Open Access Article
Open Access ArticleIonic liquid functionalized polylactides: the effect of anions on catalytic activity and carbon nanotube dispersion
Dawei
Fang
 ,
Jingyi
Wen
,
Hao
Yu
,
Jince
Zhang
,
Xiaolei
You
and
Na
Liu
,
Jingyi
Wen
,
Hao
Yu
,
Jince
Zhang
,
Xiaolei
You
and
Na
Liu
 *
*
Institute of Rare and Scattered Elements, College of Chemistry, Liaoning University, Shenyang 110036, P. R. China. E-mail: liuna@lnu.edu.cn; Fax: +86 24 62202380; Tel: +86 24 62202380
First published on 21st December 2022
Abstract
Some hydroxylated imidazolium-based ionic liquids with different anions ([ILs-OH]+[X]−) were synthesized using an anion exchange reaction. A series of novel polylactides bearing an imidazolium group with different anions (PLLA-ILs[X]−) at one chain extremity were synthesized at 25 °C by ring-opening of L-lactide, which was initiated by the hydroxylated imidazolium-based ILs ([ILs-OH]+[X]−) in the presence of organocatalysts. Nuclear magnetic resonance (NMR) and matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) confirmed the structures of the PLLA-ILs[X]−. The results showed that the hydroxylated ionic liquid is an efficient initiator for the ring-opening polymerization (ROP) of L-lactide. Carbon nanotubes (CNTs) were dispersed in a solution of PLLA-ILs[X]− in 1,4-dioxane and a significant improvement in the stability of suspension was observed by measurements of suspension absorbance. The absorbance of suspension between CNTs and PLLA-ILs[X]− followed the order: [TFSI]− > [H2PO4]− > [Cl]− > [NO3]−, and was much higher than that of untreated CNTs, indicating that the anions have an obvious effect on the interaction between CNTs and PLLA-ILs[X]−. In the scanning electron microscopy (SEM) images, it was also clearly visible that the globular aggregates of PLLA-IL[Cl]− were bound to CNTs, which was consistent with the results of absorbance.
Introduction
Carbon nanotubes (CNTs) exhibit remarkable properties, such as high electrical conductivity, excellent mechanical properties and high thermal conductivity, and have been widely utilized in the field of biosensors,1 high-strength conductive composites,2 and hydrogen storage plants.3 Most applications of CNTs require that they are well-separated (i.e. individualized or in the form of thin bundles) and well-dispersed in the liquid phase. However, CNTs are usually generated in the entangled form and hard to disperse because of intertube strong van der Waals forces and π–π stacking, which limit their utility as materials.4 To overcome this issue, two distinct approaches have been reported for the functionalization of CNTs: covalent and non-covalent functionalization.5 The covalent modification of CNTs can introduce useful functional groups onto the surface of CNTs, while some defects are inevitably introduced on the sidewalls of CNTs and some properties of CNTs may be altered.6 In contrast, non-covalent modifications, namely supermolecular interactions (van der Waals forces, π–π, hydrogen bond, etc.), involving mild reaction conditions and having the ability to retain the original structure and properties of CNTs, have attracted tremendous attention.7 Consequently, they are more attractive than covalent modifications for dispersing CNTs.Recently, ionic liquids (ILs) consisting of cations and anions have gained great attention due to their excellent properties, including non-volatility, non-flammability, high conductivity, high thermal stability, easy recycling capacity and good dissolving capacity, and have been widely applied in many fields.8 In the field of polymer chemistry, ILs are mainly utilized as a solvent or a catalyst.9 Interestingly, their affinity to CNTs attracted the interest of polymer chemists. For example, the imidazolium ring could adsorb onto the CNTs with cation-π/π–π interactions, giving rise to CNT bundle disentanglement.10 It has been broadened to poly(ionic liquid)s (PILs),11 which afford a good dispersion in aqueous and organic media. In this regard, the introduction of the imidazolium group to polymeric backbones is expected to provide an attractive and efficient strategy aiming at improving CNT interactions and dispersion within polymer matrices. This inspired us to exploit a straightforward method to synthesize novel polymeric chains containing imidazolium rings to disperse CNTs via non-covalent functionalization.
