 Open Access Article
Open Access ArticleCoordination-driven self-assembled Mn(II)-metallostar with high relaxivity and synergistic photothermal and photodynamic effects†
Huiyu
Wu‡
ab,
Zhenghui
Li‡
ad,
Yao
Liu
ac,
Xingchi
Shi
e,
Yuan
Xue
ac,
Zuhua
Zeng
ab,
Fanglin
Mi
d,
Haiying
Wang
*a and
Jiang
Zhu
 *a
*a
aMedical Imaging Key Laboratory of Sichuan province, Department of Oncology, Affiliated Hospital of North Sichuan Medical College, Maoyuan Road 1, Nanchong City, Sichuan 637000, China. E-mail: hywang@nsmc.edu.cn; zhujiang@nsmc.edu.cn
bSchool of Pharmacy, North Sichuan Medical College, Fujiang Road 234, Nanchong City, Sichuan 637000, China
cSchool of Basic Medical Sciences and Forensic Medicine, North Sichuan Medical College, Fujiang Road 234, Nanchong City, Sichuan 637000, China
dDepartment of Stomatology, North Sichuan Medical College, Fujiang Road 234, Nanchong City, Sichuan 637000, China
eDepartment of Cardiovascular Disease, School of Clinical Medicine, Affiliated Hospital of North Sichuan Medical College, Maoyuan Road 1, Nanchong City, Sichuan 637000, China
First published on 15th November 2023
Abstract
A novel Mn(II)-metallostar structure (ML3Mn3, M = Fe3+, Ti4+) was synthesized through the self-assembly of high-valence transition-metal ions (Fe3+ and Ti4+) with a heteroditopic Mn(II) chelate (MnL) bearing a catechol group. UV-vis spectroscopy and variable-temperature 17O NMR reveals pH-dependent coordination modes of the FeL3Mn3 metallostar, with tris-coordination at pH 9.0 and an equilibrium between tris- and bis-coordination at pH 7.4. The heteropolymetallic Mn(II)-metallostars (ML3Mn3) demonstrated enhanced relaxivity per Mn (more than 2-fold) compared to the monomeric Mn(II) chelate (MnL). The Fe–Mn metallostar exhibited synergistic photothermal therapy (PTT) and photodynamic therapy (PDT) effects upon 808 nm laser excitation due to ligand-to-metal charge transfer (LMCT) from the metal-catechol core, with a photothermal conversion efficiency of 20.3% and a singlet oxygen quantum yield of 24.8%. In vitro phototherapy studies showed that the Fe–Mn metallostar showed effective antitumor effects in the BxPC-3 cell line. In MRI studies in normal mice, low-dose FeL3Mn3 (25 μmol kg−1) provided a superior contrast-enhancement compared to Gd-DTPA (100 μmol kg−1) with rapid blood clearance and mixed hepatobiliary and renal excretion. In summary, we have developed a novel Mn(II)-metallostar structure with high relaxivities and synergistic NIR light-irritable PTT/PDT effects, which may be a promising theranostic agent for MRI-guided phototherapy.
Introduction
Magnetic resonance imaging (MRI) is one of the most powerful non-invasive diagnostic tools in medical imaging, offering high spatial resolution and excellent soft-tissue contrast without ionizing radiation.1 MRI is widely used for diagnosis, disease staging, treatment planning, and response evaluation. However, the diagnostic accuracy and confidence can be compromised when intrinsic contrast between abnormal and normal tissue is lacking. To address this, contrast-enhanced (CE) MR scanning is routinely used in clinical practice with the application of exogenous contrast agents (CAs) to enhance the contrast between lesions and surrounding normal tissues.2Strong paramagnetic gadolinium (Gd3+) chelates are the majority of clinically used MRI CAs, which can accelerate the longitudinal (R1) and transverse (R2) relaxation rates (defined as Ri = 1/Ti, i = 1, 2) of water protons in tissues, leading to increased MR signal intensity and improved imaging quality. Proton relaxivitiy (r1 and r2), the key parameter for evaluating the efficacy of MRI CAs, is defined as the enhancement in relaxation rate (R1 or R2) of water protons per mM concentration of CAs.3 The utilization of high relaxivity contrast agents facilitates equivalent contrast enhancement at lower doses, thereby reducing the risk of systemic toxicity resulting from limited exposure.
The T1 relaxivity of MRI contrast agents is determined by a complicated interplay of various molecular parameters that govern the magnetic dipolar interactions between water protons and unpaired electrons on the paramagnetic metal center. The following three factors are the main contributors: the number of water molecules directly coordinated to the metal center (q), the residence time of the coordinated water molecule (τm), and the rotational correlation time (τR) representing the inverse of the molecular tumbling rate of the complex in solution.4
The rapid molecular tumbling of clinically used small molecule Gd-based contrast agents (GBCAs) in solution, with a rotational correlation time (τR) on the order of tens of picoseconds, does not match the clinically relevant Larmor frequencies (<3.0 T, 127 MHz). As a result, GBCAs exhibit low relaxivity (in the range of 3–4 mM−1 s−1, at 0.47 T and 37 °C), which was far below their theoretically possible value.4,5
In order to obtain high relaxivity contrast agents, efforts have been made to made to optimize τR by conjugating Gd(III) complexes to nanoparticles,6 and macromolecular architectures7 (proteins,8,9 plolymers,10–12 and dentrimers13–15) to slow the rotation motion of the agent in solution.16
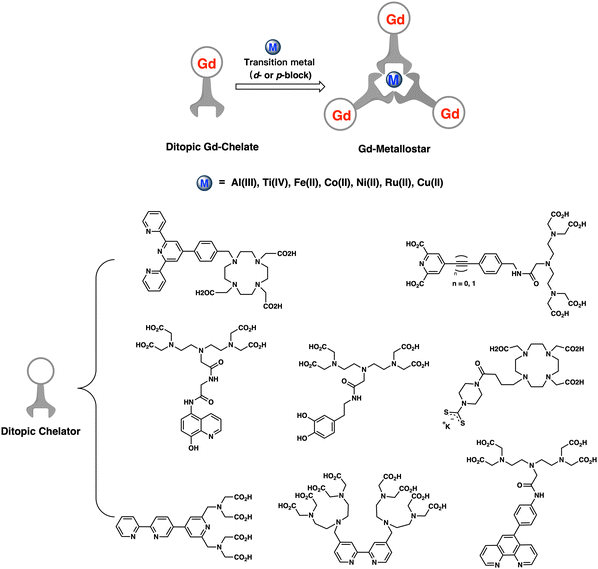
Supramolecular architectures, known as ‘metallostar’, represent another effective approach for obtaining high per Gd relaxivity contrast agents. This approach relies on the coordination-driven self-assembly of heteroditopic Gd(III) chelates in a rigid and compact space around a suitable central d- or p-block metal ion. The formation of supramolecular architectures in a rigid and compact space allows for the optimization of multiple sets of parameters related to molecular motion in solution. For example, increasing the molecular weight while restricting free rotation of the Gd center can be achieved simultaneously (see Chart 1).
