Open Access Article
Open Access ArticleBent-to-planar Si-rhodamines: a distinct rehybridization lights up NIR-II fluorescence for tracking nitric oxide in the Alzheimer's disease brain†
Qingshuang
Xu‡
a,
Yutao
Zhang‡
a,
Mingming
Zhu
b,
Chenxu
Yan
 a,
Wenle
Mao
a,
Wei-Hong
Zhu
a,
Wenle
Mao
a,
Wei-Hong
Zhu
 a and
Zhiqian
Guo
a and
Zhiqian
Guo
 *a
*a
aKey Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Shanghai Key Laboratory of Functional Materials Chemistry, Shanghai Frontier Science Research Base of Optogenetic Techniques for Cell Metabolism, Frontiers Science Center for Materiobiology and Dynamic Chemistry, Institute of Fine Chemicals, School of Chemistry and Molecular Engineering, East China University of Science & Technology, Shanghai 200237, China. E-mail: guozq@ecust.edu.cn
bDivision of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology and Hepatology, Ministry of Health, Inflammatory Bowel Disease Research Center, Renji Hospital, School of Medicine, Shanghai Institute of Digestive Disease, Shanghai Jiao Tong University, Shanghai, China
First published on 16th March 2023
Abstract
An ongoing revolution in fluorescence-based technologies has transformed the way we visualize and manipulate biological events. An enduring goal in this field is to explore high-performance fluorogenic scaffolds that show tunability and capability for in vivo analysis, especially for small-molecular near-infrared (NIR) fluorophores. We present a unique bent-to-planar rehybridization design strategy for NIR fluorogenic scaffolds, thus yielding a palette of switchable bent/planar Si-rhodamines that span from visible to NIR-II wavelengths. We demonstrate that the rehybridization of meso-nitrogen in this innovative NIR scaffold Cl-SiRhd results in flipping between the disruption and recovery of the polymethine π-electron system, thereby significantly altering the spectral wavelength with crosstalk-free responses. Using elaborately lighting-up NIR-II probes with ultra-large Stokes shifts (ca. 250 nm), we successfully achieve real-time in situ monitoring of biological events in live cells, zebrafish, and mice. Notably, for the first time, the light-up NIR-II probe makes a breakthrough in directly in situ tracking nitric oxide (NO) fluctuations in the brains of mice with Alzheimer's disease. This de novo bent-to-planar rehybridization strategy of NIR-II probes opens up exciting opportunities for expanding the in vivo imaging toolbox in both life science research and clinical applications.
Introduction
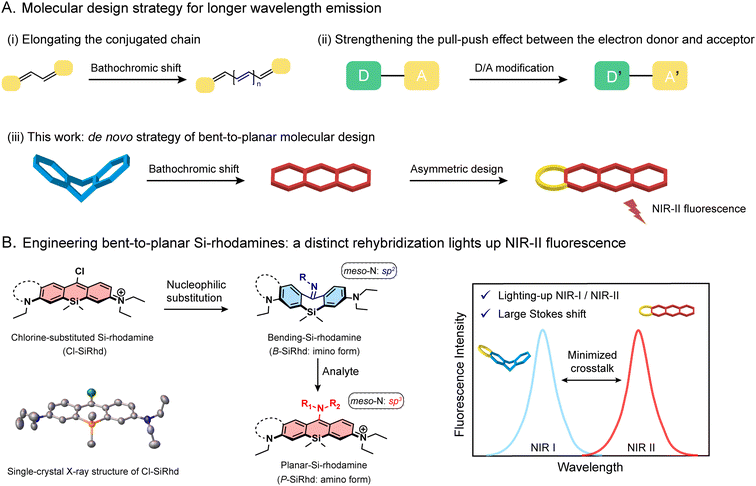
Fluorescence-based technologies have changed the way we study life science and conduct diagnostics.1–5 Most prominent amongst these enabling technologies is the utility of fluorescent probes for in vivo tracking of biological events with high resolution.6–9 Probes with near-infrared (NIR) emission are extremely important, but the primary hurdle is the lack of novel NIR-II fluorogenic scaffolds, showing powerful tunability and capability for in vivo analysis. Most efforts rely on conjugated π-expansion and donor–acceptor (D–A) adjustment (Fig. 1A).10–12 However, most of the current NIR-II fluorophores usually require difficult and laborious synthesis and inflict self-instability along with undesirable quenching.12–16 Nowadays, it is urgent to develop an innovative NIR-II fluorogenic framework for highly accurate in vivo sensing and clinical translation.Various fluorophores, including Si-rhodamines, have been widely used across a variety of fields including chemistry and life sciences.17–20 For instance, the element-substitution strategy that replaces the oxygen-bridge atom with a silicon atom in the xanthene core could alter the energy level of both HOMO and LUMO simultaneously, thereby narrowing the bandgap to afford considerable spectral red-shifts.21 Notably, Si-rhodamines afford a longer wavelength, superior brightness, and photostability, making them an outstanding candidate for in vivo bioimaging.22,23 However, this merely element-substitution skeleton modification alone limits the emission window expanding into the NIR-II window. Inspired by π-extension from molecular conformation changes, we hypothesize that one reasonable solution for developing an innovative NIR-II fluorogenic platform could be attained by tailoring the conjugated skeleton and switchable delocalization, that is greatly expanding the spectral windows and lighting up NIR fluorescence intensity.
Herein, we report a de novo design strategy to construct bent-to-planar NIR-II fluorophores based on a discovered fluorogenic platform, i.e., meso-chlorine substituted Si-rhodamine (Cl-SiRhd, Fig. 1B). Through the nucleophilic substitution at the meso-position, π-conjugation of these Si-rhodamines becomes switchable, thereby initiating a new method of analyte controlled molecular configuration changes. We expand this bent-to-planar molecular strategy on this novel Cl-SiRhd scaffold to engineer a series of Si-rhodamine probes spanning from the visible to NIR-II range. We demonstrate that the change in the hybridization state of meso-nitrogen in this Cl-SiRhd scaffold could result in flipping between the disruption and recovery of the polymethine π-electron system, thereby significantly altering the spectral wavelength. Specifically, the imino forms of sp2-hybridized meso-nitrogen generate a bent configuration and short wavelengths; the corresponding amino forms cause planar configuration with long wavelengths (Fig. 1B). Using these switchable bent/planar Si-rhodamines, we have constructed various crosstalk-free ratiometric probes, especially for highly favorable turn-on NIR-II probes with large Stokes shifts (ca. 250 nm). Notably, these configuration-dependent Si-rhodamine probes allow us to noninvasively and real-time monitor biological events in live cells, zebrafish, and mice. For the first time, the elaborately lighting-up NIR-II Si-rhodamine probes make a breakthrough in directly tracking nitric oxide (NO) fluctuation in the Alzheimer's disease (AD) brain of mice. This accessible bent-to-planar strategy opens up further exciting opportunities for developing NIR-II probes, facilitating the advancement of highly accurate analysis in vivo.
Results and discussion
Engineering meso-chlorine substituted Si-rhodamine chromophores
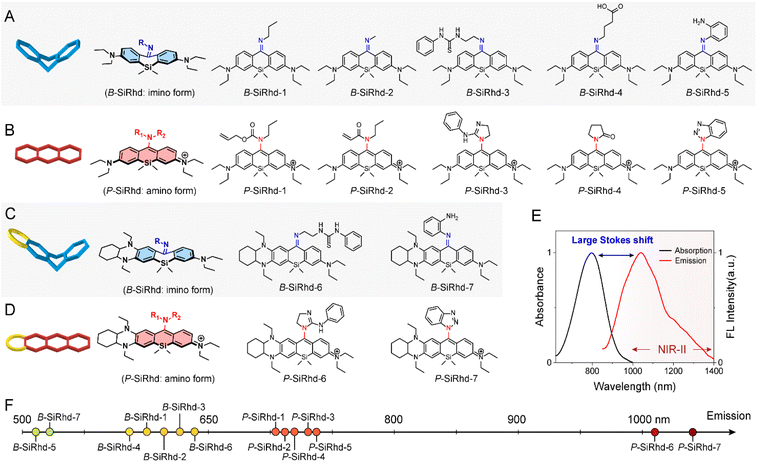
Aiming to expand the utility of fluorophores along with tailoring their emission properties for in vivo bioimaging, many research studies were devoted to Si-rhodamines.24–26 We noted that the modification of meso-substituents in xanthene and cyanine skeletons could afford significant spectral changes.27–31 For instance, amination or hydroxylation on meso-chloride cyanine dyes could significantly disturb the conjugated system.32,33 Here, we firstly discovered and obtained chemically reactive meso-chlorine substituted Si-rhodamines, i.e., Cl-SiRhd (Fig. 1B), which allows various meso-substitutions under mild conditions, thus regulating the nature of the conjugated system. Similar to Cl-cyanine, Cl-SiRhd can be substituted with nucleophiles to produce a variety of NIR fluorophores and probes, and due to the superior stability and quantum efficiency of Si-rhodamine dyes, we expect that Cl-SiRhd has great potential for the development of in vivo fluorescent diagnostic reagents. To gain further insight into the structure–property relationship of the spectral changes, via nucleophilic amination, a series of meso-nitrogen substituted Si-rhodamines were portable synthesized (Fig. 2).As is well-known, it's extremely difficult to regulate the emission spectra in a “given” Si-rhodamine scaffold with a large Stokes shift.23,26,34 However, our obtained Si-rhodamine probes show unprecedented and distinct spectral changes: (i) the resulting imino form of Si-rhodamines (such as B-SiRhd-1; λem = 600 nm) showed short-wavelength emission (Fig. 2F and S1–S3†); (ii) when meso-nitrogen becomes more electron poor, the corresponded amination of Si-rhodamines (such as P-SiRhd-1; λem = 700 nm) displayed long-wavelength emission (Fig. 2F and S4–S7†). Thus, these changes of the meso-nitrogen substituent in the conjugation play a key role in both absorption and emission spectral changes. As mentioned above, the hybridized nature of the meso-nitrogen substituent in this Cl-SiRhd scaffold responsible for the significant spectral shift is highly unusual in a “given” Si-rhodamine scaffold and needs to be further investigated.
Starting from the initial B-SiRhd-1 and P-SiRhd-1, we engineered a series of wavelength-regulable Si-rhodamines using the following guidelines (Fig. 1B): (i) starting with Cl-SiRhd as the fluorogenic motif; (ii) regulating meso-nitrogen with nucleophilic substituents. This strategy unlocks a great opportunity to rapidly establish a library of Si-rhodamines for studying the regularity of their spectra. Specifically, we employed propylamine, 1-(2-aminoethyl)-3-phenylthiourea, and 4-aminobutyric acid, thus forming imine-type structures; while allyl propylcarbamate, guanidine derivatives, and pyrrolidinone as amine-type structures (Fig. 2). To our delight, all the resulting imino forms of Si-rhodamines displayed relative short-wavelength emission (Fig. S8†), whereas their corresponding amino forms of Si-rhodamines exhibited much longer emission (Fig. S9†). More importantly, when we elaborately constructed an asymmetric Si-rhodamine fluorogenic scaffold, the resulting Si-rhodamines (P-SiRhd-6 and P-SiRhd-7) achieved unexpected maximum NIR-II emission around 1050 nm (Fig. 2E, F and S10†). As far as we know, for the first time, a simple and generalizable Si-rhodamine engineering strategy was formulated to induce large spectral shifts spanning from the visible to NIR-II range (emission from 520 nm to over 1050 nm).
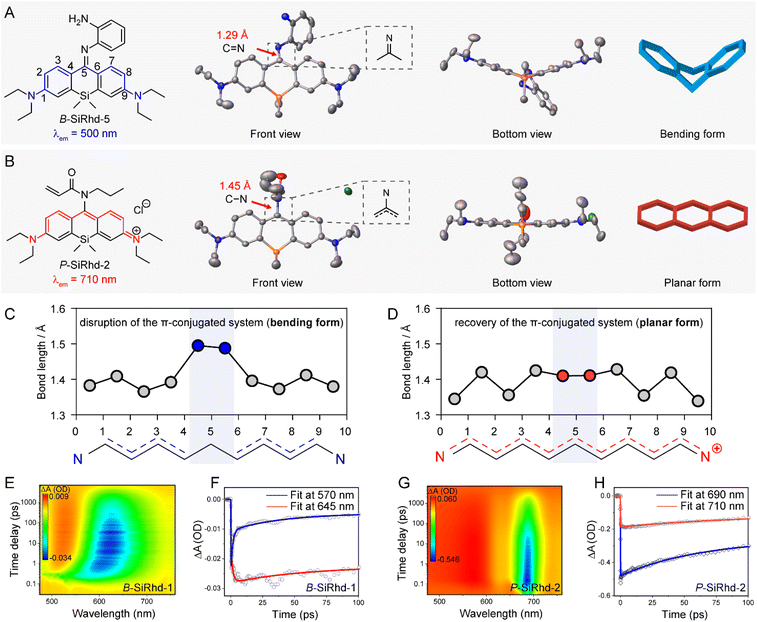
Revealing the bent-to-planar configuration changes
To get a deeper understanding of these significant spectral shifts, it's critical to obtain the molecular geometries/configuration of these Si-rhodamines. It has been revealed that modifying the amino group at the meso-position of the xanthene scaffold could significantly alter the spectral properties of Si-rhodamines.35–37 Fortunately, we acquired single crystals of B-SiRhd-5 and P-SiRhd-2 (Fig. 3A and B). Specifically, B-SiRhd-5 possesses a large dihedral angle (around 37.15°) between two benzene units (Fig. 3A). With the attachment of the amino-group with an acryloyl unit, the resulting P-SiRhd-2 exhibits an almost coplanar configuration (Fig. 3B, with merely a 5.76° dihedral angle between two benzene groups). According to single-crystal analysis, the dihedral angle between two benzene rings is distinctly different from the electron density perturbation of meso-nitrogen substituents. These results elucidated that the regulation of the meso-nitrogen substitution of these B-SiRhd/P-SiRhd chromophores plays a key role in their molecular configuration. Thus, we reasoned that B-SiRhd-5 has a bent configuration with a shorter emission due to its obviously disrupted π-conjugation system. In contrast, P-SiRhd-2 with much longer emission exhibits planar configuration, maintaining the “unbroken” polymethine π-delocalization.Subsequently, we investigated the role of meso-substituent in switching the intramolecular π-electron delocalization. Two (or more) consecutive carbon–carbon single bonds generally disrupt the π-electron delocalization in a conjugated system. Indeed, the bent B-SiRhd-5 (imino form) possesses an obvious double bond (1.29 Å) of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond between meso-nitrogen and central-carbon C5, and therefore this sp2 hybridization of the N atom leads to two consecutive single bonds C4–C5 and C5–C6 in this Si-rhodamine (Fig. 3A and C). In contrast, the planar P-SiRhd-2 (amino form) exhibits a single bond (1.45 Å) of the C–N bond between meso-nitrogen (sp3 hybridization) and central-carbon atoms. Importantly, in P-SiRhd-2, no consecutive single carbon bonds are found in its skeleton (Fig. 3B and D), thereby affording much longer emission than that of B-SiRhd-5. Collectively, these results elucidate that the hybridization of meso-nitrogen in these B-SiRhd/P-SiRhd skeletons could switch the π-electron delocalization: (i) C
N bond between meso-nitrogen and central-carbon C5, and therefore this sp2 hybridization of the N atom leads to two consecutive single bonds C4–C5 and C5–C6 in this Si-rhodamine (Fig. 3A and C). In contrast, the planar P-SiRhd-2 (amino form) exhibits a single bond (1.45 Å) of the C–N bond between meso-nitrogen (sp3 hybridization) and central-carbon atoms. Importantly, in P-SiRhd-2, no consecutive single carbon bonds are found in its skeleton (Fig. 3B and D), thereby affording much longer emission than that of B-SiRhd-5. Collectively, these results elucidate that the hybridization of meso-nitrogen in these B-SiRhd/P-SiRhd skeletons could switch the π-electron delocalization: (i) C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond formation disrupts π-electron delocalization with a bent fluorophore scaffold (Fig. 3A and C); (ii) meso-nitrogen undergoing sp3-hybridization restores π-electron delocalization with a planar fluorophore scaffold (Fig. 3B and D). Given that changing the molecular skeletal conformation could expand the π-conjugation,38,39 it is believed that tailoring the nature of the meso-position substituent in the conjugated system would play a key role in the development of NIR Si-rhodamine scaffolds.
N bond formation disrupts π-electron delocalization with a bent fluorophore scaffold (Fig. 3A and C); (ii) meso-nitrogen undergoing sp3-hybridization restores π-electron delocalization with a planar fluorophore scaffold (Fig. 3B and D). Given that changing the molecular skeletal conformation could expand the π-conjugation,38,39 it is believed that tailoring the nature of the meso-position substituent in the conjugated system would play a key role in the development of NIR Si-rhodamine scaffolds.
According to single-crystal analysis, distinctly different π-delocalization was observed from meso-nitrogen substituted Si-rhodamines due to the electron density perturbation, and therefore the configuration in the molecular solution-state should be further investigated. With this in mind, we subsequently carried out femtosecond time-resolved transient absorption (TA) spectroscopy experiments. As shown in Fig. 3E, the time-resolved experiments of B-SiRhd-1 showed a red shift stimulated emission band, which is in agreement with the flexible molecular geometry (i.e., bent configuration) of B-SiRhd-5 from the single-crystal analysis. More importantly, the transient absorption of B-SiRhd-1 kinetic traces at 570 nm and 645 nm clearly denotes an internal conversion in the excited state40–42 (Fig. 3F and S11†), further confirming that the B-SiRhd scaffold is flexibly bent in the molecular solution state. In contrast, P-SiRhd-2 displayed a still stimulated emission band over time (Fig. 3G and H), which implies that the Si-rhodamine scaffold is rigid in both the crystalline solid-state and solution-state (i.e., planar configuration). All these results further confirm that the regulation of the meso-nitrogen substituent in the Cl-SiRhd scaffold can switch intramolecular π-electron delocalization, thereby resulting in flipping between the disruption and recovery of the polymethine π-electron system.
Constructing ratiometric NIR probes with minimized spectral crosstalk
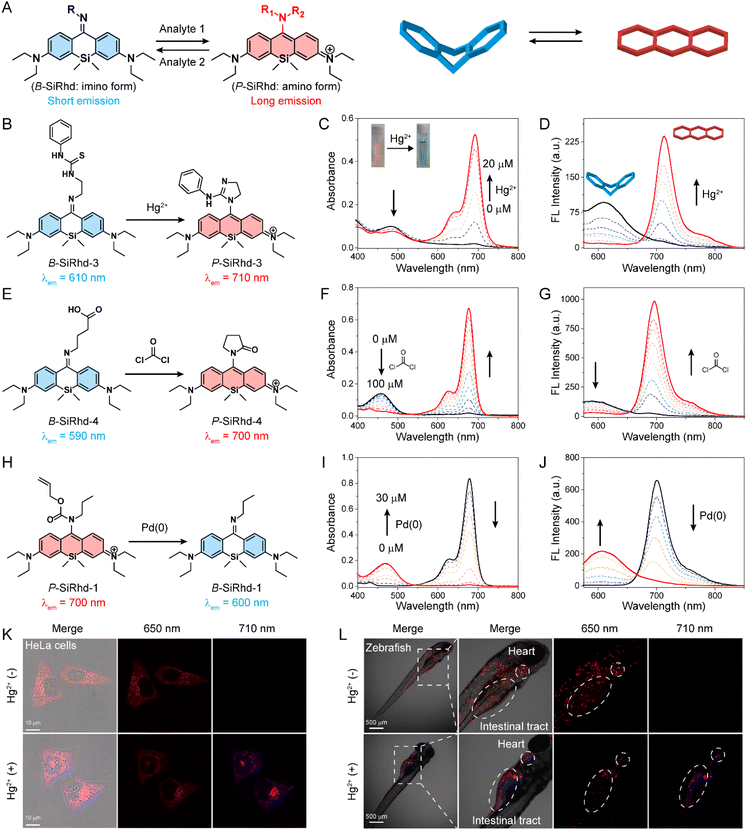
The above experiments demonstrated that the alteration of π-electron delocalization in Si-rhodamines could generate a crosstalk-free dual-channel response. We thus hypothesized that high-performance ratiometric fluorescent probes43–48 could be designed via switching Si-rhodamines between the bent and planar configurations (Fig. 4A). Consequently, the following functionalized B-SiRhd/P-SiRhd probes were designed: B-SiRhd-3 with a 1-(2-aminoethyl)-3-phenylthiourea group for sensing Hg2+, B-SiRhd-4 with a 4-aminobutyric acid group for sensing phosgene and P-SiRhd-1 with the allyl propylcarbamate group for sensing Pd(0). As shown in Fig. 4B–J and S12–S15,† the crosstalk-free ratiometric responses were observed in all test assays.For example, upon the addition of Hg2+, B-SiRhd-3 exhibited a remarkable shift in the absorption spectra, along with an obvious color change from yellow to green. Specifically, the absorption peak at 488 nm sharply decreased, and an increasing band centered at 690 nm was simultaneously observed, along with a distinct isosbestic point at around 550 nm (Fig. 4C). Concomitantly, when excited at an isosbestic point of 550 nm, an obvious decrease in the emission spectra at 610 nm and a sharp increase at 710 nm were also observed in the emission spectra (Fig. 4D, S16 and S17†). Importantly, this crosstalk-free ratiometric response not only contributes to the accurate measurement of dual-channel emission intensity, but also results in a huge ratiometric value (Fig. S18†). To our delight, similar emission profiles and crosstalk-free responses were also observed in B-SiRhd-3 and P-SiRhd-1 towards phosgene and Pd(0), respectively (Fig. S19 and S20†). Furthermore, the crosstalk-free ratiometric bioimaging in cells and zebrafish demonstrated that B-SiRhd-3 can real-time track the uptake of Hg2+ in living cells and zebrafish (Fig. 4K, L and S21†). All these above results showed that the bent/planar switchable Si-rhodamines enable crosstalk-free ratiometric analysis and serve as a generalizable platform for detecting various chemical species.
Enabling simultaneously activatable NIR-II, photoacoustic, and photothermal signals
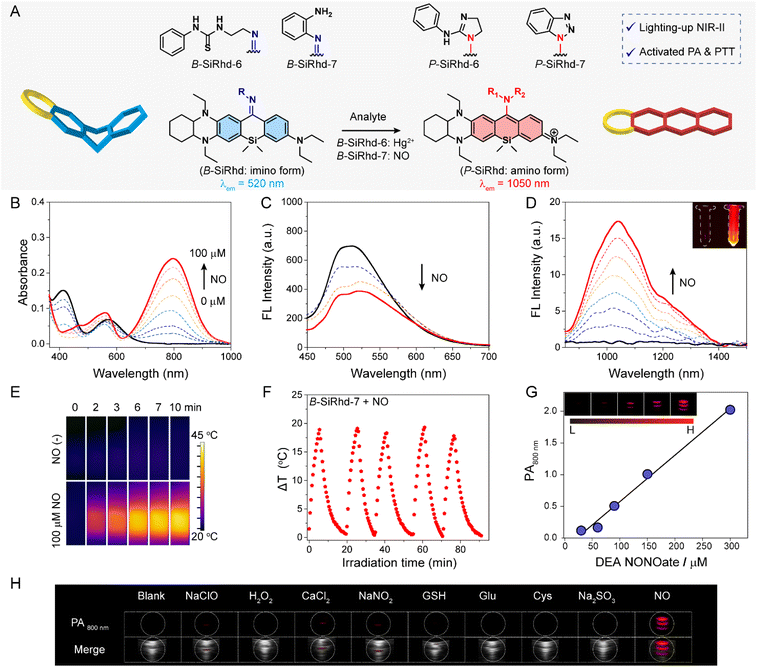
It remains quite challenging to rationally regulate absorption and emission in the NIR-II region, and these bottlenecks severely hinder the applicability of such probes for in vivo biosensing.49–54 Given that the asymmetric bent form (B-SiRhd with NIR-I emission) could be transformed to planar-form Si-rhodamines (P-SiRhd with NIR-II emission) (Fig. 2F and 5A), rational modulation of the NIR-II emission could be achieved via bent-to-planar configuration change. In this regard, various NIR-II fluorescent probes could be constructed via using our B-SiRhd/P-SiRhd chromophores.The asymmetric Si-rhodamine probes B-SiRhd-6 and B-SiRhd-7 were obtained by using a 1-(2-aminoethyl)-3-phenylthiourea moiety as the Hg2+ responsive site, and an o-phenylenediamine moiety as the nitric oxide (NO) responsive site, respectively. To our delight, both B-SiRhd-6 (Fig. S22–S26†) and B-SiRhd-7 displayed remarkable lighting-up NIR-II fluorescence in response to their respective target analytes. For example, B-SiRhd-7 initially displayed two main absorptions at 420 and 580 nm with an emission peak at 520 nm. The detection of NO resulted in a new sharply increased NIR-I absorption band centered at 800 nm (Fig. 5B), accompanied by a reduction of the original absorption bands with a limit of detection of 0.3 μM (Fig. S27†). Concomitantly, a new significantly increased emission band in the NIR-II region around 1050 nm (λex = 800 nm) was observed, along with the decrease of the 520 nm emission (Fig. 5C, D and S28–S33†). Such an obvious NIR-II fluorescence enhancement (20-fold) is attributed to the NO-induced bent-to-planar transformation of Si-rhodamine (Fig. 5A). We summarize these photophysical properties of Cl-SiRhd-based fluorophores (Table S1†). Impressively, P-SiRhd-7 exhibits excellent photostability compared to the traditional NIR-II fluorophore, which is a critical property in in vivo fluorescence imaging (Fig. S34†). All these results demonstrated that our bent-to-planar strategy enables not only the construction of dual-channel crosstalk-free ratiometric probes, but also the construction of turn-on probes with NIR-II fluorescence.
Inspired by the NO-induced sharp enhancement of the absorption at 800 nm, we reasoned that B-SiRhd-7 could be a promising chromophore for activatable photothermal (PT) therapy and photoacoustic (PA) imaging.55–60 As shown in Fig. 5E, upon treatment with NO, the probe B-SiRhd-7 exhibited a clear NIR-photothermal effect upon exposure to 808 nm NIR light laser irradiation. The repeatable temperature increases (even after five cycles of heating and cooling) indicated that this NO-activatable probe B-SiRhd-7 has excellent photo-thermal stability as a potential PTT agent (Fig. 5F and S35–S37†). Furthermore, we also explored the NO-induced PA signal from the response of B-SiRhd-7. Notably, the corresponding optoacoustic signal at approximately 800 nm is enhanced for the detection of NO with good linear quantifiability (Fig. 5G) and high selectivity (Fig. 5H and S38†). Taken together, all these spectral results including single-crystals and time-resolved transient absorption analysis clearly demonstrated that the B-SiRhd/P-SiRhd platform could serve as a generalizable method for engineering activatable NIR-II probes with PT and PA signals.
Lighting-up NIR-II fluorescence for real-time in situ tracking inflammation in the liver, intestine and AD mice brain
The diagnosis of chronic diseases is of great significance for the timely implementation of medical intervention. Chronic inflammation plays a key role in the development and progression of many chronic diseases, such as inflammatory bowel disease (IBD) and Alzheimer's disease (AD), and can lead to increases in morbidity.61,62 Therefore, accurate mapping of inflammation will provide critical information to better understand its role in the onset and progression of chronic diseases. Activatable NIR-II imaging is a powerful approach to interrogate intact living samples in real time with spatial resolution due to its deep penetration and high-fidelity imaging capabilities. It has been widely applied for non-invasive real-time sensing and disease diagnosis in living systems.Our new method of analyte-controlled molecular configuration changes for lighting-up NIR-II detection fits in well with disease diagnosis in living systems. Herein, to validate our strategy of expanding light-up NIR-II sensors with the real-time in vivo imaging capability, we chose the construction of an inflammation sensing system based on effective interaction of inflammatory factors for in situ generation of NO in deep tissue. We next assessed the capability of B-SiRhd-7 for in vivo monitoring endogenous NO levels in LPS-induced inflammation and Alzheimer's disease mice (Fig. 6). Because NO is a lipid-permeable free radical molecule, liposomal nanoparticles would be better employed to encapsulate B-SiRhd-7 for tracking NO fluctuation. With this in mind, stable lipid-based supramolecular nanoparticles were fabricated by self-assembly of colipids (phosphatidylcholine, DSPE-PEG-2000, and cholesterol) facilitated by hydrophilic and hydrophobic interactions. Indeed, hydrophobic B-SiRhd-7 occupies the spaces between the lipid bilayer constituting the B-SiRhd-7@liposome system63 (Fig. 6A). The hydrodynamic diameter was evaluated using dynamic light scattering and was found to be ca. 125 nm (Fig. S39 and S40†). Transmission electron microscopy revealed the nanoparticles to have a clear structure of the lipid bilayer (Fig. 6B). Taking the above results together, we successfully prepared spherical core–shell structured liposome nanoparticles with a homogeneous particle size.
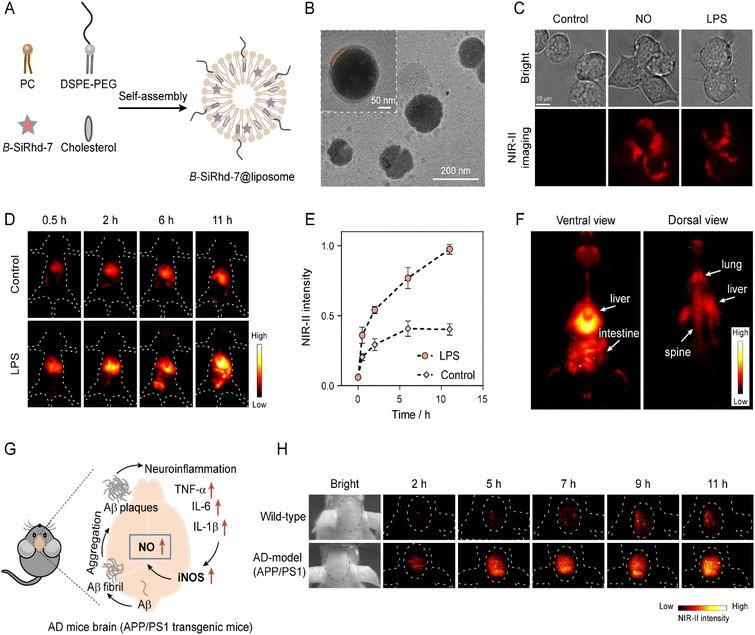 | ||
| Fig. 6 In vivo NIR-II imaging of nitric oxide in LPS-induced inflammation and Alzheimer disease (AD) mice. (A) Schematics showing the synthesis of B-SiRhd-7@liposome. Cholesterol and NO probe B-SiRhd-7 were loaded into a stable liposomal system facilitated by the self-assembly of colipids (DSPE-PEG and phosphatidyl choline (PC)). (B) TEM image showing a B-SiRhd-7@liposome. (C) NIR-II fluorescence imaging of NO in RAW264.7 cells (incubated with B-SiRhd-7@liposome, without and with exogenous or endogenous NO). Notes: λex = 808 nm, and 900 nm emission filter. (D) Representative images of mice receiving intravenously B-SiRhd-7@liposome (0.4 mM, 200 μL) pretreated with LPS or saline (control) intraperitoneally at a different time point. (E) The normalized fluorescence intensity over time in Fig. 6D. The maximum fluorescence intensity (11 h) is defined as 1.0. Data are presented as mean ± SD (n = 3). (F) Biodistribution of B-SiRhd-7@liposome in the LPS-induced mouse in the ventral view (26 h) and dorsal view (28 h) after tail-vein injection of B-SiRhd-7@liposome. (G) Purposed biological synthesis route of NO in AD mouse brains. (H) In vivo fluorescence imaging of NO in wild-type and AD-model (APP/PS1) mice brains for 11 h via intravenous injection of 200 μL of 0.4 mM B-SiRhd-7@liposomes. λex = 808 nm, and 1100 nm long-pass filter. | ||
Firstly, we evaluated the NIR-II fluorescence response ability of B-SiRhd-7@liposome towards NO in living cells, and a RAW 264.7 cell was chosen as a model cell line. As shown in Fig. 6C and S41,† when RAW264.7 cells were preloaded with B-SiRhd-7@liposome (10 μM) for 30 min, dim fluorescence was detected in cells. However, after the cells were incubated with NO (10 μM) for another 10 min, a distinct enchantment of NIR-II fluorescence (λex = 808 nm and 900 nm emission filter) of the cells was observed. Similarly, remarkable NIR-II fluorescence was found in the lipopolysaccharide (LPS, 1 μg mL−1) pretreatment group, in which LPS could induce an inflammatory response in cells to produce NO. Cellular test results indicated that B-SiRhd-7@liposome enables real-time tracking of the alteration of endogenous NO levels.
Encouraged by in vivo cellular imaging results, we further examined B-SiRhd-7@liposome for monitoring the fluctuation of NO levels in LPS-induced inflammation mice models. Mice were treated with either LPS (1 mg mL−1, 4 mg kg−1) or saline (as a control group) intraperitoneally. After 6 h, B-SiRhd-7@liposome (0.4 mM, 200 μL) was intravenously injected into the tail of mice. The images were collected upon light irradiation at 808 nm, with a 1100 nm long-pass filter. The LPS pretreated group displayed a faster liver response and clearer intestinal imaging than that of the control group (Fig. 6D, E and S42†). These results showed that B-SiRhd-7@liposome has the capability of effectively real-time monitoring of multi-organ inflammation induced by intraperitoneal LPS injection64,65 rather than just a single liver injury. Indeed, the real-time biodistribution from the probe provides important evidence to assess their biosafety for further clinical applications. Particularly, the light-up NIR-II fluorescence with deep tissue penetration makes it possible to directly visualize B-SiRhd-7@liposome's biodistribution. As illustrated in Fig. 6F, after the administration of the probe into LPS-induced inflammation models, B-SiRhd-7@liposomes are distributed especially in the mononuclear phagocyte system (MPS)-enriched organs, such as liver, intestine, lung and spine (Fig. S43–S45†). All these in vivo imaging results indicated that the light-up NIR-II fluorescence signals of B-SiRhd-7@liposomes can be used as a powerful tool for real-time in situ monitoring of multi-organ inflammation, especially in intestinal inflammation.
We further evaluated the biosensing efficacy of lighting-up NIR-II B-SiRhd-7@liposome in the Alzheimer's disease brain. Neuroinflammation has demonstrated an important role in the pathogenesis of Alzheimer's disease (AD), which is the most prevalent form of dementia.66–68 Previous work has proven that the expression of the inducible nitric oxide synthase (iNOS) level in the APP/PS1 mice brain was much higher than that of wild-type (WT) mice, thereby leading to the overproduction of NO (Fig. 6G).66 We envision that B-SiRhd-7@liposome is suitable for in vivo monitoring the fluctuation of NO in the AD brains. To confirm this feasibility, 12 month-old male AD-model (APP/PS1 transgenic) mice and age-matched wild-type WD mice were chosen to study the brain kinetics by intravenous injection of B-SiRhd-7@liposome. As shown in Fig. 6H, nearly all of the NIR-II fluorescence signals were centralized in the brain compartments and could be efficiently captured. In particular, the fluorescence intensity of B-SiRhd-7@liposome in the brain regions of the APP/PS1 mice was higher than that in the control of wild-type mice at 11 h post injection, indicative of specifically trapping NO in vivo with probe B-SiRhd-7@liposome (Fig. S46†). These directly in situ brain results evidenced that the overexpression of NO is one of the hallmarks of AD mice. The above in vivo imaging results illustrated that our bent-to-planar conformational design strategy for NIR-II Si-rhodamine probes makes a breakthrough in directly in situ mapping inflammation (neuroinflammation and LPS-induced inflammation).
Conclusions
Harnessing a concise molecular configuration-tunable strategy, i.e., bent-to-planar transformation in Si-rhodamines, we have successfully developed NIR-II Si-rhodamines with a large Stokes shift (ca. 250 nm) for directly in situ mapping inflammatory (neuroinflammation and LPS-induced inflammation). This breakthrough establishes an innovative rehybridization of NIR-I&II fluorogenic framework Cl-SiRhd and thus activates configuration change to greatly expand the Si-rhodamine spectral window. With single-crystals and transient absorption experiments, we confirmed that the imino form of meso-nitrogen with sp2 hybridization results in a bent configuration, which disrupts π-electron delocalization and accounts for a short emission wavelength. In contrast, when meso-nitrogen undergoes sp3 hybridization, the amino form leads to a planar conformation, which recovers the π-conjugation and lights up NIR I&II emissions. These unique NIR-II lighting-up Si-rhodamine probes (B-SiRhd-6 and B-SiRhd-7) with crosstalk-free responses successfully perform in vivo tracking of LPS-induced multi-organ inflammation, including intestinal inflammation in deep physiological anatomical locations. Notably, for the first time, B-SiRhd-7@liposomes with light-up NIR-II fluorescence signals makes a breakthrough in monitoring abnormal nitric oxide levels in the Alzheimer's brain. We anticipate that this de novo conformation-dependent strategy of Si-rhodamine probes paves a new way for expanding NIR-II bio-analytical toolboxes in both basic life science research and clinical applications.Data availability
Additional experimental details and data are provided in the ESI.† The accession numbers for the crystallographic data reported in this paper are CCDC: 2119838, 2119842 and 2132279. All procedures involving animals were conducted in accordance with the National Research Council Guide for Care and Use of Laboratory Animals. APP/PS1 transgenic mice (Jiangsu Huachuang Sino Pharmatech Co., Ltd; approval number: SCXK (Jiangsu) 2020-0009) or BALB/cA nude mice (Shanghai SLAC Laboratory Animal Co. Ltd; approval number: SCXK (Shanghai) 2022-0004) were used for animal experiments.Author contributions
All the experiments were conducted by Q. X., Y. Z., M. Z., C. Y., and W. M. with the supervision of W. Z. and Z. G. All authors discussed the results and co-wrote the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by NSFC/China (22225805 and 32121005), National Key Research and Development Program of China (2021YFA0910000), Shanghai Municipal Science and Technology Major Project (Grant 2018SHZDZX03, 21JC1401700), Innovation Program of Shanghai Municipal Education Commission, Shanghai Frontier Science Research Base of Optogenetic Techniques for Cell Metabolism (Shanghai Municipal Education Commission, grant 2021 Sci & Tech 03-28), and Programme of Introducing Talents of Discipline to Universities (B16017), and sponsored by Shanghai Pujiang Program (21PJD038). We thank Weimin Liu, ShanghaiTech University, for the femtosecond time-resolved transient absorption spectroscopy experimental support.Notes and references
- T. C. Pham, V. N. Nguyen, Y. Choi, S. Lee and J. Yoon, Chem. Rev., 2021, 121, 13454–13619 CrossRef CAS PubMed.
- Q. Yao, J. Fan, S. Long, X. Zhao, H. Li, J. Du, K. Shao and X. Peng, Chem, 2022, 8, 197–209 CAS.
- X. Wu, H. Li, E. Lee and J. Yoon, Chem, 2020, 6, 2893–2901 CAS.
- C. Li, G. Chen, Y. Zhang, F. Wu and Q. Wang, J. Am. Chem. Soc., 2020, 142, 14789–14804 CrossRef CAS PubMed.
- E. M. Surender, S. J. Bradberry, S. A. Bright, C. P. McCoy, D. C. Williams and T. Gunnlaugsson, J. Am. Chem. Soc., 2017, 139, 381–388 CrossRef CAS PubMed.
- J.-T. Hou, K.-K. Yu, K. Sunwoo, W. Y. Kim, S. Koo, J. Wang, W. X. Ren, S. Wang, X.-Q. Yu and J. S. Kim, Chem, 2020, 6, 832–866 CAS.
- C. Li, Y. Xu, L. Tu, M. Choi, Y. Fan, X. Chen, J. L. Sessler, J. S. Kim and Y. Sun, Chem. Sci., 2022, 13, 6541–6549 RSC.
- T. Wang, S. Wang, Z. Liu, Z. He, P. Yu, M. Zhao, H. Zhang, L. Lu, Z. Wang, Z. Wang, W. Zhang, Y. Fan, C. Sun, D. Zhao, W. Liu, J. G. Bunzli and F. Zhang, Nat. Mater., 2021, 20, 1571–1578 CrossRef CAS PubMed.
- L. Wu, J. Liu, P. Li, B. Tang and T. D. James, Chem. Soc. Rev., 2021, 50, 702–734 RSC.
- A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, Nat. Mater., 2016, 15, 235–242 CrossRef CAS PubMed.
- C. Li, Y. Xu, L. Tu, M. Choi, Y. Fan, X. Chen, J. L. Sessler, J. S. Kim and Y. Sun, Chem. Sci., 2022, 13, 6541–6549 RSC.
- E. D. Cosco, J. R. Caram, O. T. Bruns, D. Franke, R. A. Day, E. P. Farr, M. G. Bawendi and E. M. Sletten, Angew. Chem., Int. Ed., 2017, 56, 13126–13129 CrossRef CAS PubMed.
- E. D. Cosco, A. L. Spearman, S. Ramakrishnan, J. G. P. Lingg, M. Saccomano, M. Pengshung, B. A. Arus, K. C. Y. Wong, S. Glasl, V. Ntziachristos, M. Warmer, R. R. McLaughlin, O. T. Bruns and E. M. Sletten, Nat. Chem., 2020, 12, 1123–1130 CrossRef CAS PubMed.
- Y. Fang, J. Shang, D. Liu, W. Shi, X. Li and H. Ma, J. Am. Chem. Soc., 2020, 142, 15271–15275 CrossRef CAS PubMed.
- Z. Lei and F. Zhang, Angew. Chem., Int. Ed., 2021, 60, 16294–16308 CrossRef CAS PubMed.
- T. B. Ren, Z. Y. Wang, Z. Xiang, P. Lu, H. H. Lai, L. Yuan, X. B. Zhang and W. Tan, Angew. Chem., Int. Ed., 2021, 60, 800–805 CrossRef CAS PubMed.
- J. Li, Y. Dong, R. Wei, G. Jiang, C. Yao, M. Lv, Y. Wu, S. H. Gardner, F. Zhang, M. Y. Lucero, J. Huang, H. Chen, G. Ge, J. Chan, J. Chen, H. Sun, X. Luo, X. Qian and Y. Yang, J. Am. Chem. Soc., 2022, 144, 14351–14362 CrossRef CAS PubMed.
- L. Wang, M. Tran, E. D'Este, J. Roberti, B. Koch, L. Xue and K. Johnsson, Nat. Chem., 2020, 12, 165–172 CrossRef CAS PubMed.
- S. Takahashi, Y. Kagami, K. Hanaoka, T. Terai, T. Komatsu, T. Ueno, M. Uchiyama, I. Koyama-Honda, N. Mizushima, T. Taguchi, H. Arai, T. Nagano and Y. Urano, J. Am. Chem. Soc., 2018, 140, 5925–5933 CrossRef CAS PubMed.
- L. Wang, W. Du, Z. Hu, K. Uvdal, L. Li and W. Huang, Angew. Chem., Int. Ed., 2019, 58, 14026–14043 CrossRef CAS PubMed.
- M. Fu, Y. Xiao, X. Qian, D. Zhao and Y. Xu, Chem. Commun., 2008, 15, 1780–1782 RSC.
- N. Lardon, L. Wang, A. Tschanz, P. Hoess, M. Tran, E. D'Este, J. Ries and K. Johnsson, J. Am. Chem. Soc., 2021, 143, 14592–14600 CrossRef CAS PubMed.
- A. N. Butkevich, G. Lukinavicius, E. D'Este and S. W. Hell, J. Am. Chem. Soc., 2017, 139, 12378–12381 CrossRef CAS PubMed.
- J. B. Grimm, A. N. Tkachuk, L. Xie, H. Choi, B. Mohar, N. Falco, K. Schaefer, R. Patel, Q. Zheng, Z. Liu, J. Lippincott-Schwartz, T. A. Brown and L. D. Lavis, Nat. Methods, 2020, 17, 815–821 CrossRef CAS PubMed.
- Y. Koide, Y. Urano, K. Hanaoka, W. Piao, M. Kusakabe, N. Saito, T. Terai, T. Okabe and T. Nagano, J. Am. Chem. Soc., 2012, 134, 5029–5031 CrossRef CAS PubMed.
- T. B. Ren, W. Xu, W. Zhang, X. X. Zhang, Z. Y. Wang, Z. Xiang, L. Yuan and X. B. Zhang, J. Am. Chem. Soc., 2018, 140, 7716–7722 CrossRef CAS PubMed.
- C. Yan, Z. Guo, Y. Liu, P. Shi, H. Tian and W. H. Zhu, Chem. Sci., 2018, 9, 6176–6182 RSC.
- P. Horvath, P. Sebej, T. Solomek and P. Klan, J. Org. Chem., 2015, 80, 1299–1311 CrossRef CAS PubMed.
- K. H. Kim, S. Singha, Y. W. Jun, Y. J. Reo, H. R. Kim, H. G. Ryu, S. Bhunia and K. H. Ahn, Chem. Sci., 2019, 10, 9028–9037 RSC.
- L. Wu and K. Burgess, Org. Lett., 2008, 10, 1779–1782 CrossRef CAS PubMed.
- H. Zhang, J. Liu, L. Wang, M. Sun, X. Yan, J. Wang, J. P. Guo and W. Guo, Biomaterials, 2018, 158, 10–22 CrossRef CAS PubMed.
- Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- X. Peng, F. Song, E. Lu, Y. Wang, W. Zhou, J. Fan and Y. Gao, J. Am. Chem. Soc., 2005, 127, 4170–4171 CrossRef CAS PubMed.
- Y. Zhang, S. Xia, M. Fang, W. Mazi, Y. Zeng, T. Johnston, A. Pap, R. L. Luck and H. Liu, Chem. Commun., 2018, 54, 7625–7628 RSC.
- Y. Q. Sun, J. Liu, H. Zhang, Y. Huo, X. Lv, Y. Shi and W. Guo, J. Am. Chem. Soc., 2014, 136, 12520–12523 CrossRef CAS PubMed.
- M. S. Frei, P. Hoess, M. Lampe, B. Nijmeijer, M. Kueblbeck, J. Ellenberg, H. Wadepohl, J. Ries, S. Pitsch, L. Reymond and K. Johnsson, Nat. Commun., 2019, 10, 4580 CrossRef PubMed.
- T. Pastierik, P. Sebej, J. Medalova, P. Stacko and P. Klan, J. Org. Chem., 2014, 79, 3374–3382 CrossRef CAS PubMed.
- Z. Zhang, Y. S. Wu, K. C. Tang, C. L. Chen, J. W. Ho, J. Su, H. Tian and P. T. Chou, J. Am. Chem. Soc., 2015, 137, 8509–8520 CrossRef CAS PubMed.
- Z. Zhang, C. L. Chen, Y. A. Chen, Y. C. Wei, J. Su, H. Tian and P. T. Chou, Angew. Chem., Int. Ed., 2018, 57, 9880–9884 CrossRef CAS PubMed.
- W. Chi, Q. Qiao, R. Lee, W. Liu, Y. S. Teo, D. Gu, M. J. Lang, Y. T. Chang, Z. Xu and X. Liu, Angew. Chem., Int. Ed., 2019, 58, 7073–7077 CrossRef CAS PubMed.
- L. Shi, C. Yan, Z. Guo, W. Chi, J. Wei, W. Liu, X. Liu, H. Tian and W. H. Zhu, Nat. Commun., 2020, 11, 793 CrossRef CAS PubMed.
- C. Wang, W. Chi, Q. Qiao, D. Tan, Z. Xu and X. Liu, Chem. Soc. Rev., 2021, 50, 12656–12678 RSC.
- Q. Lan, P. Yu, K. Yan, X. Li, F. Zhang and Z. Lei, J. Am. Chem. Soc., 2022, 144, 21010–21015 CrossRef CAS PubMed.
- K. Xin, X. Li, Y. Guo, Y. Zhong, J. Wang, H. Yang, J. Zhao, C. Guo, Y. Huang, Z. Lei, Y.-L. Ying, X. Luo, H. Wang, X. Qian, W. Yang, X. Liang and Y. Yang, CCS Chem., 2021, 3, 2307–2315 CrossRef CAS.
- X. Zhang, Y. Xiao and X. Qian, Angew. Chem., Int. Ed., 2008, 47, 8025–8029 CrossRef CAS PubMed.
- X. Zhou, L. Lesiak, R. Lai, J. R. Beck, J. Zhao, C. G. Elowsky, H. Li and C. I. Stains, Angew. Chem., Int. Ed., 2017, 56, 4197–4200 CrossRef CAS PubMed.
- T. Zhou, Q. Wang, M. Liu, Z. Liu, Z. Zhu, X. Zhao and W. H. Zhu, Aggregate, 2021, 2, e22 CrossRef CAS.
- J. H. Kim, P. Verwilst, M. Won, J. Lee, J. L. Sessler, J. Han and J. S. Kim, J. Am. Chem. Soc., 2021, 143, 14115–14124 CrossRef CAS PubMed.
- W. Wang, Q. Yang, Y. Du, X. Zhou, X. Du, Q. Wu, L. Lin, Y. Song, F. Li, C. Yang and W. Tan, Angew. Chem., Int. Ed., 2020, 59, 2628–2633 CrossRef CAS PubMed.
- D. Liu, Z. He, Y. Zhao, Y. Yang, W. Shi, X. Li and H. Ma, J. Am. Chem. Soc., 2021, 143, 17136–17143 CrossRef CAS PubMed.
- H. Lin, S. Gao, C. Dai, Y. Chen and J. Shi, J. Am. Chem. Soc., 2017, 139, 16235–16247 CrossRef CAS PubMed.
- G. S. Hong, A. L. Antaris and H. J. Dai, Nat. Biomed. Eng., 2017, 1, 0010 CrossRef CAS.
- C. S. L. Rathnamalala, J. N. Gayton, A. L. Dorris, S. A. Autry, W. Meador, N. I. Hammer, J. H. Delcamp and C. N. Scott, J. Org. Chem., 2019, 84, 13186–13193 CrossRef CAS PubMed.
- Y. Shi, W. Yuan, Q. Liu, M. Kong, Z. Li, W. Feng, K. Hu and F. Li, ACS Mater. Lett., 2019, 1, 418–424 CrossRef CAS.
- J. Qi, J. Li, R. Liu, Q. Li, H. Zhang, J. W. Y. Lam, R. T. K. Kwok, D. Liu, D. Ding and B. Z. Tang, Chem, 2019, 5, 2657–2677 CAS.
- M. Shi, Z. Fu, W. Pan, Y. Chen, K. Wang, P. Zhou, N. Li and B. Tang, Angew. Chem., Int. Ed., 2021, 60, 13564–13568 CrossRef CAS PubMed.
- H. Gao, X. Duan, D. Jiao, Y. Zeng, X. Zheng, J. Zhang, H. Ou, J. Qi and D. Ding, Angew. Chem., Int. Ed., 2021, 60, 21047–21055 CrossRef CAS PubMed.
- Y. Wu, S. Huang, J. Wang, L. Sun, F. Zeng and S. Wu, Nat. Commun., 2018, 9, 3983 CrossRef PubMed.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- Y. Y. Zhao, L. Zhang, Z. Chen, B. Y. Zheng, M. Ke, X. Li and J. D. Huang, J. Am. Chem. Soc., 2021, 143, 13980–13989 CrossRef CAS PubMed.
- C. J. Reinhardt, E. Y. Zhou, M. D. Jorgensen, G. Partipilo and J. Chan, J. Am. Chem. Soc., 2018, 140, 1011–1018 CrossRef CAS PubMed.
- Z. He, D. Liu, Y. Liu, X. Li, W. Shi and H. Ma, Anal. Chem., 2022, 94, 10256–10262 CrossRef CAS PubMed.
- A. Ramesh, S. Kumar, A. Brouillard, D. Nandi and A. Kulkarni, Adv. Mater., 2020, 32, e2000648 CrossRef PubMed.
- M. Nighot, R. Al-Sadi, S. Guo, M. Rawat, P. Nighot, M. D. Watterson and T. Y. Ma, Am. J. Pathol., 2017, 187, 2698–2710 CrossRef CAS PubMed.
- G. Kolios, V. Valatas and S. G. Ward, Immunology, 2004, 113, 427–437 CrossRef CAS PubMed.
- P. Wang, L. Yu, J. Gong, J. Xiong, S. Zi, H. Xie, F. Zhang, Z. Mao, Z. Liu and J. S. Kim, Angew. Chem., Int. Ed., 2022, 61, e202206894 CAS.
- F. Leng and P. Edison, Nat. Rev. Neurol., 2021, 17, 157–172 CrossRef PubMed.
- A. Guerrero, B. De Strooper and I. L. Arancibia-Carcamo, Trends Neurosci., 2021, 44, 714–727 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional experimental details and data. CCDC 2119838, 2119842 and 2132279. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc00193h |
| ‡ Q. X. and Y. Z. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |