Regulating Li nucleation/deposition by bamboo-shoot like lithiophilic particles anchored on carbon cloth for a dendrite-free lithium metal anode†
Yuchi
Liu
ab,
Zhicui
Song
ab,
Zihao
Wang
ab,
Jianxiong
Xing
ab,
Wei
Zou
cd and
Jingze
Li
 *ab
*ab
aSchool of Materials and Energy, University of Electronic Science and Technology of China, Chengdu 611731, China. E-mail: lijingze@uestc.edu.cn
bYangtze Delta Region Institute (Huzhou), University of Electronic Science and Technology of China, Huzhou 313001, China
cResearch and Development Center, Tianqi Lithium Co., Ltd., Chengdu 610093, China
dLithium Resources and Lithium Materials Key Laboratory of Sichuan Province, Tianqi Lithium Corporation, Chengdu, 610000, China
First published on 21st November 2022
Abstract
The extended application of lithium (Li) anodes is restricted by volume change and Li dendrites. The addition of a “host” structure and regulation of Li deposition/dissolution behavior can significantly enhance the electrochemical performance of Li anodes. Herein, bamboo-shoot like particles of a highly lithiophilic Li22Sn5 intermetallic compound are successfully anchored on the light and flexible carbon cloth (CC) host to accommodate Li via facile thermal infusion of the molten Li-rich Li–Sn alloy. The affinity between Sn and carbon affects the phase separation behavior while the molten Li–Sn alloy is cooled down, resulting in exclusive solidification of Li22Sn5 into micro-sized particles on the carbon fibres. The strong interaction between Li22Sn5 sites and Li allows uniform Li deposition and suppresses the formation of Li dendrites in the CC host matrix, although the initial electrochemical process is Li deposition or Li dissolution. Consequently, the electrochemical performance is greatly improved, in which the symmetric cell can be stably cycled for 3500 h at 1 mA cm−2 and 1 mA h cm−2 in the ester-based electrolyte, and the LiFePO4 full cell delivers a high specific capacity of 128.6 mA h g−1 even at an increased current rate of 2C, representing a significant advance in the pursuit of practical Li metal batteries.
Introduction
The rapid development of electric vehicles and electronic products has put forward more urgent requirements for high energy density, long lifespan, light weight, compact size, and good portability of power supply devices. Lithium (Li) metal is considered as an ideal anode for future electrochemical energy storage systems due to its high specific capacity (3860 mA h g−1), low oxidation–reduction potential (−3.04 V vs. standard hydrogen electrode) and low density (0.59 g cm−3).1–3 Moreover, with the development of Li-free cathodes in Li–S batteries4–6 and Li-air batteries,7–9 Li anodes have attracted considerable attention. However, Li anodes are currently only commercially used in primary batteries. In secondary batteries, due to the formation of Li dendrites and the gradual consumption of active Li during repeated charging and discharging processes, Li metal anodes suffer from safety hazards, low Coulombic efficiency and other issues.10–12 Moreover, Li anodes have no “host” structure like graphite, show high chemical reactivity, undergo huge volume deformation and become loose and porous, leading to severe side reactions and a thick solid electrolyte interface (SEI). In the extreme case, the formed Li dendrites may pierce through the separator and cause internal short circuit overheating of the battery. Therefore, it is crucial to design advanced Li anodes and regulate the deposition/dissolution behavior of Li efficiently.Up to now, many studies have been reported on the modification of Li anodes to suppress the growth of Li dendrites. Among them, introducing a three-dimensional (3D) conductive skeleton to prepare Li composite anodes is very popular. A 3D metal framework and various carbon architectures as “host” structures can provide void space for Li deposition, limiting the volume expansion and reducing the local current density effectively. C.P. Yang et al. fabricated a 3D submicron-sized and porous Cu skeleton with a larger conductive area, which can induce Li to deposit in the pore structure and effectively inhibit the formation of Li dendrites.13 L. L. Lu et al. prepared a Cu nanowire (NW) film as the host architecture, the average Coulombic efficiency of which reached 98.6%.14 In addition, there are many reports concerning the modification of the 3D skeleton to increase its lithiophilicity, for example, Co3O4 nanoflower modified Ni foam,15 3D graphene complexed with Ni foam,16 3D hierarchical porous metal collectors,17 MXene modified Cu foam,18 ZnF2 coated 3D Li–Ni composite anodes,19 partly lithiated graphitic carbon foam,20 metal–organic framework (MOF)-coated CNT,21 coralloid carbon fiber-based Ag–Li composites,22 ZnO quantum dot decorated porous carbon scaffolds,23 Li-wicking host (denoted as GCF/Cu/Li),24 Li deposited on Cu-coated CF (Li/CuCF),25 Li–metal composite yarn (LMCY),26 etc.27–29 Apparently, multiple steps are involved in the preparation of a 3D skeleton or compositing with Li. Therefore, simplifying the fabrication process of the lithiophilic skeleton and increasing its Li affinity are the main research directions of the 3D conductive skeleton. Very recently, a Li-rich alloy, consisting of excess free Li phase and Li intermetallic compound/solid solution phase, has been proposed as a promising Li anode.30,31 Here, the latter component in situ generates a 3D skeleton network distributed in the matrix of Li metal phase uniformly via phase-segregation in the sample fabrication. The reported Li-rich alloys range from Li–Mg,32,33 Li–Ag,34 Li–B,35–37 Li–Si,38 Li–In,39 Li–Cu,40 Li–Zn,41,42 Li–Sn,37,43–45 Li–Ca,46 to Li–Al.7 It is obvious that the alloy skeleton is critical for the performance of a Li-rich alloy anode considering its roles in the charging/discharging process. First, the alloy skeleton should be mechanically robust acting as the host structure and a stable electrode structure. Second, the alloy skeleton is associated with strong Li affinity, which can change the surface energy of the alloy electrode and tune the Li nucleation and growth process for effectively inhibiting the Li dendrite. Third, since the alloy framework is a good electronic conductor, Li+ ions prefer to be reduced at the interface of the anode and the electrolyte. If the reduced Li atoms cannot be transported inside the anode, the aggregated Li metal might form a thick layer on the anode surface. Hence, the alloy framework should also be a fast Li+ ion conductor. At this moment, it is really a challenge to synthesize a perfect Li-rich alloy material meeting the mentioned three criteria well. Therefore, a hybrid of the 3D conductive skeleton with a Li-rich alloy can have the properties of both materials, where the synergistic effect may further promote the electrochemical performance.
In our previous work, LiCux alloy nanowires nested in Ni foam47 and Li–Cu2Mg composites in Cu foam48 have been successfully prepared by infiltrating a molten Li alloy into the metal foam, showing a much improved cycle stability. It should be pointed out that the metallic foam sheet is expensive, fragile and heavy, which is not suitable for practical application. Alternatively, a porous carbon sheet is cheap, robust and light, which might be the right candidate having a 3D hosting architecture. A Li-rich Li–Ca alloy composited with carbon cloth provides a secondary network composed of the CaLi2 intermetallic compound with interconnected ant-nest-like lithiophilic channels across the primary scaffold of the carbon cloth matrix and exhibits a long-term lifespan.49 Hence, a hybrid of a carbon cloth sheet with a lithiophilic Li-rich alloy fabricated by a facile way might be a promising candidate to prolong the lifespan of the Li metal anode.
