Open Access Article
Open Access ArticleComprehensive phytochemical analysis of lingonberry (Vaccinium vitis-idaea L.) from different regions of China and their potential antioxidant and antiproliferative activities†
Jian Xuab,
Han Yangab,
Chengdong Nieab,
Tao Wangab,
Xiangyu Qinab,
Jie Yangab,
Yuanhang Changab,
Siming Nieab and
Yujie Fu *c
*c
aKey Laboratory of Forest Plant Ecology, Ministry of Education, Northeast Forestry University, Harbin 150040, China
bCollege of Chemistry, Chemical Engineering and Resource Utilization, Northeast Forestry University, 150040, Harbin, China
cCollege of Forestry, Beijing Forestry University, 100083, Beijing, China. E-mail: yujie_fu@163.com
First published on 9th October 2023
Abstract
Lingonberry are underutilised due to the lack of evaluating active compounds in different parts. In this study, the phytochemical profiles, antioxidant and antiproliferative activities of lingonberry's fruits, leaves and stems from different regions of China were compared. Ninety-five bioactive compounds were rapidly identified using a molecular network based on UPLC-Q-Exactive Orbitrap mass spectrometry. The UPLC-QqQ-MS/MS method combined with principal component analysis (PCA) quantified 18 bioactive components in 6 classes. The highest content of arbutin (15 mg/100 g DW) was found in leaves of Huzhong (P6). Ursolic acid and cyanidin-3-O-galactoside were highest in fruits of Tahe (P4) (4.5 mg/100 g DW and 3.2 mg/100 g DW, respectively). Antioxidant activities determined by DPPH, ABTS+ and FRAP methods were significantly correlated with total phenolic content (TPC), total flavonoid content (TFC) and total anthocyanin content (TAC). The results indicate that the strongest antioxidant activity and antiproliferative efficacy are observed in the fruits of Tahe (P4) and leaves of Huzhong (P6), respectively. Our results provide valuable insights into lingonberry's comprehensive development and utilization.
Introduction
Plant-driven natural antioxidant-sourced foods with proven human health benefits are receiving more attention.1 Lingonberry (Vaccinium vitis-idaea L.) is a wild evergreen dwarf shrub known as a “superfood” owing to its high antioxidant content.2 It is widespread in northern and central Europe, Canada and Asia, including alpine areas such as the Greater Khingan Range and Lesser Hinggan Mountains in North-eastern China.3 The fruits and aerial parts of lingonberries are rich in nutrients, including vitamins C, A, E, fibre and minerals.4 In addition to nutrients, lingonberries are abundant in functional compounds such as polyphenols, flavonoids, anthocyanins and triterpenoids. These compounds have biological activities such as antioxidant, anti-inflammatory, antimicrobial, antitumour and vasoprotective effects.5–8 Given its nutritional and functional properties, lingonberry has high potential as a functional food.Plants have inherited genes that regulate their biological clocks and are able to respond to changes in the external environment by regulating the synthesis of biologically active compounds, thus adapting to different growing conditions.9 However, external conditions strongly influence the phytochemical content, quality and safety of plant foods. Plants exposed to changes in the external environment during the growing season. The effects of external abiotic stresses on the transport, accumulation and storage of plant secondary phytometabolites need to be elucidated. They may differ according to plant species, organ parts and metabolite types.10 Therefore, it is important to examine the relationship between the external environment and secondary metabolites from the perspective of quality.11
It has been shown that different parts of the lingonberry (fruit, leaves, and stems) are used for other purposes based on varying chemical contents.12 Lingonberry leaves are used as an important raw material for making tea, as they contain high levels of polyphenols and possess strong antimicrobial and antioxidant properties.13 Compared to other parts of the plant, the fruit of the lingonberry has a higher accumulation of triterpenoid constituents. Plants triterpenoids exist in free and bound forms with different polarities and solubilities. In particular, the low-polarity compounds are mainly present in the edible peel. The epidermis was usually removed during processing, resulting in a loss of active compounds. For this reason, lingonberries with edible peel are of particular interest when consumed fresh or frozen, and can also be processed into juices, jams, jellies and pastries. They can be considered as a dietary source rich in naturally occurring bioactives.12 Therefore, it is imperative to investigate the types of functional agents present in different parts of lingonberry. Analyzing the content and efficacy of these components is crucial for both application and assessment of biosafety in functional foods. In view of this, an effective analytical strategy must be in place for a comprehensive evaluation of the functional constituents of lingonberry in different geographical regions.14
However, current studies mainly focus on individual parts of lingonberries such as fruits, leaves or are reported for a separate group of components,3,11 polyphenols, flavonoids and triterpenes.8,12 In addition, metabolites in plants are influenced by a number of factors such as genotype, environmental conditions and geographical location.
To our knowledge, there have been no reports on the varying metabolic profile of lingonberry in different alpine regions in China. The aim of this study was to carry out the first comparative investigation of the bioactive constituents and their corresponding bioactivities in lingonberries from different geographical regions in China. The extracts of lingonberry were identified based on a comprehensive strategy of UPLC-Q-Exactive Orbitrap MS, Global Natural Products Society (GNPS) molecular network and chemical standards. The target compounds were rapidly quantified by UPLC-QqQ-MS/MS. In vitro antioxidant and antiproliferative activities of lingonberry extracts were also investigated. Overall, a comparative study of the functional components and in vitro activities of lingonberries from different geographical regions provides new insights into their comprehensive utilisation.
Experimental
Material and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, v/v), sonicated for 25 min at 4 °C and 300 W, and centrifuged at 10
70, v/v), sonicated for 25 min at 4 °C and 300 W, and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. After centrifugation for 2 times, the supernatant was collected. Subsequently, the extracts were rotary dried under vacuum at 45 °C, redissolved in 10 mL of methanol solution. Then, 100 μL were piped to a constant volume of 10 mL, and filtered through a 0.22 μm PTFE membrane, followed by injection into a UPLC-QqQ-MS/MS and UPLC-Q-Exactive Orbitrap MS system for subsequent analysis. Water
000 rpm for 10 min. After centrifugation for 2 times, the supernatant was collected. Subsequently, the extracts were rotary dried under vacuum at 45 °C, redissolved in 10 mL of methanol solution. Then, 100 μL were piped to a constant volume of 10 mL, and filtered through a 0.22 μm PTFE membrane, followed by injection into a UPLC-QqQ-MS/MS and UPLC-Q-Exactive Orbitrap MS system for subsequent analysis. Water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (30
ethanol (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, v/v) and quality control (QC) samples were also included for identification of untargeted metabolites. QC samples were prepared by mixing all samples tested. The blank and QC samples were placed at the beginning and the end of the sample sequence of the assay at 10 sample intervals.
70, v/v) and quality control (QC) samples were also included for identification of untargeted metabolites. QC samples were prepared by mixing all samples tested. The blank and QC samples were placed at the beginning and the end of the sample sequence of the assay at 10 sample intervals.| A = absorbance (A510–A700 nm) pH 1.0 − (A510–A700 nm) pH 4.5 | (1) |
| TAC (mg g−1) = (A × MW × DF ×1000)/ε × L × Wt | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600, DF = diluted factor, and Wt = sample weight (g).
600, DF = diluted factor, and Wt = sample weight (g).The MS conditions were set as follows: positive and negative ion switch mode, scanning range: m/z 80–1200; curtain gas (CUR): 40 psi; the temperature of the ion source (TEM): 550 °C or 600 °C and voltage of the ion source (IS): −3000 V or 3800 V in negative or positive modes, respectively; first order scanning: declustering potential (DP): 130 V; collision voltage (CE): 10 V; second order scanning: Q-Exactive Orbitrap Product Ion mode was used to collect MS data, collision energy (CE) was 20, 40 and 60 V.12
Thermo Compound Discoverer™ 3.0 software (Thermo Fisher Science) was used to process raw data from 54 lingonberry fruit, leaf and stem profiles (27 samples and their replicates) analysed by UPLC-Q-Exactive Orbitrap MS. The specific parameters were set as follows: the RT alignment was set to 2 min and the m/z tolerance was set to 5 ppm; the signal-to-noise ratio (S/N) for feature detection was set to 3; the intensity threshold of the target peak was set to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; and the additive ions were set to [2M + H]+, [2M − H]−, [M + H]+, [M − H]−. The following databases included in the Component Discoverer™ software were then used to identify the raw MS data: Thermo Fisher, Nature Chemical Biology, Nature Chemistry, MassBank, KEGG, Food and Agriculture Organisation of the United Nations, ChemBank. Finally, blanks were added for background filtering and gap filling to refine the data, and the sample data were normalised using the median, and the filtered data were used for further chemometric analysis.
000; and the additive ions were set to [2M + H]+, [2M − H]−, [M + H]+, [M − H]−. The following databases included in the Component Discoverer™ software were then used to identify the raw MS data: Thermo Fisher, Nature Chemical Biology, Nature Chemistry, MassBank, KEGG, Food and Agriculture Organisation of the United Nations, ChemBank. Finally, blanks were added for background filtering and gap filling to refine the data, and the sample data were normalised using the median, and the filtered data were used for further chemometric analysis.
An online workflow on the Platform (GNPS) (http://gnps.ucsd.edu) was used to construct MS/MS molecular networks.18 To accurately use high-resolution mass spectrometry MS/MS data, system annotations for GNPS were set as follows: precursor ion mass tolerance set to 2.0 Da, fragment ion mass tolerance set to 0.5 Da, minimum pairwise cos 0.6, minimum matched fragment ion 2, minimum cluster size 1.
| No. | Compound name | Formula | RT (min) | Molecular mass | Precursor ion (m/z) | Product ion (m/z) | Fragmentor (V) | Collision energy (V) | Monitoring ion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ascorbic acid | C6H8O6 | 0.43 | 176.12 | 175.00 | 115.00 | 80 | 4 | [M − H]− |
| 2 | Malic acid | C4H6O5 | 0.44 | 134.09 | 132.90 | 115.00 | 70 | 5 | [M − H]− |
| 3 | Citric acid | C6H8O7 | 0.53 | 192.12 | 191.10 | 110.20 | 85 | 5 | [M − H]− |
| 4 | Succinic acid | C4H6O4 | 0.56 | 118.09 | 117.00 | 73.00 | 70 | 12 | [M − H]− |
| 5 | Quinic acid | C7H12O6 | 1.23 | 192.17 | 191.00 | 84.90 | 83 | 24 | [M − H]− |
| 6 | Cyanidin-3-O-glucoside | C21H21O11 | 1.42 | 449.39 | 449.00 | 287.00 | 100 | 10 | [M + H]+ |
| 7 | Cyanidin-3-O-galactoside | C21H21O11 | 1.55 | 449.38 | 449.00 | 287.00 | 120 | 10 | [M + H]+ |
| 8 | Arbutin | C12H16O7 | 1.75 | 272.20 | 274.30 | 105.70 | 140 | 12 | [M − H]− |
| 9 | Cyanidin-3-O-arabinoside | C20H19O10 | 2.03 | 419.81 | 421.20 | 287.00 | 140 | 21 | [M + H]+ |
| 10 | Chlorogenic acid | C16H18O9 | 2.22 | 354.31 | 352.80 | 191.10 | 100 | 10 | [M − H]− |
| 11 | (+)-Catechin | C15H14O6 | 2.41 | 290.27 | 291.00 | 139.00 | 100 | 9 | [M + H]+ |
| 12 | (−)-Epicatechin | C15H14O6 | 2.49 | 290.27 | 291.00 | 139.00 | 110 | 13 | [M + H]+ |
| 13 | Quercetin | C15H10O7 | 2.55 | 302.24 | 301.00 | 151.00 | 148 | 23 | [M − H]− |
| 14 | P-Coumaric acid | C9H8O3 | 2.69 | 164.16 | 162.90 | 119.00 | 75 | 16 | [M − H]− |
| 15 | Astragalin | C21H20O11 | 2.87 | 448.40 | 447.00 | 284.00 | 165 | 30 | [M − H]− |
| 16 | Benzoic acid | C7H6O2 | 3.04 | 122.12 | 121.00 | 77.00 | 75 | 15 | [M − H]− |
| 17 | Oleanolic acid | C30H48O3 | 5.78 | 456.70 | 457.30 | 411.10 | 140 | 10 | [M + H]+ |
| 18 | Ursolic acid | C30H48O3 | 5.83 | 456.70 | 457.30 | 411.10 | 120 | 15 | [M + H]+ |
The (ferric ion reducing antioxidant power) FRAP test was conducted in accordance with the approach reported with a minor modification.20 In a nutshell, the FRAP solution consisted of 2.5 mL TPTZ (2,4,6-tripyridyl-s-triazine) solution (10 mM) added to HCl solution (40 mM), 2.5 mL ferric chloride (FeCl3–6H2O) (20 mM) and 25 mL acetate buffer (0.3 M). Store mixed solutions at 37 °C for use. Subsequently, the sample solution (0.1 mL) was thoroughly mixed with FRAP solution (0.9 mL) and left at room temperature for 30 min, and the absorbance was measured at 593 nm.
