Open Access Article
Open Access ArticleA Pd-catalyzed highly selective three-component protocol for trisubstituted allenes†
Can
Li
ab,
Zhengnan
Zhou
ab and
Shengming
Ma
 *ac
*ac
aState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, P. R. China. E-mail: masm@sioc.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
cResearch Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai 200433, P. R. China
First published on 22nd June 2023
Abstract
Herein we report the first example of a Pd-catalyzed highly selective three-component reaction of alkynyl-1,4-diol dicarbonates, organoboronic acids, and malonate anions for the efficient synthesis of trisubstituted 2,3-allenyl malonates not readily available by the known protocols. The reaction demonstrates an excellent regio- and chemo-selectivity for both the oxidative addition referring to the two C–O bonds and the subsequent coupling with the nucleophile with a remarkable functional group compatibility. A series of control experiments confirm a unique mechanism involving β-O elimination forming alka-1,2,3-triene and the subsequent insertion of its terminal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond into the Ar–Pd bond.
C bond into the Ar–Pd bond.
Introduction
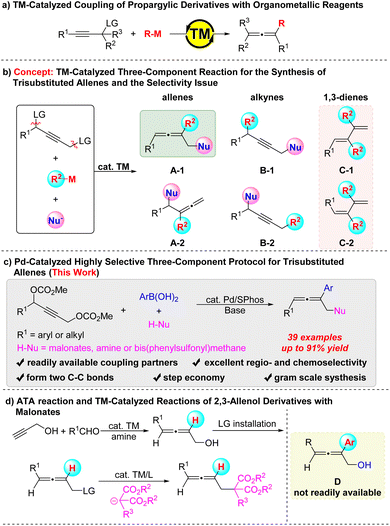
Allenes have attracted significant attention1 in the fields of natural products,2 modern organic synthesis,3,4 medicinal chemistry,5 and materials science,6 and thus, much attention has been paid to the development of new methodologies for the syntheses of allenes from readily available starting chemicals.7 Among them, a two-component reaction between readily available alkynes with an appropriate propargylic leaving group and organometallic reagents has been developed as a straightforward method for the syntheses of allenes8 (Scheme 1a). We envisioned a new concept of Pd-catalyzed reaction of readily available 2-alkynyl-1,4-diol dicarbonate with an organometallic reagent and a nucleophile for allene synthesis (Scheme 1b). As we know the Pd-catalyzed reaction of 2-alkynyl-1,4-diol dicarbonate with organometallic reagents would afford the double coupling products, 1,3-dienes C.9 Thus, the challenges of this strategy are (1) the regioselectivity of the oxidative additions of the two different C–O bonds in the dicarbonate; (2) the selectivity issue for the formation of different allenes A-1 or A-2, alkynes B-1 or B-2, or 1,3-conjugated dienes C-1 or C-2 (Scheme 1b). Herein, we report our realization of the first example of a Pd-catalyzed three-component reaction of 2-alkynyl-1,4-diol dicarbonates with organoboronic acids and malonates, affording trisubstituted allenes 4 (ref. 10 and 11) exclusively, enjoying an excellent regio- and chemo-selectivity with an unprecedented mechanism (Scheme 1c). Such A-1-type allenyl malonates have been demonstrated as highly versatile building blocks for natural allene product synthesis12 and are traditionally prepared via the transition metal catalyzed reactions of 2,3-allenol derivatives with malonates.13,14 Although the precursors for 2,4-disubstituted 2,3-allenol derivatives, 2,3-allenols, are conveniently available via allenation of the propargyl alcohol (ATA) with aldehydes15,16 (Scheme 1d), the current method is highly efficient and diverse due to the readily availability of the three starting materials and irreplaceable due to the inaccessibility of D-type allenols via the ATA reaction.Results and discussion
At the outset, we examined the reaction of 2-alkynyl-1,4-diol dicarbonate 1a, phenylboronic acid 2a, and dimethyl malonate 3a in the presence of [Pd(allyl)Cl]2, SPhos (L1),9a and K2CO3 (Table 1, entry 1) at room temperature in THF for 22 h. To our delight, the allene product 4aaa was obtained albeit in only 36% yield with 56% yield of an unexpected by-product, 2,3-allenyl methyl ether 5aa. Other allenes A-2, alkynes B-1 and B-2, or conjugated dienes C-1 and C-2 were not detected, indicating exclusive chemo- and regio-selectivity. It is quite surprising to note that the oxidative addition occurred with the sterically more crowded secondary C–O bond. The study on the ligand effect indicated that although L2 or L3 led to a better yield for allene product 4aaa, a small amount of dicarbonate 1a was also recovered (Table 1, entries 2 and 3). No reaction occurred with bulkier ligand L4 (Table 1, entry 4). Since Gorlos-Phos (L5·HBF4) and LB-Phos (L6·HBF4)17 were fully ineffective for this reaction (Table 1, entries 5 and 6), L1 was applied for further screening. The base was proven to be essential (Table 1, entry 7) and slightly increasing the loading of K2CO3 would improve the yield of 4aaa to 41% (Table 1, entry 8). By running the reaction at 50 °C, the yield of 4aaa could be improved to 77% together with 15% of 2,3-allenyl methyl ether 5aa (Table 1, entries 9–11). Solvent screening led to the observation that DCE was the best furnishing product 4aaa in 88% yield (Table 1, entries 12–16). A reaction with 1.0 mmol furnished a slightly better result with 4aaa being isolated in 91% yield (Table 1, entry 17).| Entry | Ligand | Solvent | Temp. | Yield of 4aaa/1a recovered/yield of 5aab % |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (1.0 equiv.), 3a (1.5 equiv.), [Pd(allyl)Cl]2 (2.5 mol%), ligand (12 mol%), and base in solvent unless otherwise noted. b Determined by 1H NMR analysis of the crude product using CH2Br2 as the internal standard. c The reaction was run in the absence of K2CO3. d 2.0 equiv. of K2CO3 were used. e The reaction was run for 24 h. f The reaction was carried out on a 1 mmol scale. g Isolated yield. | ||||
| 1 | L1 | THF | r.t. | 36/0/56 |
| 2 | L2 | THF | r.t. | 49/10/24 |
| 3 | L3 | THF | r.t. | 48/23/12 |
| 4 | L4 | THF | r.t. | 0/71/0 |
| 5 | L5·HBF4 | THF | r.t. | 0/90/0 |
| 6 | L5·HBF4 | THF | r.t. | 0/84/0 |
| 7c | L1 | THF | r.t. | 0/99/0 |
| 8d | L1 | THF | r.t. | 41/0/53 |
| 9d | L1 | THF | 30 °C | 46/0/49 |
| 10d | L1 | THF | 40 °C | 64/0/26 |
| 11d | L1 | THF | 50 °C | 77/0/15 |
| 12d,e | L1 | DCE | 50 °C | 88/0/0 |
| 13d,e | L1 | Toluene | 50 °C | 32/0/53 |
| 14d,e | L1 | EtOAc | 50 °C | 83/0/4 |
| 15d,e | L1 | CH3CN | 50 °C | 69/0/0 |
| 16d,e | L1 | 1,4-Dioxane | 50 °C | 80/0/0 |
| 17d,e,f | L1 | DCE | 50 °C | 94 (91)g/0/0 |

|
||||
After establishing the optimal conditions, we turned to investigate the scope of 2-alkynyl-1,4-diol dicarbonates 1 (Table 2). The reaction could be applied to a wide range of substrates 1 bearing either electron-poor (1c–1f) or electron-rich (1g–1h) aryl groups (R1) generating the corresponding allene products 4 in 67–88% yields. Interestingly, ortho-fluoro (1i) and meta-fluoro (1j) substituted substrates were accommodated to afford the multi-functionalized allene products 4iaa and 4jaa. Delightedly, commercial drug adapalene derived 2-alkynyl-1,4-diol dicarbonate 1k could also afford the desired allene 4kaa in an excellent yield. Moreover, R1 may also be an alkyl group (4laa–4naa). Next, the substrate scope of the organoboronic acid 2 was studied. Obviously, the electronic nature of the organoboronic acids has a limited impact on the reactivity affording the allene products 4aba–4aka in up to 90% yields. Bioactive molecules, such as ibuprofen and estrone, modified organoboronic acids could also be applied, delivering products 4ala and 4ama in moderate yields. The practicality was demonstrated by the gram scale reaction of 1a with 2a and 3a affording 3.5 g of product 4aaa in 90% yield under 50 °C for 24 h. The structure of 4aaa was further confirmed by single-crystal X-ray diffraction.18
| a Unless otherwise indicated, the reaction was performed with 1 mmol of 1, 1.0 equiv. of 2, 1.5 equiv. of 3, 6 or 8, 2.5 mol% of [Pd(allyl)Cl]2, 12 mol% of SPhos, and 2.0 equiv. of K2CO3 in DCE (0.1 M) at 50 °C for 24 h. Yields of isolated products are given. b 5 mol% of [Pd(allyl)Cl]2 and 24 mol% of SPhos were used. The reaction was run for 48 h. c 3.5 mol% of [Pd(allyl)Cl]2 and 15 mol% of SPhos were used. The reaction was run for 36 h. d 3.5 mol% of [Pd(allyl)Cl]2, 15 mol% of SPhos, and 2.0 equiv. of 3a were used. The reaction was run for 36 h. e The reaction was run for 36 h. f 3.5 mol% of [Pd(allyl)Cl]2 and 15 mol% of SPhos were used. The reaction was run for 48 h. g 2.0 equiv. of Cs2CO3 were used instead of K2CO3. The reaction was run for 48 h. h The reaction was performed with 1 mmol of 1, 1.0 equiv. of 2a, 2 equiv. of 3, 3.5 mol% of [Pd(allyl)Cl]2, 15 mol% of SPhos, and 2.0 equiv. of Cs2CO3 in DCE (0.1 M) at 50 °C for 48 h. |
|---|

|
With 2-substituted malonates 3, K2CO3 should be replaced with Cs2CO3 for complete transformation obviously due to the steric effect. Methyl, benzyl, allyl and 2,3-allenyl groups may be introduced into the 2-position of malonates to prepare allenes 4aab–4aac, 1,6-vinylallene 4aad, and bisallene 4aae in moderate to excellent yields. The protocol also works for 2-acetamidomalonate 3f and 2-fluoromalonate 3g. Besides, bis(phenylsulfonyl)methane 6a and dibenzylamine 8a19 could also serve as suitable nucleophiles to deliver the desired products 7 and 9 in moderate to high yields (42–73%). Diallylamine 8b could provide the desired product 9aab in 16% yield with 44% recovery of 1a. Other amines such as tetrahydroisoquinoline, morpholine, pyrrolidine, N-allylmethylamine and benzylamine were also studied; however, no corresponding allenyl amine products were obtained.
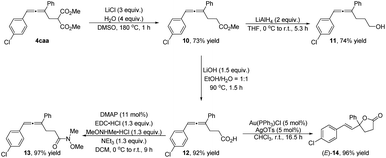
To demonstrate the synthetic potential of this methodology, synthetic transformations of 4caa have been demonstrated (Scheme 2): firstly, Krapcho decarboxylation was achieved, affording 4,5-allenoate 10 in 73% yield;20 reduction of 10 with LiAlH4 formed 4,5-allenol 11 in 74% yield; 4,5-allenoic acid 12 could be yielded by its treatment with LiOH; next, Weinreb amide 13 could be obtained in high yield by using N,O-dimethylhydroxylamine hydrochloride; gold(I)-catalyzed butyrolactone (E)-14 in 96% yield with an exclusive E-selectivity.21
To gain insight into the mechanism, a couple of control experiments were performed (Scheme 3): at first, the reaction of 2-alkynyl-1,4-diol dicarbonate 1a with 1 equiv. of phenylboronic acid 2a under the standard conditions for 24 h afforded 2,3-allenyl methyl ether 5aa in 81% yield (Scheme 3(a)); by increasing the loading of 2a to 2 equiv., the conjugated diene 16 was obtained in 36% yield (E/Z = 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) together with 60% yield of allenylic ether 5aa (Scheme 3(b)); a control experiment showed that only 4% yield (E/Z = 1
1) together with 60% yield of allenylic ether 5aa (Scheme 3(b)); a control experiment showed that only 4% yield (E/Z = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of conjugated diene 16 was formed when 2 equiv. of 2a were applied under the standard reaction conditions together with allenylic malonate 4aaa being formed in 90% yield (Scheme 3(c)); interestingly, when 1a was treated with phenylboronic acid 2a (1 equiv.) for 3 h, ether 5aa was already formed in 79% NMR yield. The subsequent addition of malonate 3a (1.5 equiv.) led to the formation of ether 5aa in 73% yield, exclusively (Scheme 3(d)); when the reaction of 2-alkynyl-1,4-diol dicarbonate 1a and malonate 3a was conducted in the absence of phenylboronic acid 2a, no products were observed with complete recovery of 1a, indicating the importance of phenylboronic acid for the transformation (Scheme 3(e)). Furthermore, the reaction of ether 5aa with 1.5 equiv. of 3a under the standard conditions failed to afford the product 4aaa, indicating that 2,3-allenyl methyl ether 5aa is certainly not the intermediate for the formation of 4aaa (Scheme 3(f)). We then deliberately prepared the envisioned intermediate, methyl 2,3-allenyl carbonate 15. Its reaction under the standard conditions afforded 6% yield of 1,3-enyne 17 with 81% recovery (Scheme 3(g)); the reaction of allenylic carbonate 15 with malonate 3a under the standard conditions in 24 h afforded 4aaa in a lower yield of 62% without the formation of 1,3-enyne 17 or allenylic ether 5aa, as compared with the three-component reaction (Scheme 3(h)). The reaction of 1a in the presence of phenylboronic acid 2a and malonate 3a was also monitored and the formation of 15 was not observed during the whole reaction time (Scheme 3(i)). These experiments (Scheme 3(g)–(i)) indicated that 15 is not the intermediate product for the formation of either 4aaa or ether 5aa under the current reaction conditions.
1) of conjugated diene 16 was formed when 2 equiv. of 2a were applied under the standard reaction conditions together with allenylic malonate 4aaa being formed in 90% yield (Scheme 3(c)); interestingly, when 1a was treated with phenylboronic acid 2a (1 equiv.) for 3 h, ether 5aa was already formed in 79% NMR yield. The subsequent addition of malonate 3a (1.5 equiv.) led to the formation of ether 5aa in 73% yield, exclusively (Scheme 3(d)); when the reaction of 2-alkynyl-1,4-diol dicarbonate 1a and malonate 3a was conducted in the absence of phenylboronic acid 2a, no products were observed with complete recovery of 1a, indicating the importance of phenylboronic acid for the transformation (Scheme 3(e)). Furthermore, the reaction of ether 5aa with 1.5 equiv. of 3a under the standard conditions failed to afford the product 4aaa, indicating that 2,3-allenyl methyl ether 5aa is certainly not the intermediate for the formation of 4aaa (Scheme 3(f)). We then deliberately prepared the envisioned intermediate, methyl 2,3-allenyl carbonate 15. Its reaction under the standard conditions afforded 6% yield of 1,3-enyne 17 with 81% recovery (Scheme 3(g)); the reaction of allenylic carbonate 15 with malonate 3a under the standard conditions in 24 h afforded 4aaa in a lower yield of 62% without the formation of 1,3-enyne 17 or allenylic ether 5aa, as compared with the three-component reaction (Scheme 3(h)). The reaction of 1a in the presence of phenylboronic acid 2a and malonate 3a was also monitored and the formation of 15 was not observed during the whole reaction time (Scheme 3(i)). These experiments (Scheme 3(g)–(i)) indicated that 15 is not the intermediate product for the formation of either 4aaa or ether 5aa under the current reaction conditions.
Based on these experimental mechanistic studies, we proposed a plausible mechanism as shown in Scheme 4: initially, the oxidative addition occurred unexpectedly with the sterically more crowded secondary C–O bond exclusively to afford the allenyl palladium species Int-I rather than its regioisomer Int-I′, most probably due to the steric effect in these two intermediates. Int-I would undergo transmetalation exclusively with boronic acid 2a to generate the intermediate Int-II. Int-II would prefer β-O elimination over reductive elimination, affording 1,2,3-butatriene-coordinated palladium intermediate Int-III. Reductive elimination could be ruled out by the monitoring experiment showing that the formation of 15 was not observed during the whole reaction time. Subsequently, highly regioselective insertion of the terminal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of the 1,2,3-butatriene into Ph-PdLnOMe formed methylene-π-allyl Pd species Int-IV. After deprotonation of malonate 3a and nucleophilic attack, product allene 4aaa was afforded and the catalytically active Pd(0) species was regenerated, finishing the catalytic cycle. In the absence of malonate, reductive elimination would afford the methyl ether 5aa.
C bond of the 1,2,3-butatriene into Ph-PdLnOMe formed methylene-π-allyl Pd species Int-IV. After deprotonation of malonate 3a and nucleophilic attack, product allene 4aaa was afforded and the catalytically active Pd(0) species was regenerated, finishing the catalytic cycle. In the absence of malonate, reductive elimination would afford the methyl ether 5aa.
Conclusions
In conclusion, a novel protocol to synthesize trisubstituted allenes from 2-alkynyl-1,4-diol dicarbonate with organoboronic acid and malonate has been developed. The method utilizes readily available coupling partners, affording trisubstituted double functionalized allenes in an excellent regio- and chemo-selectivity while enjoying a broad substrate scope and step-economy. It should be noted that such 2,2,4-trisubstituted allenyl malonates require lengthy steps via known protocols (see Scheme S1†). A unique mechanism involving β-O elimination forming 1,2,3-alkatrienes has been proposed. This reaction provides a new entity for the syntheses of multiple substituted allenes. Further studies, including the asymmetric version of this reaction, are being conducted in our laboratory.Data availability
All experimental data and detailed procedures are available in the ESI.†Author contributions
S. M. directed the research and developed the concept of the reaction with C. L., who also performed most of the experiments and prepared the ESI. Z. Z. also performed some of the experiments. C. L. and S. M. checked the experimental data. C. L. and S. M. wrote the manuscript with contributions from the other authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 21988101) and National Key R&D Program of China (No. 2021YFA1500100) is greatly appreciated. We also thank Weiyi Wang in our group for reproducing the results of 4baa, 4laa, and 4aab presented in Table 2.References
- For a recent monograph on the chemistry of allenes, see: Modern Allene Chemistry, ed. N. Krause and A. S. K. Hashmi, Wiley-VCH, Weinheim, 2004, vol. 1 and 2 Search PubMed.
- For a review on allenic natural products, see: A. Hoffmann-Roder and N. Krause, Angew. Chem., Int. Ed., 2004, 43, 1196 CrossRef PubMed.
- For selected reviews on the synthetic applications of allenes, see: (a) S. Ma, Acc. Chem. Res., 2003, 36, 701 CrossRef CAS PubMed; (b) S. Ma, Chem. Rev., 2005, 105, 2829 CrossRef PubMed; (c) M. Brasholz, H. U. Reissig and R. Zimmer, Acc. Chem. Res., 2009, 42, 45 CrossRef CAS PubMed; (d) S. Ma, Acc. Chem. Res., 2009, 42, 1679 CrossRef CAS PubMed; (e) S. Yu and S. Ma, Angew. Chem., Int. Ed., 2012, 51, 3074 CrossRef CAS PubMed; (f) T. Lu, Z. Lu, Z. X. Ma, Y. Zhang and R. P. Hsung, Chem. Rev., 2013, 113, 4862 CrossRef CAS; (g) F. Lopez and J. L. Mascarenas, Chem. Soc. Rev., 2014, 43, 2904 RSC; (h) J. Ye and S. Ma, Acc. Chem. Res., 2014, 47, 989 CrossRef CAS PubMed; (i) L. Liu, R. M. Ward and J. M. Schomaker, Chem. Rev., 2019, 119, 12422 CrossRef CAS PubMed; (j) J. M. Alonso and P. Almendros, Chem. Rev., 2021, 121, 4193 CrossRef CAS PubMed.
- For selected reviews on the synthesis of allenes, see: (a) J. Tsuji and T. Mandai, Angew. Chem., Int. Ed., 1995, 34, 2589 CrossRef CAS; (b) N. Krause and A. Hoffmann-Röder, Tetrahedron, 2004, 60, 11671 CrossRef CAS; (c) K. Brummond and J. DeForrest, Synthesis, 2007, 2007, 795 CrossRef; (d) M. Ogasawara, Tetrahedron: Asymmetry, 2009, 20, 259 CrossRef CAS; (e) S. Yu and S. Ma, Chem. Commun., 2011, 47, 5384 RSC; (f) R. K. Neff and D. E. Frantz, ACS Catal., 2014, 4, 519 CrossRef CAS; (g) J. Ye and S. Ma, Org. Chem. Front., 2014, 1, 1210 RSC; (h) W. Chu, Y. Zhang and J. Wang, Catal. Sci. Technol., 2017, 7, 4570 RSC; (i) L. Fu, S. Greßies, P. Chen and G. Liu, Chin. J. Chem., 2020, 38, 91 CrossRef CAS.
- For pharmacologically active allenes, see: (a) P. W. Collins and S. W. Djuric, Chem. Rev., 1993, 93, 1533 CrossRef CAS; (b) P. H. Nelson, E. Eugui, C. C. Wang and A. C. Allison, J. Med. Chem., 1990, 33, 833 CrossRef CAS PubMed; (c) C. Souli, N. Avlonitis, T. Calogeropoulou, A. Tsotinis, G. Maksay, T. Bíró, A. Politi, T. Mavromoustakos, A. Makriyannis, H. Reis and M. Papadopoulos, J. Med. Chem., 2005, 48, 5203 CrossRef CAS PubMed.
- For a review on the development of allene-containing functional materials, see: P. Rivera-Fuentes and F. Diederich, Angew. Chem., Int. Ed., 2012, 51, 2818 CrossRef CAS PubMed.
- For selected recent reports on the synthesis of allenes, see: (a) F. Ye, M. L. Hossain, Y. Xu, X. Ma, Q. Xiao, Y. Zhang and J. Wang, Chem.–Asian J., 2013, 8, 1404 CrossRef CAS PubMed; (b) Y. Tang, J. Xu, J. Yang, L. Lin, X. Feng and X. Liu, Chem, 2018, 4, 1658 CrossRef CAS; (c) N. J. Adamson, H. Jeddi and S. J. Malcolmson, J. Am. Chem. Soc., 2019, 141, 8574 CrossRef CAS PubMed; (d) L. Bayeh-Romero and S. L. Buchwald, J. Am. Chem. Soc., 2019, 141, 13788 CrossRef CAS PubMed; (e) F. Zhong, Q. Xue and L. Yin, Angew. Chem., Int. Ed., 2020, 59, 1562 CrossRef CAS PubMed; (f) Y. Zeng, M. Chiou, X. Zhu, J. Cao, D. Lv, W. Jian, Y. Li, X. Zhang and H. Bao, J. Am. Chem. Soc., 2020, 142, 18014 CrossRef CAS PubMed; (g) Y. Xie, X. Yang, J. Xu, H. Chai, H. Liu, J. Zhang, J. Song, Y. Gao, Z. Jin and Y. Robin Chi, Angew. Chem., Int. Ed., 2021, 60, 14817 CrossRef CAS PubMed; (h) J. Sheng Ng and T. Hayashi, Angew. Chem., Int. Ed., 2021, 60, 20771 CrossRef PubMed; (i) Y. Song, C. Fu and S. Ma, ACS Catal., 2021, 11, 10007 CrossRef CAS; (j) L. Yang, J. Ouyang, H. Zou, S. Zhu and Q. Zhou, J. Am. Chem. Soc., 2021, 143, 6401 CrossRef CAS PubMed; (k) S. Yang, Y. Wang, W. Zhao, G. Lin and Z. He, J. Am. Chem. Soc., 2021, 143, 7285 CrossRef CAS PubMed; (l) M. Plaza, J. Großkopf, S. Breitenlechner, C. Bannwarth and T. Bach, J. Am. Chem. Soc., 2021, 143, 11209 CrossRef CAS PubMed; (m) W. Chen, C. Jiang, J. Zhang, J. Xu, L. Xu, X. Xu, J. Li and C. Cui, J. Am. Chem. Soc., 2021, 143, 12913 CrossRef CAS PubMed; (n) T. J. O'Connor, B. K. Mai, J. Nafie, P. Liu and F. D. Toste, J. Am. Chem. Soc., 2021, 143, 13759 CrossRef PubMed; (o) R. Lu, T. Yang, X. Chen, W. Fan, P. Chen, Z. Lin and G. Liu, J. Am. Chem. Soc., 2021, 143, 14451 CrossRef CAS PubMed; (p) Y. Wang, S. G. Scrivener, X. Zuo, R. Wang, P. N. Palermo, E. Murphy, A. C. Durham and Y. Wang, J. Am. Chem. Soc., 2021, 143, 14998 CrossRef CAS PubMed; (q) S. A. Gonsales, Z. C. Mueller, F. Zhao, P. H. S. Paioti, L. Karmazin, J. Wan, F. Liu, K. N. Houk and A. H. Hoveyda, J. Am. Chem. Soc., 2021, 143, 20640 CrossRef CAS PubMed; (r) B. S. Schreib, M. Son, F. A. Aouane, M. Baik and E. M. Carreira, J. Am. Chem. Soc., 2021, 143, 21705 CrossRef CAS PubMed; (s) F. Zhang, X. Guo, X. Zeng and Z. Wang, Angew. Chem., Int. Ed., 2022, 61, e202117114 CAS; (t) X. Xu, M. Wang, L. Peng and C. Guo, J. Am. Chem. Soc., 2022, 144, 21022 CrossRef CAS PubMed; (u) Q. Lin, S. Zheng, L. Chen, J. Wu, J. Li, P. Liu, S. Dong, X. Liu, Q. Peng and X. Feng, Angew. Chem., Int. Ed., 2022, 61, e202203650 CAS; (v) C. Xu, J. P. Tassone, B. Q. Mercado and J. A. Ellman, Angew. Chem., Int. Ed., 2022, 61, e202202364 CAS; (w) G. Wang, L. Li, Y. Jiang, X. Zhao, X. Ban, T. Shao, Y. Yin and Z. Jiang, Angew. Chem., Int. Ed., 2023, 62, e202214838 CAS.
- For selected reports on the synthesis of allenes via TM-catalyzed coupling of propargylic derivatives with organometallic reagents, see: (a) H. Ito, Y. Sasaki and M. Sawamura, J. Am. Chem. Soc., 2008, 130, 15774 CrossRef CAS PubMed; (b) M. Guisán-Ceinos, V. Martín-Heras and M. Tortosa, J. Am. Chem. Soc., 2017, 139, 8448 CrossRef PubMed; (c) Q. Lu, S. Greßies, F. J. R. Klauck and F. Glorius, Angew. Chem., Int. Ed., 2017, 56, 6660 CrossRef CAS PubMed; (d) Y. Xu, H. Yi and M. Oestreich, Organometallics, 2021, 40, 2194 CrossRef CAS; (e) D. Posevins, A. Bermejo-López and J. Bäckvall, Angew. Chem., Int. Ed., 2021, 60, 22178 CrossRef CAS PubMed; (f) O. Bernardo, S. González-Pelayo, I. Fernández and L. A. López, Angew. Chem., Int. Ed., 2021, 60, 25258 CrossRef CAS PubMed; (g) H. Wang, H. Qian, J. Zhang and S. Ma, J. Am. Chem. Soc., 2022, 144, 12619 CrossRef CAS PubMed; (h) W. Wang, Y. Wu, H. Wang, P. Qi, W. Lan and Q. Zhang, Nat. Synth., 2022, 1, 738 CrossRef.
- (a) N. Nishioka, S. Hayashi and T. Koizumi, Angew. Chem., Int. Ed., 2012, 51, 3682 CrossRef CAS PubMed; (b) S. Hayashi, M. Kasuya, J. Machida and T. Koizumi, Tetrahedron Lett., 2017, 58, 2429 CrossRef CAS; (c) Y. M. Fan, M. J. Sowden, N. L. Magann, E. J. Lindeboom, M. G. Gardiner and M. S. Sherburn, J. Am. Chem. Soc., 2022, 144, 20090 CrossRef CAS PubMed.
- For selected reports on the synthesis of trisubstituted allenes via 1,3-enynes, see: (a) Y. Matsumoto, M. Naito and T. Hayashi, Organometallics, 1992, 11, 2732 CrossRef CAS; (b) C. Lüken and C. Moberg, Org. Lett., 2008, 10, 2505 CrossRef PubMed; (c) J. Zhao, Y. Liu, Q. He, Y. Li and S. Ma, Chem.–Eur. J., 2009, 15, 11361 CrossRef CAS PubMed; (d) Y. Xie, J. Hu, Y. Wang, C. Xia and H. Huang, J. Am. Chem. Soc., 2012, 134, 20613 CrossRef CAS PubMed; (e) H. Qian, X. Yu, J. Zhang and J. Sun, J. Am. Chem. Soc., 2013, 135, 18020 CrossRef CAS PubMed; (f) Y. Huang, J. del Pozo, S. Torker and A. H. Hoveyda, J. Am. Chem. Soc., 2018, 140, 2643 CrossRef CAS PubMed; (g) D. Gao, Y. Xiao, M. Liu, Z. Liu, M. K. Karunananda, J. S. Chen and K. M. Engle, ACS Catal., 2018, 8, 3650 CrossRef CAS PubMed; (h) S. Yu, H. L. Sang, S. Zhang, X. Hong and S. Ge, Commun. Chem., 2018, 1, 64 CrossRef; (i) F. Wang, D. Wang, Y. Zhou, L. Liang, R. Lu, P. Chen, Z. Lin and G. Liu, Angew. Chem., Int. Ed., 2018, 57, 7140 CrossRef CAS PubMed.
- For selected reports on the synthesis of trisubstituted allenes via Pd-catalyzed Heck reaction of alkynes, see: (a) W. Tao, L. Silverberg, A. Rheingold and R. Heck, Organometallics, 1989, 8, 2550 CrossRef CAS; (b) S. Pivsa-Art, T. Satoh, M. Miura and M. Nomura, Chem. Lett., 1997, 26, 823 CrossRef; (c) L. Chapman, B. Adams, L. Kliman, A. Makriyannis and C. Hamblett, Tetrahedron Lett., 2010, 51, 1517 CrossRef CAS; (d) N. Nella, E. Parker, J. Hitce, P. Larini, R. Jazzar and O. Baudoin, Chem.–Eur. J., 2014, 20, 13272 CrossRef CAS PubMed; (e) R. Neff and D. Frantz, J. Am. Chem. Soc., 2018, 140, 17428 CrossRef CAS PubMed; (f) W. Lv, Y. Chen, Z. Zhao, Si. Wen and G. Cheng, Org. Lett., 2019, 21, 7795 CrossRef CAS PubMed; (g) C. Zhu, H. Chu, G. Li, S. Ma and J. Zhang, J. Am. Chem. Soc., 2019, 141, 19246 CrossRef CAS PubMed.
- For selected reports on allenyl malonates utilized as building blocks for natural allene product synthesis, see: (a) X. Tang, X. Huang, T. Cao, Y. Han, X. Jiang, W. Lin, Y. Tang, J. Zhang, Q. Yu, C. Fu and S. Ma, Org. Chem. Front., 2015, 2, 688 RSC; (b) J. Zhou, C. Fu and S. Ma, Nat. Commun., 2018, 9, 1654 CrossRef PubMed; (c) S. Song, J. Zhou, C. Fu and S. Ma, Nat. Commun., 2019, 10, 507 CrossRef PubMed.
- For selected reports on Pd-catalyzed reactions of 2,3-allenol derivatives with malonates, see: (a) Y. Imada, K. Ueno, K. Kutsuwa and S.-I. Murahashi, Chem. Lett., 2002, 31, 140 CrossRef; (b) Y. Imada, M. Nishida, K. Kutsuwa, S. Murahashi and T. Naota, Org. Lett., 2005, 7, 5837 CrossRef CAS PubMed; (c) B. M. Trost, D. R. Fandrick and D. C. Dinh, J. Am. Chem. Soc., 2005, 127, 14186 CrossRef CAS PubMed; (d) T. Nemoto, M. Kanematsu, S. Tamura and Y. Hamada, Adv. Synth. Catal., 2009, 351, 1773 CrossRef CAS; (e) H. Tsukamoto, T. Konno, K. Ito and T. Doi, Org. Lett., 2019, 21, 6811 CrossRef CAS PubMed; (f) H. C. Liu, Y. Z. Hu, Z. F. Wang, H. Y. Tao and C. J. Wang, Chem.–Eur. J., 2019, 25, 8681 CAS.
- For selected reports on Ir-catalyzed reactions of 2,3-allenol derivatives with nucleophiles, see: (a) D. A. Petrone, M. Isomura, I. Franzoni, S. L. Rossler and E. M. Carreira, J. Am. Chem. Soc., 2018, 140, 4697 CrossRef CAS PubMed; (b) Y. Cui, Y. Zhai, J. Xiao, C. Li, W. F. Zheng, C. Huang, G. Wu, A. Qin, J. Lin, Q. Liu, H. Wang, P. Wu, H. Xu, Y. Zheng and S. Ma, Chem. Sci., 2021, 12, 11831 RSC; (c) A. Chakrabarty and S. Mukherjee, Angew. Chem., Int. Ed., 2022, 61, e202115821 CrossRef CAS PubMed.
- (a) J. Kuang and S. Ma, J. Org. Chem., 2009, 74, 1763 CrossRef CAS PubMed; (b) J. Kuang and S. Ma, J. Am. Chem. Soc., 2010, 132, 1786 CrossRef CAS PubMed; (c) X. Tang, C. Zhu, T. Cao, J. Kuang, W. Lin, S. Ni, J. Zhang and S. Ma, Nat. Commun., 2013, 4, 2450 CrossRef PubMed; (d) X. Huang, T. Cao, Y. Han, X. Jiang, W. Lin, J. Zhang and S. Ma, Chem. Commun., 2015, 51, 6956 RSC; (e) Q. Liu, X. Tang, Y. Cai and S. Ma, Org. Lett., 2017, 19, 5174 CrossRef CAS PubMed.
- X. Huang and S. Ma, Acc. Chem. Res., 2019, 52, 1301 CrossRef CAS PubMed.
- (a) B. Lü, P. Li, C. Fu, L. Xue, Z. Lin and S. Ma, Adv. Synth. Catal., 2011, 353, 100 CrossRef; (b) Y. Zheng, B. Miao, A. Qin, J. Xiao, Q. Liu, G. Li, L. Zhang, F. Zhang, Y. Guo and S. Ma, Chin. J. Chem., 2019, 37, 1003 CrossRef CAS; (c) X. Huang, B. Chen, P. Li, D. Ji, J. Liu, H. Zheng, S. Yang, Y. Hu, B. Wan, X. Hu, C. Fu, Y. Huang, J. Zheng, Q. Chen and S. Ma, Nat. Chem., 2022, 14, 1185 CrossRef CAS PubMed.
- Crystal data for 4aaa: C25H22O4, MW = 386.45, triclinic, space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , final R indices [I > 2σ(I)], R1 = 0.0361, wR2 = 0.0911; R indices (all data), R1 = 0.0397, wR2 = 0.0943; a = 8.2186(9) Å, b = 10.7453(13) Å, c = 12.2370(14) Å, α = 115.168(3)°, β = 93.669(4)°, γ = 93.606(4)°, V = 971.20(19) Å3, T = 213(2) K, Z = 2, reflections collected/unique 22564/3771 (Rint = 0.0248). ESI crystallographic data have been deposited at the Cambridge Crystallographic Data Centre, CCDC 2202991.
, final R indices [I > 2σ(I)], R1 = 0.0361, wR2 = 0.0911; R indices (all data), R1 = 0.0397, wR2 = 0.0943; a = 8.2186(9) Å, b = 10.7453(13) Å, c = 12.2370(14) Å, α = 115.168(3)°, β = 93.669(4)°, γ = 93.606(4)°, V = 971.20(19) Å3, T = 213(2) K, Z = 2, reflections collected/unique 22564/3771 (Rint = 0.0248). ESI crystallographic data have been deposited at the Cambridge Crystallographic Data Centre, CCDC 2202991. - B. M. Trost, D. R. Fandrick and D. C. Dinh, J. Am. Chem. Soc., 2005, 127, 14186 CrossRef CAS PubMed.
- A. P. Krapcho, J. F. Weimaster, J. M. Eldridge, E. G. E. Jahngen, A. J. Lovey and W. P. Stephens, J. Org. Chem., 1978, 43, 138 CrossRef CAS.
- (a) A. S. K. Hashmi, T. M. Frost and J. W. Bats, J. Am. Chem. Soc., 2000, 122, 11553 CrossRef CAS; (b) A. S. K. Hashmi, L. Schwarz, J.-H. Choi and T. M. Frost, Angew. Chem., Int. Ed., 2000, 39, 2285 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedures, characterization data, and copies of NMR spectra. CCDC 2202991. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc01849k |
| This journal is © The Royal Society of Chemistry 2023 |