Open Access Article
Open Access ArticleOxidative annulation of acetophenones and 2-aminobenzothiazoles catalyzed by reusable nickel-doped LaMnO3 perovskites†
Phuong T. Phamab,
Duyen K. Nguyenab,
Nam T. S. Phan ab,
Minh-Vien Le
ab,
Minh-Vien Le *ab and
Tung T. Nguyen
*ab and
Tung T. Nguyen *ab
*ab
aFaculty of Chemical Engineering, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet Street, District 10, Ho Chi Minh City, Vietnam
bVietnam National University Ho Chi Minh City, Linh Trung Ward, Ho Chi Minh City, Vietnam. E-mail: tungtn@hcmut.edu.vn; lmvien@hcmut.edu.vn
First published on 23rd January 2023
Abstract
Synthesis of imidazole[2,1-b]benzothiazoles often suffers from the use of pre-functionalized substrates and/or homogeneous, non-recyclable catalytic systems. Herein we report a method for direct coupling of acetophenones and 2-aminobenzothiazoles in the presence of reusable perovskites, namely LaMn0.95Ni0.05O3. Imidazole[2,1-b]benzothiazoles were obtained in moderate to good yields and contained an array of useful functionalities. Control experiments indicated that the perovskites played pivotal roles in halogenation and condensation steps.
Introduction
Imidazole[2,1-b]benzothiazoles are fused tricyclic heterocycles that are commonly found in many medicinally relevant molecules and functional materials.1–3 Traditional methods often rely on the annulation of α-bromo acetophenones and 2-aminobenzothiazoles.4,5 The first example of directly using acetophenones, without pre-functionalization of α C–H bonds, to couple with 2-aminobenzothiazoles was revealed by Hajra and co-workers.6 The reactions utilized catalytic amounts of FeCl3 and ZnI2 to facilitate the condensation. Jeong and Balwe reported a multi-component synthesis of benzo[d]imidazo[2,1-b]thiazoles from 2-aminobenzothiazoles, aldehydes, and nitromethane as a one carbon source.7 Feng, Ma, and co-workers recently developed a new method for copper-catalyzed, two-step annulation of ethylarenes and 2-aminopyridines.8 However, only one imidazole[2,1-b]benzothiazole was isolated. Notably, those methods utilized homogeneous catalytic systems. It is arguably more beneficial to use a heterogeneous, reusable catalyst.Lanthanum manganese perovskite oxide (LaMnO3) is a well-known catalyst for oxidation reactions.9–11 Notably, the doping of late transition metals, such as cobalt(II) or nickel(II) ions, to LaMnO3 was reported to increase the catalytic performances.12–15 Nevertheless, most of the available methods focus on the high-temperature, gas-phase oxidation. Herein we report our attempts to expand the application of nickel-doped LaMnO3 perovskites into liquid-phase organic transformation. The annulation of 2-aminobenzothiazoles and acetophenones occurred in the presence of catalytic amount of Ni-doped LaMnO3 and mild conditions enough to tolerate a wide range of functionalities. Our method appears to be the first method for heterogeneously catalytic condensation toward the synthesis of imidazole[2,1-b]benzothiazoles.
Experimental
General considerations
Commercially available chemicals were used as received unless otherwise noted. The crystal structures of the samples were determined by X-ray diffraction (XRD) using D2 Phaser-Bruker diffractometer using CuKα (λ = 1.54184 Å), operated at an accelerating voltage of 30 kV and intensity of 10 Ma, 2θ range 20°–80° with a step size of 0.015°. The EDX spectrum carried out on EX 350-Horiba were used to quantify the elements Ti, Si, O and N. The results of differential thermal analysis-thermogravimetric analysis (DTA-TGA) were obtained from the Labsys Evo, Setaram, which was in the environment of air at the heating rate of 5 °C min−1. FESEM (Hitachi S-4800) and TEM (JEOL JEM-2100) were applied to study morphology and particle size. The nitrogen adsorption–desorption isotherms at 77 K were run on the MicroMeritics ASAP 2010. Gas chromatographic (GC) analyses were performed using a Shimadzu GC 2010-Plus equipped with a flame ionization detector (FID) and an SPB-5 column. GC-MS analyses were carried out on a Shimadzu GCMS-QP2010 Ultra containing a ZB-5MS column. The 1H-NMR and 13C-NMR spectra were recorded on Bruker AV 500 and 600 MHz spectrometers.Preparation of LaMn0.95Ni0.05O3
The Ni-doped LaMnO3 perovskites were prepared following the known sol–gel method.16 Lanthanum nitrate hexahydrate (2.16 g, 5 mmol), manganese nitrate tetrahydrate (1.72 g, 4.75 mmol), and nickel nitrate hexahydrate (70 mg, 0.25 mmol) were mixed in a beaker containing 50 mL distilled water and 50 mL alcohol to form a homogeneous solution. Then, citric acid monohydrate (4.22 g, 20 mmol) was added and the solution was heated at 80 °C until a viscous gel was formed. The obtained gel was dried at 120 °C for 5 h (ash), pulverized, and calcined in the air at 500 °C for 3 h at a heating rate of 5 °C min−1 to evaporate the solvent. Finally, the resulting powder was calcined again at 800 °C for 3 h.General procedure for studying the annulation
For a typical reaction of optimization, the mixture of acetophenone (0.15 mmol), I2, catalyst, and solvent was added to a 12 mL screw-cap vial. The reaction tube was flushed with O2, tightly capped, and stirred at the given temperature for 12 h. Then, 2-aminobenzothiazole (0.1 mmol) and an additive were added. The tube was flushed with O2 again, capped, then stirred at the temperature identical to the first step for an additional 8 h. The mixture was cooled to room temperature and diphenyl ether (17.0 mg, 0.1 mmol) as an internal standard was added. Organic components were extracted into ethyl acetate (2 mL), washed with Na2S2O3 solution (5% in water, 1 mL), and brine (1.0 mL). The obtained organic layer was dried over anhydrous Na2SO4, filtered, and analyzed by GC with reference to diphenyl ether. For isolation, the aforementioned steps should be followed, except the addition of the internal standard. Purification of the last organic layer by column chromatography afforded the desired product. For studying the recyclability of perovskites, the material was removed by centrifugation after the reaction finished, then washed with solvents (methanol 3 × 3 mL, acetone 3 × 3 mL, and diethyl ether 3 × 3 mL), activated under vacuum for 12 h, and used for next runs.Results and discussion
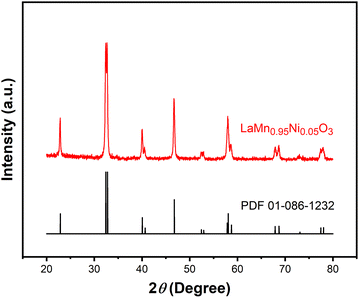
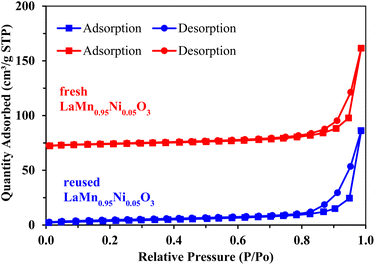
The results of characterization confirmed the successful preparation of the LaMn0.95Ni0.05O3 perovskites (see the ESI† for details). For example, the XRD pattern showed diffraction peaks of LaMn0.95Ni0.05O3 at 2θ = 23°, 32°, 40°, 47°, 53°, 58°, 68°, and 78° (Fig. 1). The peaks exhibited the rhombohedral structure of LaMnO3 perovskites (card PDF#01-086-1232).17 The EDX spectrum expressed that no foreign elements or initial synthetic precursors were detected (Fig. S2†). The exothermic peak at 677 °C in the TGA-DTA result was assigned for the formation of the perovskite phase (Fig. S3†). The SEM (Fig. S4†) and TEM (Fig. S5†) images showed that nanoparticles were obtained. The surface area of the LaMn0.95Ni0.05O3 obtained from nitrogen physisorption isotherm was 14.538 m2 g−1 (Fig. S6†).The as-prepared LaMn0.95Ni0.05O3 perovskites were firstly used for the annulation of 2-aminobenzothiazole 1a and acetophenone 2a to afford the desired imidazole[2,1-b]benzothiazole 3aa. The results of optimization studies are presented in Table 1. It should be noted that acetophenone 2a was treated with iodine, in the presence of catalyst, prior to the addition of 2-aminobenzothiazole 1a and the additive. The annulation should be run at 120 °C to obtain a reasonable yield (entries 1–3). Chlorobenzene was superior to other aromatic solvents such as toluene and p-xylene (entries 4 and 5). Polar, aprotic solvents were not suitable for the annulation (entry 6). Coupling of 1a and 2a in the presence of LaMnO3 catalyst gave only 14% yield of the desired product 3aa, somewhat confirming the crucial role of doped nickel ions (entry 7). Increasing the amount of iodine resulted in a better yield (entry 8). Among the additives attempted, NaHCO3 provided the best yield of 3aa (entries 9–12). Decreasing the amount of chlorobenzene solvent by a half afforded a 75% yield of 3aa (entry 13). Omitting the presence of Ni-doped LaMnO3 perovskites gave only 10% yield of 3aa (entry 14). The reaction under air afforded 3aa in 57% yield, which was lower than that under O2, confirming the crucial role of O2 to obtain reasonable yields (entry 15).
| Entry | Temperature (°C) | Solvent | Catalyst | Additive | Yield of 3aa (%) |
|---|---|---|---|---|---|
| a 2a (0.15 mmol), catalyst (10 μmol), I2 (0.05 mmol), and solvent (1 mL), under O2, 8 h, then 1a (0.1 mmol), additive (0.15 mmol), under O2, 12 h. Both steps were run at the same temperature. Yields are GC yields using diphenyl ether as internal standard.b I2 (0.1 mmol).c PhCl (0.5 mL).d Under air for both steps. Abbreviations: BzOH = benzoic acid, AcOH = acetic acid, PivOH = pivalic acid. | |||||
| 1 | 120 | PhCl | LaMn0.95Ni0.05O3 | BzOH | 52 |
| 2 | 110 | PhCl | LaMn0.95Ni0.05O3 | BzOH | 45 |
| 3 | 130 | PhCl | LaMn0.95Ni0.05O3 | BzOH | 50 |
| 4 | 120 | Toluene | LaMn0.95Ni0.05O3 | BzOH | 33 |
| 5 | 120 | p-xylene | LaMn0.95Ni0.05O3 | BzOH | 21 |
| 6 | 120 | DMSO | LaMn0.95Ni0.05O3 | BzOH | 16 |
| 7 | 120 | PhCl | LaMnO3 | BzOH | 14 |
| 8b | 120 | PhCl | LaMn0.95Ni0.05O3 | BzOH | 59 |
| 9b | 120 | PhCl | LaMn0.95Ni0.05O3 | AcOH | 40 |
| 10b | 120 | PhCl | LaMn0.95Ni0.05O3 | PivOH | 55 |
| 11b | 120 | PhCl | LaMn0.95Ni0.05O3 | NaHCO3 | 65 |
| 12b | 120 | PhCl | LaMn0.95Ni0.05O3 | Na2CO3 | 47 |
| 13b,c | 120 | PhCl | LaMn0.95Ni0.05O3 | NaHCO3 | 75 |
| 14b,c | 120 | PhCl | — | NaHCO3 | 10 |
| 15b,c,d | 120 | PhCl | LaMn0.95Ni0.05O3 | NaHCO3 | 57 |
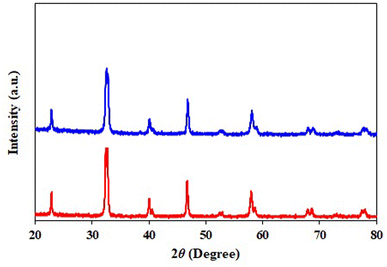
Next, we studied the recyclability of Ni-doped LaMnO3 perovskites. Notably, the yields of the annulation product 3aa after three cycles were comparable (Fig. 2). The results of XRD diffractogram (Fig. 3) and nitrogen isotherm (Fig. 4) with respect to the reused Ni-doped LaMnO3 perovskites were nearly identical to those of the fresh material, somewhat confirming that the structure of the material was still remained. Thus, the Ni-doped LaMnO3 perovskites feature a promising reusability toward the condensation of 2-aminobenzothiazoles and acetophenones.
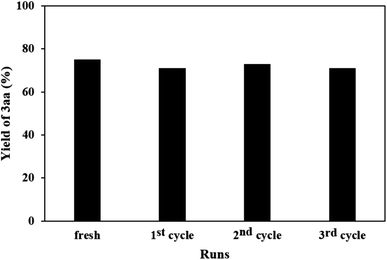 | ||
| Fig. 2 Recyclability of LaMn0.95Ni0.05O3 catalyst. Yield for each of reused runs was obtained after three independent attempts. | ||
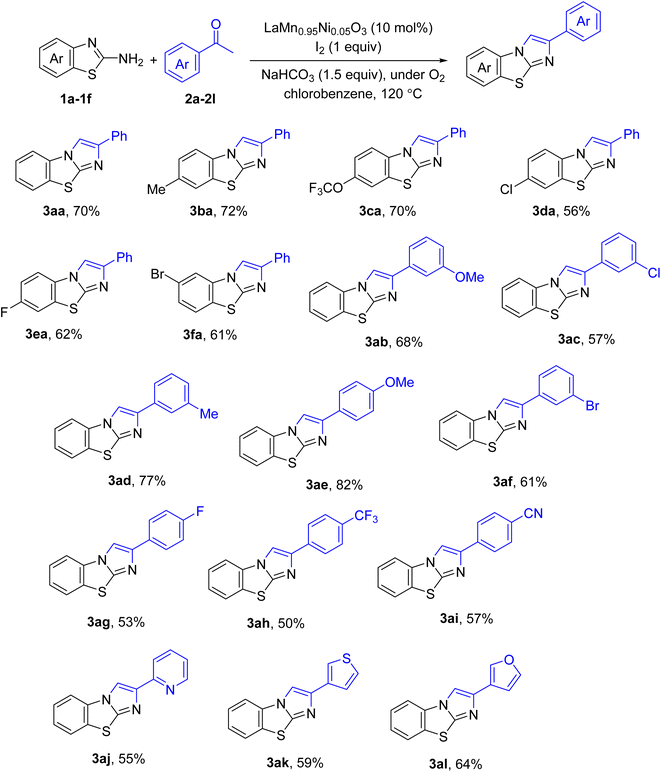
Scope of the substrates was next investigated. The result is shown in Scheme 1. Fluoro (3ea, 3ag), chloro (3da, 3ac), bromo (3af), and cyano (3ai) functionalities were all compatible with reaction conditions. Regarding acetophenones, electron-rich compounds (3ae) were more reactive than the electron-poor (3ag-3ai). The yields of imidazole[2,1-b]benzothiazoles obtained from pyridyl (3aj), thiophenyl (3ak), and furanyl (3al) ketones varied from 55% to 64%, showing the compatibility of heterocycles toward the annulation.
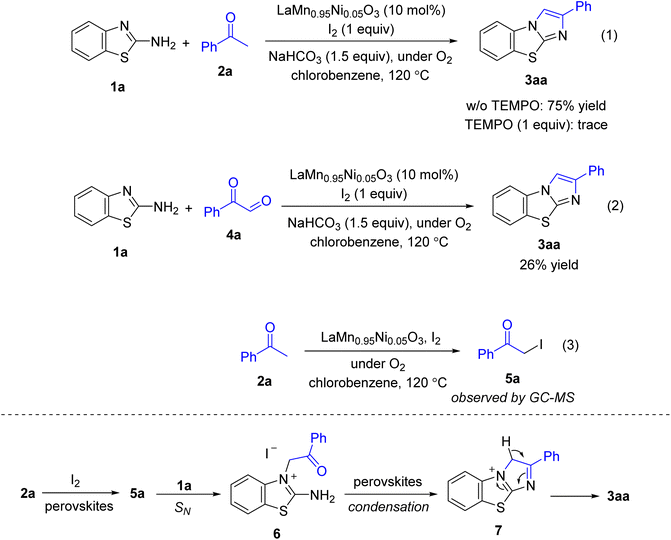
To understand the mechanism, some control experiments were carried out (Scheme 2). No product was observed if TEMPO was added (equation 1), somewhat implying the formation of radical species during the course of the reaction. Use of phenylglyoxal 4a to couple with 2-aminobenzothiazole 1a afforded a low yield of the product 3aa (equation 2), confirming that oxidation of α C–H bonds to furnish the aldehyde was unlikely the key step. Meanwhile, running the first step which included acetophenone 2a, Ni-doped LaMnO3 perovskites, and iodine gave the iodination intermediate 5a (equation 3). Based on the results that we observed as well as those previously reported,6,11 a possible mechanism was proposed (Scheme 2). Iodination of α C–H bonds in acetophenone 2a gave the adduct 5a followed by a nucleophilic substitution to afford 6. Imine condensation would yield 7 which underwent a tautomerization to finally furnish the desired product 3aa. We envisaged that Ni-doped LaMnO3 perovskites played a crucial role in the first oxidation (2a → 5a).
Conclusions
In conclusion, we have developed a method for nickel-doped LaMnO3 perovskites mediated annulation of 2-aminobenzothiazoles and acetophenones. The reactions proceeded under mild conditions that were tolerant of many useful functionalities as well as heterocycles. Characterization of the reused material regarding the results XRD and nitrogen physisorption isotherm somewhat confirmed that the structure of the LaMn0.95Ni0.05O3 was still remained, thus implying the recyclability and reusability of the perovskites.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to Vietnam National University Ho Chi Minh City (VNU-HCM) for financial support via project No. NCM2019-20-01 (for Tung T. Nguyen).Notes and references
- R. M. Kumbhare, K. V. Kumar, M. J. Ramaiah, T. Dadmal, S. N. C. V. L. Pushpavalli, D. Mukhopadhyay, B. Divya, T. A. Devi, U. Kosurkar and M. Pal-Bhadra, Eur. J. Med. Chem., 2011, 9, 4258 CrossRef PubMed.
- S. K. Maddili, Z.-Z. Li, V. K. Kannekanti, R. R. Y. Bheemanaboina, B. Tuniki, V. K. R. Tangadanchu and C.-H. Zhou, Bioorg. Med. Chem. Lett., 2018, 28, 2426 CrossRef CAS PubMed.
- H. Wang, S. Zhao, Y. Xu, L. Li, B. Li, M. Pei and G. Zhang, J. Mol. Struct., 2020, 1203, 127384 CrossRef CAS.
- M. S. Christodoulou, F. Colombo, D. Passarella, G. Ieronimo, V. Zuco, M. De Cesare and F. Zunino, Bio. Med. Chem., 2011, 19, 1649 CrossRef CAS PubMed.
- A. Kamal, F. Sultana, M. J. Ramaiah, Y. V. V. Srikanth, A. Viswanath, C. Kishor, P. Sharma, S. N. C. V. L. Pushpavalli, A. Addlagatta and M. Pal-Bhadra, ChemMedChem, 2012, 7, 292 CrossRef CAS PubMed.
- S. Mishra, K. Monir, S. Mitra and A. Hajra, Org. Lett., 2014, 16, 6084 CrossRef CAS PubMed.
- S. G. Balwe and Y. T. Jeong, RSC Adv., 2016, 6, 107225 RSC.
- Y. Wang, S. Li, X. Wang, Y. Yao, L. Feng and C. Ma, RSC Adv., 2022, 12, 5919 RSC.
- C. Zhang, Y. Guo, Y. Guo, G. Lu, A. Boreave, L. Retailleau, B. Baylet and A. Giroir-Fendler, Appl. Catal., B, 2014, 148–149, 490 CrossRef CAS.
- H. Deng, L. Lin, Y. Sun, C. Pang, J. Zhuang, P. Ouyang, Z. Li and S. Liu, Catal. Lett., 2008, 126, 106 CrossRef CAS.
- Y. Sahin, A. T. Sika-Nartey, K. E. Ercan, Y. Kocak, S. Senol, E. Ozensoy and Y. E. Türkmen, ACS Appl. Mater. Interfaces, 2021, 13, 5099 CrossRef CAS PubMed.
- Y.-C. Hou, M.-W. Ding, S.-K. Liu, S.-K. Wu and Y.-C. Lin, RSC Adv., 2014, 4, 5329 RSC.
- R. Ran, X. Wu, D. Weng and J. Fan, J. Alloys Compd., 2013, 577, 288 CrossRef CAS.
- C. Zhang, C. Wang, W. Zhan, Y. Guo, Y. Guo, G. Lu, A. Baylet and A. Giroir-Fendler, Appl. Catal., B, 2013, 129, 509 CrossRef CAS.
- C. Zhang, K. Zeng, C. Wang, X. Liu, G. Wu, Z. Wang and D. Wang, Ceram. Int., 2020, 46, 6652 CrossRef CAS.
- Y. Chen, J. Ma, D. Li, C. Zhu, W. Zhang and C. Miao, J. Alloys Compd., 2021, 888, 161555 CrossRef CAS.
- L. Vradman and A. Navrotsky, J. Am. Ceram. Soc., 2013, 96, 3202 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra08045a |
| This journal is © The Royal Society of Chemistry 2023 |