Open Access Article
Open Access ArticleA copper(II) complex containing pyridine-2-carbaldehyde and its direct binding onto ethylenediamine functionalized with Fe3O4@SiO2 nanoparticles for catalytic applications†
Masoud Karimia,
Ali Ramazani *ab,
Sami Sajjadifar
*ab,
Sami Sajjadifar c and
Sobhan Rezayati
c and
Sobhan Rezayati a
a
aDepartment of Chemistry, Faculty of Science, University of Zanjan, Zanjan 45371-38791, Iran. E-mail: aliramazani@znu.ac.ir; aliramazani@gmail.com; sobhan.rezayati@yahoo.com; sobhan.rezayati@znu.ac.ir; masoudkarimi67@gmail.com
bDepartment of Biotechnology, Research Institute of Modern Biological Techniques (RIMBT), University of Zanjan, Zanjan 45371-38791, Iran
cDepartment of Chemistry, Payame Noor University, PO BOX 19395-4697, Tehran, Iran. E-mail: ss.sajjadifar@gmail.com
First published on 4th October 2023
Abstract
In the present study, a copper(II) complex containing a pyridine-2-carbaldehyde ligand and its direct binding onto ethylenediamine functionalized with Fe3O4@SiO2 nanoparticles [Cu(II)-Schiff base-(CH2)3-SiO2@Fe3O4] as a heterogeneous magnetic nanocatalyst can be easily prepared using a multi-step method. Next, the structural and magnetic properties of the synthesized nanoparticles were identified using Fourier-transform infrared spectroscopy (FT-IR), inductively coupled plasma (ICP), vibrating-sample magnetometry (VSM), transmission electron microscopy (TEM), field-emission scanning electron microscopy (FE-SEM), thermogravimetric analysis (TGA), PXRD (Powder X-ray diffraction), Brunauer–Emmett–Teller (BET), and energy-dispersive X-ray spectrometry (EDX) techniques. TEM images reveal that the average particle size distribution was found to be in the range of 45–55 nm with spherical shape. The PXRD analysis indicated that the crystallite size was found to be 35.2 nm. The synthesized nanocatalyst exhibited a very good catalytic ability in the synthesis reaction of pyran derivatives and 2-benzylidenemalononitrile derivatives. Product 2-amino-7,7-dimethyl-4-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydrobenzo[b]pyran 4e was achieved in 97% yield with a TON of 129.3 and a TOF of 646.6 h−1 and product 2-(4-cyanobenzylidene)malononitrile 3j was achieved in 96% yield with a TON of 128 and a TOF of 984.6 h−1. In addition, the synthesized nanocatalyst was easily separated from the reaction mixture by a magnet and used 7 consecutive times without significant loss of catalytic activity. Also, leaching of copper metal from the synthesized nanocatalyst was very insignificant for this reaction.
Introduction
Green chemistry is now a widely discussed subject, attracting the attention of numerous companies and researchers alike. The goal of green chemistry is to discover eco-friendly reaction conditions as well as enhance reaction rates and the elimination of dangerous chemicals and solvents in the application of chemical products. Additionally, green chemistry decreases the harm caused by chemical substances and processes to human health and effectively eliminates environmental pollution by implementing sustainable prevention initiatives. The use of catalysts is one of the principles of green chemistry. The prevention of waste production in the synthetic process is made possible by the utilization of catalysts and non-toxic reagents. Heterogeneous catalysts have become increasingly appealing in the synthetic organic field due to their ability to simplify product isolation, facilitate simple preparation methods, provide economic advantages, allow for recycling, and contribute to green procedures. Recently, the catalytic activity of nanocatalysts in various reactions, including the formation of new carbon–heteroatom and carbon–carbon bonds, was investigated.1–3Fe3O4 nanoparticles have been extensively employed in the fields of catalysis.4,5 Recently, there has been a surge in interest in utilizing magnetically responsive materials, specifically Fe3O4 MNPs, which are combined with nano-structured transition metal heterogeneous catalysts.6 This approach has gained attention due to its environmental friendliness and the convenience it offers in terms of catalyst recyclability.7 These systems offer enhanced flexibility, allowing for improved contact between the catalyst and reactants. Consequently, the catalyst's activity is greatly increased. Fe3O4 magnetic nanoparticles (MNPs) possess a cubic inverse spinel configuration, wherein the Fe element exists in both the Fe2+ and Fe3+ cationic. Nonetheless, Fe3O4 MNPs, when synthesized, exhibit instability owing to their elevated surface energy, which tends to promote oxidation, decomposition and aggregation. To prevent these issues, a potential solution is to encase Fe3O4 NPs within a shell made of a different material. Silica functionalized magnetic nanoparticles have gained significant attention due to their numerous advantageous features, including ease of functionalization, lack of toxicity, straightforward synthesis, convenient separation from the reaction medium facilitated by an external magnet, and straightforward synthesis. Silica has become increasingly popular due to its attractive potential, making it one of the most durable and versatile surfaces. It offers numerous advantages including chemical inertness and biocompatibility, improved mechanical stability, thermal stability, optical transparency, and the ability to control chemical reactions spectroscopically.8 We were motivated to develop green and sustainable Fe3O4-based heterogeneous catalysts due to the intriguing characteristics of these special. Scientists can design highly active, selective and flexible nanocatalysts. All these benefits make industrial chemical reactions more resource efficient, enabling energy use and waste reduction, helping to offset the environmental impact of reliance on chemical processes.9,10
From the point of view of green chemistry, it is very important to design and economic chemical processes using heterogeneous catalysts for the preparation of chemical and pharmaceutical products through multicomponent reactions (MCRs).11–13 Multi-component reactions have significant advantages, such as high atom economy and bond formation efficiency, avoiding by-products, fast and simple execution, saving time and energy, and avoiding complex purification methods.14
Knoevenagel condensation is a nucleophilic addition reaction, in which a compound containing active hydrogen, such as malonates, which attacks a carbonyl group (aldehyde or ketone) and loses a water molecule to form the final product. Knoevenagel condensation is one of the most common methods used in organic chemistry for electron-less synthetic olefins, which occurs as a result of the reaction between active methylenes and carbonyl compounds.15 The α,β-unsaturated compounds which formed by Knoevenagel condensation, are one of the essential intermediates in producing chemicals, herbicides, carbohydrates, pharmaceuticals, and pesticides, dyes.16–19 Benzylidene malononitriles are regularly utilized as an intermediate molecule and a target molecule in organic synthesis. They have fascinated consideration due to special properties such as anti-fungal, anti-cancer, anti-bacterial, and anti-corrosive20–23 and used to probes of solvent friction.24
Substituted 2-amino-4H-pyran derivatives in which the Knoevenagel condensation are prepared through a three-component one-pot reaction including malononitrile, aldehyde and enolizable acids (such as dimedone, barbituric acid, α,β-naphthols, 2-hydroxy-1,4-naphthoquinone-4-hydroxycoumarin).25–27 Recently, the synthesis of this group of compounds has been revised due to the increase in their use in the field of medicinal chemistry and materials science.28 In the past, various synthetic methods for making this class of compounds, including conventional thermal reactions,29 microwave-radiation reactions30 in the presence of organic solvents and various catalysts and such reactions have been widely expanded. Substituted 2-amino-4H-pyran derivatives are synthesized in the presence of various catalysts including theophylline,31 uric acid,32 TiCl4,33 Fe3O4@SiO2@NH2,34 Fe3O4/HNTs,35 Fe3O4@SiO2-imine/phenoxy-Cu(II) core–shell MNPs,36 lipase,37 CuFe2O4@starch,38 ZnO,39 nano CeO2,40 Fe3O4@SiO2@(CH2)3-Urea,41 Fe3O4@SiO2@GPTMS@guanidine,42 Fe3O4@SiO2@(CH2)3/EDA,43 NH2@SiO2@Fe3O4,44 Au/NiAlTi LDH,45 core/shell Fe3O4@GA@isinglass.46 However, the many disadvantages of using these catalysts, such as low efficiency, long reaction time, corrosiveness, environmental pollution, etc., made chemists think of using novel catalysts to perform this reaction.
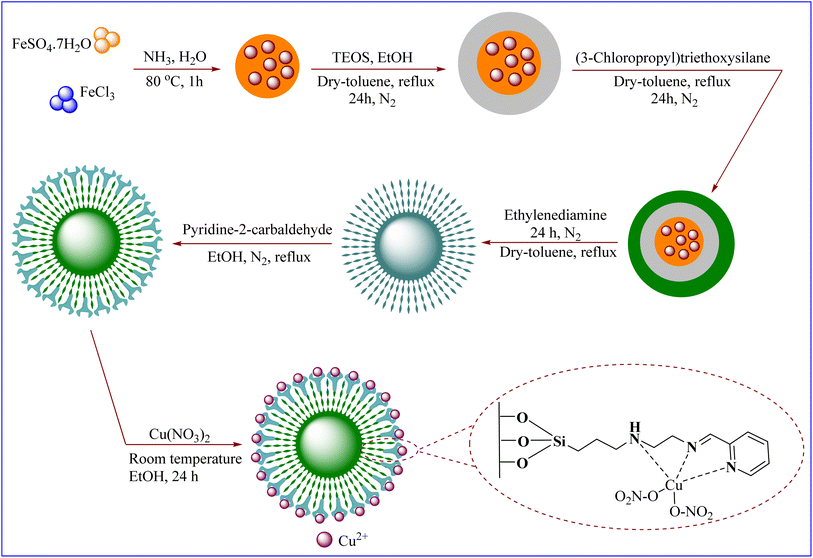
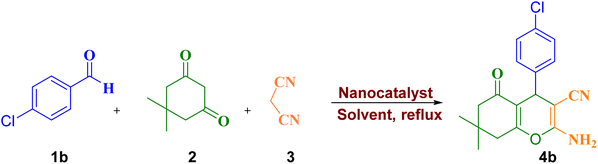
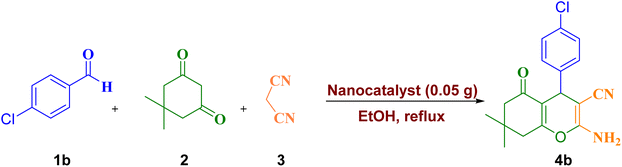
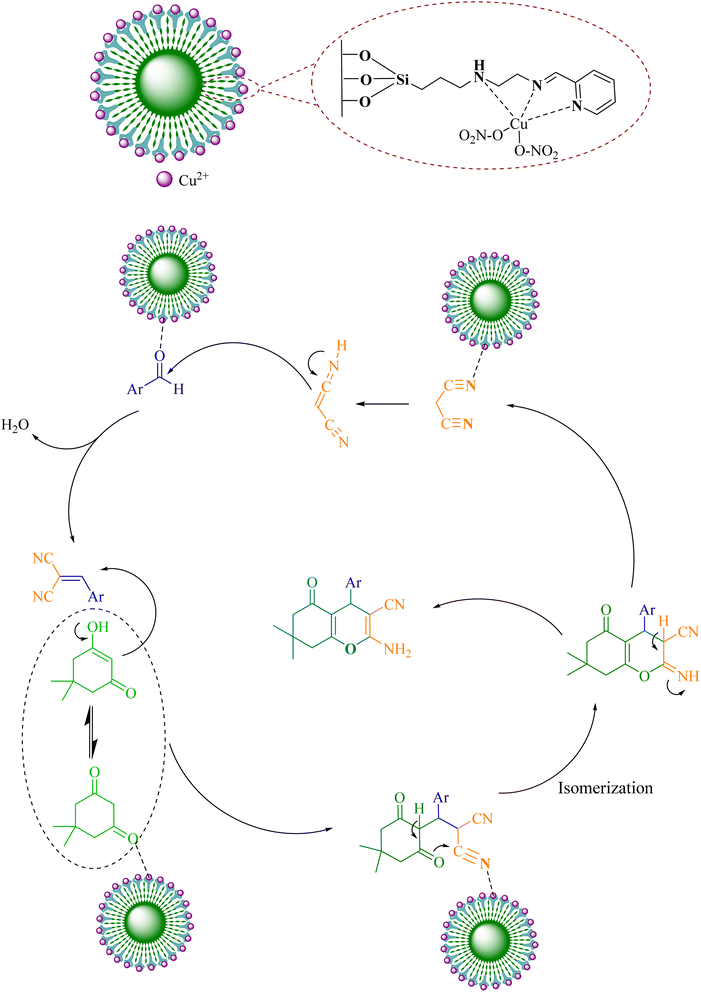
Considering the importance and benefits of MCRs with catalysts for the synthesis of heterocyclic compounds,47 in this study, we synthesized for the first time the copper(II) complex containing pyridine-2-carbaldehyde ligand and its direct binding onto ethylenediamine functionalized with Fe3O4@SiO2 nanoparticles [Cu(II)-Schiff base-(CH2)3-SiO2@Fe3O4] as a recyclable catalysts by a facile and multi-step method. Then, the catalytic activity of this nanocatalyst has been investigated for the synthesis of 2-amino-4H-pyrans and 2-benzylidenemalononitriles (Scheme 1).
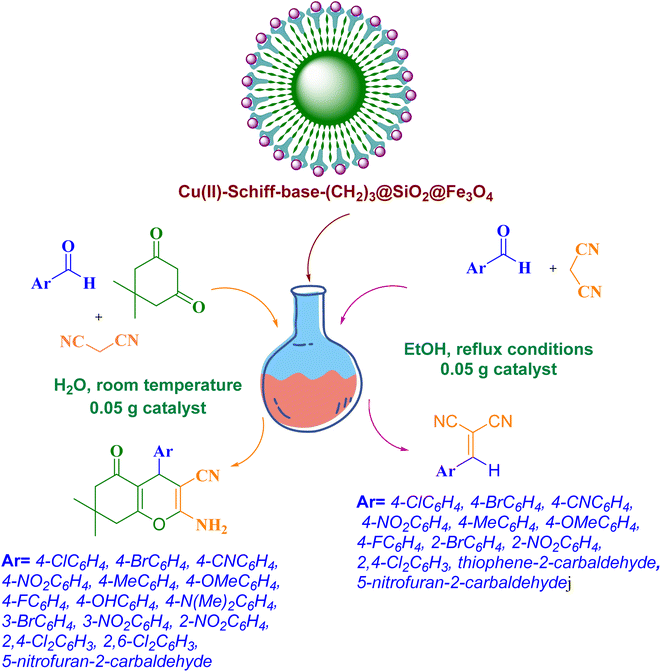 | ||
| Scheme 1 Schematic representation of the 2-amino-4H-pyrans and 2-benzylidenemalononitrile derivatives catalyzed by Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 | ||
Experimental
Tools and chemicals
All reagents and solvents such as ethanol (C2H5OH), dry methanol (CH3OH), acetone [(CH3)2CO], toluene (C6H6CH3), aqueous ammonia 25% (NH4OH), iron(II) sulfate (FeSO4·7H2O), copper(II) nitrate trihydrate [Cu(NO3)2·3H2O], iron(III) chloride (FeCl3), tetraethyl orthosilicate (TEOS, SiC8H20O4), (3-chloropropyl)silanetriol (C3H9ClO3Si), 2-picolylaldehyde (NC5H4CHO), ethylenediamine [C2H4(NH2)2], various aromatic aldehydes, malononitrile (C3H2N2), and dimedone (C8H12O2) were purchased from the Fluka (Switzerland) or Merck Company. Infrared spectra were taken with a PerkinElmer 597 devices. 1H-NMR and 13C-NMR spectra were taken with Bruker 250 MHz and FT NMR-62.5 MHz devices in pure solvents (CDCl3 and DMSO-d6). Chemical shifts with scale δ are expressed in parts per million (ppm) and coupling constant with J in Hz. The splittings of 1H-NMR peaks are characterized by singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), broad (br). Also, Electrothermal 9100 device was used to determine the melting point of the synthesized products. VSM analysis (Taban, Tehran, Iran) was used to determine the magnetic properties of the synthesized catalysts. In order to investigate the crystal structure of the prepared samples, an XRD machine (advanced diffractometer X'Pert-PRO works with 40 kV and 40 mA at room temperature) was used. In order to investigate the structure and determine the dimensions of nanoparticles prepared in this method, transmission electron microscope (TEM) model (Zeiss EM10C-80 KV) and scanning electron microscope (SEM) model (VEGA, TESCAN Czech Republic) were used. EDAX detector and X-ray map were used to determine the elemental analysis of the synthesized catalysts. Thermal gravimetric analysis (TGA) was performed by Setaram SETSYS 16/18 device from 25 to 800 °C with an increase of 10 °C per min.The preparation of Cu(II)-Schiff base-(CH2)3-SiO2@Fe3O4
Cu(II)-Schiff base-(CH2)3-SiO2@Fe3O4 was effectively synthesized to the following steps (Scheme 2).Preparation of Fe3O4 NPs
In this work for synthesis of Fe3O4, co-precipitation method was investigated. In summary, in 120 mL of de-ionized water, mixture of FeSO4·7H2O (0.9 g)/FeCl3 (0.97 g) at 80 °C was prepared. Following this, a solution comprising NH4OH 1.5 M (120 mL) was gradually added to the mixture under N2 gas. Consequently, the mixture rapidly transitioned from a yellowish hue to a black color. The synthesized Fe3O4 was detached with an external magnet. Black Fe3O4 NPs were washed with deionized water (4 × 30 mL) to pH 7.5 and once with acetone (1 × 30 mL) for 5 times. Finally, the obtained black particles were placed in an oven at 40 °C for 24 h.48Preparation of SiO2@Fe3O4
In a 250 mL round-bottom flask, one gram of magnetite nanoparticles (Fe3O4) was added in 100 mL of a mixture of ethanol and deionized water (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) for 30 min in an ultrasonic to complete its dispersion. Then, TEOS (2 mL) and 25% ammonia (2 mL) were added to the mixture. Then it was stirred with a mechanical stirrer under nitrogen gas at room temperature for 12 h. The obtained SiO2@Fe3O4 nanoparticles were washed five times with deionized water (4 × 30 mL) and finally once with ethanol (1 × 30 mL), and in each washing time, the nanoparticles were deposited using a magnet. The obtained nanoparticle was placed in an oven at a temperature of 40 °C for 24 h.48
80) for 30 min in an ultrasonic to complete its dispersion. Then, TEOS (2 mL) and 25% ammonia (2 mL) were added to the mixture. Then it was stirred with a mechanical stirrer under nitrogen gas at room temperature for 12 h. The obtained SiO2@Fe3O4 nanoparticles were washed five times with deionized water (4 × 30 mL) and finally once with ethanol (1 × 30 mL), and in each washing time, the nanoparticles were deposited using a magnet. The obtained nanoparticle was placed in an oven at a temperature of 40 °C for 24 h.48
Preparation of Cl(CH2)3@SiO2@Fe3O4
After dispersed of SiO2@Fe3O4 NPs (1 g) in 100 mL of dry toluene, 10 mmol (3-chloropropyl)triethoxysilane was added it, and refluxed under N2 for 24 h. The Cl(CH2)3@SiO2@Fe3O4 was separated by an external magnetic field and washed with dry toluene and diethyl ether each twice times (2 × 30 mL). Cl(CH2)3@SiO2@Fe3O4 dried overnight at room temperature.48Preparation of NH2(CH2)2NH-(CH2)3@SiO2@Fe3O4
After dispersed of 1 g of Cl(CH2)3@SiO2@Fe3O4 NPs in 100 mL of EtOH, the 0.72 g ethylenediamine slowly was added to it under N2 atmosphere under reflux conditions for 24 h. The NPs was separated by an external magnetic field and washed with dry toluene (2 × 30 mL) and dried overnight at room temperature.Preparation of Schiff-base-(CH2)3@SiO2@Fe3O4
After dispersed of 1 g of NH2(CH2)2NH(CH2)3@SiO2@Fe3O4 NPs in 100 mL of dry ethanol, the 10 mmol pyridine-2-carbaldehyde was added to it under N2 atmosphere under reflux conditions for 24 h. The Schiff-base-(CH2)3@SiO2@Fe3O4 was separated by an external magnetic field and washed with dry toluene (2 × 30 mL) and dried overnight at room temperature.Preparation of Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4
To prepare Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4, in a 250 mL round-bottomed flask 1 g of Schiff-base-(CH2)3@SiO2@Fe3O4 was placed in an ultrasonic bath in 30 mL of ethanol for 30 min. Then, 2.5 mmol of copper nitrate solution in 10 mL of ethanol solvent was added to the reaction mixture and stirred for 48 h by a magnetic stirrer under reflux conditions. After finishing the reaction mixture was cooling in room temperature. The next steps, the contents were transferred to a beaker. The obtained Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 were washed three times (20 × 3 mL) with ethanol, and in each washing, the nanoparticles were deposited using a magnet and the supernatant solution was overflowed. In order to dry the obtained nanoparticles, it was set at room temperature for 24 h.General procedure for the synthesis of 2-amino-4H-pyran derivatives
For this reaction, a mixture of dimedone, aromatic aldehyde, malononitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mmol ratio), 0.05 g nanocatalyst, and 3 mL ethanol was added in a round bottom flask under reflux conditions. The progress of the reaction was evaluated by thin layer chromatography (TLC). After completing the reaction, the mixture was cooled, then nanocatalyst filtered separated by external magnetic field. The 2-amino-4H-pyran derivatives can be recrystallized by hot ethanol. The structures of compounds 4a–p were apparent from the FT-IR, 1H NMR and 13C NMR spectra.
1 mmol ratio), 0.05 g nanocatalyst, and 3 mL ethanol was added in a round bottom flask under reflux conditions. The progress of the reaction was evaluated by thin layer chromatography (TLC). After completing the reaction, the mixture was cooled, then nanocatalyst filtered separated by external magnetic field. The 2-amino-4H-pyran derivatives can be recrystallized by hot ethanol. The structures of compounds 4a–p were apparent from the FT-IR, 1H NMR and 13C NMR spectra.
General procedure for the synthesis of 2-benzylidene malononitriles
For this reaction, a mixture of 1 mmol of various aromatic aldehyde, 1 mmol of malononitrile, 3 mL distilled water, and 0.05 g of nanocatalyst was added to a round-bottomed balloon and was stirred at room temperature for suitable times. The progress of the reaction was evaluated by thin layer chromatography (TLC). After completing the reaction, the mixture was cooled, then nanocatalyst filtered separated by external magnetic field. The 2-benzylidene malononitrile derivatives can be recrystallized by hot ethanol. The structures of compounds 3a–m were apparent from the FT-IR, 1H NMR and 13C NMR spectra.Results and discussions
In this study, we have synthesized the Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 with a facile and multi-step method (Scheme 2). In this regard, silica-coated iron oxide was prepared via TEOS in the presence of iron oxide. Then, the surfaces of the synthesized magnetic nanocatalyst in the pervious step are immobilized with (3-chloropropyl)triethoxysilane and ethylenediamine, respectively to afford the NH2(CH2)2NH-(CH2)3@SiO2@Fe3O4. In the following, the synthesized magnetic nanocatalyst in the pervious step reacted with pyridine-2-carbaldehyde and treatment with copper nitrate to synthesis of final catalyst.The structure and the functional groups of (a) Fe3O4, (b) SiO2@Fe3O4, (c) Cl(CH2)3@SiO2@Fe3O4, (d) NH2(CH2)2NH-(CH2)3@SiO2@Fe3O4, (e) Schiff-base-(CH2)3@SiO2@Fe3O4, and (f) Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 were approved by the FT-IR spectrum (Fig. 1). By examining layer by layer and comparing with the new layer, we can prove the synthesis of the catalyst. Fe3O4 NPs is confirmed via Fe–O bond apparition around 615 cm−1 (Fig. 1a).48 In the next step, by coating SiO2 two broad bands related to the hydroxyl groups and Si–O–Si appeared (Fig. 1b). Symmetric and antisymmetric stretching vibrations of Si–O–Si appeared about 795 and 1084 cm−1, respectively.48 Absorptions peaks of C–H group of Cl(CH2)3@SiO2@Fe3O4 appeared about 2913 cm−1 (Fig. 1c). The absorptions peaks about 1619 and 3406 cm−1 that are belong to the bending vibration of N–H and stretching frequency of N–H, respectively1 (Fig. 1d).48 After adding pyridine-2-carbaldehyde absorptions peaks C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C belong to aromatic ring appear about in 1617 cm−1 and 1623 cm−1 (Fig. 1e). In Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4, the carbonyl peak shifts from 1636 cm−1 which indicates the complexation of Cu has occurred (Fig. 1f). The absorption peaks at about 1384 cm−1 belong to the bending vibration of NO2.
C belong to aromatic ring appear about in 1617 cm−1 and 1623 cm−1 (Fig. 1e). In Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4, the carbonyl peak shifts from 1636 cm−1 which indicates the complexation of Cu has occurred (Fig. 1f). The absorption peaks at about 1384 cm−1 belong to the bending vibration of NO2.
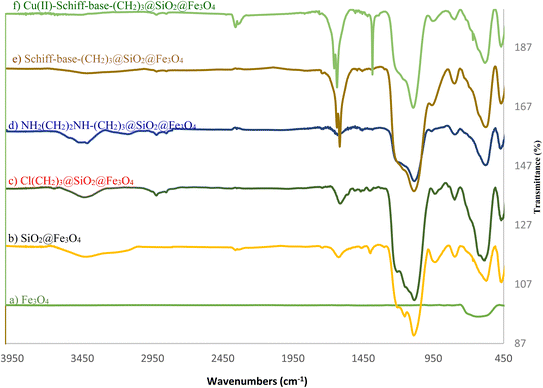 | ||
| Fig. 1 FT-IR spectra of (a) Fe3O4, (b) SiO2@Fe3O4, (c) Cl(CH2)3@SiO2@Fe3O4, (d) NH2(CH2)2NH-(CH2)3@SiO2@Fe3O4, (e) Schiff-base-(CH2)3@SiO2@Fe3O4, and (f) Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 | ||
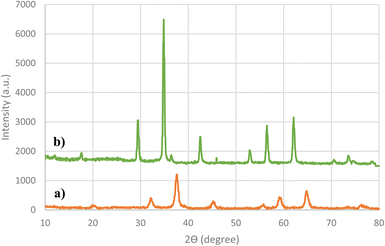
PXRD analysis was used to identify the crystallite size, inter-planer distance and Miller indices of the nanocatalyst. Fig. 2 indicated the PXRD pattern of the Fe3O4 and Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 were demonstrated in the 2θ at room temperature (range of 10–80°). Examining the X-ray diffraction pattern in the Fe3O4 shows seven index peaks at 2θ = 19.9, 32.4, 37.7, 44.9, 55.7, 59.7 and 65.1, which are respectively related to pages (1 1 1), (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1) and (4 4 0), which agrees with the spectrum of Fe3O4 (JCPDS 88-0866) and confirms its cubic structure.49 Moreover, examining the X-ray diffraction pattern in the Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 shows new two peaks at 2θ = 43.2 (2 0 0) and 74.2 (2 2 0) that agrees with the spectrum of copper (JCPDS, copper file no. 04-0836). Two new peaks confirmed that the nanoparticles successful synthesized. Additionally, we evaluated the crystallite size and inter-planer distance of the catalyst for per peak of intensity by applying the Scherrer equation [D = Kλ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)] and Bragg formula [dhkl = k/(2
θ)] and Bragg formula [dhkl = k/(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)], respectively (Table 1). The Table 1 shows that the average crystallite size was 35.2 nm and average inter-planer distance was 0.2097 nm.
