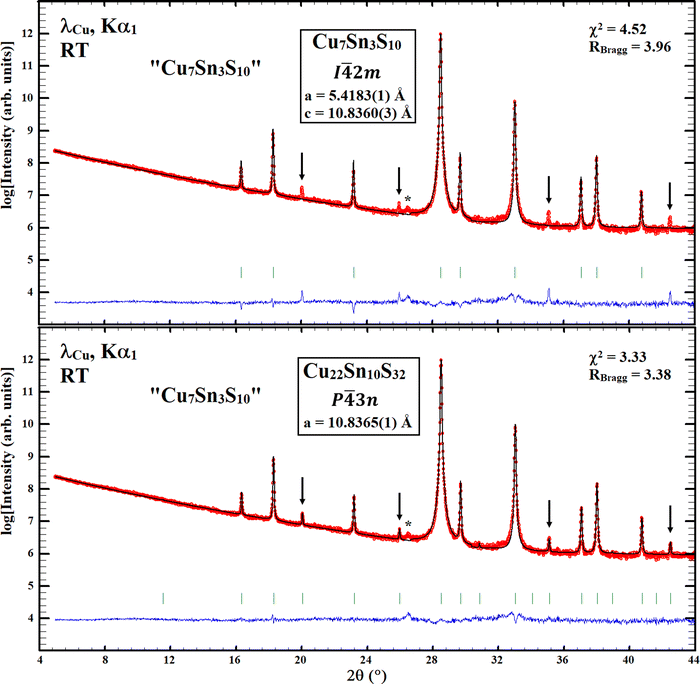 | ||
Fig. 2 Magnified Rietveld refinement of the Cu7Sn3S10 sample, with intensity in the logarithmic scale, considering (i) the tetragonal structural model (I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m) proposed by Deng et al.1 (top panel) and (ii) the cubic Cu22Sn10S32 structural model (P 2m) proposed by Deng et al.1 (top panel) and (ii) the cubic Cu22Sn10S32 structural model (P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3n) proposed by Pavan Kumar et al.2 (bottom panel). * refers to copper sulphide impurity. 3n) proposed by Pavan Kumar et al.2 (bottom panel). * refers to copper sulphide impurity. | ||
Moreover, it is worth pointing out that the local transmission electron microscopy analysis previously reported by Deng et al. (Fig. 4b in ref. 1) for “Cu7Sn3S10” is erroneous and incompatible with the proposed cell and space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m. Indeed, several low-intensity extra reflections in the [110] zone axis pattern, for example between the transmitted beam (000) and the reflection indexed 1 − 10, cannot be indexed in the I
2m. Indeed, several low-intensity extra reflections in the [110] zone axis pattern, for example between the transmitted beam (000) and the reflection indexed 1 − 10, cannot be indexed in the I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m space group, however, could be well indexed with the cubic cell (a ∼ 10.8 Å) and the space group P
2m space group, however, could be well indexed with the cubic cell (a ∼ 10.8 Å) and the space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3n (Fig. 3)
3n (Fig. 3)
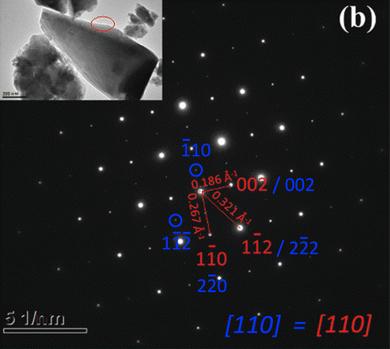 | ||
Fig. 3 Selected area electron diffraction (SAED) pattern of the sample Cu7Sn3S10 presented by Deng et al.1 in Fig. 4. The spots with high intensity (red) were indexed by Deng et al. in the tetragonal cell (I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m) with 2 × at = ct, while additional spots of weak intensity (blue) were not and cannot be indexed in this tetragonal cell. The present indexation, in blue, shows that the additional weak intensity spots are indexed in the cubic cell (P 2m) with 2 × at = ct, while additional spots of weak intensity (blue) were not and cannot be indexed in this tetragonal cell. The present indexation, in blue, shows that the additional weak intensity spots are indexed in the cubic cell (P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3n) with ac = 2 × at. 3n) with ac = 2 × at. | ||
Thus, these results demonstrate without any ambiguity that the samples of composition Cu7Sn3S10 prepared in the conditions reported by Deng et al.1 (i.e. heat treatments at 723 K, 1223 K, and 1073 K, followed by ball-milling process and SPS treatment at 923 K) are biphasic and that the observed majority phase is not tetragonal with composition Cu7Sn3S10 but cubic with composition Cu22Sn10S32.
As a consequence, the crystal chemistry results, reported by Deng et al.,1 cannot be used to explain the thermoelectric properties of the pristine Cu7Sn3S10 sample. The fact that the structure of the pristine sample is cubic and not tetragonal significantly influences the interpretation previously proposed for the lattice thermal conductivity of pristine and Cl-substituted SPS-treated Cu7Sn3S10−xClx materials, which can no more be compared on the basis of a tetragonal-cubic conversion neither the disappearance of the distorted non-cubic framework due to the Cl-substitution.1 The fact that the pristine composition exhibits lower lattice thermal conductivity than those of disordered Cl-substituted materials remains also still quite unexpected. Indeed, Cl-substituted samples exhibit a cubic fully-disordered sphalerite structure, while the pristine composition Cu7Sn3S10 (in fact cubic Cu22.2(1)Sn9.8(1)S32) has a partially ordered structure. The pristine compound should then exhibit a larger lattice thermal conductivity than the Cl-doped compounds. Full cationic disorder, induced by Cl-substitution, stoichiometry deviation, or process effect, are well known to strongly enhance phonon scattering and to reduce the lattice thermal conductivity in copper-based sphalerite derivative compounds.3,4 Attention must be drawn to the extremely low value of the lattice thermal conductivity of the pristine phase. A possible explanation may be in the estimation of the latter by the expression κL = κ − LσT, where L is the Lorenz number calculated by the single parabolic band (SPB) model.5,6 Unfortunately, this model is inadequate for L calculation due to the multiband nature of the valence band structure of this highly metallic degenerate semiconductor. It results in too high L values leading to a substantial underestimation of κL. This difficulty in determining the lattice contribution has been highlighted in the degenerate semiconductor Cu5Sn2S7,3,7 and several thermoelectric materials, such as SnTe, for which the presence of several electronic bands tends to lower the L values despite the strongly degenerate nature of the compound.8–11
To summarize, our present structural investigations clearly demonstrate that the stable structure around the composition Cu2.1Sn0.9S3 is not tetragonal with composition Cu7Sn3S10 but cubic with the space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3n and the composition Cu22Sn10S32, requiring the revision of the understanding of structure-transport properties relationships of this system. Moreover, we demonstrate once again that the order–disorder phenomena in copper sulphides are very complex and very difficult to detect. In-depth structural analyses should be then systematically performed to elucidate the subtle influence of crystal chemistry on the electrical and thermal properties of these materials.
3n and the composition Cu22Sn10S32, requiring the revision of the understanding of structure-transport properties relationships of this system. Moreover, we demonstrate once again that the order–disorder phenomena in copper sulphides are very complex and very difficult to detect. In-depth structural analyses should be then systematically performed to elucidate the subtle influence of crystal chemistry on the electrical and thermal properties of these materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the group of X. Shi for having provided a sample prepared in the conditions reported in their paper (i.e. heat treatments at 723 K, 1223 K and 1073 K, followed by ball-milling process and SPS treatment at 923 K). See their experimental section.1References
- T. Deng, T. Xing, M. K. Brod, Y. Sheng, P. Qiu, I. Veremchuk, Q. Song, T.-R. Wei, J. Yang, G. J. Snyder, Y. Grin, L. Chen and X. Shi, Energy Environ. Sci., 2020, 13, 3041–3053 RSC.
- V. Pavan Kumar, P. Lemoine, V. Carnevali, G. Guélou, O. I. Lebedev, B. Raveau, R. Al Rahal Al Orabi, M. Fornari, C. Candolfi, C. Prestipino, D. Menut, B. Malaman, J. Juraszek, K. Suekuni and E. Guilmeau, Inorg. Chem., 2021, 60, 16273–16285 CrossRef PubMed.
- G. Guélou, V. Pavan Kumar, V. Carnevali, O. I. Lebedev, B. Raveau, C. Couder, C. Prestipino, P. Lemoine, B. Malaman, J. Juraszek, C. Candolfi, B. Lenoir, R. Al Rahal Al Orabi, M. Fornari and E. Guilmeau, Chem. Mater., 2021, 33, 9425–9438 CrossRef.
- P. Lemoine, G. Guélou, B. Raveau and E. Guilmeau, Angew. Chem., Int. Ed., 2022, 61, e202108686 CrossRef CAS PubMed.
- Thermal Conductivity Theory, Properties, and Applications, ed. T. M. Tritt, Kluwer Academic/Plenum Publishers, New York, 2004 Search PubMed.
- A. F. May and J. G. Snyder, in Thermoelectrics and its energy harvesting, ed. D. M. Rowe, CRC Press, Boca Racon, 2012, ch. 11, pp. 1–18 Search PubMed.
- V. Pavan Kumar, P. Lemoine, V. Carnevali, G. Guélou, O. I. Lebedev, P. Boullay, B. Raveau, R. Al Rahal Al Orabi, M. Fornari, C. Prestipino, D. Menut, C. Candolfi, B. Malaman, J. Juraszek and E. Guilmeau, J. Mater. Chem. A, 2021, 9, 10812–10826 RSC.
- R. W. McKinney, P. Gorai, V. Stevanović and E. S. Toberer, J. Mater. Chem. A, 2017, 5, 17302–17311 RSC.
- M. Thesberg, H. Kosina and N. Neophytou, Phys. Rev. B, 2017, 95, 125206 CrossRef.
- L. V. Prokof’eva, A. A. Shabaldin, V. A. Korchagin, S. A. Nemov and Y. I. Ravich, Semiconductors, 2008, 42, 1161 CrossRef.
- S. Misra, B. Wiendlocha, J. Tobola, F. Fesquet, A. Dauscher, B. Lenoir and C. Candolfi, J. Mater. Chem. C, 2020, 8, 977–988 RSC.
| This journal is © The Royal Society of Chemistry 2023 |
