Open Access Article
Open Access ArticlePalladium-anchored donor-flexible pyridylidene amide (PYA) electrocatalysts for CO2 reduction†
Afshan Khurshid‡
 a,
Tania Tanveer‡a,
Komal Hafeez‡a,
Maqsood Ahmed
a,
Tania Tanveer‡a,
Komal Hafeez‡a,
Maqsood Ahmed b,
Zareen Akhtara and
M. Naveed Zafar
b,
Zareen Akhtara and
M. Naveed Zafar *a
*a
aDepartment of Chemistry, Quaid-i-Azam University, Islamabad, 45320, Pakistan. E-mail: mnzafar@qau.edu.pk
bMaterials Chemistry Laboratory, Department of Chemistry, The Islamia University of Bahawalpur, 63100, Pakistan
First published on 29th November 2023
Abstract
The conversion of CO2 into CO as a substitute for processing fossil fuels to produce hydrocarbons is a sustainable, carbon neutral energy technology. However, the electrochemical reduction of CO2 into a synthesis gas (CO and H2) at a commercial scale requires an efficient electrocatalyst. In this perspective, a series of six new palladium complexes with the general formula [Pd(L)(Y)]Y, where L is a donor-flexible PYA, N2,N6-bis(1-ethylpyridin-4(1H)-ylidene)pyridine-2,6-dicarboxamide, N2,N6-bis(1-butylpyridin-4(1H)-ylidene)pyridine-2,6-dicarboxamide, or N2,N6-bis(1-benzylpyridin-4(1H)-ylidene)pyridine-2,6-dicarboxamide, and Y = OAc or Cl−, were utilized as active electrocatalysts for the conversion of CO2 into a synthesis gas. These palladium(II) pincer complexes were synthesized from their respective H-PYA proligands using 1,8-diazobicyclo[5.4.0]undec-7-ene (DBU) or sodium acetate as a base. All the compounds were successfully characterized by various physical methods of analysis, such as proton and carbon NMR, FTIR, CHN, and single-crystal XRD. The redox chemistry of palladium complexes toward carbon dioxide activation suggested an evident CO2 interaction with each Pd(II) catalyst. [Pd(N2,N6-bis(1-ethylpyridin-4(1H)-ylidene)pyridine-2,6-dicarboxamide)(Cl)]Cl showed the best electrocatalytic activity for CO2 reduction into a synthesis gas under the acidic condition of trifluoracetic acid (TFA) with a minimum overpotential of 0.40 V, a maximum turnover frequency (TOF) of 101 s−1, and 58% FE of CO. This pincer scaffold could be stereochemically tuned with the exploration of earth abundant first row transition metals for further improvements in the CO2 reduction chemistry.
1 Introduction
Carbon dioxide emissions are gradually increasing due to natural and anthropogenic activities, thus leading to global warming. The reduction of carbon dioxide to carbon neutral, valuable products is of major interest for researchers to overcome global warming challenges and energy deficiencies.1 An easier alternative to complex, less efficient catalytic processes involving the direct conversion of CO2 into hydrocarbons is the electrochemical conversion of CO2 into a synthesis gas (CO and H2), followed by the Fisher Tropsch reaction. The two-electron reduction of CO2 to CO (eqn (1)) is also beneficial, since CO is used as a precursor in numerous industrial processes.2 In this instance, however, a rival reaction (eqn (2)) is the direct proton reduction into hydrogen.| CO2 + 2H+ + 2e− → CO + H2O, E0 = −0.77 V vs. SCE | (1) |
| 2H+ + 2e− → H2, E0 = −0.66 V vs. SCE | (2) |
Many scientists across the globe have worked on the CO2 reduction into CO utilising homogeneous electrocatalysts with the aim to achieve a high activity and selectivity. Following the earlier discovery of a Rh-phosphine electrocatalyst by Wagenknecht et al. in 1984,3 DuBois and co-workers4–8 focused almost solely on the development of pincer Pd-phosphine based homogeneous electrocatalysts of the type [Pd(L3)(CH3CN)](BF4)2 for the CO2-reduction reaction (CRR). They detected CO gas as a major reduction product under acidic conditions in both dimethylformamide (DMF) and acetonitrile (CH3CN) solvents.5 DuBois also proposed the reaction mechanism for the palladium-catalyzed conversion of CO2 to CO. The rate-determining step was demonstrated by the addition of a high acid concentration that led to the η1-C model of CO2. A few examples of homogeneous palladium complexes were reported by Ogura for the CRR with nitrogen donor ligands, such as pyridine, pyrazole,9 quinoline, bipyridine, and phenanthroline.10
Pincer complexes are of particular interest in the field of redox catalysis owing to their strong chelating effect, preorganized geometry, high tunability, and potential for ligand-based redox activity.11–13 Several pincer complexes have been described in literature for the electrocatalytic CO2 reduction reaction (CRR) as shown in Fig. 1.14–24 Some early examples include Abruna's catalyst; with which bis(imino)pyridine anchored cobalt14,15 yielded formic acid as a reduced CRR product. Kang et al. created a similar pincer ligand but one imino arm was replaced with rigid pyridine to access a bipyridyl unit that increased the selectivity of cobalt for formate.16 Fontecave made a more selective cobalt terpyridine-based electrocatalyst for the reduction of CO2 to CO.17,18 Jurss and co-workers created a bipyridyl phosphine based mixed-donor ligand and achieved CO2 reduction catalysis at lower potentials.19 Richeson's PNP-ligated manganese–tricarbonyl complex achieved the conversion of CO2 to CO without the use of an additional Brønsted acid.20 Several studies by Hollis, Sun, and Luca on a C^C^C pincer based on N-heterocyclic carbene showed this supporting framework to be efficient for transition metal-based CO2 reduction catalysis.21–23 Similarly, Wolf developed a series of various C^N^C pincer palladium complexes with N-heterocyclic carbene for the CRC24–28 and studied the effect of various substitutions on the ligand architecture as well as metal replacement on the CRC. His work concluded that conjugated NHC catalysts with palladium worked much better for the CRR than Ni and Pt analogues. Although these palladium complexes were electrolytically stable and showed current augmentation under CO2, the faradaic efficiency (FE) for CO generation remained below 50%.
 | ||
| Fig. 1 Some selected pincer spectator ligands for the transition metal-catalyzed CO2 electroreduction. | ||
Here in this study, we aimed to utilize the potential of the donor-flexible and pincer-shaped pyridylidene amide (PYA) ligands toward palladium-catalyzed CO2 reduction into synthesis gas. In this regard, a series of new pyridylidene amide (PYA) proligands and their respective palladium complexes were synthesized and successfully characterized with various spectroscopic techniques and single-crystal XRD. The PYA ligand existed in two major limiting resonating forms (Fig. 2) and could tune the electron donation to the metal according to the requirement during catalytic cycles (Fig. 2).29–32 The resonance adaptation, inductive effect of various substituents on the PYA spectator ligand, and effect of different labile ligands (OAc, Cl) were investigated for palladium-centered CO2 activation.
2 Results and discussion
2.1 Synthesis and characterization of proligands (1–4)
Precursor (1) was synthesized from 4-aminopyridine and pyridine-2,6-dicarbonyldichloride from a previously reported method.33 The further alkylation of 1 was carried out using various alkylating reagents, such as ethyl iodide, butyl bromide, and benzyl chloride, in acetonitrile that afforded the proligands H-PYAs (2–4) (Scheme 1).The H-PYAs were characterized using different spectroscopic and structural techniques, like 1H NMR, 13C NMR, FTIR, and X-ray diffraction single-crystal analysis (S12†). The characterization data of 1 resembled the reported values.33 The representative 1H and 13C NMR of the 2–5 are given in Table 1. The proton NMR of the proligands showed NH peaks in the range of 13.19–11.27 ppm and aromatic peaks from 7.41 to 9.06 ppm. There were no aliphatic protons in 1 while 2–5 showed the aliphatic protons in the usual aliphatic region from 5.77–1.38 ppm. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak was observed from 163.3–170.7 ppm when analyzed by 13C NMR. Aliphatic methylene carbon signals were observed in the range of 19.2–62.2 ppm while the methyl carbons were found between 13.8–16.5 ppm in the carbon NMR. In the FTIR spectra, N–H stretching band was noticed in the range of 3400–3482 cm−1. The C
O peak was observed from 163.3–170.7 ppm when analyzed by 13C NMR. Aliphatic methylene carbon signals were observed in the range of 19.2–62.2 ppm while the methyl carbons were found between 13.8–16.5 ppm in the carbon NMR. In the FTIR spectra, N–H stretching band was noticed in the range of 3400–3482 cm−1. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, aromatic C–H, aliphatic C–H, and C
O, aromatic C–H, aliphatic C–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching bands were found in the ranges of 1645–1694, 3032–3098, 2900–2998, and 1556–1652 cm−1, respectively.34
N stretching bands were found in the ranges of 1645–1694, 3032–3098, 2900–2998, and 1556–1652 cm−1, respectively.34
| Compounds | 2 | 3 | 4 | 5 | ||||
|---|---|---|---|---|---|---|---|---|
| Protons/carbons | δH | δC | δH | δC | δH | δC | δH | δC |
| NH | 11.97 | — | 12.19 | — | 13.29 | — | — | — |
| Ar–H/C | 9.02–8.59 | 151.5–116.7 | 9.07–8.56 | 151.7–116.8 | 9.14–7.54 | 152.9–117.4 | 8.77–7.94 | 161.5–125.8 |
| CO | — | 163.7 | — | 164.0 | — | 164.6 | — | 170.5 |
| CH2 (α) | 4.59 | 55.4 | 4.54 | 59.5 | 5.77 | 62.2 | 4.55 | 59.2 |
| CH2 (β) | — | — | — | 32.9 | — | — | 1.89 | 32.9 |
| CH2 (ϒ) | — | — | — | 19.2 | — | — | 1.32 | 19.2 |
| CH3 | 1.58 | 16.5 | 1.90 | 13.8 | — | — | 0.94 | 13.8 |
2.2 Synthesis and characterization of palladium(II) complexes (6–11)
Palladium complexes with different proligands (H-PYAs) were synthesized by in situ deprotonations of H-PYAs (2–4) with 1,8-diazobicyclo[5.4.0]undec-7-ene (DBU) (in 2 and 4) or sodium acetate (in case of 3). The ligands generated with the help of weak base were treated with palladium source i–e palladium acetate for 6–8 and bisbenzonitrilepalladium(II) chloride for 9–11. The synthetic protocol is summarized in Scheme 2.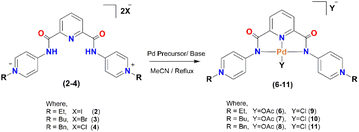 | ||
| Scheme 2 The synthesis of palladium complexes (6–11) from proligands (2–4) using Pd(OAc)2 or Pd(PhCN)2Cl2 as the palladium precursor and sodium acetate or DBU as the base. | ||
The palladium complexes were well characterized using various spectroscopic and structural techniques, like 1H NMR, 13C NMR, FTIR, and X-ray diffraction single-crystal analysis (S13†). The representative 1H and 13C NMR of the 6–11 are given in Table 2. The absence of the NH peak in 6–11 was confirmed by proton NMR. All the aromatic peaks were shifted to a more shielded region due to the resonance of amidate electrons on the whole complex in both proton and carbon NMR. The acetate ligand was revealed in 6–8 as a singlet for methyl that appeared in the range of 1.50–1.61 ppm. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks in 13C NMR were observed in the range of 162.1–170.7 ppm in all complexes, while the acetate carbonyl peaks in 6–8 appeared between 170.3–175.8 ppm. The appearance of these new peaks revealed the synthesis of these complexes. The aliphatic protons and carbons appeared in the normal range but were slightly shifted compared to the proligands. The absence of N–H band in the IR spectra supported the NMR results. The carbonyl bands of amidate and acetate appeared in their characteristic range of 1612–1658 cm−1 in the FTIR analysis.34 The Pd–N bands for the complexes were observed in the range of 511–528 cm−1.35
O peaks in 13C NMR were observed in the range of 162.1–170.7 ppm in all complexes, while the acetate carbonyl peaks in 6–8 appeared between 170.3–175.8 ppm. The appearance of these new peaks revealed the synthesis of these complexes. The aliphatic protons and carbons appeared in the normal range but were slightly shifted compared to the proligands. The absence of N–H band in the IR spectra supported the NMR results. The carbonyl bands of amidate and acetate appeared in their characteristic range of 1612–1658 cm−1 in the FTIR analysis.34 The Pd–N bands for the complexes were observed in the range of 511–528 cm−1.35
| Compounds | 6 | 7 | 8 | 9 | 10 | 11 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protons/carbons | δH | δC | δH | δC | δH | δC | δH | δC | δH | δC | δH | δC |
| OAc (CO) | 1.62 | 175.8 | 1.59 | 170.4 | 1.50 | 170.3 | — | — | — | — | — | — |
| CO | — | 170.3 | — | 162.0 | — | 162.1 | — | 170.5 | — | 170.5 | — | 170.5 |
| Ar–H/C | 8.75–7.89 | 161.7–123.0 | 8.78–7.90 | 149.4–126.6 | 8.90–7.47 | 149.4–123.2 | 8.76–7.95 | 161.5–125.7 | 8.77–7.94 | 161.5–125.7 | 8.90–7.53 | 161.8–125.9 |
| OAc (CH3) | — | 24.8 | — | 19.2 | — | 24.7 | — | — | — | — | — | — |
| CH2 (α) | 4.46 | 54.8 | 4.44 1.85 1.28 | 59.3 | 5.67 | 61.8 | 4.46 | 55.0 | 4.43 1.86 1.28 | 59.2 | 5.68 | 62.0 |
| CH2 (β) | — | — | 32.9 | — | — | — | — | 32.9 | — | — | ||
| CH2 (ϒ) | — | — | 19.2 | — | — | — | — | 19.2 | — | — | ||
| CH3 | 1.51 | 16.6 | 0.93 | 13.8 | — | — | 1.53 | 16.5 | 0.91 | 13.8 | — | — |
Attempts were made to isolate all the ligands that were produced in situ during the complex formation reactions. However, only 5 was obtained as a white powder by the deprotonation of 3 with an aqueous solution of KOH followed by its extraction in dichloromethane (Scheme 1). The disappearance of the proligand amide N–H peak was observed both in the proton NMR (Table 1) and FTIR. A comparison of 5 with 7 and 10 showed that the protons and carbon signals were slightly shifted after complex formation.
2.3 Electrochemical behavior of palladium complexes (6–11) under N2
To evaluate the redox behavior of the palladium complexes, cyclic voltammetry (CV) experiments were performed under an inert atmosphere of nitrogen. The cyclic voltammograms of 6–11 under N2 are represented as black line in Fig. 3, and some important parameters from the CV tests are given in Table 3. An H-cell with 2 mM solution of the catalyst in DMF and 0.1 M supporting electrolyte tetrabutylammonium hexafluorophosphate (TBAPF6) was used in all the measurements. The appearance of anodic and cathodic peaks represented the redox active natures of 6–11. An anodic peak for 6–8 emerged for the acetate and appeared from 0.5 V to 0.6 V, while the peaks observed at 0.3, −1.3, and −0.25 V could be related to complex oxidation. Similarly, 9–11 showed complex oxidation at 0.3, 0, and −0.2 V, respectively. Similarly, the halide oxidation occurred at 0.7, 0.8, and 0.3 V, respectively. Two to three irreversible cathodic waves appeared for all complexes (black line in Fig. 3), which could be attributed to the formation of redox species.26 The first cathodic wave, Epc1, for 6–11 ranged from −0.80 V to −0.98 V with peak current values, Ipc1 in the range of 25 μA to 150 μA. The second cathodic wave, Epc2, for the complexes appeared in the range of −1.33 V to −1.95 V with peak current values, Ipc2, ranging from 35 μA to 175 μA. The third cathodic events appeared at −1.80 V to −2.20 V with Ipc3 values up to 160 μA for the palladium complexes. The cathodic waves could be related to the reduction of Pd(II) to Pd(I) and Pd(I) to Pd(0). The current under each peak increased with the increase in the scan rate according to Randles–Sevcik eqn (S15).†36 | ||
| Fig. 3 The cyclic voltammograms of 6–11 under nitrogen (black line) and CO2 (red line). All experiments were performed at 100 mV s−1 scan rate in DMF and 0.1 M supporting electrolyte (TBAPF6). | ||
| Catalysts | Epc1 (V) | Epc2 (V) | Epc3 (V) | Epa1 (V) | Epa2 (V) |
|---|---|---|---|---|---|
| a The peak potential of complexes 6–11 [2 mM] from CV measured at 100 mV s−1 in DMF containing 0.1 M (TBAPF6) under N2. | |||||
| 6 | −0.96 | −1.80 | −2.11 | 0.30 | 0.60 |
| 7 | −0.80 | −1.86 | −1.82 | −1.30 | 0.50 |
| 8 | −0.87 | −1.33 | −1.80 | −0.25 | 0.50 |
| 9 | −0.70 | −1.75 | — | 0.30 | 0.70 |
| 10 | −0.98 | −1.60 | −1.89 | — | 0.80 |
| 11 | −0.87 | −1.95 | −2.20 | −0.20 | 0.30 |
2.4 Electrochemical behavior of palladium complexes (6–11) under CO2
To evaluate the electrochemical interactions of the palladium complexes with CO2, cyclic voltammetry experiments were performed under a saturated environment of CO2 by sparging the solution with carbon dioxide gas for 15 min before running the electrochemical reaction. The carbon dioxide bubbling in the electrolytic solution was done to not only ward-off all the reactive gases like oxygen from the solution but also to produce its reduced products. The cyclic voltammograms results under CO2 and some important parameters are given in Fig. 3 (as red lines) and Table 4, respectively. Most of the cathodic peaks showed current enhancement, particularly 9 with peak current ratios of CO2 to N2 (I/Ip0) 1.41 at Epc1 and 2.58 at Epc2. This was followed by complex 6 (acetate analogue of 9), which indicated the optimized inductive synergistic effect of the ethyl groups on the palladium center via the pendant pyridinium rings of the spectator ligand for CO2 activation. The chloride seemed a little better actor ligand compared to the acetate as complexes 9–11 showed a high cathodic peak current under the CO2 environment compared to their respective acetate analogues (6–8). The complexes 7 and 10 with butyl pendent groups showed the least activity toward CO2 activation followed by the benzylated complexes 8 and 11. These current enhancements under the CO2 environment compared to the inert gas suggested the formation of some CO2 reduced products. The palladium complex with the ethylated pendant group on the spectator ligand and chloride as the actor ligand had a lower overpotential for CO2 reduction than its acetate analogue. However, the palladium complex with the butyl substituent on the ligand and with acetate showed less overpotential compared to its chloride counterpart. However, the benzyl-bearing complex remained unaffected by the change of the labile ligand. The palladium complex upon reduction released the actor ligand (acetate or chloride in this case) and interacted with the electrophilic carbon of CO2 through its fourth coordination site (Scheme 3). The linear CO2 molecule got bent upon its binding with the electron-rich palladium center.24 The formation of [N^N^N–Pd(II)–COO˙] was reflected by the enhancement in the cathodic peak currents in the cyclic voltammograms of the complexes under the saturated CO2 environment (red lines, Fig. 3). This suggested the fastest and spontaneous interaction of CO2 with 9 compared to other catalysts. A low current enhancement upon CO2 interaction revealed the slowest kinetics for 7 and 10. The magnitude of the homogeneous complexation (K (equilibrium constant) and kf (rate constant)) or heterogeneous reduction (kr2 (rate constant)) demonstrated the peak position of Epc (CO2).25 This in turn depended on the scan rate (timeframe of the experiment) relative to the scale of K and the rates of reduction. The shift of Epc (CO2) to a more negative potential revealed a larger value of K while a shift of Epc (CO2) to a less negative potential represented a larger kr2.37,38 Shifts in the position of Epc1 under CO2 within the range of −10 mV to 60 mV were observed for all the complexes (Table 4). The maximum value of Ipc1 (CO2)/Ipc1 (N2) = 1.41, was observed at the first cathodic event for 9. This represented the acceptance of a second electron by [N^N^N–Pd+2COO˙] species. Similarly, the reduction events at Epc2 (CO2) showed a more positive potential compared to Epc2 (N2) in almost all the complexes with large Ipc2 (CO2)/Ipc2 (N2) values up to 2.58 (in the case of 9). This could be related to the larger value of K.37,38| Catalysts | Epc1 | ΔEpc1 | I/Ip0 | Epc2 | ΔEpc2 | I/Ip0 | Epc3 | ΔEpc3 |
|---|---|---|---|---|---|---|---|---|
| a Cathodic peak waves, change in potentials to N2, and the peak current ratio of CO2 to N2 specified as i/ip0 of complexes 6–11. CV data were collected at 100 mV s−1 in DMF with 2 mM complex and (TBAPF6) as the supporting electrolyte. | ||||||||
| 6 | −0.94 | −20 | 1.36 | −1.47 | 330 | 2.00 | −2.11 | 00 |
| 7 | −0.81 | −10 | 0.90 | −1.38 | 480 | 1.33 | −2.07 | −250 |
| 8 | −0.88 | 10 | 1.02 | −1.34 | −10 | 1.40 | −2.12 | −300 |
| 9 | −0.68 | 20 | 1.41 | −1.69 | 60 | 2.58 | — | — |
| 10 | −0.92 | 60 | 0.54 | −1.53 | 70 | 2.02 | −1.99 | −100 |
| 11 | −0.88 | −10 | 0.80 | −1.63 | 320 | 2.51 | −1.98 | 220 |
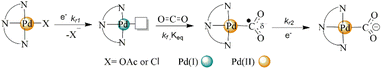 | ||
| Scheme 3 The proposed mechanism for binding of the solvated CO2 with the reduced pincer palladium catalyst. | ||
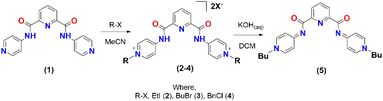
2.5 Electrocatalysis of proton-promoted CO2 reduction by palladium complex (9)
Complex 9 that showed the highest CO2 activation (as mentioned above) was further studied under the reducing environment of protons. Different acids, such as glacial acetic acid (GAA), trifluoroethanol (TFE), and trifluoracetic acid (TFA), were utilized for this study. Complex 9 showed the best results with 10 mmol of TFA in terms of the cathodic current enhancements (blue line, Fig. 4A). Two distinct cathodic peaks, Epc1 and Epc2 at −1.04 and −1.95 V with peak currents Ipc1 and Ipc2 of 679 μA and 975 μA, respectively, were noticed. The current ratio of Icat/Ip at Epc1 was 11.3 and at Epc2 was 8.2 for 9. The maximum turnover frequency (TOF) of 101 s−1 and Kcat of 561 M−1 s−1 were obtained. The minimum overpotential obtained was 0.40 V for catalyst 9. The TOF and kcat were calculated by using eqn (3) from the literature.39
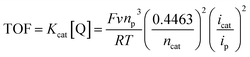 | (3) |
The bulk electrolysis experiment was performed for a period of 7200 s for 9 at a 10 mV more negative potential than the first cathodic wave. Samples were obtained from the H-cell's headspace to characterize the gaseous product. The controlled potential electrolysis yielded 58% CO and 40% H2. The CPE plot (Fig. 4B) revealed no major fluctuation in the current and proved that the catalyst was robust at 7200 s. The post-CPE cyclic voltammetry measurements were also performed and revealed no characteristic change in the respective reduction and oxidation peaks (Fig. 4C).
The best catalyst 9 in this work was compared with several other palladium complexes reported in the literature, and was found to be superior in terms of the catalytic efficiency parameters. Therrien's Pd–NHC pincer complex showed a maximum TOF of 11 s−1, Kcat of 30 M−1 s−1, and η of −640 mV.24 Wolf studied the influence of para substituents (OMe, Br, COOR) in a series of palladium–NHC complexes with general the formula [Pd(C^N^C)Cl]OTf on CO2 reduction catalysis27 and showed TOFs in the range of 1–9 s−1.
A mechanism for the conversion of CO2 into CO and H2 is proposed based on the literature25 and is shown in Scheme 4. The catalyst [PYA-Pd(II)–Cl]Cl generated [PYA-Pd(0)] species after the addition of two electrons via electroreduction from an external source. Depending on the favorable kinetics, two pathways are suggested. In the first case, the interaction of CO2 with the reduced catalyst species produces a metal–carboxylate intermediate, [PYA-Pd(II)–CO2−], that upon protonation with TFA results in the formation of CO. In the other case, the proton adds to the electron-rich [PYA-Pd(0)] and generates the hydride intermediate, [PYA-Pd(II)–H], that reacts with the next proton and generates hydrogen gas. It was noticed that with the addition of acid from 10 mmol to 40 mmol, the cathodic current increased but the ratio of CO to H2 remained unaffected. However, upon the addition of 50 mmol concentration of the TFA, hydrogen production was increased to 47% while the CO was decreased to 50%. At the same time, the overpotential moved to 20 mV more positive potential (Fig. S16†).
To rule out the idea that the high activity and selectivity of 9 were due to elemental palladium deposited from considerable Pd demetallation, a “rinse test” was carried out. To get rid of any loosely bound molecular Pd catalyst, the working electrode from the CPE experiments with 9 was gently rinsed twice with DMF after immersion overnight in DMF. The electrode was then tested to see if Pd metal had been plated onto the carbon electrode during the earlier experiment using a brand-new, catalyst-free electrolyte solution. The little CO generation and negligible current enhancements proved that the electrode was devoid of heterogenized Pd catalyst or elemental palladium.
3 Experimental section
3.1 General consideration
A typical Schlenk line apparatus was used for the synthesis of the proligand, ligands, and the respective palladium(II) complexes. The chemical reagents and solvents were used with or without additional purification or drying depending on the conditions. Analytical grade N,N-dimethylformamide (DMF), acetonitrile, acetone, dichloromethane, and diethyl ether were purchased from DaeJung, Korea, and were dried and stored over molecular sieves in Schlenk flasks before use. Triethylamine, pyridine-2,6-dicarboxylic acid, 4-aminopyridine, and triphenylphosphite were purchased from Merck Chemicals. Tetrabutylammonium hexafluorophosphate, iodoethane, 1-bromobutane, and benzyl chloride were acquired from Sigma Aldrich. Palladium(II) acetate, and bis(benzonitrile)dichloropalladium were purchased from Aldrich.1H and 13C NMR spectra were collected using a Bruker AV300 spectrometer. The Campbell Microanalytical Laboratory at the University of Otago collected the elemental analysis data of the samples for C, H, N analysis. IR data of the compounds were obtained from Thermo Scientific Nicolet-6700 FT-IR (4000–400 cm−1). A UVGL-58 UV lamp was used to monitor the reactions using TLC plates. The melting points of all the compounds were measured on a Sanyo Gallen Kamp MPD350 instrument. Electrochemical studies were performed on a CH-I760 electrochemical workstation.
3.2 Electrochemistry
Electrocatalytic activities were performed by using cyclic voltammetry and controlled potential electrolysis. An air-tight H-shaped electrochemical cell with three conventional electrodes, namely glassy carbon as the working electrode (with an area of 7 mm2), counterelectrode of Pt mesh, and Ag/AgCl (Ag wire dipped in 3.5 M KCl solution isolated from the bulk solution by a Vycor frit) as the reference electrode. An H-cell was used to put the working and Pt mesh counterelectrode in separate compartments and to avoid direct interaction of CO with Pt. Electrochemical studies were performed under inert as well as CO2 environments. The H-cell was filled with 2 mM solutions of 6–11 along with 0.1 M TBAPF6 (tetrabutylammonium hexafluorophosphate) as the supporting electrolyte in 15 mL of dimethyl formamide in the absence or presence of TFA. Gases were purged for 10 min followed by running the instrument at various scan rates. The glassy carbon electrode was polished with 1, 0.3, and 0.05 μM alumina paste successively followed by sonication for 5 min in distilled water followed by methanol. It was finally rinsed with distilled water before each experiment. The glassy carbon electrodes were eviscerated by electrochemical oxidation at +1.0 V versus Ag/AgCl followed by a thorough rinsing with methanol and acetone. Electrolyte solutions of 0.1 M TBAPF6 in anhydrous DMF with and without catalysts were sparged with N2 and CO2 for 15–20 min prior to each experiment. The sparging of CO2 for 20 min was enough to create a concentration of CO2 ∼0.18 M in DMF.24 The precursor N2,N6-di(pyridin-4-yl)pyridine-2,6-dicarboxamide (1) was prepared following the reported method.333.3 General procedure for the synthesis of proligands (2–4)
Precursor (1) and respective alkyl halide were charged to an evacuated 100 mL round-bottom two-neck flask. Acetonitrile was added and the reaction mixture was refluxed at 80 °C for an appropriate time. The reaction mixture was cooled to room temperature and the precipitates were collected (via filtration), washed with acetone and diethyl ether, and dried in vacuo to yield pure products.Yield: 90%. Color: yellow. mp: 230 °C. Anal. calculated for C21H23I2N2O5: C, 66.83; H, 6.14; N, 18.55. Found: C, 66.60; H, 6.34; N, 18.28. IR (KBr, cm−1): 3425 (NH), 3005 (Ar-CH), 2970, 2872 (Aliph-CH), 1668 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1620 (C
O), 1620 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1226 (C–N). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 11.97 (s, 2H, HNH), 9.02 (d, J = 7.2, 4H, HAr), 8.59–8.47 (m, 5H, HAr), 4.59–4.52 (m, 4H, HCH2), 1.58 (t, J = 15, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 163.7(Ar–C
N), 1226 (C–N). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 11.97 (s, 2H, HNH), 9.02 (d, J = 7.2, 4H, HAr), 8.59–8.47 (m, 5H, HAr), 4.59–4.52 (m, 4H, HCH2), 1.58 (t, J = 15, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 163.7(Ar–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 151.5, 147.6, 145.5, 141.5, 127.8, 116.7 (Ar), 55.4 (CCH2), 16.5 (CCH3).
O), 151.5, 147.6, 145.5, 141.5, 127.8, 116.7 (Ar), 55.4 (CCH2), 16.5 (CCH3).
Yield: 90%. Color: white. mp: 238 °C. Anal. calcd for C25H31Br2N5O2: C, 50.61; H, 5.27; N, 11.80. Found: C, 50.46; H, 5.08; N, 11.56. IR (KBr, cm−1): 3431 (NH), 3006 (Ar-CH), 2871 (Aliph-CH), 1692 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1638 (C
O), 1638 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1227 (C–N). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 12.19 (s, 2H, HNH), 9.07 (d, J = 6, 4H, HAr), 8.69 (d, 4H, J = 7.5, HAr) 8.56–8.45 (m, 3H, J = 7.8, 6.6, HAr), 4.54 (pseudo triplet, 4H, HCH2), 1.90 (pseudo triplet, 4H, HCH2), 1.33 (pseudo triplet, 4H, HCH2), 0.96 (pseudo triplet, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 164.0 (C
N), 1227 (C–N). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 12.19 (s, 2H, HNH), 9.07 (d, J = 6, 4H, HAr), 8.69 (d, 4H, J = 7.5, HAr) 8.56–8.45 (m, 3H, J = 7.8, 6.6, HAr), 4.54 (pseudo triplet, 4H, HCH2), 1.90 (pseudo triplet, 4H, HCH2), 1.33 (pseudo triplet, 4H, HCH2), 0.96 (pseudo triplet, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 164.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 151.7, 147.9, 145.7, 141.3, 128.0, 116.8 (Ar), 59.5, 32.9, 19.2 (CCH2), 13.8 (CCH3).
O), 151.7, 147.9, 145.7, 141.3, 128.0, 116.8 (Ar), 59.5, 32.9, 19.2 (CCH2), 13.8 (CCH3).
Yield: 90%. Color: white. mp: 276 °C. Anal. calcd for C31H27Cl2N5O2: C, 74.23; H, 5.43; N, 13.96. Found: C, 74.06; H, 5.15; N, 13.75. IR (KBr, cm−1): 3431 (NH), 3063 (Ar-CH), 2395 (Aliph-CH), 1691 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1512 (C
O), 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1267 (C–N). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 13.20 (s, 2H, HNH), 9.14 (d, J = 7.2, 4H, HAr), 8.99 (d, J = 7.2, 4H, HAr) 8.52 (d, J = 8.4, 2H, HAr) 8.49 (m, 1H, HAr). 7.54–7.44 (m, 10H, HAr), 5.77 (s, 4H, HCH2). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 164.6 (C
N), 1267 (C–N). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 13.20 (s, 2H, HNH), 9.14 (d, J = 7.2, 4H, HAr), 8.99 (d, J = 7.2, 4H, HAr) 8.52 (d, J = 8.4, 2H, HAr) 8.49 (m, 1H, HAr). 7.54–7.44 (m, 10H, HAr), 5.77 (s, 4H, HCH2). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 164.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 152.9, 148.3, 145.5, 140.9, 135.2, 129.6, 128.9, 128.2, 117.4 (Ar), 62.2(CCH2).
O), 152.9, 148.3, 145.5, 140.9, 135.2, 129.6, 128.9, 128.2, 117.4 (Ar), 62.2(CCH2).
Yield: 67%. Color: white. mp: 300 °C. Anal. calcd for C25H29N5O2: C, 69.58; H, 6.77; N, 16.23. Found: C, 69.35; H, 6.68; N, 16.05. IR (KBr, cm−1): 3053 (Ar-CH), 2930 (Aliph-CH), 1650 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1628 (C
O), 1628 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1575 (C
N), 1575 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.77 (d, J = 6.9, 4H, HAr), 8.48 (d, J = 7.8, 1H, HAr), 8.10 (d, J = 7.8, 2H, HAr), 7.94 (d, J = 6.9, 4H, HAr), 4.45 (t, J = 7.2, 4H, HCH2), 1.89–1.81 (t, J = 9, 4H, HCH2), 1.32–1.24 (m, 4H, HCH2), 0.94 (t, J = 6, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 170.5 (C
C). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.77 (d, J = 6.9, 4H, HAr), 8.48 (d, J = 7.8, 1H, HAr), 8.10 (d, J = 7.8, 2H, HAr), 7.94 (d, J = 6.9, 4H, HAr), 4.45 (t, J = 7.2, 4H, HCH2), 1.89–1.81 (t, J = 9, 4H, HCH2), 1.32–1.24 (m, 4H, HCH2), 0.94 (t, J = 6, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 170.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.5, 149.6, 143.3, 128.3, 125.8(Ar), 59.3, 32.9, 19.2 (CCH2), 13.8 (CCH3).
O), 161.5, 149.6, 143.3, 128.3, 125.8(Ar), 59.3, 32.9, 19.2 (CCH2), 13.8 (CCH3).
3.4 General procedure for the synthesis of complexes (6–11)
H2L (1.00 eq.), palladium precursor (1.10 eq.), and acetonitrile (5 mL) were combined in an evacuated 25 mL two-necked round-bottom flask. After stirring for 15 min, DBU or sodium acetate (3.00 eq.) was added, and the reaction mixture was heated under reflux (80 °C) in an atmosphere of dry nitrogen for several hours. The solution was cooled to room temperature and the precipitates formed were filtered and dried in vacuo to yield a pure product.Yield 67%. Color: brown. mp: 254 °C. Anal. calcd for C25H27N5O6Pd: C, 50.05; H, 4.54; N, 11.67. Found: C, 50.31; H, 4.30; N, 11.54. IR (KBr, cm−1): 3072 (Ar-CH), 2900 (Aliph-CH), 1650 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1618 (OCH3,C
O), 1618 (OCH3,C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1556 (C
O), 1556 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1558 (C
N), 1558 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.75 (d, J = 6.9, 4H, HAr), 8.46 (t, J = 7.8, 1H, HAr), 8.07 (d, J = 7.8, 2H, HAr), 7.89 (d, J = 6.9, 4H, HAr), 4.46 (q, J = 7.2, 4H, HCH2), 1.62 (s, 3H, HOCH3), 1.51 (t, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 175.8 (OCH3,C
C). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.75 (d, J = 6.9, 4H, HAr), 8.46 (t, J = 7.8, 1H, HAr), 8.07 (d, J = 7.8, 2H, HAr), 7.89 (d, J = 6.9, 4H, HAr), 4.46 (q, J = 7.2, 4H, HCH2), 1.62 (s, 3H, HOCH3), 1.51 (t, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 175.8 (OCH3,C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 170.3 (C
O), 170.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.7, 150.4, 143.3, 142.8, 128.3, 123.0 (Ar), 54.8 (CCH2), 24.8 (OCH3), 16.6 (CCH3).
O), 161.7, 150.4, 143.3, 142.8, 128.3, 123.0 (Ar), 54.8 (CCH2), 24.8 (OCH3), 16.6 (CCH3).
Yield: 67%. Color: orange-yellow. mp: 300 °C. Anal. calcd for C29H35N5O6Pd: C, 50.09; H, 5.38; N, 10.68. Found: C, 50.38; H, 5.50; N, 10.45. IR (KBr, cm−1): 3044 (A-CH), 2930 (Aliph-CH), 1645 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1612 (OCH3,C
O), 1612 (OCH3,C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1598 (C
O), 1598 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N),1556 (C
N),1556 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.78 (d, J = 6.3, 4H, HAr), 8.49 (t, J = 8.7, 1H, HAr), 8.09 (d, J = 7.5, 2H, HAr), 7.90 (d, J = 5.7, 4H, HAr), 4.44 (pseudo triplet, 4H, HCH2), 1.85 (pseudo triplet, J = 7.5, 4H, HCH2), 1.59 (s, 3H, HOCH3), 1.28 (q, J = 7.2, 4H, HCH2), 0.93 (pseudo triplet, J = 6.9, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 170.4 (OCH3,C
C). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.78 (d, J = 6.3, 4H, HAr), 8.49 (t, J = 8.7, 1H, HAr), 8.09 (d, J = 7.5, 2H, HAr), 7.90 (d, J = 5.7, 4H, HAr), 4.44 (pseudo triplet, 4H, HCH2), 1.85 (pseudo triplet, J = 7.5, 4H, HCH2), 1.59 (s, 3H, HOCH3), 1.28 (q, J = 7.2, 4H, HCH2), 0.93 (pseudo triplet, J = 6.9, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 170.4 (OCH3,C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 162.0 (C
O), 162.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.4, 143.3, 128.3, 126.6 (Ar), 59.3 (CCH2), 32.9 (CCH2), 19.2 (OCH3), 13.8 (CCH3).
O), 149.4, 143.3, 128.3, 126.6 (Ar), 59.3 (CCH2), 32.9 (CCH2), 19.2 (OCH3), 13.8 (CCH3).
Yield 68%. Color: olive-green. mp: 282 °C. Anal. calcd for C35H31N5O6Pd: C, 58.06; H, 4.32; N, 9.67. Found: C, 58.30; H, 4.58; N, 9.45. IR (KBr, cm−1): 3016 (Ar-CH), 2926 (Aliph-CH), 1653 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1621 (OCH3,C
O), 1621 (OCH3,C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1600 (C
O), 1600 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1575 (C
N), 1575 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.90 (d, J = 6.9, 4H, HAr), 8.41 (t, J = 7.8, 1H, HAr), 8.04 (d, J = 7.8, 2H, HAr), 7.90 (d, J = 6.6, 4H, HAr), 7.47–7.41 (m, J = 3.6, 10H, HAr), 5.67 (s, 4H, HCH2), 1.50 (s, 3H, HOCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 170.3(OCH3, C
C). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.90 (d, J = 6.9, 4H, HAr), 8.41 (t, J = 7.8, 1H, HAr), 8.04 (d, J = 7.8, 2H, HAr), 7.90 (d, J = 6.6, 4H, HAr), 7.47–7.41 (m, J = 3.6, 10H, HAr), 5.67 (s, 4H, HCH2), 1.50 (s, 3H, HOCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 170.3(OCH3, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 162.1 (C
O), 162.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 149.4, 143.7, 142.8, 135.5, 129.6, 129.5, 128.8, 128.4, 123.2 (Ar), 61.8 (CCH2), 24.7 (OCH3).
O), 149.4, 143.7, 142.8, 135.5, 129.6, 129.5, 128.8, 128.4, 123.2 (Ar), 61.8 (CCH2), 24.7 (OCH3).
Yield: 90%. Color: dark green. mp: 280 °C. Anal. calcd for C21H21Cl2N5O2Pd: C, 45.63; H, 3.83; N, 12.67. Found: C, 45.88; H, 3.60; N, 12.45. IR (KBr, cm−1): 3170 (Ar-CH), 2912,2871 (Aliph-CH), 1692 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1492 (C
O), 1492 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1227 (C–N). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.76 (d, J = 6.2, 4H, HAr), 8.46 (dd, 1H, J = 7.8, HAr), 8.08 (d, J = 0.9, 2H, HAr), 7.95 (d, J = 6.6, 4H, HAr), 4.48 (m, 4H, HCH2), 1.52 (t, J = 6, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 170.5 (C
C), 1227 (C–N). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.76 (d, J = 6.2, 4H, HAr), 8.46 (dd, 1H, J = 7.8, HAr), 8.08 (d, J = 0.9, 2H, HAr), 7.95 (d, J = 6.6, 4H, HAr), 4.48 (m, 4H, HCH2), 1.52 (t, J = 6, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 170.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.5, 149.7, 143.0, 142.7, 128.3, 125.7 (Ar), 55.0 (CCH2), 16.5 (CCH3).
O), 161.5, 149.7, 143.0, 142.7, 128.3, 125.7 (Ar), 55.0 (CCH2), 16.5 (CCH3).
Yield: 60%. Color: yellowish green. mp: 295 °C. Anal. calcd for C25H29Cl2N5O2Pd: C, 49.32; H, 4.80; N, 11.50. Found: C, 49.64; H, 4.57; N, 11.73. IR (KBr, cm−1): 3006 (Ar-CH), 2871 (Aliph-CH), 1682 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1517 (C
O), 1517 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1455 (C
N), 1455 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1376 (C–N). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.77 (pseudo triplet, 4H, HAr), 8.44 (pseudo triplet, 1H, HAr), 8.08–7.94 (m, 6H, HAr) 4.43 (pseudo triplet, 4H, HCH2), 1.86–1.64 (m, 4H, HCH2), 1.28 (pseudo triplet, 4H, HCH2), 0.91 (pseudo triplet, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 170.5 (C
C), 1376 (C–N). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.77 (pseudo triplet, 4H, HAr), 8.44 (pseudo triplet, 1H, HAr), 8.08–7.94 (m, 6H, HAr) 4.43 (pseudo triplet, 4H, HCH2), 1.86–1.64 (m, 4H, HCH2), 1.28 (pseudo triplet, 4H, HCH2), 0.91 (pseudo triplet, 6H, HCH3). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 170.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.5, 149.6, 143.8, 143.3, 128.3, 125.7 (Ar), 59.2, 32.9, 19.2 (CCH2), 13.8 (CCH3).
O), 161.5, 149.6, 143.8, 143.3, 128.3, 125.7 (Ar), 59.2, 32.9, 19.2 (CCH2), 13.8 (CCH3).
Yield: 75%. Color: yellow. mp: 254 °C. Anal. calcd for C31H25Cl2N5O2Pd: C, 55.01; H, 3.72; N, 10.35. Found: C, 55.25; H, 3.55; N, 10.13. IR (KBr, cm−1): 3058 (Ar-CH), 2994 (Aliph-CH), 1658 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1644 (C
O), 1644 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1351 (C–N). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.90 (d, J = 6.9, 4H, HAr), 8.46 (t, J = 7.8, 1H, HAr), 8.08 (d, J = 6.9, 2H, HAr), 7.96 (d, J = 6, 3H, HAr), 7.53–7.40 (m, J = 15, 11H, HAr), 5.68 (s, 4H, HCH2). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 170.5 (C
N), 1351 (C–N). 1H NMR δ (ppm)/J (Hz)/DMSO-d6/300 MHz: 8.90 (d, J = 6.9, 4H, HAr), 8.46 (t, J = 7.8, 1H, HAr), 8.08 (d, J = 6.9, 2H, HAr), 7.96 (d, J = 6, 3H, HAr), 7.53–7.40 (m, J = 15, 11H, HAr), 5.68 (s, 4H, HCH2). 13C NMR δ (ppm)/DMSO-d6/75 MHz: 170.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.8, 149.6, 143.4, 135.3, 129.6, 129.0, 128.4, 128.1, 125.9 (Ar), 62.0 (CCH2).
O), 161.8, 149.6, 143.4, 135.3, 129.6, 129.0, 128.4, 128.1, 125.9 (Ar), 62.0 (CCH2).
4 Conclusion
New N,N,N pincer complexes of palladium were synthesized and successfully characterized. The donor-flexible nature of the pyridylidene amide (PYA) spectator ligands with their X and L type limiting resonance structures were effectively exploited to facilitate palladium-catalyzed CO2 reduction reactions. The synergy of palladium with PYA bearing ethylated groups and chloride being a better labile ligand activated CO2 the most compared to the respective benzyl and butyl PYA analogues and acetate actor ligand. A minimum overpotential of 0.40 V and a maximum turnover frequency (TOF) of 101 s−1 with a 58% FE of CO was calculated for 9 under electrocatalytic conditions in TFA.Author contributions
Afshan Khurshid: investigation, visualization, writing – original draft, data curation, validation. Tania Tanveer: formal analysis, investigation, visualization, writing – original draft, data curation. Komal Hafeez: formal analysis, investigation, visualization, data curation. Maqsood Ahmed: single-crystal analysis. Zareen Akhter: resources. Muhammad Naveed Zafar: conceptualization, methodology, writing – review & editing, resources, project administration, funding acquisition, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are highly grateful to Higher education commission (HEC) Pakistan for providing research grant to principal investigator (M. N. Z).References
- E. E. Benson, C. P. Kubiak, A. J. Sathrum and J. M. Smieja, Chem. Soc. Rev., 2009, 38, 89–99 RSC.
- M. Mikkelsen, M. Jørgensen and F. C. Krebs, Energy Environ. Sci., 2010, 3, 43–81 RSC.
- S. Slater and J. H. Wagenknecht, J. Am. Chem. Soc., 1984, 106, 5367–5368 CrossRef CAS.
- M. Rakowski Dubois and D. L. Dubois, Acc. Chem. Res., 2009, 42, 1974–1982 CrossRef CAS PubMed.
- D. L. DuBois, A. Miedaner and R. C. Haltiwanger, J. Am. Chem. Soc., 1991, 113, 8753–8764 CrossRef CAS.
- D. L. DuBois and A. Miedaner, J. Am. Chem. Soc., 1987, 109, 113–117 CrossRef CAS.
- B. D. Steffey, C. J. Curtis and D. L. DuBois, Organometallics, 1995, 14, 4937–4943 CrossRef CAS.
- A. Miedaner, B. C. Noll and D. L. DuBois, Organometallics, 1997, 16, 5779–5791 CrossRef CAS.
- A. M. Hossain, T. Nagaoka and K. Ogura, Electrochim. Acta, 1996, 41, 2773–2780 CrossRef.
- A. M. Hossain, T. Nagaoka and K. Ogura, Electrochim. Acta, 1997, 42, 2577–2585 CrossRef.
- J. v. Vlugt and J. Reek, Angew Chem. Int. Ed. Engl., 2009, 48, 8832–8846 CrossRef PubMed.
- T. K. Michaelos, D. Y. Shopov, S. B. Sinha, L. S. Sharninghausen, K. J. Fisher, H. M. Lant, R. H. Crabtree and G. W. Brudvig, Acc. Chem. Res., 2017, 50, 952–959 CrossRef CAS PubMed.
- J. A. Wright, A. A. Danopoulos, W. B. Motherwell, R. J. Carroll and S. Ellwood, J. Organomet. Chem., 2006, 691, 5204–5210 CrossRef CAS.
- C. Arana, S. Yan, K. M. Keshavarz, K. T. Potts and H. D. Abruna, Inorg. Chem., 1992, 31, 3680–3682 CrossRef CAS.
- G. Chiericato Jr, C. Arana, C. Casado, I. Cuadrado and H. Abruna, Inorg. Chim. Acta, 2000, 300, 32–42 CrossRef.
- F. W. Liu, J. Bi, Y. Sun, S. Luo and P. Kang, ChemSusChem, 2018, 11, 1656–1663 CrossRef CAS PubMed.
- N. Elgrishi, M. B. Chambers, V. Artero and M. Fontecave, Phys. Chem. Chem. Phys., 2014, 16, 13635–13644 RSC.
- N. Elgrishi, M. B. Chambers and M. Fontecave, Chem. Sci., 2015, 6, 2522–2531 RSC.
- K. Talukdar, A. Issa and J. W. Jurss, Front. Chem., 2019, 7, 330 CrossRef CAS PubMed.
- G. K. Rao, W. Pell, I. Korobkov and D. Richeson, Chem. Commun., 2016, 52, 8010–8013 RSC.
- J. D. Cope, N. P. Liyanage, P. J. Kelley, J. A. Denny, E. J. Valente, C. E. Webster, J. H. Delcamp and T. K. Hollis, Chem. Commun., 2017, 53, 9442–9445 RSC.
- M. Sheng, N. Jiang, S. Gustafson, B. You, D. H. Ess and Y. Sun, Dalton Trans., 2015, 44, 16247–16250 RSC.
- T. H. Myren, A. M. Lilio, C. G. Huntzinger, J. W. Horstman, T. A. Stinson, T. Franklin, C. Moore, B. Lama, H. H. Funke and O. R. Luca, Organometallics, 2018, 38, 1248–1253 CrossRef.
- J. A. Therrien, M. O. Wolf and B. O. Patrick, Inorg. Chem., 2014, 53, 12962–12972 CrossRef CAS PubMed.
- E. E. DeLuca, Z. Xu, J. Lam and M. O. Wolf, Organometallics, 2018, 38, 1330–1343 CrossRef.
- J. Therrien, M. Wolf and B. Patrick, Dalton Trans., 2018, 47, 1827–1840 RSC.
- J. A. Therrien and M. O. Wolf, Inorg. Chem., 2017, 56, 1161–1172 CrossRef CAS PubMed.
- J. A. Therrien, M. O. Wolf and B. O. Patrick, Inorg. Chem., 2015, 54, 11721–11732 CrossRef CAS PubMed.
- J. Chang, J.-X. Mao, M. Ding, J. Zhang and X. Chen, Inorg. Chem., 2023, 62, 4971–4979 CrossRef CAS PubMed.
- P. D. Boyd, L. J. Wright and M. N. Zafar, Inorg. Chem., 2011, 50, 10522–10524 CrossRef CAS PubMed.
- M. N. Zafar, S. Masood, T. S. T. Muhammad, A. F. Dalebrook, M. F. Nazar, F. P. Malik, E. U. Mughal and L. J. Wright, Dalton Trans., 2019, 48, 15408–15418 RSC.
- M. Zafar, Development of New ligands for homogeneous Transition metal catalysts, PhD dissertation, University of Aukland, New Zealand, 2011 Search PubMed.
- A. Dorazco-González, H. Höpfl, F. Medrano and A. K. Yatsimirsky, J. Org. Chem., 2010, 75, 2259–2273 CrossRef PubMed.
- D. L. Pavia, G. M. Lampman, G. S. Kriz, J. Vyvyan and A. López Maestresalas, Introduction to Spectroscopy, 2008, vol. 153, p. 752 Search PubMed.
- I. Georgieva, N. Mintcheva, N. Trendafilova and M. Mitewa, Vib. Spectrosc., 2001, 27, 153–164 CrossRef CAS.
- P. Zanello, C. Nervi and F. F. De Biani, Inorganic Electrochemistry: Theory, Practice and Application, Royal Society of Chemistry, 2019 Search PubMed.
- J.-M. Savéant, Elements of Molecular and Biomolecular Electrochemistry: An Electrochemical Approach to Electron Transfer Chemistry, John Wiley & Sons, 2006 Search PubMed.
- R. S. Nicholson and I. Shain, Anal. Chem., 1964, 36, 706–723 CrossRef CAS.
- C. W. Machan, M. D. Sampson and C. P. Kubiak, J. Am. Chem. Soc., 2015, 137, 8564–8571 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Spectroscopic data of all compounds 1–11 (S1–S11), crystal data of 2, 4, 11 (S12 and S13) and cyclic voltammograms of 6–11 under different conditions (S13 and S14) are in ESI. CCDC 2281737–2281739. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra06477h |
| ‡ Authors contributed equally to this manuscript. |
| This journal is © The Royal Society of Chemistry 2023 |