Open Access Article
Open Access ArticleEstablishing the nexus between the coagulant for microalgae harvesting and the biomass nutrient assemblage
Toyin Dunsin
Saliu
a,
Olayinka John
Akinyeye
a,
Yetunde Irinyemi
Bulu
b,
Isiaka Ayobamidele
Lawal
c,
Isaac Ayodele
Ololade
d and
Nurudeen Abiola
Oladoja
 *a
*a
aHydrochemistry Research Laboratory, Department of Chemical Sciences, Adekunle Ajasin University, Akungba Akoko 342111, Nigeria. E-mail: bioladoja@yahoo.com; nurudeen.oladoja@aaua.edu.ng
bDepartment of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba Akoko 342111, Nigeria
cChemistry Department, Vaal University of Technology, Vanderbijlpark Campus, Boulevard 1900, Vanderbijlpark, South Africa
dEnvironmental Monitoring Unit, Department of Chemical Sciences, Adekunle Ajasin University, Akungba Akoko 342111, Nigeria
First published on 3rd April 2023
Abstract
Microalgae biomass is being studied as a potential resource for the production of renewable biofertilizer, but transforming the highly dispersed miniscule microalgae cells into harvestable biomass is challenging. Amongst the strategies for harvesting microalgae biomass, the coagulation–flocculation method is expedient. However, this method requires the use of coagulants that can interfere with the chemical composition and functionality of the biomass. Therefore, the need to establish the nexus between the harvesting coagulant and the biomass nutrient assemblage is germane. The physicochemical characteristics and the nutrient speciation of biomass harvested with either synthetic (AlCl3 and FeCl3) or naturally sourced (Moringa oleifera, gastropod shell and MargaritarIa discoidea) coagulants were evaluated. The influence of the different coagulants on the forms and patterns of nutrients (i.e., phosphorus, nitrogen and potassium) in the harvested biomass was evaluated. The physicochemical characteristics of the biomass, which influence nutrient availability, were impacted to various degrees by the different coagulants studied. The different coagulants used had no effect on the total phosphorus fraction of the harvested biomass, but impacted the available phosphorus fraction. All the coagulants investigated enhanced the available nitrogen in the biomass, as the percentage of the available nitrogen that constitute the total nitrogen fraction was >90% in all the harvested biomass samples, but less than 80% in the non-coagulant-biomass. None of the coagulants studied impacted the species of potassium in the biomass.
Sustainability spotlightMicroalgae biomass is a viable resource for the production of renewable biofertilizer, but transforming the highly dispersed miniscule microalgae cells into harvestable biomass is challenging. Amongst the strategies for harvesting microalgae biomass, the coagulation–flocculation method is expedient. However, this method requires the use of chemical coagulants that can interfere with the chemical composition and functionality of the biomass. Premised on the use of microalgae biomass as a nutrient resource in sustainable agricultural practice and the possible incorporation of the coagulant fraction in the harvested biomass, we hereby established the nexus between the harvesting coagulant and the biomass nutrient assemblage. |
1 Introduction
Consequent upon the need to ensure global food security through sustainable agricultural practice, the use of microalgae biomass is now being investigated as a green and sustainable resource substitute for conventional synthetic fertilizers and growth promoters.1–5 This emerging interest in the use of the microalgae biomass is ascribed to its nutrient-rich status, and the potential for cultivation in nutrient-rich wastewater for the dual purposes of nutrient recovery and wastewater purification.6 Microalgae biomass is endowed with both macro- and micronutrients that are crucial for crop development and growth, and are considered eco-friendly and probable substitutes for synthetic fertilizers and plant growth regulators.7 This is because a wide range of bioactive compounds, including plant-growth-promoting substances, such as phytohormones (gibberellin, auxin, cytokinin, abscisic acid, and ethylene), phycobilins, amino acids, and carotenoids, are produced from biomass.8–10 It has been reported that Cyanobacteria, microalgal species, take up the available phosphorus (P) species into the aqueous matrix and integrate them into the cell biomass. This cellular bound-P is then made available to plants by slow release, through autolysis and secretion, or decomposition of the dead algal cells.11 It was posited that Cyanobacteria improve the bioavailability of P by solubilizing organic-P through phosphatase enzyme production.11,12 Insoluble P species, such as (Ca)3(PO4)2, FePO4, AlPO4, or (Ca5(PO4)3OH), in sediments and soils are solubilized by synthesizing chelators, which aids the release of P for plant uptake.12Studies have shown that the use of Scenedesmus subspicatus, combined with humic acid, and Spirulina platensis, combined with cow dung, in separate experiments, improved the growth, yield, and content of pigments in the leaves of onions, and also elevated the levels of biochemicals and minerals in the crop.13,14 The application of microalgae biomass improved the growth of tomatoes,15–17 cucumbers,15,18 eggplants,19 peppers,20 lettuce,21,22etc.