| Inhibition rate (%) = ((Acontrol − Asample)/Acontrol) × 100%. |
Results and discussion
Total phenolic, flavonoid, and anthocyanin contents in lingonberry fruits, leaves and stems
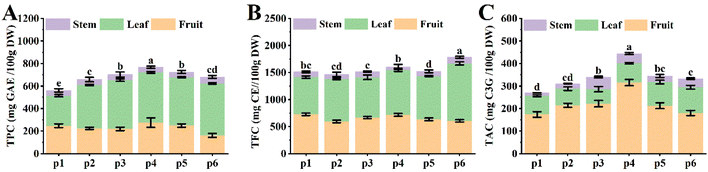
Polyphenols are widely found in a variety plants, many of which have been identified in food crops.21 In general, plant genetics affects the distribution of secondary metabolites, while external environmental factors can induce significant changes in metabolite composition.22 In this study, we found significant differences in the accumulation of phytochemical components in the different parts of the lingonberry, with TAC being the most abundant in the fruits, while TPC and TFC were accumulated to a greater extent in the leaves. Specifically, the lowest TPC in P4 stems was 44.94 mg GAE/100 g DW, whereas the highest TPC in P6 leaves was 461.55 mg GAE/100 g DW, a 10-fold difference (Fig. 2A), suggesting that plants genetically regulate phytochemical synthesis and transport to adapt to the external environment.23Moreover, altitude is an important environmental factor influencing the distribution of plant metabolites, with a gradual decrease in temperature and an increase in the intensity of visible light with increasing altitude. In bilberry berries, higher levels of TPC and TAC were found at 600 m altitude, and metabolite contents decreased sharply at both lower altitudes (450 m altitude) and excessive altitudes (from 800 m to 1500 altitude).24 In the present study, we found that TAC was highest in fruits at P4 (358.76 m altitude), whereas TPC and TFC were more in leaves at P6 (512.30 m altitude) compared to other parts of the plant (Fig. 1 and 2). Therefore, the external environment has an effect on the accumulation of most phenolic compounds, which may be a positive response to the defence mechanism against a negative external environment.3
Identification of metabolites in fruits, leaves and stems of lingonberry
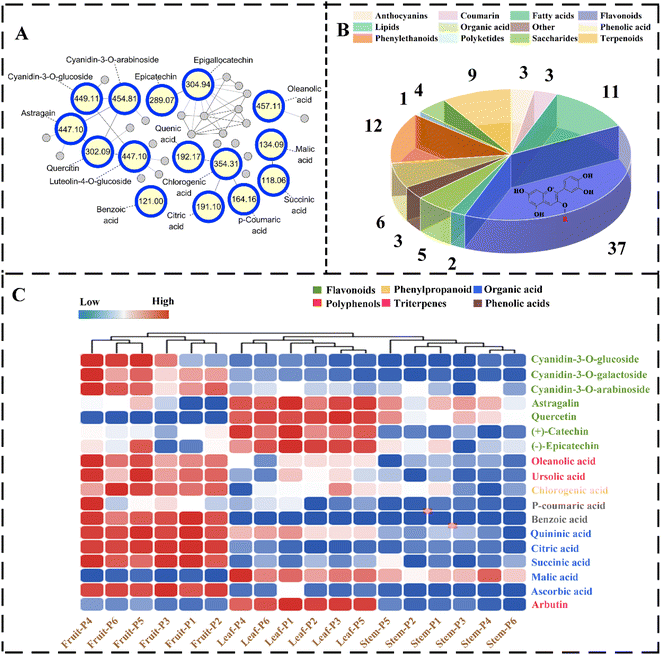
To further characterize the phytochemical composition of lingonberry fruits, leaves, and stems from different regions, UPLC-Q-Exactive Orbitrap MS was performed in a positive and negative ion mode.The metabolites were identified by comparing the precursor ions, MS2 fragmentation ions, accurate molecular weight and retention time with the standard databases of mzVault, mzCloud and BGI high resolution accurate mass plant metabolome database (BGI HRAM-PMDB). The GNPS platform is an open-access database of existing prevalent MS/MS spectral libraries that facilitate rapid compound identification based on MS/MS similarity networks by executing Proteowizard software to convert raw MS/MS files into mzXML data.18 After constructing a molecular network using raw MS/MS data of lingonberry extracts, data visualization was performed using Cytoscape 3.8.2 software, 1411 precursor ions of metabolites were observed, which were divided into 95 clusters (nodes ≥ 2) and 780 single nodes with a threshold cosine value of 0.7 (Fig. 3A).
After analysis of metabolites from various parts of lingonberries, a total of 95 metabolites were identified, as shown in Table S1.† Thereinto, 54 metabolites were identified through the GNPS library, and 23 known compounds were identified by comparison with the chemical standards. Additionally, in combination with the Compound Discovery 3.0 software mzCloud database, 20 metabolites were identified by comparing MS2 fragmentation patterns. The analysis results indicated that 37 flavonoids, 12 phenylethanoids, 11 fatty acids, 9 terpenoids, 5 phenolic acids, 5 organic acids, 4 saccharides, 3 coumarins, 3 anthocyanins, 2 lipids, 1 polyketide, and 3 compounds belonged to others (Fig. 3B).
In previous reports, investigations of lingonberry phytochemicals have focused on mixtures from localized sources and lacked criteria to assess their overall quality control.25 Based on this, this study conducted qualitative and quantitative analysis on lingonberry from different regions in China, with the aim of revealing the distribution characteristics of bioactive components in lingonberry and providing a basis for product development and biological breeding.
Quantification and distribution of the constituents in fruits, leaves and stems of lingonberry
Although 95 components were initially identified based on untargeted methods, quantification of all metabolites was challenging due to trace content and the absence of available reference substances. Therefore, to obtain accurate quantitative results, we used a targeted LC-QqQ-MS/MS method combined with chemical standards to quantify 18 target metabolites from 6 classes.First, critical parameters of chromatographic separation, such as column, mobile phase and elution efficiency, were optimized to achieve favorable peak shapes and reproducible separations, thus improving sensitivity and reliability. In addition, the full-scan MS method was used to detect in both positive and negative ionization modes. Based on this, the highest and most stable MRM leap response signal was obtained with the combination of adjusting the standard solution fragmentation voltage and collision energy to suit all analytes. Finally, the analytes were scanned and identified after manually optimising the parameters of the system's MRM mode. The calibration curve of the signal intensity (peak area) of the MRM transition to the six concentration gradients of the standard solution was plotted. The limits of LOD (S/N = 3) and LOQ (S/N = 10) results for each analyte were below 0.3 ng mL−1 and 0.7 ng mL−1, respectively. The intra-day RSD of the peak area was less than 4.21%, and the daytime RSD was less than 3.45% (Table S2†). In conclusion, the established UPLC-QqQ-MS/MS method was satisfactory linearity, sensitivity, precision, accuracy, and stability for the simultaneous determination of 18 compounds in complex lingonberry-related matrices (fruits, leaves and stems).
To evaluate the distributional capacity of different parts, a clustering heat map model was developed. The results are shown in Fig. 3C, where triplicates from several same geographical regions were successfully clustered, further demonstrating the reliability of the method.
Obviously, the results showed that 7 flavonoids (cyanidin-3-O-glucoside, cyanidin-3-O-galactoside and cyanidin-3-O-arabinoside, quercetin, (+)-catechin, (−)-epicatechin and astragalin), 1 phenylpropanoid (chlorogenic acid), 2 triterpenes (ursolic acid and oleanolic acid), 2 phenolic acids (benzoic acid and p-coumaric acid) and 5 organic acids (succinic acid, quinic acid, malic acid, citric acid, and ascorbic acid) and 1 polyphenols (arbutin) were the dominant components in lingonberry, which could be used as marker compounds for quality control. To the best of our knowledge, aerial parts of lingonberry are enriched with bioactive components, which are utilized in functional food materials.26
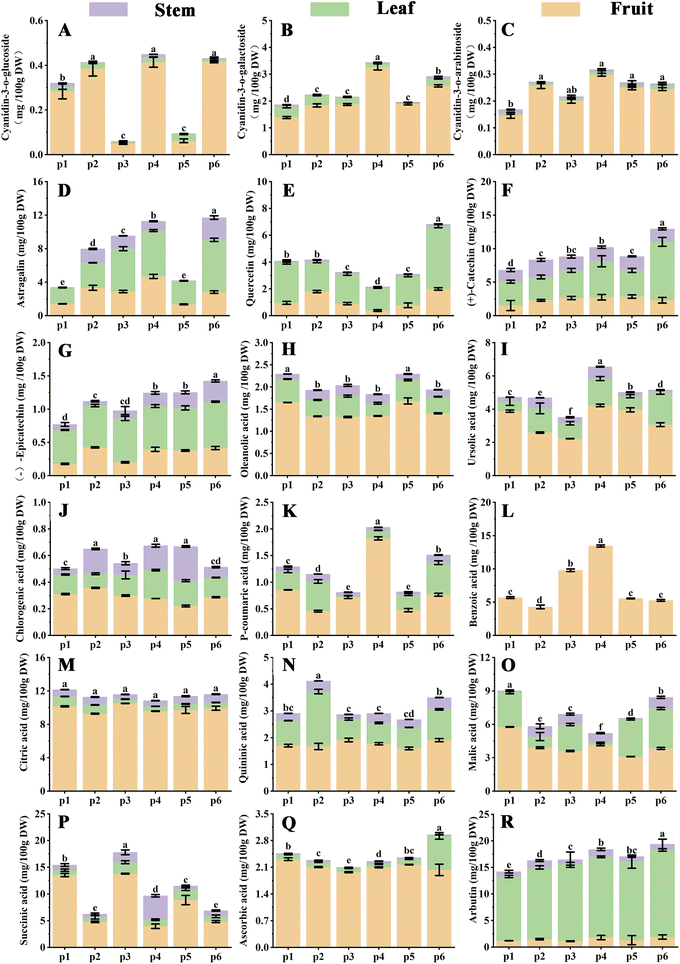
Previous findings have suggested that lingonberries contain a variety of bioactive phytochemicals including anthocyanins, flavonoids, flavanols, triterpenes and organic acids.27 The anthocyanins in lingonberry fruits are based on the parent nucleus of cyanidin with different glycoside compositions, as reported by Michiels et al.28 It has the highest content with strong antioxidant activity. In particular, we found that anthocyanidins accumulate most in fruits, with cyanidin-3-O-galactoside content up to 3.27 mg/100 g DW, and mature fruits show red cyanidin consistent with anthocyanidin causing.26 In addition, the content of triterpene acid in lingonberries had the highest percentage of the total triterpenoids and was significantly affected by the harvesting period.29 In the present study, we can conclude that the highest content of ursolic acid in the fruits (P4) was 4.23 mg/100 g DW, in contrast to chlorogenic acid (P2) which was the highest at 0.35 mg/100 g DW, indicating that the geographical location has a significant effect on the accumulation of active compounds (Fig. 4J). Furthermore, the organic acid content of the lingonberry fruits was important factor in the sensory characteristics of the fruits. It even has a significant influence on consumer acceptance.30,31 The results showed that phenolic and organic acids had the highest percentage of fruits composition, mainly dominated by citric acid (10.51 mg/100 g DW), quinic acid (1.92 mg/100 g DW), while benzoic acid (13.45 mg/100 g DW) was only detected in fruits, the main source of fruits acidity (Fig. 4L–N). In industrial production, acids are used as antioxidants, preservatives, acidulants and drug absorption modifiers.32 They are also used to maintain the quality and nutritional value of the fruits therefore, as a good source of acidic constituents for flavour and nutritional control, lingonberry fruits are an important indicator of berry quality parameters and a reasonable target for crop improvement.33
Lingonberry leaves contain a wide variety of chemical constituents compared to the fruits. Flavonoid glycosides are the most abundant phenolic compounds in lingonberry. They have astringent, antitussive, urinary antiseptic, diuretic, neuroprotective, antioxidant and anti-inflammatory effects, and possibly inhibitory effects on cancer cell growth.10 The results of this study showed that astragalin and quercetin, (+)-catechin and (−)-epicatechin were ranked in different parts: leaves > fruits > stems (Fig. 4D–G), with the highest content in the region P6. In addition, we found that the highest TFC was in the leaves (Fig. 2A), suggesting that the leaves are a potential site of application for flavonoid constituents, which correlates with the role of leaves in resistance defense.25,34 Interestingly, we found that the highest content of arbutin in leaves was up to 16.4 mg/100 g DW, which is 10 times higher than that in fruits. Lingonberry leaves are usually regarded as food waste and underutilised, with the advantage of being a potential low-cost source of food and medicine that avoids harming the plant.35
Compared to the fruits and leaves, the stems were found to contain fewer active compounds. As shown in Fig. S3A,† flavonoids were distributed in small amounts in the stems, while the chlorogenic acid content was similar to that of its parts. The bioactive compounds were mainly derived from the bark.25
In conclusion, the highest levels of anthocyanins, ursolic acid and phenolic acids were found in the fruits from the P4 region, while the leaves from the P6 region were more favourable for accumulating flavonoids and arbutin compounds, potentially serving as the best collecting point for the material.
Predominant chemical markers selection through multivariate statistical analysis
To visualize the comparison of quantitative data of the target compounds in fruits, leaves and stems, the PCA models were established (Fig. 5A). The results of the PCA indicated a strong separation trend of three parts (fruits, leaves and stems) from different geographical regions, which were clustered in three groups, indicating similarity in metabolite components and contents between the samples of the groups. Cyanidin-3-O-glucoside, cyanidin-3-O-galactoside, cyanidin-3-O-arabinoside, chlorogenic acid, ursolic acid, oleanolic acid, citric acid, quinic acid, p-coumaric acid, benzoic acid and ascorbic acid were responsible for the formation of the group I (fruits). Leaves in the group II were characterized by (+)-catechin, (−)-epicatechin, malic acid, quercetin, astragalin, succinic acid and arbutin.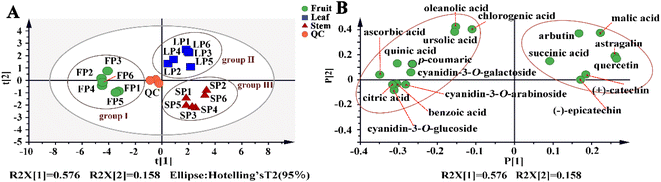 | ||
| Fig. 5 The PCA of lingonberry's fruits, stems and leaves from different regions (A) PCA score scatter plot; (B) loading plot. | ||
Clustering heat maps and projection variable importance (VIP) values were also employed to identify differences between parts. The loading plot metabolites with VIP values > 1 combined with clustering heatmaps compound abundances were considered as potential chemical makers that could be characterised for differences in phytochemical composition between fruits, leaves and stems (Fig. 5B). Among these compounds, cyanidin-3-O-galactoside, ursolic acid, (+)-catechin, arbutin, benzoic acid, citric acid and astragalin as principal chemical markers for their high abundance in the various parts.
Antioxidant activity
As potential antioxidant active substances in various sites, DPPH, ABTS+ and FRAP assays were performed to monitor their activity. The results of DPPH, ABTS+ and FRAP showed a similar trend in the following order: fruits > leaves > stems (Table 2). It has been shown that catechins and flavonols contain a catechol structure in the b-ring and three hydroxyl groups in the c-ring.36 In the present study, although the flavonoid content of the leaves was high, the antioxidant value was lower than that of the fruits. They have strong antioxidant activity. The fruits were thought to contain other antioxidant substances such as anthocyanin. Interestingly, the highest values for DPPH, ABTS+ and FRAP were all observed at the fruits in the P4 region, with 269.34 mol Trolox/g DW, 363.16 mol Trolox/g DW and 102.13 mol Trolox/g DW respectively. On the other hand, the antioxidant activity of crude plant extracts is related to the bioactive ingredients and is influenced by both external biotic and abiotic factors.20 In addition, compared to stems and leaves, the fruits represent a food and medicinal source with the advantage of avoided plant damage. Therefore, the fruits of the P4 region could be the best potential collection site for antioxidant material.| Site | Fruits | Leaves | Stems | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH (mol Trolox/g DW) | ABTS (mol Trolox/g DW) | FRAP (mol Trolox/g DW) | DPPH (mol Trolox/g DW) | ABTS (mol Trolox/g DW) | FRAP (mol Trolox/g DW) | DPPH (mol Trolox/g DW) | ABTS (mol Trolox/g DW) | FRAP (mol Trolox/g DW) | |
| a Data are presented as mean ± SD (n = 3). Different lowercase letters in each column indicate significant differences (p < 0.05). | |||||||||
| P1 | 226.88 ± 3.52b | 351.36 ± 5.66ab | 102.09 ± 0.93a | 79.07 ± 3.48d | 259.68 ± 6.75b | 78.04 ± 0.7b | 39.95 ± 8.91c | 89.15 ± 1.3c | 40.49 ± 0.58d |
| P2 | 93.14 ± 1.97e | 189.46 ± 4.3d | 64.38 ± 2.38d | 90.1 ± 9.13bc | 180.48 ± 6.65c | 62.41 ± 0.56d | 17.34 ± 4.87e | 127.95 ± 9.08ab | 32.77 ± 0.59f |
| P3 | 144.46 ± 3.97c | 349.96 ± 4.91b | 100.71 ± 0.34a | 102.73 ± 6.71ab | 336.11 ± 6.62a | 75.82 ± 0.58c | 27.26 ± 1.91d | 136.11 ± 6.66a | 43.1 ± 0.47c |
| P4 | 269.34 ± 9.74a | 363.16 ± 3.63a | 102.13 ± 1.08a | 89.41 ± 14.07bc | 345.36 ± 2.43a | 100.72 ± 0.26a | 71.45 ± 2.65a | 126.26 ± 2.3ab | 69.39 ± 0.46a |
| P5 | 109.85 ± 5.07d | 342.39 ± 6.64b | 92.91 ± 0.66b | 91.19 ± 9.38bc | 131.02 ± 9.7e | 75.21 ± 0.47c | 47.74 ± 9.09b | 107.48 ± 4.07bc | 50.38 ± 2.08b |
| P6 | 130.83 ± 3.98c | 225.42 ± 12.27c | 78.41 ± 0.56c | 120.49 ± 8.77a | 170.46 ± 6.69d | 74.87 ± 1.03c | 12.22 ± 3.27f | 140.61 ± 7.67a | 37.24 ± 0.97e |
Antiproliferative activity
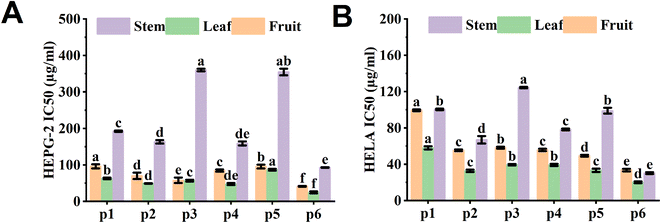