Polylactide (PLA) with excellent biodegradability and biocompatibility has been widely applied in many fields, such as packaging, agriculture materials, drug delivery, and so on.12 Ring-opening polymerization (ROP) catalyzed by metal complexes is the most efficient method to synthesize PLA. Meanwhile, the organocatalytic ROP of lactide also attracts much attention because it is more economical, and environmentally friendly and requires no removal of metals.13 Therefore, developing a new catalytic system to synthesize functional PLA is necessary and attractive.
Dubois and coworkers employed hydroxylated ionic liquid [1-(11-hydroxy-undecyl)-3-methyl-imidazolium bromide] as an initiator to initiate the ROP of L-lactide in the presence of 1,8-diazobicyclo[5.4.0] undec-7-ene (DBU) as a catalyst, obtaining polylactide bearing an imidazolium group at one chain end. When compared with CNTs in CHCl3, PLLA end-functionalized by the imidazolium ring was much more efficient for solubilizing CNTs through cation-π or π–π interactions.14 Kubisa et al.15 synthesized a series of medium molecular weight PLLAs containing imidazolium ionic liquid by cationic polymerization using a hydroxylated IL as the initiator. The stability of the suspension of CNTs was improved. It is attributed to the specific π–π interactions between the imidazolium ring and the CNTs. They also proved that after isolation of CNTs from suspensions in which an excess polymer is present in solution and thorough washing, CNTs treated with PLA are no longer stabilized whereas for PLA-IL the stabilizing effect is preserved. This clearly shows that there is indeed a relatively strong interaction of ionic liquid end-groups with CNT surfaces. These literature reviews reported that PLA end-functionalized by imidazolium ionic liquid could stabilize the CNTs by supermolecular interactions;16 however, there are no reports that systematically investigate the effect of IL anions on the dispersion of CNTs.
Herein, we prepared some hydroxyl functionalized imidazolium-based ILs with different anions via an anion exchange method. Then, a series of novel polylactides bearing an imidazolium group with different anions (PLLA-ILs[X]−) at one chain extremity were synthesized at 25 °C by the ROP of L-lactide, which was initiated by the hydroxylated imidazolium-based ILs in the presence of an organocatalyst (1,8-diazobicyclo[5.4.0] undec-7-ene/N-[3,5-bis(trifluoromethyl)phenyl]-N′-cyclohexylthiourea, DBU/TU). The effects of anions on the ROP of L-lactide and the binding abilities of anions on the dispersion of CNTs were systematically studied for the first time, providing a convenient approach for improving the dispersion of CNTs in an organic solvent.
