Open Access Article
Open Access ArticleDenitrification activity test of a V modified Mn-based ceramic filter
Lei Sun *a,
Zhenzhen Wang
*a,
Zhenzhen Wang b and
Mengxi Zangb
b and
Mengxi Zangb
aAnhui Academy for Ecological and Environmental Science Research, Hefei 230071, China. E-mail: sunlei551@qq.com
bSchool of Resource and Environmental Engineering, Hefei University of Technology, Hefei 230009, China
First published on 4th July 2023
Abstract
In view of the characteristics of high temperature denitrification and low water and sulfur resistance of single manganese-based catalysts, a vanadium–manganese-based ceramic filter (VMA(14)-CCF) was prepared by the impregnation method modified with V. The results showed that the NO conversion of VMA(14)-CCF was more than 80% at 175–400 °C. At 225–300 °C, the conversion of NO can reach 100%. High NO conversion and low pressure drop can be maintained at all face velocities. The resistance of VMA(14)-CCF to water, sulfur and alkali metal poisoning is better than that of a single manganese-based ceramic filter. XRD, SEM, XPS and BET were further used for characterization analysis. The introduction of V protects the MnOx center, promotes the conversion of Mn3+ to Mn4+, and provides abundant surface adsorbed oxygen. The development of VMA(14)-CCF greatly broadens the application range of ceramic filters in denitrification.
1 Introduction
Nitrogen oxides (NOx), including NO, NO2, and N2O, are the main air pollutants, which can cause a series of environmental problems such as acid rain, photochemical smog, and haze, posing a risk to human health.1–3 The use of various fuels and vehicles are the main sources of NOx. To improve the environment and achieve sustainable development of society, the development of new and efficient denitrification technologies has become a research focus in the field of environmental protection.4–6Catalytic ceramic filter (CCF) is a filtration material used for high-temperature gas purification.7 The dust removal and denitration in high-temperature flue gas can be carried out synchronously in the same device.8 This approach solves the issues of a lengthy purification process and large footprint associated with separate dust removal and denitrification steps in traditional processes, as well as problems such as catalyst poisoning and erosion caused by dust, alkali metals, and other factors. It can effectively prolong the lifespan of catalysts and reduce denitrification costs. CCF is a novel, promising, and efficient denitrification technology. The catalyst commonly used in CCF is V–W/Ti, and its active temperature range being 300–400 °C. It has poor low-temperature activity.9 However, V2Os exhibit excellent Selective Catalytic Reduction (SCR) activity and strong water and sulfur resistance at high temperatures.
The structure and electronic distribution of metal elements can change due to mutual doping, resulting in differences in catalytic performance under different flue gas conditions.10–13 Liu et al.14 modified the low-temperature SCR activity of V/Ti catalyst by loading Mn, and the results demonstrated that the addition of MnOx enhanced the redox capability of V. Thanh et al.15 employed a co-precipitation method to prepare Ce/TiO2, Mn–Ce/Ti, and Mn–V–Ce/Ti catalysts. They observed a significant reduction in NH3 oxidation and a substantial improvement in the N2 selectivity of the catalyst upon simultaneous addition of V and Mn to the Ce/TiO2 catalyst. Characterization results indicated that this enhancement was due to the synergistic effect between VO3+/VO2+ groups in the VOx species and Mn3+/Mn2+ groups in the MnOx species, which suppressed NH3 oxidation and thereby increased the N2 selectivity of these catalysts. Niu et al.16 employed the sol–gel method to incorporate Ce and V into MnOx/TiO2 catalysts to suppress the formation of nitric oxide (NO) at low temperatures and enhance the N2 selectivity of the catalyst. Various techniques, including selectivity, XRD, XPS, NH3-TPD, and infrared spectroscopy, were utilized for analysis. The results demonstrated that the addition of Ce improved the conversion efficiency of NO, shifting the high-performance region to lower temperatures. Meanwhile, the addition of V reduced the generation of N2O while maintaining high NO conversion efficiency and low N2O formation.
However, further research is needed to investigate the coupling of V–Mn composite catalysts with CCF, as well as the SCR reaction mechanism, water resistance, sulfur resistance, and other aspects of V–Mn-based CCF, in both depth and breadth. This study uses a small amount of V-doped Mn/γ-Al2O3 to prepare V–Mn/γ-Al2O3. V–Mn/γ-Al2O3 is loaded on ceramic filter to prepare a CCF with a wide active temperature range, good water and sulfur resistance, and strong alkali resistance, to expand the industrial application range of the CCF. In addition, characterization and analysis using XRD, SEM, EDS, and BET are conducted to investigate the influence of V on the SCR activity of catalyst, as well as the reaction mechanism and poisoning mechanism of V–Mn-based CCF.