θ)], respectively (Table 1). The Table 1 shows that the average crystallite size was 35.2 nm and average inter-planer distance was 0.2097 nm.
| Entry | 2θ | Peak width (FWHM) | Miller indices | Particle size (nm) | Inter-planer distance (nm) | ||
|---|---|---|---|---|---|---|---|
| h | k | l | |||||
| 1 | 18.42 | 0.3444 | 0 | 1 | 2 | 23.4 | 0.3686 |
| 2 | 30.16 | 0.2952 | 1 | 0 | 4 | 27.9 | 0.2703 |
| 3 | 35.51 | 0.3444 | 1 | 1 | 0 | 24.2 | 0.2519 |
| 4 | 43.25 | 0.2952 | 2 | 0 | 2 | 29.0 | 0.2080 |
| 5 | 46.65 | 0.1476 | 0 | 2 | 4 | 58.5 | 0.1842 |
| 6 | 53.59 | 0.1968 | 1 | 1 | 6 | 45.2 | 0.1696 |
| 7 | 57.17 | 0.3444 | 1 | 2 | 2 | 26.3 | 0.1601 |
| 8 | 62.75 | 0.2400 | 2 | 1 | 4 | 38.8 | 0.1487 |
| 9 | 74.23 | 0.2460 | 2 | 2 | 0 | 40.5 | 0.1259 |
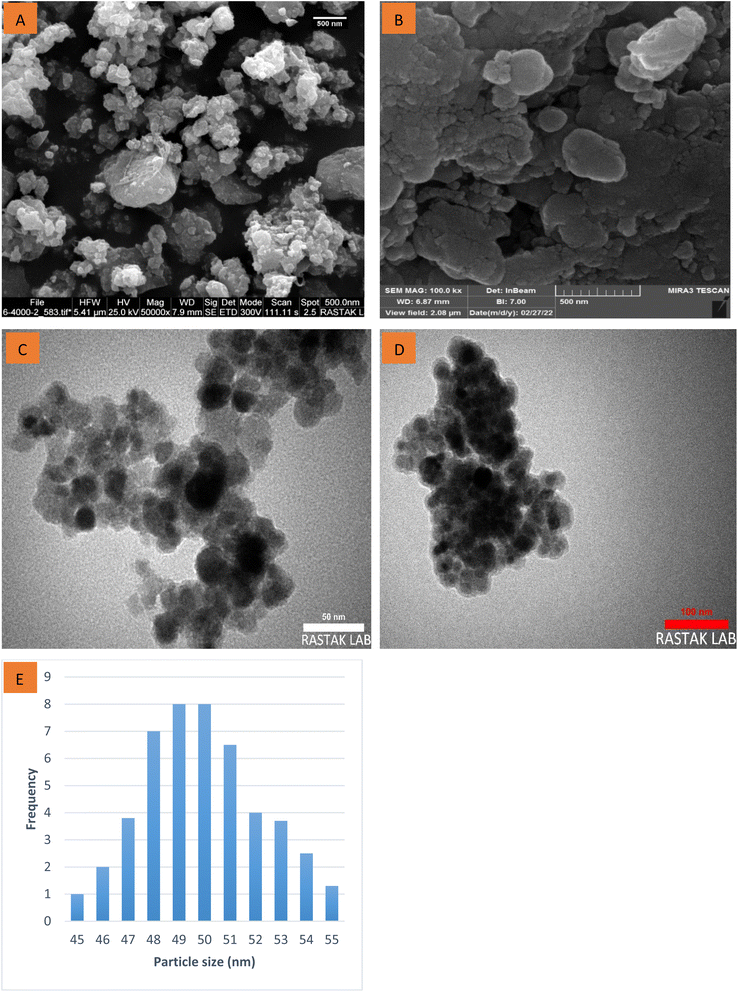
The major physical features and surface morphology of the Fe3O4 and as-prepared nanocatalyst were considered by FE-SEM images in various magnifications (Fig. 3A and B). The SEM images display that the Fe3O4 and as-prepared nanocatalyst have a spherical shape with nanoscale dimensions. After coating Fe3O4 with silica and various organic compounds, the spherical morphology of as-prepared nanocatalyst was intact. In another study, TEM images for Fe3O4 and as-prepared nanocatalyst were studied (Fig. 3C and D). The TEM analysis images confirm well and show the core–shell structure. TEM enlarged form clearly shows that silica has been successfully fixed (bright layer) on Fe3O4 magnetic nanoparticles (dark area). Moreover, the particle size distribution diagram for as-prepared nanocatalyst are almost 45–55 nm (Fig. 3E).
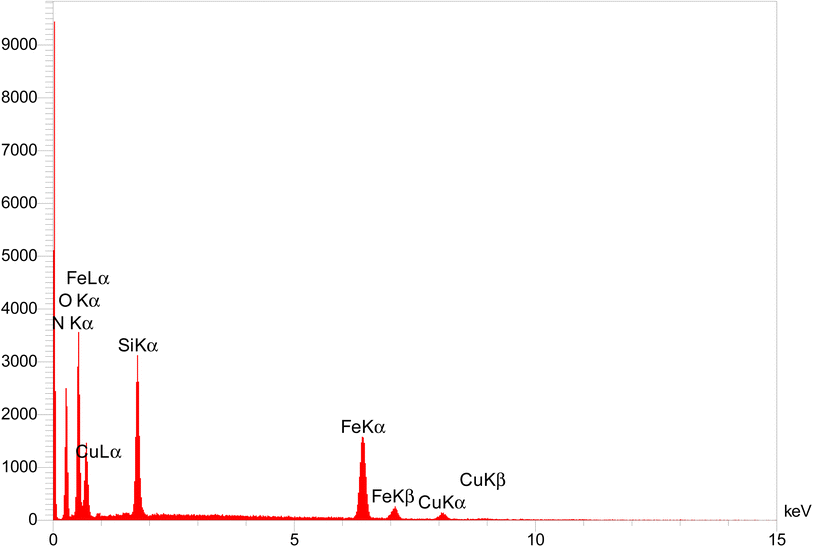
The elemental composition related to as-prepared nanocatalyst was examined by EDX analysis (Fig. 4). Hence, the presence of nanocatalyst components elements, including Fe, Cu, Si, O, C, and N and the mass percentages 14.04, 1.62, 15.40, 37.46, 29.40, and 2.08, respectively was proved as expected. This analysis show that the copper metal coated well on the surface. The results obtained from the ICP analysis show that the exact amount of copper in the structure is 1.92 w%.
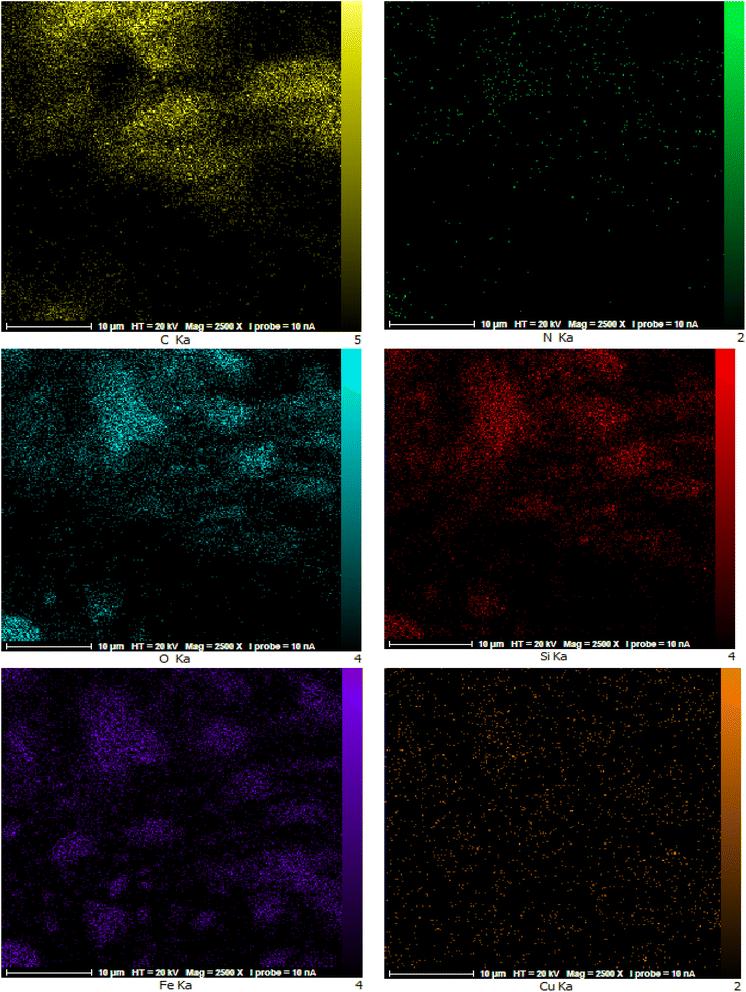
In the following, elemental mapping analysis was used for determine the elemental content of the surface of catalyst. The distribution of atoms (Fe, Cu, Si, O, C, and N elements) indicates the position of the components associated with them and, distribution of components in catalyst (Fig. 5). The mapping images obtained demonstrate the presence of a significant concentration of C, O, and Fe elements originating from the catalyst support. According to this analysis, these elements make up over 80.9% of the sample. Furthermore, the mapping images clearly demonstrate a remarkable distribution of C, Si, N, and Cu moieties across the Fe3O4 support. These photograms clearly show that the distribution patterns of these elements agree well. This indicates that the imine groups of the as-prepared nanocatalyst serve as donor nitrogen atoms to coordinate with Cu(II) ions.
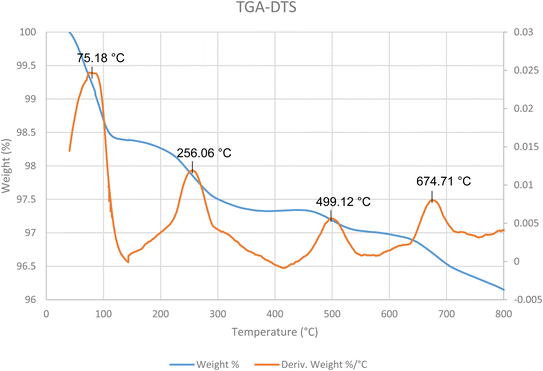
The thermal stability of as-prepared nanocatalyst was investigated by TGA-DTS curves by illustrate the nanocatalyst's weight loss and decomposition temperature (Fig. 6). As can be seen, three mass losses were confirmed for the nanocatalyst. The primary step is related to as mass-loss (1.8%) due to organic solvents or moisture at temperatures below 256 °C. The second mass-loss stage is occurred at temperatures below 500 °C due to the decomposition of organic compounds that coated on the surface. The third mass reduction occurred between 499–674 °C temperature. This decrease is because of the decomposition of silanol groups in as-prepared nanocatalyst. According to these results, the good thermal stability for the nanocatalyst was observed.
For appraise the textural properties and porosity of the Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 the BET analysis was perform at 77 K by nitrogen adsorption–desorption (Fig. 7). The BET results from the adsorption–desorption isotherm and BJH curve are listed in Table 2. The mesoporous nanocatalyst display a type IV isotherm. The mean pore diameter of as-synthesized nanocatalyst was 41.31 nm. The surface area is 41.40 m2 g−1, and the total pore volume is 0.066 cm3 g−1.
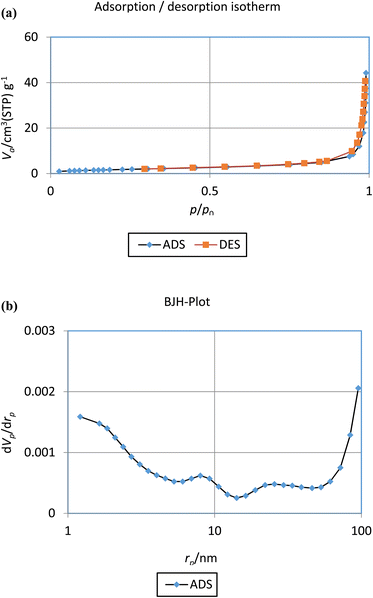 | ||
| Fig. 7 The (a) BET plot of the nitrogen adsorption–desorption and (b) pore size distribution curve of the as-prepared nanocatalyst. | ||
| Entry | Parameter | Amount |
|---|---|---|
| 1 | Pore volume | 1.4717 [cm3(STP) g−1] |
| 2 | BET surface area (as,BET) | 41.4054 [m2 g−1] |
| 3 | Total pore volume(p/p0 = 0.990) | 0.066146 [cm3 g−1] |
| 4 | Mean pore diameter | 41.306 [nm] |
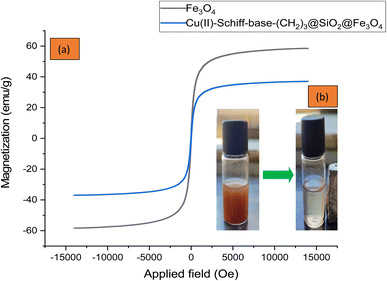
The magnetic properties of Fe3O4 and Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 nanoparticles were investigated by vibrating sample magnetometer (VSM) and are shown in Fig. 8. This analysis shows the magnetization curve of the samples at room temperature and in the fields of −10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 10
000 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe. The saturation magnetization (Ms) value of Fe3O4 and Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 complex was 58.5 and 37 emu g−1, respectively (Fig. 8a). This decrease in the saturation magnetization value is because of adding silica and other organic compounds on the surface of Fe3O4. The presence of a magnetic field shows that we have a clear decrease in the saturation magnetization of nanoparticles (Fig. 8b). This result is due to the effect of the magnetic field on the orientation of the magnetic dipoles. During the formation of magnetic nanoparticles, part of the nanoparticles aligns with the magnetic field.
000 Oe. The saturation magnetization (Ms) value of Fe3O4 and Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 complex was 58.5 and 37 emu g−1, respectively (Fig. 8a). This decrease in the saturation magnetization value is because of adding silica and other organic compounds on the surface of Fe3O4. The presence of a magnetic field shows that we have a clear decrease in the saturation magnetization of nanoparticles (Fig. 8b). This result is due to the effect of the magnetic field on the orientation of the magnetic dipoles. During the formation of magnetic nanoparticles, part of the nanoparticles aligns with the magnetic field.
Application of Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4
The use of Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 catalyst due to its special properties can lead to a tremendous progress in the use of this material for various synthesis purposes. Moreover, the very low toxicity of this substance has made its use in chemistry as a green condition for us. We investigated the role and effect of this magnetically recoverable Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 catalyst in the Knoevenagel–Michael cyclocondensation for the synthesis of 2-amino-4H-pyrans derivatives of various aromatic arylaldehyde, dimedone and malononitrile (Scheme 1). In order to optimize reaction conditions of 4b, the effect of different parameters such as various amounts of nanocatalyst (0.05–0.1 g) and solvents (EtOH, CH2Cl2, CH3CN, CH3OH, Toluene, and CHCl3) in the reaction of 4-chlorobenzaldehyde (1 mmol), malononitrile (1 mmol), and dimedone (1 mmol) was selected as model reaction. As the results of the optimization of the reaction conditions show, no product was obtained in the absence of the catalyst in ethanol after 5 h (Table 3, entry 1). The results in the presence of the catalyst were encouraging. In the presence of 0.005 g of catalyst, 55% of 4b was obtained in ethanol under refluxing conditions (Table 3, entry 2). 0.05 g of the nanocatalyst in ethanol under reflux conditions produces a higher yield of the product in a short time (Table 3, entry 5). It was observed that by increasing the amount of catalyst from 0.07 to 0.1 g, no improvement was observed (Table 3, entries 6–8). When the reaction was performed in other solvents, 4b was obtained in lower yield (Table 3, entries 9–13).| Entry | Amount of catalyst (g) | Solvent (2 mL) | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (1 mmol), dimedone (1 mmol), malononitrile (1 mmol), reflux conditions.b Isolated pure yield. | ||||
| 1 | — | Ethanol | 5 h | Nil |
| 2 | 0.005 | Ethanol | 60 | 55 |
| 3 | 0.01 | Ethanol | 60 | 71 |
| 4 | 0.03 | Ethanol | 45 | 82 |
| 5 | 0.05 | Ethanol | 40 | 94 |
| 6 | 0.07 | Ethanol | 40 | 91 |
| 7 | 0.09 | Ethanol | 40 | 90 |
| 8 | 0.1 | Ethanol | 45 | 88 |
| 9 | 0.05 | CH3CN | 100 | 66 |
| 10 | 0.05 | CHCl3 | 90 | 75 |
| 11 | 0.05 | CH2Cl2 | 90 | 76 |
| 12 | 0.05 | CH3OH | 120 | 70 |
| 13 | 0.05 | Toluene | 180 | 65 |
Most importantly, the catalytic activity was investigated with other catalysts including Schiff-base-(CH2)3@SiO2@Fe3O4, NH2(CH2)2NH-(CH2)3@SiO2@Fe3O4, Cl-(CH2)3@SiO2@Fe3O4, SiO2@Fe3O4, and Fe3O4. For this purpose, the reaction of 4-chlorobenzaldehyde (1 mmol), malonontrile (1 mmol) and dimedone (1 mmol) in the presence of 0.05 g of as-prepared nanocatalyst in ethanol under reflux conditions was investigated. The summary of the obtained results is presented in Table 4. As the table shows, Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 shows better results than other catalysts and the corresponding product was obtained in 94% efficiency.
| Entry | Catalyst | Conditions | Yielda (%) |
|---|---|---|---|
| a Isolated pure yield. | |||
| 1 | Fe3O4 | Ethanol/reflux | 50 |
| 2 | SiO2@Fe3O4 | Ethanol/reflux | 40 |
| 3 | Cl-(CH2)3@SiO2@Fe3O4 | Ethanol/reflux | Trace |
| 4 | NH2(CH2)2NH-(CH2)3@SiO2@Fe3O4 | Ethanol/reflux | 95 |
| 5 | Schiff-base-(CH2)3@SiO2@Fe3O4 | Ethanol/reflux | 22 |
| 6 | Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 | Ethanol/reflux | 94 |
The reaction of various aromatic aldehydes with malononitrile and dimedone was investigated in the presence of nanocatalyst in ethanol and under reflux conditions (Table 5). As can be seen, from the reaction between different aldehydes containing electron-donating and electron-withdrawing groups, the desired products were obtained with an excellent yield of 89–97% after 10–40 min. The presence of electron-withdrawing groups (EWG) on the aromatic ring increased the rate of reaction, whereas the electron-donating groups (EDG) decreased the rate slightly.
| Entry | Product | Time (min) | Yieldb (%) | TON | TOF (h−1) | Mp (reference) |
|---|---|---|---|---|---|---|
| a A mixture of various aldehyde (1 mmol), dimedone (1 mmol), malononitrile (1 mmol), catalyst (0.05 g, 0.75 mol%) in ethanol (2 mL) was refluxed.b Isolated pure yield. | ||||||
| 1 | 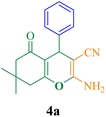 |
40 | 94 | 125.3 | 189 | 229–231 (ref. 50) |
| 2 | 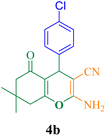 |
20 | 95 | 126.6 | 383 | 212–214 (ref. 50) |
| 3 | 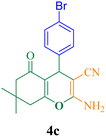 |
20 | 94 | 125.3 | 379.6 | 215–217 (ref. 50) |
| 4 | 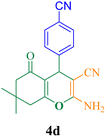 |
12 | 96 | 128 | 640 | 222–224 (ref. 50) |
| 5 | 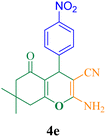 |
12 | 97 | 129.3 | 646.6 | 178–180 (ref. 50) |
| 6 |  |
40 | 94 | 125.3 | 189.8 | 215–217 (ref. 50) |
| 7 |  |
40 | 92 | 122.6 | 185.6 | 196–198 (ref. 50) |
| 8 |  |
20 | 90 | 120 | 363.6 | 188–190 (ref. 50) |
| 9 |  |
45 | 89 | 118.6 | 158.1 | 220–222 (ref. 50) |
| 10 | 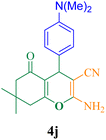 |
35 | 94 | 125.3 | 216 | 185–187 (ref. 50) |
| 11 | 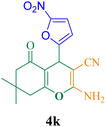 |
10 | 95 | 126.6 | 791.2 | 153–155 (ref. 50) |
| 12 | 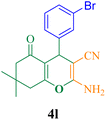 |
25 | 93 | 124 | 302.4 | 212–214 (ref. 5) |
| 13 | 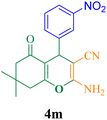 |
15 | 92 | 122.6 | 490.4 | 211–213 (ref. 50) |
| 14 |  |
12 | 96 | 128 | 640 | 222–224 (ref. 50) |
| 15 |  |
30 | 91 | 121.3 | 242.6 | 180–182 (ref. 50) |
| 16 |  |
30 | 92 | 122.6 | 245.3 | 248–250 (ref. 50) |
Various mechanisms have been proposed for this reaction in recent years. Recently, due to the increase in the use of Lewis acids and positive halogen generating sources for this synthesis, more acceptable mechanisms have been proposed. Hence, a proposed and acceptable mechanism for the synthesis of pyran derivatives using a recyclable magnetic catalyst in the condition in EtOH at reflux condition is shown in Scheme 3. What is clear is that from the first stage of the reaction to the end of the reaction, the Lewis acid makes the nucleophilic groups more active and increases their electron deficiency (I), as a result, the nucleophile attacks faster and finally leads to ring closure. In the next step Michael addition of dimedone to arylidene malononitrile gives intermediate (II). Finally, isomerization gives the corresponding product (III).51
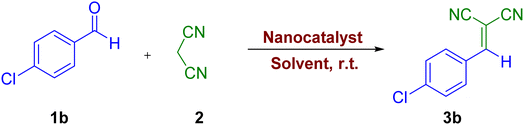
Using the good results obtained for the synthesis of 2-amino-4H-pyrans derivatives, we decided to investigate the activity of the synthesized catalyst for the synthesis of 2-benzylidenemalononitrile derivatives through the one-pot reaction of aromatic aldehydes and malonontrile. In order to optimize reaction conditions of 3b, the effect of different parameters such as various amounts of nanocatalyst (0.05–0.1 g) and solvents (distilled water, CH3CN, CHCl3, CH2Cl2, and CH3OH) in the reaction of 4-chlorobenzaldehyde (1 mmol) and malononitrile (1 mmol) was selected as model reaction. As the results of the optimization of the reaction conditions show, no product was obtained in the absence of the catalyst and solvent at room temperature after 180 min (Table 6, entry 1). The summary of the obtained results is presented in Table 6. As the table shows, the best result was obtained when the reaction was achieved using 0.05 g of the catalyst with the presence of distilled water at room temperature within 20 min with an efficiency of 95% (Table 6, entry 5). It was observed that by increasing the amount of catalyst from 0.07 to 0.1 g, no improvement was observed (Table 6, entries 6–8). The reaction was performed in the presence of other solvents, giving low yields of 3b (Table 6, entries 9–14). When the reaction mixture was stirred at a temperature of 100 °C, it was noticed that the yield decreased (Table 6, entry 15).
| Entry | Catalyst (g) | Solvent | Temperature (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: malononitrile (1 mmol), 4-chlorobenzaldehyde (1 mmol), solvent (2 mL).b Isolated pure yield. | |||||
| 1 | — | — | 25 | 180 | Trace |
| 2 | 0.005 | Distilled water | 25 | 60 | 55 |
| 3 | 0.01 | Distilled water | 25 | 30 | 78 |
| 4 | 0.03 | Distilled water | 25 | 30 | 85 |
| 5 | 0.05 | Distilled water | 25 | 20 | 95 |
| 6 | 0.07 | Distilled water | 25 | 25 | 90 |
| 7 | 0.09 | Distilled water | 25 | 25 | 89 |
| 8 | 0.1 | Distilled water | 25 | 40 | 84 |
| 9 | 0.05 | CH3CN | 25 | 50 | 60 |
| 10 | 0.05 | CHCl3 | 25 | 50 | 65 |
| 11 | 0.05 | CH2Cl2 | 25 | 50 | 74 |
| 12 | 0.05 | CH3OH | 25 | 40 | 51 |
| 13 | 0.05 | Toluene | 25 | 100 | 38 |
| 14 | 0.05 | EtOH | 25 | 30 | 85 |
| 15 | 0.05 | Distilled water | 100 (reflux) | 90 | 70 |
After obtaining the optimal conditions, different derivatives of 2-benzylidenemalononitrile derivatives were synthesized using 0.05 g of the nanocatalyst, the results of which are presented in Table 7. As can be seen, from the reaction between different aldehydes with malonontrile, the desired products were obtained with an excellent yield of 88–96% after 8–40 min.
| Entry | Product | Time (min) | Yieldb (%) | TON | TOF (h−1) | Reference |
|---|---|---|---|---|---|---|
| a A mixture of various aldehyde (1 mmol) and malononitrile (1 mmol), catalyst (0.05 g, 0.75 mol%) in distilled water (2 mL) at room temperature was stirred.b Isolated pure yield. | ||||||
| 1 |  |
20 | 95 | 126.6 | 383.8 | 80–82 (ref. 52) |
| 2 |  |
15 | 94 | 125.3 | 501.3 | 163–165 (ref. 52) |
| 3 |  |
15 | 95 | 126.6 | 506.4 | 148–150 (ref. 52) |
| 4 |  |
17 | 92 | 122.6 | 437.8 | 162–164 (ref. 52) |
| 5 |  |
10 | 93 | 124 | 775 | 180–182 (ref. 52) |
| 6 |  |
40 | 89 | 118.6 | 179.7 | 111–113 (ref. 52) |
| 7 |  |
10 | 92 | 122.6 | 766.6 | 138–140 (ref. 52) |
| 8 |  |
15 | 89 | 118.6 | 474.6 | 125–127 (ref. 52) |
| 9 |  |
40 | 88 | 117.3 | 177.7 | 132–134 (ref. 52) |
| 10 |  |
8 | 96 | 128 | 984.6 | 154–156 (ref. 52) |
| 11 |  |
8 | 95 | 126.6 | 974.3 | 160–162 (ref. 52) |
| 12 |  |
17 | 91 | 121.3 | 433.2 | 91–93 (ref. 52) |
| 13 |  |
20 | 90 | 120 | 363.3 | 100–102 (ref. 52) |
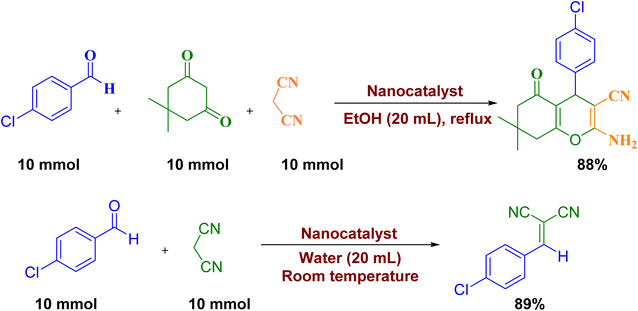
We also studied the gram scale synthesis on the reaction of 4-chlorobenzaldehyde, malononitrile and dimedone for the synthesis of derivative 4b and the reaction of 4-chlorobenzaldehyde with malononitrile for the synthesis of derivative 3b to demonstrate the ability of current method (Scheme 4). As can be seen, by stirring a mixture of 4-chlorobenzaldehyde (10 mmol), malonontrile (10 mmol) and dimedone (10 mmol) in EtOH (20 mL) and 4-chlorobenzaldehyde (10 mmol), malonontrile (10 mmol) in water (20 mL) at room temperature in the presence of catalyst, 4b and 3b can be synthesized with 88% and 89% yield, respectively.
One of the important factors in the design of environmentally friendly nanocatalysis systems is the ability to recycle and reuse the catalyst. In order to investigate the recyclability and reusability of Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4, the reaction of 4-chlorobenzaldehyde, malonontrile and dimedone for the synthesis of derivative 4b and the reaction of 4-chlorobenzaldehyde with malonontrile for the synthesis of derivative 3b was selected as the model reaction under optimal conditions. After the completion of the reaction, the reaction mixture was cooled to room temperature. Then, the nanocatalyst was separated from the reaction mixture by an external field, washed with a mixture of water and ethanol, dried in air, and used to perform the reaction again. To check its catalytic power, the reaction was repeated seven times in the vicinity of this catalyst, and after seven times, the reaction efficiency decreased to 96 to 90% for 3b and the reaction efficiency decreased to 96 to 91% for 4b, respectively (Fig. 9a). The surface morphology of recycled sample after recycling seven times were conducted by TEM analysis (Fig. 9b). As shown in Fig. 9b, after recycling seven times, the surface morphology of recycled sample had not changed and stable against repeated use. Furthermore, the leaching experiment for the synthesis of 4b after recycling over seven successive runs by ICP analysis was studied. The obtained results indicated that leaching of Cu is was very insignificant about 1.1%.
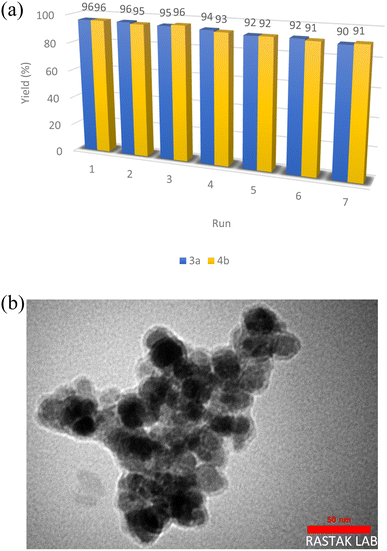 | ||
| Fig. 9 (a) Reusability of as-prepared nanocatalyst during the model reaction and (b) TEM image of as-prepared nanocatalyst after seven times reuse. | ||
Conclusion
In summary, we unveiled from the a novel heterogeneous magnetic nanocatalyst with core–shell morphology. The catalyst is easily synthesized via benign conditions and inexpensive precursors. Afterwards, the structural and magnetic characteristics of the produced nanoparticles were determined by employing using FT-IR, ICP, VSM, FE-SEM, TEM, TGA, PXRD, BET and EDX analyses. The performance of synthesized Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 magnetic nanocatalyst has been tested for the 2-amino-4H-pyrans derivatives and 2-benzylidenemalononitrile derivatives. By evaluating the outcomes of different catalytic activity tests such as substrate scope analysis, leaching examination, recyclability tests, and gram scale experiments, one can ascertain the effectiveness of the synthesized Cu(II)-Schiff-base-(CH2)3@SiO2@Fe3O4 catalyst. The advantages of these methods include easy separation and ability to recycle the catalyst after several times with high activity, use of solvent-free or green solvent conditions, use of available materials, no use of column chromatography, performing the reaction on a hot scale, short reaction time, production of products in high yield pointed out.Author contributions
S. Rezayati and A. Ramazani conceived the idea and designed the research. M. Karimi carried out the research. M. Karimi wrote the original draft. S. Rezayati and A. Ramazani reviewed the manuscript and editing. A. Ramazani was responsible for the funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present study, derived from PhD thesis by Masoud Karimi, was supported by the Iran National Science Foundation INSF and the University of Zanjan.References
- S. K. Panja, N. Dwivedi and S. Saha, RSC Adv., 2015, 5, 65526–65531 RSC.
- A. F. Trindade, P. M. Gois and C. A. Afonso, Chem. Rev., 2009, 109, 418–514 CrossRef CAS PubMed.
- A. Rai and K. V. Ranganath, J. Heterocycl. Chem., 2021, 58, 1039–1057 CrossRef CAS.
- Y. P. Yew, K. Shameli, M. Miyake, N. B. B. A. Khairudin, S. E. B. Mohamad, T. Naiki and K. X. Lee, Arabian J. Chem., 2020, 13, 2287–2308 CrossRef CAS.
- (a) E. A. Campos, D. V. B. S. Pinto, J. I. S. D. Oliveira, E. D. C. Mattos and R. D. C. L. Dutra, J. Aerosp. Technol. Manage., 2015, 7, 267–276 CrossRef CAS; (b) F. Sheikholeslami-Farahani, Asian J. Nanosci. Mater., 2022, 5, 132–143 CAS; (c) S. Tabar Maleki and S. J. Sadati, Asian J. Nanosci. Mater., 2022, 5, 98–108 Search PubMed.
- (a) H. Pourfaraj, S. Rostamzadeh Mansour, M. Zaeifizadeh and A. Vojood, Asian J. Nanosci. Mater., 2023, 6, 92–105 CAS; (b) R. Khazaei, A. Khazaei and M. Nasrollahzadeh, Appl. Organomet. Chem., 2023, 3, 123–133 Search PubMed; (c) N. Pourbahar and S. Sattari Alamdar, Asian J. Green Chem., 2023, 7, 9–16 CAS.
- (a) H. Ghafuri, M. Zargari and A. Emami, Asian J. Green Chem., 2023, 7, 54–69 CAS; (b) F. Hakimi, A. Sharifi-Zarchi and E. Golrasan, Chem. Methodol., 2023, 7, 489–498 CAS; (c) E. Ezzatzadeh, Asian J. Nanosci. Mater., 2021, 4, 125–136 CAS; (d) M. B. Swami, G. R. Nagargoje, S. R. Mathapati, A. S. Bondge, A. H. Jadhav, S. P. Panchgalle and V. S. More, Appl. Organomet. Chem., 2023, 3, 184–198 Search PubMed; (e) K. Yadollahzadeh, Asian J. Nanosci. Mater., 2021, 4, 81–94 CAS; (f) F. Hakimi, M. Taghvaee and E. Golrasan, Adv. J. Chem., Sect. A, 2023, 6, 188–197 CAS.
- Y. Zhao, Q. Zumin and J. Huang, Chin. J. Chem. Eng., 2008, 16, 451–455 CrossRef CAS.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371–3393 RSC.
- M. Bustamante-Torres, D. Romero-Fierro, J. Estrella-Nuñez, B. Arcentales-Vera, E. Chichande-Proaño and E. Bucio, Polymers, 2022, 14, 752 CrossRef CAS PubMed.
- (a) B. H. Rotstein, S. Zaretsky, V. Rai and A. K. Yudin, Chem. Rev., 2014, 114, 8323–8359 CrossRef CAS PubMed; (b) R. Muslim Muhiebes, L. Fatolahi and S. Sajjadifar, Asian J. Green Chem., 2023, 7, 121–131 Search PubMed; (c) A. R. Moosavi-Zare, H. Goudarziafshar, Z. Jalilian and F. Hosseinabadi, Chem. Methodol., 2022, 6, 571–581 CAS; (d) S. Mowlazadeh Haghighi, A. Purkhosrow, A. Khalafi-Nezhad and S. Oftadehgan, Asian J. Green Chem., 2022, 6, 203–222 Search PubMed; (e) S. Dey, P. Basak, S. Sarkar and P. Ghosh, Asian J. Green Chem., 2022, 6, 24–39 CAS.
- T. Zarganes-Tzitzikas, A. L. Chandgude and A. Dömling, Chem. Rec., 2015, 15, 981–996 CrossRef CAS PubMed.
- J. E. Biggs-Houck, A. Younai and J. T. Shaw, Curr. Opin. Chem. Biol., 2010, 14, 371–382 CrossRef CAS PubMed.
- G. Bosica and R. Abdilla, Catalysts, 2022, 12, 725 CrossRef CAS.
- R. H. Vekariya and H. D. Patel, Synth. Commun., 2014, 44, 2756–2788 CrossRef CAS.
- A. Samui, N. Kesharwani, C. Haldar and S. K. Sahu, Microporous Mesoporous Mater., 2020, 299, 110112 CrossRef CAS.
- D. B. Shinde, S. Kandambeth, P. Pachfule, R. P. Kumar and R. Banerjee, Chem. Commun., 2015, 51, 310–313 RSC.
- M. L. Gao, M. H. Qi, L. Liu and Z. B. Han, Chem. Commun., 2019, 55, 6377–6380 RSC.
- Y. X. Ma, Z. J. Li, L. Wei, S. Y. Ding, Y. B. Zhang and W. Wang, J. Am. Chem. Soc., 2017, 139, 4995–4998 CrossRef CAS PubMed.
- C. Viel and J. C. Doré, Farmaco Sci., 1972, 27, 257–312 CAS.
- A. Sidhu, J. R. Sharma and M. Rai, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2010, 49, 247–250 Search PubMed.
- S. A. Khan, A. M. Asiri, R. M. Rahman, S. A. Elroby, F. M. S. Aqlan, M. Y. Wani and K. Sharma, J. Heterocycl. Chem., 2017, 54, 3099–3107 CrossRef CAS.
- A. S. Fouda, Y. A. El-Ewady, O. M. Abo-El-Enien and F. A. Agizah, Anti-Corros. Methods Mater., 2008, 55, 317–323 CrossRef CAS.
- H. Jin, M. Liang, S. Arzhantsev, X. Li and M. Maroncelli, J. Phys. Chem. B, 2010, 114, 7565–7578 CrossRef CAS PubMed.
- Z. Tashrifi, M. Mohammadi-Khanaposhtani, H. Hamedifar, B. Larijani, S. Ansari and M. Mahdavi, Mol. Diversity, 2020, 24, 1385–1431 CrossRef CAS PubMed.
- S. Saneinezhad, L. Mohammadi, V. Zadsirjan, F. F. Bamoharram and M. M. Heravi, Sci. Rep., 2020, 10, 1–26 CrossRef PubMed.
- H. Kiyani and F. Ghorbani, Chem. Pap., 2014, 68, 1104–1112 CAS.
- M. Aghajani, S. Asghari, G. F. Pasha and M. Mohseni, Res. Chem. Intermed., 2020, 46, 1841–1855 CrossRef CAS.
- E. Kolvari, N. Koukabi, Z. Ozmaei, H. Khoshkho and F. Seidi, Curr. Res. Green Sustainable Chem., 2022, 5, 100327 CrossRef CAS.
- J. Safaei-Ghomi, A. Javidan, A. Ziarati and H. Shahbazi-Alavi, J. Nanopart. Res., 2015, 17, 1–12 CrossRef CAS.
- F. Mohammadpour, Polycyclic Aromat. Compd., 2021, 41, 160–172 CrossRef.
- M. Lashkari, F. Mohamadpour, M. T. Maghsoodlou, R. Heydari and N. Hazeri, Polycyclic Aromat. Compd., 2020, 56, 1123–1134 Search PubMed.
- B. Sunil Kumar, N. Srinivasulu, R. H. Udupi, B. Rajitha, Y. Thirupathi Reddy, P. Narsimha Reddy and P. S. Kumar, J. Heterocycl. Chem., 2006, 43, 1691–1693 CrossRef.
- P. Singh, P. Yadav, A. Mishra and S. K. Awasthi, ACS Omega, 2020, 5, 4223–4232 CrossRef CAS PubMed.
- A. Maleki and Z. Hajizadeh, Silicon, 2019, 11, 2789–2798 CrossRef CAS.
- H. Ebrahimiasl and D. Azarifar, Appl. Organomet. Chem., 2020, 34, e5359 CrossRef CAS.
- Z. J. Yang, Q. T. Gong, Y. Wang, Y. Yu, Y. H. Liu, N. Wang and X. Q. Yu, Mol. Catal., 2020, 491, 456–470 Search PubMed.
- Saigal, M. Irfan, P. Khan, M. Abid and M. M. Khan, ACS Omega, 2019, 4, 16794–16807 CrossRef CAS PubMed.
- R. Muslim Mhaibes, Z. Arzehgar, M. Mirzaei Heydari and L. Fatolahi, Asian J. Green Chem., 2023, 7, 1–8 Search PubMed.
- B. Baghernejad and S. M. Hojati, Asian J. Green Chem., 2022, 6, 194–202 CAS.
- K. Yadollahzadeh, Asian J. Nanosci. Mater., 2022, 5, 144–158 CAS.
- H. Taherkhani, A. Ramazani, S. Sajjadifar, H. Aghahosseini, A. Rezaei and S. Rezayati, ChemistrySelect, 2021, 6, 1–14 CrossRef.
- S. Rezayati, G. Dinmohammadi, A. Ramazani and S. Sajjadifar, Polycyclic Aromat. Compd., 2023, 43, 5869–5891 CrossRef CAS.
- P. Singh, P. Yadav, A. Mishra and S. K. Awasthi, ACS Omega, 2020, 5, 4223–4232 CrossRef CAS PubMed.
- G. Rathee, S. Kohli, S. Panchal, N. Singh, A. Awasthi, S. Singh, A. Singh, S. Hooda and R. Chandra, ACS Omega, 2020, 5, 23967–23974 CrossRef CAS PubMed.
- E. Pourian, S. Javanshir, Z. Dolatkhah, S. Molaei and A. Maleki, ACS Omega, 2018, 3, 5012–5020 CrossRef CAS PubMed.
- (a) S. Rezayati, F. Kalantari and A. Ramazani, RSC Adv., 2023, 13, 12869 RSC; (b) F. Kalantari, S. Rezayati and A. Ramazani., Appl. Organomet. Chem., 2023, 37, e7064 CrossRef CAS; (c) G. Mansouri, M. Abdizad, A. R. Abbasi and S. Rezayati, Appl. Organomet. Chem., 2022, 36, e6866 CrossRef CAS; (d) S. Rezayati, H. Haghighat and A. Ramazani, Silicon, 2023, 15, 2679–2692 CrossRef CAS; (e) S. Rezayati, Y. Ahmadi and A. Ramazani, Inorg. Chim. Acta, 2023, 544, 121203 CrossRef CAS; (f) S. Rezayati, Z. Naserifar and A. Ramazani, Phosphorus, Sulfur Silicon Relat. Elem., 2022, 197, 1016–1025 CrossRef CAS; (g) S. Rezayati, F. Kalantari, A. Ramazani and E. Ezzatzadeh, J. Sulfur Chem., 2021, 42, 575–590 CrossRef CAS; (h) S. Rezayati, R. Hajinasiri and Z. Erfani, Res. Chem. Intermed., 2016, 42, 2567–2576 CrossRef CAS; (i) S. Rezayati, M. Mehmannavaz, E. Salehi, Sh. Haghi, R. Hajinasiri and S. Afshari Sharif Abad, J. Sci., Islamic Repub. Iran, 2016, 27, 51–63 Search PubMed; (j) S. Rezayati, E. Rezaee Nezhad and R. Hajinasiri, Chin. Chem. Lett., 2016, 27, 974–978 CrossRef CAS; (k) S. Rezayati and S. Sajjadifar, J. Sci., Islamic Repub. Iran, 2014, 25, 329–337 Search PubMed.
- S. Rezayati, A. Ramazani, S. Sajjadifar, H. Aghahosseini and A. Rezaei, ACS Omega, 2021, 6, 25608–25622 CrossRef CAS PubMed.
- M. Mirzaee, B. Bahramian, P. Gholampour, T. Teymouri and T. Khorsand, Appl. Organomet. Chem., 2019, 33, e4792 CrossRef.
- F. Kalantari, A. Ramazani, M. R. Poor Heravi, H. Aghahosseini and K. Ślepokura, Inorg. Chem., 2021, 60, 15010–15023 CrossRef CAS PubMed.
- S. Amirnejat, A. Nosrati, R. Peymanfar and S. Javanshir, Res. Chem. Intermed., 2020, 46, 3683–3701 CrossRef CAS.
- F. Kalantari, S. Rezayati, A. Ramazani, H. Aghahosseini, K. Ślepokura and T. Lis, ACS Appl. Nano Mater., 2022, 5, 1783–1797 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of FT-IR, 1H NMR (250 MHz, CDCl3 or DMSO-d6) and 13C NMR (62.5 MHz, CDCl3 or DMSO-d6) spectra of synthesized compounds. See DOI: https://doi.org/10.1039/d3ra05649j |
| This journal is © The Royal Society of Chemistry 2023 |