Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluation of adhesion strength, corrosion, and biological properties of the MWCNT/TiO2 coating intended for medical applications
Dorota Rogala-Wielgus *a,
Beata Majkowska-Marzec
*a,
Beata Majkowska-Marzec a,
Andrzej Zieliński
a,
Andrzej Zieliński a,
Katarzyna Roszek
a,
Katarzyna Roszek b and
Malwina Liszewska
b and
Malwina Liszewska c
c
aDivision of Biomaterials Technology, Institute of Manufacturing and Materials Technology, Faculty of Mechanical Engineering and Ship Technology, Gdansk University of Technology, 11 Narutowicza Str., 80-233 Gdańsk, Poland. E-mail: dorota.wielgus@pg.edu.pl
bFaculty of Biological and Veterinary Sciences, Nicolaus Copernicus University in Toruń, Lwowska 1 Str., 87-100 Toruń, Poland
cInstitute of Optoelectronics, Military University of Technology, Kaliskiego 2 Str., 00-908 Warsaw, Poland
First published on 16th October 2023
Abstract
Multi-wall carbon nanotube (MWCNT) coatings are gaining increasing interest because of their special properties used in many science fields. The titania coatings are known for their improvement of osteoblast adhesion, thus changing the surface architecture. Bi-layer coatings comprising 0.25 wt% of the MWCNTs and 0.30 wt% of titania (anatase structure) were synthesized in a two-stage procedure using the electrophoretic deposition method (EPD). The MWCNT and TiO2 coatings were deposited with voltage and time parameters, respectively, of 20 V and 0.5 min, and 50 V and 4 min. EDS, AFM, SEM, Raman spectroscopy, nano-scratch test, potentiodynamic corrosion tests, wettability studies, and cytotoxicity determined with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) test on human dermal fibroblasts (HDF) and mouse osteoblast precursors (MC3T3), and lactate dehydrogenase (LDH) activity test were carried out on examined surfaces. The prepared MWCNT/TiO2 coating is uniformly distributed by MWCNTs and agglomerated by TiO2 particles of size ranging from 0.1 to 3 μm. Raman spectroscopy confirmed the anatase structure of the TiO2 addition and showed typical peaks of the MWCNTs. The MWCNT/TiO2 coating had higher roughness, higher adhesion strength, and improved corrosion resistance compared to the MWCNT basic coating. The results of biological tests proved that physicochemical properties of the surface, such as high porosity and wettability of MWCNT/TiO2-coated material, would support cell adhesion, but toxic species could be released to the culture medium, thus resulting in a decrease in proliferation.
1 Introduction
Among nanomaterials, carbon allotropes of carbon nanotubes (CNTs) have gained significant attention owing to their unique properties: chemical inertness, exceptionally high mechanical strength, significant electrical conductivity, and often content-related optical properties. Therefore, construction and functional materials containing CNTs have been developed in various fields of interest. The last reviews on such materials have shown the great potential of CNTs, in medicine as reinforced polymer-based composites,1 in tissue engineering,2 and as optical contrast agents for cell imaging,3 in electronics for the construction of CNT-based nanogenerators,4 supercapacitors, and high-performance batteries,5 in industry for the development of construction reinforced polymers6 and reinforcement of cement concrete,7,8 and in chemistry for adsorptive removal of metal ions,9 and as catalysts in hydrogenation processes.10CNTs have seldom been proposed as coating components and exceptionally as single layers. Recently, such implementations have resulted in abrasion-resistant, photothermal, and superhydrophobic anti-icing coatings,11–14 anticorrosion and mechanically resistant coatings,15–18 antifouling coating,19 and coatings designed especially for heat exchangers and microwaves.20,21
CNT-based coatings deposited on titanium and its alloys, including NiTi, are infrequent and highly diversified. The carboxylic multi-wall carbon nanotube (MWCNT) coating on Ti alloy with CNT content from 0.05 to 0.2 wt% was designed to decrease friction and wear rates.14 The most popular ones are hydroxyapatite (HAp) coatings reinforced with CNTs in amounts of up to 2 wt%22 or 0.01 to 0.1 wt%,23 which are expected to increase hardness and adhesion strength. The HAp–Ti coatings doped with 1 wt% of MWCNTs on NiTi alloy24 and (Ce, Sr)HAp–agar–chitosan–MWCNTs (an amount unknown) on Ti25 were proposed for their better hardness, adhesion, and biological behavior. The tantalum oxide and CNTs were obtained by applying the sol–gel method to increase corrosion resistance, adhesion, and bioactivity.26,27 MWCNTs deposited on Ti in amounts of 5, 10, and 20 μg cm−2 were planned to enhance osseointegration.28 All these coatings were specially designed and investigated as potential candidates for surface modifications of titanium implants. Moreover, the self-lubricating coatings Al3Ti–3CNTs–3Cu–7SiC were produced by laser melting for light material processing.29 The CNT–polysiloxane coating was proposed as chemically resistant, durable, and highly hydrophobic,30 and epoxy resin vapor-deposited with CNT coatings for aircraft applications31 was developed.