Considering the physicochemical characteristics (e.g., microscopic nature and high surface charge density) of the microalgae cells, which inhibit aggregation in an aqueous system, harvesting the biomass for industrial and agricultural application is difficult. Since the harvested microalgae biomass is used in agriculture, directly, or indirectly after some valuable constituents have been extracted, the biomass constituents can impact the nutrient availability. Therefore, a careful choice of coagulant for harvesting microalgae biomass is apposite, because the constituents of the chosen coagulant are prone to altering the physicochemical characteristics of the biomass, impeding nutrient availability, and providing an avenue for the entry of undesirable biomass constituents into the food chain. It was posited that in the design of a sustainable phosphorus-recovery system, the choice of reactive metal species is vital.23 This is because phosphorus that is strongly bound to the reactive metal species cannot be recovered for either industrial or agricultural usage. For example, Al3+ and Fe3+ show higher affinity for P capture, but their use for phosphorus recovery purposes is discouraged because the captured phosphorus is obstinately bound to the metal phase. Moreover, aluminum is noxious to many plants and some soil organisms.24–26
The major microalgae biomass harvesting methods comprise filtration, flotation, centrifugation, sedimentation, coagulation–flocculation, electrocoagulation, or hybrids of these processes.27–30 Coagulation–flocculation is a simple harvesting method for bulk microalgae biomass production, but the coagulant of choice is bound to impact the physicochemical characteristics of the biomass produced.31,32 The issue of aluminium salt causing cell lysis, where the outer membrane of the microalga is ruptured, subsequently releasing the desired intracellular compounds into the water, has been reported.33 It has been observed that a significant difference occurred between the yield of biofuel produced from microalgae biomass harvested with ferric salt and that from a non-coagulant centrifugation method.34 A difference in the biomass yield from microalgae harvested with aluminum salt and that harvested with cationic starch35 has been documented. Aluminum salt was found to inhibit transesterification reactions and negatively impacted biodiesel production.36 It was postulated that the inorganic fractions of the coagulant that is embedded in the harvested biomass caused an increase in ash content, thereby reducing the calorific value of the biomass.36 These embedded inorganic fractions are also capable of serving as a catalyst, catalyst promoter, or catalyst support, that can promote thermochemical conversion or act as adsorbents for product cleanup.37,38 Consequently, it was posited that the incorporation of chemical species during the coagulation–flocculation process and the implications for the end-use or products should be systematically interrogated.39
Premised on the use of microalgae biomass as a nutrient resource in sustainable agricultural practice and the possible incorporation of the coagulant fraction in the harvested biomass, it is hereby hypothesised that the coagulant fraction can tinker with the nutrient availability. In order to validate this hypothesis, the effects of both synthetic (AlCl3 and FeCl3) and naturally sourced (M. oleifera (MO) seed extract, gastropod shell and M. discoidea fruit seed extract (MDFE)) coagulants on the physicochemical characteristics (i.e., pH value, electrical conductivity (EC) value, salt index (SI), and E3/E5 ratio), the elemental composition (i.e., carbon, hydrogen, nitrogen, and sulfur), the surface functional groups, and nutrient (i.e., N, P and K) speciation of the biomass harvested through different coagulant regimes were investigated.
2 Material and methods
2.1 Collection of eutrophicated water
The microalgae were sampled from a eutrophicated fish pond located at a village close to the university (Ayegunle-Akoko, latitude; 7° 20′ 43′′ N, and longitude: 5° 41′ 39′′ E). The microalgae composition has been previously identified and described.40 The pH of the eutrophicated water was 7.2, the microalgae biomass concentration was 5.7 g L−1, and the species of microalgae identified in the eutrophicated system included Apatococcus lobatus, Arthronema africanum, and Aphanocapsa sp.402.2 Determination of optimum coagulant dosage
The synthetic coagulants were prepared from both aluminium (AlCl3) and ferric (FeCl3) alum. Three different coagulants obtained from natural sources were prepared thus:(i) MO: The seeds were obtained from dry pods collected from the university farm, pulverised in a laboratory grinding machine and stored in an air-tight plastic container pending usage. Premised on findings that the active coagulating ingredient of the MO seeds is best extracted in a solution of high ionic strength,41,42 the extraction was carried out as follows: 5 g of the MO seed powder was dispersed in 100 mL of 1.0 M NaCl solution, and stirred on a magnetic stirrer for 30 min, at ambient tropical temperature, to extract the active coagulating component of the seed powder. The suspension was filtered using Whatman grade 1 filter paper, and the filtrate was used as the primary coagulant. The extraction of the active coagulant fraction of MO was carried out afresh for each use.
(ii) Gastropod shell (CGS): The coagulant from GS was prepared as previously reported.43 The raw GS was subjected to thermal treatment in a furnace at 1000 °C for 2 h to obtain CGS. The coagulant suspension was prepared by the dispersion of 0.5 g of CGS in 10 mL of water.
(iii) MDFE: The fresh fruits were collected and the extraction of the coagulant fraction was carried out by adopting the procedure described in ref. 40 and 44. The coagulant was extracted by grinding 50 g of the fruits in 25 mL of distilled water, for a total period of 3 min. The suspension was filtered using a Whatman grade 1 filter paper, and the filtrate was used as the primary coagulant for microalgae harvesting. Extraction from the fresh fruit seed was carried out daily for the coagulation–flocculation experiment.
(iv) As a control, the microalgae biomass was harvested using a non-coagulant approach via centrifugation (CG), at 3000 g for 10 min.
The optimum dosage for the respective coagulants was determined in a fixed volume (500 mL) of the microalgae suspension placed in a 1 L beaker, at a fixed optical density of the microalgae solution. Varying dosages of the respective coagulant were added in each case: for AlCl3 and FeCl3, the dosage ranged between 25 and 100 mg L−1, for MDFE, the dosage ranged between 1 and 8 mL, for MO, the dosage was 5 to 15 mL, and for CGS, the dosage was 0.3–1.6 g L−1.
After the addition of the respective coagulant dosages, the mixture was stirred vigorously at 200 rpm for 2 min, and then slowly at 50 rpm for a period of 30 min. Subsequently, the mixture was allowed to settle for a period of 1 h, before the sample was withdrawn and the optical density (OD) value was determined at λmax = 680 mm (i.e., the λmax value obtained from the spectra of UV/Visible spectrophotometry scanned between 400 and 800 nm) using a UV-Visible spectrophotometer.
The harvesting efficiency (HE%) was calculated using eqn (1):
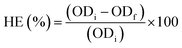 | (1) |
The coagulant dose with the highest HE (%) value represents the optimum coagulant dose.
2.3 Biomass harvesting
The microalgae biomass harvesting was carried out, using the respective optimum coagulant dosage, in a 15 L plastic container filled with 10 L of microalgae suspension. The solutions were stirred vigorously for 2 min and slowly for 30 min and allowed to settle for 60 min. The wet microalgae biomass was separated from the solution and dried in a laboratory oven at 70 °C. The control microalgae biomass sample, with no harvesting coagulant, was obtained by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min.
000 rpm for 10 min.
2.4 Biomass characterization
The physicochemical characteristics (i.e., pH value, EC, SI, and E3/E5 ratio) of the dried algae biomass (DAB) were determined using the methods described by Li et al.45 The pH value was determined after 0.25 g of DAB was completely dispersed in 250 mL of deionised water in a 500 mL beaker, using a pre-calibrated pH meter. The EC of the biomass suspension was determined by accurately weighing 1 g of DAB into 400 mL of deionised water in a 500 mL beaker. The mixture was stirred for 10 min, and the EC was determined using an EC meter.The SI value was determined by dispersing accurately weighed NaNO3 (1 g) in 400 mL of deionised water in a 500 mL beaker and stirring for 10 min. An equal weight (1 g) of DAB was also dispersed in 400 mL of deionised water and vigorously stirred, before the EC value was determined. The SI value was calculated using eqn (2):
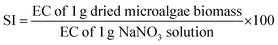 | (2) |
The UV-VIS absorbance ratio between λmax = 350 nm and 550 nm (E3/E5) of the DAB was determined by adding 0.2 g of the biomass into 10 mL of 0.05 N NaHCO3. Thereafter, the absorbance of the mixture was taken at 350 nm and 550 nm, respectively, using a UV-Visible spectrophotometer. E3/E5 was calculated by dividing the absorbance value at 350 nm by that at 550 nm.
The surface functional groups on the DAB were determined using Fourier-transform infrared spectroscopy (FTIR). The DAB (1.5 mg) was ground with 300 mg of KBr and vacuum compressed to form a testing disc. The FTIR spectra were recorded on an FTIR spectrophotometer (PerkinElmer Spectrum 100 with ATR unit), with a resolution of 2 cm−1, for a wavenumber range of 4000 to 400 cm−1, after 64 scans. The elemental composition (wt% oven-dry weight) (i.e., CHNS) was determined for the DAB, using an elemental analyzer (Vario MACRO cube, Germany).
2.5 Nutrient speciation in biomass
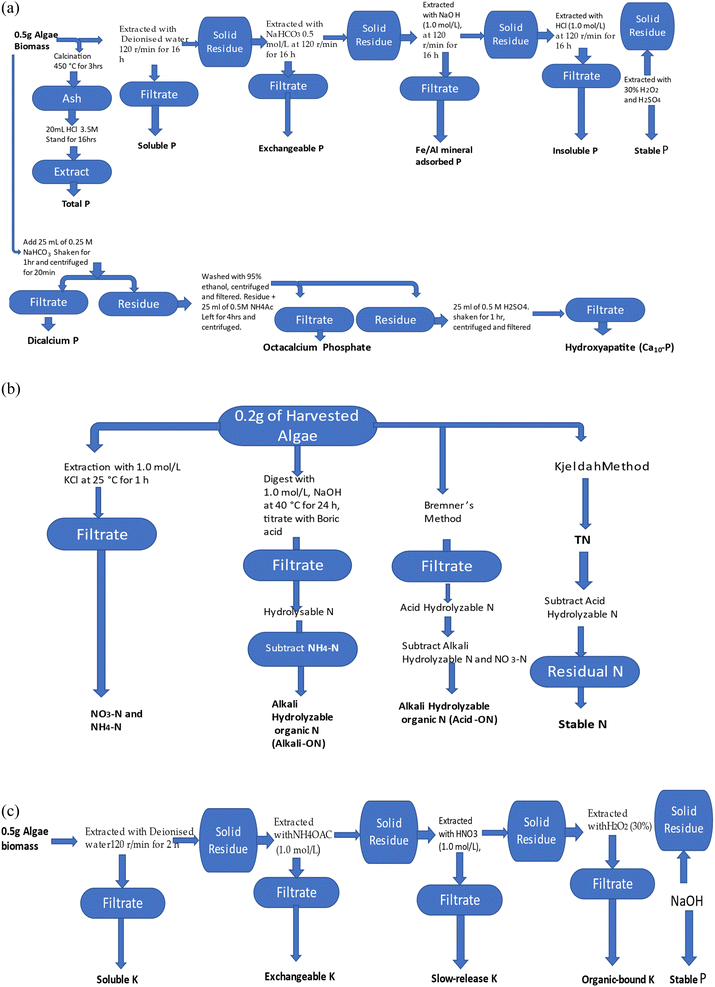 | ||
| Fig. 1 (a) Determination of P speciation in DAB. (b) Determination of N speciation in DAB. (c) Determination of K speciation in DAB. | ||
In order to determine the TP fraction in the DAB, 0.50 g of DAB was calcined for 3 hours at 450 °C, before 20 mL of 3.5 M HCl was added to the cooled ash and then agitated for 16 hours. The TP value was determined after the mixture was centrifuged for 15 min and filtered. The P fractions present in 0.5 g of DAB were sequentially extracted by water, 0.5 mol L−1 NaHCO3, 0.1 mol L−1 NaOH and 1.0 mol L−1 HCl, 30% H2O2 with H2SO4 to obtain the SP, EP, AP, ISP, and residual P (RP). For each extraction, the mixture was oscillated at 120 rpm for 16 h, before the mixture was centrifuged and then filtered with a 0.45 μm filter, as presented in Fig. 1. In each case, the different P fraction was determined by digesting the filtrate with potassium persulfate (K2S2O8), and then the concentration of P was determined by the molybdenum-blue ascorbic acid method with a UV-VIS spectrophotometer.48
The Ca-bound P species fractionating protocol, initially proposed by Chang and Jackson,49 and subsequently modified by Gu and Jiang,50 was adopted for the determination of Ca-bound P speciation. This is a method that is used to fractionate P in calcareous soil, which includes different operationally defined phosphorus fractions. 0.50 g of accurately weighed DAB was added to 50 mL polyethylene centrifuge tubes holding 25 mL of 0.25 M NaHCO3 (pH 7.5). The mixture was agitated on an orbital shaker at 25 °C for 1 h and centrifuged at 5000g for 20 min. The filtrate was analysed for dicalcium phosphate (Ca2-P). The residue was washed twice with 95% ethanol (12.5 mL for each wash), the mixture was centrifuged and the filtrate discarded before 25 mL of 0.5 M NH4Ac (pH 4.2) was added. The mixture was left to stand for 4 h, to enable complete dispersion of the residue. The evenly dispersed mixture was agitated for 1 h at 25 °C, followed by centrifugation at 5000g for 20 min, and the filtrate was analysed for octacalcium phosphate (Ca8-P). Ten-calcium phosphate (Ca10-P) was determined by adding 25 mL of 0.5 M H2SO4 to the residue from the determination of Ca8-P. The mixture was agitated for 1 h at 25 °C, followed by centrifugation and filtration, before analysis was carried out for phosphate, as Ca10-P.
The concentration of acid-hydrolyzable N in each DAB sample was measured according to the Bremner method: exactly 0.2 g of DAB was extracted with 6 mol L−1 hydrochloric acid, followed by digestion with sulfuric acid and a catalyst (K2SO4–CuSO4–Se powder). The value of the acid-hydrolyzable N was finally obtained with distilled/semi-micro titration.52 The concentration of residual N (RN) was obtained by subtracting the value for the acid-hydrolyzable N from the TN value.
2.6 Process and measurement reproducibility
For microalgae harvesting using different coagulants, a grab sample of the eutrophicated water was collected from the pond to form a single system from which all the experiments were conducted. The physicochemical characterization of biomass and the determination of the harvested biomass nutrient profiles were conducted in triplicate. All data obtained were subjected to one-way ANOVA. Statistical means were separated with the new Duncan's multiple range test at 95% level of significance using the Statistical Package for Social Sciences (SPSS) software, version 24.0.3 Results and discussion
3.1 Microalgae biomass harvesting
Maximum harvesting efficiencies of 96.5%, 96.3%, 95.9%, 94.8%, and 91.7% were achieved using 25 mg L−1 AlCl3, 50 mg L−1 FeCl3, 0.8 g L−1 CGS, 16 mL L−1 MDFE and 12 mL L−1 MO, respectively. The results obtained in this study were similar to those achieved by Wang et al.,55 with 97% harvesting efficiency of Coccomyxa sp. and Chlorella vulgaris using 30 mg L−1 AlCl3. The optimum dosage of FeCl3 (50 mg L−1) obtained in the present study was much lower than that employed for some freshwater microalgal strains (150 mg L−1 FeCl3).56 The observed gap in the optimum coagulant dosage between our microalgae sample and those reported in the literature could be attributed to the difference in the physicochemical characteristics, which have a great influence on the coagulation efficiency. It was reported that the alkalinity/pH of a medium affects the hydrochemistry of the coagulants, predominantly speciation transformation, as well as the distribution of the coagulants after dosing.57 The pH of the raw microalgae sample used in this study was near neutral (pH value = 7.2), and required a rather low FeCl3 dosage for favourable coagulation. Furthermore, the coagulation efficiency of different coagulants can be affected by microalgae species, cell sizes, cell surface features, cell densities, zeta potentials, and cell stability.55,58,59 It has been reported that 30 mg L−1 AlCl3 harvested 97% of both Coccomyxa sp. K.J. and Chlorella vulgaris; however, 1000 mg L−1 AlCl3 was required to achieve the same harvesting efficiency (%) for Chlamydomonas reinhardtii.553.2 Physicochemical characteristics of harvested biomass
The dry weight percentages of CHNS in the different DAB samples are presented in Table 1. All the DAB contained CHN in various proportions, but sulfur was undetectable (Table 1). The carbon content (%) in the various DAB varied, and CG-DAB (43.42%), MO-DAB (43.02%) and MDFE-DAB (35.52%) gave the highest percentage carbon content amongst the coagulants studied. The presence of sulfur in harvested microalgae biomass has either been reported at relatively low concentrations (<1%),34,43,60,61 or has been undetectable.40,62| Element (%) | MO-DAB | MDFE-DAB | AlCl3-DAB | FeCl3-DAB | CGS-DAB | CG-DAB |
|---|---|---|---|---|---|---|
| a UND = undetectable. b N.B: MO-DAB (MO-harvested DAB), MDFE-DAB (MDFE-harvested DAB), AlCl3-DAB (aluminium-chloride-harvested DAB), FeCl3-DAB (iron-chloride-harvested DAB), CGS-DAB (CGS-harvested DAB), CG-DAB (centrifuged DAB). | ||||||
| Carbon | 43.1 | 35.5 | 30.3 | 30.9 | 26.1 | 43.4 |
| Hydrogen | 6.8 | 5.6 | 5.2 | 5.0 | 3.2 | 6.7 |
| Nitrogen | 6.4 | 4.2 | 6.3 | 6.8 | 4.4 | 9.8 |
| Sulfur | UNDa | UNDa | UNDa | UNDa | UNDa | UNDa |
As expected, the natural-based coagulants gave biomass with the highest carbon content, because of the contribution from the carbonaceous fraction of the coagulant. This observation aligned with the report of Soares et al.62 that showed that a tannin-based polymer produced biomass with relatively higher carbon content (47.7%) than the other inorganic-based coagulants investigated. The higher carbon content biomass harvested with a natural coagulant could also be ascribed to the effectiveness of these coagulants in the removal of the carbonaceous fraction of the eutrophicated water.
Carbon is indisputably regarded as the most important regulator of soil fertility, with an impact on a wide range of soil parameters that boost crop performance. Organic carbon influences soil physicochemical performance by promoting healthy soil structure, which improves tilth, porosity, and water-holding capacity. The high percentage of carbon content in the harvested DAB showed that they have potential applications as biofertilizers, to boost and enhance soil productivity. All the DAB samples contained an appreciable amount of N (range = 9.8–4.2%) (Table 1).
The C/N ratio (%) calculated for the different DAB was 6.8 for MO-DAB, 8.6 for MDFE-DAB, 4.8 for AlCl3-DAB, 4.5 for FeCl3-DAB, 5.9 for CGS-DAB, and 4.4 for CG-DAB. The ratio of the carbon and nitrogen (C/N) constituents of biomass has been identified as a salient parameter that determines the rate of biochemical conversion of biomass in soil. Therefore, biomass with a C/N ratio > 25 is not easily degraded by microbial action.32 Furthermore, the C/N ratio of commercial organic amendment must be <30 to enhance nitrogen availability to plants. For the respective DAB, the C/N ratios ranged between 8.6 and 4.4, which is comparable with the values reported (ranging between 3 and 7) for other biomass-based fertilizers.32,63,64
The EC values of the different coagulant-DAB samples were investigated to evaluate the degree of dissolution of ions from the DAB and the nutrient availability potentials (Table 2). The EC values obtained ranged between 305.7 and 489.4 μs cm−1. The highest value of EC (489.4 μs cm−1) was obtained for FeCl3-DAB. The observed value can be ascribed to the relatively high concentration of FeCl3 (50 mg L−1) (i.e., the required optimum coagulant dosage) used for the harvesting process, which dissolved in the aqueous matrix and enhanced the ionic activity of the medium. The EC values of AlCl3-DAB (447 μs cm−1), MO-DAB (402.4 μs cm−1) and MDFE-DAB (412.5 μs cm−1) were also relatively high. CG gave the lowest EC value (305.7 μs cm−1).
| Parameter | MO-DAB | MDFE-DAB | AlCl3-DAB | FeCl3-DAB | CG-DAB | CGS-DAB |
|---|---|---|---|---|---|---|
| EC (μs cm−1) | 402.4 ± 1.2 | 412.5 ± 1.5 | 447.0 ± 0.9 | 489.4 ± 1.6 | 305.7 ± 1.4 | 387.5 ± 2.1 |
| pH | 7.4 ± 0.5 | 6.4 ± 0.7 | 6.6 ± 0.2 | 6.4 ± 0.3 | 7.2 ± 0.3 | 7.9 ± 0.2 |
| SI | 28.2 ± 0.45 | 25.4 ± 0.27 | 40.5 ± 0.11 | 46.7 ± 1.1 | 20.3 ± 0.91 | 40.2 ± 0.23 |
| E3/E5 | 1.9 ± 0.1 | 1.7 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.4 | 1.4 ± 0.1 | 1.6 ± 0.3 |
The soil pH value significantly affects nutrient availability for plant uptake. Nutrients such as N, P, K, are mostly available within the neutral and alkaline pH value range of 6.5 to 8. The pH value range (i.e., 6.4–7.9) of the different harvested microalgae was within the desirable neutral–alkaline range that promotes nutrient availability (Table 2). MDFE-DAB gave the lowest pH value of 6.4, while CGS-DAB gave the highest pH value of 7.9. The pH value exhibited by each of the DAB is attributed to the influence of each coagulant used for harvesting.
The SI value of a fertilizer is the degree of salt concentration that the fertilizer produces in a soil solution.65 It is also defined as a proportion of the increase in osmotic pressure of the salt solution produced by the fertilizer to the osmotic pressure of the same weight of sodium nitrate (NaNO3).66 The SI values obtained were higher for FeCl3-DAB (46.7), AlCl3-DAB (40.5) and CGS-DAB (40.3), than for MO-DAB (28.2) or MDFE-DAB (25.4) (Table 2). The relatively higher values of SI in FeCl3-DAB, AlCl3-DAB and CGS-DAB were attributed to the chemical profiles of each coagulant. The SI values obtained were comparable with the values obtained for the solid residue from the ammonium sulfite process (58.4) and Kraft pulping process (39.1).32 The values were also comparable with commercial fertilizers such as ammonium polyphosphate (20.0), MAP (26.7), DAP (29.2), and ammonia (47.1).67 Fertilizers with high SI is an indication of a high concentration of soluble salts, which may not necessarily be soluble nutrient fractions. As soon as these salts dissolve in the soil, they cause an upsurge in the salt concentration of the soil solution and subsequently increase the solution's osmotic potential. The higher the osmotic potential of the soil solution, the more difficult it is for plant seeds or plants to extract the soil water required for growth.
A UV-VIS absorbance ratio between 350 nm and 550 nm is a pointer to the degree of humification and the relative molecular size of organic fertilizer.32 The value of E3/E5 obtained for the different DAB samples ranged between 1.3 and 1.9 (Table 2). A high E3/E5 ratio shows that the material has a small molecular size and a low content of condensed aromatic rings,68 while a low value indicates the presence of a high content of aromatic rings. The E3/E5 values obtained for the different harvested algae are low (range = 1.3–1.9), compared with the E3/E5 values obtained for residues from the ammonium sulfite process (13.9) or Kraft pulping process (7.2).32 The E3/E5 values obtained for MO-DAB (1.9) and MDFE-DAB (1.7) were higher than those obtained for the other coagulants, but the values were far smaller than the E3/E5 values for humic acid (6.0) or fulvic acid (8.0) obtained by.68 This suggests that MO-DAB and MDFE-DAB have small molecular sizes, followed by DAB-CGS (1.6) but not as small as the molecular size of humic acid. Fertilizers with small molecules are absorbed more easily through plant roots, stems, and leaves than fertilizers with larger molecules.69 With reference to the E3/E5 values obtained in this study, nutrient fraction absorption from the biomass by a plant will decrease in the order MO-DAB > MDFE-DAB > CGS-DAB > CG-DAB > FeCl3-DAB > AlCl3-DAB.
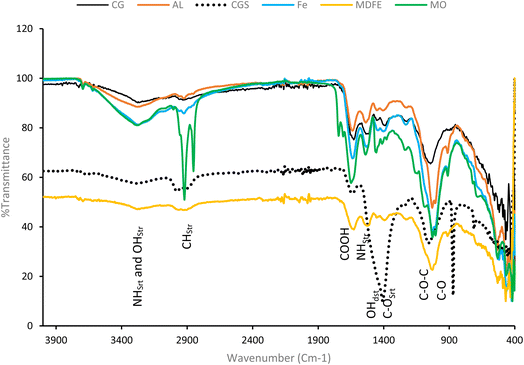
The FTIR spectra of the different coagulant-DAB samples (Fig. 2) showed different peaks that revealed the presence of protein and polysaccharides. Apart from the difference in the peak intensities, the peak positions shown by both the synthetic and non-synthetic harvested DAB were similar. The % transmittance values of the diagnostic peaks in the coagulant-DBA samples were lower than that of CG-DBA, which made the peaks appear to be more prominent in coagulant-DBA than in CG-DBA. The FTIR spectra of all the DAB showed broad peaks, with different intensities, at 3300–3200 cm−1, which has been attributed to the stretching vibrations of N–H and OH.10,70 The two sharp peaks between 2900 and 2800 cm−1, which are more prominent in the MO-DAB spectra, are assigned to C–H stretching. The occurrence of a relatively strong absorption around 1600 cm−1 present in all the samples is attributed to the characteristic peak of the carboxylic group.71 The prominent peaks that appeared between 1650 and 1580 cm−1 were assigned to N–H stretching72 and the 1500−1400 cm−1 peaks were characteristics of C–C stretching in aromatic rings. The peak at 1446–1372 cm−1 present in all the coagulant-DAB samples is attributed to –OH distortion and C–O stretching of phenolic OH. The sharp peaks between 1200 and 1000 cm−1 were assigned to the presence of C–O–C and C–O functional groups present in all the DAB. The presence of Cyanobacteria (Aphanocapsa sp.) in the microalgae colony was attributed to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric vibration peaks that appeared around 1246 cm−1.
O asymmetric vibration peaks that appeared around 1246 cm−1.
3.3 Phosphorus speciation in biomass
The forms and patterns of the phosphorus species in the different coagulant-DAB samples are presented in Table 3. The values of the total phosphorus (TP) for the different coagulant-DAB samples varies significantly (p ≤ 0.05) as follows: MDFE-DAB (5457.4 ± 27.4 mg kg−1) > MO-DAB (5360.8 ± 17.3 mg kg−1) = AlCl3-DAB (5301.7 ± 15.6 mg kg−1) > FeCl3-DAB (5227.1 ± 15.8 mg kg−1) = CGS-DAB (5210.4 ± 18.9 mg kg−1) > CG-DAB (5072.4 ± 22.0 mg kg−1). The TP contents in the different DAB were higher than the values detected in Chlorella vulgaris (2500–4000 mg kg−1) cultivated in municipal wastewater,73 but lower than the TP content of harvested microalgae (8000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1) grown in synthetic wastewater.74 Studies have shown that microalgae adjust the N and P concentrations in their system with the nutrient concentration in the surrounding water.75,76 Although the biomass P accumulation is influenced by the external supply of P and N, N accumulation is independent of P.75,76 This is related to the fact that nitrogen is used primarily for protein synthesis, while P is incorporated into ribosomal RNA.75
000 mg kg−1) grown in synthetic wastewater.74 Studies have shown that microalgae adjust the N and P concentrations in their system with the nutrient concentration in the surrounding water.75,76 Although the biomass P accumulation is influenced by the external supply of P and N, N accumulation is independent of P.75,76 This is related to the fact that nitrogen is used primarily for protein synthesis, while P is incorporated into ribosomal RNA.75
| Soluble P | Exchangeable P | Fe/Al-adsorbed P | Residual P | Insoluble P | Total P | |
|---|---|---|---|---|---|---|
| a Values are mean ± standard error for 3 replicates. Means with the same letters on the same column are not significantly different from each other at p ≥ 0.05 (Duncan's multiple range test). | ||||||
| AlCl3 | 704.3 ± 4.6b | 800.2 ± 7.6a | 1877.1 ± 8.5f | 881.90 ± 5.2c,d | 1038.2 ± 10.5c | 5301.6 ± 15.6c |
| FeCl3 | 733.3 ± 7.6b | 784.9 ± 6.5a | 1705.6 ± 7.8e | 654.96 ± 3.3b | 1348.3 ± 8.7e | 5227.1 ± 15.8b |
