Open Access Article
Open Access ArticleA novel portable immuno-device for the recognition of lymphatic vessel endothelial hyaluronan receptor-1 biomarker using GQD–AgNPrs conductive ink stabilized on the surface of cellulose†
Ahmad Mobed‡
 abc,
Fereshteh Kohansal‡d,
Sanam Dolati
abc,
Fereshteh Kohansal‡d,
Sanam Dolati *c and
Mohammad Hasanzadeh
*c and
Mohammad Hasanzadeh *ad
*ad
aPharmaceutical Analysis Research Center, Tabriz University of Medical Sciences, Tabriz 51664, Iran. E-mail: hasanzadehm@tbzmed.ac.ir
bAging Research Institute, Faculty of Medicine, Tabriz University of Medical Sciences, Iran
cPhysical Medicine and Rehabilitation Research Center, Aging Research Institute, Faculty of Medicine, Tabriz University of Medical Sciences, Iran. E-mail: sanam.dolati@gmail.com
dNutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
First published on 23rd October 2023
Abstract
Lymphatic vessel endothelium expresses various lymphatic marker molecules. LYVE-1, the lymphatic vessel endothelial hyaluronan (HA) receptor, a 322-residue protein belonging to the integral membrane glycoproteins which is found on lymph vessel wall and is completely absent from blood vessels. LYVE-1 is very effective in the passage of lymphocytes and tumor cells into the lymphatics. As regards cancer metastasis, in vitro studies indicate LYVE-1 to be involved in tumor cell adhesion. Researches show that, in neoplastic tissue, LYVE-1 is limited to the lymphovascular and could well be proper for studies of tumor lymphangiogenesis. So, the monitoring of LYVE-1 level in human biofluids has provided a valuable approach for research into tumor lymphangiogenesis. For the first time, an innovative paper-based electrochemical immune-platform was developed for recognition of LYVE-1. For this purpose, graphene quantum dots decorated silver nanoparticles nano-ink was synthesized and designed directly by writing pen-on paper technology on the surface of photographic paper. This nano-ink has a great surface area for biomarker immobilization. The prepared paper-based biosensor was so small and cheap and also has high stability and sensitivity. For the first time, biotinylated antibody of biomarker (LYVE-1) was immobilized on the surface of working electrode and utilized for the monitoring of specific antigen by simple immune-assay strategy. The designed biosensor showed two separated linear ranges in the range of 20–320 pg ml−1 and 0.625–10 pg ml−1, with the acceptable limit of detection (LOD) of 0.312 pg ml−1. Additionally, engineered immunosensor revealed excellent selectivity that promises its use in complex biological samples and assistance for biomarker-related disease screening in clinical studies.
1. Introduction
Cancer is among the most critical health issues worldwide.1 Treatment of cancer poses considerable difficulties such as resistance to multiple drugs and a scarcity of therapies that target tumors specifically while causing minimal adverse effects.2 Recent advances in cancer immunotherapy have focused on enhancing the body's natural antitumor immune response. This can be achieved through various approaches, such as checkpoint inhibitors, which block proteins that inhibit immune responses, adoptive T cell therapy3–5 and peptide-based therapy2,6,7 which is new strategy in cancer treatment. The predominant mode of metastasis in cancer cells is through the blood and lymphatic vessels, resulting in the colonization of distant organs and lymph nodes, which ultimately leads to unfavorable prognosis. Consequently, curtailing the dissemination of cancer cells through the blood and lymphatic systems has been a central area of interest in cancer research for many years.8Lymphatic vessels have an important role in maintaining tissue-fluid homeostasis, immune surveillance and metastasis.9 LYVE-1 is a receptor for hyaluronan and has been known as a potent marker for lymphatic endothelium.10 It is structurally related to CD44 and other HA-binding proteins. The most significant feature of LYVE-1 is its pattern of tissue expression.11 LYVE-1 is selectively expressed in the endothelia of lymphatic capillaries and also expressed in spleen endothelium, alveolar lining epithelium, liver sinusoidal endothelial cells (LSEC), and activated tissue macrophages.12,13 On the other hand, LYVE-1 is a promising diagnostic and prognostic biomarker in several types of cancer including gastric, lung, liver, pancreas, and breast cancer.14–17 LYVE-1 is likely to play a role in either hyaluronan homeostasis or in the regulation of cellular trafficking to the lymph nodes.18 The lymphatics are an important route for early metastasis in cancer. The LYVE-1 has been extensively used for the detection of tumor-associated lymphatic vessels in different types of tumors.19 During cancer progression, expression of LYVE-1 is increased in lymphatic endothelial cells. A high density of lymph vessels expressing LYVE-1 is associated with a high frequency of regional lymph node metastases.20 Immunohistochemical intensity of LYVE-1 expression is beneficial for monitoring of lymphatic invasion or lymphangiogenesis in different kinds of cancers, such as gastric, pulmonary, colon, mammary, endometrial/testicular cancers, and neuroblastomas/vascular tumors.21,22
The detection of LYVE-1 as a precise biomarker for lymphatic vasculature provides a possible minimally-invasive method for predicting disease progress.23 The detection and quantification of LYVE-1 can provide important information valuable for clinical cancer diagnosis. There are various approaches for detection of the LYVE-1 molecule as an integral membrane glycoprotein, such as immunofluorescence microscopy, flow cytometry and immunohistochemistry.24,25 During the last decade, a growing number of scientists have focused on developing rapid techniques. However, the growing sensitivity of detection methods poses a challenge in differentiating between insignificant alterations and lesions that have the potential to progress to malignant cancer.26
Biosensors as excellent tools in medical analysis allows accurate detection of biomarkers of diseases. Biosensors are efficient tools for the rapid, qualitative and quantitative measurement of biomarkers in animal/human biofluids.27,28 Biosensors with sensitive and specific features could give advantage to the progress of LYVE-1 detection platforms. The results of this investigation contribute to the detection of LYVE-1 by biosensing system.
Immunosensors are commonly employed across various domains due to their exceptional sensitivity. However, the utilization of these biosensors necessitates multiple stages of preparation, skilled technicians, and expensive equipment.29 In order to address this issue, paper-based immunoassays have been developed and are being explored by numerous researchers.30 Paper, owing to its advantageous properties, emerges as a highly favorable material for this purpose.31 It boasts attributes such as ease of manipulation, absence of the need for specialized tools, reusability, cost-effectiveness, and a hydrophilic platform that allows the passage of liquid substances without the requirement for external power sources.30 Consequently, paper plays a pivotal role in the fabrication of paper-based immunosensors, serving as a substantial and economical substrate for the immobilization of reagents and their subsequent reactions. The fibrous and porous structure inherent to paper offers significant advantages for immobilizing reagents and facilitating their reactions. Capitalizing on capillary forces, paper efficiently directs aqueous liquid streams, providing an extensive surface area for reagent immobilization and a substantial region for ensuing reagent reactions.32
Recently, the practice of creating conductive patterns on paper has emerged as a cost-effective and straightforward method for crafting paper-based sensors.33 This approach facilitates the economical and expedient production of customized electrodes. Consequently, paper-based sensors represent an attractive alternative to conventional electrochemical biosensors.34 Given the critical demand within healthcare facilities such as clinical laboratories and medical research centers for the early detection of cancer, there exists a compelling need for these novel biosensors employing nano-inks. Nano-inks play a pivotal role in enhancing the efficiency of electrochemical immunosensors, enabling the rapid and facile detection of cancer.35 For example, a paper-based genosensor by immobilization of ssDNA on the surface of AgNPs with graphene quantum was fabricated for detection of microorganism.36,37 Also, a flexible label-free electrochemical biosensor was developed for the screening of miRNA-21 using Ag@Au/GQD core–shell as nano-ink stabilized on the surface of photographic paper.37,38 Created novel platform was low-cost and performed as a paper-based laboratory technology.39 Similarly, a flexible paper based electrochemical biosensor as a moveable device was advanced for recognition of ractopamine in animal feed. Planned biosensor was cost-effective and applicable for food quality control and analysis.40 This study's outcomes offer novel insights into detection of LYVE-1 in complex samples, by using biosensor technology.
For the first time, novel conductive nano-ink based on GQDs–silver nanoprisms (GQDs–AgNPrs) was synthesized and used to preparation of three-electrode template on the surface of photographic paper by pen-on-paper technology. Due to quantum imprisonment and boundary effects due to changes in electronic distribution, and exhibit a bandgap responsible for their photoluminescence properties and changes in conductivity, which can be adjusted depending on the size and structure of the GQD.41,42 GQDs have also been positively used in electrochemical sensors, which utilize redox reactions and other charge transfer phenomena to detect and quantify target analytes.43 GQDs application in electrochemistry is also popular due to their ease of manufacture and the ability to be doped or modified for specific sensing requests.44,45 These features enable GQD-based nanomaterials to have higher sensitivity and lower detection limits. In several cases, GQD can also be combined with other materials to create GQD-based nanocomposites with the purpose of creating a synergistic effect that facilitates the catalytic reaction with the target analyte.45,46 QDs in some cases consider as a toxic material but the adverse effects caused by the first generations of QDs will likely be reduced by newer generations of QDs. The search for new nanoparticles that are as effective as current QDs but without proven toxic elements has been intensified, and very promising new luminescent NPs have been discovered in recent years.47,48 Recently, luminescent carbon nanodots (C-dots), GQDs, and nanometer-sized graphene flakes with excellent optical and electronic properties have been discovered recently. These NPs are very promising due to their chemical inertness, high specific surface area, and good surface grafting ability. Primary discoveries indicate that C-dots and GQD exhibit minimal cytotoxicity for exposed cells.47,48
Silver nanoparticles (AgNPs) exhibit exceptional properties and high electrical conductivity which making them a key component in enhancing the performance of conductive inks compared to other metal nanoparticles. The advantages of silver include outstanding electrical, optical, thermal characteristics, biocompatibility, low toxicity, and support for electrocatalytic activity.49 However, the challenge arises from the tendency of AgNPs to aggregate and the subsequent solvent evaporation, which can lead to gaps in printed patterns and reduced conductivity.50 Consequently, there is a pressing need to develop novel types of conductive ink.33,49
To address this issue, hybrid inks have been formulated by combining AgNPs with carbon based nanoparticles, as these compounds can effectively bridge the gaps between aggregated AgNPs, creating efficient electrical pathways.50 Within this context, graphene quantum dots (GQDs) have gained prominence as zero-dimensional graphene nanocrystals, finding applications in optical51 and electrochemical sensors,52 lithium-ion batteries,53 and bio-imaging.54 These nanoparticles are exceptionally small, measuring just a few nanometers, and possess strong quantum confinement effects along with various functional groups.49,55 Importantly, studies have revealed that the electronic properties of GQDs undergo significant changes as a result of their functionalization.
For the first time, the engineered interface utilized for the construction of novel paper-based immunosensor using immobilization of antibody on the working electrode zone of three-electrode system. So, based on immune-complex of antibody–antigen, the target biomolecule (LYVE-1) was identified by using electrochemical techniques. Interestingly a novel and powerful portable bioassay was proposed for the monitoring of LYVE-1 in human plasma samples, which promises its application in early-stage diagnosis of cancer.
2. Methods and materials
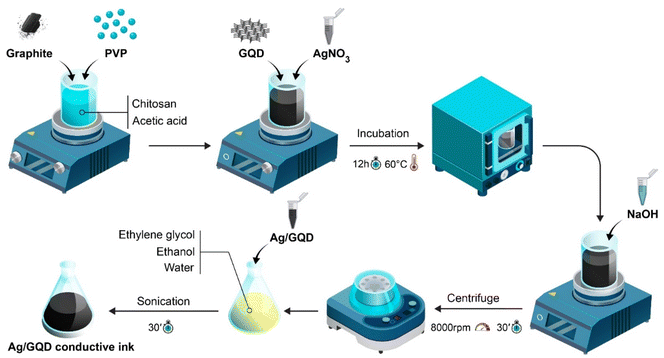
The ELISA kit containing antibody and antigen receptor, and dilution solution were purchased from ZellBio Co., Germany, polyvinylpyrrolidone K-30 (PVP) (molecular weight ≈ 300 g), graphite powder, chitosan, acetic acid, graphene quantum dots (GQD), ethanol, ethylene glycol, silver nitrate, sodium hydroxide, trisodiumcitrate (TSC), hydrogen peroxide, sodium borohydride (NaBH4), potassium ferricyanide K3Fe(CN)6 and potassium ferrocyanide K4Fe(CN)6 were purchased from Sigma Aldrich (Ontario, Canada). Also, potassium chloride (KCl), N-hydroxysuccinimide (NHS), bovine serum albumin (BSA), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) were obtained from Merck KGaA, Germany. Humana plasma samples were gotten from the Iranian Blood Transfusion Center (Tabriz, Iran).To begin, a PVP solution containing 0.06 g of PVP in 3 ml of water, was introduced into 200 ml of deionized water (DW). Then, a solution of AgNO3 with a concentration of 0.01 M and a volume of 4 ml was added and mixed vigorously. Following that, a solution of TSC with a concentration of 75 mM and a volume of 8 ml was added to the mixture. Subsequently, 1 l of hydrogen peroxide was added and stirred. Afterward, a solution of NaBH4 with a concentration of 100 mM and a volume of 3.2 l was introduced into the mixture. The inclusion of NaBH4 as a reducing agent led to the formation of small AgNPrs, resulting in a highly intense yellow coloration of the solution. Within a few seconds, the color of the colloidal solution transitioned to a light-yellow shade. The resultant liquid was stirred at room temperature for 30 minutes, causing the hue to change to blue. The resulting colloidal mixture was then stored at a temperature of 4 °C for future utilization. To do this, 2 ml of 0.1 M acetic acid was used to dissolve 0.0113 g of chitosan before being sonicated. Chitosan was fully dissolved in the acid before being combined with 0.6 g of graphite and 0.2 g of PVP using a magnetic stirrer. 2 ml of pre-prepared GQDs were then added and given time to dissolve entirely. After that, 600 μl of silver nanoparticles were added. It is recommended to formerly set the oven to 60 °C. The resultant solution has to be incubated for 12 to 18 hours with its container completely sealed at 60 °C. Following this step, the solution remained for 3 minutes at 80 °C. The magnetic stirrer was deactivated after 3 minutes and a shaker or stirrer was switched on instead. Then, 0.12 g of sodium hydroxide dissolved in 400 μl of deionized water and added to the mixture, it was blended for three minutes using a magnetic stirrer. Finally, the mixture was washed three times with DW and alcohol for purification and utilization of electrodes preparation. Scheme 1 shows all of preparation steps of AgNPrs–GQDs conductive nano-ink.
The PalmSens 4c system was employed to carry out electrochemical measurements, and it was operated using the PSTrace software on a laptop. The measurements for chronoamperometry were taken with a 2 second equilibrium time and a voltage amplitude of 10 millivolts. AgNPrs–GQDs nano-ink was utilized to create paper electrodes, which were employed as a working electrode, a reference electrode, and a counter electrode. It is worth mentioning that, we synthesized and prepared the AgNPrs–GQDs nano-ink, then utilized a high-resolution field emission scanning electron microscope (FE-SEM) called Hitachi SU8020 from Czech to examine the morphology of the electrode surface. Additionally, we used energy dispersive spectroscopy (EDS) to analyze the chemical compounds that were formed on the surface of the electrode. For measuring the electrical resistance of nano-ink estimate, ohmmeter, XIOLE, XL830L, China, multi-meter employed. Falling ball viscometer (Anton Paar-AMVn, Germany) was utilized to measure the viscosity of nano-ink. In this study, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) techniques were applied for determination of the behavior in different fabrication steps and also selectivity of the engineered immunosensor, where CV (Tequilibration: 0 s, Ebegin: −1.0 V, Evertex1: −1.0 V, Evertex2: 1.0 V, Estep: 0.01 V, scan rate: 0.1 V s−1), electrochemical impedance spectroscopy EIS (Tequilibration: 0 s, scan type: fixed, Edc: 0.25 V, Eac: 0.01 V, max-frequency: 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz, min-frequency: 0.1 Hz). Square wave voltammetry (SWV) and DPV applied for measuring reproducibility, repeatability and stability. The SWV technique data info (Tequilibration: 0 s, Ebegin: −1.0 V, Eend: 1.0 V, Estep: 0.005 V, amplitude: 0.02 V, frequency: 10 Hz), and DPV technique data info (Tequilibration: 2 s, Ebegin: −1.0 V, Eend: 1.0 V, Estep: 0.05 V, Epulse: 0.1 V, Tpulse: 0.01 s, scan rate: 0.1 V s−1). Chronoamperometry (ChA) method was used for analytical studies, ChA data info (Tequilibration: 2 s, Edc: 0.3 V, t interval: 0.1 s, t run: 100 s).
000 Hz, min-frequency: 0.1 Hz). Square wave voltammetry (SWV) and DPV applied for measuring reproducibility, repeatability and stability. The SWV technique data info (Tequilibration: 0 s, Ebegin: −1.0 V, Eend: 1.0 V, Estep: 0.005 V, amplitude: 0.02 V, frequency: 10 Hz), and DPV technique data info (Tequilibration: 2 s, Ebegin: −1.0 V, Eend: 1.0 V, Estep: 0.05 V, Epulse: 0.1 V, Tpulse: 0.01 s, scan rate: 0.1 V s−1). Chronoamperometry (ChA) method was used for analytical studies, ChA data info (Tequilibration: 2 s, Edc: 0.3 V, t interval: 0.1 s, t run: 100 s).
3. Results and discussion
3.1. AgNPrs/GQD nano-ink conductivity analysis
In order to analysis of conductivity of nano-ink on the surface of paper, electrical conductivity was checked using an LED lamp, and also, an ohmmeter which was utilized to examine the resistance of nano-ink stabilized on the surface of a photographic paper. This was achieved by producing conductive tracks on the paper surface. The resulting measurement of resistance yielded a value of approximately 6.46 mΩ. To generate an electric current, it is essential to employ a battery holder of equivalent dimensions to the battery being utilized, to ensure proper integration of a battery within a circuit. After inserting the battery into the holder, the next step involves establishing electrical connections between the two wires emanating from the holder and the wire of the LED lamp source. The other unconnected wire of the LED was then connected to the designated surface coated with AgNPrs–GQD nano-ink, resulting in the illumination of the LED (Fig. S1 and S2) and video file.†3.2. Characterization of synthesized nano-ink using TEM, XRD and Raman spectra
The TEM images (Fig. S1 (see ESI)†) of the bare AgNPrs were recorded and similar results were obtained according to previous reports.23 It is found that, average particle sized is 10–50 nm. Also, bulk AgNPrs/GQDs nano-ink were recorded for extra confirmation, indicating proper ink synthesis (Fig. S1 (see ESI)†). It is noteworthy that the large-scale sheets are due to the presence of graphite, and silver nanoparticles are scattered on these sheets.3.3. Fabrication of the immunosensor
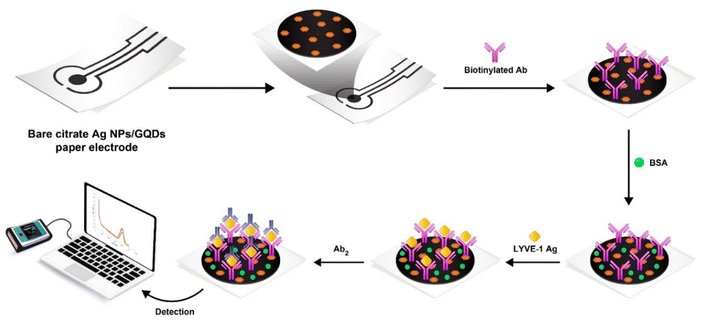
Photographic paper was used as appropriate substrate to prepare the electrochemical biosensor. At first, photographic papers with dimensions of 2 × 3 centimeters were prepared. In the initial phase, paper-based electrodes (PBE) were fabricated by promptly applying conductive lines onto the surface of photographic paper using direct writing of nano-ink (AgNPs–GQDs). This application was achieved through the utilization of pen-on-paper technology, followed by a subsequent drying period at room temperature for a duration of 5 minutes (Scheme 2). It is important to point out that, the electrode is designed for one-time use or if it can be reused for multiple experiments. We use a Kapton polyimide film to minimize electrolyte absorption.For activation of the –COOH groups of antibodies, 10 μl of biotinylated antibody was mixed with EDC/NHS and incubated for 20 min at 25 °C.
In pursuit of this objective, a mixture composed of EDC/NHS solution and biotinylated antibody was prepared in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Next, a 5 μl of biotinylated antibody was immobilized on the surface of working electrode (central zone of three electrode template and incubated for 2 hours in room temperature). For deactivation of unreacted areas, 5 μl of BSA 10% solution was used and incubated for 30 minutes. Finally, 5 μl of standard LYVE-1 antigen was added, incubated for 2 hours (see Scheme 2 for more information). For the electrochemical evaluation of sensor performance, a 0.1 M solution of ferrocene in the presence of 0.1 M KCl was used. Also, first comparative electro-analysis was done by CV and EIS techniques, which will be discussed on the next part of this report.
1 ratio. Next, a 5 μl of biotinylated antibody was immobilized on the surface of working electrode (central zone of three electrode template and incubated for 2 hours in room temperature). For deactivation of unreacted areas, 5 μl of BSA 10% solution was used and incubated for 30 minutes. Finally, 5 μl of standard LYVE-1 antigen was added, incubated for 2 hours (see Scheme 2 for more information). For the electrochemical evaluation of sensor performance, a 0.1 M solution of ferrocene in the presence of 0.1 M KCl was used. Also, first comparative electro-analysis was done by CV and EIS techniques, which will be discussed on the next part of this report.
3.4. Characterization of different stages of the immunosensor's fabrication
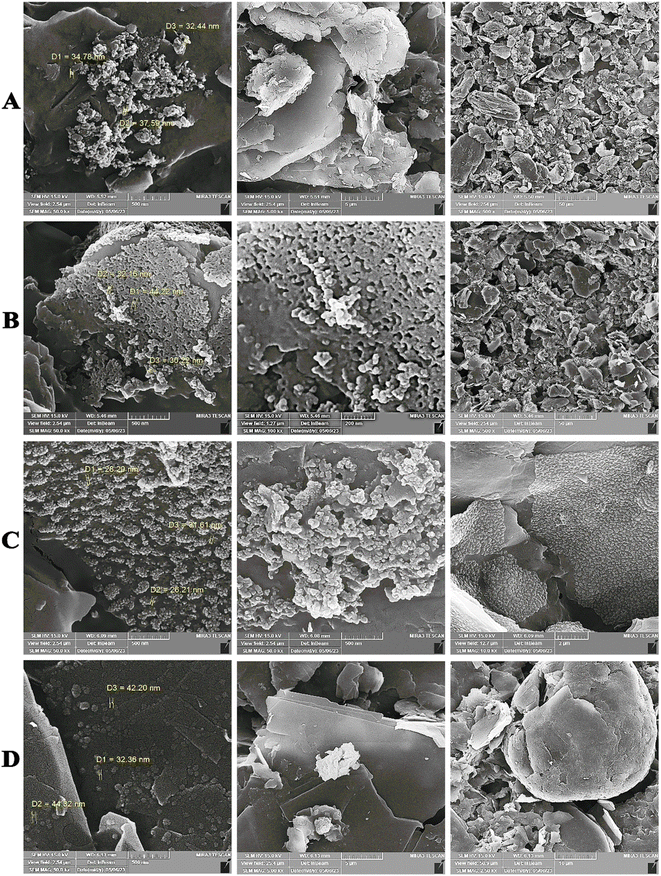
As shown in Fig. S3 and S5 (see ESI),† the synthesized AgNPrs–GQD nano-ink composites were successfully stabilized on the surface of paper. In addition, the binding of the synthesized AgNPrs–GQD nanoink to Ab–BSA and the electrode surfaces was imaged by energy dispersion. According to the obtained results, the different arrangement of the nanoparticles can be clearly seen. Also, at the second step (immobilization of Ab on the AgNPrs–GQDs modified paper), the morphology of particles was generally changed and confirmed successful interaction (binding) of Ab to AgNPrs of conductive ink nanocomposite based on Ag–Ab bioaffinity. The final step is construction of Ab–Ag immunocomplex towards detection of biomarker. As can be seen, after interaction of biotinylated Ab with LYVE-1, the structure of working electrode interface was totally changed, which confirmed appropriate immobilization of antigen on the Ab–BSA–(AgNPrs–GQDs nano-ink). In summary, the FESEM technique has provided valuable images regarding the different steps of the immunosensor, using paper-based sensor strategy. All of these results were confirmed by EDS analysis (Fig. S4 (see ESI)†).
3.5. Electro-analytical study
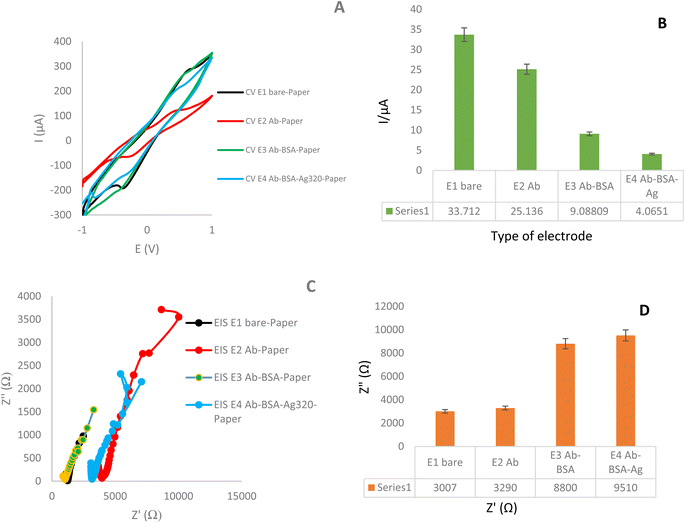
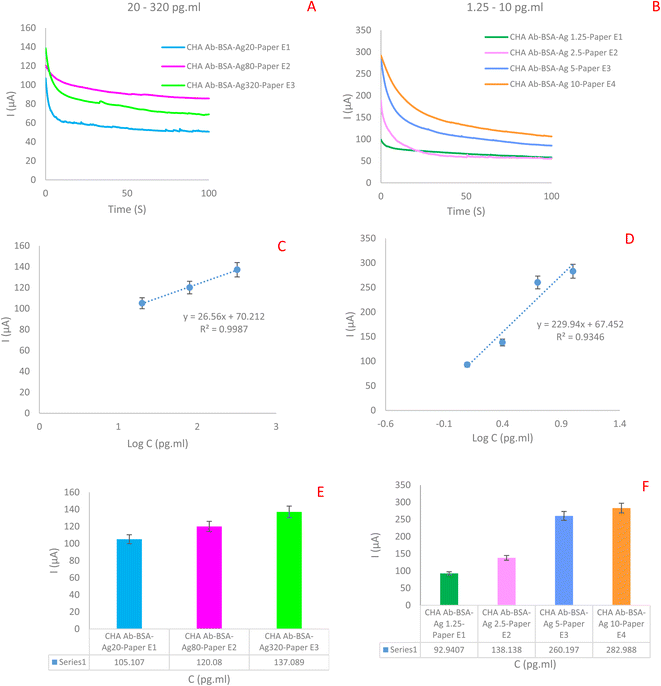
Below, we describe the most important measurements of this study, which were performed as part of the development of an appropriate biosensor for immune-analysis.As illustrated in Fig. 3, the electrochemical response of the immunosensor was examined at various stages of its assembly. Notably, there were distinct electrochemical characteristics observed between the bare electrode and the antibody-modified electrode. Subsequently, when the antibody–antibody (Ag–Ab) modified electrodes were introduced in the next step, they exhibited a different electrochemical profile. According to the obtained results, the highest current intensity is related to the paper-based electrodes modified with AgNPrs/GQD nano-ink (33.71 μA). Further, with the addition of antibody, the current intensity was decreased significantly (25.13 μA). Also, with the addition of the LYVE-1 Ag, the current intensity was decreased from 25.13 μA to 4.065 μA. In this way, the decreasing trend in flow intensity and peak height is clearly evident. These differences refer to Ab–Ag interaction with electrode surface and also, related to used nanomaterials and their affinity to Ab–Ag–electrode.
The diameter of the semicircle of the Nyquist plot represented 3007 Ω resistance, related to paper-based electrodes modified with AgNPrs/GQD nano-ink. The impedance has increased to 3290 Ω by adding Ab on the electrode modified with AgNPrs/GQD nano-ink. The semicircle of the Nyquist plot related to the AgNPrs/GQD nano-ink–Ab–BSA was started at 8800 Ω, but the semicircle of Nyquist plot of AgNPrs/GQD nano-ink–Ab–BSA–LYVE-1, was started at 9510 Ω.
According to the obtained results, the electroconductive layer of AgNPrs–GQD nano-ink on the surface of paper lead to high electrical conductivity and a large specific surface area, allowed more (Fe(CN)6)3−/4− to reach its surface and electron transfer to occur at a higher rate. So, the prepared interface shows high electrocatalytically behavior for the electron transfer process of electrolyte towards high speed of redox process based on increased the rate of electron transfer and demonstrated appropriate electroactivity. Therefore, the AgNPrs–GQD are promising candidates for forming an antibody-active substrate. However, when the biotinylated antibody was assembled on the conductive ink-modified paper via the AgNPrs reaction, the peak currents of CV obviously decreased, which led to a decrease in the peak height. This result illustrated that as a biological species, antibody (bioreceptors) was successfully stabilized on the surface of AgNPrs–GQDs modified-paper. Because antibody as macromolecule occupy a large portion of the electrode surface, it inhibits redox process of Fe(III) to Fe(IV) which reduces the electron transfer of ferro/ferri (supporting electrolytes). So, antibody function as an insulator, slowing down electron transmission. Finally, in the presence of antigen (target), it was observed that the anodic peak current decrease again which point to the presence of analyte, as a result of the immune-complex on the surface of the electrode. So, the presence of analyte lead to hindrance of redox process Fe(III) to Fe(IV) based on low speed of electro-oxidation behavior mediator. All of these results were confirmed by EIS. So, it is clear that the designed immunosensor is capable of monitoring of antigen LYVE-1.
In the second step, the constructed immunosensor was applied for the measurement of low concentration of analyte which regression equation is 0.93. Using these results there are two linear range for this immunosensor. Also, low limit of quantification (LLOQ) was about 0.312 pg ml−1. Experimental data obtained on 4 different electrodes (dots) and linear regression (line) are presented. Linear regression: I (μA) = 229.94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C(LYVE-1) + 67.452, R2 = 0.9346.
C(LYVE-1) + 67.452, R2 = 0.9346.
Several effective methods exist for determination of hyaluronic acid. However, these methods primarily depend on the hydrolysis of HA and come with various limitations, including discontinuity, strict detection conditions, and time-consuming procedures. In response to these challenges, certain researchers have explored alternative techniques involving various such as the turbidity assay,58 ELISA,59,60 colorimetry,61 resonance Rayleigh scattering,62 and lateral flow immunoassay,63 fluorescence signals,64 and high-performance liquid chromatography (HPLC).65 As present in Table 1, there has not been a fully established methodology for the quantification of LYVE-1 or characterization of hyaluronan.
| Detection method | Sample | Detection time | Detection range | Limit of detection | Adv/disadv | Ref. |
|---|---|---|---|---|---|---|
| Turbidity assay | Cultured broth | NR | 2.35 ± 0.04 mg ml−1 | NR | It is a fast process and does not harm bacteria/it does not distinguish between living and dead cells | 58 |
| ELISA | White rabbits' blood | NR | 28 ± 17 ng ml−1 | NR | High specificity/sophisticated techniques and expensive culture media are required | 59 and 60 |
| Colorimetry | Complex samples | 25–60 min | 3–2000 mg l−1 | 0.3 mg l−1 | Simple operation, quick response, adaptable sensitivity/fairly expensive, some surfaces reflect light, making it difficult to take measurements, cannot be used colorless compound | 61 |
| Resonance Rayleigh scattering | Eye drop | NR | 0.4–48.0 μg ml−1 | 0.096 μg ml−1 | Rapid analysis, high sensitivity and wide detection range/its stability is easily affected by the product | 62 |
| Lateral flow immunoassay | Carbohydrate-binding module (CBM) SrCBM70 | 5 min | NR | 0.1 μg ml−1 | Simple, user-friendly operation difficult to miniaturize sample volume, high sensitivity and specificity multiplexing can be challenging, low sample volume required/unclear patent situation in some instances | 63 |
| Fluorescence signals | Human serum | Lower than 5 h | 0.2 and 500.0 μg l−1 | 0.2 μg l−1 | Superior image clarity over fluorescence microscopy/prolonged exposure to fluorescent light can result in bleaching and loss of fluorescence intensity | 64 |
| High-performance liquid chromatography (HPLC) | Pharmaceutical formulations | NR | 320–480 μg ml−1 | NR | Complexity, expensive, time consuming | 65 |
| Paper-based immunosensor | Human plasma | NR | 20–320 pg ml−1 and 0.625–10 pg ml−1 | 0.312 pg ml−1 | Cost-effective, portable, fast, sensitive | This work |
As it has been determined in the comparison of recent studies with the biosensor developed in this work, the most important feature and innovation of the current technique is the type of synthesized nanocomposite, low-cost, fast operation, mobility as well as good sensitivity and specificity. It seems that the appropriate sensitivity of the engineered system in this study is directly related to the synthesized nanocomposite.
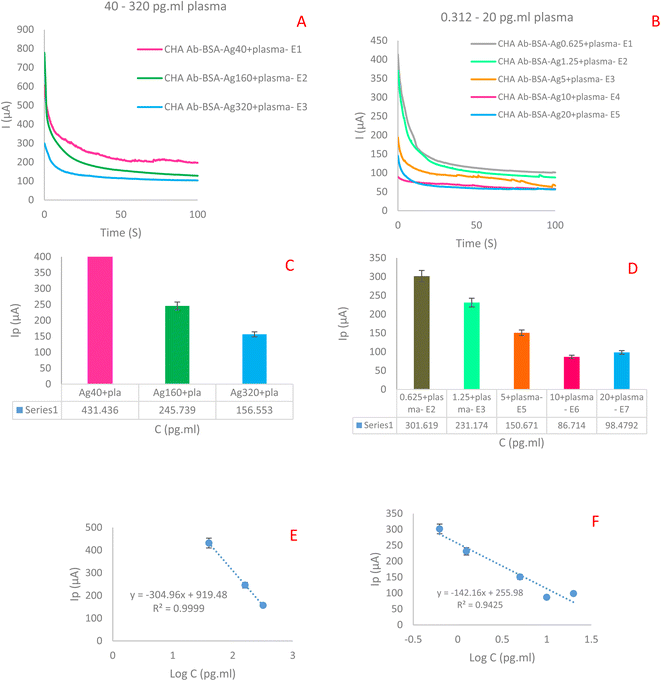
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CLYVE-1 + 255.98, R2 = 0.9425. Based on ChA measurements, a pulse height of 300 μA and a width of 300–800 μA were used along a step height of 0.25 V and a step time of t = 100 s.
CLYVE-1 + 255.98, R2 = 0.9425. Based on ChA measurements, a pulse height of 300 μA and a width of 300–800 μA were used along a step height of 0.25 V and a step time of t = 100 s.
4. Conclusion
The primary goal of this investigation was to provide a novel portable electrochemical biosensing platform for the quantitative detection of LYVE-1 in human plasma samples which was constructed based on AgNPrs and conductive nano-ink stabilized on the surface of paper using suitable three electrode template. Material applied for the quantitative determination of LYVE-1 plasma samples was effortlessly and cost-effective. The remarkable achievement of this study is the construction of a new immunosensor for the sensitive detection of the LYVE-1. The designed sensor showed good linear ranges of 20–320 pg ml−1 and 0.625–10 pg ml−1, with the low detection limit (LOD) of 0.312 pg ml−1. It is lower than that of most of the existing developed methods. Moreover, planned nanosensor showed excellent selectivity that promises its use in the monitoring of LYVE-1 in complex samples and assistance for biomarker-related disease screening which is necessary for early-stage diagnosis of cancer.Abbreviations
| LYVE-1 | Lymphatic vessel endothelial hyaluronan receptor-1 |
| LOD | Low detection limit |
| LLOQ | Lower limit of quantitation |
| QDs | Quantum dots |
| ssDNA | Single strand DNA |
| AgNPrs | Silver nanoprisms |
| ELISA | Enzyme-linked immunosorbent assay |
| CV | Cyclic voltammetry |
| SWV | Square wave voltammetry |
| DPV | Differential pulse voltammetry |
| PVP | Polyvinylpyrrolidone |
| TSC | Trisodiumcitrate |
| KCl | Potassium chloride |
| NHS | N-Hydroxysuccinimide |
| BSA | Bovine serum albumin |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| FE-SEM | Field emission scanning electron microscope |
| EDS | Energy dispersive spectroscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors would like to thank the Iran National Science Foundation (INSF) for funding this work, through a grant no. 99028845.References
- R. L. Siegel, K. D. Miller and A. Jemal, Ca-Cancer J. Clin., 2018, 68(1), 7–30 CrossRef PubMed.
- N. Faraji, S. S. Arab, A. Doustmohammadi, N. L. Daly and A. Y. Khosroushahi, Sci. Rep., 2022, 12, 21341 CrossRef CAS PubMed.
- M. Morotti, A. Albukhari, A. Alsaadi, M. Artibani, J. D. Brenton, S. M. Curbishley, T. Dong, M. L. Dustin, Z. Hu and N. McGranahan, Br. J. Cancer, 2021, 124, 1759–1776 CrossRef PubMed.
- J. A. Rath and C. Arber, Cells, 2020, 9, 1485 CrossRef CAS PubMed.
- S. Mardiana, B. J. Solomon, P. K. Darcy and P. A. Beavis, Sci. Transl. Med., 2019, 11(495), eaaw2293 CrossRef CAS PubMed.
- K.-Y. Huang, Y.-J. Tseng, H.-J. Kao, C.-H. Chen, H.-H. Yang and S.-L. Weng, Sci. Rep., 2021, 11, 13594 CrossRef CAS PubMed.
- F. Grisoni, C. S. Neuhaus, M. Hishinuma, G. Gabernet, J. A. Hiss, M. Kotera and G. Schneider, J. Mol. Model., 2019, 25, 1–10 CrossRef CAS PubMed.
- D. Jones, Cells, 2020, 9, 762 CrossRef CAS PubMed.
- M. X. Luong, J. Tam, Q. Lin, J. Hagendoorn, K. J. Moore, T. P. Padera, B. Seed, D. Fukumura and R. Kucherlapati, J. Cell. Physiol., 2009, 219, 430–437 CrossRef CAS PubMed.
- V. Brezovakova and S. Jadhav, J. Comp. Neurol., 2020, 528, 2021–2032 CrossRef CAS PubMed.
- D. G. Jackson, APMIS, 2004, 112, 526–538 CrossRef CAS PubMed.
- T. Wrobel, P. Dziegiel, G. Mazur, M. Zabel, K. Kuliczkowski and A. Szuba, Lymphology, 2005, 38, 107–110 CAS.
- C. M. Carreira, S. M. Nasser, E. di Tomaso, T. P. Padera, Y. Boucher, S. I. Tomarev and R. K. Jain, Cancer Res., 2001, 61, 8079–8084 CAS.
- F. Ozmen, M. M. Ozmen, E. Ozdemir, M. Moran, S. Seçkin, D. Guc, E. Karaagaoglu and E. J. Kansu, World J. Gastroenterol., 2011, 17, 3220–3228 CAS.
- M. Koukourakis, A. Giatromanolaki, E. Sivridis, C. Simopoulos, K. Gatter, A. Harris and D. J. Meinhardt, Colorectal Cancer, 2005, 58, 202–206 CAS.
- M. E. Karar, N. El-Fishawy and M. Radad, J. Biol. Eng., 2023, 17, 28 CrossRef CAS PubMed.
- C. Sethy, K. Goutam, B. Das, S. R. Dash and C. N. Kundu, Vasc. Pharmacol., 2021, 140, 106865 CrossRef CAS PubMed.
- R. Prevo, S. Banerji, D. J. Ferguson, S. Clasper and D. G. Jackson, J. Biol. Chem., 2001, 276, 19420–19430 CrossRef CAS PubMed.
- P. Ramani, J. V. Dungwa and M. T. May, Virchows Arch., 2012, 460, 183–191 CrossRef CAS PubMed.
- P. Bono, V.-M. Wasenius, P. i. Heikkilä, J. Lundin, D. G. Jackson and H. Joensuu, Clin. Cancer Res., 2004, 10, 7144–7149 CrossRef CAS PubMed.
- Y. Gao, Z. Liu, F. Gao and X.-y. Meng, BMC Cancer, 2010, 10, 1–8 CrossRef PubMed.
- K. Nunomiya, Y. Shibata, S. Abe, S. Inoue, A. Igarashi, K. Yamauchi, T. Kimura, Y. Aida, T. Nemoto and M. Sato, J. Cancer, 2014, 5, 242 CrossRef PubMed.
- A. S. Jauch, S. A. Wohlfeil, C. Weller, B. Dietsch, V. Häfele, A. Stojanovic, M. Kittel, H. Nolte, A. Cerwenka and M. Neumaier, Cancer Cell Int., 2022, 22, 1–17 CrossRef PubMed.
- H. Y. Lim, S. Y. Lim, C. K. Tan, C. H. Thiam, C. C. Goh, D. Carbajo, S. H. S. Chew, P. See, S. Chakarov and X. N. Wang, Immunity, 2018, 49(2), 326–341 CrossRef CAS PubMed.
- A. Fujimoto, Y. Ishikawa, Y. Akishima-Fukasawa, K. Ito, Y. Akasaka, S. Tamai, T. Maehara, H. Kiguchi, K. Ogata and C. Nishimura, Am. J. Clin. Pathol., 2007, 127, 82–88 CrossRef PubMed.
- R. C. Fitzgerald, A. C. Antoniou, L. Fruk and N. Rosenfeld, Nat. Med., 2022, 28, 666–677 CrossRef CAS PubMed.
- S. Dolati, J. Soleymani, S. K. Shakouri and A. Mobed, Clin. Chim. Acta, 2021, 514, 107–121 CrossRef CAS PubMed.
- A. Mobed, F. Kohansal, S. Dolati and M. Hasanzadeh, RSC Adv., 2022, 12, 28473–28488 RSC.
- T. Mazzu-Nascimento, G. G. Morbioli, L. A. Milan, F. C. Donofrio, C. A. Mestriner and E. Carrilho, Anal. Chim. Acta, 2017, 950, 156–161 CrossRef CAS PubMed.
- F. Bahavarnia, A. Saadati, S. Hassanpour, M. Hasanzadeh and A. Hassanzadeh, Int. J. Biol. Macromol., 2019, 138, 744–758 CrossRef CAS PubMed.
- X. Li, D. R. Ballerini and W. Shen, Biomicrofluidics, 2012, 6(1), 11301–1130113 CrossRef PubMed.
- F. Farshchi, A. Saadati and M. Hasanzadeh, Heliyon, 2020, 6, e04327 CrossRef PubMed.
- S. Hassanpour, M. Hasanzadeh, A. Saadati, N. Shadjou, J. Soleymani and A. Jouyban, Microchem. J., 2019, 146, 345–358 CrossRef CAS.
- F. A. Alatraktchi, J. S. Noori, G. P. Tanev, J. Mortensen, M. Dimaki, H. K. Johansen, J. Madsen, S. Molin and W. E. Svendsen, PLoS One, 2018, 13, e0194157 CrossRef PubMed.
- S. Hassanpour, M. Hasanzadeh, A. Saadati, N. Shadjou, J. Soleymani and A. Jouyban, Microchem. J., 2019, 146, 345–358 CrossRef CAS.
- J. Rajendran, J. Hazard. Mater., 2023, 449, 130979 CrossRef CAS PubMed.
- Y. Niu, Y. Han, H. Cheng, Z. Xiong, B. Luo, T. Ma, L. Li, S. Liu, X. Chen and C. Yi, J. Mater. Res. Technol., 2023, 25, 873–886 CrossRef CAS.
- Z. Duan, Z. Yuan, Y. Jiang, L. Yuan and H. Tai, J. Mater. Chem. C, 2023, 11, 5585 RSC.
- F. Farshchi, A. Saadati, N. Fathi, M. Hasanzadeh and M. Samiei, Anal. Methods, 2021, 13, 1286–1294 RSC.
- H. K. Kordasht, A. Saadati and M. Hasanzadeh, Food Chem., 2022, 373, 131411 CrossRef CAS PubMed.
- G. Jie, Q. Zhou and G. Jie, Talanta, 2019, 194, 658–663 CrossRef CAS PubMed.
- Y. Yan, J. Gong, J. Chen, Z. Zeng, W. Huang, K. Pu, J. Liu and P. Chen, Adv. Mater., 2019, 31, 1808283 CrossRef PubMed.
- M. Ozhukil Valappil, V. Pillai and S. Alwarappan, Mater. Today, 2017, 9, 350–371 CrossRef.
- P. Tian, L. Tang, K. Teng and S. Lau, Mater. Today Chem., 2018, 10, 221–258 CrossRef CAS.
- M. Li, T. Chen, J. J. Gooding and J. Liu, ACS Sens., 2019, 4, 1732–1748 CrossRef CAS PubMed.
- C. A. Ballesteros, L. A. Mercante, A. D. Alvarenga, M. H. Facure, R. Schneider and D. S. Correa, Mater. Chem. Front., 2021, 5, 7419–7451 RSC.
- F. M. Winnik and D. Maysinger, Acc. Chem. Res., 2013, 46, 672–680 CrossRef CAS PubMed.
- Y. Wang, R. Hu, G. Lin, I. Roy and K.-T. Yong, ACS Appl. Mater. Interfaces, 2013, 5, 2786–2799 CrossRef CAS PubMed.
- M. Jafari, M. Hasanzadeh, E. Solhi, S. Hassanpour, N. Shadjou, A. Mokhtarzadeh, A. Jouyban and S. Mahboob, Int. J. Biol. Macromol., 2019, 126, 1255–1265 CrossRef CAS PubMed.
- L. Xu, G. Yang, H. Jing, J. Wei and Y. Han, Nanotechnology, 2014, 25, 055201 CrossRef CAS PubMed.
- Q. Lu, W. Wei, Z. Zhou, Z. Zhou, Y. Zhang and S. Liu, Analyst, 2014, 139, 2404–2410 RSC.
- L. Ruiyi, J. Yuanyuan, Z. Xiaoyan, L. Zaijun, G. Zhiguo, W. Guangli and L. Junkang, Electrochim. Acta, 2015, 178, 303–311 CrossRef.
- K. Lijuan, Y. Yongqiang, L. Ruiyi and L. Zaijun, Electrochim. Acta, 2016, 198, 144–155 CrossRef.
- X. Tan, Y. Li, X. Li, S. Zhou, L. Fan and S. Yang, Chem. Commun., 2015, 51, 2544–2546 RSC.
- Q. Lu, Y. Zhang and S. Liu, J. Mater. Chem. A, 2015, 3, 8552–8558 RSC.
- Z. Fattahi, A. Y. Khosroushahi and M. Hasanzadeh, Biomed. Pharmacother., 2020, 132, 110850 CrossRef CAS PubMed.
- H. K. Kordasht and M. Hasanzadeh, Anal. Methods, 2020, 12, 2795–2811 RSC.
- J.-M. Song, J.-H. Im, J.-H. Kang and D.-J. Kang, Carbohydr. Polym., 2009, 78, 633–634 CrossRef CAS.
- P. Kongtawelert and P. Ghosh, Anal. Biochem., 1989, 178, 367–372 CrossRef CAS PubMed.
- R. S. Yahya, A. A. El-Bindary, H. A. El-Mezayen, H. M. Abdelmasseh and M. A. Eissa, Clin. Lab., 2014, 60, 1115–1121 CAS.
- S. Pepeliaev, R. Hrudíková, J. Jílková, J. Pavlík, D. Smirnou, Z. Černý and L. Franke, Eur. Polym. J., 2017, 94, 460–470 CrossRef CAS.
- H. Q. Luo, N. B. Li and S. P. Liu, Biosens. Bioelectron., 2006, 21, 1186–1194 CrossRef CAS PubMed.
- X. Mei, M. Sun, Y. Zhang, J. Shen, J. Li, C. Xue and Y. Chang, Int. J. Biol. Macromol., 2022, 223, 1180–1185 CrossRef CAS PubMed.
- J. R. Martins, C. C. Passerotti, R. M. Maciel, L. O. Sampaio, C. P. Dietrich and H. B. Nader, Anal. Biochem., 2003, 319, 65–72 CrossRef CAS PubMed.
- K. Ruckmani, S. Z. Shaikh, P. Khalil, M. Muneera and O. Thusleem, J. Pharm. Anal., 2013, 3, 324–329 CrossRef CAS PubMed.
- F. Kohansal, A. Mobed, N. Aletaha, K. Ghaseminasab, S. Dolati and M. Hasanzadeh, Microchem. J., 2023, 190, 108649 CrossRef CAS.
- M. Song, X. Lin, Z. Peng, S. Xu, L. Jin, X. Zheng and H. Luo, Front. Mater., 2021, 7, 583739 CrossRef.
- D.-W. Hwang, S. Lee, M. Seo and T. D. Chung, Anal. Chim. Acta, 2018, 1033, 1–34 CrossRef CAS PubMed.
- B. Bucur, C. Purcarea, S. Andreescu and A. Vasilescu, Sensors, 2021, 21, 3038 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06025j |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2023 |