Gd-metallostars, as pioneered by Toth, Merbarch, Desreux and others, are based on various heteroditopic ligands that incorporate two different complexing moieties, such as standard polyaminocarboxylate-based ligands for Gd(III) and diverse bidentate or tridentate ligands for central transition metal ions (Al(III), Ti(IV), Fe(II), Co(II), Ni(II), Ru(II), and Cu(II)) including derivatives of bipyridine, terpyridine, 8-hydroxylquinoline, phenanthroline, dipicolinic acid, and dithiocarbamate and catechol (Chart 1).17–19
In recent years, the safety of GBCAs has been questioned due to the emergence of nephrogenic systemic fibrosis (NSF)20,21 and brain gadolinium retention,22–24 triggering investigation into alternatives to Gd-based CAs. In particular, good biocompatible Mn(II)-based chelates with excellent thermodynamic stability and kinetic inertness have been designed and developed as contrast agents.25,26
However, small molecule manganese-based contrast agents (MBCAs) also suffer from low relaxivity for the same reasons as clinically used GBCAs, as discussed above. Moreover, Mn(II) (S = 5/2) is less paramagnetic compared to Gd(III) (S = 7/2). In this context, by taking advantage of the established metal-catecholate chemistry,27–29 we designed a novel Mn(II)-metallostar contrast agent in which three Mn(II) complexes are assembled around high-valence transition metal (Fe(III), Ti(IV)) ions to form a tetrametallic species (ML3Mn3). As shown in Chart 2, the ditopic ligand (L) derived from L-Dopa contains an EDTA moiety and a catechol group, serving as the complexing unit for the Mn(II) ion and transition metal ions (M = Ti4+ or Fe3+), respectively.
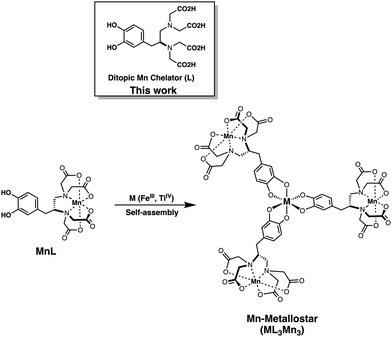 | ||
| Chart 2 The structures of ditopic ligand (L), heteroditopic Mn(II) chelate (MnL), and Mn-metallostar (ML3Mn3) discussed in this paper. | ||
Additionally, as demonstrated in precedent studies like in the Fe(III)-Tiron system,30 the Fe(III)-catechol core can provide a relatively intense and broad absorption band in the visible to near-infrared (NIR) region due to ligand-to-metal charge transfer (LMCT) and ligand-localized π–π* electronic transitions, providing this Mn(II)-metallostar with potential for NIR-responsive phototherapy properties.
Phototherapy, including photothermal therapy (PTT) and photodynamic therapy (PDT), has emerged as a promising therapeutic modality for cancer in recent years. It has attracted increasing attention due to its advantages of non-invasiveness, low side effects, and remote controllability.31–35 Irritation light in windows I and II of NIR ranging from 650 to 950 and 1000 to 1350 nm, respectively, possess remarkable benefits, including deeper penetration of biological tissue, less tissue scattering and absorption, and low photo-damage. Therefore, photosensitizers that can be irritated in the so-called biological transparency windows (NIR I and NIR II) are highly desired for achieving efficient phototherapy in vivo.
Based on the attractive properties of high relaxivity and NIR optical absorption of the metallostar structure discussed above, herein we report the synthesis and characterization of a new type of Mn(II)-metallostar (ML3Mn3, Chart 2). This metallostar exhibits high per Mn relaxivities and NIR-responsive synergistic effects of PTT and PDT, making it an attractive theranostic agent for MRI-guided phototherapy.
Results and discussion
Synthesis and characterization
The synthesis of the catechol-EDTA-based ligand (L) and the corresponding Mn(II) complex (MnL) was described in our previous study for the development of a T1 − T2 dual-modal MRI contrast agent based on superparamagnetic iron oxide nanocrystals with a surface coated with MnL.36 In the present study, saturated tris-coordination Mn(II)-metallostars (Mn: M = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, M = Fe(III), Ti(IV)) were prepared at pH = 8.5 due to the presence of the mixed coordination form of bis and tris-coordination complexes at pH < 7.8 when Fe(III) was used as the central metal ion.37
1, M = Fe(III), Ti(IV)) were prepared at pH = 8.5 due to the presence of the mixed coordination form of bis and tris-coordination complexes at pH < 7.8 when Fe(III) was used as the central metal ion.37
With the addition of iron(III) acetylacetonate (Fe(acac)3) or titanium(IV) oxide acetylacetonate (TiO(acac)2) to MnL aqueous solution, the color of the solution changed from light yellow to purple-black or orange-red, respectively. Pronounced changes in the color of the above reaction solutions indicated the coordination of the catechol group with metal ions.38 Pure dark violet (Fe(III)) or orange-red (Ti(IV)) solid products were precipitated from the aqueous solution by adding isopropyl alcohol or acetone. High-resolution mass spectrometry (HRMS, ESI+) revealed significant peaks at 1455.0683 and 1446.0740 m/z (Fig. 1), in good agreement with the calculated mass of H+ adducts [C51H48Mn3FeN6O30H10]+ (1455.0684 m/z) and [C51H48Mn3TiN6O30H9]+ (1446.0741 m/z), respectively. The results confirmed a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Mn2+versus Fe3+/Ti4+ in these complexes. Therefore, the structure of the metallostars can be represented as FeL3Mn3 or TiL3Mn3.
1 ratio of Mn2+versus Fe3+/Ti4+ in these complexes. Therefore, the structure of the metallostars can be represented as FeL3Mn3 or TiL3Mn3.
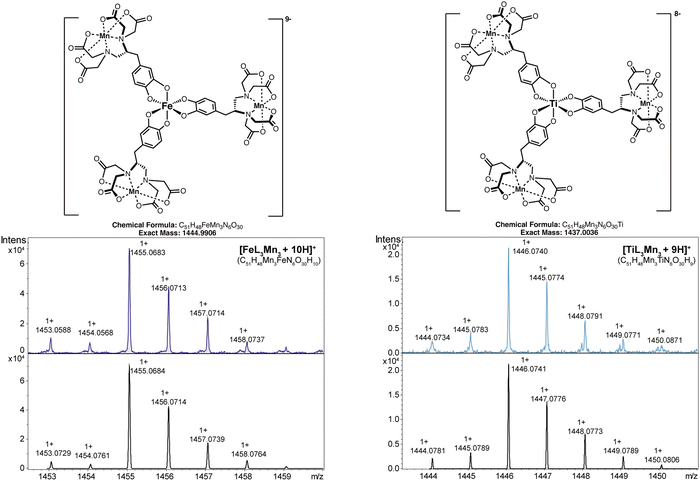 | ||
| Fig. 1 Measured and calculated isotope distribution for the single charged ions of FeL3Mn3 and TiL3Mn3 (high resolution mass (HRMS, ESI+)). | ||
Photophysical study
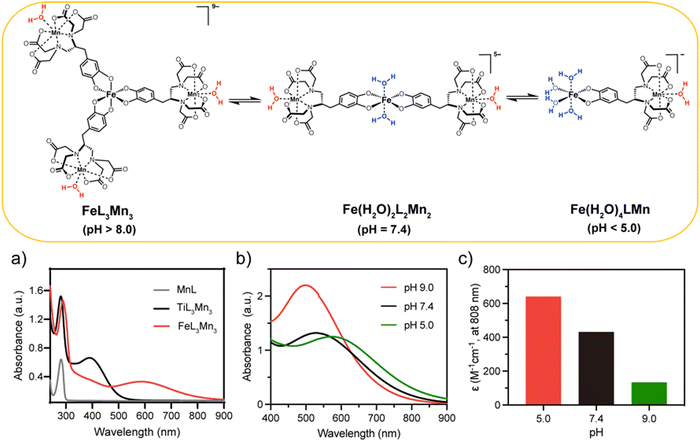
Water solubility is high for all three complexes (MnL, TiL3Mn3 and FeL3Mn3) and their aqueous solutions exhibit lavender, yellow and blue colors, respectively (Fig. S1, ESI†). The absorption spectrum of aqueous solutions of MnL, TiL3Mn3 and FeL3Mn3 (in HEPES buffer, pH = 7.4, 0.1 M) is shown in Fig. 2(a). The characteristic strong absorption peaks of MnL and TiL3Mn3 located at 281 nm are attributed to the n–π* and π–π* intra-ligand electronic transitions, while a slight red-shift to 289 nm is seen for FeL3Mn3. In addition, due to the presence of ligand-to-metal charge transfer (LMCT) in the 3d metal-catecholate structure in the Mn(II)-metallostar core, new broader absorption bands were observed for metallostars than for monomeric MnL, ranging from 400 to 850 nm for FeL3Mn3 and 350 to 550 nm for TiL3Mn3 (Fig. 2a). Therefore, the low-energy LMCT absorption band extending into the near-infrared (NIR) region makes FeL3Mn3 a potential photosensitizer for exploring the photothermal/photodynamic effects triggered by single-wavelength NIR light (808 nm) and the performance of TiL3Mn3 will not be discussed further in this study.As reported by Nucera et al.30 and Charkoudian et al.,37 Fe(III)-catechol (Cat) type complexes demonstrated pH-dependent coordination equilibria, existing as mono-, bis-, and tris-Cat coordination forms with different Fe(III)-hydrated states ([Fe(Cat)x(H2O)6−2x], x = 1, 2, 3) when the pH varied from acidic to alkaline values. In this work, as shown in Fig. 2(b) (and Fig. S2, ESI†), the tris-coordinated FeL3Mn3 (λmax ∼ 493 nm) predominated at pH > 8.0, while the bis-coordinated [FeL2Mn2(H2O)2] (λmax ∼ 530 nm) and mono-coordinated [FeLMn(H2O)4] (λmax ∼ 600 nm) are predominant in the pH range of 5.0–7.0 and at pH < 5.0, respectively. At pH 7.4, the Mn-metallostar system existed as an equilibrium of tris-/bis-coordination species, with heavy overlap in their absorption spectrum from ∼400–620 nm.
Interestingly, the pH-dependent optical absorption displayed by the Fe(III)–Mn(II) metallostar is analogous to that of the Fe(III) complex (Fe-ZDS) reported by Liang et al.39 As depicted in Fig. 2(c), the molar absorption coefficient of metallostar at 808 nm varies significantly with values of 641, 432 and 134 M−1 cm−1 at pH 5.0, 7.4 and 9.0, respectively. This pH-responsive optical absorption could facilitate precise photothermal tumor therapy, since it enables selective activation of the photothermal effect in the acidic tumor microenvironment,40 minimizing damage to normal cells. Moreover, FeL3Mn3 exhibited good stability in FBS solution or PBS solution (Fig. S3, ESI†), suggesting that FeL3Mn3 is promising for in vivo MR imaging.
Variable-temperature 17O-NMR study
Variable-temperature 17O-NMR is a powerful technique to directly probe the coordination environments including the hydration state and dynamic parameters of paramagnetic complexes of interest for MRI applications.41 For Mn(II) complexes, the observed maximum water 17O transverse relaxivity (rO2max, mM−1 s−1) is extremely sensitive to the number of inner-sphere water ligands (q) coordinated to the Mn(II) ion (q = r02max/510 ± 100 mM−1 s−1).42 As shown in Fig. 3, the Fe–Mn Metallostar at pH 9.0 has a temperature-dependent r02 curve similar to that of monomeric MnL, with the measured r02max values of 450 and 480 mM−1 s−1, respectively. This revealed a tris-coordinated form for metallostar ([FeL3Mn3]) with one bound water on Mn(II) and no hydration on Fe(III) at pH 9.0. However, an increased r02 for the metallostar with an r02max of 560 mM−1 s−1 was observed within the same temperature range at pH 7.4. This may indicate the equilibrium of tris-/bis-coordination forms for metallostar at pH 7.4 and a hydrated state of Fe(III) center with two bound water molecules in the bis-coordinated form ([FeL2(H2O)2Mn2]).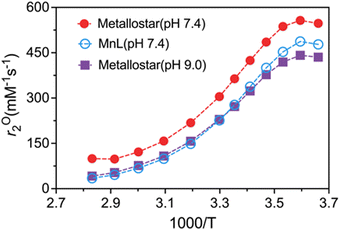 | ||
| Fig. 3 Variable-temperature 17O NMR studies of monomeric MnL and Fe(III)–Mn(II) metallostar at pH 9.0 and 7.4. | ||
Therefore, the combination of the 17O NMR and UV-vis experiments provides definitive evidence that the metallostar adopts a tris-coordinated form at pH 9.0 and an equilibrium between tris-and bis-coordination at pH 7.4 (Fig. 2).
Relaxometric study
In HEPES buffer (pH = 7.4, 0.1 M) and at 0.47 T, 32 °C, the per paramagnetic metal ion relaxivities (r1) of the metallostars FeL3Mn3 and TiL3Mn3 were determined to be 6.06 and 7.87 mM−1 s−1, respectively. This is substantially higher than the r1 of the monomeric MnL (3.66 mM−1 s−1). The lower r1 of the Fe–Mn metallostar compared to the Ti(IV) analogue suggests the Fe(III) center exhibits an equilibrium between tris-and bis-coordination (lower molecular weight) at pH 7.4 based on the pH-dependent coordination chemistry elucidated by NMR and UV-vis spectroscopy. In contrast, the Ti–Mn metallostar maintains a high molecular weight ML3Mn3 structure at pH 7.4, consistent with its higher per paramagnetic metal ion relaxivity. In addition to the improved relaxivities, the observed low r2/r1 ratio close to 1.00 (1.19 for FeL3Mn3 and 1.25 for TiL3Mn3) indicated that Mn-metallostars are favorable T1 agents for T1-weighted imaging.Compared to the relaxivities of the tri-/bis-coordination equilibrium system of the Fe(III)–Mn(II) metallostar measured at pH = 7.4 (in HEPES buffer, 0.1 M), tris-coordination FeL3Mn3 (pH = 9.0, in Tris buffer, 0.1 M) showed reduced relaxivity (r1 = 5.29 mM−1 s−1 and r2 = 6.83 mM−1 s−1). The relaxivity changes can be attributed to the different hydrated states of the Fe(III) center in bis- and tri-coordination of Fe(III)–Mn(II) metallostar. Specifically, two-water molecules coordinate to the Fe(III) center in the bis-coordinated form, while no water molecule coordinates in the tris-coordinated form of the Fe(III)–Mn(II) metallostar (Fig. 2 and Table 1).
Photothermal effect of Fe–Mn metallostar
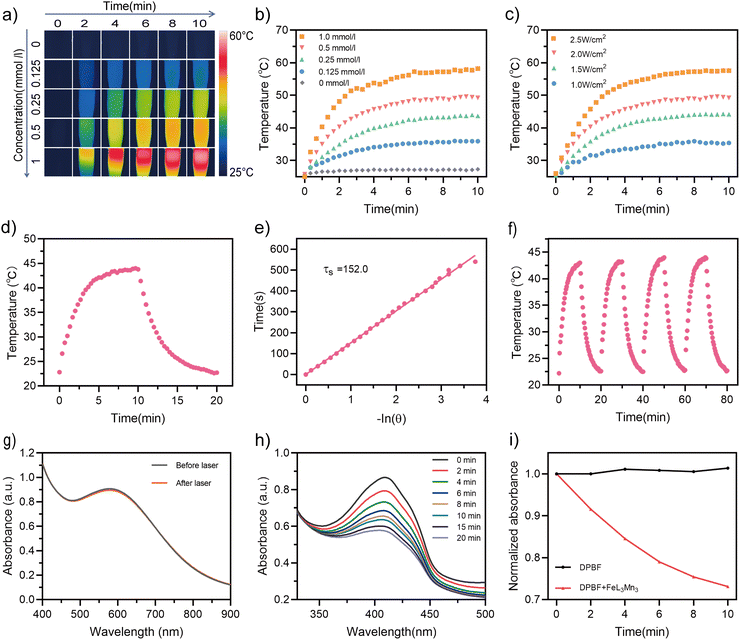
The NIR absorption of the Fe–Mn metallostar enables us to systematically investigate its photothermal effect with varying FeL3Mn3 concentration and laser power density under 808 nm. Fig. 4(a) shows infrared thermographs of the temperature elevation for Fe–Mn metallostar solution at different concentrations (in HEPES buffer, pH = 7.4, 0.1 M) irritated at a fixed laser power density (2.0 W cm−2). Under these conditions, when irritated for 10 minutes, the heating curves showed a concentration-dependent photothermal effect with a maximum temperature increment of 23.4 °C for the 0.5 mM Fe–Mn metallostar solution compared to 1.6 °C for the control solution without photosensitizer (Fig. 4b). As Fig. 4(c) indicates, the heating curves also showed a laser power-dependent photothermal effect with the highest temperature reaching 57.6 °C under an NIR (808 nm) laser power density of 2.5 W cm−2 when the Fe–Mn metallostar concentration was set to 0.5 mM.To assess the photothermal performance, we exposed the Fe–Mn metallostar solution (0.25 mM) to laser irritation (808 nm, 2.0 W cm−2) for 10 min until it reached a temperature plateau, and then allowed it to cool naturally for 10 min until it returned to room temperature (Fig. 3d). The photothermal conversion efficiency (η) was calculated to be 20.3% using the reported method.43 The time constant was obtained using a linear regression curve between the cooling stage and the negative natural logarithm of the thermal driving force temperature of the solution (Fig. 4e).
In addition, under on–off 808 nm laser irritation (2.0 W cm−2), the Fe–Mn metallostar solution temperature maintained a coincident reciprocation of the rising and cooling process (Fig. 4f), suggesting the excellent photothermal stability of FeL3Mn3. This was further confirmed by the negligible changes in the absorption spectrum measured before and after on–off 808 nm laser irritation (Fig. 4g).
Singlet oxygen-generating capability of Fe–Mn metallostar
Given the intense LCMT band (600–800 nm) and the excellent photothermal stability of Fe–Mn metallostar, we further explored its efficacy as a NIR light photodynamic therapy (PDT) agent. In this study, 1,3-diphenylisobenzofuran (DPBF) was used as a singlet-oxygen (1O2) trapping agent to evaluate the ability of Fe–Mn metallostar to produce 1O2 by monitoring the change in the absorption intensity at 410 nm.44,45 As shown in Fig. 4(h) and (i), the degradation rate of DPBF exhibited a linear positive correlation with the irritation time (808 nm, 1.5 W cm−2), indicating that 1O2 generated by FeL3Mn3 can continuously consume DPBF when exposed to laser light. In addition, the 1O2 quantum yield was calculated to be 24.8% using indocyanine green (ICG) (ΦΔ = 0.14 in water)46 as the standard reference (Fig. S5, ESI†).47Photocytotoxicity on BxPC-3 cells
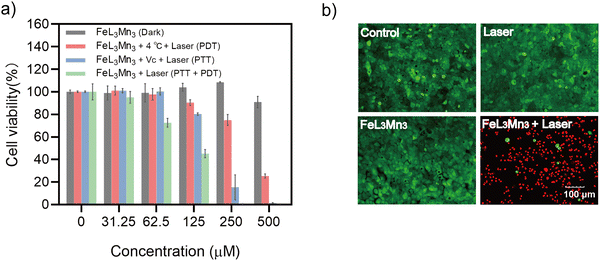
Encouraged by Fe–Mn metallostar's promising synergistic PTT and PDT effects, we investigated its photocytotoxicity effect on the BxPC-3 cell line. As shown in Fig. S6 (ESI†), in the cell culture medium loaded with 0.5 mM Fe–Mn metallostar, the temperature rapidly increased by 28.6 °C over 6 minutes of 808 nm laser irradiation (2 W cm−2), whereas it only increased by 2.7 °C in the absence of Fe–Mn metallostar. These results suggested that Fe–Mn metallostar could effectively produce photocytotoxicity with a large amount of heat combined with ROS (1O2) to induce cell death under 808 nm laser irritation.Indeed, the phototherapy effect of Fe–Mn metallostar was confirmed by in vitro dark cytotoxicity and photocytotoxicity tests (Fig. 5a). CCK-8 assays showed that Fe–Mn Metallostar had low cytotoxicity and excellent biocompatibility, with cell viability higher than 90% after 24 h of incubation in the dark with up to 500 μM Fe–Mn metallostar alone. However, when 250 μM of Fe–Mn metallostar was loaded and irritated under 808 nm laser light at a power density of 2.0 W cm−2, Fe–Mn metallostar induced dose-dependent cell death and the survival rate was close to zero (Fig. 5a).
To further clarify the relative contributions of photothermal and photodynamic effects to the cytotoxicity, two control experiments were performed. To investigate the photodynamic effect alone, the environmental temperature was maintained at 4 °C using an ice bath to suppress hyperthermia. To study the photothermal effect alone, sodium ascorbate was added to scavenge singlet oxygen to shield the photodynamic effect (Fig. 5a and Fig. S7, ESI†). These control experiments suggested Fe–Mn metallostar can generate synergistic photodynamic and photothermal cytotoxic effects, while the photothermal effect on BxPC-3 cells was more potent than the photodynamic effect under the conditions tested.
The occurrence of rapid cell death induced by phototoxicity was further confirmed by the live/dead cell staining assay, in which only the Fe–Mn metallostar-loaded group irritated with an 808 nm laser showed significant cell death when living cells were stained with calcein acetoxymethyl ester (calcein-AM, green fluorescence) and the dead cells were stained with propidium iodide (PI, red fluorescence) (Fig. 5b).