The coatings are prepared by employing different techniques. They include mostly plasma spraying,32–34 electrophoretic deposition method (EPD),35–37 laser cladding,38 and electrostatic spraying.39 However, electrophoretic deposition (EPD) is also widely used to prepare CNT coatings40,41 because it enables the manipulation of the CNTs to deposit on surfaces with variable shapes, including flat or balk, gives control over coating parameters, is low-cost and time-saving.42
The addition of CNTs to the coatings deposited on titanium and its alloys can improve several properties important for implants. However, CNTs can form agglomerates because of the significant van der Waals chemical force, which adversely affects the properties of the coatings. The proposed techniques include covalent or non-covalent functionalization in a liquid rather than in a solid.43 Recently, modification of MWNCT surfaces by ZrO2 nanoparticles was shown to enhance the dispersion of MWCNTs and increase their adhesion to the epoxy matrix and its mechanical and fracture behavior.44 Among them are mechanical properties enhanced by CNTs present as the adhesion strength from 18.5 to 24.2 MPa at 1 wt%,22 from 17.2 MPa to 32.1 MPa at 1 wt%,24 from 21 to 29 MPa,25 and from 17.5 to 32.1 MPa37 and observed only qualitatively by the homogenous dispersion of CNTs in the matrix for CNT content in the range of 2–6 wt%.34 The hardness was elevated from 6.12 to 7.22 GPa at 2 wt%22 and from 72 HV to 405 HV at 1 wt%.24 Young's modulus increased from 115 to 135 MPa at 2 wt%,22 and also from 70 to 400 MPa.37 The deposition of MWNCTs on titanium caused about a 10% decrease under dry conditions, but even a 90% decrease in SBF (simulated body fluid),14 and a moderate change in wear volume from 14.14 × 106 to 10.6 × 106 mm3 was found after adding CNTs to TiO2 in the sprayed-made coating.32 A decreasing current density was noticed for the CNT–Hap coating from 0.54 to 0.05 μA cm−2 at 1 wt% CNTs.22 On the contrary, after adding MWCNTs to HAp–Ta2O coating, corrosion current density increased from 0.011 to 0.021 μA cm−2.27 The contact angle changed from 40° for Ti to 31° for Ti–Ta2O5.27
Biological behavior has often been reported to be positively affected by the addition of CNTs. The coating composed of tantalum oxide and CNTs modified with phosphonic acid26,27 improved bioactivity, and the titanium substrate electrochemically anodized and coated with CNTs increased the proliferation of MC3T3 cells and induced the formation of hydroxyapatite, making the surface proper for application in dental implants.45 The HAp–Ti–MWCNT composite coating was claimed to improve cellular proliferation and growth on the surface of NiTi alloy.37 For Ti and its alloys as substrates, in hydroxyapatite coatings, the addition of CNTs increased cell viability.22 In more complex carrageenan–chitosan–(Ce, Sr)HAp coatings, the MWCNTs also positively affected cell adsorption in in vivo tests,25 for collagen–CNTs, enhanced cell proliferation was observed,28 and for HAp–SCNTs, improved bioactivity determined by MTT and ALP essays, cell morphology and proliferation appeared.23
The basic mechanical properties of the multi-wall carbon nanotube coating with titania (MWCNT/TiO2), which is the object of this research, were examined and discussed in our previous work.36 Herein, the presented results include adhesion strength, corrosion resistance, and biological behavior, which together strongly justify the potential application of the developed coating for implantology, particularly endoprosthesis and dental implants. The literature shows that a mixture of TiO2 polymorphs at a concentration of 80% in anatase and 20% in rutile is the most effective in biomedical applications.46,47 Thus, TiO2 in crystalline anatase form is more active than TiO2 in rutile form, and at the same time, it is more effective for antimicrobial purposes.47 However, the anatase TiO2 polymorph shows worse corrosion resistance48 and cytotoxicity properties47 compared to the rutile form. In this study, we used the anatase; it has a large Young's modulus of 177.24 GPa49 and a small share modulus of 42.69 GPa,49 which are properties demanded by coatings intended for the endoprosthesis.
2 Experimental
2.1 Preparation of the substrate surface
The round-shaped specimens of 20 mm diameter and 4 mm thick were prepared from Ti13Nb13Zr alloy (Xi'an SATE Metal Materials Development Co., Ltd., Xi'an, China) of the following composition: 13.18 wt% Nb, 13.49 wt% Zr, 0.085 wt% Fe, 0.035 wt% C, 0.004 wt% H, 0.078 wt% O, <0.001 wt% S, 0.055 wt% Hf and remaining Ti. The surface preparation was further described in ref. 35 and included surface grounding with SiC paper of up to #800 grit; cleaning in acetone (Chempur, Piekary Śląskie, Poland) for 2 min; distilled water for 2 min; etching in 5% solution of hydrofluoric acid (Chempur, Piekary Śląskie, Poland); and rinsing in distilled water. This prepared substrate was assigned an MR value (native material).2.2 Preparation of CNT coatings
The MWCNT (–COOH modified, 3D-Nano, Krakow, Poland) coatings were prepared using EPD with parameters and bath composition, as shown in Table 1.The MWCNT EPD bath was composed of 0.25 wt% of MWCNTs suspended in distilled water. Before the deposition process, the suspension was ultrasonically dispersed for 1 h in an ultrasonic bath (MKD-8, MKD Ultrasonic, Warsaw, Poland), with power and frequency of 300 W and 25 kHz, respectively. The MWCNT/TiO2 coating was prepared in a two-stage process. First, the MWCNT layer was formed by EPD in the bath of the composition described above. Then, the second, TiO2 (3D-Nano, Krakow, Poland) layer was created by EPD in the bath comprising 0.30 wt% of TiO2, isopropyl alcohol as a solvent, and 1 wt% of polysorbate 20 (Tween 20, Sigma-Aldrich, Poznan, Poland). The suspension was then ultrasonically dispersed for 6 h, and the power and frequency were set the same as those observed during the dispersion of the MWCNT suspension.
The EPD for the MWCNT coating was conducted with Ti13Nb13Zr as a positive electrode and stainless steel as a negative electrode, while for the MWCNT/TiO2 coating, the electrodes were converted. The distance between the electrodes was about 0.5 cm.
2.3 Chemical composition and topography
The surface topography was evaluated using an atomic force microscope (AFM NaniteAFM, Nanosurf, Bracknell, Great Britain) in non-contact mode at 20 mN force. The average roughness index Sa values were estimated based on 512 lines made in the area of 80.4 × 80.4 μm.A high-resolution scanning electron microscope (SEM JEOL JSM-7800F, Tokyo, Japan) with an LED detector was used at a 5 kV acceleration voltage to observe the surface morphology.
An X-ray energy dispersive spectrometer (EDS) (Octane Elite 25, EDAX Ametek, Berwyn, PA, USA) was used to evaluate the chemical composition of the MWCNT/TiO2 coating.
2.4 Chemical structure and crystallography
The measurements were carried out using a Raman microscope (Renishaw InVia Plc., Wotton-under-Edge, UK) equipped with an EMCCD detector (Andor Technology Ltd., Oxford Instruments, Belfast, UK) and the objective lens set at 20×. The wavelength of the laser radiation during Raman spectroscopy tests was 532 nm; the measurement time, the measurement counts at significant points, and the laser radiation power of the MWCNT sample were 1 s, 5, and ca. 0.2 mW, respectively, and those of the MWCNT/TiO2 coating were 0.5 s, 10, and ca. 1 mW, respectively. Raman spectra measurements were prepared as maps consisting of the mean values of 100 points and the standard deviation of the signal sample. The collected Raman spectra were processed in WiRE 5.5 software and then averaged using CasaXPS software.2.5 Adhesion determination
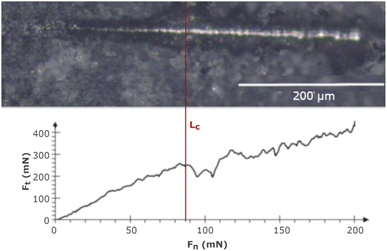
Nano-scratch tests were carried out using the NanoTest™ Vantage (Micro Materials, Wrexham, Great Britain) in increasing load mode from 0 to 200 mN at a distance of 500 μm, with a loading rate of 1.3 mN s−1. The adhesion strength was evaluated based on the critical load (Lc), which was determined by the abrupt change in critical friction (Ft) in the Ft to critical force (Fn) relation graph. In the end, the scratches were investigated using a light microscope (BX51, OLYMPUS, Tokyo, Japan), and the results were shown as mean ± SD (n = 5).2.6 Corrosion behavior
Corrosion tests were carried out using a potentiostat (Atlas 0531, Atlas Sollich, Gdańsk, Poland), with the AtlasCorr05 software, by calculating the corrosion potential (Ecorr) and corrosion current density (jcorr) based on Tafel extrapolation. The sample served as the working electrode, a platinum rod as the counter electrode, and a saturated calomel electrode as the reference electrode, immersed in Ringer's solution (composition: NaCl, 8.6; CaCl2, 0.33; KCl, 0.30 g L−1) at a temperature of 37 °C. The open circuit potential (OCP) was stabilized for 1 h, and potentiodynamic measurements were performed from −1.0 V to 1.0 V at a scan rate of 1 mV s−1.2.7 Wettability
The water contact angle (CA) was evaluated using a goniometer (Contact Angle Goniometer, Zeiss, Oberkochen, Germany) with the pendant drop mode. Wettability was measured for 10 s after the drop fell down the surface, and the CA result for each surface, shown as a mean ± SD (n = 3), was red after 5 s.2.8 Biological characterization
Cytotoxicity studies were conducted using a human dermal fibroblast (HDF, Biokom, Poland) cell line and mouse osteoblast precursors (MC3T3, Sigma-Aldrich, Germany). The HDF cells were grown in DMEM-LG (Dulbecco's Modified Eagle's Medium, Low Glucose) and MC3T3 in EMEM (Eagle's Minimum Essential Medium), both supplemented with 10% Fetal Bovine Serum (FBS) according to ref. 50. Before the experiment, the approximately 1 × 104 cells in culture media of 5 μL were seeded on the tested materials, different specimens in separate wells of a 12-well plate, and left for 3 h for adhesion. Then, the culture medium was added and incubated for 24 h and 72 h in a direct test. In an indirect test, the examined specimens were immersed in culture media for 72 h, and then as prepared suspension was used to HDF and MC3T3 cell culture seeded 24 h before in a 12-well plate. Cell viability was assessed based on an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich, Germany) assay, which showed cell ability to reduce MTT. The absorbance of the reduced formazan was measured at 570 nm using a Synergy HT Multi-detection reader (BioTek Instruments, Winooski, VT, USA).To check the integrity of the cell membranes, a lactate dehydrogenase (LDH) activity test was performed. The decrease in NADH (nicotinamide adenine dinucleotide, reduced disodium salt) (Sigma-Aldrich, Germany), indicating an increase in the number of damaged cells in the culture medium, was measured after 24 h and 72 h. To 150 μL of culture media, 25 μL of NADH (2.5 mg mL−1) and 25 μL of sodium pyruvate (2.5 mg mL−1) (Sigma-Aldrich, Germany) were added. The LDH activity was assessed spectrophotometrically (Synergy HT Multi-detection Reader, BioTek Instruments, Winooski, VT, USA) by measuring absorbance at 340 nm. The result for each sample was shown as a percentage of positive control samples treated with 1% Triton X-100 and labeled as 100% damaged cells.
2.9 Statistical data
The experimental values were provided as mean ± standard deviation (SD), and the statistical significance of differences between each specimen was evaluated utilizing Origin 8 by one-way analysis of variance (ANOVA), as depicted in figures (*).3 Results and discussion
3.1 Chemical composition and topography
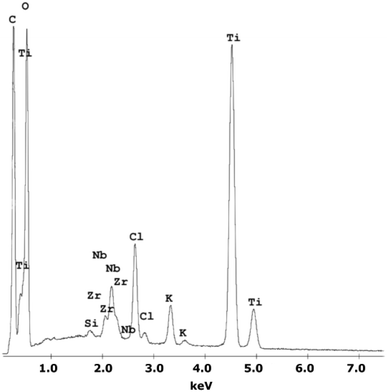
A chemical EDS-based analysis of the MWCNT/TiO2 coating, shown in Fig. 1, was conducted to detect the elements present in the coating. Titanium, zirconium, and niobium are the elements that originate from the substrate material, and carbon, titanium, and oxygen originate from the coating. Additionally, some elements, Cl and K, are impurities that appear in the deposition process. The presence of high peaks of carbon and titanium proves the deposition of the MWCNT/TiO2 layer.Table 2 shows the surface roughness Sa values for all examined surfaces estimated with an experimental error of less than 0.05 μm.
The roughness of the MWCNT coating increased more than 5.5-fold with the addition of titania nanoparticles. A similar effect was observed in our previous work.36 This is caused by the small TiO2 nanoparticles agglomerating on the MWCNT surface, as observed in Fig. 2, showing the SEM topography of the MWCNT and MWCNT/TiO2 coatings.
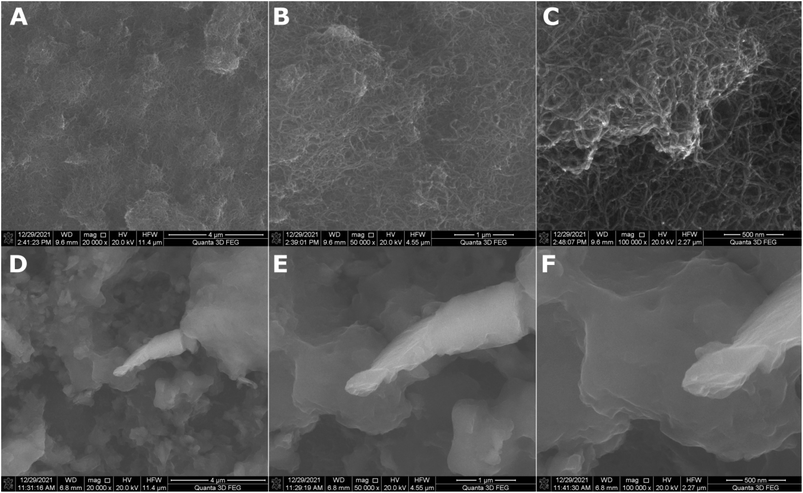 | ||
| Fig. 2 SEM surface topography of the MWCNT coating (A–C) and the MWCNT/TiO2 coating (D–F) demonstrated in different resolutions. | ||
The MWCNT coating (Fig. 2A–C) is uniformly distributed. The difference in thickness in particular areas of the coatings was previously estimated at 0.55 μm.36 Fig. 2D–F demonstrates the SEM topography of the MWCNT/TiO2 coating. The MWCNT coating is transparent and thoroughly covered by titania agglomerates of different shapes and sizes ranging from 0.1 to 3 μm. In our previous study, the average TiO2 aggregate surface area was approximately 1.5 μm2.36 The lamellar structure of each agglomerate can be observed, probably resulting from the coating's synthesis method, where coatings are built layer by layer during the EPD deposition process. The coating thickness measured earlier was 2.016 μm.36
3.2 Chemical structure and crystallography
The chemical characterization of the coatings was based on Raman spectroscopy. The Raman spectra for the MWCNT and the MWCNT/TiO2 coating are shown in Fig. 3. The values of the bands, with appropriate assignments for the characteristics of the bands, are listed in Tables 3 and 4, for MWCNT and MWCNT/TiO2 coatings, respectively.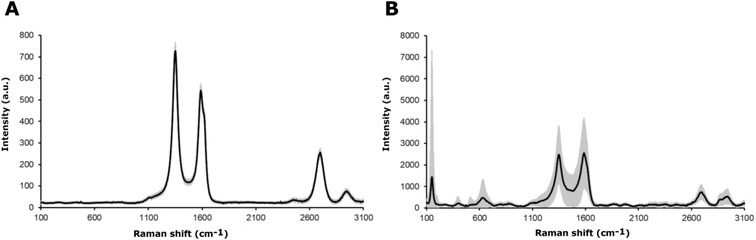 | ||
| Fig. 3 Raman spectra of (A) the MWCNT coating and (B) the MWCNT/TiO2 coating, where the black line presents an average Raman spectrum and the gray area is a standard deviation of the signal. | ||
| Eg(1) (cm−1) | Eg(2) (cm−1) | B1g(1) (cm−1) | A1g(1) + B1g(2) (cm−1) | Eg(3) (cm−1) | D band (cm−1) | G band (cm−1) | G′ band (2D band) (cm−1) | G′ band (2D band) (cm−1) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| MWCNT/TiO2 | 150 | 200 | 401 | 519 | 630 | 1348 | 1587 | 2687 | 2938 | — |
| MWCNT/TiO2 composite | 144 | 195.5 | 397 | 514 | 637 | 1356 | 1598.5 | Doesn't indicated | — | 51 |
| TiO2 (anatase) | 146 | 194 | 395 | 514 | 636 | — | — | — | — | 55 |
For the MWCNT coating, the values of each band (Fig. 3A) are similar to those shown in the literature, except for an extra Raman shift at 2942 cm−1 (second 2D (G′) band). The MWCNT coating exhibits three characteristic bands: disordered mode (D band), tangential mode (G band), and G′ band. The D mode, which appears for the MWCNTs coating at 1350 cm−1, represents the disorder in sp2-hybridized carbon atoms (graphene, which creates carbon nanotubes), the extent of sidewall defects or applied functionalization.51 The G band appears at 1588 cm −1 and the G′ band, called the first overtone of the D band, at 2699 cm−1, which are called the two modes representing graphitic materials. The G mode is related to the stretching of the C–C bond, and the G′ mode can be distinguished for non-defect sp2 carbon materials.51 The second shift for the G′ band is found at 2942 cm−1, as observed several times for single-wall carbon nanotubes (SWCNTs) and double-wall carbon nanotubes (DWCNTs), and assigned to the tubes inside CNTs, whose diameter is smaller than the outer ones or resonance with the incident and scattered light or presence of structural defects, which might be eliminated after thermal treatment.52 The D and G band intensity (ID/IG) ratios are used to identify the degree of structural defects or the level of functionalization of MWCNTs.51,53 The lowest ID/IG ratio occurs when more structural defects appear, which are the characteristics of the MWCNTs compared to those of SWCNTs and DWCNTs. The ID/IG ratio for the MWCNT coating is about 1.34. The literature reports an ID/IG ratio of 1.1437 (ref. 51) or 1.46 (ref. 54), according to our results.
The values of Raman shifts of the MWCNT/TiO2 coating (Fig. 3B) are close to those shown in the literature for the anatase phase of the titania, as illustrated in Table 4. Five optical phonon modes can be distinguished, and the assignment of the bands indicated is based on ref. 55 and 56. All of them represent five modes in the range of 150–630 cm−1 in accordance with Kamil et al. results,51 as shown in Table 4. Fig. 3B also demonstrates the mode characteristics of the MWCNT coating, which shifted and broadened and possessed a lower ID/IG ratio of 0.97. It is evident that the addition of titania to the MWCNT coating resulted in more structural defects than the bare MWCNT coating. The shift of the D and G bands is due to the interaction between the TiO2 nanoparticles and the MWCNT coatings,51,57 with the ID/IG ratio slightly higher than that shown by David et al. for the MWCNT/TiO2 film, which is 0.842.57 The archived values of the modes for both the MWCNTs and the MWCNT/TiO2 coating are slightly different from those reported in the literature, presumably owing to the effect of some impurities or a substrate.
3.3 Adhesion determination
Fig. 4 shows the adhesion nano-scratch test result for the MWCNT/TiO2 coating, with the indicated Lc value demonstrating the moment of the coating delamination, where Fn is the normal force and Ft is the friction force.Table 5 shows the estimated values of Lc and critical friction Fc for the examined coatings. The MWCNT/TiO2 coating demonstrates an almost 3.5-fold higher Lc value than the MWCNT coating, showing an improved adhesion strength. The adhesion strength measured by the shear strength test (according to ASTM standard F1044-99) for the MWCNT/TiO2/HAp coating demonstrated the adhesion strength for the coating EPD-deposited at 50 V for 1 min as 11.9 ± 3.3 MPa.58 The literature values of adhesion strength are then comparable to our results, within the limits of an experimental error. The adhesion can depend on mechanical properties, such as hardness, Young's modulus, and coating thickness, thus not only the coating composition determines the interface between a coating and a substrate. Therefore, the hardness of the tough TiO2 agglomerates on the surface of the MWCNT coating, together with its good fit to the substrate material, allows the coating to adhere better to the Ti13Nb13Zr substrate. The hardness of TiO2 can be taken as 1 GPa and that of the MWCNTs as 0.204 GPa.59,60 Owing to the higher hardness of TiO2 nanoparticles, the indenter tip encountered the titania particles on its way during the nano-scratch test and passed through the MWCNT/TiO2 coating at a higher Lc than in the MWCNT coating.
| Material | Critical load (Lc) (mN) | Critical friction (Fc) (mN) |
|---|---|---|
| MWCNTs | 25.3 ± 1.9 | 41.2 ± 2.1 |
| MWCNTs/TiO2 | 88.3 ± 1.8 | 248.5 ± 3.7 |
The adhesion strength also depends on coating thickness, coating structure, substrate architecture (evaluated using surface preparation), and method of synthesis.63 As regards the MWCNT/TiO2 coating thickness, it was assessed previously at 2.016 μm,36 while the thickness of the TiO2 coatings below 3 μm was reported to promote the loss of adhesion originating from the presence of titania agglomerates.63
There is one more parameter describing the adhesion strength of the coating, which is the ratio of hardness to reduced Young's modulus (H/Er), reported previously in ref. 36, which describes the coating endurance for substrate deflections under load. The results of the H/Er ratio for the MWCNT and MWCNT/TiO2 coatings were 0.005 and 0.013, respectively,36 which agree with the present results of the nano-scratch test, demonstrating the positive effect of the use of titania.
3.4 Corrosion behavior
The corrosion resistance test's results are shown in Table 6. The MR and MWCNT corrosion resistance parameters have been discussed previously.64The addition of TiO2 to MWCNT coatings lowers the jcorr and Ecorr of the MWCNT coating, thus improving its corrosion resistance. The same result was reported for CNT/TiO2 coating deposited on other substrates, such as Mg–Zn–Ca alloy,65 MgZn alloy,66 and HA–Ti–MWCNTs on TiNi alloy.37 Compared to the MR material's corrosion parameters, the application of MWCNT/TiO2 coating weakens the corrosion resistance of the substrate Ti13Nb13Zr alloy. For composite material comprising MWCNTs and TiO2, excellent corrosion resistance was observed,67 but the MWCNT coating had a porous structure that enhanced the transport of aggressive ions into the substrate and localized corrosion. Moreover, adding TiO2 into the coating decreases its porosity by filling pores and voids, thus increasing the corrosion resistance of the MWCNT coating, as observed in other reports.65,66
3.5 Wettability
Fig. 5 and Table 7 show the wettability measurement results for the examined surfaces. All of the surfaces are hydrophilic and both the MWCNTs and the MWCNT/TiO2 coatings demonstrate a contact angle between 50 and 60°, which is required for biomedical applications. The achieved results for the MWCNT coating are in accordance with our previous data shown in ref. 68. For the MWCNT/TiO2 materials, the literature demonstrates different results. For the MgZn/5TiO2–0.5MWCNT composite (composed of 5 wt% TiO2 and 0.5 wt% MWCNTs), the CA was reported as 87.0 ± 2.1°.66 Here, the difference might result in the effect of MgZn in the composite. | ||
| Fig. 5 Results of water contact angle measurements for MR (A), the MWCNT coating (B), the MWCNT/TiO2 coating (C), where the results are presented as mean ± SD (n = 3). | ||
| Material | CA left (°) | CA right (°) | Mean CA (°) |
|---|---|---|---|
| MR | 74.22 ± 4.96 | 73.09 ± 8.46 | 73.65 ± 6.58 |
| MWCNTs | 55.02 ± 1.02 | 55.84 ± 1.63 | 55.43 ± 1.29 |
| MWCNTs/TiO2 | 57.16 ± 2.01 | 59.46 ± 1.29 | 58.31 ± 1.60 |
Ho et al. reported the CA for the MWCNT/TiO2 membrane of 45.55 ± 1.13°,69 which is slightly lower than the CA of our MWCNT/TiO2 coating.
3.6 Biological characterization
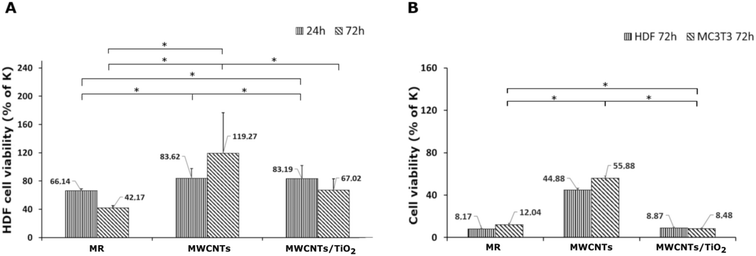
The results of the in vitro cytotoxicity studies are shown in Fig. 6 and 7. The viability of the HDF and MC3T3 cells was assayed using the MTT test. The highest cell viability for both HDF and MC3T3, and in both the direct and indirect tests, was observed for the MWCNT coating.The MWCNT coating in the direct test demonstrated a slight decrease in HDF cell viability after 24 h, followed by an increase from 83.62% to 119.27% of the control after 72 h of incubation (Fig. 6A). It can be underpinned by the topography of the MWCNT coating, which promotes cell adhesion on a porous structure during the first 24 h; then, it also supports cell proliferation. There are reports on the carboxylated MWCNT–chitosan material's ability to achieve a porous microstructure, improving cell adhesion.70 However, the HDF cell viability of the MWCNT/TiO2 coating in the direct test after 24 h of incubation was negligibly lower than that for the MWCNT coating, while after 72 h, the HDF cell viability for the MWCNT/TiO2 coating considerably decreased to 67.02% of the control but was still higher than that of the pure substrate material. Herein, we can conclude that the topography of the MWCNT/TiO2 coating is as desirable as that of the MWCNT coating for cell adhesion, while after 72 h, the TiO2 nanoparticles started to have a stronger toxic effect on HDF cells and decreased their proliferation rate. According to the literature, the cytotoxicity of MWCNT/TiO2 films is dose-dependent, but there are also reports about the non-toxic effect of MWCNTs decorated with TiO2 after 24 h and 72 h, where the HDF cell viability was similar to that of the control, and the MWCNT_TiO2 composite concentrations were 0.02 and 0.05%.57 However, the strong toxic influence of crystal phase TiO2 and a decrease in A549 and MCF-7 cell viability have also been reported in the literature.71
The HDF and MC3T3 cell viability in an indirect test (Fig. 6B) was surprisingly low, suggesting that the tested specimens may have a toxic effect on the cells. In the case of the MWCNT coating, HDF cell viability in an indirect test is the highest and reaches 44.88% of the control but is still too low for successful application in biomedicine. The MC3T3 cells in the indirect test exhibited a viability of 55.88% of the control, but it is still unsatisfactory. These observations can be explained by toxic substances (e.g. some impurities) released from the material surface or with nutrients and growth factor depletion through their adsorption on the coating surface. Undoubtedly, the processes underlying this phenomenon deserve further elucidation. According to the literature, a carboxylated MWCNT–chitosan composite sol–gel material with osteogenic growth peptide (OGP(10–14)) showed potential for use in bone regeneration.70 Additionally, MWCNT scaffolds showed higher MC3T3 cell viability contrary to poly(lactic-co-glycolic) acid (PLGA),72 and the MWCNT layer on collagen-coated titanium plates supported cell proliferation.73 The anodized TiO2 nanoparticles (NPs) on titanium EPD coated with CNTs showed higher MC3T3 proliferation than TiO2 NPs,74 whereas a similar MWCNT coating reported by Park et al. demonstrated approximately 25% lower cell proliferation after 5 days than pure titanium.75
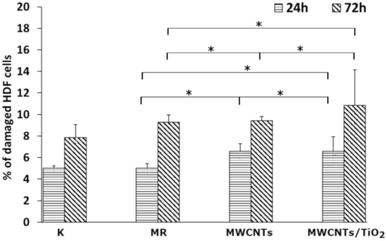
The LDH activity in the culture media (Fig. 7) was determined to check whether the MWCNT and MWCNT/TiO2 coatings (or substances released by them) have the ability to damage cell membranes and induce necrosis of HDF cells. After 24 h, both the MWCNT and the MWCNT/TiO2 coatings demonstrated a higher percentage of damaged HDF cells than the substrate material, but these values did not exceed 11% of the cells. However, after 72 h, the amount of damaged HDF cells was almost the same for the MWCNT coating (9.43%) and substrate material (9.29%), while the MWCNT/TiO2 coating achieved 10.86% of the damaged cells. The literature shows that LDH release from MWCNTs is dose- and time-dependent. The MWCNT dose of 40 μg mL−1 and the longer exposure time of HDF cells to carbon nanotubes induced cell death. However, we can see that the percentage of damaged cells exposed to MWCNTs is time dependent and much lower than that reported in the literature.76,77
4 Conclusions
In this study, employing the EPD method, we obtained a uniformly distributed MWCNT coating agglomerated with titania particles (of anatase structure, confirmed by Raman spectroscopy) deposited on the Ti13Nb13Zr substrate material to check its adhesion strength, corrosion resistance, wettability and biocompatibility with HDF and MC3T3 cells.The adhesion strength of the MWCNT/TiO2 coating was 3.5-fold improved compared to that of the MWCNT coating owing to the tough and good fitting of titania to the substrate material, which was confirmed by calculating the H/Er nanoindentation test parameter, reported previously in ref. 36.
The addition of titanium dioxide to the MWCNT coating resulted in more than 3-fold higher corrosion resistance than the basic MWCNT coating. TiO2 particles fill the inside of the MWCNT coating pores, making them denser. The MWCNT/TiO2 coating still exhibited worse corrosion resistance compared to the substrate material owing to its relatively high porosity, which is, however, the advantage of coatings intended for medical applications.
The results of biological tests confirmed that the improved mechanical and physicochemical properties of the surface, such as high porosity and wettability, in MWCNTs alone and MWCNT/TiO2-coated material support cell adhesion. The modified coatings may also release toxic substances into the culture medium, thus resulting in a decrease in proliferation.
The characterized coatings may be promising for biomedical applications, but they undoubtedly require further research and improvement, for example by reduction of the TiO2 content in the coating.
Author contributions
D. Rogala-Wielgus: conceptualization, methodology, visualization, investigation, formal analysis, resources, writing – original draft, writing – review & editing; B. Majkowska-Marzec: conceptualization, visualization, project administration; A. Zieliński: conceptualization, investigation, supervision, writing – review & editing; K. Roszek: investigation, resources, validation; M. Liszewska: investigation, resources, validation. All authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to show our sincere gratitude to Dr Grzegorz Gajowiec for his support in the evaluation of the results.References
- O. K. Abubakre, R. O. Medupin, I. B. Akintunde, O. T. Jimoh, A. S. Abdulkareem, R. A. Muriana, J. A. James, K. O. Ukoba, T.-C. Jen and K. O. Yoro, J. Sci.: Adv. Mater. Devices, 2023, 8, 100557 CAS.
- L. Bao, X. Cui, M. Mortimer, X. Wang, J. Wu and C. Chen, Nano Today, 2023, 49, 101784 CrossRef CAS.
- H. Ijaz, A. Mahmood, M. M. Abdel-Daim, R. M. Sarfraz, M. Zaman, N. Zafar, S. Alshehery, M. M. Salem-Bekhit, M. A. Ali, L. B. Eltayeb and Y. Benguerba, Inorg. Chem. Commun., 2023, 155, 111020 CrossRef CAS.
- N. Afsarimanesh, A. Nag, M. Eshrat e Alahi, S. Sarkar, S. Mukhopadhyay, G. S. Sabet and M. E. Altinsoy, Sens. Actuators, A, 2022, 344, 113743 CrossRef CAS.
- M. R. Zakaria, M. F. Omar, M. S. Z. Abidin, H. M. Akil and M. M. A. B. Abdullah, Composites, Part A, 2022, 154, 106756 CrossRef CAS.
- A. Ali, S. S. R. Koloor, A. H. Alshehri and A. Arockiarajan, J. Mater. Res. Technol., 2023, 24, 6495–6521 CrossRef CAS.
- B. Y. Jayakumari, E. N. Swaminathan and P. Partheeban, Constr. Build. Mater., 2023, 367, 130344 CrossRef.
- X. Zhang, Z. Mo, R. Arenal, W. Li and C. Wang, Appl. Surf. Sci., 2023, 609, 155208 CrossRef CAS.
- R. H. Krishna, M. N. Chandraprabha, K. Samrat, T. P. K. Murthy, C. Manjunatha and S. G. Kumar, Appl. Surf. Sci. Adv., 2023, 16, 100431 CrossRef.
- M. D. Yadav, H. M. Joshi, S. V. Sawant, K. Dasgupta, A. W. Patwardhan and J. B. Joshi, Chem. Eng. Sci., 2023, 272, 118586 CrossRef CAS.
- Y. Liu, Y. Shao, Y. Wang and J. Wang, Colloids Surf., A, 2022, 648, 129335 CrossRef CAS.
- T. Mao, C. Li, F. Mao, Z. Xue, G. Xu and A. Amirfazli, Diamond Relat. Mater., 2022, 129, 109370 CrossRef CAS.
- Z. Zhu, S. Kang, H. Chen, Q. Zhao, Z. Huo, P. Li, J. Kang and Y. Yin, Diamond Relat. Mater., 2022, 129, 109351 CrossRef CAS.
- H. Cao, P. Tian, J. Deng, Y. Li, C. Wang, S. Han and X. Zhao, J. Mech. Behav. Biomed. Mater., 2023, 142, 105825 CrossRef CAS PubMed.
- S. Singh and C. Srivastava, Electrochim. Acta, 2023, 439, 141639 CrossRef CAS.
- S. P. Vinodhini and J. R. Xavier, Mater. Sci. Eng., B, 2023, 295, 116621 CrossRef CAS.
- S. Yan, J. Li, J. Shi, X. Gao and K. Yu, Mater. Chem. Phys., 2023, 307, 128133 CrossRef CAS.
- X. Li, L. Li, W. Zhang, Y. Li, D. Ma, Q. Lei, S. Yu, J. Wang, Z. Wang and G. Wei, Colloids Surf., A, 2023, 670, 131548 CrossRef CAS.
- K. Shaikh, S. N. Kazi, M. N. M. Zubir, K. Wong, S. A. B. M. Yusoff, W. A. Khan, M. S. Alam, S. Abdullah and M. H. B. M. Shukri, Therm. Sci. Eng. Prog., 2023, 42, 101878 CrossRef CAS.
- H. Sun, S.-Q. Yi, N. Li, K.-K. Zou, J. Li, L. Xu, Y.-Y. Wang, D.-X. Yan and Z.-M. Li, J. Colloid Interface Sci., 2023, 649, 501–509 CrossRef CAS PubMed.
- H. Seok, C. Han, D. Lee and Y. Kim, Appl. Therm. Eng., 2023, 231, 120938 CrossRef CAS.
- D. Gopi, E. Shinyjoy, M. Sekar, M. Surendiran, L. Kavitha and T. S. S. Kumar, Corros. Sci., 2013, 73, 321–330 CrossRef CAS.
- X. Pei, Y. Zeng, R. He, Z. Li, L. Tian, J. Wang, Q. Wan, X. Li and H. Bao, Appl. Surf. Sci., 2014, 295, 71–80 CrossRef CAS.
- H. Maleki-Ghaleh and J. Khalil-Allafi, Surf. Coat. Technol., 2019, 363, 179–190 CrossRef CAS.
- M. Chen, H. Zhang, S. Shan, Y. Li, X. Li and D. Peng, J. King Saud Univ., Sci., 2020, 32, 1175–1181 CrossRef.
- A. Maho, S. Detriche, J. Delhalle and Z. Mekhalif, Mater. Sci. Eng., C, 2013, 33, 2686–2697 CrossRef CAS PubMed.
- A. Maho, S. Linden, C. Arnould, S. Detriche, J. Delhalle and Z. Mekhalif, J. Colloid Interface Sci., 2012, 371, 150–158 CrossRef CAS.
- J. E. Park, I.-S. Park, M. P. Neupane, T.-S. Bae and M.-H. Lee, Appl. Surf. Sci., 2014, 292, 828–836 CrossRef CAS.
- Z. Ye, J. Li, L. Liu, F. Ma, B. Zhao and X. Wang, Opt Laser. Technol., 2021, 139, 106957 CrossRef CAS.
- J. Marchewka, P. Jeleń, E. Długoń, M. Sitarz and M. Błażewicz, J. Mol. Struct., 2020, 1212, 128176 CrossRef CAS.
- F. Cheng, Y. Xu, J. Zhang, L. Wang, H. Zhang, Q. Wan, W. Li, L. Wang and Z. Lv, Surf. Coat. Technol., 2023, 457, 129296 CrossRef CAS.
- P. He, H. Wang, S. Chen, G. Ma, M. Liu, Z. Xing, Y. Wang, S. Ding, D. He and X. Chen, J. Alloys Compd., 2020, 819, 153009 CrossRef CAS.
- P. Daram, C. Banjongprasert, W. Thongsuwan and S. Jiansirisomboon, Surf. Coat. Technol., 2016, 306, 290–294 CrossRef CAS.
- G. M. T. Basha, A. Srikanth and B. Venkateshwarlu, Mater. Today: Proc., 2020, 20, 191–194 CrossRef.
- D. Rogala-Wielgus, B. Majkowska-Marzec, A. Zieliński and B. J. Jankiewicz, Appl. Sci., 2021, 11, 7862 CrossRef CAS.
- D. Rogala-Wielgus, B. Majkowska-Marzec, A. Zieliński, M. Bartmański and B. Bartosewicz, Materials, 2021, 14, 2905 CrossRef CAS PubMed.
- H. Maleki-Ghaleh and J. Khalil-Allafi, Mater. Corros., 2019, 70, 2128–2138 CrossRef CAS.
- Q. H. Li, M. M. Savalani, Q. M. Zhang and L. Huo, Surf. Coat. Technol., 2014, 239, 206–211 CrossRef CAS.
- E. J. T. Pialago, J. Yoo, X. Zheng, B. R. Kim, S. J. Hong, O. K. Kwon and C. W. Park, Int. J. Heat Mass Transfer, 2020, 147, 118958 CrossRef CAS.
- A. Wesełucha-Birczyńska, E. Stodolak-Zych, W. Piś, E. Długoń, A. Benko and M. Błażewicz, J. Mol. Struct., 2016, 1124, 61–70 CrossRef.
- A. Frączek-Szczypta, E. Dlugon, A. Wesełucha-Birczyńska, M. Nocun and M. Blazewicz, J. Mol. Struct., 2013, 1040, 238–245 CrossRef.
- M. Atiq Ur Rehman, Q. Chen, A. Braem, M. S. P. Shaffer and A. R. Boccaccini, Int. Mater. Rev., 2021, 66, 533–562 CrossRef CAS.
- C. Gao, M. Guo, Y. Liu, D. Zhang, F. Gao, L. Sun, J. Li, X. Chen, M. Terrones and Y. Wang, Carbon, 2023, 212, 118233 CrossRef.
- A. Rathi and S. I. Kundalwal, Polym. Compos., 2020, 41, 2491–2507 CrossRef CAS.
- Y. Bai, I. Park, T. Bae, K. Kim, F. Watari, M. Uo and M. Lee, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2011, 26, 867–871 CrossRef CAS.
- H. N. Pantaroto, J. M. Cordeiro, L. T. Pereira, A. B. de Almeida, F. H. Nociti Junior, E. C. Rangel, N. F. A. Neto, J. H. D. da Silva and V. A. R. Barão, Mater. Sci. Eng., C, 2021, 119, 111638 CrossRef CAS PubMed.
- S. Jafari, B. Mahyad, H. Hashemzadeh, S. Janfaza, T. Gholikhani and L. Tayebi, Int. J. Nanomed., 2020, 15, 3447–3470 CrossRef CAS PubMed.
- M. T. Acar, H. Kovacı and A. Çelik, Mater. Today Commun., 2022, 33, 104396 CrossRef CAS.
- X. Liu and J. Fu, Optik, 2020, 206, 164342 CrossRef CAS.
- M. Fandzloch, W. Bodylska, K. Roszek, K. Halubek-Gluchowska, A. Jaromin, Y. Gerasymchuk and A. Lukowiak, Nanoscale, 2022, 14, 5514–5528 RSC.
- A. M. Kamil, F. H. Hussein, A. F. Halbus and D. W. Bahnemann, Int. J. Photoenergy, 2014, 2014, 1–8 CrossRef.
- I. O. Maciel, M. A. Pimenta, M. Terrones, H. Terrones, J. Campos-Delgado and A. Jorio, Phys. Status Solidi B, 2008, 245, 2197–2200 CrossRef CAS.
- S. Costa, E. Borowiak-Palen, M. Kruszyńska, A. Bachmatiuk and R. J. Kaleńczuk, Mater. Sci., 2008, 26, 433–441 CAS.
- M. S. Tehrani, P. A. Azar, P. Ehsaninamin and S. M. Dehaghi, J. Appl. Environ. Biol. Sci., 2014, 4, 316–326 Search PubMed.
- L. Kernazhitsky, V. Shymanovska, T. Gavrilko, V. Naumov, L. Fedorenko, V. Kshnyakin and J. Baran, Ukr. J. Phys., 2014, 59, 246–253 CrossRef CAS.
- R. Palomino-Merino, P. Trejo-Garcia, O. Portillo-Moreno, S. Jiménez-Sandoval, S. A. Tomás, O. Zelaya-Angel, R. Lozada-Morales and V. M. Castaño, Opt. Mater., 2015, 46, 345–349 CrossRef CAS.
- M. E. David, R. M. Ion, R. M. Grigorescu, L. Iancu, A. M. Holban, F. Iordache, A. I. Nicoara, E. Alexandrescu, R. Somoghi, S. Teodorescu and A. I. Gheboianu, Nanomaterials, 2022, 12, 239 CrossRef CAS PubMed.
- O. Albayrak, O. El-Atwani and S. Altintas, Surf. Coat. Technol., 2008, 202, 2482–2487 CrossRef CAS.
- Å. K. Jämting, J. M. Bell, M. V. Swain, L. S. Wielunski and R. Clissold, Thin Solid Films, 1998, 332, 189–194 CrossRef.
- C. Zheng, W. Chen and X. Ye, Opt. Mater., 2012, 34, 1042–1047 CrossRef CAS.
- B. Majkowska-Marzec, P. Tęczar, M. Bartmański, B. Bartosewicz and B. J. Jankiewicz, Materials, 2020, 13, 3991 CrossRef CAS PubMed.
- M. Daavari, M. Atapour, M. Mohedano, R. Arrabal, E. Matykina and A. Taherizadeh, Surf. Interfaces, 2021, 22, 100850 CrossRef CAS.
- J. Ramier, N. Da Costa, C. J. G. Plummer, Y. Leterrier, J. A. E. Månson, R. Eckert and R. Gaudiana, Thin Solid Films, 2008, 516, 1913–1919 CrossRef CAS.
- D. Rogala-Wielgus, B. Majkowska-Marzec and A. Zieliński, Mater. Today Commun., 2023 Search PubMed , under review.
- H. R. Bakhsheshi-Rad, M. Abdellahi, E. Hamzah, M. Daroonparvar and M. Rafiei, RSC Adv., 2016, 6, 108498–108512 RSC.
- M. T. Amirzade-Iranaq, M. Omidi, H. R. Bakhsheshi-Rad, A. Saberi, S. Abazari, N. Teymouri, F. Naeimi, C. Sergi, A. F. Ismail, S. Sharif and F. Berto, Materials, 2023, 16, 1919 CrossRef CAS PubMed.
- E. J. Kim, K. h. Kim, J. Bak, K. Lee and E. Cho, RSC Adv., 2022, 12, 35943–35949 RSC.
- B. Majkowska-Marzec, D. Rogala-Wielgus, M. Bartmański, B. Bartosewicz and A. Zieliński, Coatings, 2019, 9, 643 CrossRef CAS.
- K. C. Ho, S. M. Raffi and Y. H. Teow, Int. J. Nanoelectron. Mater., 2022, 15, 207–222 Search PubMed.
- J. Zhong, J. Huang, L. Chen and J. Duan, RSC Adv., 2022, 12, 31663–31670 RSC.
- V. De Matteis, M. Cascione, V. Brunetti, C. C. Toma and R. Rinaldi, Toxicol. In Vitro, 2016, 37, 201–210 CrossRef CAS PubMed.
- G. Lalwani, A. Gopalan, M. D'Agati, J. S. Sankaran, S. Judex, Y.-X. Qin and B. Sitharaman, J. Biomed. Mater. Res., Part A, 2015, 103, 3212–3225 CrossRef CAS PubMed.
- M. Terada, S. Abe, T. Akasaka, M. Uo, Y. Kitagawa and F. Watari, Bio-Med. Mater. Eng., 2009, 19, 45–52 Search PubMed.
- J. E. Park, I. S. Park, T. S. Bae and M. H. Lee, Bioinorg. Chem. Appl., 2014, 2014, 1–7 CrossRef.
- J.-E. Park, Y.-S. Jang, T.-S. Bae and M.-H. Lee, Coatings, 2018, 8, 159 CrossRef.
- A. Patlolla, B. Patlolla and P. Tchounwou, Mol. Cell. Biochem., 2010, 338, 225–232 CrossRef CAS PubMed.
- C. L. Ursini, D. Cavallo, A. M. Fresegna, A. Ciervo, R. Maiello, G. Buresti, S. Casciardi, F. Tombolini, S. Bellucci and S. Iavicoli, Toxicol. In Vitro, 2012, 26, 831–840 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)