Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A pillar[5]arene-based planar chiral charge-transfer dye with enhanced circularly polarized luminescence and multiple responsive chiroptical changes†
Jin-Fa
Chen
 *a,
Qing-Xiu
Gao
a,
Lijie
Liu
c,
Pangkuan
Chen
*a,
Qing-Xiu
Gao
a,
Lijie
Liu
c,
Pangkuan
Chen
 b and
Tai-Bao
Wei
b and
Tai-Bao
Wei
 a
a
aKey Laboratory of Eco-Environment-Related Polymer Materials, Ministry of Education of China, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu 730070, P. R. China. E-mail: chenjinfa@nwnu.edu.cn; Fax: +86 9317973191; Tel: +86 9317973191
bSchool of Chemistry and Chemical Engineering, Beijing Institute of Technology of China, Beijing 102488, P. R. China
cCollege of Science, Henan Agricultural University, Zhengzhou, Henan 450002, P. R. China
First published on 3rd January 2023
Abstract
The fabrication of circularly polarized luminescent (CPL) organic dyes based on macrocyclic architecture has become an importantly studied topic in recent years because it is of great importance to both chiral science and supramolecular chemistry, where pillar[n]arenes are emerging as a promising class of planar chiral macrocyclic hosts for CPL. We herein synthesized an unusual planar chiral charge-transfer dye (P5BB) by covalent coupling of triarylborane (Ar3B) as an electron acceptor to parent pillar[5]arene as an electron donor. The intramolecular charge transfer (ICT) nature of P5BB not only caused a thermally responsive emission but also boosted the luminescence dissymmetry factor (glum). Interestingly, the specific binding of fluoride ions changed the photophysical properties of P5BB, including absorption, fluorescence, circular dichroism (CD), and CPL, which could be exploited as an optical probe for multi-channel detection of fluoride ions. Furthermore, the chiroptical changes were observed upon addition of 1,4-dibromobutane as an achiral guest.
Introduction
Chirality, as one of the most significant phenomena, is ubiquitous in life and the environment, and determines the daily physiological activities and metabolism of life.1 Chiral science unarguably promotes the development of life, medicine and materials science.2 Chiroptical functional materials with circularly polarized luminescence (CPL) have drawn great attention in the last decade,3 not only for the understanding of the inherent principles of chirality but also owing to their wide potential applications in chiral sensing,4 photoelectric devices,5 3D displays,6 asymmetric catalysis7 and so forth. Generally, CPL is generated due to the molecules or supramolecular aggregates having both chiral features and luminescent properties.8 Therefore, it has become an effective strategy to achieve CPL activity by connecting luminophores with chiral fragments (e.g., binaphthyls, helicenes, and [2.2]paracyclophane).9 Recently, CPL-active systems based on planar chiral analogues have attracted increasing attention,10 because their inherent macrocyclic skeletons have more important potential in the field of chiral supramolecular chemistry (e.g., chiral network gelation and chiral host–guest recognition).Pillararenes,11 as an important type of macrocyclic arene, have attracted extensive studies and made significant contributions in host–guest recognition and self-assembly because of their unique structure and easy synthesis.12 Pillararenes possess planar chirality, which comes from the different orientations of 1,4-alkoxyphenyl units.13 Although the enantiomers (pS and pR) are easy to interconvert due to the dynamic rotations of phenyl units, stable chiral configurations can still be achieved by reasonable molecular functionalization of the parent pillararenes. For example, introducing 10 cyclohexylmethyl groups at both rims of pillar[5]arene could prevent the rotations of benzene rings.14 Stoddart et al. also developed an effective strategy to obtain separable enantiomers by introducing bulky π-conjugated units at the A1/A2 positions of pillar[5]arene.15 Recently, pillararenes have been used to prepare CPL-active molecules by integrating with appropriate fluorophores.16 For example, Chen et al. reported two π-conjugated CPL-active systems (P5NN and P5BN) through axial functionalization of pillar[5]arene with sterically bulky triarylamine (Ar3N) and triarylborane (Ar3B) (Scheme 1); however, the glum values were only a 10−4 order because the luminescence largely depended on axial π-conjugated fluorophores and hence limited the transfer of chirality.17 In 2022, Ogoshi et al. reported a series of rim-differentiated C5-symmetric pillar[5]arenes with improved glum values, but it was difficult to obtain proper fluorescence efficiency while improving the asymmetry factor.18 Therefore, the design and synthesis of CPL-active pillararenes at the molecular level with a good balance between glum factors and luminescence efficiency are highly anticipated.
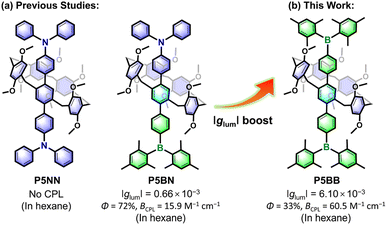 | ||
| Scheme 1 Research foundation and design strategy of pillararene-based planar chiral charge transfer dye with amplified glum values. | ||
Theoretically, the glum factor is simply approximated using 4|m|cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/|μ|, where m, μ and θ are the magnetic transition dipole moment, electric transition dipole moment and the angle between m and μ, respectively.19 If organic systems are appropriately designed to have weaker electric dipole transition and stronger magnetic dipole transition, it could lead to CPL-active materials with high glum. In fact, a large number of chiral organic molecules show relatively low glum values because of their electric dipole-allowed but magnetic dipole-forbidden transitions.20 In contrast, because charge transfer (CT) systems possess relatively small |μ| and large |m|, the larger glum values obtained from the CT state are hypothesized.21 As a typical electron acceptor, Ar3B has extensive application prospects in organic optoelectronic materials and stimulus-responsive materials.22 Notably, Ar3B are enabled to show distinctive CT emission once they are electronically coupled with electron donors.23 Pillararenes possess electron rich macrocyclic structures and can be used as a kind of electron donor. As one of our continuous pursuits of functionalized pillar[5]arenes,24 we herein propose a facile methodology to amplify glum values through functionalizing the pillar[5]arene parent to construct planar chiral CT dye (P5BB) with Ar3B. Sterically bulky Ar3B fluorophores not only allow the enantiomeric resolution but also promote intramolecular charge transfer (ICT) from pillar[5]arene to Ar3B. Based on the inherent host–guest nature of pillar[5]arene and the stimulus-responses of Ar3B, the chiroptical response behaviors of this chiral system were further studied. The details are presented herein.
