Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Surface engineering on a microporous metal–organic framework to boost ethane/ethylene separation under humid conditions†
Xiao-Jing
Xie
a,
Ying
Wang
 a,
Qi-Yun
Cao
a,
Rajamani
Krishna
a,
Qi-Yun
Cao
a,
Rajamani
Krishna
 b,
Heng
Zeng
b,
Heng
Zeng
 *a,
Weigang
Lu
*a,
Weigang
Lu
 *a and
Dan
Li
*a and
Dan
Li
 a
a
aCollege of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou 510632, P. R. China. E-mail: zengheng90@163.com; weiganglu@jnu.edu.cn
bVan't Hoff Institute for Molecular Sciences, University of Amsterdam, Science Park 904, Amsterdam 1098 XH, Netherlands
First published on 9th October 2023
Abstract
Recently, examples of metal–organic frameworks (MOFs) have been identified displaying ethane (C2H6) over ethylene (C2H4) adsorption selectivity. However, it remains a challenge to construct MOFs with both large C2H6 adsorption capacity and high C2H6/C2H4 adsorption selectivity, especially under humid conditions. Herein, we reported two isoreticular MOF-5 analogues (JNU-6 and JNU-6-CH3) and their potential applications in one-step separation of C2H4 from C2H6/C2H4 mixtures. The introduction of CH3 groups not only reduces the pore size from 5.4 Å in JNU-6 to 4.1 Å in JNU-6-CH3 but also renders an increased electron density on the pyrazolate N atoms of the organic linker. JNU-6-CH3 retains its framework integrity even after being immersed in water for six months. More importantly, it exhibits large C2H6 adsorption capacity (4.63 mmol g−1) and high C2H6/C2H4 adsorption selectivity (1.67) due to the optimized pore size and surface function. Breakthrough experiments on JNU-6-CH3 demonstrate that C2H4 can be directly separated from C2H6/C2H4 (50/50, v/v) mixtures, affording benchmark productivity of 22.06 and 18.71 L kg−1 of high-purity C2H4 (≥99.95%) under dry and humid conditions, respectively.
As one of the seven world-changing chemical separations, olefin/paraffin separation accounts for more than 0.3% of global energy consumption.1 Ethylene (C2H4) is an important chemical feedstock in petrochemical industries, with a global production capacity of over 200 million tons in 2023.2 At present, C2H4 is mainly produced via steam cracking of ethane (C2H6) in industry, which would inevitably leave a certain amount of C2H6 in the obtained C2H4. Given that the C2H6 impurity may interfere with the polymerization process, further purification is required and the polymer-grade (≥99.95%) C2H4 is highly desired in the manufacture of value-added chemicals. Owing to their very similar physicochemical properties and molecular sizes (3.81 × 4.08 × 4.82 Å3 and 3.28 × 4.18 × 4.84 Å3 for C2H6 and C2H4, respectively), the industrial C2H4/C2H6 separation relies on cryogenic distillation, which is energy intensive and requires high distillation towers with many trays in order to achieve high reflux ratios.3
Compared to traditional distillation, non-thermal separation technologies using porous materials are of great significance to energy-efficient separation economy.4,5 Metal–organic frameworks (MOFs), also known as porous coordination polymers (PCPs),6–8 have been extensively investigated in hydrocarbon separation due to their highly tunable pore geometry and surface chemistry. With regard to C2H4/C2H6 separation, MOFs can be categorized into two types: C2H6-selective and C2H4-selective. For C2H4-selective MOFs, desorption by heat or purge is necessary in order to obtain C2H4, which likely would result in C2H6 contamination. For example, the benchmark C2H4/C2H6 sieving MOF, UTSA-280,9 can realize complete exclusion of large-sized C2H6 molecules and an infinite C2H4 over C2H6 selectivity, yet C2H4 with only 99.1% purity was reported upon desorption.
By contrast, C2H6-selective MOFs allow for direct production of C2H4 in a single adsorption step, which could potentially save ca. 40% of energy consumption (0.4 to 0.6 GJ ton−1 of C2H4) for C2H4/C2H6 separation.10 Considering the numbers of hydrogen atoms on the surface of C2H6 and C2H4 molecules (6 vs. 4), controlled surface engineering with polar functions (e.g., N- and O-containing groups) on the pore walls may facilitate non-classic hydrogen bonding and stronger affinity toward C2H6 than C2H4.5,11–16 Nevertheless, water vapor may compete for the interactions with those polar functional groups, leading to substantially reduced separation potential under humid conditions. For example, the benchmark C2H6-selective MOF, Fe2(O2)(dobdc),17 demonstrated an excellent C2H6 over C2H4 selectivity with a record separation factor of ca. 4.4. The material itself, however, is extremely sensitive to moisture and has to be handled in a glove box. Recent studies show that nonpolar pore environments can prevent moisture from entering inside the frameworks and therefore retain the C2H4/C2H6 separation potential even under humid conditions. More importantly, nonpolar pore surfaces may still facilitate C2H6 over C2H4 selectivity due to their slightly different polarizability (C2H6: 44.7 × 1025 cm3, C2H4: 42.5 × 1025 cm3). For instance, the MOF FJI-H11-Me-(des),18 featuring nonpolar pore surfaces comprised of aromatic rings and alkyl groups, exhibits a stable C2H6 over C2H4 separation capacity in a wide range of relative humidities (RHs). However, the overall separation potential was limited due to its moderate adsorption capacity for C2H6 (2.58 mmol g−1). Until now, it remains a challenge to construct MOFs with both large C2H6 adsorption capacity and high C2H6 over C2H4 adsorption selectivity to break the adsorption/selectivity trade-off limitation, especially under humid conditions.
Isoreticular chemistry allows for the design and synthesis of MOFs with tailor-made pore dimensions and functions for selective binding of one over the other in C2H4/C2H6 separation. The methyl (CH3) group is electron-donating, and its effect on gas adsorption and separation has been well documented.19–21 In addition, the CH3 group is considered strongly hydrophobic, and the MOF decorated with CH3 groups usually exhibits low water adsorption capacity even at high RH, which could effectively suppress the competition of water vapor for adsorption sites. Herein, we selected Zn4O(PyC)3 (termed here as JNU-6, H2PyC = pyrazole-4-carboxylic acid), an isoreticular MOF-5 analogue,22–24 as the platform for surface functionalization via linker methylation. We found that the introduction of CH3 groups not only reduces the pore size from 5.4 Å in JNU-6 to 4.1 Å in JNU-6-CH3 but also renders an increased electron density on the pyrazolate N atoms of the organic linker. As a result, JNU-6-CH3 retains its framework integrity even after being immersed in water for six months. More importantly, it exhibits large C2H6 adsorption capacity (4.63 mmol g−1) and high C2H6/C2H4 adsorption selectivity (1.67) due to the optimized pore size and surface function. Breakthrough experiments on JNU-6-CH3 demonstrate benchmark productivity of 22.06 and 18.71 L kg−1 of high-purity C2H4 (≥99.95%) from a C2H6/C2H4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) mixture under dry and humid conditions, respectively.
50) mixture under dry and humid conditions, respectively.
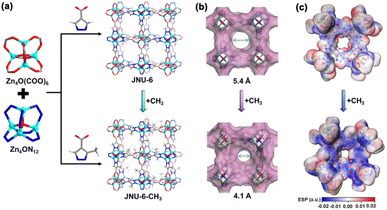
To apply reticular chemistry and address the separation challenge of C2H6/C2H4 under humid conditions, it is crucial to find a C2H6-selective MOF that can be easily functionalized. In this work, we selected an isoreticular analogue of MOF-5 as the platform for the introduction of CH3 groups. A solvothermal reaction of Zn(NO3)2 with pyrazole-4-carboxylic acid or 3-methylpyrazole-4-carboxylic acid in a mixed solution of DEF/H2O afforded high-quality block crystals of JNU-6 and JNU-6-CH3, respectively. Single crystal X-ray diffraction (SCXRD) analyses reveal that JNU-6 and JNU-6-CH3 are of cubic crystal structure isoreticular to MOF-5. It should be pointed out that both JNU-6 and JNU-6-CH3 were reported by Zhong and co-workers recently for n-C4H10/iso-C4H10 separation during our preparation of this paper.25 In the crystal structures, two types of octahedral Zn4O SBUs (secondary building units, Zn4ON12 and Zn4O(COO)6) are connected by ditopic organic linkers to form a 3-dimensional (3D) network with interconnected cubic-shaped cages (Fig. 1a). The introduction of CH3 groups on the pore surface decreases the aperture size from 5.4 Å to 4.1 Å (Fig. 1b), making it more comparable to the kinetic diameters of C2H6 and C2H4 (C2H6 = 4.44 Å, C2H4 = 4.16 Å).26 Density functional theory (DFT) calculations were carried out to generate the mapping of electrostatic potential (ESP) on JNU-6 and JNU-6-CH3. As shown in Fig. 1c, an increased electron density was observed on the pyrazole rings of JNU-6-CH3, particularly around the N atoms, owing to the electron-donating effect of the CH3 groups. Such an electrostatic potential in JNU-6-CH3 indicates an increased surface dipole, which may potentially facilitate the discrimination of C2H6 from C2H4 due to their slightly different polarizability.
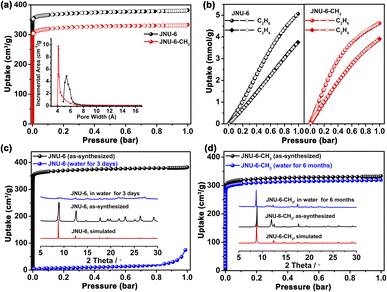
The phase purity and crystallinity of the bulk JNU-6 and JNU-6-CH3 samples were checked by powder X-ray diffraction (PXRD) analyses, showing good agreement with the ones simulated from their respective crystal structures. N2 adsorption/desorption isotherms at 77 K were measured to investigate the porosity of JNU-6 and JNU-6-CH3. As shown in Fig. 2a, both of them exhibit type-I adsorption/desorption isotherms characteristic of microporous materials. Due to the introduction of CH3 groups, the calculated Brunauer–Emmett–Teller (BET) surface area of JNU-6-CH3 is slightly decreased from 1411 m2 g−1 in JNU-6 to 1270 m2 g−1, and the calculated pore volume is also decreased from 0.59 cm3 g−1 in JNU-6 to 0.51 cm3 g−1. Further, the pore size distribution was determined by the Horvath–Kawazoe model and the dominant pore diameters exhibit the same trend, with values decreasing from 5.4 Å in JNU-6 to 4.1 Å in JNU-6-CH3 (Fig. 2a, inset).
Single-component adsorption isotherms of JNU-6 and JNU-6-CH3 for C2H6 and C2H4 were measured at 298 K. As exhibited in Fig. 2b, the C2H6 adsorption capacity is substantially larger than C2H4 in the entire pressure range (0–1 bar) for both JNU-6 and JNU-6-CH3. The maximum uptakes for C2H4 are 84.4 cm3 g−1 (3.77 mmol g−1) and 88.1 cm3 g−1 (3.93 mmol g−1) on JNU-6 and JNU-6-CH3, respectively, while the uptakes for C2H6 can reach up to 113.6 cm3 g−1 (5.07 mmol g−1) and 103.7 cm3 g−1 (4.63 mmol g−1) on JNU-6 and JNU-6-CH3, respectively. The C2H6 uptakes on JNU-6 and JNU-6-CH3 are comparable to or larger than those of most of the C2H6-selective MOFs, such as Cu(Qc)2 (1.84 mmol g−1),27 MUF-15 (4.69 mmol g−1),28 NKMOF-8-Br (4.22 mmol g−1),29 FJI-H11-Me-(des) (2.58 mmol g−1),18 Ni(IN)2 (3.05 mmol g−1),30 AzoleTh-1 (4.47 mmol g−1),31 and NPU-1 (4.5 mmol g−1).32 We applied the ideal adsorbed solution theory (IAST) to calculate the adsorption selectivity, and the IAST selectivity of JNU-6-CH3 for a C2H6/C2H4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) mixture at 298 K can reach up to 1.67 (Fig. S4–S9†), which is comparable to those of the reported benchmark MOF adsorbents, such as MUF-15 (1.96),28 NKMOF-8-Br (2.65),29 NKMOF-8-Me (1.88),29 Ni(IN)2 (2.44),30 AzoleTh-1 (1.46),31 and NPU-1 (1.32).32 Isosteric heat of adsorption (Qst) was calculated by fitting adsorption isotherms at 273, 283, and 298 K using the dual-site Langmuir–Freundlich model (Fig. S10–S19†). At 298 K, the Qst of JNU-6 at zero loading was determined to be 24.0 kJ mol−1and 20.9 kJ mol−1 for C2H6 and C2H4, respectively, while the Qst of JNU-6-CH3 at zero loading was determined to be 24.7 kJ mol−1vs. 23.9 kJ mol−1 for C2H6 and C2H4, respectively. The data confirm the stronger thermodynamic affinity toward C2H6 than C2H4 in both materials. Moreover, the reduced pore size in JNU-6-CH3 may allow for an increased host–guest interaction between the framework and gas molecules, resulting in adsorption affinity stronger than JNU-6 for both C2H6 and C2H4. Meanwhile, the Qst values of both JNU-6 and JNU-6-CH3 are much lower than those of Fe2(O2)(dobdc) (67 kJ mol−1),17 IRMOF-8 (52.5 kJ mol−1),33 PAF-40-Fe (47.8 kJ mol−1),34 Zn-atz-ipa (45.8 kJ mol−1),35 and MAF-49 (60 kJ mol−1).12 The relatively low Qst value may facilitate easy regeneration and low energy consumption during the desorption process, reflecting the advantages of pore surface engineering with nonpolar functional groups. Furthermore, ten continuous adsorptions for C2H6 and C2H4 were carried out on an ASAP2020 gas sorption instrument. As shown in Fig. S20–S23,† both JNU-6 and JNU-6-CH3 retain adsorption capacity over ten cycles, indicating that the samples can be fully regenerated by vacuum at room temperature.
50) mixture at 298 K can reach up to 1.67 (Fig. S4–S9†), which is comparable to those of the reported benchmark MOF adsorbents, such as MUF-15 (1.96),28 NKMOF-8-Br (2.65),29 NKMOF-8-Me (1.88),29 Ni(IN)2 (2.44),30 AzoleTh-1 (1.46),31 and NPU-1 (1.32).32 Isosteric heat of adsorption (Qst) was calculated by fitting adsorption isotherms at 273, 283, and 298 K using the dual-site Langmuir–Freundlich model (Fig. S10–S19†). At 298 K, the Qst of JNU-6 at zero loading was determined to be 24.0 kJ mol−1and 20.9 kJ mol−1 for C2H6 and C2H4, respectively, while the Qst of JNU-6-CH3 at zero loading was determined to be 24.7 kJ mol−1vs. 23.9 kJ mol−1 for C2H6 and C2H4, respectively. The data confirm the stronger thermodynamic affinity toward C2H6 than C2H4 in both materials. Moreover, the reduced pore size in JNU-6-CH3 may allow for an increased host–guest interaction between the framework and gas molecules, resulting in adsorption affinity stronger than JNU-6 for both C2H6 and C2H4. Meanwhile, the Qst values of both JNU-6 and JNU-6-CH3 are much lower than those of Fe2(O2)(dobdc) (67 kJ mol−1),17 IRMOF-8 (52.5 kJ mol−1),33 PAF-40-Fe (47.8 kJ mol−1),34 Zn-atz-ipa (45.8 kJ mol−1),35 and MAF-49 (60 kJ mol−1).12 The relatively low Qst value may facilitate easy regeneration and low energy consumption during the desorption process, reflecting the advantages of pore surface engineering with nonpolar functional groups. Furthermore, ten continuous adsorptions for C2H6 and C2H4 were carried out on an ASAP2020 gas sorption instrument. As shown in Fig. S20–S23,† both JNU-6 and JNU-6-CH3 retain adsorption capacity over ten cycles, indicating that the samples can be fully regenerated by vacuum at room temperature.
To test their water stability, JNU-6 and JNU-6-CH3 were soaked in water for days and then subjected to PXRD and gas adsorption measurements. As shown in Fig. 2c, JNU-6 lost most of the crystallinity and porosity after being soaked in water for three days. In contrast, the crystallinity and structural integrity of JNU-6-CH3 can be well retained after being soaked in water for six months (Fig. 2d). Water vapor adsorption measurements for JNU-6 and JNU-6-CH3 were carried out and both of them show S-shaped adsorption isotherms characteristic of pore filling (Fig. 4b), and the limited water uptake at low pressure suggests that the water affinity on the MOF surface is relatively low. With the linker methylation, higher water vapor pressure is required to induce the pore filling, indicating further increased hydrophobicity of MOF pores from JNU-6 and JNU-6-CH3. Overall, the introduction of CH3 groups renders JNU-6-CH3 with an optimized pore size, increased surface dipole, and improved hydrolytic stability, which prompted us to further study its potential for C2H6/C2H4 separation under humid conditions.
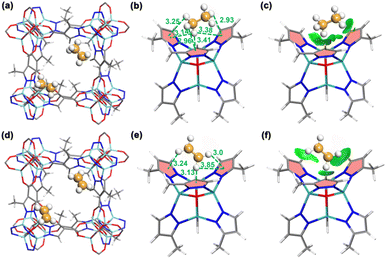
To verify the preferential adsorption of C2H6 over C2H4 on JNU-6 and JNU-6-CH3, we first performed computational modeling studies using grand canonical Monte Carlo (GCMC) simulations.35,36 The simulated C2H6 and C2H4 adsorption isotherms are in good agreement with the experimental ones at 298 K and 1 bar, and the probability density distributions of C2H6 and C2H4 reveal that both C2H6 and C2H4 are preferentially adsorbed at the corners of the cubic-shaped cages in both JNU-6 and JNU-6-CH3 (Fig. S24–S27†). Take JNU-6-CH3 as an example, there are six C–H⋯π interactions between the H atoms of C2H6 and the pyrazole rings of the linkers with H⋯π distances from 2.93 to 3.41 Å. In comparison, there are fewer C–H⋯π interactions between C2H4 and the pyrazole rings of the linkers with H⋯π distances from 3.0 to 3.85 Å (Fig. 3b and e). Further, an independent gradient model based on Hirshfeld partition (IGMH) analysis on the optimized structures was developed. As shown in Fig. 3c and f, multiple green isosurfaces were observed for both C2H6 and C2H4, indicating the existence of vdW interactions between the gas molecules and the three pyrazole rings. The static binding energies (ΔE) for C2H6 on JNU-6 and JNU-6-CH3 were calculated to be 18.04 and 22.23 kJ mol−1, respectively, higher than those for C2H4 (17.22 and 20.15 kJ mol−1). These values further confirm the weak vdW nature of the host–guest interactions between gas molecules and the nonpolar pore surfaces, which favors the adsorption of C2H6 over C2H4.
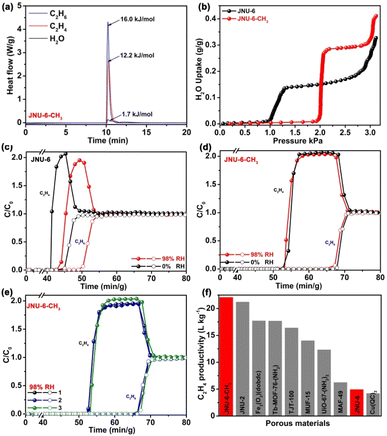
To evaluate the actual separation capability of JNU-6 and JNU-6-CH3 for C2H6/C2H4 mixtures, we first performed dynamic column breakthrough experiments in which a C2H6/C2H4 (50/50, v/v) mixture was introduced over the activated JNU-6 or JNU-6-CH3 at a flow rate of 2 mL min−1 and 298 K. As shown in Fig. 4c, JNU-6 can separate C2H6 from the C2H6/C2H4 mixture with an estimated productivity of 4.92 L kg−1 of high-purity C2H4 (≥99.95%) under dry conditions. Surprisingly, JNU-6-CH3 exhibits significantly improved separation capacity under similar conditions, and the data are in good agreement with the simulated breakthrough curve (Fig. S29†). As shown in Fig. 4d, C2H4 and C2H6 were detected to break through the column at the time points of 52.7 min g−1and 67.9 min g−1, respectively. During the above time period, high-purity C2H4 (≥99.95%) can be collected with an estimated C2H4 productivity of 22.06 L kg−1, which is about four times that of JNU-6 and the highest among those of the reported MOFs under similar conditions, including JNU-2 (21.2 L kg−1),13 Fe2(O2)(dobdc) (17.7 L kg−1),17 Tb-MOF-76-(NH2) (17.66 L kg−1),37 TJT-100 (16.38 L kg−1),38 MUF-15 (14 L kg−1),28 UiO-67-(NH2)2 (12.32 L kg−1),5 MAF-49 (6.23 L kg−1),12 and Cu(Qc)2 (4.0 L kg−1)27 (Fig. 4f).
To further examine the moisture effect on the separation capability for C2H6/C2H4, we performed differential scanning calorimetry (DSC) measurements of heat flow upon introducing water vapor, C2H4, and C2H6 on JNU-6 and JNU-6-CH3. For JNU-6, the experimental Qst for water vapor, C2H4, and C2H6 is 0.2, 10.2, and 15.7 kJ mol−1, respectively (Fig. S38†), while for JNU-6-CH3, the experimental Qst for water vapor, C2H4, and C2H6 is 1.7, 12.2, and 16.0 kJ mol−1, respectively (Fig. 4a), both indicative of significantly stronger binding affinity for C2H6 and C2H4 than for water vapor. This, together with water vapor adsorption measurements (Fig. 4b), suggests that JNU-6-CH3 may be able to maintain the high separation capability for C2H6/C2H4 mixtures under humid conditions. Breakthrough experiments were thus performed on JNU-6 and JNU-6-CH3 for a C2H6/C2H4 (50/50, v/v) mixture under 98% RH conditions. As revealed in Fig. 4c and d, the purity of C2H4 dropped from 99.95% to 99.2% for JNU-6, likely due to its hydrolytic instability (Fig. 2c), whereas the purity of C2H4 remained over 99.95% with only slightly dropped productivity (18.71 L kg−1) for JNU-6-CH3. The results confirm that the introduction of CH3 groups in the framework can indeed improve separation capability, especially under humid conditions. Furthermore, continuous breakthrough experiments under humid conditions were carried out, revealing the retained separation performance of JNU-6-CH3 over three cycles (Fig. 4e and S31†).
To further study the effect of methylation degree on adsorption separation performance, we synthesized JNU-6-(CH3)2 with 3,5-dimethylpyrazole-4-carboxylic acid. JNU-6-(CH3)2 also shows preferential adsorption of C2H6 over C2H4, especially in the low-pressure range (Fig. S33†). However, its C2H6 and C2H4 adsorption amounts at 0.5 bar are almost the same, and the adsorption of C2H6 and C2H4 on JNU-6-(CH3)2 is significantly lower than those on JNU-6-CH3 and JNU-6 in the high-pressure range, likely due to the reduced porosity of JNU-6-(CH3)2 (Fig. S32a†). As a result, dynamic column breakthrough experiments on JNU-6-(CH3)2 reveal a poor separation for a C2H6/C2H4 (50/50, v/v) mixture at a flow rate of 2.0 mL min−1 and 298 K (Fig. S32d†). On the other hand, JNU-6-CF3 was synthesized by using 5-trifluoromethyl-4-carboxylic acid as a ligand. JNU-6-CF3 also shows preferential adsorption of C2H6 over C2H4. The maximum uptake of C2H6 on JNU-6-CF3 is 3.49 mmol g−1 (Fig. S34b†), which is nearly 25% less than that of JNU-6-CH3, likely due to the reduced porosity of JNU-6-CF3 (Fig. S35a†). The water vapor adsorption isotherm of JNU-6-CF3 displays almost no water uptake over the entire pressure range (Fig. S34c†), reflecting its extremely high hydrophobicity. We evaluated dynamic column breakthrough experiments on JNU-6-CF3 for a C2H6/C2H4 (50/50, v/v) mixture at a flow rate of 2.0 mL min−1 and 298 K. As shown in Fig. S34d,† a clean separation of C2H6 from the C2H6/C2H4 mixture can be realized under either dry or 98% RH conditions with no obvious decrease in separation performance. Based on the breakthrough curves, ca. 10.5 L kg−1 of high-purity C2H4 (≥99.95%) can be recovered from the C2H4/C2H6 (50/50) mixture in a single breakthrough operation, which is about half of that of JNU-6-CH3. The results indicate that further increase of methylation degree or introducing more hydrophobic CF3 groups may not be necessarily favorable for the C2H6/C2H4 separation, and both adsorption capacity and adsorption selectivity have to be considered to achieve high separation efficiency.
In summary, we have successfully demonstrated a surface engineering strategy to boost the separation potential of C2H4 from C2H6/C2H4 mixtures under either dry or humid conditions. The introduction of CH3 groups on an isoreticular MOF-5 analogue (JNU-6) renders the obtained JNU-6-CH3 with enhanced hydrolytic stability and a more suitable pore environment for C2H6/C2H4 separation. JNU-6-CH3 retains its framework integrity even after being immersed in water for six months, and it exhibits large C2H6 adsorption capacity (4.63 mmol g−1) and high C2H6/C2H4 adsorption selectivity (1.67) due to the optimized pore size and surface function. Breakthrough experiments reveal benchmark productivity of 22.06 and 18.71 L kg−1 of high-purity C2H4 (≥99.95%) from a C2H6/C2H4 (50/50, v/v) mixture under dry and humid conditions, respectively. This work offers a promising approach for designing MOFs to overcome the adsorption/selectivity trade-off limitation in paraffin/olefin separation.
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request. The X-ray crystallographic coordinates for structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2259108, 2258075, and 2286047.†https://www.ccdc.cam.ac.uk/data_request/cifAuthor contributions
H. Z., W. L., and D. L. conceived and designed the research. X.-J. X., H. Z., and W. L. co-wrote the manuscript. X.-J. X. and Q.-Y. C. planned and executed the synthesis, characterization, and gas separation studies. Y. W. and R. H. performed the theoretical simulations. X.-J. X. carried out the structural analyses. All authors participated in and contributed to the preparation of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21731002, 21975104, 22150004, 22271120, and 22301102), the Ministry of Science and Technology of China (2021ZD0303102), the Guangdong Basic and Applied Basic Research Foundation (2023A1515010952), the Innovation Team Project in Guangdong Colleges and Universities (2021KCXTD009), and the National Postdoctoral Program for Innovative Talent (BX20220132).Notes and references
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed.
- https://www.fortunebusinessinsights.com/ethylene-market-104532 .
- S. Chu, Y. Cui and N. Liu, Nat. Mater., 2017, 16, 16–22 CrossRef PubMed.
- H. Zeng, M. Xie, T. Wang, R.-J. Wei, X.-J. Xie, Y. Zhao, W. Lu and D. Li, Nature, 2021, 595, 542–548 CrossRef CAS PubMed.
- X. W. Gu, J. X. Wang, E. Wu, H. Wu, W. Zhou, G. Qian, B. Chen and B. Li, J. Am. Chem. Soc., 2022, 144, 2614–2623 CrossRef CAS PubMed.
- S. Furukawa, J. Reboul, S. Diring, K. Sumida and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5700–5734 RSC.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- Z. Chen, K. O. Kirlikovali, P. Li and O. K. Farha, Acc. Chem. Res., 2022, 55, 579–591 CrossRef CAS PubMed.
- R. B. Lin, L. Li, H. L. Zhou, H. Wu, C. He, S. Li, R. Krishna, J. Li, W. Zhou and B. Chen, Nat. Mater., 2018, 17, 1128–1133 CrossRef CAS PubMed.
- Y. P. Li, Y. N. Zhao, S. N. Li, D. Q. Yuan, Y. C. Jiang, X. Bu, M. C. Hu and Q. G. Zhai, Adv. Sci., 2021, 8, 2003141 CrossRef CAS PubMed.
- A. A. Lysova, D. G. Samsonenko, K. A. Kovalenko, A. S. Nizovtsev, D. N. Dybtsev and V. P. Fedin, Angew. Chem., Int. Ed., 2020, 59, 20561–20567 CrossRef CAS PubMed.
- Q. Liao, W. X. Zhang, J. P. Zhang and X. M. Chen, Nat. Commun., 2015, 6, 8697–8705 CrossRef PubMed.
- H. Zeng, X. J. Xie, M. Xie, Y. L. Huang, D. Luo, T. Wang, Y. Zhao, W. Lu and D. Li, J. Am. Chem. Soc., 2019, 141, 20390–20398 CrossRef CAS PubMed.
- H. G. Hao, Y. F. Zhao, D. M. Chen, J. M. Yu, K. Tan, S. Q. Ma, Y. Chabal, Z. M. Zhang, J. M. Dou, Z. H. Xiao, G. Day, H. C. Zhou and T. B. Lu, Angew. Chem., Int. Ed., 2018, 57, 16067–16071 CrossRef CAS PubMed.
- G. D. Wang, Y. Z. Li, W. J. Shi, L. Hou, Y. Yu. Wang and Z. H. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202205427 CrossRef CAS PubMed.
- J. W. Cao, S. Mukherjee, T. Pham, Y. Wang, T. Wang, T. Zhang, X. Jiang, H. J. Tang, K. A. Forrest, B. Space, M. J. Zaworotko and K.-J. Chen, Nat. Commun., 2021, 12, 6507 CrossRef CAS PubMed.
- L. Li, R. B. Lin, R. Krishna, H. Li, S. Xiang, H. Wu, J. Li, W. Zhou and B. Chen, Science, 2018, 362, 443–446 CrossRef CAS PubMed.
- Z. Di, C. Liu, J. Pang, S. Zhou, Z. Ji, F. Hu, C. Chen, D. Yuan, M. Hong and M. Wu, Angew. Chem., Int. Ed., 2022, 134, e202210343 CrossRef.
- C. X. Chen, Z. W. Wei, J. J. Jiang, S.-P. Zheng, H.-P. Wang, Q.-F. Qiu, C.-C. Cao, D. Fenske and C.-Y. Su, J. Am. Chem. Soc., 2017, 139, 6034–6037 CrossRef CAS PubMed.
- Z. Di, C. Liu, J. Pang, C. Chen, F. Hu, D. Yuan, M. Wu and M. Hong, Angew. Chem., Int. Ed., 2021, 60, 10828–10832 CrossRef CAS PubMed.
- S. Xing, J. Liang, P. Brandt, F. Schäfer, A. Nuhnen, T. Heinen, I. Boldog, J. Möllmer, M. Lange, O. Weingart and C. Janiak, Angew. Chem., Int. Ed., 2021, 60, 17998–18005 CrossRef CAS PubMed.
- C. Montoro, F. Linares, E. Quartapelle Procopio, I. Senkovska, S. Kaskel, S. Galli, N. Masciocchi, E. Barea and J. A. R. Navarro, J. Am. Chem. Soc., 2011, 133, 11888–11891 CrossRef CAS PubMed.
- B. Tu, Q. Pang, D. Wu, Y. Song, L. Weng and Q. Li, J. Am. Chem. Soc., 2014, 136, 14465–14471 CrossRef CAS PubMed.
- C. Feng, H. Zhao and Z.-Q. Li, J. Solid State Chem., 2018, 258, 841–844 CrossRef CAS.
- L. Wang, W. Xue, H. Zhu, X. Guo, H. Huang and C. Zhong, Angew. Chem., Int. Ed., 2023, 135, e202218596 CrossRef.
- Z. Zhang, S. B. Peh, Y. Wang, Y. Wang, C. Kang, W. Fan and D. Zhao, Angew. Chem., Int. Ed., 2020, 132, 19089–19094 CrossRef.
- R. B. Lin, H. Wu, L. Li, X. L. Tang, Z. Li, J. Gao, H. Cui, W. Zhou and B. L. Chen, J. Am. Chem. Soc., 2018, 140, 12940–12946 CrossRef CAS PubMed.
- O. T. Qazvini, R. Babarao, Z.-L. Shi, Y.-B. Zhang and S. G. Telfer, J. Am. Chem. Soc., 2019, 141, 5014–5020 CrossRef CAS PubMed.
- S. B. Geng, E. Lin and X. Li, J. Am. Chem. Soc., 2021, 143, 8654–8660 CrossRef CAS PubMed.
- M. Kang, S. Yoon, S. Ga, D. W. Kang, S. Han, J. H. Choe, H. Kim, D. W. Kim, Y. G. Chung and C. S. Hong, Adv. Sci., 2021, 8, 2004940 CrossRef CAS PubMed.
- Z. Xu, X. Xiong, J. Xiong, R. Krishna, L. Li, Y. Fan, F. Luo and B. Chen, Nat. Commun., 2020, 11, 3163 CrossRef CAS PubMed.
- B. Zhu, J. Cao, S. Mukherjee, T. Pham, T. Zhang, T. Wang, X. Jiang, K. A. Forrest and M. J. Zaworotko, J. Am. Chem. Soc., 2021, 143, 1485–1492 CrossRef CAS PubMed.
- J. Pires, M. L. Pinto and V. K. Saini, ACS Appl. Mater. Interfaces, 2014, 6, 12093–12099 CrossRef CAS PubMed.
- S. Meng, H. Ma, L. Jiang, H. Ren and G. Zhu, J. Mater. Chem. A, 2014, 2, 14536–14541 RSC.
- K. J. Chen, D. G. Madden, S. Mukherjee, T. Pham, K. A. Forrest, A. Kumar, B. Space, J. Kong, Q. Y. Zhang and M. J. Zaworotko, Science, 2019, 366, 241–246 CrossRef CAS PubMed.
- D. Dubbeldam, S. Calero, D. E. Ellis and R. Q. Snurr, Mol. Simul., 2016, 42, 81–101 CrossRef CAS.
- G. D. Wang, R. Krishna, Y. Z. Li, W. J. Shi, L. Hou, Y. Y. Wang and Z. H. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202213015 CrossRef CAS PubMed.
- H.-G. Hao, Y.-F. Zhao, D.-M. Chen, J.-M. Yu, K. Tan, S. Ma, Y. Chabal, Z.-M. Zhang, J.-M. Dou, Z.-H. Xiao, G. Day, H.-C. Zhou and T.-B. Lu, Angew. Chem., Int. Ed., 2018, 130, 16299–16303 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2258075, 2286047 and 2259108. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc04119k |
| This journal is © The Royal Society of Chemistry 2023 |