The antiproliferative activity of lingonberry from various origins was evaluated by MTT method. The results are shown in Fig. 6A and B, the lingonberry extracts showed strong antiproliferative effects on both HeLa and HepG-2 cell lines with IC50 values less than 400 μg mL−1. Notably, the leaves have the strongest antiproliferation effect, followed by the fruits and then the stems. It has been shown that arbutin and flavonoids are inhibitors of cancer cell proliferation.37 Of all the regions, we found the lowest IC50 values for leaves in the P6 region. This correlates with the high levels of arbutin and flavonoids in this region. As a result, potential pharmaceutical and healthcare products are also collected at the P6 site.Pearson correlation between bioactive compounds and bioactivities
It has been suggested that polyphenol content in plants has a positive correlation with antioxidant capacity.14 In this study, Pearson correlation analysis was performed to reveal the potential correlation between bioactive compounds and antioxidant activity in order to characterise the important antioxidant properties in different parts. As shown in Table 3, TPC, TAC, and TFC were significantly and positively correlated with DPPH, ABTS+ and FRAP activities in fruits, leaves and stems, in agreement with previous reports.11| Fruit | Leaf | Stem | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | HEPG2 (IC50) | HELA (IC50) | DPPH | ABTS | FRAP | HEPG2 (IC50) | HELA (IC50) | DPPH | ABTS | FRAP | HEPG2 (IC50) | HELA (IC50) | |
| a Asterisks indicate significant differences (*P < 0.05, **P < 0.01). Anthocyanins contain cyanidin-3-O-glucoside, cyanidin-3-O-galactoside and cyanidin-3-O-arabinoside; flavonols contain astragalin and quercetin; flavanols contain (+)-catechin and (−)-epicatechin; phenylpropanoids include chlorogenic acid; triterpene contain oleanolic acid and ursolic acid; phenolic acid contain p-coumaric and benzoic acid; organic acid contain quinic acid, citric acid, succinic acid and malic acid; flavonoids contain anthocyanins, flavonols and flavanols; polyphenols contain arbutin, flavonoids, phenylpropanoids and phenolic acids; TCM contains polyphenols, triterpenes and organic acids. | |||||||||||||||
| TPC | 0.793** | 0.785** | 0.529* | 0.489* | 0.358 | 0.482* | 0.839** | 0.609** | 0.141 | 0.063 | 0.529* | 0.486* | 0.533* | 0.311 | 0.376 |
| TFC | 0.662** | 0.700** | 0.426 | 0.454 | 0.346 | 0.352 | 0.695** | 0.521* | −0.143 | −0.118 | 0.589* | 0.505* | 0.532* | 0.466 | 0.508* |
| TAC | 0.775** | 0.850** | 0.586* | 0.491* | 0.327 | 0.677** | 0.536* | 0.552* | 0.155 | 0.130 | 0.452 | 0.505* | 0.568* | −0.414 | −0.528* |
| Anthocyanin | 0.746** | 0.641** | 0.855** | −0.283 | −0.147 | 0.615** | 0.749** | 0.689** | −0.024 | −0.037 | −0.159 | −0.352 | −0.295 | 0.281 | 0.296 |
| Flavonols | 0.150 | 0.220 | 0.152 | 0.131 | 0.174 | 0.095 | 0.567* | 0.448 | −0.071 | −0.067 | 0.560* | 0.406 | 0.432 | 0.293 | 0.238 |
| Flavanols | 0.302 | 0.631** | 0.521* | −0.201 | −0.283 | 0.649** | 0.634** | 0.737** | −0.324 | −0.297 | −0.076 | −0.609 | −0.147 | −0.091 | −0.026 |
| Flavonoids | 0.548* | 0.430 | 0.594** | −0.129 | −0.010 | 0.550* | 0.756** | 0.556* | −0.544** | −0.448* | 0.549* | 0.530* | 0.534* | −0.082 | −0.018 |
| Phenylpropanoids | −0.118 | 0.178 | 0.104 | −0.032 | −0.369 | −0.235 | 0.183 | 0.148 | −0.161 | −0.116 | 0.479* | 0.216 | 0.463 | −0.323 | −0.207 |
| Phenolic acid | 0.477* | 0.597** | 0.476* | −0.046 | −0.147 | 0.435 | 0.125 | 0.234 | −0.415 | −0.360 | 0.207 | 0.082 | 0.199 | −0.114 | −0.075 |
| Polyphenols | 0.588* | 0.547* | 0.659** | −0.132 | −0.073 | 0.580* | 0.663** | 0.493* | −0.041 | −0.035 | 0.652** | 0.621** | 0.734** | −0.095 | −0.027 |
| Triterpene | 0.472* | 0.560* | 0.520* | 0.242 | 0.133 | −0.251 | 0.310 | 0.165 | −0.055 | −0.078 | 0.157 | 0.009 | 0.076 | −0.438 | −0.565* |
| Organic acid | 0.067 | 0.106 | −0.167 | 0.061 | 0.080 | −0.606** | −0.226 | −0.329 | −0.181 | −0.224 | 0.231 | 0.321 | 0.276 | −0.117 | −0.282 |
| TCM | 0.509* | 0.492* | 0.470* | 0.145 | 0.100 | −0.005 | 0.597** | 0.397 | −0.012 | −0.030 | 0.684** | 0.655** | 0.766** | −0.128 | −0.083 |
It has been shown that anthocyanins have good antioxidant effects and have a protective effect on plants.38 In the fruits, the levels of anthocyanins (cyanidin-3-O-glucoside, cyanidin-3-O-galactoside and cyanidin-3-O-arabinoside), phenolic acids (p-coumaric and benzoic acid) and triterpenes (oleanolic acid and ursolic acid) were high and significantly and positively correlated with antioxidant activity, indicated that lingonberry fruit has antioxidant activity associated with a variety of bioactive compounds, which is consistent with previous reports.14 Altitude is an important environmental factor affecting the accumulation of metabolic substances in plants. In this study, we found that antioxidant activity (DPPH, ABTS+, FRAT) was strongest in lingonberry fruits at P4 (Table 2), and correlation analyses showed that phenolic and anthocyanin components were significantly and positively correlated with antioxidant activity (DPPH, ABTS+, FRAT) (Table 3), suggesting that altitude affects antioxidant activity of resistant plants through the accumulation of phytochemicals, which is in accordance with previous reports.39
Similarly, the flavonoids in lingonberry leaves showed a significant positive correlation with antioxidant capacity, while the DPPH assay revealed a weak correlation (r = 0.352), indicating that there were other undetected compounds (Table 3). In comparison, flavanols (catechins and epicatechins) in the leaves were significantly positively correlated with antioxidant activity, in agreement with previous reports.40
Flavonoids found in berries have been shown to penetrate the cell membranes of cancer cells and have a powerful antiproliferative effect.41 Flavonoids exhibited a significant negative correlation with IC50 values of HeLa and HepG-2 cell lines (r = −0.544, −0.448), while flavanols showed a weak negative correlation (r = −0.324, −0.297), indicating the existence of other antiproliferative constituents in the leaves. Furthermore, arbutin in the leaves showed cytotoxic activity and non-toxicity.42
Conclusions
This study provides the first insight into the phytochemical composition and bioactivities of lingonberry fruits, leaves and stems from different regions based on an integrated strategy. The results of comparative analyses showed that habitat influenced the distribution of secondary metabolites in lingonberry. Flavanols and arbutin were higher in leaves, and anthocyanins, triterpenoids and organic acids were mainly present in fruits. In particular, the highest arbutin content was present in Huzhong (P6) at 15 mg/100 g DW. The highest content of ursolic acid and cyanidin-3-O-galactoside (4.5 mg/100 g DW and 3.2 mg/100 g DW, respectively) was found in the fruits of Tahe (P4). In addition, antioxidant and antiproliferative assays revealed that fruits (P4) exhibited the strongest antioxidant properties, while leaves (P6) had antiproliferative activity. In conclusion, this work identifies potential applications for lingonberries in the food and pharmaceutical industries. Further studies will continue to assess toxicity and explore key compounds with antioxidant activity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial supports by the National Natural Science Foundation of China (32271805), National Natural Science Foundation of China (31930076), Fundamental Research Funds for the Central Universities (2572020DR07), The 111 Project (B20088), Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).References
- R. Dhalaria, R. Verma, D. Kumar, S. Puri, A. Tapwal, V. Kumar, E. Nepovimova and K. Kuca, Antioxidants, 2020, 9, 1–38 CrossRef.
- B. E. Stefanescu, L. F. Calinoiu, F. Ranga, F. Fetea, A. Mocan, D. C. Vodnar and G. Crisan, Antioxidants, 2020, 9, 649 CrossRef.
- G. Vilkickyte and L. Raudone, Plants, 2021, 10, 1986 CrossRef CAS PubMed.
- C. Mane, M. Loonis, C. Juhel, C. Dufour and C. Malien-Aubert, J. Agric. Food Chem., 2011, 59, 3330–3339 CrossRef CAS PubMed.
- P. Vyas, N. H. Curran, A. U. Igamberdiev and S. C. Debnath, Can. J. Plant Sci., 2015, 95, 663–669 CrossRef CAS.
- T. Kostka, J. J. Ostberg-Potthoff, J. Starke, C. Guigas, S. Matsugo, V. Mirceski, L. Stojanov, S. K. Velickovska, P. Winterhalter and T. Esatbeyoglu, Antioxidants, 2022, 11, 467 CrossRef CAS PubMed.
- P. Z. Liu, A. Lindstedt, N. Markkinen, J. Sinkkonen, J. P. Suomela and B. R. Yang, J. Agric. Food Chem., 2014, 62, 12015–12026 CrossRef CAS PubMed.
- A. Szakiel, C. Paczkowski, H. Koivuniemi and S. Huttunen, J. Agric. Food Chem., 2012, 60, 4994–5002 CrossRef CAS.
- S. E. Sanchez and S. A. Kay, Cold Spring Harbor Perspect. Biol., 2016, 8, a027748 CrossRef PubMed.
- S. Dragovic, V. Dragovic-Uzelac, S. Pedisic, Z. Cosic, M. Friscic, I. Elez Garofulic and Z. Zoric, Food Technol. Biotechnol., 2020, 58, 303–314 CrossRef PubMed.
- G. Vilkickyte and L. Raudone, Foods, 2021, 10, 2243 CrossRef CAS PubMed.
- G. Vilkickyte, V. Motiekaityte, R. Vainoriene and L. Raudone, J. Food Compos. Anal., 2022, 114, 104796 CrossRef CAS.
- M. V. Dragana, R. P. Miroslav, B. R. G. Branka, D. S. Olgica, M. V. Sava and R. Č. Ljiljana, Afr. J. Microbiol. Res., 2013, 7, 5130–5136 CrossRef.
- O. C. Bujor, C. Le Bourvellec, I. Volf, V. I. Popa and C. Dufour, Food Chem., 2016, 213, 58–68 CrossRef CAS PubMed.
- R.-Y. Gan, Z.-Q. Deng, A.-X. Yan, N. P. Shah, W.-Y. Lui, C.-L. Chan and H. Corke, Lwt, 2016, 73, 168–177 CrossRef CAS.
- Z. Z. Bai, J. M. Tang, J. Ni, T. T. Zheng, Y. Zhou, D. Y. Sun, G. N. Li, P. Liu, L. X. Niu and Y. L. Zhang, Food Res. Int., 2021, 148, 110609 CrossRef CAS PubMed.
- S. J. Krithika, B. Sathiyasree, E. B. Theodore, R. Chithiraikannu and K. Gurushankar, Crit. Rev. Food Sci. Nutr., 2020, 62(9), 1–8 Search PubMed.
- M. Wang, J. J. Carver, V. V. Phelan, L. M. Sanchez, N. Garg, Y. Peng, D. D. Nguyen, J. Watrous, C. A. Kapono, T. Luzzatto-Knaan, C. Porto, A. Bouslimani, A. V. Melnik, M. J. Meehan, W. T. Liu, M. Crusemann, P. D. Boudreau, E. Esquenazi, M. Sandoval-Calderon, R. D. Kersten, L. A. Pace, R. A. Quinn, K. R. Duncan, C. C. Hsu, D. J. Floros, R. G. Gavilan, K. Kleigrewe, T. Northen, R. J. Dutton, D. Parrot, E. E. Carlson, B. Aigle, C. F. Michelsen, L. Jelsbak, C. Sohlenkamp, P. Pevzner, A. Edlund, J. McLean, J. Piel, B. T. Murphy, L. Gerwick, C. C. Liaw, Y. L. Yang, H. U. Humpf, M. Maansson, R. A. Keyzers, A. C. Sims, A. R. Johnson, A. M. Sidebottom, B. E. Sedio, A. Klitgaard, C. B. Larson, C. A. Boya, D. Torres-Mendoza, D. J. Gonzalez, D. B. Silva, L. M. Marques, D. P. Demarque, E. Pociute, E. C. O’Neill, E. Briand, E. J. N. Helfrich, E. A. Granatosky, E. Glukhov, F. Ryffel, H. Houson, H. Mohimani, J. J. Kharbush, Y. Zeng, J. A. Vorholt, K. L. Kurita, P. Charusanti, K. L. McPhail, K. F. Nielsen, L. Vuong, M. Elfeki, M. F. Traxler, N. Engene, N. Koyama, O. B. Vining, R. Baric, R. R. Silva, S. J. Mascuch, S. Tomasi, S. Jenkins, V. Macherla, T. Hoffman, V. Agarwal, P. G. Williams, J. Dai, R. Neupane, J. Gurr, A. M. C. Rodriguez, A. Lamsa, C. Zhang, K. Dorrestein, B. M. Duggan, J. Almaliti, P. M. Allard, P. Phapale, L. F. Nothias, T. Alexandrov, M. Litaudon, J. L. Wolfender, J. E. Kyle, T. O. Metz, T. Peryea, D. T. Nguyen, D. VanLeer, P. Shinn, A. Jadhav, R. Muller, K. M. Waters, W. Shi, X. Liu, L. Zhang, R. Knight, P. R. Jensen, B. O. Palsson, K. Pogliano, R. G. Linington, M. Gutierrez, N. P. Lopes, W. H. Gerwick, B. S. Moore, P. C. Dorrestein and N. Bandeira, Nat. Biotechnol., 2016, 34, 828–837 CrossRef CAS PubMed.
- Q. Q. Yang, D. Zhang, A. K. Farha, X. Yang, H. B. Li, K. W. Kong, J. R. Zhang, C. L. Chan, W. Y. Lu, H. Corke and R. Y. Gan, Ind. Crops Prod., 2020, 143, 111928 CrossRef CAS.
- Z. Z. Bai, J. Ni, J. M. Tang, D. Y. Sun, Z. G. Yan, J. Zhang, L. X. Niu and Y. L. Zhang, Food Chem., 2021, 343, 128444 CrossRef CAS.
- D. Li, B. Li, Y. Ma, X. Sun, Y. Lin and X. Meng, J. Food Compos. Anal., 2017, 62, 84–93 CrossRef CAS.
- K. Karppinen, L. Zoratti, N. Nguyenquynh, H. Haggman and L. Jaakola, Front. Plant Sci., 2016, 7, 655 Search PubMed.
- Z. Alam, H. R. Morales and J. Roncal, Botany, 2016, 94, 509–521 CrossRef CAS.
- G. Rieger, M. Muller, H. Guttenberger and F. Bucar, J. Agric. Food Chem., 2008, 56, 9080–9086 CrossRef CAS PubMed.
- O. C. Bujor, C. Ginies, V. I. Popa and C. Dufour, Food Chem., 2018, 252, 356–365 CrossRef CAS PubMed.
- K. Kowalska, Int. J. Mol. Sci., 2021, 22, 5126 CrossRef CAS PubMed.
- P. Trivedi, N. Nguyen, L. Klavins, J. Kviesis, E. Heinonen, J. Remes, S. Jokipii-Lukkari, M. Klavins, K. Karppinen, L. Jaakola and H. Häggman, Food Chem., 2021, 354, 129517 CrossRef CAS PubMed.
- J. A. Michiels, C. Kevers, J. Pincemail, J. O. Defraigne and J. Dommes, Food Chem., 2012, 130, 986–993 CrossRef CAS.
- E. Hajazimi, R. Landberg and G. Zamaratskaia, Lwt, 2016, 74, 128–134 CrossRef CAS.
- B. M. Silva, P. B. Andrade, G. C. Mendes, R. M. Seabra and M. A. Ferreira, J. Agric. Food Chem., 2002, 50, 2313–2317 CrossRef CAS PubMed.
- E. Ermis, C. Hertel, C. Schneider, R. Carle, F. Stintzing and H. Schmidt, Int. J. Food Microbiol., 2015, 204, 111–117 CrossRef.
- H. G. Daood, P. A. Biacs, M. A. Dakar and F. Hajdu, J. Chromatogr. Sci., 1994, 32, 481–487 CAS.
- Z. Liang, M. Sang, P. Fan, B. Wu, L. Wang, W. Duan and S. Li, J. Food Sci., 2011, 76, C1231–C1238 CrossRef CAS.
- Y. Tian, J. Liimatainen, A. L. Alanne, A. Lindstedt, P. Liu, J. Sinkkonen, H. Kallio and B. Yang, Food Chem., 2017, 220, 266–281 CrossRef CAS.
- X. Zhu, Y. Tian, W. Zhang, T. Zhang, C. Guang and W. Mu, Appl. Microbiol. Biotechnol., 2018, 102, 8145–8152 CrossRef CAS PubMed.
- K. Csepregi, S. Neugart, M. Schreiner and E. Hideg, Molecules, 2016, 21, 208 CrossRef PubMed.
- S. Sarli and N. Ghasemi, Eurasian Chem. Commun., 2020, 2, 302–318 CrossRef CAS.
- Z. Liang, H. Liang, Y. Guo and D. Yang, Int. J. Mol. Sci., 2021, 22, 2261 CrossRef CAS PubMed.
- M. Mikulic-Petkovsek, V. Schmitzer, A. Slatnar, F. Stampar and R. Veberic, J. Sci. Food Agric., 2015, 95, 776–785 CrossRef CAS PubMed.
- Y. Zhang, X. Zhou, W. Tao, L. Li, C. Wei, J. Duan, S. Chen and X. Ye, J. Funct. Foods, 2016, 27, 645–654 CrossRef CAS.
- V. Rupasinghe, S. V. Neir and I. Parmar, Funct. Foods Health Dis., 2016, 6, 754–768 Search PubMed.
- Ö. Hazman, A. Sarıova, M. F. Bozkurt and İ. H. Ciğerci, Mol. Cell. Biochem., 2020, 76, 349–360 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra05698h |
| This journal is © The Royal Society of Chemistry 2023 |