In this work, a composite anode marked as SnLi@CC is fabricated by infiltrating commercial carbon cloth (CC) with the molten Li-rich SnLi90 alloy at 500 °C wherein the atomic ratio of Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li is 1
Li is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, followed by a natural cooling treatment. In the cooling process, the Li22Sn5 intermetallic compound preferentially nucleates at the surface of carbon fibers and forms bamboo-shoot like particles which is different from the rod-like network structure in the pristine Li-rich SnLi90 alloy.45 Remarkably, although the uppermost CC sheet does not come in contact with the molten Li–Sn alloy, Li22Sn5 bamboo-shoots can appear on the top surface of the sheet, indicating the strong interaction between Sn and carbon fibers. Obviously, Li22Sn5 particles can endow the CC host with super lithiophilicity for enhanced wettability of molten Li in the process of thermal infusion. Furthermore, the introduction of bamboo-shoot like Li22Sn5 particles on the carbon fibers is the key point to guide uniform Li nucleation/growth in the CC matrix in the subsequent cycling measurement. Compared to Li and Li@CC anodes, the electrochemical performance of SnLi@CC is greatly improved by the combination of the CC “host” architecture and the regulation effect of Li22Sn5 lithiophilic sites. As a result, the SnLi@CC alloy composite electrode in the symmetric configuration demonstrates an ultralong lifespan of up to 3500 h at 1 mA cm−2 and 1 mA h cm−2 in the ester-based electrolyte, and the full cell pairing with the LiFePO4 (LFP, mass loading of 17.1 mg cm−2) cathode exhibits excellent rate capacity and cycle stability, offering a valuable way for practical application of advanced Li metal batteries.
90, followed by a natural cooling treatment. In the cooling process, the Li22Sn5 intermetallic compound preferentially nucleates at the surface of carbon fibers and forms bamboo-shoot like particles which is different from the rod-like network structure in the pristine Li-rich SnLi90 alloy.45 Remarkably, although the uppermost CC sheet does not come in contact with the molten Li–Sn alloy, Li22Sn5 bamboo-shoots can appear on the top surface of the sheet, indicating the strong interaction between Sn and carbon fibers. Obviously, Li22Sn5 particles can endow the CC host with super lithiophilicity for enhanced wettability of molten Li in the process of thermal infusion. Furthermore, the introduction of bamboo-shoot like Li22Sn5 particles on the carbon fibers is the key point to guide uniform Li nucleation/growth in the CC matrix in the subsequent cycling measurement. Compared to Li and Li@CC anodes, the electrochemical performance of SnLi@CC is greatly improved by the combination of the CC “host” architecture and the regulation effect of Li22Sn5 lithiophilic sites. As a result, the SnLi@CC alloy composite electrode in the symmetric configuration demonstrates an ultralong lifespan of up to 3500 h at 1 mA cm−2 and 1 mA h cm−2 in the ester-based electrolyte, and the full cell pairing with the LiFePO4 (LFP, mass loading of 17.1 mg cm−2) cathode exhibits excellent rate capacity and cycle stability, offering a valuable way for practical application of advanced Li metal batteries.
Results and discussion
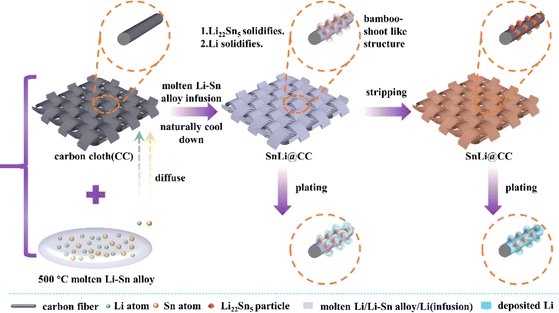
Fig. 1 shows a schematic diagram of the fabrication process of the SnLi@CC electrode and its cycling process. SnLi@CC is obtained by putting carbon cloth (CC) on the molten Li–Sn alloy at 500 °C. By virtue of the improved wettability of the Li-rich alloy toward carbon, Li and Sn atoms may diffuse along the carbon fibre and infiltrate the CC. In the subsequent cooling process, the Li22Sn5 intermetallic compound nucleates and forms a bamboo-shoot like structure at the carbon fibre since Li22Sn5 has a higher solidification point than that of the Li metal. When the temperature continues to decrease, the Li metal phase will solidify at the surface of the Li22Sn5 alloy and in the void spaces of the CC membrane. This hierarchical structure contains a primary CC framework and secondary lithiophilic bamboo-shoot shaped Li22Sn5 alloy particles, which enables uniform Li deposition and suppresses the formation of Li dendrites, despite that the initial electrochemical process is Li deposition or Li dissolution.The thickness of the pristine CC sheet is about 206 and 316 μm at the narrowest and widest points, respectively, and the carbon fibres are very uniform with a diameter of around 9 μm (Fig. S1, ESI†). After infiltrating with molten Li, the thickness of Li@CC is around 301 and 313 μm, respectively, and the average diameter of the fibres on the top surface of the CC sheet is enlarged up to 11 μm (Fig. S2, ESI†). The maximum film thickness of Li@CC is slightly thinner than that of the pristine CC, and the possible reason for this is ascribed to the volume shrinkage from the molten Li liquid to the condensed solid-state metal. Besides, the carbon fibre has competent flexibility.
Fig. 2(a–c) and (g–i) show the top-view and section-view SEM images of SnLi@CC, respectively. By virtue of the improved wettability of the Li-rich alloy toward carbon, after infiltrating with the molten SnLi90 alloy45 (Li-rich Li–Sn alloy wherein the atomic ratio of Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li is 1
Li is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90), the thickness of the SnLi@CC is increased to about 309 and 391 μm (Fig. 2(g)). In terms of the phase diagram of the Li–Sn binary alloy50 as shown in Fig. S3 (ESI†), the phase separation process of the molten SnLi90 generates Li22Sn5 intermetallic compound and Li metal phases while cooling down to room temperature. Since the solidification temperature of Li22Sn5 is much higher than that of the Li metal, the Li22Sn5 alloy phase self-assembles into a lithiophilic rod-like framework in the pristine SnLi90 alloy. However, while the molten SnLi90 alloy is composited with CC, the situation is different. When the temperature begins to decrease, the Li22Sn5 intermetallic compound preferentially nucleates at the surface of carbon fibres and forms bamboo-shoot like particles as shown in Fig. 2(a–c). Herein, the morphology of Li22Sn5 is distinct from rod-like shape observed in the pristine SnLi90 alloy. As the temperature further decreases, the metallic Li phase solidifies at the surface of the Li22Sn5 particles and in the void spaces of the CC. In contrast, the morphology of the phase-separated Li–Ca binary alloy is almost identical to that of the composite of the Li–Ca alloy and CC, which is synthesized by the same method.46
90), the thickness of the SnLi@CC is increased to about 309 and 391 μm (Fig. 2(g)). In terms of the phase diagram of the Li–Sn binary alloy50 as shown in Fig. S3 (ESI†), the phase separation process of the molten SnLi90 generates Li22Sn5 intermetallic compound and Li metal phases while cooling down to room temperature. Since the solidification temperature of Li22Sn5 is much higher than that of the Li metal, the Li22Sn5 alloy phase self-assembles into a lithiophilic rod-like framework in the pristine SnLi90 alloy. However, while the molten SnLi90 alloy is composited with CC, the situation is different. When the temperature begins to decrease, the Li22Sn5 intermetallic compound preferentially nucleates at the surface of carbon fibres and forms bamboo-shoot like particles as shown in Fig. 2(a–c). Herein, the morphology of Li22Sn5 is distinct from rod-like shape observed in the pristine SnLi90 alloy. As the temperature further decreases, the metallic Li phase solidifies at the surface of the Li22Sn5 particles and in the void spaces of the CC. In contrast, the morphology of the phase-separated Li–Ca binary alloy is almost identical to that of the composite of the Li–Ca alloy and CC, which is synthesized by the same method.46
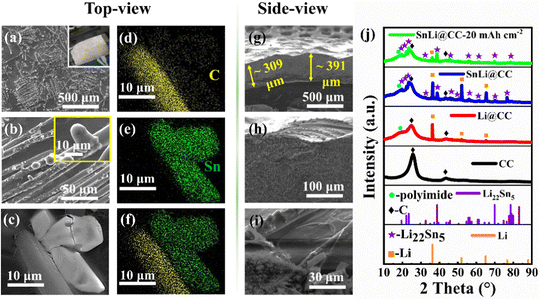 | ||
| Fig. 2 (a–c) Surface morphology of SnLi@CC. (d–f) EDS analysis of (c). (g–i) Side-views of SnLi@CC. (j) XRD images of CC, Li@CC, SnLi@CC and SnLi@CC after delithiation of 20 mA h cm−2. | ||
Surprisingly, although the uppermost CC sheet does not contact the molten Li–Sn alloy in the infusion process, Li22Sn5 bamboo-shoots appear on the top surface of the sheet (top-view in Fig. 2(a–c) and side-view in Fig. 2(g–i)), indicating strong interaction between Sn and carbon fibres. Fig. S4 (ESI†) also shows the improved wettability of SnLi@CC. Alternatively, the superb lithiophilicity and wettability of metallic Sn toward Li have already been proved in SnLi9045 and other Li–Sn alloy-based reports.37,43,44 Taking into account the fact that the Li22Sn5 phase is self-assembled as a rod-like network structure in the pristine Li-rich Li–Sn binary alloys, it can be derived that the formation of a bamboo-shoot like Li22Sn5 intermetallic compound is driven by the competitive effect among Sn, Li and C. These bamboo-shoot like particles have a maximum diameter of around 10 μm and a height of about 20 μm. Fig. 2(d–f) are the corresponding EDS element analysis results of Fig. 2(c). The EDS mapping images confirm that Sn elements are dominated and uniformly distributed in the bamboo-like structure. XRD profiles (Fig. 2(j)) prove that the phase components of SnLi@CC consist of Li, Li22Sn5, and C. Hence, it is easily deduced that the bamboo-shoot like structure is majorly composed of Li22Sn5. A broad diffraction peak of the polyimide protective film occurs between 10° and 30°.41
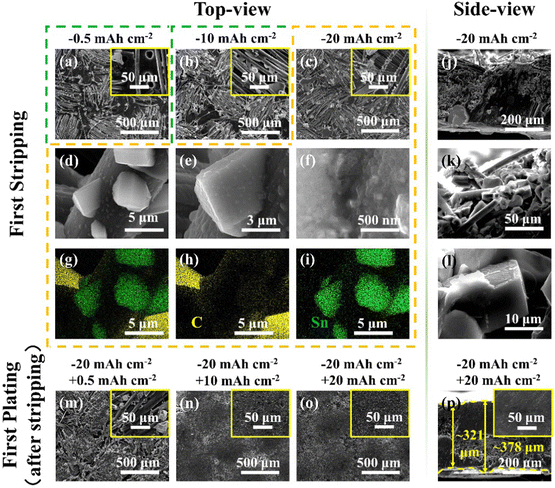
As the “host” structure of the anode, many factors of the skeleton may affect the deposition/dissolution behaviour of Li, such as the mechanical strength, electrochemical stability, morphology, surface properties, and intrinsic physicochemical characteristics. Fig. 3(a–f) show the SEM images of SnLi@CC after having removed Li of 0.5, 10, and 20 mA h cm−2 at a current density of 1 mA cm−2, respectively. When a small amount of Li is etched off, some pores far from the carbon fibres appear on the surface, reflecting that the dissolution process is not very uniform during the initial stage since the separation distance among the carbon fibres is relatively large. As the delithiation capacity increases, the carbon fibres and alloy frameworks that are originally covered by metallic Li are gradually exposed. There are many particles randomly decorated on the carbon fibres. And the zoomed-in images illustrate that these particles seem to grow from the fibres, which are quite similar to the bamboo-shoot structure observed on the top-most composite surface. In light of EDS mapping images (Fig. 3(g–i) and XRD curves (Fig. 2(j))), SnLi@CC after delithiation of 20 mAh cm−2 is still composed of C, Li, and Li22Sn5, whereas there is no other Li–Sn alloy phase formed. This means that Li22Sn5 particles retain electrochemical “inertness” in this Li stripping process, where the Li metal phase is active for the capacity contribution. The cross-sectional images of SnLi@CC after delithiation of 20 mA h cm−2 (Fig. 3(j–l)) display that a large number of alloy particles are anchored to carbon fibers, further proving that the bamboo-shoot shaped Li22Sn5 architectures are uniformly distributed within the CC skeleton.
To further validate the mechanical and electrochemical performances of the skeleton, Li in SnLi@CC (including free Li and Li in the Li22Sn5 alloy) was completely removed at a current density of 1 mA cm−2. Fig. S5 (ESI†) shows the capacity-voltage curve of SnLi@CC completely delithiated to 0.5 V, and Table S1 (ESI†) exhibits the mass of the SnLi@CC electrode sheet and its theoretical and actual areal capacities. The ignorable difference between the actual areal capacity and the theoretical areal capacity is caused by the formed SEI (solid electrolyte interphase) layer, which is not considered in the calculation process. In the initial stage, the redox potential difference between the Li22Sn5 alloy and Li metal phases guarantees the preferential dissolution of free Li and maintains electrochemical “inertness” of the alloy skeleton. After complete chemical delithiation by reaction with water, the bamboo-shoot shaped Li22Sn5 structure is collapsed and the pulverized Sn particles are left on the carbon fibres (Fig. S6, ESI†). The pulverized particles on carbon cloth are mainly Sn and a small amount of SnO2 (Fig. S7 and S8, ESI†). Owing to the well-preserved primary framework CC and Sn decoration, the 3D “host” architecture can still ensure good mechanical stability and Li affinity.
The Coulombic efficiency test was performed on the remaining framework after chemical delithiation (reacting with water to remove Li). As illustrated in Fig. S9 (ESI†), the Coulombic efficiency of the Li@CC chemically delithiated framework is higher than that of the SnLi@CC chemically delithiated framework in the initial few cycles, which is attributed to the capacity loss induced by part of the electrically “inactive” Sn particles.51–53 After that, the Coulombic efficiencies of both frameworks are almost the same since the alloying/dealloying reaction reversibility is high enough for the residual “active” Sn particles. However, the Li@CC chemically delithiated framework is seriously fluctuated. That is to say, the formed lithiophilic alloy particles are favourable for Li nucleation/deposition compactly. Although the alloy structure is partly destroyed due to the volume shrinkage and the pulverized alloy particles are not uniformly distributed on the carbon fibers, the superior Li affinity of these residual alloy particles can still make Li deposit densely inside the skeleton of the carbon cloth, which can further regulate the behavior of Li electroplating and provide enough free space to accommodate the deposited Li (1 or 5 mA h cm−2). Therefore, the composite skeleton of SnLi@CC after reacting with water can achieve a better performance in the Coulombic efficiency test.