θ/|μ|, where m, μ and θ are the magnetic transition dipole moment, electric transition dipole moment and the angle between m and μ, respectively.19 If organic systems are appropriately designed to have weaker electric dipole transition and stronger magnetic dipole transition, it could lead to CPL-active materials with high glum. In fact, a large number of chiral organic molecules show relatively low glum values because of their electric dipole-allowed but magnetic dipole-forbidden transitions.20 In contrast, because charge transfer (CT) systems possess relatively small |μ| and large |m|, the larger glum values obtained from the CT state are hypothesized.21 As a typical electron acceptor, Ar3B has extensive application prospects in organic optoelectronic materials and stimulus-responsive materials.22 Notably, Ar3B are enabled to show distinctive CT emission once they are electronically coupled with electron donors.23 Pillararenes possess electron rich macrocyclic structures and can be used as a kind of electron donor. As one of our continuous pursuits of functionalized pillar[5]arenes,24 we herein propose a facile methodology to amplify glum values through functionalizing the pillar[5]arene parent to construct planar chiral CT dye (P5BB) with Ar3B. Sterically bulky Ar3B fluorophores not only allow the enantiomeric resolution but also promote intramolecular charge transfer (ICT) from pillar[5]arene to Ar3B. Based on the inherent host–guest nature of pillar[5]arene and the stimulus-responses of Ar3B, the chiroptical response behaviors of this chiral system were further studied. The details are presented herein.
Results and discussion
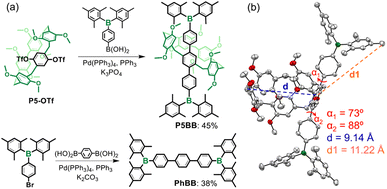
The key synthetic process of P5BB is shown in Scheme 2a and the ESI.† The core planar chiral block P5-OTf was directly obtained via the previous report,25 and then Pd-catalyzed Suzuki coupling by the reaction of P5-OTf with 2.0 equiv. Mes2B-containing phenylboronic acid led to the formation of P5BB in 45% yield. Similarly, PhBB was also obtained by standard Suzuki coupling of (4-bromophenyl)dimesitylborane with 1,4-phenylenediboronic acid in 38% yield. The chemical structures of P5BB and PhBB were fully characterized by 1H, 13C, and 11B NMR and high-resolution mass spectrometry (HRMS). A single crystal of rac-P5BB for X-ray diffraction analysis was collected by slowly evaporating the solution of acetone/MeOH (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In the crystalline form, a highly twisted π-conjugated skeleton was observed, as confirmed by dihedral angles (α1 and α2) that are measured to be 73° and 88°, respectively (Scheme 2b). Molecular size measurement revealed that the Ar3B-substituents (d1 = 11.22 Å) are greater than the cavity diameter of pillar[5]arene (d = 9.14 Å), so the racemization of enantiomers is sufficient to be inhibited through Ar3B-substituents. The equimolar enantiomers of pS-P5BB and pR-P5BB are packed in a unit cell via C–H⋯O, C–H⋯π and C–H⋯C interactions (Fig. S8†).
1). In the crystalline form, a highly twisted π-conjugated skeleton was observed, as confirmed by dihedral angles (α1 and α2) that are measured to be 73° and 88°, respectively (Scheme 2b). Molecular size measurement revealed that the Ar3B-substituents (d1 = 11.22 Å) are greater than the cavity diameter of pillar[5]arene (d = 9.14 Å), so the racemization of enantiomers is sufficient to be inhibited through Ar3B-substituents. The equimolar enantiomers of pS-P5BB and pR-P5BB are packed in a unit cell via C–H⋯O, C–H⋯π and C–H⋯C interactions (Fig. S8†).
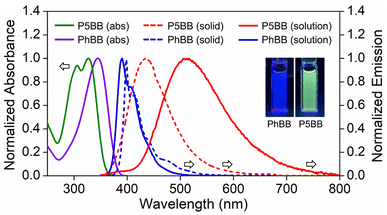
The photophysical properties of P5BB and PhBB were investigated in THF solution and the solid state (Fig. 1 and Table 1). PhBB shows a strong absorption band of π–π* transition at 345 nm. However, P5BB exhibits two absorption peaks at 306 nm and 328 nm, corresponding to the local π–π* transitions in the pillar[5]arene motif and in the axial conjugated skeleton, respectively (Fig. S9†).22i In comparison to PhBB, P5BB showed significantly red-shifted emission in both the solid state and solution, which was ascribed to the ICT between the pillar[5]arene donor and Ar3B acceptor. The ICT nature could be verified by a visible solvatochromic emission in various polar solvents (Fig. S11†). Owing to the temperature dependence of the equilibrium between the local excited (LE) state and the ICT excited state,26 we explored the thermally responsive emission of P5BB in 2-methyltetrahydrofuran as a low melting point solvent (Fig. S12†). At low temperature (150 K), P5BB showed a significantly dual emission band with a main ICT emission at 552 nm (τ = 33.9 ns) slightly overlapped with a minor LE emission at 405 nm (τ = 2.3 ns). With temperature increasing from 150 to 330 K, the main emission band of P5BB experienced an apparent hypsochromic shift, and the emission color change from yellow to blue was monitored using CIE coordinates. There is a good linear relationship of the maximum emission wavelength versus temperature with a correlation coefficient of 0.976. It is noteworthy that the above thermochromic response is completely reversible. The good accuracy and reversibility suggested that the system is an ideal candidate for high-performance fluorescent thermometers.
| λ abs (nm) | λ em (nm) | Φ L (%) | Φ S (%) | τ ave (ns) | E HOMO (eV) | E LUMO (eV) | E gap(DFT) (eV) | E ox (V) | E red (V) | |
|---|---|---|---|---|---|---|---|---|---|---|
| a Measured in THF (1.0 × 10−5 mol L−1) at room temperature. b Fluorescence quantum yield (ΦL) measured in THF. c Φ S measured in the solid state. d Average fluorescence lifetime in THF at room temperature. e HOMO and LUMO energy levels obtained using DFT calculations (B3LYP, 6-31G(d,p)). f E gap(DFT) = ELUMO − EHOMO (B3LYP, 6-31G(d,p)). g The first half-wave potentials of oxidation and reduction processes. h See ref. 17. | ||||||||||
| P5NN | 306 | 393 | 61 | 19 | 1.2 | −4.88 | −0.64 | 4.24 | +0.52 | — |
| P5BN | 303, 330 | 493 | 99 | 57 | 6.4 | −4.89 | −1.67 | 3.22 | +0.59 | −2.49 |
| P5BB | 306, 328 | 510 | 12 | 16 | 20.5 | −4.91 | −1.77 | 3.14 | +0.61 | −2.46 |
| PhBB | 345 | 390 | 99 | 70 | 1.8 | −5.83 | −1.94 | 3.89 | — | — |
In order to further understand the correlation between the molecular structures and the photophysical properties of P5BB and PhBB, the electronic structure calculations were performed using DFT (B3LYP, 6-31G(d,p)) and TD-DFT (B3LYP, 6-31G(d)). TD-DFT computations revealed that the absorption of PhBB is mainly attributed to the π–π* transition to the S1 state (HOMO → LUMO, f = 1.2994) (Fig. S14 and Table S5†). In P5BB, the HOMO is fully located on the electron-donor pillar[5]arene backbone; however, the LUMO is delocalized over the B-conjugated π-extension (Fig. S15†). By means of TD-DFT calculations, it was found that the first three CT transitions are the results of vertical excitations from the pillar[5]arene-localized HOMO, HOMO−1 and HOMO−2, to the LUMO (Fig. S16†). The higher excited states (S4 and S5) are excitations to the LUMO+1 level from the HOMO and HOMO−1. Cyclic voltammetry (CV) of P5BB showed a reversible reduction potential at −2.46 V (vs. Fc+/Fc, in THF), representing the reduction of the electron-deficient Ar3B segments (Fig. S17†). Three reversible oxidation curves with the first oxidation potentials at +0.61 V for P5BB (vs. Fc+/Fc, in CH2Cl2) were identified, corresponding to the oxidation of the pillar[5]arene skeleton. The electrochemical gap (3.07 eV) is almost consistent with the HOMO–LUMO gap (3.14 eV) via the DFT calculations. As expected, the first oxidation potential of P5BB is slightly higher than that of compounds with the N donor (P5NN, +0.52 V; P5BN, +0.59 V), which is completely consistent with the slightly lower HOMO energy level (P5BB, −4.91 eV; P5NN, −4.88 eV; P5BN, −4.89 V).
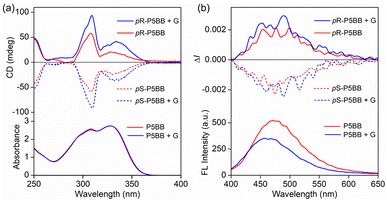
The definite enantiomeric configuration inspired us to prepare their optically pure forms for studying the chiroptical properties. Two separated peaks with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 area were observed by the initial injection of rac-P5BB into a chiral HPLC with a Daicel Chiralpak IB N-5 column (hexane/2-propanol = 96/4, v/v). After each fraction was well isolated with an enantiomeric excess (>99% ee, Fig. S18†), the CD spectra of the enantiomers exhibited mirror-image relationships with strong CD absorption peaks in various organic solvents (Fig. 2a). The CD signals belonging to pillar[5]arene cores are clearly observed at 310 nm,27 and the absorption dissymmetry factor |gabs| was calculated to be 1.53 × 10−3 in hexane. On the basis of a comparison of the experimental CD absorption signals with the results of previous reports,27 the pS configurations correspond to the first peak and the second peak correspond to pR configurations in HPLC traces. In the CPL spectra, the enantiomers exhibited almost mirror-imaged signals in various solvents (Fig. 2b). The |glum| reached 10−3 in solution as well as in the solid state (Table 2), which is significantly higher than that of P5NN and P5BN. The CPL spectra of P5BB gradually redshifted with the increase in solvent polarity, and the positions of CPL signals were almost consistent with that of fluorescence. In fact, the enantiomers of P5NN and P5BN did not show apparent CPL signals in various solvents (except for hexane), suggesting that the chiral transfer did not occur effectively in this case. These phenomena indicated that the ICT character of P5BB not only amplifies the glum factors but also adjusts the color of CPL via the selection of different solvents. With all the necessary photophysical and chiral optical data in hand, the CPL brightness (BCPL: defined as BCPL = ε × Φ × |glum|/2) in solutions was further calculated to evaluate the overall performance of the CPL dyes. The BCPL of pS-P5BB was calculated to be 60.5 M−1 cm−1 in hexane, which is significantly higher than that of pS-P5BN (15.9 M−1 cm−1), indicating that P5BB possesses excellent chiroptical properties for future CPL applications.
1 area were observed by the initial injection of rac-P5BB into a chiral HPLC with a Daicel Chiralpak IB N-5 column (hexane/2-propanol = 96/4, v/v). After each fraction was well isolated with an enantiomeric excess (>99% ee, Fig. S18†), the CD spectra of the enantiomers exhibited mirror-image relationships with strong CD absorption peaks in various organic solvents (Fig. 2a). The CD signals belonging to pillar[5]arene cores are clearly observed at 310 nm,27 and the absorption dissymmetry factor |gabs| was calculated to be 1.53 × 10−3 in hexane. On the basis of a comparison of the experimental CD absorption signals with the results of previous reports,27 the pS configurations correspond to the first peak and the second peak correspond to pR configurations in HPLC traces. In the CPL spectra, the enantiomers exhibited almost mirror-imaged signals in various solvents (Fig. 2b). The |glum| reached 10−3 in solution as well as in the solid state (Table 2), which is significantly higher than that of P5NN and P5BN. The CPL spectra of P5BB gradually redshifted with the increase in solvent polarity, and the positions of CPL signals were almost consistent with that of fluorescence. In fact, the enantiomers of P5NN and P5BN did not show apparent CPL signals in various solvents (except for hexane), suggesting that the chiral transfer did not occur effectively in this case. These phenomena indicated that the ICT character of P5BB not only amplifies the glum factors but also adjusts the color of CPL via the selection of different solvents. With all the necessary photophysical and chiral optical data in hand, the CPL brightness (BCPL: defined as BCPL = ε × Φ × |glum|/2) in solutions was further calculated to evaluate the overall performance of the CPL dyes. The BCPL of pS-P5BB was calculated to be 60.5 M−1 cm−1 in hexane, which is significantly higher than that of pS-P5BN (15.9 M−1 cm−1), indicating that P5BB possesses excellent chiroptical properties for future CPL applications.
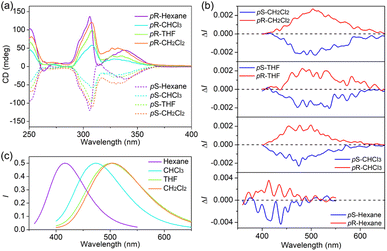 | ||
| Fig. 2 (a) CD, (b) CPL and (c) fluorescence spectra of enantiomers of P5BB in different solvents (c = 5.0 × 10−5 mol L−1, λex = 306 nm). | ||
| λ abs (nm) | ε (M−1 cm−1) | λ em (nm) | Φ (%) | g lum (10−3) | B CPL (M−1 cm−1) | ||
|---|---|---|---|---|---|---|---|
| a Molar absorption coefficient (ε) at a given wavelength. b Fluorescence quantum yield (Φ). c Luminescence dissymmetry factor (glum). d The CPL brightness (BCPL). e See ref. 17. | |||||||
| pS-P5NNe | Hexane | 306 | 7.31 × 104 (306) | 391 | 52 | — | — |
| Solid | — | — | 398 | 19 | +0.22 | — | |
| pS-P5BNe | Hexane | 303, 330 | 6.71 × 104 (303) | 403 | 72 | +0.66 | 15.9 |
| Solid | — | — | 455 | 57 | +0.88 | — | |
| pS-P5BB | Hexane | 305, 329 | 6.01 × 104 (305) | 422 | 33 | −6.10 | 60.5 |
| CHCl3 | 308, 328 | 5.98 × 104 (308) | 473 | 23 | −3.64 | 25.1 | |
| THF | 306, 328 | 6.08 × 104 (306) | 510 | 12 | −3.34 | 12.2 | |
| CH2Cl2 | 306, 327 | 6.41 × 104 (306) | 512 | 12 | −4.62 | 17.8 | |
| Solid | — | — | 435 | 16 | −5.13 | — | |
| pS-P5BB + G | CHCl3 | 308, 328 | 6.00 × 104 (308) | 460 | 18 | −4.16 | 22.4 |
| pS-P5BB + F− | THF | 295 | 3.35 × 104 (295) | 365 | 56 | −5.08 | 47.6 |
In order to further evaluate the chiroptical changes induced through host–guest chemistry, we selected the neutral small molecule 1,4-dibromobutane (G) as the representative guest to bind P5BB by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation.28 As shown in Fig. S19,† upon the addition of excess 1,4-dibromobutane to P5BB in CDCl3 solution, the proton signals of H1–3 and H5–11 on P5BB were shifted downfield, and meanwhile, all signal peaks on the guest were found to shift upfield in 1H NMR spectra. Additionally, the proton peaks of the guest were substantially reduced after complexation due to inclusion-induced shielding effects. The association constant (Ka) was determined to be 187.8 M−1 using the nonlinear data fitting of 1H NMR titrations (Fig. S20 and S21†). This implies that host–guest recognition between P5BB and 1,4-dibromobutane has occurred. Notably, the addition of a guest results in dramatic enhancement of CD signals at 310 nm and 328 nm in CHCl3, while the absorption does not change significantly, implying that the host–guest complexation decreases the configuration rotation of the pillar[5]arene skeleton of P5BB (Fig. 3). The addition of excessive 1,4-dibromobutane led to a decrease in fluorescence intensity, and meanwhile, the CPL signals showed a mild enhancement, indicating the host–guest recognition further affected the excited state chiral conformation of P5BB.
1 complexation.28 As shown in Fig. S19,† upon the addition of excess 1,4-dibromobutane to P5BB in CDCl3 solution, the proton signals of H1–3 and H5–11 on P5BB were shifted downfield, and meanwhile, all signal peaks on the guest were found to shift upfield in 1H NMR spectra. Additionally, the proton peaks of the guest were substantially reduced after complexation due to inclusion-induced shielding effects. The association constant (Ka) was determined to be 187.8 M−1 using the nonlinear data fitting of 1H NMR titrations (Fig. S20 and S21†). This implies that host–guest recognition between P5BB and 1,4-dibromobutane has occurred. Notably, the addition of a guest results in dramatic enhancement of CD signals at 310 nm and 328 nm in CHCl3, while the absorption does not change significantly, implying that the host–guest complexation decreases the configuration rotation of the pillar[5]arene skeleton of P5BB (Fig. 3). The addition of excessive 1,4-dibromobutane led to a decrease in fluorescence intensity, and meanwhile, the CPL signals showed a mild enhancement, indicating the host–guest recognition further affected the excited state chiral conformation of P5BB.
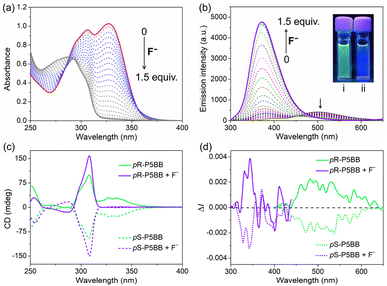
Considering the Lewis acidity of Ar3B, another interesting thing is the response behavior of P5BB toward small Lewis bases.29 Herein, the optical responses of four common halogen anions (including F−, Cl−, Br− and I−) as Lewis bases to P5BB were primarily investigated in THF solution. In the UV-vis absorption spectra (Fig. 4a), the absorption bands at around 306 nm and 328 nm of P5BB gradually decrease with the addition of F− anions, which is due to the fact that the conjugation of the Ar3B moiety was broken by the formation of tetra-coordinated boron complexing with F− anions (Scheme S1 and Fig. S24†). The limit of detection (LOD) was further calculated to be 43.3 nM based on the 3σ/S values (Fig. S22†). Remarkably, other halogen ions (Cl−, Br− and I−) could not cause any significant changes in absorption spectra, which was likely ascribed to the smaller steric size of the F− anion. Furthermore, the strong affinity between fluoride and boron also played a crucial role. As displayed in Fig. 4b, as the concentration of F− increased, the emission peak at 510 nm of P5BB slowly declined, while the emission peak at 365 nm rapidly enhanced. Quite evidently, the emission spectrum exhibited a blue shift (∼145 nm) with the emission color changing from green to purple, and the fluorescence intensity increased dramatically with Φ up to 56%. These phenomena indicated that the binding of F− anions with the Ar3B unit prevented the ICT process in the excited state, leading to the enhancement of LE-state emission.29 Moreover, in the presence of an excess amount of F− anions, the CD signals of the enantiomers at 328 nm disappeared, while the signals at 310 nm were effectively enhanced. Even after complexation with F− anions, significant CPL signals with a blue-shift were also detected. The |glum| values were calculated to reach 5.08 × 10−3 with a BCPL of 47.6 in this case and were much higher than those of chiral pillararene derivatives reported in ref. 16b (Scheme S2†). These investigations confirmed that the P5BB system could realize UV-vis absorption/fluorescence/CD/CPL quadruple-mode sensing of F− anions.
Conclusions
In summary, we have developed an effective strategy to design and synthesize a π-conjugated planar chiral CT dye P5BB through integration of pillar[5]arene with organoborane. The ICT character in P5BB not only amplified the glum values but also could tune the CPL color by the selection of various solvents with different polarities. Approximately 10-fold enhancements in glum were observed from P5BB to P5BN in hexane, which was ascribed to the fact that the photo-responsive unit itself contains planar chiral pillar[5]arene in P5BB. Inclusion of the achiral guest 1,4-dibromobutane directly enhanced chiral optical signals, including CD and CPL. Furthermore, the coordination of F− anions with boron leads to remarkable changes in absorption, fluorescence, CD and CPL signals of the P5BB system. Consequently, the P5BB platform could specifically detect F− anions with high sensitivity and favorable selectivity. The current design strategy to fabricate a CPL-active ICT dye with the amplification of the glum factor is expected to promote the development of future planar chiral pillararene materials. We envision that this work will catalyze the future application of pillararene-based CPL-active systems in chiroptical sensing, chiral supramolecular chemistry and CPL-based photoelectric devices.Data availability
All the data supporting this article have been included in the main text and the ESI.†Author contributions
J.-F. C. initiated and coordinated the study. J.-F. C., Q.-X. G. and T.-B W. designed all experiments, analyzed the data, and wrote the manuscript. L. L. carried out the computational work. P. C. and T.-B. W. supervised and administrated the project. All authors approved the final version.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (No. 21772012; 22001214; 22165027; and 22061039). We are greatly thankful to Dr Xu-Sheng Du at the Chinese Academy of Sciences for help in CPL measurements. We thank the Analysis & Testing Centre at the Beijing Institute of Technology for advanced facilities.Notes and references
- (a) G. Gonzalez-Rubio and L. Liz-Marzan, Nature, 2018, 556, 313 CrossRef CAS PubMed; (b) U. S. Goksen, S. Sarigul, P. Bultinck, W. Herrebout, I. Dogan, K. Yelekci, G. Ucar and N. G. Kelekci, Chirality, 2019, 31, 21 CrossRef CAS PubMed.
- (a) A. Accetta, Top. Curr. Chem., 2010, 300, 175 CrossRef PubMed; (b) W. Xiao, K. H. Ernst, K. Palotas, Y. Zhang, E. Bruyer, L. Peng, T. Greber, W. A. Hofer, L. T. Scott and R. Fasel, Nat. Chem., 2016, 8, 326 CrossRef CAS PubMed; (c) Q. Gan, X. Wang, B. Kauffmann, F. Rosu, Y. Ferrand and I. Huc, Nat. Nanotechnol., 2017, 12, 447 CrossRef CAS PubMed.
- (a) T. Mori, Chem. Rev., 2021, 121, 2373 CrossRef CAS PubMed; (b) T. Zhao, J. Han, P. Duan and M. Liu, Acc. Chem. Res., 2020, 53, 1279 CrossRef CAS PubMed; (c) K. Takaishi, K. Iwachido and T. Ema, J. Am. Chem. Soc., 2020, 142, 1774 CrossRef CAS PubMed; (d) Z.-L. Gong, X. Zhu, Z. Zhou, S.-W. Zhang, D. Yang, B. Zhao, Y.-P. Zhang, J. Deng, Y. Cheng, Y.-X. Zheng, S.-Q. Zang, H. Kuang, P. Duan, M. Yuan, C.-F. Chen, Y. S. Zhao, Y.-W. Zhong, B. Z. Tang and M. Liu, Sci. China: Chem., 2021, 64, 2060 CrossRef CAS; (e) Z.-P. Yan, L. Yuan, Y. Zhang, M.-X. Mao, X.-J. Liao, H.-X. Ni, Z.-H. Wang, Z. An, Y.-X. Zheng and J.-L. Zuo, Adv. Mater., 2022, 34, 2204253 CrossRef CAS PubMed; (f) L. Arrico, L. D. Bari and F. Zinna, Chem.–Eur. J., 2021, 27, 2920 CrossRef CAS PubMed.
- (a) K. Takaishi, M. Yasui and T. Ema, J. Am. Chem. Soc., 2018, 140, 5334 CrossRef CAS PubMed; (b) H. Maeda, Y. Bando, K. Shimomura, I. Yamada, M. Naito, K. Nobusawa, H. Tsumatori and T. Kawai, J. Am. Chem. Soc., 2011, 133, 9266 CrossRef CAS PubMed; (c) J.-L. Ma, Q. Peng and C.-H. Zhao, Chem.–Eur. J., 2019, 25, 15441 CrossRef CAS PubMed.
- (a) M. Li, S.-H. Li, D. Zhang, M. Cai, L. Duan, M.-K. Fung and C.-F. Chen, Angew. Chem., Int. Ed., 2018, 57, 2889 CrossRef CAS PubMed; (b) F. Zinna, S. Voci, L. Arrico, E. Brun, A. Homberg, L. Bouffier, T. Funaioli, J. Lacour, N. Sojic and L. Di Bari, Angew. Chem., Int. Ed., 2019, 58, 6952 CrossRef CAS PubMed; (c) S.-Y. Yang, Y.-K. Wang, C.-C. Peng, Z.-G. Wu, S. Yuan, Y.-J. Yu, H. Li, T.-T. Wang, H.-C. Li, Y.-X. Zheng, Z.-Q. Jiang and L.-S. Liao, J. Am. Chem. Soc., 2020, 142, 17756 CrossRef CAS PubMed; (d) Y.-P. Zhang, X. Liang, X.-F. Luo, S.-Q. Song, S. Li, Y. Wang, Z.-P. Mao, W.-Y. Xu, Y.-X. Zheng, J.-L. Zuo and Y. Pan, Angew. Chem., Int. Ed., 2021, 60, 8435 CrossRef CAS PubMed.
- (a) F. Zinna, U. Giovanella and L. D. Bari, Adv. Mater., 2015, 27, 1791 CrossRef CAS PubMed; (b) J. R. Brandt, F. Salerno and M. J. Fuchter, Nat. Rev. Chem., 2017, 1, 0045 CrossRef CAS.
- (a) Y. Tang and A. E. Cohen, Science, 2011, 332, 333 CrossRef CAS PubMed; (b) C. He, G. Yang, Y. Kuai, S. Shan, L. Yang, J. Hu, D. Zhang, Q. Zhang and G. Zou, Nat. Commun., 2018, 9, 5117 CrossRef PubMed.
- (a) K. Takaishi, S. Hinoide, T. Matsumoto and T. Ema, J. Am. Chem. Soc., 2019, 141, 11852 CrossRef CAS PubMed; (b) C. Zhang, Z.-P. Yan, X.-Y. Dong, Z. Han, S. Li, T. Fu, Y.-Y. Zhu, Y.-X. Zheng, Y.-Y. Niu and S.-Q. Zang, Adv. Mater., 2020, 32, 2002914 CrossRef CAS PubMed; (c) W.-L. Zhao, M. Li, H.-Y. Lu and C.-F. Chen, Chem. Commun., 2019, 55, 13793 RSC.
- (a) X. Liang, T.-T. Liu, Z.-P. Yan, Y. Zhou, J. Su, X.-F. Luo, Z.-G. Wu, Y. Wang, Y.-X. Zheng and J.-L. Zuo, Angew. Chem., Int. Ed., 2019, 58, 17220 CrossRef CAS PubMed; (b) Z.-B. Sun, J.-K. Liu, D.-F. Yuan, Z.-H. Zhao, X.-Z. Zhu, D.-H. Liu, Q. Peng and C.-H. Zhao, Angew. Chem., Int. Ed., 2019, 58, 4840 CrossRef CAS PubMed; (c) T. Katayama, S. Nakatsuka, H. Hirai, N. Yasuda, J. Kumar, T. Kawai and T. Hatakeyama, J. Am. Chem. Soc., 2016, 138, 5210 CrossRef CAS PubMed; (d) H. Shang, Z. Ding, Y. Shen, B. Yang, M. Liu and S. Jiang, Chem. Sci., 2020, 11, 2169 RSC.
- (a) Y. Morisaki, M. Gon, T. Sasamori, N. Tokitoh and Y. Chujo, J. Am. Chem. Soc., 2014, 136, 3350 CrossRef CAS PubMed; (b) J. Nogami, Y. Tanaka, H. Sugiyama, H. Uekusa, A. Muranaka, M. Uchiyama and K. Tanaka, J. Am. Chem. Soc., 2020, 142, 9834 CAS; (c) X.-N. Han, Y. Han and C.-F. Chen, J. Am. Chem. Soc., 2020, 142, 8262 CrossRef CAS PubMed; (d) P. D. Sala, R. D. Regno, C. Talotta, A. Capobianco, N. Hickey, S. Geremia, M. D. Rosa, A. Spinella, A. Soriente, P. Neri and C. Gaeta, J. Am. Chem. Soc., 2020, 142, 1752 CrossRef PubMed.
- (a) T. Ogoshi, T. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937 CrossRef CAS PubMed; (b) T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022 CrossRef CAS PubMed; (c) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294 CrossRef CAS PubMed; (d) Z.-Y. Li, Y. Zhang, C.-W. Zhang, L.-J. Chen, C. Wang, H. Tan, Y. Yu, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2014, 136, 8577 CrossRef CAS PubMed; (e) X.-Y. Lou and Y.-W. Yang, Adv. Mater., 2020, 32, 2003263 CrossRef CAS PubMed.
- (a) R. Wang, Y. Sun, F. Zhang, M. Song, D. Tian and H. Li, Angew. Chem., Int. Ed., 2017, 56, 5294 CrossRef CAS PubMed; (b) Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542 CrossRef CAS PubMed; (c) S. Fa, K. Egami, K. Adachi, K. Kato and T. Ogoshi, Angew. Chem., Int. Ed., 2020, 59, 20353 CrossRef CAS PubMed; (d) Y. Wu, J. Zhou, E. Li, M. Wang, K. Jie, H. Zhu and F. Huang, J. Am. Chem. Soc., 2020, 142, 19722 CrossRef CAS PubMed; (e) L.-L. Tan, H. Li, Y. Tao, S. X.-A. Zhang, B. Wang and Y.-W. Yang, Adv. Mater., 2014, 26, 7027 CrossRef CAS PubMed; (f) D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721 CrossRef CAS PubMed; (g) B. Li, Z. Meng, Q. Li, X. Huang, Z. Kang, H. Dong, J. Chen, J. Sun, Y. Dong, J. Li, X. Jia, J. L. Sessler, Q. Meng and C. Li, Chem. Sci., 2017, 8, 4458 RSC.
- J.-F. Chen, J.-D. Ding and T.-B. Wei, Chem. Commun., 2021, 57, 9029 RSC.
- T. Ogoshi, K. Masaki, R. Shiga, K. Kitajima and T. Yamagishi, Org. Lett., 2011, 13, 1264 CrossRef CAS PubMed.
- N. L. Strutt, D. Fairen-Jimenez, J. Iehl, M. B. Lalonde, R. Q. Snurr, O. K. Farha, J. T. Hupp and J. F. Stoddart, J. Am. Chem. Soc., 2012, 134, 17436 CrossRef CAS PubMed.
- (a) J.-F. Chen, G. Tian, K. Liu, N. Zhang, N. Wang, X. Yin and P. Chen, Org. Lett., 2022, 24, 1935 CrossRef CAS PubMed; (b) J.-F. Chen, X. Yin, K. Zhang, Z. Zhao, S. Zhang, N. Zhang, N. Wang and P. Chen, J. Org. Chem., 2021, 86, 12654 CrossRef CAS PubMed; (c) T. Ogoshi, D. Yamafuji, T. Akutsu, M. Naito and T. Yamagishi, Chem. Commun., 2013, 49, 8782 RSC; (d) S. Fa, T. Tomita, K. Wada, K. Yasuhara, S. Ohtani, K. Kato, M. Gon, K. Tanaka, T. Kakuta, T. Yamagishi and T. Ogoshi, Chem. Sci., 2022, 13, 5846 RSC; (e) W.-J. Li, Q. Gu, X.-Q. Wang, D.-Y. Zhang, Y.-T. Wang, X. He, W. Wang and H.-B. Yang, Angew. Chem., Int. Ed., 2021, 60, 9507 CrossRef CAS PubMed; (f) K. Kato, S. Ohtani, M. Gon, K. Tanaka and T. Ogoshi, Chem. Sci., 2022, 13, 13147 RSC.
- J.-F. Chen, X. Yin, B. Wang, K. Zhang, G. Meng, S. Zhang, Y. Shi, N. Wang, S. Wang and P. Chen, Angew. Chem., Int. Ed., 2020, 59, 11267 CrossRef CAS PubMed.
- K. Kato, Y. Kurakake, S. Ohtani, S. Fa, M. Gon, K. Tanaka and T. Ogoshi, Angew. Chem., Int. Ed., 2022, 61, e202209222 CAS.
- (a) F. S. Richardson and J. P. Riehl, Chem. Rev., 1977, 77, 773 CrossRef CAS; (b) J. P. Riehl and F. S. Richardson, Chem. Rev., 1986, 86, 1 CrossRef CAS.
- E. M. Sanchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. de la Moya, Chem.–Eur. J., 2015, 21, 13488 CrossRef CAS PubMed.
- (a) J. Han, D. Yang, X. Jin, Y. Jiang, M. Liu and P. Duan, Angew. Chem., Int. Ed., 2019, 58, 7013 CrossRef CAS PubMed; (b) J. Jimenez, F. Moreno, B. L. Maroto, T. A. Cabreros, A. S. Huy, G. Muller, J. Banuelos and S. de la Moya, Chem. Commun., 2019, 55, 1631 RSC; (c) Z. Dominguez, R. Lopez-Rodriguez, E. Alvarez, S. Abbate, G. Longhi, U. Pischel and A. Ros, Chem.–Eur. J., 2018, 24, 12660 CrossRef CAS PubMed.
- (a) L. Ji, S. Griesbeck and T. B. Marder, Chem. Sci., 2017, 8, 846 RSC; (b) C. R. Wade, A. E. J. Broomsgrove, S. Aldridge and F. P. Gabbaï, Chem. Rev., 2010, 110, 3958 CrossRef CAS PubMed; (c) A. Iida, S. Saito, T. Sasamori and S. Yamaguchi, Angew. Chem., Int. Ed., 2013, 52, 3760 CrossRef CAS PubMed; (d) S. K. Mellerup and S. Wang, Chem. Soc. Rev., 2019, 48, 3537 RSC; (e) X.-Y. Wang, F.-D. Zhuang, R.-B. Wang, X.-C. Wang, X.-Y. Cao, J.-Y. Wang and J. Pei, J. Am. Chem. Soc., 2014, 136, 3764 CrossRef CAS PubMed; (f) B. Meng, Y. Ren, J. Liu, F. Jäkle and L. X. Wang, Angew. Chem., Int. Ed., 2018, 57, 2183 CrossRef CAS PubMed; (g) W. Zhang, G. Li, L. Xu, Y. Zhuo, W. Wan, N. Yan and G. He, Chem. Sci., 2018, 9, 4444 RSC; (h) P. Chen, X. Yin, N. Baser-Kirazli and F. Jäkle, Angew. Chem., Int. Ed., 2015, 54, 10768 CrossRef CAS; (i) Z. Wu, J. Nitsch, J. Schuster, A. Friedrich, K. Edkins, M. Loebnitz, F. Dinkelbach, V. Stepanenko, F. Würthner, C. M. Marian, L. Ji and T. B. Marder, Angew. Chem., Int. Ed., 2020, 59, 17137 CrossRef CAS PubMed.
- (a) G. Zhou, M. Baumgarten and K. Müllen, J. Am. Chem. Soc., 2008, 130, 12477 CrossRef CAS PubMed; (b) A. G. Bonn and O. S. Wenger, J. Org. Chem., 2015, 80, 4097 CrossRef CAS; (c) M. Numata, T. Yasuda and C. Adachi, Chem. Commun., 2015, 51, 9443 RSC; (d) K. Suzuki, S. Kubo, K. Shizu, T. Fukushima, A. Wakamiya, Y. Murata, C. Adachi and H. Kaji, Angew. Chem., Int. Ed., 2015, 54, 15231 CrossRef CAS PubMed; (e) Z.-H. Zhao, X. Liang, M.-X. He, M.-Y. Zhang and C.-H. Zhao, Org. Lett., 2019, 21, 9569 CrossRef CAS; (f) G. Meng, L. Liu, Z. He, D. Hall, X. Wang, T. Peng, X. Yin, P. Chen, D. Beljonne, Y. Olivier, E. Zysman-Colman, N. Wang and S. Wang, Chem. Sci., 2022, 13, 1665 RSC.
- (a) J.-F. Chen, Q. Lin, Y.-M. Zhang, H. Yao and T.-B. Wei, Chem. Commun., 2017, 53, 13296 RSC; (b) Y.-Y. Chen, X.-M. Jiang, G.-F. Gong, H. Yao, Y.-M. Zhang, T.-B. Wei and Q. Lin, Chem. Commun., 2021, 57, 284 RSC; (c) J.-F. Chen, G. Meng, Q. Zhu, S. Zhang and P. Chen, J. Mater. Chem. C, 2019, 7, 11747 RSC; (d) J.-F. Chen, Q. Lin, H. Yao, Y.-M. Zhang and T.-B. Wei, Mater. Chem. Front., 2018, 2, 999 RSC; (e) Q. Lin, Y.-Q. Fan, G.-F. Gong, P.-P. Mao, J. Wang, X.-W. Guan, J. Liu, Y.-M. Zhang, H. Yao and T.-B. Wei, ACS Sustainable Chem. Eng., 2018, 6, 8775 CrossRef CAS.
- X. Li, Z. Li and Y.-W. Yang, Adv. Mater., 2018, 30, 1800177 CrossRef PubMed.
- (a) J. Feng, K. Tian, D. Hu, S. Wang, S. Li, Y. Zeng, Y. Li and G. Yang, Angew. Chem., Int. Ed., 2011, 50, 8072 CrossRef CAS PubMed; (b) N. A. Sayresmith, A. Saminathan, J. K. Sailer, S. M. Patberg, K. Sandor, Y. Krishnan and M. G. Walter, J. Am. Chem. Soc., 2019, 141, 18780 CrossRef CAS PubMed; (c) H. Naito, K. Nishino, Y. Morisaki, K. Tanaka and Y. Chujo, Angew. Chem., Int. Ed., 2017, 56, 254 CrossRef CAS PubMed.
- (a) E. Lee, H. Ju, I. H. Park, J. H. Jung, M. Ikeda, S. Kuwahara, Y. Habata and S. S. Lee, J. Am. Chem. Soc., 2018, 140, 9669 CrossRef CAS PubMed; (b) C. Xiao, W. Wu, W. Liang, D. Zhou, K. Kanagaraj, G. Cheng, D. Su, Z. Zhong, J. J. Chruma and C. Yang, Angew. Chem., Int. Ed., 2020, 59, 8094 CrossRef CAS PubMed; (c) H. Zhu, Q. Li, B. Shi, H. Xing, Y. Sun, S. Lu, L. Shangguan, X. Li, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2020, 142, 17340 CrossRef CAS PubMed; (d) J. Yao, W. Wu, W. Liang, Y. Feng, D. Zhou, J. J. Chruma, G. Fukuhara, T. Mori, Y. Inoue and C. Yang, Angew. Chem., Int. Ed., 2017, 56, 6869 CrossRef CAS PubMed.
- X. Shu, J. Fan, J. Li, X. Wang, W. Chen, X. Jia and C. Li, Org. Biomol. Chem., 2012, 10, 3393 RSC.
- (a) Z. M. Hudson and S. Wang, Acc. Chem. Res., 2009, 42, 1584 CrossRef CAS PubMed; (b) C.-J. Sun, G. Meng, Y. Li, N. Wang, P. Chen, S. Wang and X. Yin, Inorg. Chem., 2021, 60, 1099 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2215838. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc06000k |
| This journal is © The Royal Society of Chemistry 2023 |