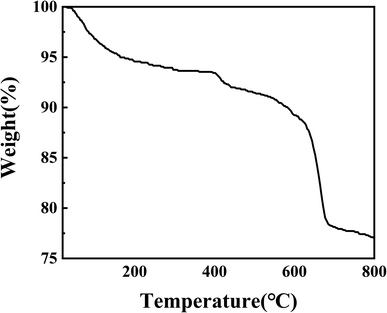
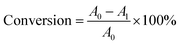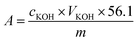Open Access Article
Open Access ArticleLow-cost diatomite supported binary transition metal sulfates: an efficient reusable solid catalyst for biodiesel synthesis†
Weiqing Chen a,
Zhaoji Wua,
Ruoxue Penga,
Wenjuan Wua,
Xiaonan Lia,
Dan Caoa,
Zhigang Zhang*ab and
Kui Niu*ab
a,
Zhaoji Wua,
Ruoxue Penga,
Wenjuan Wua,
Xiaonan Lia,
Dan Caoa,
Zhigang Zhang*ab and
Kui Niu*ab
aCollege of Chemical Engineering, Hebei Normal University of Science & Technology, Qinhuangdao, China 066600. E-mail: zgzhang333@163.com
bHebei Key Laboratory of Active Components and Functions in Natural Products, Qinhuangdao, China 066600. E-mail: niukui007@163.com
First published on 17th February 2023
Abstract
Using a simple method of impregnation and then calcination, diatomite supported binary transition metal sulfates (Fe and Zr, designated as Fe2(SO4)3&Zr(SO4)2@diatomite) were prepared and used as a catalyst in the preparation of renewable biofuels. The synthesised Fe2(SO4)3&Zr(SO4)2@diatomite catalyst (Fe2(SO4)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr(SO4)2
Zr(SO4)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diatomite = 1
diatomite = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
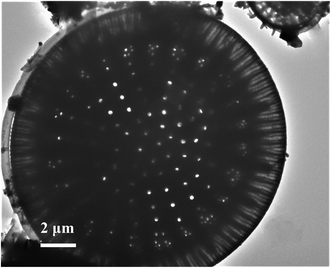
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, mass ratio) was thoroughly characterised using transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier-transform infrared (FTIR) spectroscopy, microbeam X-ray fluorescence (μ-XRF) spectroscopy and thermogravimetric analysis (TG). The results demonstrated that the sulfate was successfully loaded onto the diatomite with a uniform distribution. The N2 adsorption/desorption analysis indicated that the catalyst's specific surface area was 1.54 m2 g−1. The catalyst exhibited outstanding performance in the preparation of renewable biofuel (biodiesel) from waste fatty acids and the optimal parameters were methanol-to-oil 1.25
6, mass ratio) was thoroughly characterised using transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier-transform infrared (FTIR) spectroscopy, microbeam X-ray fluorescence (μ-XRF) spectroscopy and thermogravimetric analysis (TG). The results demonstrated that the sulfate was successfully loaded onto the diatomite with a uniform distribution. The N2 adsorption/desorption analysis indicated that the catalyst's specific surface area was 1.54 m2 g−1. The catalyst exhibited outstanding performance in the preparation of renewable biofuel (biodiesel) from waste fatty acids and the optimal parameters were methanol-to-oil 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, reaction temperature 70 °C, catalyst concentration 10 wt%, reaction time 4 h. The conversion was found to reach 98.90% under optimal parameters, which is better than that of Fe2(SO4)3·xH2O, Zr(SO4)2·4H2O, Fe2(SO4)3@diatomite and Zr(SO4)2@diatomite. Moreover, the catalyst can be recycled by simple filtration and reused for three cycles after regeneration without noticeable reduction in catalytic activity.
1, reaction temperature 70 °C, catalyst concentration 10 wt%, reaction time 4 h. The conversion was found to reach 98.90% under optimal parameters, which is better than that of Fe2(SO4)3·xH2O, Zr(SO4)2·4H2O, Fe2(SO4)3@diatomite and Zr(SO4)2@diatomite. Moreover, the catalyst can be recycled by simple filtration and reused for three cycles after regeneration without noticeable reduction in catalytic activity.
1 Introduction
With increasing energy demand, the consumption of fossil fuels, and their negative impact on the environment, such as environmental pollution, global warming, and rising sea levels, great attention has been given to researching renewable and clean biofuels.1–4 Biodiesel, a green, biodegradable, renewable and sustainable biofuel, is usually made from waste vegetable oils and animal fats. The composition of biodiesel is alkyl esters of fatty acids derived from vegetable oils and fats. Compared with traditional fuels, it has many advantages, such as it is nontoxic, biodegradable, has a low amount of sulfur, has a high energy density, excellent combustion performance, less particulate pollutants, less greenhouse gases (mainly CO2 emissions) and carbon monoxide emissions, it is safer to handle, and so on.5–7 In particular, biodiesel can be applied directly in diesel engines or blended with fossil fuel diesel in any proportion.8 Hence, a lot of researchers are investigating biodiesel because from a strategic standpoint it is one of the most significant fossil fuel alternatives.Biodiesel is usually made from animal and vegetable oils using the chemical processes of esterification and transesterification with methanol or ethanol in the presence of a catalyst.9 In production processes, catalysts always play a central role and affect the biodiesel yield and the long-term viability of the industrial process.10,11 They are often classified into homogeneous catalysts or heterogeneous catalysts.12–14 Homogeneous catalysts, such as sulfuric acid (H2SO4), H3PO4, KOH, and potassium hydroxide (NaOH), have many disadvantages, such as the difficulty of separation, and inability to be reused, generation of wastewater and corrosion of the reactor.15–17 Heterogeneous catalysts, including heterogeneous acids and bases, are considered as a potential alternative because of their easy separation from the reaction mixture, good stability, excellent reactivity and easy recovery.16,18–21
However, waste vegetable oils and fats usually contain high levels of fatty acids due to them becoming rancid during collection and processing. When employing base catalysts, these fatty acids cannot be transformed into fatty acid alkyl esters (biodiesel) by esterification, resulting in low yields. Therefore, heterogeneous acids are considered appropriate for the catalysis of biodiesel manufacture from waste vegetable oils and fats, because they can stimulate esterification of the fatty acids and transesterification of the triglycerides into alkyl esters simultaneously. Many types of heterogeneous acids, including solid superacid (especially sulfated metal oxide),22–25 cation exchange resin,26–28 supported heteropoly acid,29–31 sulfonated carbon-based solid acids32–34 and so on, have been widely studied by researchers during the past decade. However, few of them have been widely used in industrial production for various factors such as high cost, complexity and the generation of pollutants during preparation. For industrial biodiesel manufacture using waste vegetable oils and fats, it is crucial to design heterogeneous acidic catalysts with high efficiency, stability, acceptable reusability, low cost and environmental benefits.
Transition metal sulfates such as ferric sulfate (Fe2(SO4)3) and zirconium sulfate (Zr(SO4)2), as solid Lewis acids, exhibited excellent catalytic performance in the catalysis of esterification.35–38 Compared with Zr(SO4)2, Fe2(SO4)3 is inexpensive and readily available, but it is susceptible to moisture absorption which affects its catalytic performance in the esterification reaction. In addition, although Fe2(SO4)3 and Zr(SO4)2 are insoluble in oil, they can be slightly soluble in methanol and will dissolve in the water formed during esterification, which will lead to loss of the catalyst and inferior reusability.35 To address these problems, immobilising them on suitable carrier was a good strategy to avoid the dissolution, and loss of the catalytic active substance, greatly improving their catalytic activity and stability.
Diatomite is a typical natural siliceous rock mainly composed of amorphous silica with excellent properties, such as low cost, porosity, chemical inertness, compatibility and availability for surface modification.39,40 Hence, diatomite has been considered as a promising support/template and used for broad range of applications including energy conversion and storage,41–44 water remediation,45–48 and drug delivery.49–51 Furthermore, diatomite has also been used as a desirable catalyst support in many organic chemical reactions.52,53 Some diatomite supported basic heterogeneous compounds, such as diatomite supported CaO/MgO and diatomite-supported KOH, have been developed for biodiesel preparation.54–56
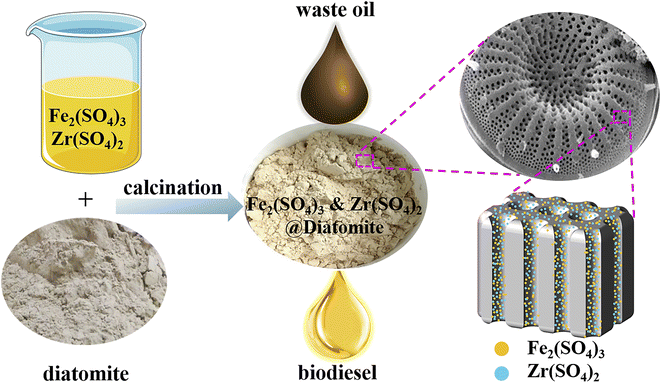
In addition, the cost of diatomite is usually much lower than that of other supports such as zeolite,57,58 alumina,59,60 activated carbon61,62 and so on. The price of diatomite reported online is about RMB 2200 per ton, whereas activated carbon is usually RMB 4500–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per ton, alumina is about RMB 7200 per ton, and zeolite is about RMB 9000 per ton.63,64 Consequently, immobilising Fe2(SO4)3 and Zr(SO4)2 on diatomite may be a good strategy. In this study, the Fe2(SO4)3&Zr(SO4)2@diatomite catalyst was prepared using simple impregnation and calcination (Scheme 1). The method for the synthesis of this novel catalyst is not only simple but also economical, which is beneficial for industrial applications.
000 per ton, alumina is about RMB 7200 per ton, and zeolite is about RMB 9000 per ton.63,64 Consequently, immobilising Fe2(SO4)3 and Zr(SO4)2 on diatomite may be a good strategy. In this study, the Fe2(SO4)3&Zr(SO4)2@diatomite catalyst was prepared using simple impregnation and calcination (Scheme 1). The method for the synthesis of this novel catalyst is not only simple but also economical, which is beneficial for industrial applications.
2 Materials and methods
2.1 Materials
Ferric sulfate hydrate (Fe2(SO4)3·xH2O, Fe content 21–23 wt%), zirconium sulfate tetrahydrate (Zr(SO4)2·4H2O, AR), methanol (99.5% purity), and diatomite (median diameter: 19.6 μm) were purchased from Shanghai Aladdin Bio-Chem Technology (Shanghai, China). The H2SO4 (98% purity) and potassium hydroxide (KOH, ≥85% purity) were purchased from Institute of Tianjin Chemical Reagent Research Institute (Tianjin, China). Waste animal and vegetable oils (moisture content 2.28 wt%, ash content 0.52 wt%) were collected from a municipal sewer. Waste fatty acids (acid value 195.50 mg KOH per g, saponification value 205.82 mg KOH per g) were extracted from waste animal and vegetable oils. The composition of the waste fatty acids was: palmitic acid (C16:0) 22.99 wt%, stearic acid (C18:0) 5.62 wt%, oleic acid (C18:1) 36.68 wt%, linoleic acid (C18:2) 26.19 wt%, and dietary linolenic acid (C18:3) 2.86 wt%.2.2 Methods
where A0 is the acid value of the waste fatty acids, and A1 is the acid value of the product.
where cKOH is the concentration of the KOH solution, VKOH is the volume of the KOH solution consumed during titration, 56.1 is the molecular weight of KOH, and m is the weight of the sample.
3 Results and discussion
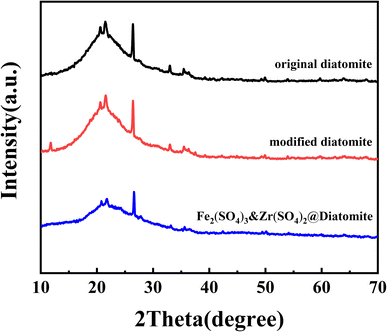
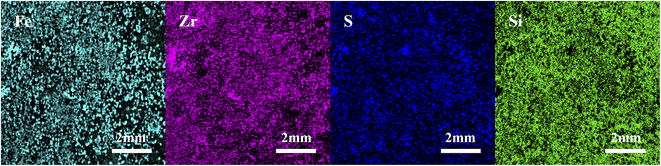
3.1 Catalyst characterisation
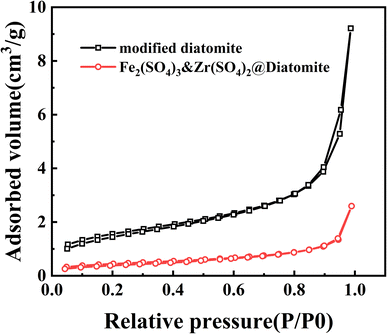 | ||
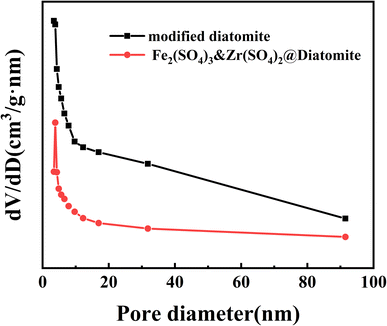
| Fig. 5 The N2 adsorption/desorption isotherms of modified diatomite and the Fe2(SO4)3&Zr(SO4)2@diatomite catalyst. | ||
3.2 Optimum conditions in the esterification process
The effects of the methanol/oil ratio, catalyst concentration, reaction temperature, and reaction time on the esterification rate were investigated using orthogonal experiments (see the ESI for the Experimental details†). The optimum conditions obtained were: methanol/oil ratio: 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, catalyst concentration: 10%, reaction temperature: 70 °C, and reaction time: 4 h.
1, catalyst concentration: 10%, reaction temperature: 70 °C, and reaction time: 4 h.
3.3 Catalytic activity comparison
We also synthesised Fe2(SO4)3@diatomite and Zr(SO4)2@diatomite according to the method described in Section 2.2.2 for further investigation of the catalytic property of Fe2(SO4)3&Zr(SO4)2@diatomite. The catalytic effects of the following catalysts: Fe2(SO4)3·xH2O, Zr(SO4)2·4H2O, Fe2(SO4)3@diatomite, Zr(SO4)2@diatomite and Fe2(SO4)3&Zr(SO4)2@diatomite in the esterification of waste fatty acids and methanol were compared using the method described in Section 2.2.4. Table 1 shows the results, and it was observed that the conversions of Fe2(SO4)3·xH2O, Zr(SO4)2·4H2O, Fe2(SO4)3@diatomite and Zr(SO4)2@diatomite were 94.40%, 97.75%, 95.22% and 98.56%, respectively, whereas that of Fe2(SO4)3&Zr(SO4)2@diatomite was 98.90% when the identical reaction parameters were adopted. It was quite clear that the composited Fe2(SO4)3&Zr(SO4)2@diatomite showed a better catalytic performance than Fe2(SO4)3·xH2O, Zr(SO4)2·4H2O, Fe2(SO4)3@diatomite and Zr(SO4)2@diatomite.| Trial number | Catalyst | Conversion (%) |
|---|---|---|
| a Waste fatty acids 5 g, methanol 6.25 g, reaction temperature 70 °C, reaction time 4 h. | ||
| 1 | Fe2(SO4)3·xH2O (0.2 g) | 94.40% ± 0.14 |
| 2 | Zr(SO4)2·4H2O (0.2 g) | 97.75% ± 0.13 |
| 3 | Fe2(SO4)3@diatomite (0.5 g) | 95.22% ± 0.19 |
| 4 | Zr(SO4)2@diatomite (0.5 g) | 98.56% ± 0.12 |
| 5 | Fe2(SO4)3&Zr(SO4)2@diatomite (0.5 g) | 98.90% ± 0.16 |
3.4 Reusability of the catalyst
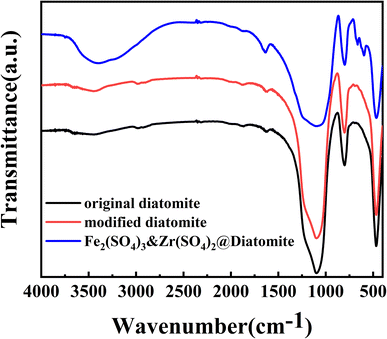
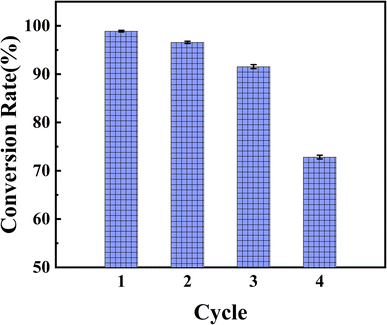
Reusability is a pivotal property of solid catalysts and is closely tied to their affordability and potential applications. The reusability of Fe2(SO4)3&Zr(SO4)2@diatomite was investigated by carrying out successive batch cycles using the same catalyst in the preparation of biodiesel using waste fatty acids, under the optimal parameters obtained from the orthogonal experiment (see ESI†). At the end of each batch run, the Fe2(SO4)3&Zr(SO4)2@diatomite was recycled using simple filtration. The recycled catalyst was washed with methanol to remove the residual oils, and then dried in a vacuum oven at 40 °C, and then reused in the next esterification experiment. The experiments were carried out for four cycles and the outcomes are displayed in Fig. 8. This demonstrated that the conversion decreased slightly after three successive catalytic cycles, but it was still 91.56% after three cycles. Therefore, it was concluded that the Fe2(SO4)3&Zr(SO4)2@diatomite catalyst was reusable, and had the potential to be applied as an efficient solid catalyst in biodiesel production. However, a significant decrease was observed after four cycles. The stability and water resistance of the diatomite supported Fe2(SO4)3 and Zr(SO4)2 were significantly improved, but it might still dissolve slightly in water, a by-product of the esterification reaction. Considering the existence of the Fe–O–Si and Zr–O–Si bonds in the catalyst, which were demonstrated by the FTIR spectra, it was speculated that the diminishing of the catalytic activity may be mainly caused by the loss of SO42−, which was soluble in water, rather than the loss of Zr4+ and Fe3+.3.5 Properties of biodiesel prepared from waste fatty acids
In order to collect enough samples to determine the properties of biodiesel, a larger experiment was carried out under optimal conditions (see Section 3.2) according to the method described in Section 2.2.4. During the industrial process, biodiesel prepared from waste oils usually required refining (vacuum distillation) to ensure its properties met those of the relevant standards. Hence, vacuum distillation (temperature: 250 °C, vacuum degree: 0.097 MPa) was performed before determining the properties of the biodiesel prepared from waste fatty acids. The acid value of refined biodiesel measured according to standard EN 14104 was 0.44 mg KOH per g, the density at 15 °C obtained by using a hydrometer according to standard EN ISO 3675 was 882 kg m−3, and the flash point detected by using the Pensky–Martens closed cup method according to standard EN ISO 2719 was 179 °C. These properties met the requirements of European standard EN 14214: which states that the acid value should not exceed 0.5 mg KOH per g, density (15 °C) should be in the range of 860–900 kg m−3, and the flash point should be higher than 101 °C.4 Conclusions
We synthesised a Fe2(SO4)3&Zr(SO4)2@diatomite catalyst by a simple impregnation and calcination process, and applied it in the esterification of waste fatty acids with methanol as a solid acid catalyst. The catalytic activity of the synthesised diatomite supported catalyst is superior to that of neat Fe2(SO4)3·xH2O and neat Zr(SO4)2·4H2O due to the loading of the active ingredient onto diatomite, which improves the dispersion and water-resistance of the active ingredient. Moreover, the catalytic activity of this catalyst is superior to that of Fe2(SO4)3@diatomite and Zr(SO4)2@diatomite, benefiting from the synergistic effect of Fe2(SO4)3 and Zr(SO4)2. The conversion of waste fatty acids catalysed by the Fe2(SO4)3&Zr(SO4)2@diatomite reached 98.90% under optimal parameters (MeOH to oil ratio 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; catalyst concentration 10 wt%, reaction temperature 70 °C, reaction time 4 h). These optimal parameters obtained may be useful in the actual biodiesel production process.
1; catalyst concentration 10 wt%, reaction temperature 70 °C, reaction time 4 h). These optimal parameters obtained may be useful in the actual biodiesel production process.
The catalyst can also be recycled simply from reaction system via filtration, and then reapplied for multiple cycles without a noticeable decrease in catalytic activity, and the conversion remained at 91.56% after three cycles.
The catalyst in this work is simple to prepare, highly efficient and economical, which is beneficial for industrial applications. In addition, some reported diatomite-supported catalysts, such as diatomite-supported CaO/MgO,54 diatomite-supported KOH55 and diatomite-supported CaO,56 cause saponification when the waste oils contain high amounts of fatty acids. The Fe2(SO4)3&Zr(SO4)2@diatomite as an acid heterogeneous catalyst can catalyse the esterification and transesterification reactions simultaneously. Hence, it may be suited to the catalysis of biodiesel production from waste oils containing high amounts of fatty acids.
Author contributions
Weiqing Chen: conceptualisation, methodology, investigation, data curation and writing – original draft. Zhaoji Wu: visualisation. Ruoxue Peng and Wenjuan Wu: formal analysis. Xiaonan Li and Dan Cao: validation. Zhigang Zhang: conceptualisation, methodology, writing – review and editing, funding acquisition and supervision. Kui Niu: funding acquisition and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by a project for the Hebei Provincial Central Government Guides Local Science and Technology Development (Grant No. 206Z2801G).References
- S. K. R. Devasani, S. Vodnala, D. Singarapu and J. N. Nair, Environ. Sci. Pollut. Res., 2021, 29, 51083–51094 CrossRef PubMed.
- P. S. Nigam and A. Singh, Prog. Energy Combust. Sci., 2011, 37, 52–68 CrossRef CAS.
- H. H. Mardhiah, H. C. Ong, H. H. Masjuki, S. Lim and H. V. Lee, Renewable Sustainable Energy Rev., 2017, 67, 1225–1236 CrossRef CAS.
- N. Pranyoto, Y. D. Susanti, I. J. Ondang, A. E. Angkawijaya, F. E. Soetaredjo, S. P. Santoso, M. Yuliana, S. Ismadji and S. B. Hartono, Nanomaterials, 2022, 12, 245 CrossRef CAS PubMed.
- C. D. M. de Araújo, C. C. de Andrade, E. D. S. e Silva and F. A. Dupas, Renewable Sustainable Energy Rev., 2013, 27, 445–452 CrossRef.
- B. A. Oni and D. Oluwatosin, Renewable Energy, 2020, 149, 725–734 CrossRef CAS.
- Y. Ulusoy, Environ. Sci. Pollut. Res., 2019, 27, 500–509 CrossRef PubMed.
- J. Chang, X. Guan, S. Pan, M. Jia, Y. Chen and H. Fan, New J. Chem., 2018, 42, 13074–13080 RSC.
- P. Maheswari, M. B. Haider, M. Yusuf, J. J. Klemeš, A. Bokhari, M. Beg, A. Al-Othoman, R. Kumar and A. K. Jaiswal, J. Cleaner Prod., 2022, 355, 131588 CrossRef.
- T. Tamoradi, A. R. Kiasat, H. Veisi, V. Nobakht and B. Karmakar, New J. Chem., 2021, 45, 21116–21124 RSC.
- A. Pramanik and S. Bhar, New J. Chem., 2021, 45, 16355–16388 RSC.
- M. Mohadesi, B. Aghel, M. Maleki and A. Ansari, Renewable Energy, 2019, 136, 677–682 CrossRef CAS.
- M. Hamza, M. Ayoub, R. B. Shamsuddin, A. Mukhtar, S. Saqib, I. Zahid, M. Ameen, S. Ullah, A. G. Al-Sehemi and M. Ibrahim, Environ. Technol. Innovation, 2021, 21, 101200 CrossRef CAS.
- J. Gupta, M. Agarwal and A. K. Dalai, J. Ind. Eng. Chem., 2020, 88, 58–77 CrossRef CAS.
- I. Raheem, M. N. B. Mohiddin, Y. H. Tan, J. Kansedo, N. M. Mubarak, M. O. Abdullah and M. L. Ibrahim, J. Ind. Eng. Chem., 2020, 91, 54–68 CrossRef CAS.
- M. O. Faruque, S. A. Razzak and M. M. Hossain, Catalysts, 2020, 10, 1025 CrossRef CAS.
- B. Changmai, R. Rano, C. Vanlalveni and L. Rokhum, Fuel, 2021, 286, 119447 CrossRef CAS.
- A. Wang, P. Sudarsanam, Y. Xu, H. Zhang, H. Li and S. Yang, Green Chem., 2020, 22, 2977–3012 RSC.
- L. F. Man, T. L. Kwong, W. T. Wong and K. F. Yung, Nanomaterials, 2021, 11, 2690 CrossRef CAS PubMed.
- M. Dionicio-Navarrete, C. D. Arrieta-Gonzalez, A. Quinto-Hernandez, M. Casales-Diaz, J. Zuñiga-Diaz, J. Porcayo-Calderon and L. Martinez-Gomez, Nanomaterials, 2019, 9, 1545 CrossRef CAS PubMed.
- F. Núñez, L. F. Chen, J. A. Wang, S. O. Flores, J. Salmones, U. Arellano, L. E. Noreña and F. Tzompantzi, Catalysts, 2022, 12, 900 CrossRef.
- C. C. Huang, S. H. Ho, J. S. Chang and P. J. Gao, New J. Chem., 2020, 44, 13669–13684 RSC.
- B. Lowe, J. Gardy and A. Hassanpour, Catalysts, 2022, 12, 223 CrossRef CAS.
- Y. Li, X. D. Zhang, L. Sun, J. Zhang and H. P. Xu, Appl. Energy, 2010, 87, 156–159 CrossRef CAS.
- D. Guan, M. Fan, J. Wang, Y. Zhang, Q. Liu and X. Jing, Mater. Chem. Phys., 2010, 122, 278–283 CrossRef CAS.
- Y. Feng, A. Zhang, J. Li and B. He, Bioresour. Technol., 2011, 102, 3607–3609 CrossRef CAS PubMed.
- J. Fu, L. Chen, P. Lv, L. Yang and Z. Yuan, Fuel, 2015, 154, 1–8 CrossRef CAS.
- N. Siddique, M. Suzue, M. Kato, K. Hiromori and N. Shibasaki-Kitakawa, Fuel, 2021, 289, 119884 CrossRef CAS.
- M. A. Hanif, S. Nisar and U. Rashid, Catal. Rev., 2017, 59, 165–188 CrossRef CAS.
- F. Esmi, V. B. Borugadda and A. K. Dalai, Catal. Today, 2022, 404, 19–34 CrossRef CAS.
- W. Xie, C. Gao and J. Li, Renewable Energy, 2021, 168, 927–937 CrossRef CAS.
- R. Leesing, S. Siwina and K. Fiala, Renewable Energy, 2021, 171, 647–657 CrossRef CAS.
- R. R. C. Bastos, A. P. Luz Corrêa, P. T. S. Luz, G. N. Rocha Filho, J. R. Zamian and L. R. V. Conceição, Energy Convers. Manage., 2020, 205, 112457 CrossRef CAS.
- M. A. Farabi, M. L. Ibrahim, U. Rashid and Y. H. Taufiq-Yap, Energy Convers. Manage., 2019, 181, 562–570 CrossRef CAS.
- P. Yu, C. Chen, G. Li, Z. Wang and X. Li, Catalysts, 2020, 10, 384 CrossRef CAS.
- M. I. Senoymak Tarakcı and O. Ilgen, Chem. Eng. Technol., 2018, 41, 845–852 CrossRef.
- R. Nakamura, K. Komura and Y. Sugi, Catal. Commun., 2008, 9, 511–515 CrossRef CAS.
- F. P. Martins, F. A. Rodrigues and M. J. Silva, Energies, 2018, 11, 1263 CrossRef.
- Y. Pan, Y. Liu and C. Liu, J. Power Sources, 2015, 285, 169–177 CrossRef CAS.
- U. T. Uthappa, V. Brahmkhatri, G. Sriram, H. Y. Jung, J. Yu, N. Kurkuri, T. M. Aminabhavi, T. Altalhi, G. M. Neelgund and M. D. Kurkuri, J. Controlled Release, 2018, 281, 70–83 CrossRef CAS PubMed.
- X. W. Sun, Y. X. Zhang and D. Losic, J. Mater. Chem. A, 2017, 5, 8847–8859 RSC.
- P. A. Zong, D. Makino, W. Pan, S. Yin, C. Sun, P. Zhang, C. Wan and K. Koumoto, Mater. Des., 2018, 154, 246–253 CrossRef CAS.
- C. Li, M. Wang, Z. Chen and J. Chen, J. Energy Storage, 2021, 34, 102171 CrossRef.
- F. Jiang, Z. Ge, X. Ling, D. Cang, L. Zhang and Y. Ding, Renewable Energy, 2021, 179, 327–338 CrossRef CAS.
- D. B. Jiang, X. Liu, Y. Yuan, L. Feng, J. Ji, J. Wang, D. Losic, H. C. Yao and Y. Z. Zhang, Chem. Eng. J., 2020, 383, 123156 CrossRef CAS.
- G. Sriram, U. T. Uthappa, D. Losic, M. Kigga, H. Y. Jung and M. D. Kurkuri, Appl. Sci., 2020, 10, 2285 CrossRef CAS.
- Y. Tan, S. Zheng, Y. Di, C. Li, R. Bian and Z. Sun, J. Mater. Sci. Technol., 2021, 95, 57–69 CrossRef CAS.
- L. Bengotni, B. Trari, B. Lebeau, L. Michelin, L. Josien, A. Bengueddach and R. Hamacha, New J. Chem., 2021, 45, 13463–13474 RSC.
- U. T. Uthappa, G. Sriram, O. R. Arvind, S. Kumar, H. Y. Jung, G. M. Neelgund, D. Losic and M. D. Kurkuri, Appl. Surf. Sci., 2020, 528, 146974 CrossRef CAS.
- I. Ruggiero, M. Terracciano, N. M. Martucci, L. De Stefano, N. Migliaccio, R. Tatè, I. Rendina, P. Arcari, A. Lamberti and I. Rea, Nanoscale Res. Lett., 2014, 9, 1–7 CrossRef CAS PubMed.
- M. Terracciano, M. A. Shahbazi, A. Correia, I. Rea, A. Lamberti, L. De Stefano and H. A. Santos, Nanoscale, 2015, 7, 20063–20074 RSC.
- N. Ezzatahmadi, T. Bao, H. Liu, G. J. Millar, G. A. Ayoko, J. Zhu, R. Zhu, X. Liang, H. He and Y. Xi, RSC Adv., 2018, 8, 7687–7696 RSC.
- G. Zhang, A. Peyravi, Z. Hashisho, Z. Sun, Y. Liu, S. Zheng and L. Zhong, Catal. Sci. Technol., 2020, 10, 2378–2388 RSC.
- A. M. Rabie, M. Shaban, M. R. Abukhadra, R. Hosny, S. A. Ahmed and N. A. Negm, J. Mol. Liq., 2019, 279, 224–231 CrossRef CAS.
- E. Modiba, C. Enweremadu and H. Rutto, Chin. J. Chem. Eng., 2015, 23, 281–289 CrossRef CAS.
- R. Shan, C. Zhao, H. Yuan, S. Wang and Y. Wang, Energy Convers. Manage., 2017, 138, 547–555 CrossRef CAS.
- S. M. Pavlović, D. M. Marinković, M. D. Kostić, I. M. Janković-Častvan, L. V. Mojović, M. V. Stanković and V. B. Veljković, Fuel, 2020, 267, 117171 CrossRef.
- L. Du, S. Ding, Z. Li, E. Lv, J. Lu and J. Ding, Energy Convers. Manage., 2018, 173, 728–734 CrossRef CAS.
- J. K. Karo, H. Husin, F. Nasution, F. T. Yani, S. Maliki, D. D. Prayuda and F. Hasfita, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 980, 012058 CrossRef CAS.
- A. S. Yusuff, J. O. Owolabi and S. Afr, J. Chem. Eng., 2019, 30, 42–49 Search PubMed.
- Z. Helwani, I. Zahrina, S. Z. Amraini, R. I. Sianturi, G. M. Idroes and R. Idroes, IOP Conf. Ser.: Mater. Sci. Eng., 2021, 1087, 012053 CAS.
- L. J. Konwar, J. Boro and D. Deka, Energy Sources, Part A, 2018, 40, 601–607 CrossRef CAS.
- The price of diatomite and activated carbon online, https://b2b.baidu.com/, accessed January 2023 Search PubMed.
- The price of alumina and zeolite online, http://jsx888.51sole.com/companynewsdetail_134118715.htm, accessed January 2023 Search PubMed.
- L. Ma, Y. Han, K. Sun, J. Lu and J. Ding, Energy Convers. Manage., 2015, 98, 46–53 CrossRef.
- M. Guo, W. Jiang, C. Chen, S. Qu, J. Lu, W. Yi and J. Ding, Energy Convers. Manage., 2021, 229, 113745 CrossRef CAS.
- S. Yusan, K. Korzhynbayeva, S. Aytas, S. Tazhibayeva and K. Musabekov, J. Alloys Compd., 2014, 608, 8–13 CrossRef CAS.
- J. C. Juan, J. Zhang and M. A. Yarmo, Appl. Catal., A, 2008, 347, 133–141 CrossRef CAS.
- L. Chen, J. Xu and J. Hu, J. Radioanal. Nucl. Chem., 2013, 297, 97–105 CrossRef CAS.
- D. L. Zhao, S. J. Feng, C. L. Chen, S. H. Chen, D. Xu and X. K. Wang, Appl. Clay Sci., 2008, 41, 17–23 CrossRef CAS.
- G. Yao, J. Lei, X. Zhang, Z. Sun, S. Zheng and S. Komarneni, Mater. Res. Bull., 2018, 107, 132–138 CrossRef CAS.
- S. G. Jeong, J. Jeon, J. H. Lee and S. Kim, Int. J. Heat Mass Transfer, 2013, 62, 711–717 CrossRef CAS.
- I. Cacciotti, M. Rinaldi, J. Fabbrizi and F. Nanni, J. Mater. Res. Technol., 2019, 8, 1737–1745 CrossRef CAS.
- L. Jiang, L. Liu, S. Xiao and J. Chen, Chem. Eng. J., 2016, 284, 609–619 CrossRef CAS.
- H. Hamad, M. A. El-Latif, A. E. H. Kashyout, W. Sadik and M. Feteha, New J. Chem., 2015, 39, 3116–3128 RSC.
- S. Kongwudthiti, P. Praserthdam, W. Tanakulrungsank and M. Inoue, J. Mater. Process. Technol., 2003, 136, 186–189 CrossRef CAS.
- P. Salas, J. A. Wang, H. Armendariz, C. Angeles-Chavez and L. F. Chen, Mater. Chem. Phys., 2009, 114, 139–144 CrossRef CAS.
- Y. Xia, F. Li, Y. Jiang, M. Xia, B. Xue and Y. Li, Appl. Surf. Sci., 2014, 303, 290–296 CrossRef CAS.
- Q. Liu, C. Q. Wang, J. Tan, Z. L. Yin, Q. Y. Chen, Z. Liao, P. M. Zhang and Y. Liu, J. Cent. South Univ., 2015, 22, 1256–1263 CrossRef CAS.
- S. M. Pérez-Moreno, M. J. Gázquez, A. G. Barneto and J. P. Bolívar, Thermochim. Acta, 2013, 552, 114–122 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07947j |
| This journal is © The Royal Society of Chemistry 2023 |