Consequently, the Coulombic efficiency of the Li@CC chemically delithiated framework decays to 62.4% after 362 cycles at 1 mA h cm−2, whereas it is as high as 99.6% even after 530 cycles for the SnLi@CC chemically-delithiated framework. The positive role of the decorated Li–Sn particles is highlighted at the elevated capacity of 5 mA h cm−2, where the Coulombic efficiency of the SnLi@CC chemically delithiated framework is 96.4% after 58 cycles, compared with 84.0% after 33 cycles for the Li@CC chemically delithiated framework.
Following that, the deposition behaviour of Li on the skeleton exposed by electrochemical delithiation of 20 mA h cm−2 was explored. Fig. 3(m–o) demonstrate the surface morphology images of re-embedding Li of 0.5, 10 and 20 mA h cm−2 at 1 mA cm−2. After re-embedding Li of 0.5 mA h cm−2, the bamboo-shoot shaped particles become irregular, indicating that the reduced Li prefers to be deposited on the surface of the Li22Sn5 alloy particles. As the deposited Li increases up to 10 mA h cm−2, there are a number of pores on the electrode surface, which are not occupied by the Li metal. After inserting back Li of 20 mA h cm−2, the surface is covered by a dense Li layer, showing no signal of Li dendrite formation. Moreover, the side-view images evidence that the plated Li in the interior of the electrode is mainly accumulated in a compact way (Fig. 3(p)). The electrode thickness is about 321 μm to 378 μm. Apparently, the newly deposited Li is not as dense as the thermally infiltrated Li, and the hierarchical lithiophilic framework in SnLi@CC can induce uniform Li deposition/dissolution and inhibit the formation of Li dendrites.
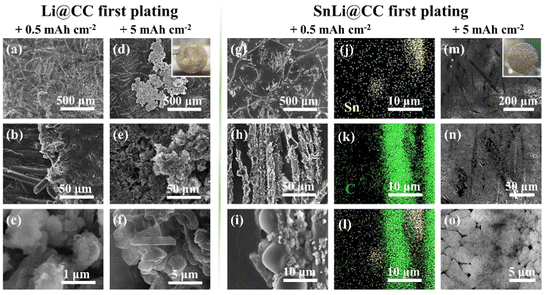
Currently, the cathode in all of the commercial Li-ion batteries contains the Li element, and the first operation step is to plate Li toward the anode side. Herein, the SnLi@CC composite anode has a unique structure, in which Li22Sn5 bamboo shoot-like particles can stand on the electrode surface. It seems that our anode with the reserved headspace is suitable for the accommodation of Li deposition directly. In contrast, an obvious uneven distribution of the plated Li occurs for Li@CC even at 0.5 mA h cm−2, where the deposited Li is loose, and the stratification phenomenon is recorded as shown in Fig. 4(a–c). After depositing Li of 5 mA h cm−2 on Li@CC (Fig. 4(d–f)), the uneven distribution is aggravated, and a large amount of Li dendrites is generated. The corresponding optical image in Fig. 4(d) also reflects that there are white areas due to the appearance of the Li dendrite block. Fig. 4(g–i) and Fig. 4(m–o) show the surface topography of Li deposited at 0.5 mA h cm−2 and 5 mA h cm−2 on SnLi@CC, respectively, and Fig. 4(j–l) show the corresponding EDS elemental analysis of Fig. 4(i). When 0.5 mA h cm−2 of Li is deposited (Fig. 4(g–i)), the electrode surface morphology is changed, and the deposited Li is preferably grown on the bamboo-shoot shaped Li22Sn5 particles due to lithiophilicity. In accordance with EDS mapping (Fig. 4(j–l)) analysis, the signal of the Sn element in the volume-expanded and vertically aligned bamboo-shoot particles is greatly weakened, suggesting that these particles are covered with a thick layer of Li metal. When the deposition capacity is promoted to 5 mA h cm−2 (Fig. 4(m–o)), the plated Li is uniformly spread among the carbon fibres, rendering a smooth surface without the formation of Li dendrites. The inset of the optical image (Fig. 4(m)) further depicts that the electrode remains homogeneous at a macroscopic level. Taking into account the above results, the unique hierarchical structure of SnLi@CC, consisting of the CC primary framework and secondary lithiophilic bamboo-shoot shaped Li22Sn5 alloy particles, allows uniform Li deposition and suppresses the formation of Li dendrites, despite that the initial electrochemical process is Li deposition or Li dissolution.
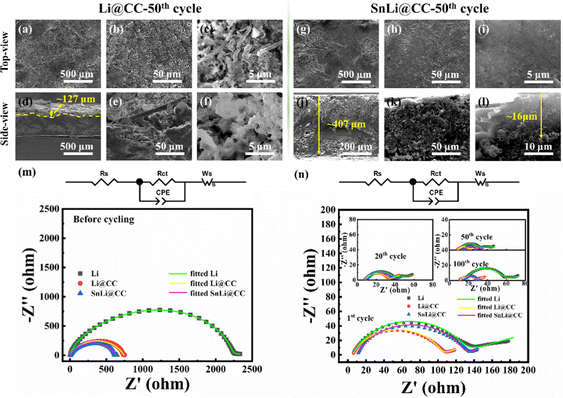
Fig. 5(a–c) and Fig. 4(d–f) show the surface morphology and cross-view morphology of Li@CC at 3 mA cm−2 and 1 mA h cm−2 for 50 cycles, respectively. Massive Li dendrites are formed and can be observed from both the surface and the cross-section. Obvious delamination occurred in the cross-section, and the upper layer Li is loose and cracked with a large thickness of about 127 μm. Under the same test conditions, after 50 cycles, the surface morphology of SnLi@CC (Fig. 5(g–i)) is dense without Li dendrites. The thickness of the SnLi@CC electrode is about 407 μm, and the upper layer Li of SnLi@CC is about 16 μm (Fig. 5(j–l)), which is much smaller than that of Li@CC. Before cycling, the charge transfer resistance (Rct) is 2294 ohms for Li, 738.2 ohms for Li@CC and 622.6 ohms for SnLi@CC, respectively (Fig. 5(m)). The Rct of SnLi@CC is smaller than that of Li@CC and Li, which is assigned to the improved lithiophilicity and enlarged surface area of the sub-level Li22Sn5 alloy skeleton. The EIS test results of Li, Li@CC and SnLi@CC after cycling are given in Fig. 5(n). After 1 cycle, Rct decreases for all the three electrodes (115.2 ohms for Li; 97.0 ohms for Li@CC; 117.0 ohms for SnLi@CC), owing to the destroyed native passivation layer on the anode and the exposure of the fresh Li. After 20 cycles, Rct continues to decrease for all the three electrodes (19.4 ohms for Li; 23.9 ohms for Li@CC; 29.9 ohms for SnLi@CC). The change of SnLi@CC is the smallest, thanks to the uniform Li deposition/dissolution and the slightest volume change during cycling. While the stripping/depositing process is up to 50 cycles, Rct of Li is increased to 35.6 ohms, representing the fast consumption of the electrolyte due to the side reaction and gradual formation of dead lithium. The Rct of Li@CC is 7.1 ohms and the Rct of SnLi@CC is 7.4 ohms for 50th cycle. The impedance of the pristine Li (44.64 ohms) at 100th cycle increases substantially due to the formation of dendrites and accumulation of “dead” Li. In contrast, the resistance of Li@CC and SnLi@CC at 100th cycle does not increase too much (17.7 ohms for Li@CC and 17.0 ohms for SnLi@CC), and SnLi@CC shows the smallest value and the smallest changes induced by the excellent mechanical stability and uniform Li deposition/dissolution behaviour.
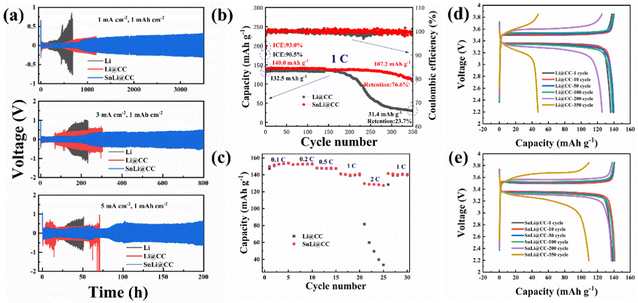
Li||Li, Li@CC||Li@CC, SnLi@CC||SnLi@CC symmetrical batteries, Li@CC||LFP and SnLi@CC||LFP batteries are assembled to evaluate the electrochemical performance of the SnLi@CC electrode. In symmetrical batteries (Fig. 6(a) and Fig. S10, ESI†), SnLi@CC demonstrates the longest lifespan and smallest polarization voltage with respect to the pristine Li and Li@CC, while the current density is varied from 1 to 5 mA cm−2 and the areal capacity is changed from 1 to 5 mA h cm−2. The lifespan of SnLi@CC (3500 h at 1 mA cm−2 and 1 mA h cm−2; 800 h at 3 mA cm−2 and 1 mA h cm−2) is more than twice that of SnLi9045 (1500 h at 1 mA cm−2 and 1 mA h cm−2; 350 h at 3 mA cm−2 and 1 mA h cm−2). The overpotential fluctuation between 50 and 100 h when cycling at 5 mA cm−2 and 1 mA h cm−2 is caused by the fluctuation of the environment temperature (the constant temperature system went ill for a while). Such a phenomenon can be observed in batteries tested at the same time (overpotential fluctuation between 1880 and 1930 h when cycling at 1 mA cm−2 and 1 mA h cm−2). Since the cycle time is much longer when cycling at 1 mA cm−2 and 1 mA h cm−2, the impact is almost negligible. The good recovery after the fluctuation also illustrates the rapid lithium-ion transport of the LiSn alloy. According to the above-mentioned SEM images, the lithiophilic bamboo-shoot like particles stemmed from the carbon fibres either on the surface or in the interior of the CC structure sheet, which can efficiently regulate the Li deposition/dissolution behaviour, inhibiting the growth of Li dendrites and improving the electrochemical performance.
The performances of the Li@CC and SnLi@CC electrodes in the LFP (areal capacity: 2.45 mA h cm−2) full battery are demonstrated in Fig. 6(b and c). After the full cell was activated at a small current of 0.1C for 2 cycles, the current ratio was promoted to 1C. The SnLi@CC||LFP battery shows an initial capacity of 140.0 mA h g−1. It could cycle stably for more than 350 cycles and the specific capacity at 350th cycle is 107.2 mA h g−1. The capacity retention rate is 76.6%. The Li@CC||LFP battery shows a slightly lower initial capacity of 132.5 mA h g−1. It could cycle stably only for 150 cycles, then the specific capacity reduces rapidly and the specific capacity at 350th cycle is 31.4 mA h g−1, which is much lower than that of SnLi@CC. The capacity retention rate is 23.7%. The cycling stability with LFP does not seem to be very good when compared with the literature,54 which did not use lithiophilic modification on CC. This is because the capacity of the LFP is 2.45 mA h cm−2, which is larger than that in the reference (2.05 mA h cm−2). The cell was tested at 1C (2.45 mA cm−2), which is larger than that in the reference (1 mA cm−2). The ether-based electrolyte was used in the literature and the ester-based electrolyte was used in this article. The first electrochemical step of the LFP battery is the removal of Li. Hence, the internal space of the anode is of vital importance. The pre-Li@CC (prelithiated carbon cloth) not only provides part of Li capacity but also retains more internal space of the CC (compared to Li@CC prepared by thermal infusion in this article). While Li@CC in this article is Li-rich, it is favorable for application to Li-free cathodes. In the rate performance test, the batteries are cycled at 0.1C, 0.2C, 0.5C, 1C and 2C each for 5 cycles and then tested at 1C for another 5 cycles. The specific capacity of SnLi@CC was always higher than that of Li@CC at different rates as the charge rate was elevated from 0.1 to 1C, but the difference was ignorable at these small current densities because both SnLi@CC and Li@CC have a CC skeleton offering a robust “host” structure for Li deposition/stripping. When the test rate increased to 2C, the performance of the SnLi@CC||LFP battery was significantly better than that of the Li@CC||LFP battery. The SnLi@CC||LFP battery demonstrated a relatively stable capacity of 128.6 mA h g−1. In contrast, the specific capacity of the Li@CC||LFP battery decayed rapidly down to 33.6 mA h g−1. When the current rate was back to 1C, both cells illustrate stable performance, which is the same as the previous recording at 1C. The rate performance testing indicates that the highest current rate of 2C does not physically destroy the electrode structure. Fig. 6(d) and (e) show the capacity–voltage curves of different cycles for the Li@CC||LFP (LiFePO4) battery and the SnLi@CC||LFP battery at 1C, respectively. SnLi@CC has smaller polarization than that of Li@CC at the same cycle number. Apparently, the lithiophilic bamboo-shoot like Li22Sn5 particles in the SnLi@CC can accelerate Li+ ion transport44 and enlarge the actual surface area for promoted electrochemical reaction kinetics. Furthermore, the hierarchical structure of the CC framework and Li22Sn5 skeleton allows Li to be deposited/dissolved uniformly at any current rate, leading to a prolonged cyclic lifespan for the symmetric cell and the full cell.
Experimental
Fabrication of SnLi@CC and Li@CC
Commercial carbon cloth (Caltech Co., Ltd) was washed with acetone, alcohol and deionized water three times each, and then it was dried in a vacuum oven at 60 °C. After the platitudinous reaction and stirring of Sn particles and Li foil with an atomic ratio of Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li of 1
Li of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 on the 500 °C heating table, the treated carbon cloth was disposed on the surface of the liquid SnLi90 alloy. The molten alloy rapidly diffused along the carbon fibres and filled the inside of the carbon cloth with an adsorption time of 3 minutes. After that, the sample was naturally cooled down to room temperature to obtain an alloy composite material hereinafter referred to as SnLi@CC. The sample was pressed into electrode sheets with a diameter of 10 mm. The pure molten Li compounding with CC for the same adsorption time of 3 minutes referred to as Li@CC was used for a comparison.
90 on the 500 °C heating table, the treated carbon cloth was disposed on the surface of the liquid SnLi90 alloy. The molten alloy rapidly diffused along the carbon fibres and filled the inside of the carbon cloth with an adsorption time of 3 minutes. After that, the sample was naturally cooled down to room temperature to obtain an alloy composite material hereinafter referred to as SnLi@CC. The sample was pressed into electrode sheets with a diameter of 10 mm. The pure molten Li compounding with CC for the same adsorption time of 3 minutes referred to as Li@CC was used for a comparison.
Characterization
The sample's composition was determined by X-ray diffraction with Cu Kα (λ = 0.154056 nm) radiation and the scanning speed was 10° min−1. The entire process of sample preparation was carried out in a glove box filled with Ar gas. To prevent the sample from oxidizing during the test, it was encapsulated and protected with a polyimide film to isolate the sample from direct contact with oxygen and water in air. The electrode samples after electrochemical cycling were washed using diethyl carbonate (DEC) to remove Li salts and impurities on the surface and then dried at 80 °C for 2 h to release residual DEC, finally encapsulated and protected with a polyimide film. After removing metallic Li from the Li-rich alloy using the chemical method (using H2O to react with Li), the as-obtained skeleton was washed with deionized water and absolute ethanol, and then dried at 80 °C for 2 h. The morphology of the sample was observed using a scanning electron microscope (SEM, Hitachi, S3400N). An element analysis test was carried out using an energy dispersive spectroscope (EDS, AXIS Supra).Electrochemical performance
All electrode sheets are assembled into CR2032 button cells in a glove box filled with Ar gas. 1 mol L−1 Li hexafluorophosphate (LiPF6) in ethylene carbonate (EC) and diethyl carbonate (DEC) mixed organic solution (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 5% fluoroethylene carbonate (FEC) additive by volume was employed as the electrolyte. The amount of the electrolyte in all cells was 120 μL. Celgard 2325 (diameter of 19 mm) was used as the separator. Li||Li, Li@CC||Li@CC and SnLi@CC||SnLi@CC symmetrical batteries as well as Li@CC||LiFePO4(LFP) and SnLi@CC||LFP batteries were assembled, respectively. Li||CC (obtained from Li@CC after the Li removal treatment using the chemical method) and Li||Sn@CC (obtained from SnLi@CC after the Li removal treatment using the chemical method) were assembled to test the Coulombic efficiency. The rate performance test of LFP batteries was performed at 0.1C, 0.2C, 0.5C, 1C and 2C each for 5 cycles (1C = 170 mA h g−1) and then operated at 1C for several cycles. The charge–discharge voltage of the LFP battery was between 2.2 and 3.85 V. The active material loading of the LFP electrode was about 17.1 mg cm−2 and the areal capacity is about 2.45 mA h cm−2. The diameters of the LFP cathode and the anodes were 10 mm. The LAND battery test system is employed for constant current charging and discharging tests. Each battery was rested for 6 hours before testing. All batteries were subjected to electrochemical cycling tests with different current densities and areal capacities at a room temperature of 25 °C. A Shanghai Chenhua CHI660C electrochemical workstation was used to perform electrochemical impedance spectroscopy (EIS) tests in the frequency range of 1 MHz to 1 mHz and a perturbation voltage amplitude of 5 mV.
1) with 5% fluoroethylene carbonate (FEC) additive by volume was employed as the electrolyte. The amount of the electrolyte in all cells was 120 μL. Celgard 2325 (diameter of 19 mm) was used as the separator. Li||Li, Li@CC||Li@CC and SnLi@CC||SnLi@CC symmetrical batteries as well as Li@CC||LiFePO4(LFP) and SnLi@CC||LFP batteries were assembled, respectively. Li||CC (obtained from Li@CC after the Li removal treatment using the chemical method) and Li||Sn@CC (obtained from SnLi@CC after the Li removal treatment using the chemical method) were assembled to test the Coulombic efficiency. The rate performance test of LFP batteries was performed at 0.1C, 0.2C, 0.5C, 1C and 2C each for 5 cycles (1C = 170 mA h g−1) and then operated at 1C for several cycles. The charge–discharge voltage of the LFP battery was between 2.2 and 3.85 V. The active material loading of the LFP electrode was about 17.1 mg cm−2 and the areal capacity is about 2.45 mA h cm−2. The diameters of the LFP cathode and the anodes were 10 mm. The LAND battery test system is employed for constant current charging and discharging tests. Each battery was rested for 6 hours before testing. All batteries were subjected to electrochemical cycling tests with different current densities and areal capacities at a room temperature of 25 °C. A Shanghai Chenhua CHI660C electrochemical workstation was used to perform electrochemical impedance spectroscopy (EIS) tests in the frequency range of 1 MHz to 1 mHz and a perturbation voltage amplitude of 5 mV.
Conclusions
In summary, a composite anode SnLi@CC with a hierarchical structure was prepared by adsorbing a molten Li-rich Li–Sn alloy on a light and flexible carbon cloth. During the cooling process, due to the difference of solidification points between Li and Li22Sn5, Li22Sn5 first forms lithiophilic bamboo-shoot like alloy particles on the carbon fiber surface, and when the temperature is further reduced, Li solidifies at the lithiophilic alloy surface and in the voids of the carbon fiber skeleton. Carbon cloth constitutes a flexible main frame, and lithiophilic Li22Sn5 alloy particles form a secondary lithiophilic skeleton. Remarkably, the Li22Sn5 phase is phase-segregated as the bamboo-shoot like particles’ array on the carbon fibers via the interaction among Sn, Li and C, which is quite different from the phase separation behavior of the conventional Li-rich Li–Sn binary alloy. During cycling, the hierarchical lithiophilic framework of Li22Sn5 particles anchored in the CC sheet can induce uniform Li deposition/dissolution if the initial electrochemical process is to remove or deposit Li. Consequently, the electrochemical performance of the as-assembled cells is greatly promoted. In a symmetrical configuration, the cell can be cycled stably for 3500 h at 1 mA cm−2 and 1 mA h cm−2. The rate performance of the SnLi@CC-based LFP full battery with a large mass loading of 17.1 mg cm−2 is superior to that of Li@CC. In particular, at the highest current rate of 2C, the SnLi@CC full cell can stably deliver 128.6 mA h g−1, whereas the Li@CC cell cannot work properly. Hence, our work provides a good demonstration to optimize the structure of the Li composite anode wherein a facile thermal infusion method can unexpectedly produce a lithiophilic Li22Sn5 bamboo-shoot like nanoparticle array on the flexible carbon cloth skeleton for improved electrochemical performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is partly supported by the National Natural Science Foundation of China (No. 21673033, 52172184) and the Suining Science and Technology Program (2019ZDCGZH003).Notes and references
- J. Lang, L. Qi, Y. Luo and H. Wu, High performance lithium metal anode: Progress and prospects, Energy Storage Mater., 2017, 7, 115–129 CrossRef.
- Z. Peng, N. Zhao, Z. Zhang, H. Wan, H. Lin, M. Liu, C. Shen, H. He, X. Guo, J.-G. Zhang and D. Wang, Stabilizing Li/electrolyte interface with a transplantable protective layer based on nanoscale LiF domains, Nano Energy, 2017, 39, 662–672 CrossRef CAS.
- X.-B. Cheng and Q. Zhang, Dendrite-free lithium metal anodes: stable solid electrolyte interphases for high-efficiency batteries, J. Mater. Chem. A, 2015, 3, 7207–7209 RSC.
- B. Duan, W. Wang, H. Zhao, A. Wang, M. Wang, K. Yuan, Z. Yu and Y. Yang, Li-B Alloy as Anode Material for Lithium/Sulfur Battery, ECS Electrochem. Lett., 2013, 2, A47–A51 CrossRef CAS.
- Y. X. Yin, S. Xin, Y. G. Guo and L. J. Wan, Lithium-sulfur batteries: electrochemistry, materials, and prospects, Angew. Chem., Int. Ed., 2013, 52, 13186–13200 CrossRef CAS PubMed.
- D. Wang, A. Zhou, Z. Yao, X. Xia and Y. Zhang, Confined Polysulfides in N-Doped 3D-CNTs Network for High Performance Lithium-Sulfur Batteries, Materials, 2021, 14, 6131 CrossRef CAS PubMed.
- H. Guo, G. Hou, D. Li, Q. Sun, Q. Ai, P. Si, G. Min, J. Lou, J. Feng and L. Ci, High Current Enabled Stable Lithium Anode for Ultralong Cycling Life of Lithium-Oxygen Batteries, ACS Appl. Mater. Interfaces, 2019, 11, 30793–30800 CrossRef CAS PubMed.
- H. Kim, G. Jeong, Y. U. Kim, J. H. Kim, C. M. Park and H. J. Sohn, Metallic anodes for next generation secondary batteries, Chem. Soc. Rev., 2013, 42, 9011–9034 RSC.
- E. Yoo and H. Zhou, Enhanced Cycle Stability of Rechargeable Li-O2 Batteries by the Synergy Effect of a LiF Protective Layer on the Li and DMTFA Additive, ACS Appl. Mater. Interfaces, 2017, 9, 21307–21313 CrossRef CAS PubMed.
- Z. Li, J. Huang, B. Yann Liaw, V. Metzler and J. Zhang, A review of lithium deposition in lithium-ion and lithium metal secondary batteries, J. Power Sources, 2014, 254, 168–182 CrossRef CAS.
- M. S. Park, S. B. Ma, D. J. Lee, D. Im, S. G. Doo and O. Yamamoto, A highly reversible lithium metal anode, Sci. Rep., 2014, 4, 3815 CrossRef.
- R. Miao, J. Yang, Z. Xu, J. Wang, Y. Nuli and L. Sun, A new ether-based electrolyte for dendrite-free lithium-metal based rechargeable batteries, Sci. Rep., 2016, 6, 21771 CrossRef CAS PubMed.
- C. P. Yang, Y. X. Yin, S. F. Zhang, N. W. Li and Y. G. Guo, Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes, Nat. Commun., 2015, 6, 8058 CrossRef CAS.
- L. L. Lu, J. Ge, J. N. Yang, S. M. Chen, H. B. Yao, F. Zhou and S. H. Yu, Free-Standing Copper Nanowire Network Current Collector for Improving Lithium Anode Performance, Nano Lett., 2016, 16, 4431–4437 CrossRef CAS.
- H. Geaney, D. McNulty, J. O’Connell, J. D. Holmes and C. O’Dwyer, Assessing Charge Contribution from Thermally Treated Ni Foam as Current Collectors for Li-Ion Batteries, J. Electrochem. Soc., 2016, 163, A1805–A1811 CrossRef CAS.
- K. Xie, W. Wei, K. Yuan, W. Lu, M. Guo, Z. Li, Q. Song, X. Liu, J.-G. Wang and C. Shen, Toward Dendrite-Free Lithium Deposition via Structural and Interfacial Synergistic Effects of 3D Graphene@Ni Scaffold, ACS Appl. Mater. Interfaces, 2016, 8, 26091–26097 CrossRef CAS PubMed.
- Z. Luo, C. Liu, Y. Tian, Y. Zhang, Y. Jiang, J. Hu, H. Hou, G. Zou and X. Ji, Dendrite-free lithium metal anode with lithiophilic interphase from hierarchical frameworks by tuned nucleation, Energy Storage Mater., 2020, 27, 124–132 CrossRef.
- Y. Zhao, Q. Li, Z. Liu, L. Fan, J. Li, Z. Ma, X. Qin and G. Shao, Stable Electrochemical Li Plating/Stripping Behavior by Anchoring MXene Layers on Three-Dimensional Conductive Skeletons, ACS Appl. Mater. Interfaces, 2020, 12, 37967–37976 CrossRef CAS.
- W. Jia, Y. Wang, S. Qu, Z. Yao, Y. Liu, C. Li, Z. Wang and J. Li, ZnF2 coated three dimensional Li-Ni composite anode for improved performance, J. Materiomics, 2019, 5, 176–184 CrossRef.
- T. Chen, W. Jia, Z. Yao, Y. Liu, X. Guan, K. Li, J. Xiao, H. Liu, Y. Chen, Y. Zhou, D. Sun and J. Li, Partly lithiated graphitic carbon foam as 3D porous current collectors for dendrite-free lithium metal anodes, Electrochem. Commun., 2019, 107, 106535 CrossRef CAS.
- J. Noh, W. Chen, P. Wu, Y. Huang, J. Tan, H. C. Zhou and C. Yu, Large Cumulative Capacity Enabled by Regulating Lithium Plating with Metal–Organic Framework Layers on Porous Carbon Nanotube Scaffolds, Adv. Funct. Mater., 2021, 31, 2104899 CrossRef CAS.
- R. Zhang, X. Chen, X. Shen, X.-Q. Zhang, X.-R. Chen, X.-B. Cheng, C. Yan, C.-Z. Zhao and Q. Zhang, Coralloid Carbon Fiber-Based Composite Lithium Anode for Robust Lithium Metal Batteries, Joule, 2018, 2, 764–777 CrossRef CAS.
- C. Jin, O. Sheng, J. Luo, H. Yuan, C. Fang, W. Zhang, H. Huang, Y. Gan, Y. Xia, C. Liang, J. Zhang and X. Tao, 3D lithium metal embedded within lithiophilic porous matrix for stable lithium metal batteries, Nano Energy, 2017, 37, 177–186 CrossRef CAS.
- J. Chang, H. Hu, J. Shang, R. Fang, D. Shou, C. Xie, Y. Gao, Y. Yang, Q. N. Zhuang, X. Lu, Y. K. Zhang, F. Li and Z. Zheng, Rational Design of Li-Wicking Hosts for Ultrafast Fabrication of Flexible and Stable Lithium Metal Anodes, Small, 2022, 18, e2105308 CrossRef PubMed.
- J. Chang, J. Shang, Y. Sun, L. K. Ono, D. Wang, Z. Ma, Q. Huang, D. Chen, G. Liu, Y. Cui, Y. Qi and Z. Zheng, Flexible and stable high-energy lithium-sulfur full batteries with only 100% oversized lithium, Nat. Commun., 2018, 9, 4480 CrossRef.
- Y. Gao, H. Hu, J. Chang, Q. Huang, Q. Zhuang, P. Li and Z. Zheng, Realizing High-Energy and Stable Wire-Type Batteries with Flexible Lithium-Metal Composite Yarns, Adv. Energy Mater., 2021, 11, 2101809 CrossRef CAS.
- L. Hu, J. Deng, Q. Liang, J. Wu, B. Ge, Q. Liu, G. Chen and X. Yu, Engineering current collectors for advanced alkali metal anodes: A review and perspective, EcoMat, 2022, 36, 386–401 Search PubMed.
- D. Li, C. Xie, Y. Gao, H. Hu, L. Wang and Z. Zheng, Inverted Anode Structure for Long-Life Lithium Metal Batteries, Adv. Energy Mater., 2022, 12, 1–8 CrossRef.
- L. Wang, J. Shang, Q. Huang, H. Hu, Y. Zhang, C. Xie, Y. Luo, Y. Gao, H. Wang and Z. Zheng, Smoothing the Sodium-Metal Anode with a Self-Regulating Alloy Interface for High-Energy and Sustainable Sodium-Metal Batteries, Adv. Mater., 2021, 33, e2102802 CrossRef PubMed.
- C. Jin, O. Sheng, M. Chen, Z. Ju, G. Lu, T. Liu, J. Nai, Y. Liu, Y. Wang and X. Tao, Armed lithium metal anodes with functional skeletons, Mater. Today Nano, 2021, 13, 100103 CrossRef CAS.
- R. Pathak, Y. Zhou and Q. Qiao, Recent Advances in Lithiophilic Porous Framework toward Dendrite-Free Lithium Metal Anode, Appl. Sci., 2020, 10, 4185 CrossRef CAS.
- C. Wu, H. Huang, W. Lu, Z. Wei, X. Ni, F. Sun, P. Qing, Z. Liu, J. Ma, W. Wei, L. Chen, C. Yan and L. Mai, Mg Doped Li-LiB Alloy with In Situ Formed Lithiophilic LiB Skeleton for Lithium Metal Batteries, Adv. Sci., 2020, 7, 1902643 CrossRef CAS.
- L.-L. Kong, L. Wang, Z.-C. Ni, S. Liu, G.-R. Li and X.-P. Gao, Lithium-Magnesium Alloy as a Stable Anode for Lithium-Sulfur Battery, Adv. Funct. Mater., 2019, 29, 1808756 CrossRef.
- S. Jin, Y. Ye, Y. Niu, Y. Xu, H. Jin, J. Wang, Z. Sun, A. Cao, X. Wu, Y. Luo, H. Ji and L. J. Wan, Solid-Solution-Based Metal Alloy Phase for Highly Reversible Lithium Metal Anode, J. Am. Chem. Soc., 2020, 142, 8818–8826 CrossRef PubMed.
- H. Zhong, Y. Wu, F. Ding, L. Sang and Y. Mai, An artificial Li-Al interphase layer on Li-B alloy for stable lithium-metal anode, Electrochim. Acta, 2019, 304, 255–262 CrossRef CAS.
- L. Fu, M. Wan, B. Zhang, Y. Yuan, Y. Jin, W. Wang, X. Wang, Y. Li, L. Wang, J. Jiang, J. Lu and Y. Sun, A Lithium Metal Anode Surviving Battery Cycling Above 200 degrees C, Adv. Mater., 2020, e2000952, DOI:10.1002/adma.202000952.
- C. Wang, H. Xie, L. Zhang, Y. Gong, G. Pastel, J. Dai, B. Liu, E. D. Wachsman and L. Hu, Universal Soldering of Lithium and Sodium Alloys on Various Substrates for Batteries, Adv. Energy Mater., 2018, 8, 1701963 CrossRef.
- T. Zhang, M. Hong, J. Yang, Z. Xu, J. Wang, Y. Guo and C. Liang, A high performance lithium-ion-sulfur battery with a free-standing carbon matrix supported Li-rich alloy anode, Chem. Sci., 2018, 9, 8829–8835 RSC.
- S. Liu, Y. Ma, Z. Zhou, S. Lou, H. Huo, P. Zuo, J. Wang, C. Du, G. Yin and Y. Gao, Inducing Uniform Lithium Nucleation by Integrated Lithium-rich Li-In Anode with Lithiophilic 3D Framework, Energy Storage Mater., 2020, 33, 423–431 CrossRef.
- W. Jia, Y. Liu, Z. Wang, F. Qing, J. Li, Y. Wang, R. Xiao, A. Zhou, G. Li, X. Yu, Y.-S. Hu, H. Li, Z. Wang, X. Huang and L. Chen, Low-temperature fusion fabrication of Li-Cu alloy anode with in situ formed 3D framework of inert LiCu nanowires for excellent Li storage performance, Sci. Bull., 2020, 65, 1907–1915 CrossRef CAS.
- Z. Yao, W. Jia, Z. Wang, J. Ruan, X. Kong, X. Guan, Z. Wang, J. Li, Y. Wang, W. Zou and F. Zhou, Fast ion/electron conducting scaffold of Li-Zn dual-phase alloy enable uniform deposition of Li metal at high current densities, J. Energy Chem., 2020, 51, 285–292 CrossRef.
- Y. Ouyang, C. Cui, Y. Guo, Y. Wei, T. Zhai and H. Li, In Situ Formed LiZn Alloy Skeleton for Stable Lithium Anodes, ACS Appl. Mater. Interfaces, 2020, 12, 25818–25825 CrossRef CAS.
- H. Qiu, T. Tang, M. Asif, W. Li, T. Zhang and Y. Hou, Stable lithium metal anode enabled by lithium metal partial alloying, Nano Energy, 2019, 65, 103989 CrossRef CAS.
- M. Wan, S. Kang, L. Wang, H. W. Lee, G. W. Zheng, Y. Cui and Y. Sun, Mechanical rolling formation of interpenetrated lithium metal/lithium tin alloy foil for ultrahigh-rate battery anode, Nat. Commun., 2020, 11, 829 CrossRef CAS PubMed.
- Y. Liu, T. Chen, J. Xue, Z. Wang, J. Xing, A. Zhou and J. Li, Three-dimensional lithiophilic Li22Sn5 alloy skeleton for dendrite-free and ultrahigh-capacity Li metal anode, Electrochim. Acta, 2022, 405, 139787 CrossRef CAS.
- W. Jia, Z. Wang, J. Li, X. Yu, Y. Wei, Z. Yao, Y. Liu, Y. Wang, A. Zhou, W. Zou, F. Zhou and H. Li, A dual-phase Li-Ca alloy with a patternable and lithiophilic 3D framework for improving lithium anode performance, J. Mater. Chem. A, 2019, 7, 22377–22384 RSC.
- Z. Wang, J. Xue, Y. Liu, J. Xing, A. Zhou, J. Li, W. Zou, F. Zhou and H. Li, LixCu alloy nanowires nested in Ni foam for highly stable Li metal composite anode, Sci. China Mater., 2021, 65, 69–77 CrossRef.
- T. Chen, S. Jianjian, J. Xing, Y. Liu, Z. Wang, J. Xiao, H. Liu, Y. Chen, X. Sun and J. Li, Self-Formed Lithiophilic Alloy Buffer Layer on Copper Foam Framework for Advanced Lithium Metal Anodes, ACS Appl. Energy Mater., 2021, 4, 4879–4886 CrossRef CAS.
- Z. Wang, Y. Liu, J. Xing, Z. Song, A. Zhou, W. Zou, F. Zhou and J. Li, Li-Ca Alloy Composite Anode with Ant-Nest-Like Lithiophilic Channels in Carbon Cloth Enabling High-Performance Li Metal Batteries, Research, 2022, 2022, 1–11 Search PubMed.
- R. A. Huggins, Lithium alloy negative electrodes, J. Power Sources, 1999, 81, 13–19 CrossRef.
- H. Xu, S. Li, C. Zhang, X. Chen, W. Liu, Y. Zheng, Y. Xie, Y. Huang and J. Li, Roll-to-roll prelithiation of Sn foil anode suppresses gassing and enables stable full-cell cycling of lithium ion batteries, Energy Environ. Sci., 2019, 12, 2991–3000 RSC.
- J. Liu, Y. Wen, P. A. van Aken, J. Maier and Y. Yu, Facile synthesis of highly porous Ni-Sn intermetallic microcages with excellent electrochemical performance for lithium and sodium storage, Nano Lett., 2014, 14, 6387–6392 CrossRef CAS PubMed.
- M. G. Park, D. H. Lee, H. Jung, J. H. Choi and C. M. Park, Sn-Based Nanocomposite for Li-Ion Battery Anode with High Energy Density, Rate Capability, and Reversibility, ACS Nano, 2018, 12, 2955–2967 CrossRef CAS.
- C. Xie, J. Chang, J. Shang, L. Wang, Y. Gao, Q. Huang and Z. Zheng, Hybrid Lithium-Ion/Metal Electrodes Enable Long Cycle Stability and High Energy Density of Flexible Batteries, Adv. Funct. Mater., 2022, 32, 2203242 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qm00888b |
| This journal is © the Partner Organisations 2023 |