MR imaging study
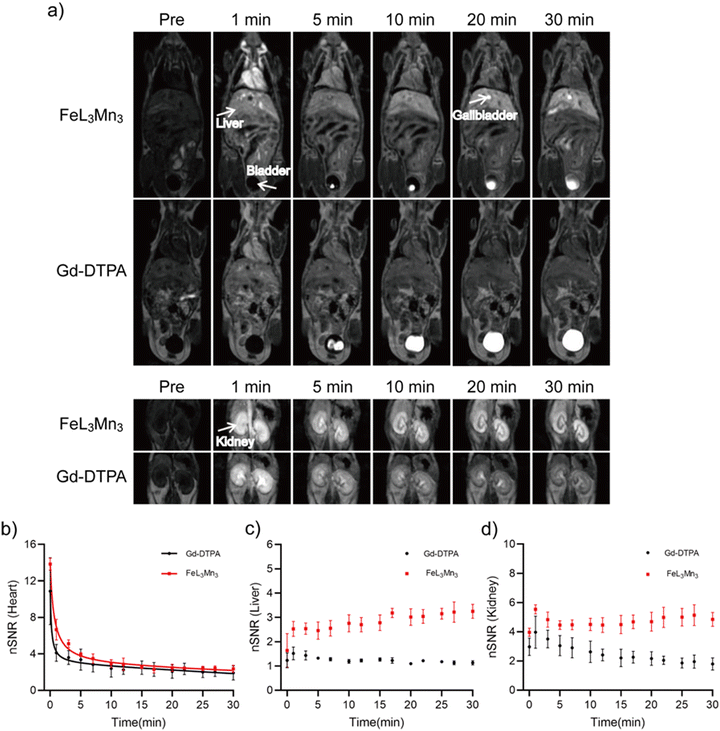
The results above suggest that FeL3Mn3 holds great promise as a theranostic agent for MR imaging-guided phototherapy based on its high relaxivity per Mn ion and NIR responsive synergistic PTT/PDT effects. Finally, we conducted in vivo MRI studies in normal mice to evaluate the contrast enhancement, distribution, and elimination of FeL3Mn3 compared to the commercially available Gd-DTPA. During the arterial phase (1 min post-injection) as shown in Fig. 6(a), a low-dose of Fe–Mn metallostar (25 μmol kg−1) produced strong intravascular contrast enhancement, with the heart, carotid arteries, abdominal aortal, and renal arteries more clearly delineated compared to Gd-DTPA (100 μmol kg−1). Dynamic MR imaging showed that Fe–Mn metallostar undergoes a combination of renal and hepatobiliary clearance, with significant enhancements of the bladder and gallbladder observed at 5 min and 20 min post-injection, respectively. Fig. 6(b)–(d) depict the change in normalized signal-to-noise (nSNR) for the heart (blood), liver, and kidney within 30 min post-injection, indicating a low-dose of FeL3Mn3 produced better enhancement in the heart (blood), liver, and kidney compared to Gd-DTPA, with a higher nSNR value measured during imaging time. The plasma pharmacokinetics of Fe–Mn Metallostar, estimated based on Fig. 6(b), indicate that Fe–Mn metallostar has a similar plasma pharmacokinetic to Gd-DTPA as a typical biexponential plasma clearance with a half-life of distribution as t1/2,α = 0.56 ± 0.14 min and a half-life of elimination as t1/2,β = 25.71 ± 0.69 min. Importantly, the highly negatively charged anion nature of Fe–Mn metallostar may potentially enable tumor accumulation despite its rapid blood clearance due to the tumor's unique pathophysiological microenvironment. However, further in vivo studies are still needed to validate this hypothesis experimentally.Experimental
Materials and apparatus
All experiments were executed in accordance with the Guidelines for Care and Use of Laboratory Animals and were approved by the Ethics Committee of North Sichuan Medical College (NSMC, Appl. No. 202155). Iron(III) acetylacetonate (Fe(acac)3) was purchased from ACROS (New jersey, USA). Titanium(IV) oxide acetylacetonate (TiO(acac)2) was purchased from Alfa-Asia (Shanghai, China). 1,3-Diphenylisobenzofuran (DPBF) was purchased from Macklin (Shanghai, China). Cell counting Kit-8 (CCK-8) and calcein/PI cell viability/cytotoxicity assay kit were purchased from beyotime (Shanghai, China). Ultrapure water in all experiments was obtained using an ultrapure purification system. High resolution mass spectroscopy (HRMS) was performed on an Agilent QTOF 6500. The Mn concentrations were determined using an inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer Nexion 350×, USA) system. The zeta potential was determined with dynamic light scattering (DLS, Malvern Zetasizer Nano ZS 90, UK).Preparation of metallostars
The synthetic route for MnL was derived from a previous work.36 Next, the MnL (149.4 mg, 0.20 mmol) was dissolved in pure water (10 mL), and the pH of the ML solution was adjusted to ∼9.0 through the addition of NaOH solution (1 M). Fe(acac)3 (31.9 mg, 0.09 mmol; acac = acetylacetonate) dissolved in CH2Cl2 (10 mL) was added to the above solution and the solution was stirred for 12 h under a N2 atmosphere. The unreacted Fe(acac)3 was repeatedly extracted (2–3 times) with CH2Cl2, and then the CH2Cl2 layer was removed. After lyophilization, the crude product was obtained. The crude product was redissolved in pure water (5 mL) and the pH of the solution was adjusted carefully to ∼8.5 with NaOH solution (1 M). The complex was then precipitated with isopropanol (40 mL) and the purplish-black solid product was obtained by centrifugation and drying. Yield: 74%. HRMS m/z: for C51H48Mn3FeN6O30, calcd: 1455.0684 [M + 10H]+; found: 1455.0683 [M + 10H]+. The TiL3Mn3 was prepared with some slight modifications. Briefly, TiO(acac)2 (6.0 mg, 0.023mmol) were added to the ML (49.8 mg, 0.067 mmol) aqueous solution. The TiL3Mn3 was synthesized according to the procedures above, and finally the orange-red solid product was obtained by precipitation with acetone. Yield: 89%. HRMS m/z: for C51H48Mn3TiN6O30, calcd: 1446.0741 [M + 9H]+; found: 1446.0740 [M + 9H]+.Relaxivity
Relaxivities of MnL, FeL3Mn3 and TiL3Mn3 were measured with a 0.47 T NMR contrast agent relaxation rate analyzer (PQ001-20-015V, Shanghai Newmag Electronic Technology Co., Ltd). Sample solutions (in HEPES buffer, pH = 7.4, 0.1 M) with different manganese ion concentrations (0.0625, 0.125, 0.25, 0.5 and 1.0 mM) were prepared first. Longitudinal (T1) relaxation and transverse (T2) relaxation were acquired via an IR (inversion recovery) sequence and a CPMG (Carr–Purcell–Meiboon–Gill) pulse sequence, respectively. Then, the slope of the linear fit of manganese ion concentrations with T1 and T2 relaxation times were used for calculating the longitudinal (r1) and transverse (r2) relaxivities.Photothermal performances of Fe–Mn metallostar
The temperature elevation of Fe–Mn metallostar solutions (0.3 mL, in HEPES buffer, pH = 7.4, 0.1 M) under 808 nm laser irritation (VCL-808nmM1-7W, China) was measured at different concentrations (0, 0.125, 0.25, 0.5 and 1 mM with a power density of 2 W cm−2) and at different laser power densities (1.0, 1.5, 2.0 and 2.5 W cm−2 with a concentration of 0.5 mM). The irritation time was 10 min and all the temperature changes were monitored by infrared thermography (Fotric226s, China) every 20 s. The PCE of the Fe–Mn metallostar was detected by irradiating (808 nm, 2 W cm−2) the sample solution (0.3 mL, in HEPES buffer, pH = 7.4, 0.1 M) of 0.25 mM for 10 min and then cooling for 10 min. To evaluate the photostability of FeL3Mn3 under laser irritation, a 0.25 mM Fe–Mn metallostar solution (0.3 mL, in HEPES buffer, pH = 7.4, 0.1 M) underwent four laser (808 nm, 2.0 W cm−2) on/off cycles. In each cycle, the laser was turned on to irritate the Fe–Mn metallostar and turned off for 10 min, respectively, and the absorption spectrum was measured before and after laser irritation.Reactive oxygen species (1O2) detection
