Open Access Article
Open Access ArticleMolecular insights into the interfacial adhesion mechanism between carbon nanotubes and epoxy resin
Songyue Chai,
Jiao Liu,
Dongshuai Hou and
Pan Wang*
and
Pan Wang*
Department of Civil Engineering, Qingdao University of Technology, Qingdao 266033, China. E-mail: wangpan@qut.edu.cn
First published on 23rd October 2023
Abstract
In recent years, carbon nanotubes (CNTs) have garnered widespread attention and have been deemed the preferred option for the creation of epoxy composites, owing to their outstanding mechanical properties. Despite this, the interaction between pure CNTs and epoxy resin is primarily dependent on van der Waals forces and therefore, the interfacial forces are weak, making it challenging for effective load transfer. To enhance the mechanical properties of the composites, surface functionalization is often deemed a more favorable method for improving interfacial bond strength. This study employs molecular dynamics simulations to examine the interfacial bonding characteristics between functionalized CNTs and epoxy resin. The results demonstrate that functional group modification can significantly improve the interfacial adhesion between CNTs and epoxy resin, and the incorporation of functional groups can enhance the crosslinking degree of the epoxy resin at the interface. The hydrogen bond network established between the CNTs and epoxy resin after functional group modification, and the high stability of the bond cooperation, are factors contributing to the excellent interfacial performance.
1 Introduction
Epoxy resin is a three-dimensional crosslinked thermosetting material composed of a bisphenol-A epoxy resin monomer and a suitable curing agent, characterized by its excellent stability, heat resistance, favorable chemical corrosion resistance, and electrical insulation.1–3 The material has seen widespread applications in industries such as aerospace, building materials, and electronic communications. However, despite its high crosslinking density, epoxy resin is known for its low absolute strength, poor fracture toughness, and high brittleness, leading to weak impact resistance and limited lifetime and application range of the composites.4,5 Therefore, it becomes imperative to investigate methods to improve the mechanical performance of epoxy resin.The advancement of nanotechnology has presented a new approach to enhance the mechanical properties of polymer systems. Numerous studies have demonstrated that the mechanical performance of epoxy resin can be improved through the doping of nanoparticles, such as TiO2,6–8 silica,9,10 and carbon nanotubes.11,12 Carbon nanotubes (CNTs) have garnered extensive interest due to their unique physical and chemical properties13 since Iijima et al.14 published the first article on them in 1991. CNTs are typical one-dimensional nanoparticles with a high specific surface area and light weight,15,16 and possess excellent tensile strength along their longitudinal axis.17,18 Utilizing CNTs as nanomaterials to improve the mechanical properties of composites has garnered interest in both experimental and theoretical studies.19–21 Experimental studies have demonstrated that incorporating CNTs into epoxy resins has a positive impact on the mechanical properties of composites, such as the elastic modulus and tensile strength. For instance, Chen et al.22 found that grafted polymers on CNTs improved the compatibility between CNTs and epoxy resins, leading to strong interfacial chemical bonding, and a 68% increase in tensile strength was achieved through the modification of epoxy resins with CNTs after grafting, attributed to the formation of a CNT network that transfers part of the external load. Bansal et al.12 suggested that uniform dispersion of CNTs within the polymer, even in small amounts, can result in a significant improvement in the mechanical properties of epoxy composites. This conclusion provides a valuable direction for the advancement of the field of CNT composites. The high interfacial area and bridging mechanism properties of MWCNTs are effective in suppressing the growth of nano-pores and inhibiting crack propagation in the substrate, thus enhancing the properties of the composites.23 In addition to the above experimental studies, molecular dynamics (MD) techniques are widely utilized across various fields, including polymer composites. A comprehensive systematic investigation of nanoparticle-reinforced epoxy resins has been conducted. Hou et al.24 employed atomic simulations to examine the mechanism behind the impact of graphene oxide on the bonding mechanism at the interface between epoxy resin and calcium silicate hydrate (CSH). They proposed that strong interfacial bonding cooperation is the key factor responsible for the enhanced bonding effect. Sul et al.25 performed simulations of carbon nanotubes (CNTs) in epoxy resin and suggested that CNTs can increase the elastic modulus of composites and decrease the glass transition temperature. The poor solubility of nanomaterials in polymers can result in inadequate mechanical properties of composites.
However, it should be noted that extensive research has demonstrated a significant relationship between the performance of epoxy resin composite materials and the interfacial bonding ability of epoxy resin, regardless of the type of nanomaterial incorporated. Baudot et al.26 found that incorporating carbon nanotubes into the epoxy resin matrix during the polymerization process leads to improved mechanical strength and enhanced heat transfer. Hou et al.27 discovered that the mechanical properties of epoxy resin modified with graphene oxide (GO) depend on the bonding strength between GO and epoxy resin. Wang et al.28 suggested that the interfacial strength and adsorption capacity of epoxy resin significantly affect the strength of concrete structures. Mao et al.29 also observed that the bonding interaction between carbon nanotubes and the epoxy resin matrix effectively enhances the properties of the nanocomposites.