2 Materials and methods
2.1 Preparation of catalyst ceramic filter
The precursor Mn(CH3COO)2·4H2O (99.9%, Guang Fu, China) was selected for this study. It was combined with γ-Al2O3 (AR, Sinopharm Chemical Reagent, China) in a beaker, followed by the addition of a specific quantity of deionized water. After vigorous stirring for 2 h, the mixture was allowed to age for 12 h at a controlled temperature of 40 °C. Subsequently, the impregnated product was subjected to a drying process and then calcined at 400 °C in a muffle furnace, yielding the pristine Mn/γ-Al2O3 catalyst. To prepare the V-modified catalyst, the precursor NH4VO3 (AR, Aladdin, China) was introduced using the same methodology, resulting in the formation of V–Mn/γ-Al2O3.For experimental purposes, the ceramic filter (Anhui Yiyi Environmental Protection Equipment Co., Ltd) was cut into cylindrical shapes with a length of 20 mm and a diameter of 16 mm. The ceramic filter (CF) was then subjected to a series of treatments including water rinsing, acid treatment, and alkaline treatment. Afterward, CF was placed in an oven and dried at 100 °C, followed by calcination in a muffle furnace at 400 °C for 3 h. The treated CF was carefully weighed and kept for further use.
To prepare the catalyst, it was dissolved in a water solution and polyethylene glycol (PEG) was added. The mixture was then sonicated for 5 min at 40 °C. The catalyst was coated onto the pre-treated CF, which is referred to as the first loading. The CCF prepared using Mn/γ-Al2O3 as the catalyst is denoted as MA(x)-CCF, where x represents the loading amount of Mn/γ-Al2O3. Similarly, the CCF prepared using V–Mn/γ-Al2O3 as the catalyst is denoted as VMA(y)-CCF, where y represents the loading amount of V–Mn/γ-Al2O3.
2.2 Sample characterization
The catalyst and the CCF were determined using a Dandong Haoyuan DX-2700 X-ray diffraction (XRD). Since the CCF is solid, the XRD requires the samples to be in powder form. Therefore, the CCF was ground in a mortar and sieved through a 200-mesh sieve. Cu Kα radiation was used, with experimental conditions set at 40 kV (1 kV per step) for tube voltage, 30 mA for tube current, and a scanning rate of 4° min−1. Intensity data was collected in the range of 2θ from 5° to 75°.The loaded state of the catalyst on the CCF was observed using a scanning electron microscope (SU8020, Hitachi, Japan) with a voltage range of 0.1–30 kV. During the sample preparation process, the CCF was cut into thin slices of 8 mm × 8 mm and attached to a sample stage with black conductive adhesive. Vacuum gold sputtering was performed due to the good insulation properties of CCF. Energy-dispersive spectroscopy (EDS) was used in conjunction to obtain the element composition and content of the designated area of the tested samples. Since the content of the catalyst in the CCF was only about 10–20%, and the content was low, XPS was directly used to determine the catalyst sample.
The elemental valence state content on the surface of the catalyst sample was determined using an ESCALAB250Xi (Thermo, America) with Al Kα radiation. The XPS spectra were deconvoluted using XPSPEAK4.1. By normalizing the peak areas, surface concentrations of each atom and the proportion of different valence states of each element were calculated.
The specific surface area and pore volume of the catalyst were determined using a BET (Brunauer–Emmett–Teller) surface area and pore size analyzer (3H-2000PS2) by nitrogen adsorption isotherm measurements at liquid nitrogen temperature (77 K). The pore structure information was obtained using the BJH (Barrett–Joyner–Halenda) method.
2.3 Catalytic performance test
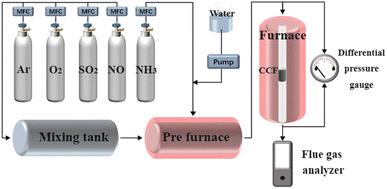
The catalytic denitration performance of the CCF was studied in a homemade fixed-bed reactor, as shown in Fig. 1. The flue gas composition used was 500 ppm NH3, 500 ppm NO, 3 vol% O2, 100 or 300 ppm SO2 (when used), and 10 vol% H2O (when use), with Ar as the balance gas. The outlet gas was analyzed using a flue gas analyzer, and the pressure drop was measured using a differential pressure gauge. The NO conversion is calculated as:
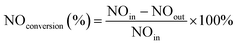 | (1) |
3 Results and discussion
3.1 The optimal preparation of catalyst ceramic filter
V–Mn/γ-Al2O3 was loaded onto the CCF using a single-step coating method with PEG as the binder, as shown in Table 1. The actual loading rate of the catalyst is calculated by calculating the quality difference of CF load before and after operation. The loading rate was 8.8% after one coating, 14% after two coatings, and 19% after three coatings.| Sample | VMA(8.8)-CCF | VMA(14)-CCF | VMA(19)-CCF |
|---|---|---|---|
| The loading number of times | 1 | 2 | 3 |
| The loading rate (%) | 8.8 | 14 | 19 |
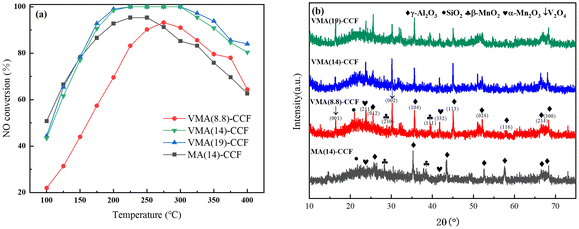
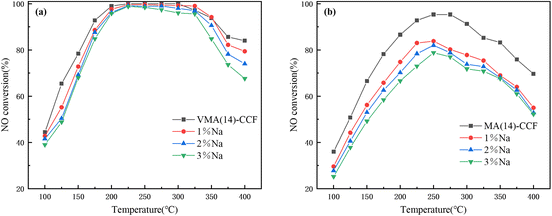
The samples with different loading rates were tested for denitration activity, as shown in Fig. 2(a). As the loading amount of V–Mn/γ-Al2O3 increased, the denitration efficiency of VMA(y)-CCF gradually increased. However, the increase in NO conversion of VMA(19)-CCF compared to VMA(14)-CCF was not significant, and increasing the number of loadings would increase production costs and filter pressure drop. Therefore, VMA(14)-CCF was chosen as the research object. The NO conversion was greater than 80% at 175–400 °C, and could reach 100% at 225–300 °C. VMA(14)-CCF exhibited excellent denitration efficiency over a wide temperature range. The denitration performance of MA(14)-CCF, which was prepared with the same mass fraction of Mn/γ-Al2O3, was slightly higher than VMA(14)-CCF at 100–150 °C, and significantly lower than VMA(14)-CCF at 150–400 °C. This indicates that the addition of V can improve the denitration efficiency of the catalyst at medium to high temperatures.
3.2 Characterization
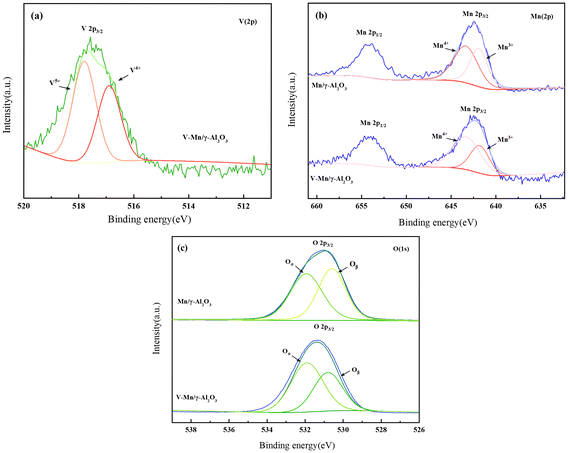
Fig. 4(a) shows the XPS spectra of V2p, where the peak at 515.2–517.7 eV can be attributed to V4+, while the peak at around 516.2–518.7 eV corresponds to V5+.23–25 The main forms of V2Os species are V2O5 and V2O4, with a higher content of V5+ than V4+. However, no diffraction peaks of V2O5 were detected in XRD, indicating that V2O5 is highly dispersed on the surface of the γ-Al2O3 support in an amorphous state. The V2O9H8 structure formed by the combination of V2O5 with H2O exhibits more Brønsted acid sites.24
The binding energy spectra of Mn 2p for the samples are shown in Fig. 4(b). The spectra include two characteristic peaks, Mn 2p1/2 and Mn 2p3/2. The Mn 2p3/2 peaks correspond to Mn3+ (642.03–643.38 eV) and Mn4+ (643.53–644.38 eV) species,26,27 indicating that MnOx in the catalyst mainly exists as a combination of Mn3+ and Mn4+, which is consistent with the XRD results. According to Table 2, the percentage of Mn4+ in V–Mn/γ-Al2O3, indicates a strong interaction between V and Mn, and the doping of V promotes the conversion of Mn3+ to Mn4+. Liu et al.14 loaded Mn on V/Ti catalyst to modify its low-temperature SCR activity, and the results showed that the addition of MnOx enhanced the REDOX ability of V. Mn4+–O2−–V5+ ↔ Mn4+–O2−–V4+ ↔ Mn3+–O2−–V5+ ↔ Mn4+–O2−–V5+ + e. This cycle can well control the electron movement between Mn and V, and ensure the REDOX capacity of the active species. The SCR activity at low temperature was improved and the original SCR activity at high temperature was maintained. Kapteijn et al.28,29 reported that the NO conversion of pure manganese oxides follows the order: MnO2 > Mn5O8 > Mn2O3 > Mn3O4. Since Mn4+ in manganese oxides has a higher oxidation state, it can promote the conversion of NO to NO2, thereby facilitating the rapid SCR reaction.30,31
| Test samples | V4+/(V4+ + V5+) | Mn4+/(Mn4+ + Mn3+) | Oα/(Oα + Oβ) |
|---|---|---|---|
| Mn/γ-Al2O3 | — | 57.1% | 53.3% |
| V–Mn/γ-Al2O3 | 41.6% | 70.5% | 61.1% |
Fig. 4(c) shows the O ls spectra of Mn/γ-Al2O3 and V–Mn/γ-Al2O3. Generally, there are two types of oxygen species on the catalyst surface, surface adsorbed oxygen and lattice oxygen (O2−), denoted as Oα and Oβ.32–34 Surface adsorbed oxygen can further be classified into physically adsorbed oxygen and chemically adsorbed oxygen, which have higher mobility and reactivity compared to lattice oxygen, such as O2, oxygen vacancies (O2−, O22−), or hydroxyl groups (OH−).35 The binding energy corresponding to Oα of V–Mn/γ-Al2O3 is 532 eV, shifted 0.1 eV to the left compared to Oα of Mn/γ-Al2O3. The binding energy corresponding to Oβ is 530.9 eV, shifted 0.4 eV to the left compared to the lattice oxygen peak of Mn/γ-Al2O3. This indicates that V changes the electron binding energy of some oxygen atoms, causing the peaks of lattice oxygen and surface adsorbed oxygen to shift to higher binding energies.36,37 Meanwhile, the addition of V increases the proportion of Oα in the catalyst, which has higher mobility and activity compared to Oβ. In a word, the presence of V promotes electron transfer and redox capability of the catalyst.
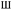 isotherms. The adsorption branch line on the curve does not overlap with the desorption branch line, which is caused by the presence of hysteresis loops and is usually associated with capillary condensation. The existence of hysteresis loop suggests that the catalysts are mainly composed of mesopores or micropores. The H4 hysteresis loops of VMA(14)-CCF and MA(14)-CCF indicate the presence of many micropores.
isotherms. The adsorption branch line on the curve does not overlap with the desorption branch line, which is caused by the presence of hysteresis loops and is usually associated with capillary condensation. The existence of hysteresis loop suggests that the catalysts are mainly composed of mesopores or micropores. The H4 hysteresis loops of VMA(14)-CCF and MA(14)-CCF indicate the presence of many micropores.
 | ||
| Fig. 5 N2 absorption and desorption curves and pore size distribution of VMA(14)-CCF and MA(14)-CCF. | ||
| Sample | Specific surface area/(m2 g−1) | Pore volume/(cm3 g−1) | Average pore size/(nm) |
|---|---|---|---|
| VMA(14)-CCF | 47.45 | 0.17 | 8.20 |
| MA(14)-CCF | 48.22 | 0.18 | 8.54 |
A larger specific surface area is favorable for gas adsorption, and a well-defined pore structure promotes the transfer of reactants between gas and catalyst, thereby enhancing the SCR reaction rate and significantly influencing the denitration activity of the catalysts.38 The specific surface area, pore volume, and pore size of VMA(14)-CCF are slightly smaller than those of MA(14)-CCF. And there is no big difference, because the catalyst content of CCF is only 14%.
3.3 Catalytic activity test of the VMA(14)-CCF
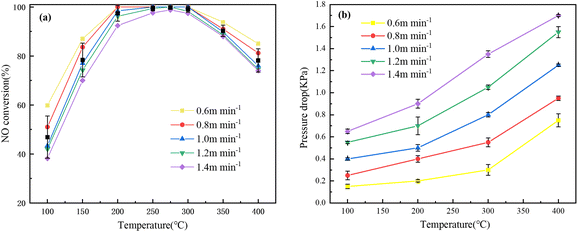
The increase in temperature will cause the gas molecules to move faster, which will increase the pressure drop of the filter. Excessively high FV will also increase the pressure drop across the filter, not only causing resistance to dust removal, but also increasing the risk of unstable operation of the equipment. As shown in Fig. 6(b), the pressure drop of VMA(14)-CCF at various FV is relatively low. This may be due to the smaller particle size of V–Mn/γ-Al2O3 under the same loading amount, which is not easy to block the pores of the ceramic fibers, as confirmed in SEM. When the FV is 0.6 m min−1, the pressure drop at 400 °C is only 0.75 kPa. The actual FV used in industrial factories is generally around 1 m min−1, and the pressure drop of VMA(14)-CCF is 0.4 kPa at 100 °C, 0.5 kPa at 200 °C, 0.8 kPa at 300 °C, and 1.25 kPa at 400 °C. At 400 °C, the maximum pressure drop of VMA(14)-CCF is 1.7 kPa, greatly increasing the applicability of this filter.
 | ||
| Fig. 7 (a) NO conversion and (b) XRD spectrum of VMA(14)-CCF and MA(14)-CCF in the presence of SO2 and H2O at 225 °C. | ||
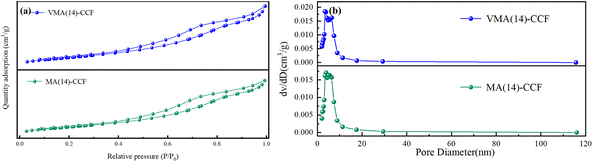
Since VMA(14)-CCF shows good sulfur resistance under 100 ppm SO2 concentration, and some industries such as power generation, steelmaking, and building materials are prone to high-temperature oxygen-rich environments in their production processes, resulting in higher SO2 content in flue gas. Therefore, the denitrification performance of VMA(14)-CCF was tested under 300 ppm SO2. Within 1 h, VMA(14)-CCF still maintained 100% NO conversion, and then gradually decreased to 86.8% within 140 min. After cutting off the supply of SO2, it recovered to 98.6% within 60 min, which was consistent with the performance before introduction. MA-CCF with the same catalyst loading amount of 14% was selected for the 300 ppm SO2 test, and the NO conversion decreased rapidly within the first 100 min, reaching a minimum of 70.6%. Afterward, the decrease was slower, with a minimum of 63.2%. It was 23.6 percentage points lower than that of VMA(14)-CCF under the same environment. This indicates that MnOx is more affected by SO2, and manganese-based catalysts are not suitable for application in high-sulfur environments. V2Os has good sulfur resistance, and the introduction of vanadium can broaden the applicable range of MnOx.
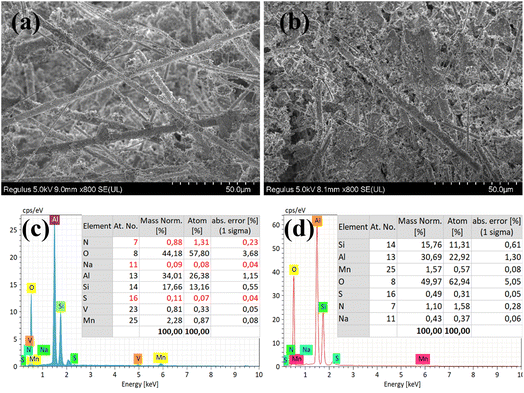
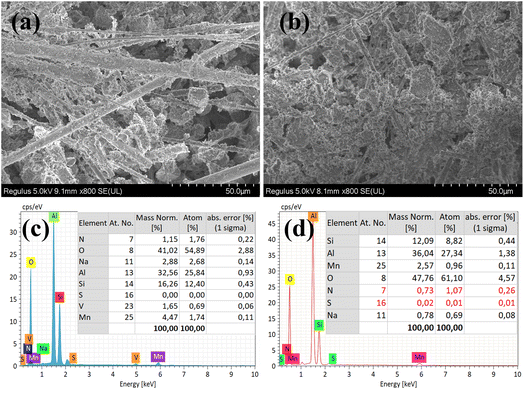
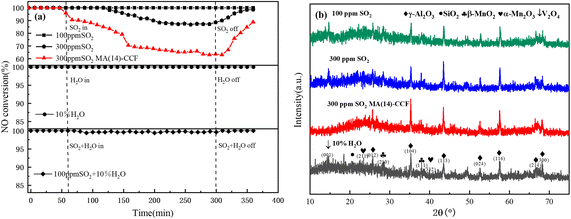
The VMA(14)-CCF after environmental testing with 100 ppm SO2 and 10% H2O was characterized by XRD, and the results showed no significant changes compared to the VMA(14)-CCF before the experiment (Fig. 7(b)). Higher Mn4+ concentration plays a critical role in the SCR activity, as evidenced by XPS characterization results indicating that the introduction of V promotes the conversion of Mn3+ to Mn4+. Higher Mn4+ concentration enhances the catalyst's catalytic reduction ability and improves its resistance to sulfur and water.41 The impact of SO2 on the catalyst can be summarized in three aspects: first, SO2 reacts with NH3 to form (NH4)2SO4 or NH4HSO4, which blocks the active sites of the catalyst and hinders the reaction between NOx and the active sites;31,42 second, SO2 competes with NO for adsorption, leading to a decrease in the conversion rate of nitrogen oxides by the catalyst;43,44 third, SO2 reacts with active components to form inert metal sulfates, which reduces the quantity of catalyst available for gas reactions and leads to a decrease in denitration performance.39,45 XRD and SEM characterization of VMA(14)-CCF and MA(14)-CCF after anti-sulfur experiments with 300 ppm revealed that the MnOx peak disappeared in MA(14)-CCF, while the MnOx in VMA(14)-CCF showed no significant change compared to before the experiment (Fig. 8). SEM combined with EDS results showed block-like substances larger than the catalyst particle size in MA(14)-CCF, with S content of 0.32%. In contrast, particles on the ceramic fibers of VMA(14)-CCF were still uniformly distributed, with only a small amount of small block-like substances and S content of only 0.07%. Combined with XPS results, the addition of V2Os provides more surface adsorbed oxygen for the catalyst, allowing NH3 to react with the abundant surface adsorbed oxygen even in the presence of SO2, resulting in less formation of (NH4)2SO4 or NH4HSO4. On the other hand, in the presence of SO2, active V2Os preferentially react with SO2, protecting the active centers of MnOx and reducing the degree of catalyst poisoning by SO2.
EDS and BET characterization were performed on MA(14)-CCF and VMA(14)-CCF after treatment with 3% Na, and the results are shown in Fig. 10 and Table 4. The content of Na in both samples increased, and block-like substances were observed to adhere to the surface in the electron microscope images. Moreover, the specific surface area and pore volume of MA(14)-CCF and VMA(14)-CCF decreased. This indicates the sodium compound is enriched and deposited on the surface of the catalyst's active sites, blocking the pores and reducing the activity centers for gas treatment. In addition, the addition of alkali metals generates basic centers after the reaction, which enhances the alkalinity of the catalyst surface and reduces its adsorption capacity for NH3.48 At the same time, the addition of alkali metals also weakens the catalytic reducibility of the catalyst, and multiple factors lead to a decrease in denitration efficiency of the catalyst, consistent with the experimental results.49 Although the specific surface area of the two samples is similar, the alkali resistance of VMA(14)-CCF is significantly better than that of MA(14)-CCF, due to the close relationship between the redox performance and alkali resistance of the catalyst. The presence of V2Os results in a more abundant surface adsorbed oxygen and Mn4+, which enhances the surface acidity and redox properties of the catalyst, making VMA(14)-CCF exhibit excellent alkali resistance and maintain high NO conversions in experiments with different alkali metal mass fractions.
| Sample | Specific surface area/(m2 g−1) | Pore volume/(cm3 g−1) | Average pore size/(nm) |
|---|---|---|---|
| 3% Na-VMA(14)-CCF | 46.41 | 0.11 | 9.54 |
| 3% Na-MA(14)-CCF | 40.56 | 0.10 | 8.34 |
4 Conclusions
A novel ceramic filter for selective catalytic reduction of NOx catalyst was developed. The ceramic fibers retain their original shape. The catalyst particles are uniformly distributed on CF, and the denitration effect is not affected by the loading process. The feasibility of Mn-based CCF modified by V is proved. When the FV is 1 m min−1, the NO conversion rate of VMA(14)-CCF reaches more than 80% at 175–400 °C, and 100% at 225–300 °C. It shows excellent denitration efficiency over a wide temperature range. High NO conversion and low pressure drop can be maintained at various filtration speeds. It has excellent resistance to water, sulfur and alkali metal poisoning. The possibility of practical application and popularization of VMA(14)-CCF is greatly increased. Its strong anti-poisoning ability is due to the introduction of V not only protects the active center of MnOx, but also promotes the transformation of Mn3+ to Mn4+. This provides abundant surface adsorbed oxygen for the catalyst, and enhances the adsorption of NH3 and NO by the catalyst, thus improving the surface acidity and oxidation reducibility of the catalyst.Author contributions
Lei Sun: writing – investigation, original draft, methodology, date curation, formal analysis, funding acquisition, visualization. Zhenzhen Wang: methodology, writing – review & editing, software. Mengxi Zang: formal analysis.Conflicts of interest
The authors declared that they have no conflicts of interest to this work.Acknowledgements
The authors thank for the financial support from Emission standard of air pollutants for the glass industry of Anhui Province of China (ZXBZ2020-27).References
- T. Wang, C. Zhu, H. Liu, Y. Xu, X. Zou, B. Xu and T. Chen, Environ. Technol., 2018, 39, 317–326 CrossRef CAS PubMed
.
- Z. Wang, S. Peng, C. Zhu, B. Wang, B. Du, T. Cheng, Z. Jiang and L. Sun, RSC Adv., 2023, 13, 344–354 RSC
.
- W. Zhang, K. Xie, Y. Tang, S. Cheng, M. Qing, Y. Xuan, C. Qin, M. Dong, Y. Zhou and J. Li, RSC Adv., 2022, 12, 22881–22892 RSC
.
- J. Kim, D. W. Kwon, S. Lee and H. P. Ha, Appl. Catal., B, 2018, 236, 314–325 CrossRef CAS
.
- X. Yang, L. Kang, C.-J. Wang, F. Liu and Y. Chen, Chem. Commun., 2021, 57, 7176–7179 RSC
.
- S. Youn, S. Jeong and D. H. Kim, Catal. Today, 2014, 232, 185–191 CrossRef CAS
.
- L. Zhao, K. Li, R. Wu, H. Zhang and J. Jin, Mater. Res. Express, 2020, 7, 125502 CrossRef CAS
.
- Y.-s. Zhang, C. Li, C. Wang, J. Yu, G. Xu, Z.-g. Zhang and Y. Yang, Ind. Eng. Chem. Res., 2019, 58, 828–835 CrossRef CAS
.
- H. Lin, Z. Chen, C. Li, X. Yao, Z. Yao, G. Xu, S. Gao, X. Huang and J. Yu, Catalysts, 2020, 10, 2073–4344 Search PubMed
.
- M. Zhu, J.-K. Lai, U. Tumuluri, M. E. Ford, Z. Wu and I. E. Wachs, ACS Catal., 2017, 7, 8358–8361 CrossRef CAS
.
- Z. Fan, J.-W. Shi, C. Gao, G. Gao, B. Wang, Y. Wang, C. He and C. Niu, Chem. Eng. J., 2018, 348, 820–830 CrossRef CAS
.
- L. Xu, S. Niu, C. Lu, Q. Zhang and J. Li, Fuel, 2018, 219, 248–258 CrossRef CAS
.
- S. Zhang, Y. Zhao, J. Yang, J. Zhang and C. Zheng, Chem. Eng. J., 2018, 348, 618–629 CrossRef CAS
.
- Z. Liu, Y. Li, T. Zhu, H. Su and J. Zhu, Ind. Eng. Chem. Res., 2014, 53, 12964–12970 CrossRef CAS
.
- T. H. Vuong, S. Bartling, U. Bentrup, H. Lund, J. Rabeah, H. Atia, U. Armbruster and A. Brückner, Catal. Sci. Technol., 2018, 8, 6360–6374 RSC
.
- Y. Niu, T. Shang, S. Hui, X. Zhang, Y. Lei, Y. Lv and S. Wang, Fuel, 2016, 185, 316–322 CrossRef CAS
.
- W. Li, X. Du, Z. Li, Y. Tao, J. Xue, Y. Chen, Z. Yang, J. Ran, V. Rac and V. Rakić, Process Saf. Environ. Prot., 2022, 159, 213–220 CrossRef CAS
.
- G. Zhai, Z. Han, X. Wu, H. Du, Y. Gao, S. Yang, L. Song, J. Dong and X. Pan, J. Taiwan Inst. Chem. Eng., 2021, 125, 132–140 CrossRef CAS
.
- R. Li, Y. Liang, Z. Zhang, Q. Huang, X. Jiang, R. Yang, L. Yu and J. Jiang, Catal. Today, 2022, 405–406, 125–134 CrossRef CAS
.
- T. Qiao, Z. Liu, C. Liu, W. Meng, H. Sun and Y. Lu, Appl. Catal., A, 2021, 617, 118128 CrossRef CAS
.
- X. Shi, J. Guo, T. Shen, A. Fan, S. Yuan and J. Li, Chem. Eng. J., 2021, 421, 129995 CrossRef CAS
.
- R. Xiao, T. Gao, X. Cui, Y. Ji, Y. Zhang, X. Chuai, Z. Xiong, Y. Liao, H. Gu, J. Yang, J. Zhang and Y. Zhao, Fuel, 2022, 310, 122219 CrossRef CAS
.
- N. Zhu, W. Shan, Z. Lian, Y. Zhang, K. Liu and H. He, J. Hazard. Mater., 2020, 382, 120970 CrossRef CAS PubMed
.
- S. Soyer, A. Uzun, S. Senkan and I. Onal, Catal. Today, 2006, 118, 268–278 CrossRef CAS
.
- A. Abubakar, C. Li, L. Huangfu, S. Gao and J. Yu, Korean J. Chem. Eng., 2020, 37, 633–640 CrossRef CAS
.
- Z. Li, Y. Gao and Q. Wang, J. Cleaner Prod., 2022, 348, 131152 CrossRef CAS
.
- K. Zheng, Z. Zhou, Y. Wang, Z. Xin, Z. Zhao, J. Zhang, T. Bo, T. Lin, B. Zhang and L. Shao, Catal. Sci. Technol., 2020, 10, 3450–3457 RSC
.
- F. Kapteijn, L. Singoredjo, A. Andreini and J. A. Moulijn, Appl. Catal., B, 1994, 3, 173–189 CrossRef CAS
.
- S. Boxiong, M. Hongqing, H. Chuan and Z. Xiaopeng, Fuel Process. Technol., 2014, 119, 121–129 CrossRef
.
- B. Tian, S. Ma, Y. Zhan, X. Jiang and T. Gao, Appl. Surf. Sci., 2021, 541, 148460 CrossRef CAS
.
- J. Chen, P. Fu, D. Lv, Y. Chen, M. Fan, J. Wu, A. Meshram, B. Mu, X. Li and Q. Xia, Chem. Eng. J., 2021, 407, 127071 CrossRef CAS
.
- Y. Zhang, C. Hao, J. Zhang, J. Wu, Y. Yue, Y. Xu and G. Qian, Colloids Surf., A, 2022, 635, 128080 CrossRef CAS
.
- J.-K. Lai, N. R. Jaegers, B. M. Lis, M. Guo, M. E. Ford, E. Walter, Y. Wang, J. Z. Hu and I. E. Wachs, ACS Catal., 2021, 11, 12096–12111 CrossRef CAS
.
- S. Ji, P. Li, X. Zhao, S. Wei, X. Cheng, L. Wu, Y. Ye, K. Ma, Y. Cai and C. Liang, Sens. Actuators, B, 2023, 383, 133595 CrossRef CAS
.
- T. Cheng, B. Du, H. Zhou, Z. Jiang, Q. Xie and C. Zhu, Environ. Sci. Pollut. Res., 2023, 30, 36294–36310 CrossRef CAS PubMed
.
- S. Dong, H. Wang, L. Gong, R. Hu and Z. Qu, Surf. Interfaces, 2022, 35, 102411 CrossRef CAS
.
- Z. Lyu, S. Niu, K. Han, C. Lu and Y. Li, Appl. Catal., A, 2021, 610, 117968 CrossRef CAS
.
- X. Zhou, X. Huang, A. Xie, S. Luo, C. Yao, X. Li and S. Zuo, Chem. Eng. J., 2017, 326, 1074–1085 CrossRef CAS
.
- S. W. Jeon, I. Song, H. Lee, J. Kim, Y. Byun, D. J. Koh and D. H. Kim, Chem. Eng. J., 2022, 433, 133836 CrossRef CAS
.
- L. Xie, C. Liu, Y. Deng, F. Liu and W. Ruan, Ind. Eng. Chem. Res., 2022, 61, 8698–8707 CrossRef CAS
.
- B. Du, Y. Hu, T. Cheng, Z. Jiang, Z. Wang and C. Zhu, RSC Adv., 2023, 13, 6378–6388 RSC
.
- B. Wang, B. Yao, F. Qi, S. Wang and B. Shen, J. Fuel Chem. Technol., 2022, 50, 503–512 CrossRef CAS
.
- F. Wang, B. Shen, S. Zhu and Z. Wang, Fuel, 2019, 249, 54–60 CrossRef CAS
.
- Y. Shu, T. Aikebaier, X. Quan, S. Chen and H. Yu, Appl. Catal., B, 2014, 150–151, 630–635 CrossRef CAS
.
- C. Tang, H. Wang, S. Dong, J. Zhuang and Z. Qu, Catal. Today, 2018, 307, 2–11 CrossRef CAS
.
- P. Raghunath and M. C. Lin, J. Phys. Chem. C, 2008, 112, 8276–8287 CrossRef CAS
.
- H. Si-Ahmed, M. Calatayud, C. Minot, E. L. Diz, A. E. Lewandowska and M. A. Bañares, Catal. Today, 2007, 126, 96–102 CrossRef CAS
.
- J. Due-Hansen, S. Boghosian, A. Kustov, P. Fristrup, G. Tsilomelekis, K. Ståhl, C. H. Christensen and R. Fehrmann, J. Catal., 2007, 251, 459–473 CrossRef CAS
.
- K. Guo, J. Ji, W. Song, J. Sun, C. Tang and L. Dong, Appl. Catal., B, 2021, 297, 120388 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2023 |