Results and discussion
ROP of L-LA catalysed by organocatalysts/[ILs-OH]+[X]−
All hydroxyl [ILs-OH]+[X]− were synthesized according to the reported procedures through an anion exchange reaction as illustrated in Scheme 1. First, we explored the ROP of L-LA catalyzed by either 1,5,7-Triazabicyclo[4.4.0]dec-5-ene (TBD) or DBU at room temperature in CH2Cl2 in combination with hydroxyl functional ionic liquids ([ILs-OH]+[X]−) with different anions as initiators, and the results are summarized in Table 1. In the presence of 1 equiv. of [IL-OH]+[Cl]−, TBD or TBD/TU could not efficiently initiate the ROP of L-lactide, which was attributed to the stronger basicity (Table 1, entries 1 and 2). In contrast, DBU alone can initiate the ROP of L-lactide but in an uncontrollable manner. The resultant PLLA has a molecular weight higher than the theoretical value, coupled with a broad molecular weight distribution (PDI = 1.51) (Table 1, entry 3). With addition of an equimolar amount of TU as a cocatalyst to DBU, the polymerization conversion reached up to 94% for 24 h, leading to higher catalytic activity and narrower molecular weight distribution (PDI = 1.35) (Table 1, entry 4). Therefore, TU as a cocatalyst was utilized in combination with DBU. Fixing the [LA]0/[[ILs-OH]+[X]−]0/[DBU]0 ratios (20/1/1) and further increasing the amount of TU from 1 to 4 did not bring out an improvement in polymerization control (Table 1, entries 4–6), indicating that the most suitable feed ratio of DBU/TU was 1/1.| Entry | IL-OH[X] | [LA]0/[ILOH]0/[DBU]0/[TU]0 | Conv. (%)b | M n,cal (103) | M n,exp (103) | M w/Mnd |
|---|---|---|---|---|---|---|
| a Conditions: DBU: 100 μmol, time: 24 h, solvent: CH2Cl2, Tp: 25 °C. b Determined by 1H NMR spectroscopy. c M n,calcd = [LA]0/[IL-OH]0 × 144.13 × Conv. + M[ILs-OH]+[X]−. d Determined by size exclusion chromatography calibrated vs. polystyrene standards and corrected by a factor of 0.58 according to literature recommendations. e TBD: 100 μmol. f Determined by 1H NMR by comparing the intensity of characteristic signals of head groups (based on the integration ratios between the methenyl protons of the repeat unit and the protons of methylene end groups). g DBU: 20 μmol. | ||||||
| 1e | [HOEMIm][Cl] | 20/1/1/0 | Trace | — | — | — |
| 2e | [HOEMIm][Cl] | 20/1/1/1 | Trace | — | — | — |
| 3 | [HOEMIm][Cl] | 20/1/1/0 | 92 | 2.81 | 3.17f | 1.51 |
| 4 | [HOEMIm][Cl] | 20/1/1/1 | 94 | 2.87 | 2.88f | 1.35 |
| 5 | [HOEMIm][Cl] | 20/1/1/2 | 93 | 2.84 | 3.46f | 1.37 |
| 6 | [HOEMIm][Cl] | 20/1/1/4 | 92 | 2.81 | 2.59f | 1.42 |
| 7g | [HOEMIm][Cl] | 25/1/1/1 | 85 | 3.23 | 7.36 | 1.29 |
| 8g | [HOEMIm][Cl] | 50/1/1/1 | 91 | 6.72 | 7.45 | 1.17 |
| 9g | [HOEMIm][Cl] | 75/1/1/1 | 97 | 10.65 | 13.97 | 1.25 |
| 10g | [HOEMIm] [NO3] | 50/1/1/1 | 84 | 6.24 | 6.68 | 1.26 |
| 11g | [HOEMIm] [NO3] | 75/1/1/1 | 90 | 9.92 | 12.07 | 1.17 |
| 12g | [HOEMIm] [H2PO4] | 50/1/1/1 | 93 | 6.93 | 12.22 | 1.59 |
| 13g | [HOEMIm] [H2PO4] | 75/1/1/1 | 97 | 10.71 | 16.45 | 1.33 |
| 14g | [HOEMIm] [TFSI] | 50/1/1/1 | 91 | 6.97 | 23.75 | 1.42 |
| 15g | [HOEMIm] [TFSI] | 75/1/1/1 | 94 | 10.57 | 40.82 | 1.20 |
Increasing the feed ratio of [LA]0/[[IL-OH]+[Cl]−]0 from 25 to 75, the molecular weights of the resultant PLLAs increased from 7.36 × 103 to 13.97 × 103, consistent with the calculated ones (Table 1, entries 7–9). The molecular weight distributions were still narrow. This characterization indicated the successful implementation of ROP through DBU/TU and [IL-OH]+[Cl]−. Inspired by the above results, we set [IL-OH]+[Cl]− as the basic module to investigate the steric effect of anions on the catalytic activity of the ROP of L-lactide. Thus, [IL-OH]+[NO3]− was chosen as the initiator. Under the same conditions, DBU/TU showed similar activity towards L-lactide to that of [IL-OH]+[Cl]− (Table 1, entries 10 and 11). The catalytic activity was a little lower than that of [IL-OH]+[Cl]−, but the polymerization control was compared to that of [IL-OH]+[Cl]−. When further increasing the steric effect of anions to [IL-OH]+[H2PO4]−, the activities were similar to those of [IL-OH]+[Cl]−, but the molecular weights of the resultant polymers were higher than that of calculated ones and the molecular weight distributions were broader than that of [IL-OH]+[Cl]−, indicating the polymerization control was poor (Table 1, entries 12 and 13). Moreover, the polymerization of [IL-OH]+[TFSI]− became uncontrollable since the molecular weights of the afforded PLLA were much higher than the theoretical ones (Table 1, entries 14 and 15), which might be attributed to the bulky steric effect. This result suggested that the anions have an obvious effect on the polymerization catalytic activity and controllability. To further confirm the active species, the low-molecular-weight PLLA (Table 1, entry 4) was used to analyze the end-group fidelity that is crucial to evaluate the efficiency of a catalyst system for the ROP of cyclic esters. The polymer chains are capped by the hydroxyl group at one end evidenced by giving the resonance at 2.70 ppm. The other end of the polymer chain is the imidazole proton by giving the multiplets at 9.16 and 7.76 ppm assignable from initiator [HOEMIm][Cl], which is a typical feature of a polymer obtained by activated-monomer mechanism. The 1H NMR spectra showed wide peaks at 1.57 ppm (CH3) and 5.16 ppm for (CHOC(O)O), which is typical for PLLA (Fig. 1).
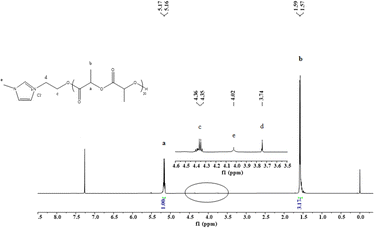 | ||
| Fig. 1 1H NMR (300 MHz, CDCl3, 25 °C) spectra of the afforded PLLA sample (Table 1, entry 4) | ||
The incorporation of [IL-OH]+[Cl]− into the polymer chain is indicated by the transformation of the hydroxyl groups in the ILs into the ester groups in the polymers. The peak for NCH2CH2O appears at 4.60 ppm, which is characteristic of a polyester chain. The signals 6.69–7.99 ppm are specific to hydrogen from an aromatic ring which corresponds to the imidazolium protons of [IL-OH]+[Cl]−. More importantly, the number average molecular weight (Mn,NMR) of the obtained polymer estimated from the 1H NMR measurement fairly agreed with the target molecular weight calculated (Mn,theo) based on the initial ratio of M0/I0 and monomer conversion.
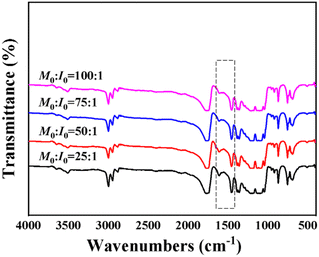
FTIR spectrometry was also carried out on imidazolium end-functionalized PLLA with different anions to determine the structure. From the FT-IR spectral results (Fig. 2), the skeleton vibration of the imidazole ring at 1607.26 cm−1 and 1457.30 cm−1 indicated that [ILs-OH]+[X]− was introduced into the PLLA. The characteristic components generated from the ionic liquid ascertained the successful grafting of ionic liquid on the PLLA main chain. The FTIR spectra are given in the characteristic peaks of the N–H stretch and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch shift to higher wavenumber, implying weakened hydrogen bond strength. This is due to the existence of bulky grafting cations of ionic liquid on PLLA chains.
O stretch shift to higher wavenumber, implying weakened hydrogen bond strength. This is due to the existence of bulky grafting cations of ionic liquid on PLLA chains.
This result was further corroborated by MALDI-TOF mass spectroscopy (Fig. 3), which provided direct evidence that the ROP of LA was initiated by [IL-OH]+[Cl]−. As shown in Fig. 3, only macromolecules containing ionic liquid head groups and hydroxyl terminal groups are observed. In the spectrum, signals of the main series are separated by 72 units. LA is a cyclic dimer of lactic acid; hence, its polymerization should lead to polymers composed of an even number of lactoyl (lactic acid) units. However, polymerization is accompanied by transesterification, leading to a redistribution of chain units and formation of macromolecules composed of an odd number of lactoyl units (but not affecting the nature of end groups). Such side reactions likely also rationalize the slightly broad polydispersity values of the produced PLLA-IL. All the characterization and analysis for PLLA-IL proved that polymerization was initiated by the designed [ILs-OH]+[X]− initiator.
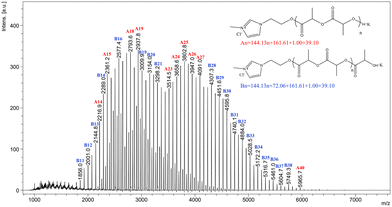 | ||
| Fig. 3 MALDI-TOF mass spectra (K+) of imidazolium end-functionalized PLLA sample (Table 1, entry 4). | ||
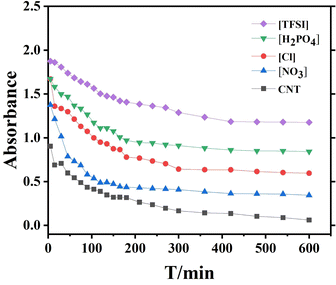
According to the literature,17 ILs have affinity for the carbon nanotube surface (CNTs) due to cation-π and/or π–π interactions between the imidazolium ring and CNTs. However, no reports have been related to the interaction between PLLAs end-functionalized by an imidazolium ring containing different anions and CNTs. Therefore, a systematical investigation of the interaction between PLLA-IL and CNTs is very attractive. The dispersion of CNTs with PLLA-ILs containing different anions was investigated. The results are shown in Fig. 4. The absorbance of untreated CNT suspension decreased rapidly within 3 h. The supernatant became colorless and transparent after 3 h by visual observation but there was still some absorbance recorded by the instrument. The changes in absorbance of the suspension were extremely small for 10 h indicating that the suspension is stable within this time-scale. The absorbance of suspension between CNTs and PLLA-IL[X]− followed the order: [TFSI]− > [H2PO4]− > [Cl]− > [NO3]−, which was much higher than that of untreated CNTs. This phenomenon indicated the anions have an obvious effect on the interaction between CNTs and PLLA-IL[X]. This might be attributed to the interaction ability between imidazolium ring cations and anions.18 When the steric effect and radii were increased, the interaction between cations and anions decreased. Thus, the order of interaction between cations and anions should be [TFSI]− < [H2PO4]− < [NO3]− < [Cl]−. However, there exists higher H-bond interaction between cation and [NO3]− than that of [Cl−]. Therefore, the interaction between cations and anions follows the order [TFSI]− < [H2PO4]− < [Cl]− < [NO3]−. This leads to the following order of interaction between cations and CNTs: [TFSI]− > [H2PO4]− > [Cl]− > [NO3]−.
This conclusion that PLLA containing IL end-groups was indeed permanently bound to CNT, which was further verified by SEM. The CNT images before and after the combination with PLA-IL were significantly different. From Fig. 5d, it is visible that the globular with a diameter up to about 36 nm aggregates of PLLA-IL[Cl]− are bound to the CNTs surface, whilst this phenomenon didn’t appear in pure CNTs (Fig. 5a and b). Meanwhile, polymer (PLLA-IL[Cl]−) was not uniformly dispersed on the surfaces of CNTs, but it still improved the intertwining between CNTs.
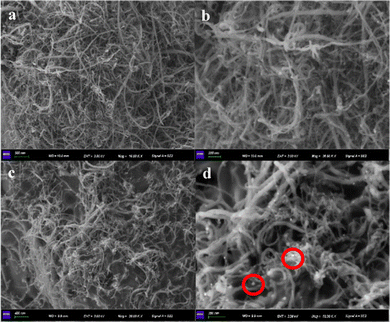 | ||
Fig. 5 SEM images. (a and b) untreated CNT (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 36 000 and 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 magnification); (c and d) CNT treated with PLA-IL[Cl] (20 000 magnification); (c and d) CNT treated with PLA-IL[Cl] (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 36 000 and 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 magnification). 000 magnification). | ||
Conclusions
In this paper, some hydroxylated imidazolium-based ionic liquids with different anions ([ILs-OH]+[X]−) were synthesized by an anion exchange method. A series of novel polylactides bearing an imidazolium group with different anions (PLLA-ILs[X]−) at the chain end were synthesized by the ROP of L-lactide for the first time, which was initiated by the hydroxylated imidazolium-based ILs in the presence of organocatalysts (DBU/TU). The results showed that these hydroxylated ionic liquids are an efficient initiator for the ROP of L-lactide, and the polymerization activity and controllability were obviously affected by the steric effect of anions. The dispersion of CNTs in 1,4-dioxane was improved by the addition of PLLA-ILs[X]−. The absorbance of suspension between CNTs and PLLA-ILs[X]− followed the order: [TFSI]− > [H2PO4]− > [Cl]− > [NO3]−, indicating that the anions have an obvious effect on the interaction between CNTs and PLLA-IL[X]−. This might be attributed to the interaction between imidazolium cations and anions. These interesting results will shed new light on designing catalytic initiators for the ROP of L-lactide and provide new supramolecular tools for the synthesis of CNT-based biomaterials.Experimental
General procedure
All reactions were carried out in a dry nitrogen atmosphere using Schlenk techniques. CH2Cl2 was dried over calcium hydride with stirring for 24 h and distilled before use. Glassware and flasks used in the polymerization were dried in an oven at 115 °C overnight and exposed to a vacuum-nitrogen cycle three times. 1,8-Diazabicyclo [5.4.0] undecano-7-ene (DBU) was purchased from Macklin, N-[3,5-bis(trifluoromethyl)phenyl]-N′-cyclohexylthiourea (TU) was synthesized according to the previous literature,19 and 1-(2-hydroxyethyl)-3-methylimidazolium chloride ([HOEMIm][Cl]) was purchased from Aladdin and dried over by calcium hydride before distillation. L-LA was recrystallized by dry ethyl acetate three times. The molecular weight and weight distribution of polymers were measured using a TOSOH HLC 8220 GPC instrument at 40 °C with THF as eluent against polystyrene standards. 1H and 13C NMR spectra were recorded on a Bruker AV400 spectrometer. The MALDI-TOF mass spectra were recorded using a Bruker Daltonic MicroFlex LT at the National Analytic Research Center of the Changchun Institute of Applied Chemistry (CIAC). Fourier transform infrared (FT-IR) analysis was measured using a Nicolet 6700 spectrometer equipped with a DGTS detector.Synthesis of hydroxylated ([ILs-OH]+[X]−)
A typical procedure for ROP of L-LA by organocatalysts/[ILs-OH]+[X]−
Polymerizations of L-LA were carried out in a 25 mL flask under a nitrogen atmosphere. A typical polymerization procedure can be described as follows (Table 1, entry 4). A flask was charged with a solution of DBU/TU and [HOEMIm][Cl], prepared previously by the addition of [HOEMIm][Cl] (0.0163 g, 0.1 mmol) to DBU/TU (100 μmol/100 μmol) in 2 mL of CH2Cl2. Next, the solution was added to a stirred solution of L-LA (0.288 g, 2 mmol) in 3 mL of CH2Cl2 at 25 °C. The polymerization was stirred for 24 h and then quenched by adding several drops of acidified ethanol. Then, polymers were precipitated with abundant ethanol, collected and dried at 45 °C under a vacuum to a constant weight. The conversion was determined by 1H NMR spectroscopy.Dispersion experiment
The CNT dispersion ability of new poly(L-lactide) incorporating an imidazolium as an end-group was evaluated in 1,4-Dioxane. The experimental steps are as follows: 3.0 mg of CNT was added to 50 mL of 1,4-dioxane. Then, the flask containing suspension was placed in an ultrasonic bath (SB-120 DT, ultrasonic frequency 80 kHz) for 1 h at room temperature. The dispersion was stirred for 24 h with a magnetic stirrer. Immediately after stirring was stopped, part of the dispersion was transferred to a quartz cell (optical path 1 cm), and the cell was placed in the compartment of a UV-VIS Lambda 35 spectrometer (PerkinElmer Corp., USA). A certain amount of polymer was added to the remaining dispersion (25 mg of PLLA-IL for 5 mL of CNT dispersion), stirred again for 24 h and the change of absorbance was recorded. The resulting mixtures showed dramatic variations of CNT dispersion, without requiring any sonication.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We are thankful for the financial support from the National Natural Science Foundation of China for the project no. 22173039 and Liaoning Province Doctor Startup Fund for the project no. 2021-BS-084.Notes and references
- (a) R. Villalonga, M. L. Villalonga, P. Díez and J. M. Pingarrón, J. Mater. Chem., 2011, 21, 12858–12864 RSC; (b) M. Eguílaz, C. J. Venegas, A. Gutiérrez, G. A. Rivas and S. Bollo, Microchem. J., 2016, 128, 161–165 CrossRef.
- (a) X. Li, Y. Tang, J. Song, W. Yang, M. Wang, C. Zhu, W. Zhao, J. Zheng and Y. Lin, Carbon, 2018, 129, 236–244 CrossRef CAS; (b) K. Lota, I. Acznik, A. Sierczynska and G. Lota, J. Appl. Polym. Sci., 2020, 137, 48867 CrossRef CAS; (c) P. Qin, C. Huang, B. Gao, C. Pi, J. Fu, X. Zhang, K. Huo and P. K. Chu, Appl. Surf. Sci., 2020, 503, 144293 CrossRef CAS; (d) R. Jiang, B. Zeng, J. Liu, X. Chai, T. Chen, Z. Wu, X. Zhang and K. Yang, J. Alloys Compd., 2020, 816, 152669 CrossRef CAS.
- L. Zhang, Z. Sun, Z. Cai, N. Yan, X. Lu, X. Zhu and L. Chen, Appl. Surf. Sci., 2020, 504, 144465 CrossRef CAS.
- K. Singh, S. Chaudhary and R. Venugopal, Polym. Eng. Sci., 2019, 59, E248–E261 CrossRef CAS.
- L. Meng, C. Fu and Q. Lu, Prog. Nat. Sci., 2009, 19, 801–810 CrossRef CAS.
- G. Diaz Costanzo, S. Ledesma, I. a Mondragon and S. Goyanes, J. Phys. Chem. C, 2010, 114, 14347–14352 CrossRef CAS.
- (a) M. S. Saha and A. Kundu, J. Power Sources, 2010, 195, 6255–6261 CrossRef CAS; (b) L. Du, F. Kong, G. Chen, C. Du, Y. Gao and G. Yin, Chin. J. Catal., 2016, 37, 1025–1036 CrossRef CAS; (c) W. Yuan and M. B. Chan-Park, ACS Appl. Mater. Interfaces, 2012, 4, 2065–2073 CrossRef CAS PubMed.
- (a) D. M. Fox, W. H. Awad, J. W. Gilman, P. H. Maupin, C. Hugh and P. C. Trulove, Green Chem., 2003, 5, 724–727 RSC; (b) M. Galiński, A. Lewandowski and I. Stępniak, Electrochim. Acta, 2006, 51, 5567–5580 CrossRef; (c) K. Fujie and H. Kitagawa, Coord. Chem. Rev., 2016, 307, 382–390 CrossRef CAS.
- A. A. Elgharbawy, F. A. Riyadi, M. Z. Alam and M. Moniruzzaman, J. Mol. Liq., 2018, 251, 150–166 CrossRef CAS.
- (a) J. Wang, H. Chu and Y. Li, ACS Nano, 2008, 2, 2540–2546 CrossRef CAS PubMed; (b) T. Fukushima, A. Kosaka, Y. Ishimura, T. Yamamoto, T. Takigawa, N. Ishii and T. Aida, Science, 2003, 300, 2072–2074 CrossRef CAS PubMed.
- X. Zhou, T. Wu, K. Ding, B. Hu, M. Hou and B. Han, Chem. Commun., 2010, 46, 386–388 RSC.
- J.-M. Raquez, R. Ramy-Ratiarison, M. Murariu and P. Dubois, Poly (lactic acid) science and technology: processing, properties, additives and applications, 2014, vol. 101 Search PubMed.
- L. Mezzasalma, A. P. Dove and O. Coulembier, Eur. Polym. J., 2017, 95, 628–634 CrossRef CAS.
- F. Meyer, J.-M. Raquez, O. Coulembier, J. De Winter, P. Gerbaux and P. Dubois, Chem. Commun., 2010, 46, 5527–5529 RSC.
- T. Biedroń, Ł. Pietrzak and P. Kubisa, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 5239–5244 CrossRef.
- (a) P. C. Barbosa, S. Mickalev, A. Barros-Timmons and F. M. Figueiredo, J. Phys. Chem. C, 2021, 126, 551–562 CrossRef; (b) M. J. Park, J. K. Lee, B. S. Lee, Y.-W. Lee, I. S. Choi and S.-g Lee, Chem. mater., 2006, 18, 1546–1551 CrossRef CAS.
- (a) Y. Ren, Z. Zhou, G. Yin, G.-X. Chen and Q. Li, RSC Adv., 2016, 6, 31351–31358 RSC; (b) Y. C. A. Sokolovicz, C. M. L. Schrekker, F. Hild, L. de Oliveira Bodo, J. L. Couto, J. S. Klitzke, T. Maraschin, N. R. de Souza Basso, J. H. Z. dos Santos and S. Dagorne, New J. Chem., 2019, 43, 16367–16373 RSC.
- (a) M. Lalitha and S. Lakshmipathi, J. Mol. Liq., 2017, 236, 124–134 CrossRef CAS; (b) M. Shakourian-Fard, Z. Jamshidi, A. Bayat and G. Kamath, J. Phys. Chem. C, 2015, 119, 7095–7108 CrossRef CAS; (c) B. D. Wu, S. W. Wang, L. Yang, T. L. Zhang, J. G. Zhang and Z. N. Zhou, Z. Anorg. Allg. Chem., 2011, 637, 2252–2259 CrossRef CAS.
- B. G. Lohmeijer, R. C. Pratt, F. Leibfarth, J. W. Logan, D. A. Long, A. P. Dove, F. Nederberg, J. Choi, C. Wade and R. M. Waymouth, Macromolecules, 2006, 39, 8574–8583 CrossRef CAS.
- S. Zhang, X. Qi, X. Ma, L. Lu and Y. Deng, J. Phys. Chem. B, 2010, 114, 3912–3920 CrossRef CAS PubMed.
- T. Zaoui, M. Debdab, B. Haddad, E. H. Belarbi, Y. Chaker, M. Rahmouni, S. Bresson and V. Baeten, J. Mol. Struct., 2021, 1236, 130264 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |