Copper-catalyzed formal [4 + 1] annulation of N-propargyl ynamides with diketones†
Hao-Jin
Xu‡
a,
Cui-Ting
Li‡
a,
Can-Ming
Chen
a,
Jie
Chen
a,
Xin-Qi
Zhu
*a,
Bo
Zhou
a and
Long-Wu
Ye
 *ab
*ab
aKey Laboratory for Chemical Biology of Fujian Province and State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China. E-mail: longwuye@xmu.edu.cn; xinqizhu@xmu.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
First published on 24th November 2022
Abstract
An efficient copper-catalyzed formal [4 + 1] annulation of N-propargyl ynamides with diketones is described. This protocol enables practical and atom-economic synthesis of valuable pyrrole-substituted dioxoles in generally moderate to excellent yields under mild reaction conditions. Thus, two new five-membered heterocycles can be constructed in one step through this vinyl cation chemistry.
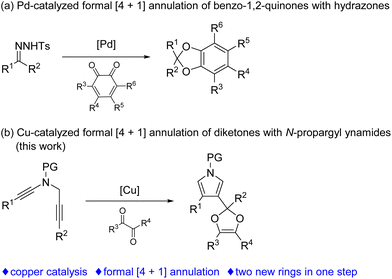
Formal [4 + 1] annulation has attracted extensive interest, since this approach can offer a concise way for the construction of five-member ring cyclic compounds, such as dihydrofurans, isoxazolines, pyrrolines, dihydropyrazoles and cyclopentenones, which are popular structural motifs in a range of natural products and bioactive compounds.1 However, formal [4 + 1] annulation involving the formation of two C–O bonds on the carbenic carbon has been rarely explored. In 2018, an important breakthrough was achieved in this regard by Jiang and coworkers,2 who demonstrated an elegant protocol on the palladium-catalyzed [4 + 1] annulation of N-tosylhydrazones with benzo-1,2-quinones via carbonyl ylides for the synthesis of useful benzodioxoles (Scheme 1a).3 Therefore, the exploration of novel formal [4 + 1] annulation involving the formation of two C–O bonds is still in high demand.
 | ||
| Scheme 1 Transition metal-catalyzed formal [4 + 1] annulations involving the formation of two C–O bonds. | ||
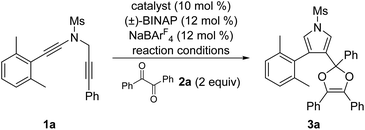
In the past decade, the chemistry of vinyl cations has received particular attention because of their unique carbene-like reactivity.4 During our recent studies on the copper-catalyzed diyne cyclization,5,6 we very recently disclosed a copper-catalyzed asymmetric formal [2 + 1] and [4 + 1] annulations of N-propargyl ynamides with ketones via carbonyl ylides, leading to the divergent, practical and atom-economical synthesis of a range of chiral oxiranes and dihydrofurans in moderate to excellent yields with generally excellent enantioselectivities.6g Importantly, this protocol represents the first reaction of vinyl cations with carbonyl compounds. Inspired by the above results and by our recent work on developing ynamide chemistry for N-heterocycle synthesis,7,8 we envisioned that vinyl cations generated from the above diyne cyclization might be trapped by diketones involving the formation of two new C–O bonds, thus affording synthetically useful pyrrole-substituted dioxoles through formal [4 + 1] annulation. Herein, we report such a copper-catalyzed formal [4 + 1] annulation of N-propargyl ynamides with diketones, enabling a novel and practical protocol to a variety of pyrrole-substituted dioxoles in generally moderate to excellent yields (Scheme 1b).
We commenced our studies by choosing 2,6-dimethylphenyl-substituted N-propargyl ynamide 1a, which could not undergo the background C–H insertion reaction,6a and benzil 2a (2 equiv.) as the model substrates, and selected results are shown in Table 1. By using 10 mol% of Cu(MeCN)4PF6 or Cu(MeCN)4BF4 as the catalyst, we were delighted to find that the desired pyrrole-substituted dioxole 3a could be formed in 58% yield (entries 1 and 2). Subsequent screening of other copper catalysts (entries 3–6) revealed that CuBr was the best catalyst to deliver the expected product 3a in 72% yield (entry 5). Notably, the use of other typical solvents such as CHCl3, DCE and toluene led to significantly decreased yields (entries 7–9). In addition, lowering the temperature also failed to improve the yield (entry 10). Gratifyingly, increasing the amount of benzil to 5 equiv. or 10 equiv. resulted in a significant increase on the yield, affording the desired 3a in 84% yield (entries 11 and 12). Without NaBArF4, the reaction failed to produce even a trace of 3a (entry 13).
| Entry | Catalyst | Reaction conditions | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.05 mmol), 2a (0.1 mmol), catalyst (0.005 mmol), (±)-BINAP (0.006 mmol), NaBArF4 (0.006 mmol), solvents (2.5 mL), 40–60 °C, 15–48 h, in Schlenk tubes. b Measured by 1H NMR using 1,3,5-trimethoxybenzene as the internal reference. c 5 equiv. of 2a was used. d 10 equiv. of 2a was used. e In the absence of NaBArF4. | |||
| 1 | Cu(CH3CN)4PF6 | DCM, 60 °C, 15 h | 58 |
| 2 | Cu(CH3CN)4BF4 | DCM, 60 °C, 15 h | 58 |
| 3 | CuOTf | DCM, 60 °C, 48 h | 40 |
| 4 | CuCl | DCM, 60 °C, 15 h | 59 |
| 5 | CuBr | DCM, 60 °C, 23 h | 72 |
| 6 | CuI | DCM, 60 °C, 15 h | 61 |
| 7 | CuBr | CHCl3, 60 °C, 48 h | 8 |
| 8 | CuBr | DCE, 60 °C, 48 h | 17 |
| 9 | CuBr | Toluene, 60 °C, 48 h | <1 |
| 10 | CuBr | DCM, 40 °C, 48 h | 46 |
| 11 | CuBr | DCM, 60 °C, 23 h | 84 |
| 12d | CuBr | DCM, 60 °C, 23 h | 84 |
| 13e | CuBr | DCM, 60 °C, 48 h | <1 |
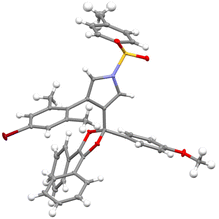
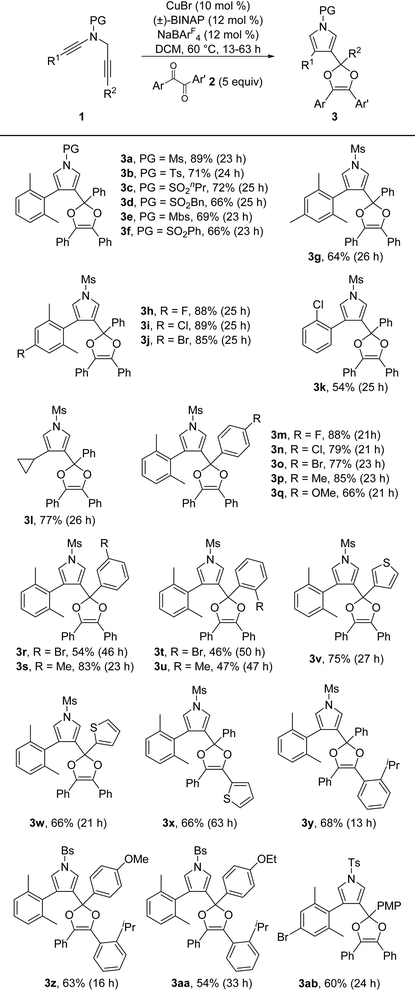
With the optimized reaction conditions in hand (Table 1, entry 11), the reaction scope of this copper-catalyzed formal [4 + 1] annulation was then exploited. As depicted in Table 2, the reaction proceeded smoothly for different N-protected ynamides, leading to the corresponding pyrrole-substituted dioxoles 3a–3f in 66–89% yields. In addition, ynamides bearing both electron-donating and -withdrawing groups on the aromatic ring were suitable for this reaction, furnishing the desired products 3g–3j in 64–89% yields. Of note, monochlorophenyl-substituted ynamide was also compatible with the reaction conditions to deliver the expected 3k in 54% yield, although the background C–H insertion product 3k′ was formed in 29% yield.9 Other types of monosubstituted ynamides such as 1-naphthyl ynamide 1aa and meta-substituted ynamides 1ab–1ac were also tested, but the reaction only led to the formation of complicated mixtures.9,10 Furthermore, the reaction was also suitable to the cyclopropyl-substituted ynamide to afford the desired product 3l in 77% yield. Subsequently, a wide range of aryl-substituted N-propargyl ynamides bearing both electron-withdrawing and -donating groups were explored, and could be converted into the corresponding products 3m–3u in 46–88% yields. Interestingly, the reaction was also extended to the heteroaromatic-substituted N-propargyl ynamides to generate the expected products 3v and 3w in 75% and 66% yields, respectively. Finally, it was found that various diaryl 1,2-diketones 2 were also tolerated, thus leading to the desired dioxoles 3x–3aa in 54–68% yields. It should be mentioned that our attempts to extend the reaction to the alkyl-substituted diketones have been unsuccessful as yet. The structure of product 3ab was further confirmed by X-ray diffraction analysis (Fig. 1).11
| a Reaction conditions: 1 (0.1 mmol), 2 (0.5 mmol), CuBr (0.01 mmol), (±)-BINAP (0.012 mmol), NaBArF4 (0.012 mmol), DCM (5 mL), 60 °C, 13–63 h, in Schlenk tubes; isolated yields are reported. |
|---|
|
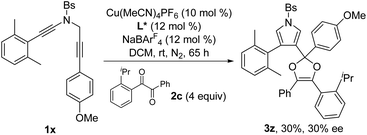
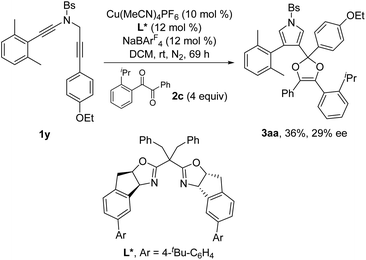
Next, the enantioselective copper-catalyzed formal [4 + 1] annulation reaction was investigated. Screening of various chiral bis(oxazoline) (BOX) ligands and biphosphine ligands revealed that the chiral copper complex-catalyzed annulation of N-propargyl ynamides 1x and 1y with 1,2-diketone 2c could afford the desired chiral dioxoles 3z and 3aa in 30% and 36% yields, respectively, with promising enantioselectivity (29–30% ee) in the presence of Tang's SaBOX ligand12 as the chiral ligand (eqn (1) and (2)).
 | (1) |
 | (2) |
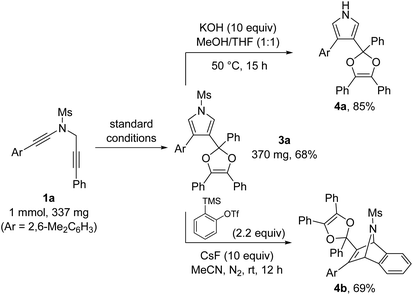
To further demonstrate the utility of this annulation reaction, we carried out several synthetic transformations of the pyrrole-substituted dioxole 3a, as illustrated in Scheme 2. First, facile deprotection of 3a, which was prepared in 68% yield on a preparative scale under standard conditions, could afford the corresponding 1H-pyrrole 4a in 85% yield. In addition, [4 + 2] cycloaddition between the pyrrole ring of 3a and the in situ generated benzyne allowed the formation of the bridged product 4b in 69% yield.
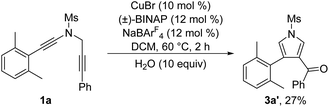
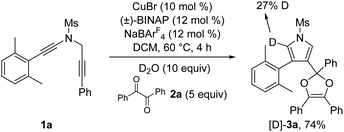
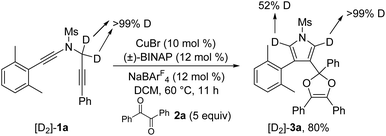
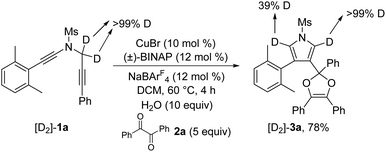
To further probe the reaction mechanism, several control experiments were then conducted. First, it was found that in the presence of 10 equiv. of water, the formation of the corresponding pyrrole-ketone product was observed in 27% yield, which indicates that the generated vinyl cation intermediate was trapped by H2O (eqn (3)).9 In addition, we tested the reaction by employing 10 equiv. of D2O under the standard conditions, and found that the 27% deuterium incorporation into the pyrrole partner of the desired product was observed (eqn (4)). Moreover, we synthesized the substrate [D2]-1a and subjected it under the standard conditions and in the presence of 10 equiv. of H2O, and found that only 52% deuterium and 39% deuterium were retained on one of the α-position pyrrole, respectively (eqn (5) and (6)). These results are consistent with our previous protocol on the copper-catalyzed cyclization of alkenyl diynes,6c where H2O-assisted 1,4-proton transfer was presumably involved.
 | (3) |
 | (4) |
 | (5) |
 | (6) |
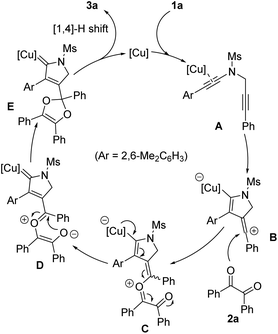
On the basis of the above experimental results and our previous study on the copper-catalyzed cyclization of N-propargyl ynamides,6 a plausible reaction mechanism to rationalize the formation of pyrrole-substituted dioxole 3a is shown in Scheme 3. First, the Cu(I) catalyst preferentially coordinates with the electron-richer amide-tethered C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond of 1a to lead to the precursor A. Subsequently, precursor A undergoes intramolecular trapping by another C–C triple bond, providing the key vinyl cation intermediate B, which is further trapped by 1,2-diketone 2a to form the copper catalyst-tethered oxonium intermediate C. Next, intermediate C is successively converted into donor/donor copper carbene intermediates D and E, which finally undergo [1,4]-H shift and demetallation to generate the corresponding product 3a. On the other hand, the use of chiral copper catalyst leads to the chiral product through asymmetric annulation by a remote control of enantioselectivity.6
C bond of 1a to lead to the precursor A. Subsequently, precursor A undergoes intramolecular trapping by another C–C triple bond, providing the key vinyl cation intermediate B, which is further trapped by 1,2-diketone 2a to form the copper catalyst-tethered oxonium intermediate C. Next, intermediate C is successively converted into donor/donor copper carbene intermediates D and E, which finally undergo [1,4]-H shift and demetallation to generate the corresponding product 3a. On the other hand, the use of chiral copper catalyst leads to the chiral product through asymmetric annulation by a remote control of enantioselectivity.6
In summary, we have developed an efficient copper-catalyzed formal [4 + 1] annulation of N-propargyl ynamides with diketones, allowing novel, practical and atom-economic synthesis of pyrrole-substituted dioxoles in generally moderate to excellent yields with broad substrate scope under mild conditions. Particularly, two new five-membered heterocycles can be constructed in one step through the formation of two new C–O bonds, thus significantly expanding the application of vinyl cation chemistry. Furthermore, the possibility of such an asymmetric formal [4 + 1] annulation via chiral copper catalysis also emerges.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from MOST (2021YFC2100100), the National Natural Science Foundation of China (22125108, 22101238, 92056104 and 21772161), the China Postdoctoral Science Foundation (2020M680087), the President Research Funds from Xiamen University (20720210002), and NFFTBS (J1310024).References
- For recent selected reviews, see: (a) P. Y. Ushakov, S. L. Ioffe and A. Y. Sukhorukov, Recent Advances in the Application of Ylide-Like Species in [4+1]-Annulation Reactions: An Update Review, Org. Chem. Front., 2022, 9, 5358 RSC; (b) H. Zhang and R. Zhou, Recent Advances in Phosphine-Promoted (4+1) Annulation Reactions, Eur. J. Org. Chem., 2020, 4098 CrossRef CAS; (c) C. Zhu, C.-Q. Wang and C. Feng, Recent Advance in Transition-Metal-Catalyzed Oxidant-Free 4+1 Annulation through C–H Bond Activation, Tetrahedron, 2018, 59, 430 CrossRef CAS; (d) T. Kaur, P. Wadhwa, S. Bagchi and A. Sharma, Isocyanide Based [4+1] Cycloaddition Reactions: An Indispensable Tool in Multi-Component Reactions (MCRs), Chem. Commun., 2016, 52, 6958 RSC; (e) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Formal [4+1] Annulation Reactions in the Synthesis of Carbocyclic and Heterocyclic Systems, Chem. Rev., 2015, 115, 5301 CrossRef CAS; (f) C. Zhu, Y. Ding and L.-W. Ye, Ylide Formal [4+1] Annulation, Org. Biomol. Chem., 2015, 13, 2530 RSC.
- H. Jiang, F. Chen, C. Zhu, R. Zhu, H. Zeng, C. Liu and W. Wu, Two C−O Bond Formations on a Carbenic Carbon: Palladium-Catalyzed Coupling of N-Tosylhydrazones and Benzo-1,2-quinones To Construct Benzodioxoles, Org. Lett., 2018, 20, 3166 CrossRef CAS PubMed.
- For recent selected reviews on the carbonyl ylide chemistry, see: (a) A. Padwa, Use of Nitrogen and Oxygen Dipole Ylides for Alkaloid Synthesis, ARKIVOC, 2018, 4, 23 Search PubMed; (b) A. Padwa, Cycloaddition Chemistry of Carbonyl Ylides for Alkaloid Synthesis, Russ. Chem. Bull., 2016, 65, 2183 Search PubMed; (c) Y. Liu, Recent Advances in Catalytic Selective Synthesis of Epoxide and Aziridine via Diazocarbonyl Compound, Curr. Org. Chem., 2016, 20, 19 CrossRef CAS; (d) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Modern Organic Synthesis with α-Diazocarbonyl Compounds, Chem. Rev., 2015, 115, 9981 CrossRef CAS PubMed; (e) A. Padwa, Domino Reactions of Rhodium(II) Carbenoids for Alkaloid Synthesis, Chem. Soc. Rev., 2009, 38, 3072 RSC; (f) Z. Zhang and J. Wang, Recent Studies on the Reactions of α-Diazocarbonyl Compounds, Tetrahedron, 2008, 64, 6577 CrossRef CAS.
- For recent selected reviews, see: (a) X.-J. Liu, Y. Xu, C. Tang, P.-C. Qian and L.-W. Ye, Unactivated C(sp3)–H Functionalization via Vinyl Cations, Sci. China: Chem., 2022, 65, 20 CrossRef CAS; (b) M. Niggemann and S. Gao, Are Vinyl Cations Finally Coming of Age?, Angew. Chem., Int. Ed., 2018, 57, 16942 CrossRef CAS PubMed.
- For recent reviews on the diyne cyclization, see: (a) F. Gagosz, Gold Vinylidenes as Useful Intermediates in Synthetic Organic Chemistry, Synthesis, 2019, 1087 CAS; (b) A. M. Asiri and A. S. K. Hashmi, Gold-Catalysed Reactions of Diynes, Chem. Soc. Rev., 2016, 45, 4471 RSC; (c) D. P. Day and P. W. H. Chan, Gold-Catalyzed Cycloisomerizations of 1,n-Diyne Carbonates and Esters, Adv. Synth. Catal., 2016, 358, 1368 CrossRef CAS; (d) A. S. K. Hashmi, Dual Gold Catalysis, Acc. Chem. Res., 2014, 47, 864 CrossRef CAS; (e) I. Braun, A. M. Asiri and A. S. K. Hashmi, Gold Catalysis 2.0, ACS Catal., 2013, 3, 1902 CrossRef CAS.
- (a) F.-L. Hong, Z.-S. Wang, D.-D. Wei, T.-Y. Zhai, G.-C. Deng, X. Lu, R.-S. Liu and L.-W. Ye, Generation of Donor/Donor Copper Carbenes through Copper-Catalyzed Diyne Cyclization: Enantioselective and Divergent Synthesis of Chiral Polycyclic Pyrroles, J. Am. Chem. Soc., 2019, 141, 16961 CrossRef CAS PubMed; (b) F.-L. Hong, Y.-B. Chen, S.-H. Ye, G.-Y. Zhu, X.-Q. Zhu, X. Lu, R.-S. Liu and L.-W. Ye, Copper-Catalyzed Asymmetric Reaction of Alkenyl Diynes with Styrenes by Formal [3+2] Cycloaddition via Cu-Containing All-Carbon 1,3-Dipoles: Access to Chiral Pyrrole-Fused Bridged [2.2.1] Skeletons, J. Am. Chem. Soc., 2020, 142, 7618 CrossRef CAS; (c) X.-Q. Zhu, P. Hong, Y.-X. Zeng, Y.-Y. Zhen, F.-L. Hong, X. Lu and L.-W. Ye, Copper-Catalyzed Asymmetric Cyclization of Alkenyl Diynes: Method Development and New mechanistic Insights, Chem. Sci., 2021, 11, 9466 RSC; (d) G.-Y. Zhu, T.-Y. Zhai, X. Li, C.-Y. Shi, X.-Q. Zhu and L.-W. Ye, Copper-Catalyzed Cyclization of N-Propargyl Ynamides with Borane Adducts through B−H Bond Insertion, Org. Lett., 2021, 23, 8067 CrossRef CAS; (e) E.-H. Huang, Y.-Q. Zhang, D.-Q. Cui, X.-Q. Zhu, X. Li and L.-W. Ye, Copper-Catalyzed Si−H Bond Insertion Reaction of N-Propargyl Ynamides with Hydrosilanes, Org. Lett., 2022, 24, 196 CrossRef CAS PubMed; (f) F.-L. Hong, C.-Y. Shi, P. Hong, T.-Y. Zhai, X.-Q. Zhu, X. Lu and L.-W. Ye, Copper-Catalyzed Asymmetric Diyne Cyclization via [1,2]-Stevens-type Rearrangement for the Synthesis of Chiral Chromeno[3,4-c]pyrroles, Angew. Chem., Int. Ed., 2022, 61, e202115554 CAS; (g) L.-J. Qi, C.-T. Li, Z.-Q. Huang, J.-T. Jiang, X.-Q. Zhu, X. Lu and L.-W. Ye, Enantioselective Copper-Catalyzed Formal [2+1] and [4+1] Annulations of Diynes with Ketones via Carbonyl Ylides, Angew. Chem., Int. Ed., 2022, 61, e202210637 CAS.
- For recent selected reviews on the ynamide chemistry, see: (a) Y.-C. Hu, Y. Zhao, B. Wan and Q.-A. Chen, Reactivity of Ynamides in Catalytic Intermolecular Annulations, Chem. Soc. Rev., 2021, 50, 2582 RSC; (b) C. C. Lynch, A. Sripada and C. Wolf, Asymmetric Synthesis with Ynamides: Unique Reaction Control, Chemical Diversity and Applications, Chem. Soc. Rev., 2020, 49, 8543 RSC; (c) Y.-B. Chen, P.-C. Qian and L.-W. Ye, Brønsted Acid-Mediated Reactions of Ynamides, Chem. Soc. Rev., 2020, 49, 8897 RSC; (d) F.-L. Hong and L.-W. Ye, Transition Metal-Catalyzed Tandem Reactions of Ynamides for Divergent N-Heterocycle Synthesis, Acc. Chem. Res., 2020, 53, 2003 CrossRef CAS PubMed; (e) J. Luo, G.-S. Chen, S.-J. Chen, J.-S. Yu, Z.-D. Li and Y.-L. Liu, Exploiting Remarkable Reactivities of Ynamides: Opportunities in Designing Catalytic Enantioselective Reactions, ACS Catal., 2020, 10, 13978 CrossRef CAS; (f) B. Zhou, T.-D. Tan, X.-Q. Zhu, M. Shang and L.-W. Ye, Reversal of Regioselectivity in Ynamide Chemistry, ACS Catal., 2019, 9, 6393 CrossRef CAS; (g) G. Evano, C. Theunissen and M. Lecomte, Ynamides: Powerful and Versatile Reagents for Chemical Synthesis, Aldrichimica Acta, 2015, 48, 59 CAS; (h) X.-N. Wang, H.-S. Yeom, L.-C. Fang, S. He, Z.-X. Ma, B. L. Kedrowski and R. P. Hsung, Ynamides in Ring Forming Transformations, Acc. Chem. Res., 2014, 47, 560 CrossRef CAS PubMed.
- For recent selected examples by our group, see: (a) G.-Y. Zhu, J.-J. Zhou, L.-G. Liu, X. Li, X.-Q. Zhu, X. Lu, J.-M. Zhou and L.-W. Ye, Catalyst-Dependent Stereospecific [3,3]-Sigmatropic Rearrangement of Sulfoxide-Ynamides: Divergent Synthesis of Chiral Medium-Sized N,S-Heterocycles, Angew. Chem., Int. Ed., 2022, 61, e202204603 CAS; (b) Z.-S. Wang, L.-J. Zhu, C.-T. Li, B.-Y. Liu, X. Hong and L.-W. Ye, Synthesis of Axially Chiral N-Arylindoles via Atroposelective Cyclization of Ynamides Catalyzed by Chiral Brønsted Acids, Angew. Chem., Int. Ed., 2022, 61, e202201436 CAS; (c) Y.-Q. Zhang, Y.-B. Chen, J.-R. Liu, S.-Q. Wu, X.-Y. Fan, Z.-X. Zhang, X. Hong and L.-W. Ye, Asymmetric Dearomatization Catalysed by Chiral Brønsted Acids via Activation of Ynamides, Nat. Chem., 2021, 13, 1093 CrossRef CAS; (d) P.-F. Chen, B. Zhou, P. Wu, B. Wang and L.-W. Ye, Brønsted Acid Catalyzed Dearomatization by Intramolecular Hydroalkoxylation/Claisen Rearrangement: Diastereo- and Enantioselective Synthesis of Spirolactams, Angew. Chem., Int. Ed., 2021, 60, 27164 CrossRef CAS; (e) Y.-Q. Zhang, Y.-P. Zhang, Y.-X. Zheng, Z.-Y. Li and L.-W. Ye, Rapid and Practical Access to Diverse Quindolines by Catalyst-Free and Regioselectivity-Reversed Povarov Reaction, Cell Rep. Phys. Sci., 2021, 2, 100448 CrossRef CAS; (f) B.-H. Zhu, Y.-X. Zheng, W. Kang, C. Deng, J.-M. Zhou and L.-W. Ye, Catalytic Hydrative Cyclization of Aldehyde-Ynamides with Water for Synthesis of Medium-Sized Lactams, Sci. China: Chem., 2021, 64, 1985 CrossRef CAS; (g) Z.-S. Wang, Y.-B. Chen, H.-W. Zhang, Z. Sun, C. Zhu and L.-W. Ye, Ynamide Smiles Rearrangement Triggered by Visible-Light-Mediated Regioselective Ketyl-Ynamide Coupling: Rapid Access to Functionalized Indoles and Isoquinolines, J. Am. Chem. Soc., 2020, 142, 3636–3644 CrossRef CAS; (h) X. Liu, Z.-S. Wang, T.-Y. Zhai, C. Luo, Y.-P. Zhang, Y.-B. Chen, C. Deng, R.-S. Liu and L.-W. Ye, Copper-Catalyzed Azide-Ynamide Cyclization to Generate α-Imino Copper Carbenes: Divergent and Enantioselective Access to Polycyclic N-Heterocycle, Angew. Chem., Int. Ed., 2020, 59, 17984 CrossRef CAS; (i) E.-H. Huang, Z.-X. Zhang, S.-H. Ye, Y.-B. Chen, W.-F. Luo, P.-C. Qian and L.-W. Ye, Copper-Catalyzed Carbocyclization of Silyl Enol Ether Tethered Ynamides for Efficient and Practical Synthesis of 2-Azabicyclo[3.2.0] Compounds, Chin. J. Chem., 2020, 38, 1086 CrossRef CAS; (j) H.-H. Li, S.-H. Ye, Y.-B. Chen, W.-F. Luo, P.-C. Qian and L.-W. Ye, Efficient and Divergent Synthesis of Medium-Sized Lactams through Zinc-Catalyzed Oxidative Cyclization of Indoly Ynamides, Chin. J. Chem., 2020, 38, 263 CrossRef CAS.
- For details, please see the ESI.†.
- For the structures of ynamides 1aa–1ac, see below:
. - CCDC 2203937 (3ab) contains the supplementary crystallographic data for this paper.†.
- S. Liao, X.-L. Sun and Y. Tang, Side Arm Strategy for Catalyst Design: Modifying Bisoxazolines for Remote Control of Enantioselection and Related, Acc. Chem. Res., 2014, 47, 2260 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2203937. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qo01786e |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2023 |