Therefore, enhancing the interfacial adhesion between the polymer and carbon nanotubes will greatly improve the performance of epoxy resin composite materials. To address the aforementioned challenges, covalent functionalization of carbon nanotubes has been identified as a viable solution for improving the interfacial interactions.30–33 Wang et al.34 employed the grafting of polymers onto multi-walled carbon nanotubes as a means to toughen epoxy composites, and the results revealed that the modified nanotubes resulted in uniform dispersion of CNTs, which were hindered from lateral interactions and led to enhanced interfacial bonding between CNTs and the epoxy resin. Ma et al.35 conducted amination treatment on the surface of CNTs, and observed a significant improvement in the stability of CNTs. Jung et al.36 verified through experiments and simulations that improving interfacial adhesion is crucial for enhancing the mechanical properties of polymer composites.
Nevertheless, most researchers have primarily focused on the impact of CNTs on the mechanical properties of composites, and only a few have briefly discussed the effect of functional groups. The mechanism behind the impact of functional groups on interfacial bonding remains unclear, impeding the progress of epoxy resin-reinforced materials.
Considering the strong correlation between mechanical properties of composites and interfacial adhesion, this paper investigates the effect of functional groups on the interfacial bonding mechanism of epoxy resin composites using molecular dynamics. Three composites reinforced with single wall CNTs, including unfunctionalized, hydroxyl-functionalized, and carboxy-functionalized groups, were established to observe differences in interfacial bonding properties through pull-out simulations of CNTs. This study is expected to offer a novel understanding of the principles of CNT-reinforced composites and provide a new perspective for the advancement of nanoreinforced composites.
2 Computational methods
2.1 Model construction
The two main components of a crosslinked epoxy are bisphenol A (DGEBA) as the monomer and isophorone diamine (IPD) as the crosslinker. The atomic structure of these components is depicted in Fig. 1(a). In this study, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of DGEBA and IPD were crosslinked to produce epoxy resin, resulting in a final crosslinking degree of 86%, satisfying the requirement for cross-linking determined by experimental studies (≥80%).37,38 The cell density of the epoxy was maintained at 1.131 g cm−3, which is in close agreement with the reported epoxy resin density values of 1.07–1.34 g cm−3 provided in the ref. 38 and 39. The crosslinking procedure was based on the methods proposed by Jian40 and Jeyranpour.41 The crosslinking process involved placing DGEBA and IPD in a predefined three-dimensional box. The reactive atom of DGEBA was designated as R1 and the reactive atom of IPD as R2. The reactive sites were identified within the reactive radius. For instance, the reactive carbon atom of DGEBA was selected, and then the reactive nitrogen atom of IPD was sought in proximity to the carbon atom. If R1 and R2 were within a specified reaction cutoff radius, covalent bonds between C–N atoms were formed, corresponding to the ring-opening of epoxy resin and the addition reaction of the curing agent. The cut-off radius range was set at 3.5–8 Å, with a 0.5 Å interval. The crosslinking temperature was maintained at 500 K, and the target crosslinking degree was 100%. Finally, the system was optimized over a short term to mitigate any adverse interactions resulting from bonding.
1 molar ratio of DGEBA and IPD were crosslinked to produce epoxy resin, resulting in a final crosslinking degree of 86%, satisfying the requirement for cross-linking determined by experimental studies (≥80%).37,38 The cell density of the epoxy was maintained at 1.131 g cm−3, which is in close agreement with the reported epoxy resin density values of 1.07–1.34 g cm−3 provided in the ref. 38 and 39. The crosslinking procedure was based on the methods proposed by Jian40 and Jeyranpour.41 The crosslinking process involved placing DGEBA and IPD in a predefined three-dimensional box. The reactive atom of DGEBA was designated as R1 and the reactive atom of IPD as R2. The reactive sites were identified within the reactive radius. For instance, the reactive carbon atom of DGEBA was selected, and then the reactive nitrogen atom of IPD was sought in proximity to the carbon atom. If R1 and R2 were within a specified reaction cutoff radius, covalent bonds between C–N atoms were formed, corresponding to the ring-opening of epoxy resin and the addition reaction of the curing agent. The cut-off radius range was set at 3.5–8 Å, with a 0.5 Å interval. The crosslinking temperature was maintained at 500 K, and the target crosslinking degree was 100%. Finally, the system was optimized over a short term to mitigate any adverse interactions resulting from bonding.
 | ||
| Fig. 1 (a) Epoxy molecule (b) functionalized carbon nanotubes (c) CNTs/epoxy interface model schematic diagram. | ||
The atomic structure of single wall CNTs is illustrated in Fig. 1(b). The CNTs are constructed as follows: the CNT cell (x = 11.483 Å, y = 11.483 Å, z = 2.459 51 Å, x = y = 90°, z = 120°) is selected with n = m = 6 and a diameter of 8.14 Å. This cell is then expanded by 15 in the z direction to match the size of the epoxy resin. Hydroxyl (–OH) and carboxyl (–COOH) functional groups are then placed in the same position on the CNTs, with 16 functional groups in total. This placement of the functional groups at the same position helps to reduce the influence of positional differences.
The interface between carbon nanotubes (CNTs) modified with different functional groups and epoxy resin is simulated through the construction of three models: CNT/epoxy, CNT-OH/epoxy, and CNT-COOH/epoxy. As depicted in Fig. 1(c), the carbon nanotubes are centered within a box of dimensions 40 Å × 40 Å × 100 Å. To simulate the process of CNT failure at the crack location within the loaded epoxy resin matrix as shown in Fig. 1(c) due to pulling.
2.2 Computational details
The Consistent Valence Force Field (CVFF) is applied to model the interatomic potential between epoxy molecules and CNTs. This force field has been extensively used in the simulation of epoxy-CNT interface bonding, as evidenced by various studies.40–42 The MD simulations were performed using the LAMMPS software.43 To obtain the global and local minimum energy configurations, eliminate irrational structures, and reduce internal stress, the initial structure underwent geometric optimization and dynamic relaxation. The calculation of the static structure was divided into two stages. The whole dynamics process was carried out using the NPT ensemble. In the first stage, the ambient temperature was set to 300 K and the time step was 1 fs. In the second stage, a dynamics simulation of 2 ns was performed, with a collection interval of 1 ps for the atomic trajectories. 2000 atomic trajectories were obtained to analyze the microstructure and dynamic properties of CNTs/epoxy composites. In the dynamic simulation process, the first two stages of the pull-out process were identical. In the third stage, the external force applied to the carbon nanotube during the pull-out process was extracted, and a force-displacement curve was generated. The pull-out force acted on the hydrogen atom closest to the vacuum layer, and the external force was calculated using the following formula:| Fpull = K(z0 + v × t) − Kzcom | (1) |
| E = Etotal − Eepoxy − Ecnts | (2) |
3 Results and discussion
3.1 Influence of functionalized-CNTs on the microstructures of epoxy resin
 | (3) |
The values of V0, Vf, and FFV for various systems are computed and presented in Table 1. These values are employed to analyze the impact of functionalization on the fractional free volume of the system. As shown in Fig. 5(a), the free volume diagram of the CNT-OH/epoxy resin system with a 86% crosslinking degree at 300 K is depicted. The gray and blue areas represent the free volume surface area and free volume, respectively.
| Systems | CNT/epoxy | CNT-OH/epoxy | CNT-COOH/epoxy |
|---|---|---|---|
| Occupied volume (Å3) | 69774.58 | 71615.99 | 72085.25 |
| Free volume (Å3) | 10225.42 | 8384.01 | 7914.75 |
| FFV (%) | 12.78% | 10.48% | 9.89% |
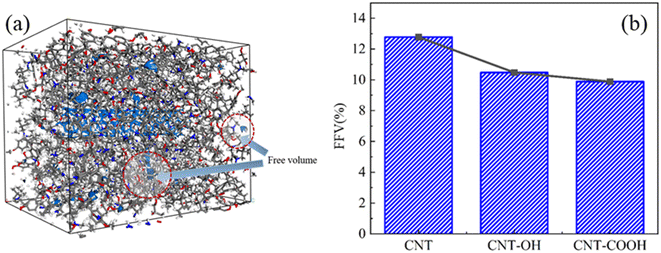 | ||
| Fig. 5 (a) The free volume diagram of CNT-OH/epoxy resin system with 86% crosslinking degree; (b) the FFV of different functionalized epoxy resin systems. | ||
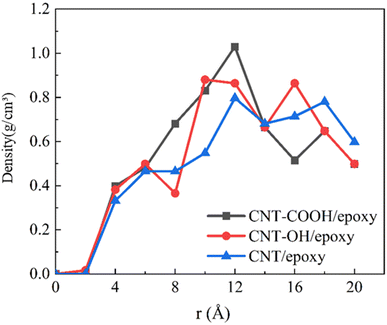
The FFV of various functionalized epoxy resin systems are determined. As seen in Fig. 5(b), the FFV exhibits a decreasing trend with the incorporation of functional groups on the CNTs. Upon the addition of functional groups on the CNTs, a significant decrease in the FFV is observed, and the greatest reduction is noted in the CNT-COOH system. This trend indicates that functionalization leads to a denser accumulation of polymer in the system, suggesting that functionalization will increase the intermolecular interaction between CNTs and epoxy resin. This could also account for the effect of partial functionalization, as discussed in 3.1.1, on the density distribution of epoxy resin. Given that functionalization results in a decrease in the FFV and a more compact arrangement of the system, the density distribution of epoxy resin near the interface is observed to follow the order: CNT-COOH > CNT-OH > CNT.
3.2 Enhancing mechanism of functionalized-CNTs on the interfacial bonding properties
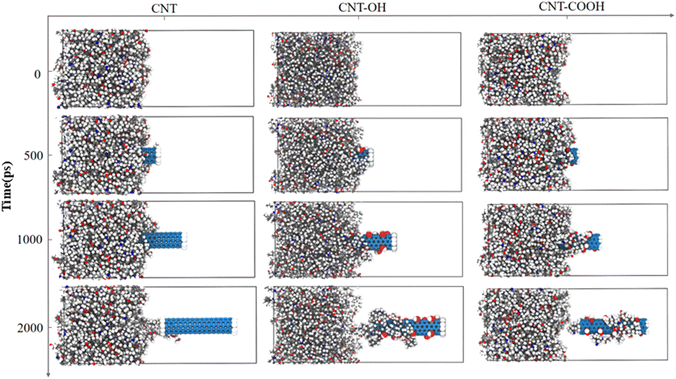
The pull-out process of CNTs can be described as follows: firstly, an initial configuration is optimized through geometric optimization and dynamic relaxation to attain an acceptable equilibrium configuration. Fixing a portion of the epoxy is crucial in preventing the rigid motion of the epoxy resin. Secondly, an external force is applied to a subset of CNT atoms. The formula for calculating external force is listed in 2.2. Finally, the structure with the lowest energy is obtained through relaxation. After repeated loading, the CNTs are completely detached from the epoxy resin. The representative snapshot in Fig. 6 displays the dynamic pull-out simulation, which is employed to investigate the interfacial bonding performance. At 0 ps, a dynamic equilibrium between the CNTs and epoxy resin is established. Furthermore, at this simulation time, the pull-out distance of CNTs in the CNT-COOH/epoxy system is the smallest among all systems, which, in conjunction with the observation that a small amount of epoxy resin remains adhered to the CNTs after full detachment, suggests the existence of robust interfacial adhesion between the CNTs and epoxy resin in the CNT-COOH/epoxy system. Additionally, it is noted that most epoxy resins adhere to functional groups, suggesting that functionalization can improve the interfacial adhesion between epoxy resin and CNTs.
Fig. 7(a) depicts the force–displacement relationship of various CNTs during the pull-out simulation. The load–displacement curve displays a periodic multi-peak phenomenon, which is strongly related to the breaking and reforming of the interface chemical bonds. The maximum shearing force that the CNTs can sustain is also closely correlated with the interface interaction. The maximum pulling force applied to the CNTs can be extracted from the force–displacement curve, as shown in Fig. 7(b). The maximum external force applied to the CNTs in the CNT-COOH/epoxy system is 5.25 × 10−9 N, while the maximum force applied in the CNT-OH/epoxy system is 4.49 × 10−9 N. In contrast, only 1.344 × 10−9 N is applied to the CNT/epoxy system, which is the lowest and coincides with the polarity of the functional groups. The results indicate that functionalization enhances the interfacial adhesion between the CNTs and epoxy resin. To further investigate this effect, the work of pulling force applied on the CNTs during the simulation was calculated, as shown in Fig. 8(a). The work done by the pulling force follows the sequence: CNT-COOH/epoxy > CNT-OH/epoxy > CNT/epoxy, which is consistent with the magnitude of the pulling force. This suggests that functionalization constitutes an effective approach to improve the interfacial adhesion between epoxy resin and CNTs. The microscopic mechanism underlying this improvement in mechanical properties will be explored in the subsequent section.
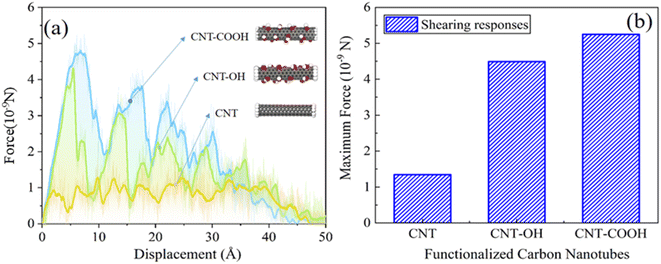 | ||
| Fig. 7 (a) The load–displacement of various systems. (b) The maximum pulling force applied to the carbon nanotubes. | ||
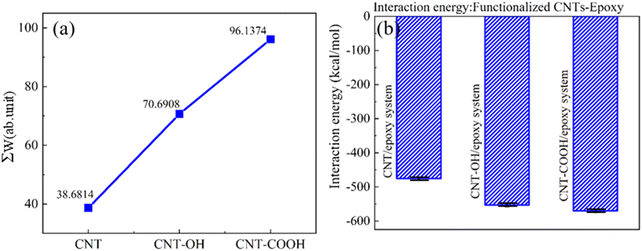 | ||
| Fig. 8 (a) The work done by pulling force. (b) The interaction energy between functionalized CNT-epoxy. | ||
Fig. 8(b) presents the interaction energy between epoxy resin and carbon nanotubes in various systems at an equilibrium stage. The interaction energy between the CNTs and epoxy resin is estimated to be approximately −475 kcal mol−1 in the absence of functionalization. The CNT-OH/epoxy system exhibits an interaction energy of −552 kcal mol−1, representing an increase of approximately 16.2%. In the CNT-COOH/epoxy system, the interaction energy is further increased by approximately 20%. The functionalization of CNTs leads to varying degrees of increased interaction energy between the epoxy resin and CNTs, suggesting that functionalized CNTs can enhance the interaction between the two materials. This observation helps explain why CNTs exhibit varying levels of adhesion to the epoxy resin when completely extracted from the resin. High interaction energy confers a strong adhesive property at the composite interface, and constitutes compelling evidence for the enhanced mechanical properties of CNT-reinforced epoxy resin.
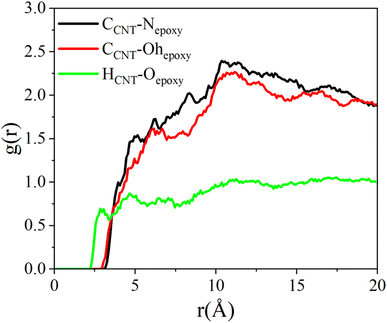
The enhancement of interfacial strength between functionalized CNTs and epoxy resin is closely tied to the chemical bonds present at the interface. This section explains the reason behind the difference in force–displacement curves by analyzing the changes in ionic and hydrogen bonds at the interface of functionalized CNTs. These chemical bonds are analyzed qualitatively using radial distribution functions (RDFs), which serve to reflect the spatial relationships between atoms. For the sake of simplicity, hydroxyl hydrogen (hoCNT), hydroxyl oxygen (hoCNT), and double bond oxygen (hoCNT) represent the atoms of CNTs, while hydroxyl hydrogen (hoepoxy), hydroxyl oxygen (ohepoxy) and nitrogen atoms (Nepoxy) represent the atoms of epoxy resin. As shown in Fig. 9, for the CNT/epoxy composite, the RDF of CCNT–Nepoxy, CCNT–Ohepoxy and HCNT–Oepoxy did not display a clear peak, indicating that ion pairs and hydrogen bonds cannot form between epoxy resin and CNTs, which is consistent with the findings of Zhang et al.45 This inability to form chemical bonds at the interface leads to low interfacial bond strength, which is in agreement with the conclusions drawn from previous pull-out simulations. Given the absence of chemical bonds at the interface of CNT-epoxy composites, the following section will not delve further into this topic.
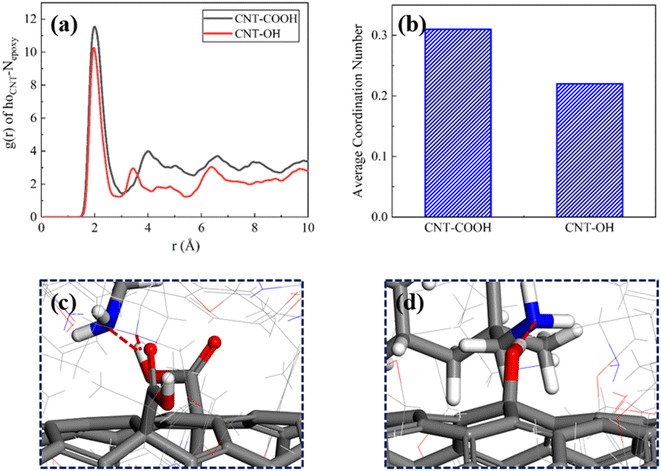
For CNT-OH/epoxy composite, the RDF between Nepoxy and hoCNT displays the obvious peaks at 2.05 Å, indicating that ions pairs can be developed between epoxy resins and CNTs. And the same ion pair is formed in CNT-COOH/epoxy composite, indicating that ion pairs can be formed at the interface of the two systems, as dispicted in Fig. 10(a). However, the intensity of the RDF peak (Nepoxy–hoCNT) in CNT-OH/epoxy composite is lower than that of the counterpart in CNT-COOH/epoxy composite. The low strength of RDF peak indicates that the attraction between Nepoxy atom (epoxy) and hoCNT atom (CNT) in CNT-OH/epoxy composites is weak. As shown in Fig. 10(b), this trend can be quantified by the average coordination number of Nepoxy–hoCNT pairs. By comparing the configuration snapshots of the interface region (Fig. 10(c) and (d)) in CNT-COOH/epoxy composite and CNT-OH/epoxy composite, the epoxy resin is attracted to the CNTs, which contributes to the improvement of interfacial bonding performance. At the same time, it also explains from the perspective of bonding that CNTs COOH and epoxy resin matrix have formed better interfacial bonding performance and shear strength in other works.46
The RDF between Nepoxy and hoCNT in the CNT-OH/epoxy composite reveals clear peaks at 2.05 Å, demonstrating the presence of ion pairs between the epoxy resin and carbon nanotubes (CNTs). A similar ion pair is observed in the CNT-COOH/epoxy composite, implying that ion pairs can be formed at the interface between the two systems, as depicted in Fig. 10(a). However, the intensity of the RDF peak (Nepoxy–hoCNT) in the CNT-OH/epoxy composite is lower than that in the CNT-COOH/epoxy composite. This low intensity suggests a weak attraction between the Nepoxy (epoxy) and hoCNT atoms in the CNT-OH/epoxy composite. As shown in Fig. 10(b), this trend can be quantified by the average coordination number of Nepoxy–hoCNT pairs. Comparison of the configuration snapshots of the interface region in the CNT-COOH/epoxy composite and CNT-OH/epoxy composite (Fig. 10(c) and (d)) reveals that the epoxy resin is attracted to the CNTs, enhancing interfacial bonding performance.
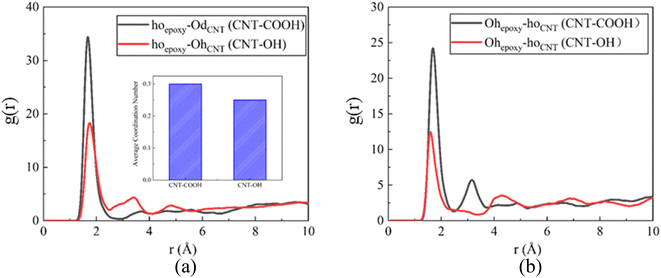
Besides the H–N bond, the hydrogen bond networks established between epoxy resin and carbon nanotubes are also substantial contributors to the interfacial adhesion properties. Fig. 11 quantifies the hydrogen bond networks between the epoxy resin and CNTs when the epoxy resins act as hydrogen donors and acceptors. As depicted in Fig. 11(a), the hydroxyl hydrogen atom of the epoxy resin and the double bond oxygen atom of the CNTs form a significant peak at 1.85 Å, indicating the formation of hydrogen bonds between the epoxy resin and CNTs. Furthermore, the appearance of the first peak occurs at a position smaller than the hydrogen bond formation threshold, demonstrating the epoxy resin's provision of hydrogen as a hydrogen bond donor to establish a hydrogen bond network with the CNTs. However, the RDF peak of the hydrogen bond in the composite system of carboxyl-functionalized CNTs is higher compared to that of hydroxyl-functionalized CNTs. This trend can be quantified by coordination numbers (CN). The CN of hoepoxy–OdCNT in the CNT-COOH/epoxy composite is 0.31, higher than that of the CNT-OH/epoxy composite. This trend is also discernible in Fig. 11(b). As a hydrogen acceptor, the epoxy resin forms hydrogen bonds with the CNTs, and the RDF peak intensity of CNT-COOH is higher than that of CNT-OH, which aligns with the trend indicated in the force-displacement curve. Thus, the incorporation of functional groups not only enhances the formation of ion pairs between the epoxy resin and CNTs but also strengthens the hydrogen bond networks.
The RDF analysis qualitatively captures the functionalization's promotion of interaction between the CNTs and epoxy resin, while the average coordination number quantitatively describes the interaction between the two atomic bonds. The time correlation function (TCF) is utilized to evaluate the dynamic behavior of the atoms. In the following section, the TCF is employed to evaluate the dynamic stability of the ion pairs.
4 Conclusions
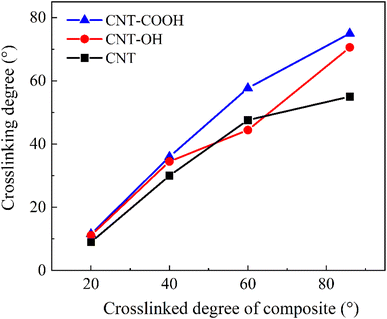
In this study, the impact of functionalized CNTs on the interfacial adhesion between epoxy resin and CNTs was investigated. The force–displacement curves were determined, and the bond interaction and dynamic properties of the functionalized CNTs-epoxy resin system were evaluated. This molecular dynamics analysis provides insight into the reinforcement of epoxy resin by nanomaterials. The conclusions can be summarized as follows:1. Functionalization enhances the density distribution and crosslinking degree of the epoxy resin at the interface. The improvement effect of functionalization increases with an increase in the crosslinking degree of the composite. Additionally, functionalization reduces the free volume fraction and increases the density of the composite.
2. Simulation of pull-out tests reveals that functionalization enhances the bonding between the epoxy resin and CNTs. The peak value of external force in the CNT-COOH/epoxy resin system was slightly higher than in the CNT-OH/epoxy resin system, indicating that carboxyl functionalized CNTs have improved bonding properties. Complete stripping of the epoxy resin revealed that functionalized CNTs can adhere to some epoxy resin, providing evidence of improved bonding performance.
3. Micro analysis highlights the reasons for the improved interfacial adhesion. The strong interaction between the epoxy resin and CNTs is the primary factor contributing to the good bonding performance of the composite. Additionally, functional CNTs form hydrogen bond networks with the epoxy resin. The polar functional groups on the CNTs attract more epoxy resin to the interface, thereby improving the interfacial bonding performance. Analysis of the bonding stability revealed that the strong stability of hydrogen bonds and ionic bonds contributes to the high interfacial bonding performance.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Financial support from National Natural science foundation of China under Grant U2006224, 52178221, 51978352, 51908308, Natural science foundation of Shandong Province under Grant ZR2020JQ25, ZR2022YQ55, 2019KJG010, Taishan Scholars of Shandong Province under Grant tsqn.201812090, are gratefully acknowledged.References
- W. Liu, Y. Wang, P. Wang, Y. Li, Q. Jiang, X. Hu, Y. Wei, Y. Qiu, S. I. S. Shahabadi and X. Lu, Composites, Part B, 2017, 113, 197–205 CrossRef CAS.
- S.-C. Shiu and J.-L. Tsai, Composites, Part B, 2014, 56, 691–697 CrossRef CAS.
- A. Kumar, P. K. Ghosh, K. L. Yadav and K. Kumar, Composites, Part B, 2017, 113, 291–299 CrossRef CAS.
- A. J. Kinloch, S. H. Lee and A. C. Taylor, Polymer, 2014, 55, 6325–6334 CrossRef CAS.
- N. Parhizkar, B. Ramezanzadeh and T. Shahrabi, Appl. Surf. Sci., 2018, 439, 45–59 CrossRef CAS.
- Z. Zheng, Y. Li, S. A. He, X. Ma, X. Zhu and S. Li, Constr. Build. Mater., 2019, 197, 319–330 CrossRef CAS.
- S.-Y. Guo, X. Zhang, J.-Z. Chen, B. Mou, H.-S. Shang, P. Wang, L. Zhang and J. Ren, Constr. Build. Mater., 2020, 264, 319–330 Search PubMed.
- S.-Y. Guo, X. Zhang, J. Ren, J.-Z. Chen, T.-J. Zhao, T.-W. Li and L. Zhang, Constr. Build. Mater., 2021, 272, 960 CrossRef.
- A. Dadrasi, G. A. Farzi, M. Shariati, S. Fooladpanjeh and V. Parvaneh, Eng. Fract. Mech., 2020, 232 CrossRef.
- P. S. Yadav, R. Purohit and A. Kothari, Mater. Today: Proc., 2019, 18, 5530–5539 CrossRef CAS.
- P. Ma, G. Jiang, Q. Chen, H. Cong and X. Nie, Composites, Part B, 2015, 69, 526–533 CrossRef CAS.
- S. A. Bansal, V. Khanna, A. P. Singh and S. Kumar, Mater. Today: Proc., 2021, 275–279 Search PubMed.
- J. Goclon, T. Panczyk and K. Winkler, Appl. Surf. Sci., 2018, 433, 213–221 CrossRef CAS.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- B. Hao, Q. Ma, S. Yang, E. Mäder and P.-C. Ma, Compos. Sci. Technol., 2016, 129, 38–45 CrossRef CAS.
- Y. Li, S. Wang, Q. Wang and M. Xing, Composites, Part B, 2018, 133, 35–41 CrossRef CAS.
- H. Khoramishad and M. Khakzad, J. Adhes., 2016, 94, 15–29 CrossRef.
- S. Yu, M. N. Tong and G. Critchlow, Mater. Des., 2010, 31, S126–S129 CrossRef CAS.
- S. Aodkeng, S. Sinthupinyo, B. Chamnankid, W. Hanpongpun and A. Chaipanich, Constr. Build. Mater., 2022, 320, 212 CrossRef.
- W.-M. Qian, M. H. Vahid, Y.-L. Sun, A. Heidari, R. Barbaz-Isfahani, S. Saber-Samandari, A. Khandan and D. Toghraie, J. Mater. Res. Technol., 2021, 12, 1931–1945 CrossRef CAS.
- J. Patil, H. Patil, R. Sankpal, D. Rathod, K. Patil, P. R. Kubade and H. B. Kulkarni, Mater. Today: Proc., 2021, 46, 7182–7186 CrossRef CAS.
- X. Chen, F. Peng, C. Wang, H. Zhou, X. Lin, W. Liu and A. Zhang, Appl. Surf. Sci., 2022, 576, 765 Search PubMed.
- B. Suresha, N. M. Indushekhara, C. A. Varun, D. Sachin and K. Pranao, Mater. Today: Proc., 2021, 43, 1478–1484 CrossRef CAS.
- D. Hou, Q. Yang, Z. Jin, P. Wang, M. Wang, X. Wang and Y. Zhang, Appl. Surf. Sci., 2021, 568, 896 CrossRef.
- J.-H. Sul, B. Gangadhara Prusty and D. W. Kelly, Adv. Manuf.: Polym. Compos. Sci., 2015, 1, 94–104 Search PubMed.
- C. Baudot and C. M. Tan, Int. J. Nanotechnol., 2009, 6, 618–627 CrossRef CAS.
- D. S. Hou, Q. R. Yang, Z. Q. Jin, P. Wang, M. H. Wang, X. P. Wang and Y. Zhang, Appl. Surf. Sci., 2021, 568, 11 CrossRef.
- P. Wang, Q. R. Yang, Z. Q. Jin, D. S. Hou and M. H. Wang, J. Mater. Sci., 2021, 56, 16475–16490 CrossRef CAS.
- Z. Q. Mao, W. Wu, Y. Cheng, C. Xie, D. M. Zhang and X. Q. Jiang, J. Nanosci. Nanotechnol., 2011, 11, 5169–5178 CrossRef CAS PubMed.
- Z. Abousalman-Rezvani, P. Eskandari, H. Roghani-Mamaqani and M. Salami-Kalajahi, Adv. Colloid Interface Sci., 2020, 278, 102126 CrossRef CAS PubMed.
- M. Liebscher, T. Gärtner, L. Tzounis, M. Mičušík, P. Pötschke, M. Stamm, G. Heinrich and B. Voit, Compos. Sci. Technol., 2014, 101, 133–138 CrossRef CAS.
- N. T. Dintcheva, R. Arrigo, E. Morici, C. Gambarotti, S. Carroccio, F. Cicogna and G. Filippone, Composites, Part B, 2015, 82, 196–204 CrossRef CAS.
- N. G. Sahoo, S. Rana, J. W. Cho, L. Li and S. H. Chan, Prog. Polym. Sci., 2010, 35, 837–867 CrossRef CAS.
- L. Wang, Y. Tan, X. Wang, T. Xu, C. Xiao and Z. Qi, Chem. Phys. Lett., 2018, 706, 31–39 CrossRef CAS.
- P.-C. Ma, S.-Y. Mo, B.-Z. Tang and J.-K. Kim, Carbon, 2010, 48, 1824–1834 CrossRef CAS.
- H. Jung, H. K. Choi, S. Kim, H.-S. Lee, Y. Kim and J. Yu, Composites, Part A, 2017, 103, 17–24 CrossRef CAS.
- C. Hirschl, M. Biebl-Rydlo, M. DeBiasio, W. Mühleisen, L. Neumaier, W. Scherf, G. Oreski, G. Eder, B. Chernev, W. Schwab and M. Kraft, Sol. Energy Mater. Sol. Cells, 2013, 116, 203–218 CrossRef CAS.
- L. H. Tam and D. Lau, RSC Adv., 2014, 4, 33074–33081 RSC.
- W.-Y. Chen, Y.-Z. Wang, S.-W. Kuo, C.-F. Huang, P.-H. Tung and F.-C. Chang, Polymer, 2004, 45, 6897–6908 CrossRef CAS.
- Y. L. Yaphary, Z. Yu, R. H. W. Lam, D. Hui and D. Lau, Composites, Part B, 2017, 131, 165–172 CrossRef CAS.
- O. Buyukozturk, M. J. Buehler, D. Lau and C. Tuakta, Int. J. Solids Struct., 2011, 48, 2131–2140 CrossRef CAS.
- F. Sanchez and L. Zhang, Carbon, 2010, 48, 1210–1223 CrossRef CAS.
- S. Plimpton, P. Crozier and A. Thompson, Sandia Natl. Lab., 2007, 18, 43 Search PubMed.
- T. G. Fox and P. J. Flory, J. Appl. Phys., 1950, 21, 581–591 CrossRef CAS.
- W. Q. Zhang, Y. Qing, W. H. Zhong, G. Sui and X. P. Yang, React. Funct. Polym., 2017, 111, 60–67 CrossRef CAS.
- W. Zhang, X. Deng, G. Sui and X. Yang, Carbon, 2019, 145, 629–639 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |