Open Access Article
Open Access ArticleSb(III)/Gum Arabic composite as a new natural-based environmentally green catalyst for the one-pot pseudo-four-component synthesis of 2H-indazolo[2,1-b] phthalazinetriones†
Mina Keihanfara,
Bi Bi Fatemeh Mirjalili *a and
Abdolhamid Bamoniri
*a and
Abdolhamid Bamoniri b
b
aDepartment of Chemistry, College of Science, Yazd University, Yazd, Iran
bDepartment of Organic Chemistry, Faculty of Chemistry, University of Kashan, Kashan, Iran. E-mail: fmirjalili@yazd.ac.ir; Fax: +98 3538210644; Tel: +983531232672
First published on 14th June 2023
Abstract
Sb(III)–Gum Arabic composite as a unique natural-based and nontoxic catalyst was synthesized and characterized by FT-IR, XRD, TGA, ICP, BET, EDX and mapping. A four-component reaction of phthalic anhydride, hydrazinium hydroxide, aldehyde, and dimedone has been carried out in the presence of Sb(III)/Gum Arabic composite to synthesise 2H-indazolo[2,1-b] phthalazine triones. The advantages of the present protocol are the appropriate reaction times, eco-friendly nature and high yields.
Introduction
Recently, science and technology are shifting emphasis toward green and environmental friendly organic processes.1 Catalysis is one of the pillars of green chemistry. Indeed, one of the main strategies for the minimization of the amount of unwanted by-products is the replacement of stoichiometric chemical procedures by efficient catalytic protocols. Organic reactions under solvent-free conditions have attracted much interest from chemists, particularly from the viewpoint of green chemistry.2 In this regard, bio-supported and solid heterogeneous catalysts for the synthesis of fine chemicals have attracted considerable interest for both environmental and economical reasons.3 Gum Arabic (GA) is a natural polymer that consists mainly of high molecular weight polysaccharides and is widely used as a stabilizer, thickening agent and hydrocolloid emulsifier. It is used in the food, textile, pottery, lithography, cosmetics and pharmaceutical industries.4 The existence of OH groups in Gum Arabic are suitable moieties for bonding of elemental cations to form supported Lewis acids. Heterocyclic compounds containing phthalazine moiety are of interest because they show some biological and pharmacological activities. Moreover, these compounds can be used as new fluorescence probes or luminescent materials.5 In the past decade, several methods for the synthesis of 2H-indazolo[2,1-b] phthalazinetriones using different catalysts such as silica sulfuric acid,6 phosphosulfonic acid,7 N-halosulfonamides,8 cyanuric chloride,9 H2SO4,10 dodecylphosphonic acid,11 and [PVPH]ClO4 (ref. 12) have been reported.The above mentioned catalysts have high yields,6–8,11 short reaction times,7,10,11 easy work-up procedure,7,10,11 solvent-free condition6,11 and reusability of catalyst.12
Some procedures have disadvantages such as strong acid condition10 and no reusability catalyst.8–10
Sb(III) is a good Lewis acid for activation of carbonyl compound.
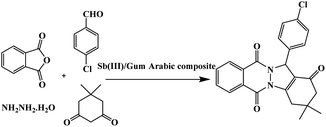
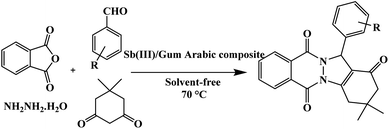
Herein, we have supported Sb(III) on Gum Arabic to preparation of Sb(III)/Gum Arabic as a heterogonous acid catalyst. Thus, we report an eco-friendly protocol for the synthesis of 2H-indazolo[2,1-b] phthalazinetriones in the presence of Sb(III)/Gum Arabic composite as a new natural-based green reusable catalyst via the reaction of dimedone, phthalic anhydride, hydrazinium hydroxide and aldehydes (Scheme 1).
Experimental
Materials
Chemicals were purchased from Merck, Fluka and Aldrich Chemical Companies.Fourier transform infrared (FT-IR) spectra recorded by ATR method on a Bruker (EQUINOX 55) spectrometer. The nuclear magnetic resonance (NMR) spectra were recorded in DMSO-d6 on Bruker (DRX-400, Avance) NMR 400 MHz. Melting points were determined by a Büchi B-540 instrument. Field emission scanning electron microscopy (FESEM) apparatus (Mira 3-XMU) was used for recording of FESEM images. The XRD graph of catalyst was obtained by X-ray diffractometer (XRD, Bruker-binary V3) using a Cu kα anode (k = 1.54 Å, radiation at 36 kV and 36 mA) in the 2θ range from 10° to 80°. Energy-dispersive X-ray spectrometer (EDS) and maps of catalyst were recorded by Phenom pro X. Thermal gravimetric analysis (TGA) was done using “BÄHR-(model: STA 503)” instrument. BELSORP MINI II nitrogen adsorption apparatus (Japan) was used for recording of Brunauer–Emmett–Teller (BET) specific surface area of catalyst at 77 K. Inductively coupled plasma mass spectrometry (ICP-MS) was recorded by AGILENT 7500 apparatus. All IR and 1H NMR spectra data are available in ESI (Fig. S1–S20).†
Catalytic test
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (8
ethyl acetate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)), 5 mL of hot ethanol was added to the mixture of reaction and the catalyst was separated by filtration. By adding H2O (5 mL) to filtrate, the solid product was appeared and then recrystallized from ethanol to give the pure product.
2)), 5 mL of hot ethanol was added to the mixture of reaction and the catalyst was separated by filtration. By adding H2O (5 mL) to filtrate, the solid product was appeared and then recrystallized from ethanol to give the pure product.Results and discussion
Synthesis and characterization of Sb(III)/Gum Arabic composite
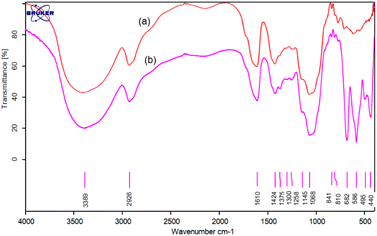
For the synthesis of Sb(III)/Gum Arabic, a mixture of Gum Arabic (0.5 g), Sb(OAc)3 (0.5 g) and dichloromethane (10 mL) was refluxed for 2 hours. Then solid catalyst was filtered, washed with dichloromethane and dried at room temperature.The structure of prepared Sb(III)/Gum Arabic composite was studied using FTIR, XRD, BET, FE-SEM, TGA, EDX(EDS), elemental mapping and ICP. Fourier transform infrared (FT-IR) spectroscopy spectra of Gum Arabic and Sb(III)/Gum Arabic composite were shown in Fig. 1. In Gum Arabic spectrum, the characteristic absorption bands at 1068 and 1145 cm−1 (C–O, stretch), 1610 (O–H, bending), 2926 cm−1 (C–H, stretch), and 3389 cm−1 (O–H, stretch) can be observed. In Sb(III)/Gum Arabic composite spectrum, the additional bands at 586 and 682 are assigned to the Sb–O stretching vibrations.
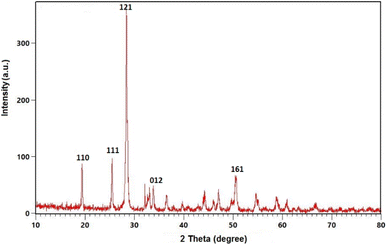
The XRD pattern of Sb(III)/Gum Arabic composite in 2θ = 10–80° is shown in Fig. 2. The broad peak at 2θ = 19.43 relates to the amorphous Gum Arabic and 2θ = 25.46, 28.41 attributed to Sb(III).
The surface morphology of Sb(III)/Gum Arabic composite was characterized using FESEM analysis (Fig. 3). According to the FESEM images, the particles are spherical with the size in the range of nanomaterial. The histogram of the size distribution detected by MIRA 3 TESCAN software in FESEM.
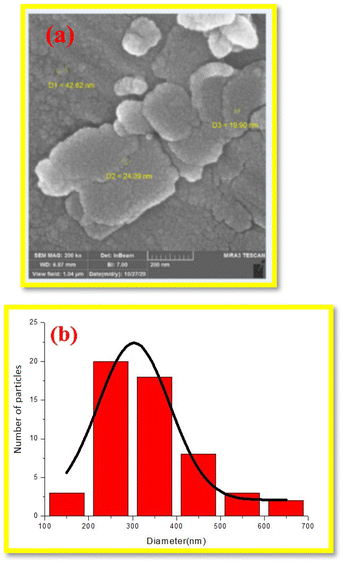 | ||
| Fig. 3 FESEM image (a) and particle size distribution histogram of Sb(III)/Gum Arabic composite (b). | ||
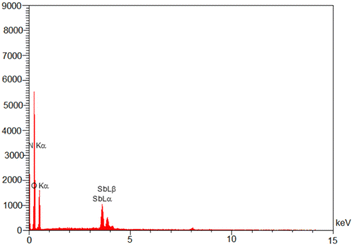
Energy-dispersive X-ray spectroscopy EDS (EDX) analysis was applied for the identification of elemental composition in Sb(III)/Gum Arabic composite (Fig. 4). The EDX data confirmed the existence of N, O and Sb elements in the catalyst.
The weight percentages of Sb(III) in catalyst was studied by inductively coupled plasma (ICP). According to obtained data, the weight percentage of Sb(III) is 43% (Fig. S21, ESI†).
The elemental mapping of Sb(III)/Gum Arabic composite was shown in (Fig. S22, ESI†) which confirmed homogenous distribution of C, N, O, Sb in catalyst.
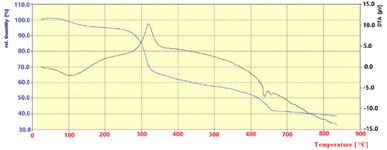
Fig. 5 shows the thermal gravimetric analysis (TG-DTA) of the Sb(III)/Gum Arabic composite in 20–900 °C. The obtained results indicated that there were three weight loss steps. The first observed weight loss of sample occurred at 100–200 °C due to removal of the catalyst moisture. The second step of weight loss, in 280–320 °C, was a result of dehydration and decarboxylation of Gum Arabic. The third step of weight loss occurs in the 343–663 °C range, most likely due to complete decomposition of the glycoprotein of Gum Arabic. Finally, the high weight of ash can be improved the presence of Sb(III) ion in catalyst.
The nitrogen adsorption–desorption graph, BET plot, Langmuir plot, t plot and BJH plot were shown in (Fig. S23, ESI†), which were used to determine the specific surface area, volume and distribution of pores. Nitrogen adsorption was measured on gas-depleted samples at 77 K. The adsorption–desorption isotherm is the type of III while indicating the presence of broader pore size distributions with H3 type hysteresis loop for Sb(III)/Gum Arabic composite, which is consistent with the IUPAC classification. The Brunauer–Emmett–Teller surface area (SBET), total pore volume and mean pore diameter of Sb(III)/Gum Arabic composite were determined as 2.2 m2 g−1, 0.006 cm3 g−1 and 10.5 nm, respectively (Table 1).
| BET plot | |
|---|---|
| Vmicro, (cm3 g−1) | 0.5 |
| S(BET), (m2 g−1) | 2.2 |
| V (Σ), (cm3 g−1) | 0.006 |
| Dpore, (nm) | 10.5 |
Catalytic properties of Sb(III)/Gum Arabic composite
In order to optimize reaction conditions for synthesis of 2H-indazolo[2,1-b] phthalazinetrione in the presence of Sb(III)/Gum Arabic composite, the condensation of dimedone, 4-chlorobenzaldehyde, phthalic anhydride and hydrazinium hydroxide was selected as a model reaction. The obtained results of this reaction such as temperature, solvent and amount of catalyst were summarized in Table 2.| Entry | Solvent | Conditions | Sb(III)/Gum Arabic composite (g) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Phthalic anhydride (1 mmol), hydrazinium hydroxide (1.2 mmol), 4-chlorobenzaldehyde (1 mmol) and dimedone (1 mmol).b Isolated yield. | |||||
| 1 | — | R.T | 0.08 | 120 | 28 |
| 2 | — | 70 °C | 0.08 | 30 | 97 |
| 3 | — | Grinding/60 °C | 0.08 | 80 | 60 |
| 4 | — | 50 °C | 0.08 | 85 | 40 |
| 5 | — | 65 °C | 0.08 | 25 | 75 |
| 6 | EtOH | Reflux | 0.08 | 50 | 62 |
| 7 | H2O | Reflux | 0.08 | 120 | 54 |
| 8 | — | 70 °C | 0.07 | 40 | 80 |
| 9 | — | 70 °C | 0.06 | 45 | 74 |
Efficiency of Sb(III)/Gum Arabic composite
According to obtained optimal conditions, the synthesis of 2H-indazolo[1,2-b] phthalazinetriones were done in the presence of Sb(III)/Gum Arabic composite at 70 °C and solvent-free conditions, which the results are summarized in Table 3.| Entry | R | Time (min) | Yieldb (%) | m.p. (°C) |
|---|---|---|---|---|
| a Reaction conditions: aldehyde (1 mmol), phthalic anhydride (1 mmol), hydrazinium hydroxide (1.2 mmol) and dimedone (1 mmol), Sb(III)/Gum Arabic composite (0.08 g) solvent-free and 70 °C.b Isolated yield. | ||||
| 1 | 4-HCOO | 120 | 94 | 265–267 |
| 2 | 4-Cl | 30 | 97 | 263–265 |
| 3 | 3-Cl | 35 | 95 | 185–187 |
| 4 | 4-OH | 35 | 90 | 262–264 |
| 5 | 3-NO2 | 32 | 91 | 270 |
| 6 | 4-CH3 | 40 | 89 | 232–234 |
| 7 | 4-OCH3 | 43 | 90 | 214–216 |
| 8 | 2-OH-5-Br | 37 | 92 | 265 |
| 9 | 4-NO2 | 30 | 93 | 228–230 |
| 10 | 4-Isopropyl | 60 | 87 | 258–260 |
According to Hammet correlation, the presence of electron-withdrawing group in para positon of aromatic aldehyde (4-COOH σpara = 0.45, 4-Cl σpara = 0.23, 4-NO2 σpara = 0.78) caused increasing of the yield of reaction and electron-donating group in para position (4-OH σpara = −0.37, 4-CH3 σpara = −0.17, 4-OCH3 σpara = −0.27) reduced the yield of reaction.
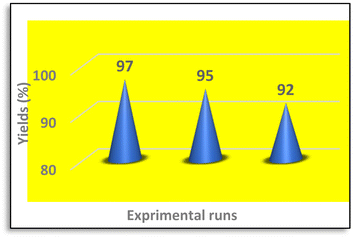
Reusability of Sb(III)/Gum Arabic composite
The reusability of the catalyst was examined in the model reaction for three times. The result showed low decrease of catalytic activity (Fig. 6).The proposed mechanism for the synthesis of 2H-indazolo[2,1-b] phthalazinetrione is shown in Scheme 2. It was anticipated that, at first, the Sb(III) Lowis acid in Sb(III)/Gum Arabic composite activates the carbonyl group of aldehyde to condensation with dimedone to form adduct (I). In continue, phthalhydrazide react with adduct (I) via a Michael-type addition to produce the intermediate (II). Finally, the OH group in intermediate (II) was activated by catalyst to production of product (III) via an intramolecular cyclization.
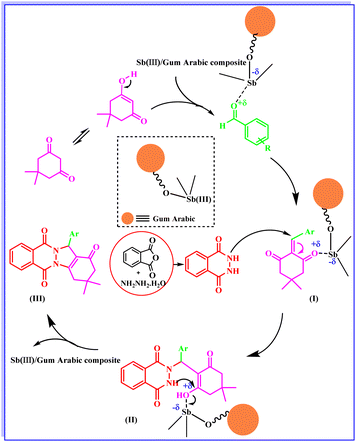 | ||
| Scheme 2 A plausible mechanism for the formation of 2H-indazolo[2,1-b] phthalazinetrione in the presence of Sb(III)/Gum Arabic composite. | ||
The comparison between our reported protocol with others was done and the results were tabulated in Table 4.
| Ent. | Catal. | Cond. | Time (min) | Yield (%) [ref.] | Product (mmol)/catalyst (g) | Catalyst (g)/reaction mixture (g) |
|---|---|---|---|---|---|---|
| a A: poly phosphoric acid–SiO2, B: Fe3O4@SiO2–ZrCl2-MNPs, C: Sb(III)/Gum Arabic composite. | ||||||
| 1 | CAN (5 mol%) | PEG 400, 50 °C | 120 | 94 (ref. 13) | 0.94/0.027 | 0.027/0.44 |
| 2 | H2SO4 (0.15 mmol) | [bmim]BF4, 80 °C | 30 | 92 (ref. 10) | 0.92/0.015 | 0.015/0.44 |
| 3 | Iodine (0.1 g) | Sonic bath | 10 | 92 (ref. 14) | 0.92/0.1 | 0.1/0.44 |
| 4 | A (5 mol%) | S.F. 100 °C | 6 | 93 (ref. 15) | 0.93/0.1 | 0.1/0.44 |
| 5 | B (0.02 g) | S.F. 110 °C | 10 | 95 (ref. 16) | 0.95/0.02 | 0.02/0.44 |
| 6 | C (0.08 g) | S.F. 70 °C | 30 | 97 [this work] | 0.097/0.08 | 0.08/0.44 |
Conclusions
In conclusion, an easy and green method for the synthesis of 2H-indazolo[2,1-b] phthalazinetriones using an eco-friendly and reusable catalyst was described. Some advantages of this protocol is mild reaction condition, solvent free and high yields of products.Data availability
All data generated or analysed during this study are included in this published article.Author contributions
MK, BFM and AB designed and performed the research, analysed the data, interpreted the results, and prepared the manuscript. MK performed the assay and conducted the optimization, and purification of compounds. All authors read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by Yazd University. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. The authors thank the Research Council of Yazd University for the support of this research.Notes and references
- W. Zhang and W. Cue Berkeley, Green techniques for organic synthesis and medicinal chemistry, 2012 Search PubMed.
- R. G. Vaghei, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Amiri and M. Ghavidel, Tetrahedron, 2011, 67, 1930–1937 CrossRef.
- A. Kumar and R. A. Maurya, Tetrahedron, 2007, 63, 1946–1952 CrossRef CAS.
- E. Sohouli, N. Irannejad, A. Ziarati, H. Ehrlich, M. Rahimi-Nasrabadi, F. Ahmadi and R. Luque, Environ. Chem. Lett., 2022, 20, 3789–3809 CrossRef CAS.
- H. Wu, X. M. Chen, Y. Wan, H. Q. Xin, H. H. Xu, R. Ma, C. H. Yue and L. L. Pang, Lett. Org. Chem., 2009, 6, 219–223 CrossRef CAS.
- H. R. Shaterian, M. Ghashang and M. Feyzi, Appl. Catal., A, 2008, 345, 128–133 CrossRef CAS.
- A. R. Kiasat, A. Mouradzadegun and S. J. Saghanezhad, J. Serb. Chem. Soc., 2013, 78, 469–476 CrossRef CAS.
- R. Ghorbani-Vaghei, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Amiri and M. Ghavidel, Tetrahedron, 2011, 67, 1930–1937 CrossRef CAS.
- X. Wang, W. Ma, L. Wu and F. L. Yan, J. Chin. Chem. Soc., 2010, 57, 1341–1345 CrossRef CAS.
- J. M. Khurana and D. Magoo, Tetrahedron Lett., 2009, 50, 7300–7303 CrossRef CAS.
- M. Kidwai, A. Jahan, R. Chauhan and N. K. Mishra, Tetrahedron Lett., 2012, 53, 1728–1731 CrossRef CAS.
- M. Mousapour, F. Shirini and M. Abedini, Polycyclic Aromat. Compd., 2019, 41, 419–426 CrossRef.
- K. Mazaahir, C. Ritika and J. Anwar, Chin. Sci. Bull., 2012, 57, 2273–2279 CrossRef CAS.
- A. Varghese, A. Nizam, R. Kulkarni and L. George, Eur. J. Chem., 2013, 4, 132–137 CrossRef CAS.
- H. R. Shaterian, A. Hosseinian and M. Ghashang, ARKIVOC, 2009, 2, 59–67 Search PubMed.
- F. Kamali and F. Shirini, New J. Chem., 2017, 41, 11778–11791 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02556j |
| This journal is © The Royal Society of Chemistry 2023 |