The 1O2 detection of FeL3Mn3 was performed using 1,3-diphenylbenzofuran (DPBF) as a probe. 1.5 mL of DPBF solution (40 μM, dissolved in ethanol) was mixed with 1.5 mL of Fe–Mn metallostar solution (160 μM, dissolved in water), irritated with an 808 nm laser (1.5 W cm−2), and the absorbance changes of DPBF at 410 nm were recorded using a UV-Vis spectrophotometer (UV-1900i, Japan) every 2 min.In vitro cytotoxicity assay
BxPC-3 cells were seeded in 96-well plates (100 μL, 1 × 104 cells per well) and incubated in RPMI-1640 culture medium with 10% (v/v) fetal bovine serum and 1% (v/v) streptomycin/penicillin at 37 °C in 5% CO2 for 24 h. Afterwards, each group was co-incubated with Fe–Mn metallostar at different concentrations (0, 31.25, 62.5, 125, 250 and 500 μM, respectively) for another 24 h. After washing with PBS, the fresh medium (100 μL) containing Cell Counting Kit-8 (CCK-8) solution (10 μL per well) was added to each well and the absorbance of each well was measured at 450 nm using a multifunctional microplate reader (Varioskan LUX, Singapore) after 1 h of incubation to calculate cell viability.In vitro phototherapy
To evaluate photocytotoxicity, BxPC-3 cells seeded in 96-well plates (100 μL, 1 × 104 cells per well) were incubated at 37 °C in 5% CO2 for 24 h. Afterwards, various concentrations of Fe–Mn metallostar (0, 31.25, 62.5, 125, 250 and 500 μM, respectively) were incubated with cells for 4 h and then each well was irritated with an 808 nm laser (2.0 W cm−2) for 10 min. Subsequently, the cells were incubated continuously for another 24 h. Cell survival was calculated using a standard CCK-8 assay. Two control experiments were conducted to further investigate the relative contributions of photothermal therapy and photodynamic therapy to the cytotoxicity. For PDT only: all the experiments were the same as the above and BxPC-3 cells were irritated with NIR laser (808 nm, 2.0 W cm−2, 10 min) at 4 °C to suppress temperature elevation. For PTT only: all the experiments were the same as the above and BxPC-3 cells were co-incubated with a ROS scavenger Vitamin C (0.5 mM) to exclude the PDT effect, followed by NIR (808 nm, 2.0 W cm−2) irritation for 10 min.Live and dead cell assay
BxPC-3 cells were plated in a confocal Petri dish (1 mL, 1 × 105 cells per dish) and incubated at 37 °C in 5% CO2 for 24 h. Then, BxPC-3 cells were divided into four groups: (1) control, (2) laser irritation only (808 nm, 2 W cm−2, 10 min), (3) Fe–Mn metallostar only (500 μM) and (4) Fe–Mn metallostar (500 μM) + laser irritation (808 nm, 2 W cm−2, 10 min). After 24 h of co-cultivation, different treatment groups were co-stained with the AM/PI staining method for 30 min, and then cell imaging was performed using an Olympus laser scanning confocal microscope.MR imaging protocol
Swiss mice (Male, 20–25 g, purchased from Laboratory Animal Center of NSMC, Sichuan, China) were maintained under anesthesia by inhalation of isoflurane (RWD Life Science, Shenzhen, China) at an anesthetic concentration of 0.8–1.5% and 20–30 mL O2 per kg. Baseline imaging was performed using a system-equipped mice coil, under a 3.0 T MR scanner (Discovery MR750, GE Medical System, Milwaukee, WI) and multi-slice 2D T1WI-FSPGR sequence, followed by injection of FeL3Mn3 (25 μmol kg−1) or Gd-DTPA (100 μmol kg−1) to obtain contrast-enhanced images of mice (n = 3, per group). The MRI parameters were as follows: TE = 2.3 ms, TR = 6.1 ms, FA = 30°, FOV = 9.0 × 6.3 cm2, matrix size = 256 × 256, slices = 40, slice thickness = 1.0 mm, NEX = 1.00, and bandwidth = 62.50 Hz. The normalized signal-to-noise ratio (nSNR) was calculated according to the formula: nSNR = SNRpost/SNRpre and SNR = SI/SDair, where SI stands for the signal intensity and SDair stands for the standard deviation of the noise estimated from the background air.Conclusions
The study presented here demonstrates a unique multifunctional Mn(II)-metallostar based on coordination-driven self-assembly, in which the metallostar with the core of Fe(III)-catechol exhibits high per Mn relaxivity and good NIR-irritable PTT/PDT effects. Cellular phototoxicity studies confirmed the good biosafety and phototherapeutic effects of FeL3Mn3. The in vivo MRI study on mice demonstrated that a low-dose of Fe–Mn metallostar (25 μmol kg−1) can provide superior contrast enhancement compared to Gd-DTPA (100 μmol kg−1) with rapid blood clearance and a mixed route of hepatobiliary and renal excretion, which considerably reduces the potential side effects of the metallostar complex. Our findings may provide useful guidance for the future development of non-gadolinium-based metallostars with attractive theranostic potential.Abbreviations
| AM | Acetoxymethyl ester |
| Cas | Contrast agents |
| CCK-8 | Cell counting kit-8 |
| CE | Contrast enhanced |
| CPMG | Carr–Purcell–Meiboon–Gill |
| DLS | Dynamic light scattering |
| DPBF | 1,3-Diphenylisobenzofuran |
| DTPA | Diethylenetriaminetetraacetic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| GBCAs | Gadolinium-based contrast agents |
| HRMS | High-resolution mass spectrometry |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| IR | Inversion recovery |
| LMCT | Ligand-to-metal charged transfer |
| MRI | Magnetic resonance imaging |
| NIR | Near-infrared |
| NMR | Nuclear magnetic resonance |
| nSNR | Normalized signal-to-noise ratio |
| NSF | nephrogenic systemic fibrosis |
| PDT | Photodynamic therapy |
| PI | Propidium iodide |
| PTT | Photothermal therapy |
| ROS | Reactive oxidative species |
Author contributions
The manuscript was written through contributions of all authors. H.-Y. W. and J. Z. conceived the project. J. Z., H.-Y. W. and Z.-H. L. designed the experiments. H.-Y. W. and Z.-H. L. conducted the synthesis and characterization of all compounds. H.-Y. W., Y. X., Y. L. and Z.-H. H. carried out imaging study. J. Z. and H.-Y. W. prepared the manuscript. All authors have approved the final version of the manuscript. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors acknowledge the financial support of the National Natural Science Foundation of China (81671675, 21172025), and the Science and Technology Project of Municipal School Strategic Cooperation, Nanchong (NSMC20170101, 18SXHZ0091,18SXHZ0379, 20SXQT0180).References
- V. S. Vassiliou, D. Cameron, S. K. Prasad and P. D. Gatehouse, Magnetic resonance imaging: Physics basics for the cardiologist, JRSM Cardiovasc Dis, 2018, 7, 2048004018772237 Search PubMed
.
- E. Kanal, Gadolinium based contrast agents (GBCA): Safety overview after 3 decades of clinical experience, Magn. Reson. Imaging, 2016, 34, 1341–1345 CrossRef CAS PubMed
.
- R. B. Lauffer, Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: theory and design, Chem. Rev., 1987, 87, 901–927 CrossRef CAS
.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS PubMed
.
- K. Hanaoka, A. J. Lubag, A. Castillo-Muzquiz, T. Kodadek and A. D. Sherry, The detection limit of a Gd3+-based T1 agent is substantially reduced when targeted to a protein microdomain, Magn. Reson. Imaging, 2008, 26, 608–617 CrossRef CAS PubMed
.
- L. M. Manus, D. J. Mastarone, E. A. Waters, X. Q. Zhang, E. A. Schultz-Sikma, K. W. Macrenaris, D. Ho and T. J. Meade, Gd(III)-nanodiamond conjugates for MRI contrast enhancement, Nano Lett., 2010, 10, 484–489 CrossRef CAS PubMed
.
- T. Barrett, H. Kobayashi, M. Brechbiel and P. L. Choyke, Macromolecular MRI contrast agents for imaging tumor angiogenesis, Eur. J. Radiol., 2006, 60, 353–366 CrossRef PubMed
.
- Z. Zhang, M. T. Greenfield, M. Spiller, T. J. McMurry, R. B. Lauffer and P. Caravan, Multilocus binding increases the relaxivity of protein-bound MRI contrast agents, Angew. Chem., Int. Ed., 2005, 44, 6766–6769 CrossRef CAS PubMed
.
- P. Caravan, G. Parigi, J. M. Chasse, N. J. Cloutier, J. J. Ellison, R. B. Lauffer, C. Luchinat, S. A. McDermid, M. Spiller and T. J. McMurry, Albumin binding, relaxivity, and water exchange kinetics of the diastereoisomers of MS-325, a gadolinium(III)-based magnetic resonance angiography contrast agent, Inorg. Chem., 2007, 46, 6632–6639 CrossRef CAS PubMed
.
- Y. Li, M. Beija, S. Laurent, L. V. Elst, R. N. Muller, H. T. T. Duong, A. B. Lowe, T. P. Davis and C. Boyer, Macromolecular Ligands for Gadolinium MRI Contrast Agents, Macromolecules, 2012, 45, 4196–4204 CrossRef CAS
.
- M. Younis, V. Darcos, C. Paniagua, P. Ronjat, L. Lemaire, B. Nottelet, X. Garric, Y. Bakkour, J. H. El Nakat and J. Coudane, MRI-visible polymer based on poly(methyl methacrylate) for imaging applications, RSC Adv., 2016, 6, 5754–5760 RSC
.
- T. R. Berki, J. Martinelli, L. Tei, H. Willcock and S. J. Butler, Polymerizable Gd(III) building blocks for the synthesis of high relaxivity macromolecular MRI contrast agents, Chem. Sci., 2021, 12, 3999–4013 RSC
.
- Z. Xiong, Y. Wang, J. Zhu, Y. He, J. Qu, C. Effenberg, J. Xia, D. Appelhans and X. Shi, Gd-Chelated poly(propylene imine) dendrimers with densely organized maltose shells for enhanced MR imaging applications, Biomater. Sci., 2016, 4, 1622–1629 RSC
.
- C. Sun, H. Lin, X. Gong, Z. Yang, Y. Mo, X. Chen and J. Gao, DOTA-Branched Organic Frameworks as Giant and Potent Metal Chelators, J. Am. Chem. Soc., 2020, 142, 198–206 CrossRef CAS PubMed
.
- S. Zhang, V. Lloveras, D. Pulido, F. Liko, L. F. Pinto, F. Albericio, M. Royo and J. Vidal-Gancedo, Radical Dendrimers Based on Biocompatible Oligoethylene Glycol Dendrimers as Contrast Agents for MRI, Pharmaceutics, 2020, 12, 772 CrossRef CAS PubMed
.
- J. Bryson, J. W. Reineke and T. M. Reineke, Macromolecular Imaging Agents Containing Lanthanides: Can Conceptual Promise Lead to Clinical Potential?, Macromolecules, 2012, 45, 8939–8952 CrossRef CAS PubMed
.
- V. Comblin, D. Gilsoul, M. Hermann, V. Humblet, V. Jacques, M. Mesbahi, C. Sauvage and J. F. Desreux, Designing new MRI contrast agents: a coordination chemistry challenge, Coord. Chem. Rev., 1999, 185–186, 451–470 CrossRef CAS
.
- L. Moriggi, A. Aebischer, C. Cannizzo, A. Sour, A. Borel, J. C. Bunzli and L. Helm, A ruthenium-based metallostar: synthesis, sensitized luminescence and (1)H relaxivity, Dalton Trans., 2009, 2088–2095, 10.1039/b818599a
.
- M. Ceulemans, E. Debroye, L. Vander Elst, W. De Borggraeve and T. N. Parac-Vogt, Luminescence and Relaxometric Properties of Heteropolymetallic Metallostar Complexes with Selectively Incorporated Lanthanide(III) Ions, Eur. J. Inorg. Chem., 2015, 4207–4216 CrossRef CAS
.
- P. Marckmann, L. Skov, K. Rossen, A. Dupont, M. B. Damholt, J. G. Heaf and H. S. Thomsen, Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging, J. Am. Soc. Nephrol., 2006, 17, 2359–2362 CrossRef PubMed
.
- J.-M. Idée, N. Fretellier, C. Robic and C. Corot, The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update, Crit. Rev. Toxicol., 2014, 44, 895–913 CrossRef PubMed
.
- T. Kanda, T. Fukusato, M. Matsuda, K. Toyoda, H. Oba, J. Kotoku, T. Haruyama, K. Kitajima and S. Furui, Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy, Radiology, 2015, 276, 228–232 CrossRef PubMed
.
- J. Ramalho, R. C. Semelka, M. Ramalho, R. H. Nunes, M. AlObaidy and M. Castillo, Gadolinium-Based Contrast Agent Accumulation and Toxicity: An Update, AJNR Am. J. Neuroradiol., 2016, 37, 1192–1198 CrossRef CAS PubMed
.
- V. M. Runge, Critical Questions Regarding Gadolinium Deposition in the Brain and Body After Injections of the Gadolinium-Based Contrast Agents, Safety, and Clinical Recommendations in Consideration of the EMA's Pharmacovigilance and Risk Assessment Committee Recommendation for Suspension of the Marketing Authorizations for 4 Linear Agents, Invest. Radiol., 2017, 52, 317–323 CrossRef CAS PubMed
.
- E. M. Gale, I. P. Atanasova, F. Blasi, I. Ay and P. Caravan, A Manganese Alternative to Gadolinium for MRI Contrast, J. Am. Chem. Soc., 2015, 137, 15548–15557 CrossRef CAS PubMed
.
- D. Ndiaye, M. Sy, A. Pallier, S. Meme, I. de Silva, S. Lacerda, A. M. Nonat, L. J. Charbonniere and E. Toth, Unprecedented Kinetic Inertness for a Mn(2+)-Bispidine Chelate: A Novel Structural Entry for Mn(2+)-Based Imaging Agents, Angew. Chem., Int. Ed., 2020, 59, 11958–11963 CrossRef CAS PubMed
.
- V. L. Pecoraro, G. B. Wong and K. N. Raymond, Gallium and indium imaging agents. 2. Complexes of sulfonated catechoylamide sequestering agents, Inorg. Chem., 1982, 21, 2209–2215 CrossRef CAS
.
- B. A. Borgias, S. R. Cooper, Y. B. Koh and K. N. Raymond, Synthetic, structural, and physical studies of titanium complexes of catechol and 3,5-di-tert-butylcatechol, Inorg. Chem., 1984, 23, 1009–1016 CrossRef CAS
.
- X. Tu, S. Luo, X. Luo, Y. Zhao, L. Feng and J. Li, Metal chelate affinity to immobilize horseradish peroxidase on functionalized agarose/CNTs composites for the detection of catechol, Sci. China: Chem., 2011, 54, 1319–1326 CrossRef CAS
.
- A. Nucera, F. Carniato, Z. Baranyai, C. Platas-Iglesias and M. Botta, Characterization of the Fe(III)-Tiron System in Solution through an Integrated Approach Combining NMR Relaxometric, Thermodynamic, Kinetic, and Computational Data, Inorg. Chem., 2023, 62, 4272–4283 CrossRef CAS PubMed
.
- X. Huang, X. Sun, W. Wang, Q. Shen, Q. Shen, X. Tang and J. Shao, Nanoscale metal-organic frameworks for tumor phototherapy, J. Mater. Chem. B, 2021, 9, 3756–3777 RSC
.
- R. Xing, Q. Zou, C. Yuan, L. Zhao, R. Chang and X. Yan, Self-Assembling Endogenous Biliverdin as a Versatile Near-Infrared Photothermal Nanoagent for Cancer Theranostics, Adv. Mater., 2019, 31, 1900822 CrossRef PubMed
.
- L. Zhao, Y. Liu, R. Xing and X. Yan, Supramolecular Photothermal Effects: A Promising Mechanism for Efficient Thermal Conversion, Angew. Chem., Int. Ed., 2020, 59, 3793–3801 CrossRef CAS PubMed
.
- S. Li, W. Zhang, R. Xing, C. Yuan, H. Xue and X. Yan, Supramolecular Nanofibrils Formed by Coassembly of Clinically Approved Drugs for Tumor Photothermal Immunotherapy, Adv. Mater., 2021, 33, 2100595 CrossRef CAS PubMed
.
- R. Chang, Q. Zou, L. Zhao, Y. Liu, R. Xing and X. Yan, Amino-Acid-Encoded Supramolecular Photothermal Nanomedicine for Enhanced Cancer Therapy, Adv. Mater., 2022, 34, 2200139 CrossRef CAS PubMed
.
- C. Wu, T. Chen, L. Deng, Q. Xia, C. Chen, M. Lan, Y. Pu, H. Tang, Y. Xu, J. Zhu, C. Xu, C. Shen and X. Zhang, Mn(II) chelate-coated superparamagnetic iron oxide nanocrystals as high-efficiency magnetic resonance imaging contrast agents, Nanoscale Adv., 2020, 2, 2752–2757 RSC
.
- L. K. Charkoudian and K. J. Franz, Fe(III)-Coordination Properties of Neuromelanin Components:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5,6-Dihydroxyindole and 5,6-Dihydroxyindole-2-carboxylic Acid, Inorg. Chem., 2006, 45, 3657–3664 CrossRef CAS PubMed
5,6-Dihydroxyindole and 5,6-Dihydroxyindole-2-carboxylic Acid, Inorg. Chem., 2006, 45, 3657–3664 CrossRef CAS PubMed .
- M. Albrecht, S. Mirtschin, M. de Groot, I. Janser, J. Runsink, G. Raabe, M. Kogej, C. A. Schalley and R. Fröhlich, Hierarchical Assembly of Helicate-Type Dinuclear Titanium(IV) Complexes, J. Am. Chem. Soc., 2005, 127, 10371–10387 CrossRef CAS PubMed
.
- G. Liang, J. Han and D. Xing, Precise Tumor Photothermal Therapy Guided and Monitored by Magnetic Resonance/Photoacoustic Imaging using A Safe and pH-Responsive Fe(III) Complex, Adv. Healthcare Mater., 2021, 10, e2001300 CrossRef PubMed
.
- X. Zhang, Y. Lin and R. J. Gillies, Tumor pH and its measurement, J. Nucl. Med., 2010, 51, 1167–1170 CrossRef CAS PubMed
.
- D. H. Powell, O. M. N. Dhubhghaill, D. Pubanz, L. Helm, Y. S. Lebedev, W. Schlaepfer and A. E. Merbach, Structural and Dynamic Parameters Obtained from 17O NMR, EPR, and NMRD Studies of Monomeric and Dimeric Gd3+ Complexes of Interest in Magnetic Resonance Imaging:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) An Integrated and Theoretically Self-Consistent Approach1, J. Am. Chem. Soc., 1996, 118, 9333–9346 CrossRef CAS
An Integrated and Theoretically Self-Consistent Approach1, J. Am. Chem. Soc., 1996, 118, 9333–9346 CrossRef CAS .
- E. M. Gale, J. Zhu and P. Caravan, Direct measurement of the Mn(II) hydration state in metal complexes and metalloproteins through 17O NMR line widths, J. Am. Chem. Soc., 2013, 135, 18600–18608 CrossRef CAS PubMed
.
- D. K. Roper, W. Ahn and M. Hoepfner, Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles, J. Phys. Chem. C, 2007, 111, 3636–3641 CrossRef CAS PubMed
.
- T. Entradas, S. Waldron and M. Volk, The detection sensitivity of commonly used singlet oxygen probes in aqueous environments, J. Photochem. Photobiol., B, 2020, 204, 111787 CrossRef CAS PubMed
.
- C. Sui, R. Tan, Y. Chen, G. Yin, Z. Wang, W. Xu and X. Li, MOFs-Derived Fe–N Codoped Carbon Nanoparticles as O2-Evolving Reactor and ROS Generator for CDT/PDT/PTT Synergistic Treatment of Tumors, Bioconjugate Chem., 2021, 32, 318–327 CrossRef CAS PubMed
.
- J. Y. Chen, K. K. Wen, H. Chen, S. Jiang, X. X. Wu, L. Lv, A. D. Peng, S. M. Zhang and H. Huang, Achieving High-Performance Photothermal and Photodynamic Effects upon Combining D–A Structure and Nonplanar Conformation, Small, 2020, 16, e2000909 CrossRef PubMed
.
- J. R. Deng, M. Q. Yang, C. Li, G. Y. Liu, Q. Sun, X. G. Luo and F. S. Wu, Single molecular-based nanoparticles with aggregation-induced emission characteristics for fluorescence imaging and efficient cancer phototherapy, Dyes Pigm., 2021, 187, 7 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available: Photographs of the aqueous solutions, photographs at different pH conditions, the molar absorption coefficient (ε), stability in different mediums, photothermal conversion efficiency, 1O2 quantum yield and hyperthermia heating curves of BxPC-3 cell media for FeL3Mn3 at 808 nm, and an ascorbate shielding effect for the photodynamics of FeL3Mn3 metallostars. See DOI: https://doi.org/10.1039/d3ma00762f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif)