Open Access Article
Open Access ArticleA facile access to 1-substituted and unsubstituted 3-isoquinolinones via Mannich or Sn2 initiated cascade reactions under catalyst-free conditions†
Lorenzo Serusi,
Antonia Di Mola* and
Antonio Massa *
*
Dipartimento di Chimica e Biologia “A. Zambelli”, Università degli Studi di Salerno, Via Giovanni Paolo II, 84084 Fisciano, Italy. E-mail: adimola@unisa.it; amassa@unisa.it
First published on 24th February 2023
Abstract
Herein we report new cascade processes for the easy access to 1-substituted and C-unsubstituted 3-isoquinolinones. The Mannich initiated cascade reaction led to the synthesis of novel 1-substituted 3-isoquinolinones under catalyst-free conditions in the presence of nitromethane and dimethylmalonate as nucleophiles without the use of any solvent. The optimization of the synthesis of the starting material in a more environmentally benign manner, allowed the identification of a common intermediate useful for the synthesis of C-unsubstituted 3-isoquinolinones as well. The synthetic utility of 1-substituted 3-isoquinolinones was also demonstrated.
1 Introduction
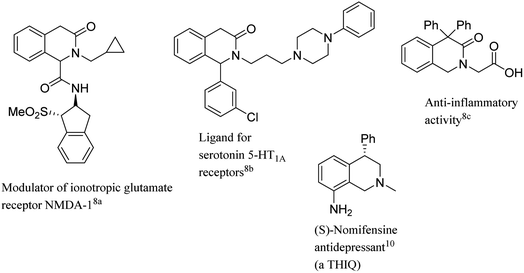
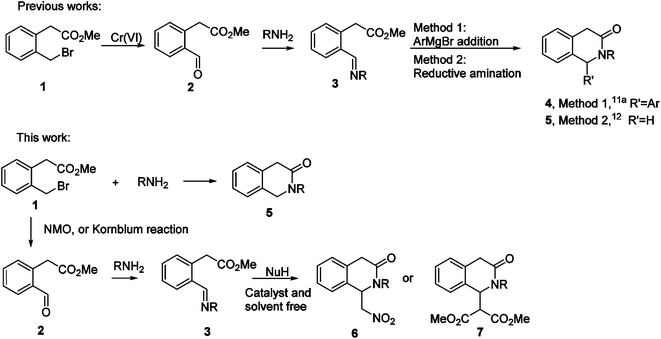
Multicomponent and cascade type reactions are powerful tools in the construction of complex molecular architectures under mild conditions,1 respecting many paradigms of the green chemistry like step-, pot- and atom-economy.2 Cascade reactions are particularly useful in the construction of heterocyclic compounds usually achieved by readily available starting materials.3 One of the main requisites to achieve these goals is indeed the design of suitable starting materials bearing proper combinations of functional groups. In this context, our group endeavored in the development of diverse cascade and multicomponent reactions for the access to valuable heterocyclic compounds like isoindolinones,4 isoquinolin-1,3,4-triones,5 isobenzofuranones,6 isoxazole-5-ones.7 In particular, nucleophilic additions to 2-formylbenzonitriles4a or to imines4b and α-amidosulfones4c derived from readily available 2-formylbenzoate4b,c triggered effective cascade processes in the construction of the γ-lactam ring of diversely decorated 3-substituted isoindolinones. In the present investigation our attention focused on the homologous building block 2-formylphenyl acetate with the aim of the construction of the δ-lactam ring of diversified 3-isoquinolinones. 3-Isoquinolinones (1,4-dihydro-3(2H)-isoquinolinones, also named isoquinolin-3-ones) belong to a class of δ-lactams of large interest in organic and medicinal chemistry, showing a wide range of biological activities (Fig. 1).8 These compounds also offer the possibility to be easily transformed into related heterocycles to give for example tetrahydroisoquinolines,9 a moiety found in many bioactive alkaloid derivatives and natural products (THIQ, see Fig. 1).10The introduction of substituents in position 1 of the ring is an important goal for the obtaining of substituted THIQs as well. Surprisingly, relatively few methodologies have been reported to achieve this goal,11 limited to the addition of organometallic reagents to the imine derived from 2-formylphenyl acetate, followed by in situ lactamization (Scheme 1, R′ = Ar).11a 2-Formylphenyl acetate has been used in the synthesis of 3-isoquinolinones by Lewis acid-catalyzed domino Strecker-lactamization,11b in Ni(II)-catalyzed reactions by in situ prepared aldimines.11c Recently, dearomatization of 3-chloroisoquinolines also proved a promising strategy.11d Reductive amination/cyclization performed on imine 3 is a viable method for the obtaining of C-unsubstituted 3-isoquinolinones (Scheme 1, R′ = H).12 A limited number of synthetic methodologies to give C-unsubstituted 3-isoquinolinones has been reported as well. C-unsubstituted 3-isoquinolinones are mainly obtained by reacting 3-isochromanones with aromatic imines under very harsh reaction conditions13 or in the presence of transition metal catalysts at high temperature.14
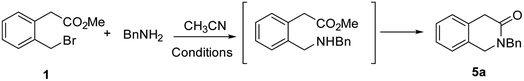
With this background in mind, we believed that the synthetic utility of the imines derived from 2-formylphenyl acetate has been underestimated. The use of nucleophiles weaker than organometallic reagents could allow the development of new cascade reactions for the access to new classes of 1-substituted 3-isoquinolinones (Scheme 1). Surprisingly, to our knowledge this strategy is unexplored. The synthetic route to the imine 3 is already reported.11a 2-Formylphenyl acetate can be obtained by an unusual oxidation of the 2-bromomethylphenyl acetate 1 performed by an excess of harmful Cr(VI)-based reagent, (nBu4)2NCr2O7 (TBADC).11a In addition, C-unsubstituted 3-isoquinolinones could be obtained by a cascade reaction based on bromide displacement in 1 by an amine, shortening existing routes (Scheme 1).12 Also in this case, to our knowledge this simpler approach is little investigated.15
Therefore, herein we describe new cascade reactions for the synthesis of 1-substituted and C-unsubstituted 3-isoquinolinones from a common building block, the 2-bromomethylphenyl acetate 1. Improvements of the synthesis of the aldehyde 2 with the replacement of the toxic TBADC reagent with more benign NMO (N-methyl morpholine N-oxide) or by application of modified Kornblum reaction were also achieved, allowing an easier access to the respective imines 3 also in high scale and consequently to 1-substituted 3-isoquinolinones.
2 Results and discussion
2.1 Cascade Mannich/lactamization reaction in the synthesis of 1-substituted 3-isoquinolinones
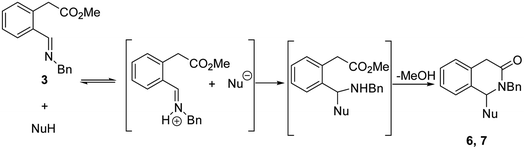
The optimization of aldehyde synthesis by the oxidation of the 2-bromomethylphenyl acetate 1, highlighted the possibility to replace the oxidant bis(tetrabutylammonium) dichromate (TBADC)11a alternatively with NMO16 or applying modified Kornblum conditions.17 Even though conversion was quantitative with both these methods, we preferred NMO oxidation to scale up the process to avoid the use of excess of DMSO and a more tedious work-up procedure (see Experimental part for details). The aldehyde 2 was, then, converted into the imines 3 in methanol which were used without purification. The reactivity of the imines was investigated in the presence of readily available carbon nucleophiles, starting with the use of nitromethane, aiming to a cascade process consisting of aza-Mannich/lactamization reaction (Table 1). To find the most suitable reaction conditions, we firstly tested standardized base-promoted conditions in acetonitrile for the generation of nitronate anion (entry 1). Even though this system proved to be particularly effective in conceptually analog cascade reactions in the synthesis of 3-substituted isoindolinones4a–c when applied to the system herein investigated it was ineffective. Therefore, we considered a recent method of aza-Mannich reaction developed under catalyst-free conditions and without any other solvent apart from a slight excess of the nitromethane (10 eq.).18 Intrigued by this greener approach, we soon verified its effectiveness and after a quick optimization of the reaction conditions (entries 2–4), the novel 1-nitromethyl-2-benzyl 3-isoquinolinone was obtained in very high yield at a reaction temperature of 50 °C (entry 4). Since the actual nucleophilic species is the nitronate anion, the imine should be enough basic to deprotonate nitromethane (pKa = 17.2 in DMSO).19 This proton exchange will increase the electrophilicity of the imine as well by iminium ion formation (see scheme of Table 1). Therefore, readily available pronucleophiles belonging to the class of double activated methylene compounds like dimethylmalonate (DMM, pKa = 15.9),19 acetylacetone (pKa = 13.3)19 and malononitrile (pKa = 11.0)19 were investigated. The less acidic acetophenone (pKa = 24.7)19 was also tested for comparison under the same catalyst-free conditions and without any other solvent. DMM gave the expected 1-substituted 3-isoquinolinone in good yield (entry 5). Malononitrile gave a complex mixture of unidentified products (entry 6). Acetylacetone gave the expected 3-isoquinolinone only in traces together with products in which the main one was identified as the respective 1-substituted 3-isochromanone (entry 7). In the presence of acetophenone we recovered the starting materials unreacted (entry 8). Other reaction conditions were not tested.| Entry | Nucleophile | Base (1 eq.) | Time (h) | T (°C) | Solvent | Yielda (%) |
|---|---|---|---|---|---|---|
| a Isolated yields by chromatography. | ||||||
| 1 | CH3NO2 | K2CO3 | 96 | rt | CH3CN | — |
| 2 | CH3NO2 | — | 48 | rt | — | Complex mixture |
| 3 | CH3NO2 | — | 18 | 50 | — | 6a, 80 |
| 4 | CH3NO2 | — | 20 | 50 | — | 6a, 99 |
| 5 | DMM | — | 17 | 50 | — | 7a, 80 |
| 6 | Malononitrile | — | 17 | 50 | — | Complex mixture |
| 7 | Acetylacetone | — | 24 | 50 | — | Complex mixture |
| 8 | Acetophenone | — | 24 | 50 | — | No reaction |
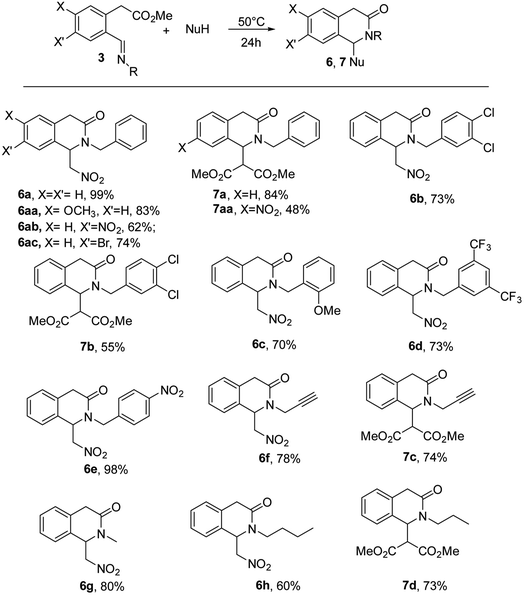
Then, the scope of the reaction was briefly analyzed using nitromethane and DMM in the presence of different N-benzylimines and N-alkylimines (Table 2). In most of the cases we obtained the expected new products from good to high yields, irrespective of the presence of both electron-donating and electron-withdrawing groups on the benzylamine part of the substrates. Only the N-α-methyl benzylimine did not react probably because of the increased steric hindrance and aniline was not tested because the synthesis of the respective imine cannot be carried out.12 Aldehyde 2b bearing a methoxy group in 6 position was synthesized by NMO oxidation of the respective 2-bromomethylphenyl acetate in quantitative yield. The resulting imine led to the 3-isoquinolinone 6aa in good yield as well. Conversely, the oxidation of the 2-bromomethylphenyl acetate bearing a nitro group in 6 position was not achieved, because of its low reactivity. Both NMO, Kornblum procedure and TBADC were tested with this substrate. On the other hand, the imine bearing a nitro group in 7 position was obtained by nitration of the aldehyde 2 (see ESI† for further details) and it was used with both nitromethane and dimethylmalonate leading to the 3-isoquinolinones 6ab and 7aa in moderate yields. The product 6ac was obtained exploiting the same synthetic route. The Mannich reactions were also scaled at 1 mmol leading to comparable 90% and 84% yields respectively with nitromethane and dimethylmalonate in the presence of imine 3a (see ESI†) (Table 2).
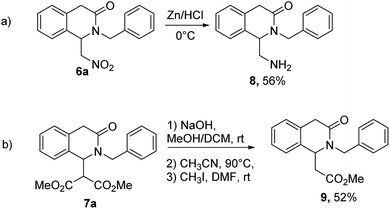
In order to demonstrate the versatility of the obtained products, the reactivity of 6a and 7a was investigated. In particular, reduction of nitro group of 6a was carried out under mild conditions in the presence of Zn and HCl, leading to the 1-aminomethyl derivative in 56% yield (Scheme 2a). Basic hydrolysis of 7a followed by heating the dicarboxylic acid in CH3CN led to the monocarboxylic acid which was converted in the methyl ester and isolated in good overall yield of 52% without the purification of the intermediates (Scheme 2b), demonstrating the utility of the synthesized products to afford new potential building blocks.
2.2 Cascade Sn2/lactamization reaction in the synthesis of C-unsubstituted 3-isoquinolinones
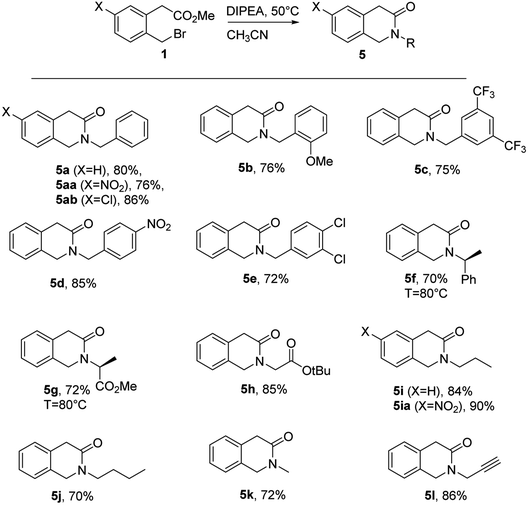
In this section the 2-bromomethylphenyl acetate 1 was reacted with benzylamine under several reaction conditions (Table 3). The use of 1 eq. of additional base was envisioned to be useful to neutralize the HBr by-product, favoring the deprotonation of the aminic acyclic intermediate. The best results were obtained using the organic base DIPEA (diisopropylethylamine) at 50 °C in acetonitrile (entry 5), while inorganic bases like NaHCO3 or K2CO3 led to unsatisfactory results (entries 3 and 4). When only 1 eq. of BnNH2 was used, a precipitate was observed, conversion was low with isolated yield of about 30% at 50 °C (entries 1 and 2). The effect of the temperature was important to achieve cyclization since at rt in the presence of DIPEA we detected in the crude the acyclic intermediate as the main product (entry 7). The effect of medium concentration was also investigated, highlighting that as one may suppose the higher the concentration the quicker is the reaction (entry 5 vs. 6). However, the reaction performed under solvent free conditions led to disappointing results even at rt (entries 8 and 9). The Sn2/lactamization reaction was scaled up at 1 mmol leading to comparable 78% yield (see ESI†).| Entry | Base (1 eq.) | M | T (°C) | t | Yielda/notes |
|---|---|---|---|---|---|
| a Isolated yields by chromatography. | |||||
| 1 | — | 0.20 | rt | 96 h | Sm in complex mixture |
| 2 | — | 0.20 | 50 | 24 h | 30% |
| 3 | NaHCO3 | 0.20 | 50 | 48 h | No react. |
| 4 | K2CO3 | 0.20 | 50 | 24 h | 45% |
| 5 | DIPEA | 0.20 | 50 | 24 h | 80% |
| 6 | DIPEA | 0.10 | 50 | 24 h | 57% |
| 7 | DIPEA | 0.20 | rt | 24 h | Sm and acyclic intermediate |
| 8 | DIPEA | Solvent free | rt | 24 h | Sm and acyclic intermediate |
| 9 | DIPEA | Solvent free | 50 | 24 h | Complex mixture of products |
Under the optimized reaction conditions several primary amines were reacted to analyze the scope of the method (Table 4). Both aliphatic, as the volatile methylamine (used as 1 M solution in THF) and substituted benzylamines bearing both EWG and EDG gave very good yields. t-Butyl ester of glycine and methyl ester of L-alanine gave good yields as well. (S)-α-Methylbenzylamine required higher reaction temperature as for L-alanine probably due to the increased steric hindrance, while aniline did not react at all. Since both glycine t-butyl ester and L-alanine methyl ester were used as HCl salts, 2 eq. of DIPEA were necessary. The presence of a nitro group or a chlorine in 6-position of the aromatic ring did not affect the reactivity, leading to the isolation of 5aa, 5ia and 5ab in good yields as well (Table 4).
3 Conclusions
In this article two cascade methodologies for the synthesis of 3-isoquinolinones have been described. In particular, Mannich initiated cascade reaction of imines derived from 2-formylphenyl acetate in the presence of nitromethane and dimethylmalonate led to novel 1-substituted-3-isoquinolinones from good to high yield under catalyst- and solvent-free conditions. The second reactivity of the obtained products was also investigated, demonstrating their utility to give useful new building blocks. In addition, the use of imine precursor 2-bromomethylphenyl acetate allowed the synthesis of C-unsubstituted isoquinolin-3-ones more conveniently than already reported methods. 2-Formylphenyl acetate synthesis was also optimized, introducing more environmentally friendly oxidations than the reported Cr(VI)-based reagent using NMO or modified Kornblum oxidation. Other studies are in course aiming to expand the scope of the reported methodologies.4 Experimental section
4.1 General methods
Unless otherwise noted, all chemicals, reagents and solvents for the performed reactions are commercially available and were used without further purification. All the reactions were monitored by thin layer chromatography (TLC) on precoated silica gel plates (0.25 mm) and visualized by fluorescence quenching at 254 nm. Flash chromatography was carried out using silica gel 60 (70–230 mesh, Merck, Darmstadt, Germany). Yields are given for isolated products showing one spot on a TLC plate and no impurities were detectable in the NMR spectrum. The NMR spectra were recorded on Bruker DRX 600, 400, and 300 MHz spectrometers (600 MHz, 1H, 125 MHz, 13C; 400 MHz, 1H, 100.6 MHz; 13C, 300 MHz, 1H, 75.5 MHz, 13C). Internal reference was set to the residual solvent signals (δH 7.26 ppm, δC 77.16 ppm for CDCl3). The 13C NMR spectra were recorded under broad-band proton-decoupling. Spectra are reported only for unknown compounds. The following abbreviations are used to indicate the multiplicity in NMR spectra: s-singlet, d-doublet, t-triplet, q-quartet, dd-doublet of doublets, m-multiplet, brs-broad signal. Coupling constants (J) are quoted in Hertz. High resolution mass spectra (HRMS) were acquired using a Bruker SolariX XR Fourier transform ion cyclotron resonance mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) equipped with a 7 T refrigerated actively-shielded superconducting magnet. For ionization of the samples electrospray ionization (ESI) or MALDI was applied. IR spectra were recorded on IR Bruker Vertex 70v. Methyl 2-(2-(bromomethyl)phenyl)acetate 1a–1c and methyl-2-(2-formylphenyl) acetate 2a–2b were prepared as reported in ESI.†4.2 General procedure for Mannich/lactamization reaction
Methyl 2-(2-formylphenyl)acetate 2a (X = H, 0.15 mmol, 1.0 eq.) was treated with the amine (0.15 mmol, 1.0 eq.) at rt in MeOH (0.3 mL) for 18 h. After solvent evaporation, the crude product imine 3 was reacted without further purification with the pronucleophile NuH (1.5 mmol, 10.0 eq.) at 50 °C (oil bath) for 24 h. Then the product was directly purified by chromatography on silica gel (hexane/ethyl acetate, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) affording the corresponding products 6 and 7.
3) affording the corresponding products 6 and 7.
4.3 General procedure for Sn2/lactamization reaction
To a solution of methyl 2-(2-(bromomethyl)phenyl)acetate 1a (X = H, 30 mg, 0.125 mmol) in acetonitrile (0.6 mL) was added an equimolar amount of DIPEA (22 μL) and RNH2 (0.125 mmol) and the mixture was stirred for 24 h at 50 °C (oil bath) in the dark and under nitrogen atmosphere. The solvent was removed in vacuo and the resulting product 5 was purified by silica gel chromatography (ethyl acetate/hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9).
9).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank MUR (Ministero Università e Ricerca) and University of Salerno (FARB) for financial support. We want also to thank Mr Felice De Piano for preliminary work in the aldehyde synthesis.References
- (a) D. E. Fogg and E. N. dos Santos, Tandem catalysis: a taxonomy and illustrative review, Coord. Chem. Rev., 2004, 248, 2365–2379 CrossRef CAS; (b) L. F. Tietze and U. Beifuss, Sequential Transformations in Organic Chemistry: A Synthetic Strategy with a Future, Angew. Chem. Int. Ed. Engl., 1993, 32, 131–163 (Angew. Chem., 1993, 105, 137–170) CrossRef.
- Y. Hayashi, Pot economy and one-pot synthesis, Chem. Sci., 2016, 7, 866–880 RSC.
- C. Grondal, M. Jeanty and D. Enders, Organocatalytic cascade reactions as a new tool in total synthesis, Nat. Chem., 2010, 2, 167–178 CrossRef CAS PubMed.
- (a) A. Macchia, F. F. Summa, A. Di Mola, C. Tedesco, G. Pierri, A. R. Ofial, G. Monaco and A. Massa, Base-Promoted Cascade Reactions for the Synthesis of 3,3-Dialkylated Isoindolin-1-ones and 3-Methyleneisoindolin-1-one, J. Org. Chem., 2021, 86, 15128–15138 CrossRef CAS PubMed; (b) L. Serusi, A. Massa, C. Tedesco, A. Capobianco and L. Palombi, The First Highly Enantioselective Synthesis of 3-Sulfinyl-Substituted Isoindolinones Having Adjacent Carbon and Sulfur Stereocenters, J. Org. Chem., 2021, 86, 10630–10639 CrossRef CAS PubMed; (c) L. Serusi, L. Palombi, G. Pierri, A. Di Mola and A. Massa, Asymmetric Cascade Aza-Henry/Lactamization Reaction in the Highly Enantioselective Organocatalytic Synthesis of 3-(Nitromethyl)isoindolin-1-ones from α-Amido Sulfones, J. Org. Chem., 2022, 87, 8420–8428 CrossRef CAS PubMed.
- A. Di Mola, C. Tedesco and A. Massa, Metal-Free Air Oxidation in a Convenient Cascade Approach for the Access to Isoquinoline-1,3,4(2H)-triones, Molecules, 2019, 24(11), 2177 CrossRef PubMed.
- A. Di Mola, F. Scorzelli, G. Monaco, L. Palombi and A. Massa, Highly diastereo- and enantioselective organocatalytic synthesis of new heterocyclic hybrids isoindolinone-imidate and isoindolinone-phthalide, RSC Adv., 2016, 6, 60780–60786 RSC.
- A. Macchia, F. F. Summa, G. Monaco, A. Eitzinger, A. R. Ofial, A. Di Mola and A. Massa, Access to β-Alkylated γ-Functionalized Ketones via Conjugate Additions to Arylideneisoxazol-5-ones and Mo(CO)6-Mediated Reductive Cascade Reactions, ACS Omega, 2022, 7, 8808–8818 CrossRef CAS PubMed.
- (a) M. P. Epplin, A. Mohan, L. D. Harris, Z. Zhu, K. L. Strong, J. Bacsa, P. Le, D. S. Menaldino, S. F. Traynelis and D. C. Liotta, Metabolic and Pharmaceutical Aspects of Fluorinated Compounds, J. Med. Chem., 2020, 63, 7569–7600 CrossRef CAS PubMed; (b) J. L. Mokrosz, A. J. Bojarski, M. Mackowiak, Z. Bielecka and J. Boksa, Structure-activity relationship studies of CNS agents. Part 10(1): 1-aryl-2-[3-(4-aryl-1-piperazinyl)propyl]-1,4-dihydro-3(2H)-isoquino linones: two modes of the interaction with the 5-HT1A receptor site, Die Pharmazie, 1994, 49, 328–333 CAS; (c) J. Gunzinger and K. Leander, US Pat., US2009099229A1, 2009 Search PubMed.
- (a) J. Finkelstein and A. Brossi, The synthesis of tetrahydroisoquinolines from 1,4-dihydro-3(2H)-isoquinolones, J. Heterocycl. Chem., 1967, 4, 315–318 CrossRef CAS; (b) Z. Li, J. Chen, L. Wu, A. Ren, P. Lu and Y. Wang, Preparation of 4-Diazoisoquinolin-3-ones via Dimroth Rearrangement and Their Extension to 4-Aryltetrahydroisoquinolin-3-ones, Org. Lett., 2020, 22, 26–30 CrossRef CAS PubMed; (c) L. Li, B. Zhou, Y. H. Wang, C. Shu, Y. F. Pan, X. Lu and L. W. Ye, Zinc-Catalyzed Alkyne Oxidation/C-H Functionalization: Highly Site-Selective Synthesis of Versatile Isoquinolones and β-Carbolines, Angew. Chem., Int. Ed., 2015, 54, 8245–8249 CrossRef CAS PubMed; (d) X. Bao, Y. X. Cao, W. D. Chu, H. Qu, J. Y. Du, X. H. Zhao, X. Y. Ma, C. T. Wang and C. A. Fan, Bioinspired Total Synthesis of Montanine-Type Amaryllidaceae Alkaloids, Angew. Chem., Int. Ed., 2013, 52, 14167–14172 CrossRef CAS PubMed.
- (a) M. A. M. Subbaiah, Triple Reuptake Inhibitors as Potential Therapeutics for Depression and Other Disorders: Design Paradigm and Developmental Challenges, J. Med. Chem., 2018, 61, 2133–2165 CrossRef CAS PubMed; (b) E. Anakabe, D. Badía, L. Carrillo, E. Reyes and J. L. Vicario, in Targets in Heterocyclic Systems, ed. O. A. Attanasi and D. Spinelli, Italian Society of Chemistry, 2002, vol. 6, pp. 270–311 Search PubMed.
- (a) W. Zhou, Y.-X. Zhang, X.-D. Nie, C.-M. Si, X. Sun and B.-G. Wei, Approach to Chiral 1-Substituted Isoquinolone and 3-Substituted Isoindolin-1-one by Addition–Cyclization Process, J. Org. Chem., 2018, 83, 9879–9889 CrossRef CAS PubMed; (b) S. Dhanasekaran, A. Suneja, V. Bisai and V. K. Singh, Approach to Isoindolinones, Isoquinolinones, and THIQs via Lewis Acid-Catalyzed Domino Strecker-Lactamization/Alkylations, Org. Lett., 2016, 18, 634–637 CrossRef CAS PubMed; (c) R. Karmakar, A. Suneja, V. Bisai and V. K. Singh, Ni(II)-Catalyzed Highly Stereo- and Regioselective Syntheses of Isoindolinones and Isoquinolinones from in Situ Prepared Aldimines Triggered by Homoallylation/Lactamization Cascade, Org. Lett., 2015, 17, 5650–5653 CrossRef CAS PubMed; (d) J. Shi, X. Chen, J. Huang, Y. Lu, Z. Yang, M. Zhong, Z. Zhu and C. Xiuwen, Direct Synthesis of Disubstituted 3-Isoquinolinone Derivatives through the Construction of C−N, C=O, and C(sp2)−C(sp3) Bonds under Transition-Metal-Free Conditions, Eur. J. Org. Chem., 2022, e202201231 Search PubMed.
- M. J. O'Sullivan, R. J. D. Hatley, C. R. Wellaway, S. P. Bew and C. J. Richards, Synthetic approaches to N- and 4-substituted 1,4-dihydro-3(2H)-isoquinolone derivatives, Tetrahedron, 2021, 100, 132455–132467 CrossRef.
- (a) Y. Shvo, E. C. Taylor, K. Mislow and M. Raban, Chemical shift nonequivalence of diastereotopic protons due to restricted rotation around aryl-nitrogen bonds in substituted amides, J. Am. Chem. Soc., 1967, 89, 4910–4917 CrossRef CAS; (b) C. Y. Cheng, H. B. Tsai and M. S. Lin, Synthetic approaches to 2-substituted 1-oxo-and 3-oxotetrahydroisoquinolines, J. Heterocycl. Chem., 1995, 32, 73–77 CrossRef CAS.
- K. Kim and S. H. Hong, Iridium-Catalyzed Single-Step N-Substituted Lactam Synthesis from Lactones and Amines, J. Org. Chem., 2015, 80, 4152–4156 CrossRef CAS PubMed.
- (a) N. Philippe, V. Levacher, G. Dupas, G. Queguiner and J. Bourguignon, Diastereoselective Protonation of Lactam Enolates Derived from (R)-Phenylglycinol. A Novel Asymmetric Route to 4-Phenyl-1,2,3,4-tetrahydroisoquinolines, Org. Lett., 2000, 2, 2185–2187 CrossRef CAS PubMed; (b) R. E. Dolle, C. MacLeod, B. Martinez-Teipel, W. Barker, P. R. Seida and T. Herbertz, Solid/Solution-Phase Annulation Reagents: Single-Step Synthesis of Cyclic Amine Derivatives, Angew. Chem., Int. Ed., 2005, 44, 5830–5833 CrossRef CAS PubMed.
- W. P. Griffith, J. M. Jolliffe, S. V. Ley, K. F. Springhorn and P. D. Tiffin, Oxidation of Activated Halides to Aldehydes and Ketones by N-Methylmorpholine-N-oxide, Synth. Commun., 1992, 22, 1967–1971 CrossRef CAS.
- N. Kornblum, J. W. Powers, G. J. Anderson, W. J. Jones, H. O. Larson, O. Lbvand and W. M. Weaver, A new and elective method for oxidation, J. Am. Chem. Soc., 1957, 79, 6562 CrossRef CAS.
- A. Pelagalli, L. Pellacani, E. Scandozza and S. Fioravanti, Aza-Henry Reactions on C-Alkyl Substituted Aldimines, Molecules, 2016, 21, 723 CrossRef PubMed.
- F. G. Bordwell, Equilibrium acidities in dimethyl sulfoxide solution, Acc. Chem. Res., 1988, 21, 456 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Spectroscopic data and copies of 1H and 13CNMR spectra. See DOI: https://doi.org/10.1039/d3ra00378g |
| This journal is © The Royal Society of Chemistry 2023 |