| CGS | 609.2 ± 8.7a | 2179.8 ± 7.9e | 432.9 ± 7.3a | 869.99 ± 6.8c | 1118.6 ± 12.3d | 5210.4 ± 18.9b |
| MDFE | 1498.1 ± 27.2d | 1693.9 ± 7.1d | 524.5 ± 5.1b | 897.89 ± 7.5d | 843.0 ± 4.5b | 5457.4 ± 27.4d |
| MO | 1408.4 ± 11.4c | 1529.9 ± 7.3c | 750.2 ± 5.3d | 877.60 ± 10.0c,d | 794.6 ± 5.3a | 5360.8 ± 17.3c |
| CG | 1470.5 ± 10.1d | 1115.5 ± 9.8b | 550.4 ± 4.7c | 525.56 ± 6.9a | 1410.5 ± 9.9f | 5072.4 ± 22.0a |
The concentrations of soluble P in MDFE-DAB (1498.1 ± 27.1 mg kg−1) and CG-DAB (1470.5 ± 10.1 mg kg−1) were not significantly different from each other, but were significantly higher than the DAB harvested with metal salts, AlCl3 (704.3 ± 4.6) and FeCl3 (733.3 ± 7.6) (Table 3). The soluble P obtained in FeCl3-DAB, AlCl3-DAB and CGS-DAB were equivalent to 14%, 13.3% and 11.6% of their TP, respectively, while the soluble P in MDFE-DAB and MO-DAB represent 27.4% and 26.3% of their total phosphorus, respectively. The relatively lower amount of soluble P recorded in the DAB harvested with metal ions and CGS was attributed to the formation of insoluble Al–P, Fe–P and Ca–P species. Phasey et al.77 suggested that conventional coagulants (i.e., iron and aluminium salts) contaminate the harvested biomass and lock up the embedded phosphorus, thereby preventing their beneficial reuse as biofertilizer. Saliu et al.40 confirmed the formation of hydroxyapatite, a less soluble Ca–P species, when thermally treated GS was used for P recovery from human urine. The higher soluble P obtained in the plant-based-DAB samples showed that the P fraction will be readily available for plant uptake but may suffer nutrient loss through run-off.
Significantly high values of insoluble phosphorus were obtained from CG-DAB (1410.5 ± 9.9 mg kg−1), FeCl3-DAB (1348.3 ± 8.7 mg kg−1) and CGS-DAB (1118.6 ± 12.3 mg kg−1) compared to the significantly lower values of MDFE-DAB (842.9 ± 4.5 mg kg−1) and MO-DAB (794.6 ± 5.3 mg kg−1). Significantly high values of Fe/Al-adsorbed P were recorded in AlCl3-DAB (1877.1 ± 8.5 mg kg−1) and FeCl3-DAB (1705.6 ± 7.8 mg kg−1), compared with MO-DAB (750.2 3 mg kg−1), MDFE-DAB (524.5 ± 5.1 mg kg−1), CGS-DAB (432.9 ± 7.3 mg kg−1) and the centrifuged-DAB (550.4 ± 4.7 mg kg−1) (Table 3). Studies have shown that P availability for plant uptake decreases when P is bound to Fe and Al.78,79
The determination of Ca–P species in DAB-CGS (Table 4) showed the prevalence of Ca10-P (i.e., hydroxyapatite), which further confirmed the higher insoluble P fraction. Dicalcium P, a more soluble species, was significantly higher in CGS-DAB (619.3 ± 14.5 mg kg−1), compared with MDFE-DAB (140.3 ± 12.9 mg kg−1), MO-DAB (102.3 ± 4.9 mg kg−1) or AlCl3-DAB (134.2 mg kg−1). Dicalcium-P, being a more soluble P species, is an indication that the P fraction will be readily available for plant growth.
| Sample | Dicalcium P | Ca8-P | Ca10-P |
|---|---|---|---|
| a Values are mean ± standard error for 3 replicates. Means with the same letters on the same column are not significantly different from each other at p ≥ 0.05 (Duncan's multiple range test). | |||
| AlCl3 | 134.2 ± 7.6a,b | 103.9 ± 6.8b | 166.0 ± 4.0d |
| FeCl3 | 280.3 ± 13.4c | 260.9 ± 6.7c | 140.8 ± 4.7c |
| CGS | 619.3 ± 14.5d | 477.7 ± 10.0d | 986.7 ± 6.7e |
| MDFE | 140.3 ± 12.9b | 120.0 ± 5.0b | 73.2 ± 3.8b |
| MO | 102.3 ± 4.9a | 58.3 ± 3.8a | 66.5 ± 3.3a,b |
| CG | 105.4 ± 7.2a | 74.6 ± 3.0a | 55.2 ± 1.9a |
3.4 Nitrogen speciation in biomass
The N species present in the different coagulant-DAB samples are presented in Table 5. The TN fraction for the harvested DAB varies significantly (p ≤ 0.05) as follows; 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 633 ± 47.9 mg kg−1 (MDFE-DAB) > 96
633 ± 47.9 mg kg−1 (MDFE-DAB) > 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 594 ± 45.6 mg kg−1 (MO-DAB) > 81
594 ± 45.6 mg kg−1 (MO-DAB) > 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 971 ± 47.9 mg kg−1 (FeCl3-DAB) > 81
971 ± 47.9 mg kg−1 (FeCl3-DAB) > 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 799 ± 46.8 mg kg−1 (CG-DAB) = 81
799 ± 46.8 mg kg−1 (CG-DAB) = 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 754 ± 1.7 mg kg−1 (CGS-DAB) > 81
754 ± 1.7 mg kg−1 (CGS-DAB) > 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 179 ± 52.0 mg kg−1 (AlCl3-DAB). The TN contents of the different DAB were within the range of TN for the biomass of algae grown in municipal wastewater (20
179 ± 52.0 mg kg−1 (AlCl3-DAB). The TN contents of the different DAB were within the range of TN for the biomass of algae grown in municipal wastewater (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–90
500–90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1),73 but higher than those reported for microalgae biomass from horticultural wastewater (60
000 mg kg−1),73 but higher than those reported for microalgae biomass from horticultural wastewater (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 mg kg−1)80 and 70
800 mg kg−1)80 and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 mg kg−1.81 The nutrient concentration of the culture medium could be ascribed to the variations in the TN values observed. This is a pointer to the fact that a microalgae culture medium with higher concentration of TN has the potential to produce microalgae biomass with a higher TN fraction.
190 mg kg−1.81 The nutrient concentration of the culture medium could be ascribed to the variations in the TN values observed. This is a pointer to the fact that a microalgae culture medium with higher concentration of TN has the potential to produce microalgae biomass with a higher TN fraction.
| Sample | NO3 | NH4 | Alkali-ON | Acid-ON | RN | TN |
|---|---|---|---|---|---|---|
| a Values are mean ± standard error for 3 replicates. Means with the same letters on the same column are not significantly different from each other at p ≥ 0.05 (Duncan's multiple range test). | ||||||
| AlCl3 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 761.0 ± 9.2c 761.0 ± 9.2c |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 776.0 ± 16.7c 776.0 ± 16.7c |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 701.0 ± 9.8a 701.0 ± 9.8a |
3977.0 ± 5.2b | 6964.0 ± 11.0e | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 179.0 ± 52.0a 179.0 ± 52.0a |
| FeCl3 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 789.0 ± 16.7d 789.0 ± 16.7d |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 904.0 ± 12.1d 904.0 ± 12.1d |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 506.0 ± 7.5c 506.0 ± 7.5c |
4151.0 ± 4.6d | 2621.0 ± 6.9c | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 971.0 ± 47.9c 971.0 ± 47.9c |
| GS | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 058.0 ± 6.4b 058.0 ± 6.4b |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 118.0 ± 9.8e 118.0 ± 9.8e |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 699.0 ± 7.5b 699.0 ± 7.5b |
4017.0 ± 7.5c | 2862.0 ± 5.2d | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 754.0 ± 1.7b 754.0 ± 1.7b |
| MD | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 295.0 ± 16.2f 295.0 ± 16.2f |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 713.0 ± 6.4f 713.0 ± 6.4f |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 302.0 ± 13.3f 302.0 ± 13.3f |
3916.0 ± 8.7a | 1407.0 ± 3.5b | 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 633.0 ± 47.9e 633.0 ± 47.9e |
| MO | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 014.0 ± 13.9e 014.0 ± 13.9e |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 295.0 ± 9.8b 295.0 ± 9.8b |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 155.0 ± 8.7e 155.0 ± 8.7e |
4751.0 ± 6.9e | 1379.0 ± 6.4a | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 594.0 ± 45.6d 594.0 ± 45.6d |
| CG | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 067.00 ± 11.0a 067.00 ± 11.0a |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 905.0 ± 8.1a 905.0 ± 8.1a |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732.0 ± 9.8d 732.0 ± 9.8d |
8573.0 ± 8.1f | 9522.0 ± 9.8f | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 799.0 ± 46.8b 799.0 ± 46.8b |
Inorganic nitrogen, such as NH4–N and NO3–N, may be readily absorbed and utilized by plants, and it is often used as nitrogen fertilizer because of the high N availability.47 The highest inorganic N fraction (i.e., NO3 plus NH4) was detected in MDFE-DAB and MO-DAB (66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 008 mg kg−1 and 54
008 mg kg−1 and 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 309 mg kg−1, respectively), which accounted for 59.3% and 56.2% of the TN, respectively, than in CGS-DAB (54
309 mg kg−1, respectively), which accounted for 59.3% and 56.2% of the TN, respectively, than in CGS-DAB (54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 176 mg kg−1), FeCl3-DAB (53
176 mg kg−1), FeCl3-DAB (53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 693 mg kg−1), or AlCl3-DAB (50
693 mg kg−1), or AlCl3-DAB (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 537 mg kg−1), which accounted for 66.3%, 65.5%, and 62.3% of TN, respectively. Although the magnitude of the readily available N is higher in the plant-based-DAB than in the other coagulant-DAB samples, the relative percentage to TN was higher in the other coagulant-DAB samples than in the plant-based-DAB samples. The non-coagulant approach (i.e., CG-DAB) gave the lowest magnitude of available N (40
537 mg kg−1), which accounted for 66.3%, 65.5%, and 62.3% of TN, respectively. Although the magnitude of the readily available N is higher in the plant-based-DAB than in the other coagulant-DAB samples, the relative percentage to TN was higher in the other coagulant-DAB samples than in the plant-based-DAB samples. The non-coagulant approach (i.e., CG-DAB) gave the lowest magnitude of available N (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 972 mg kg−1) and the relative percentage of inorganic-N to TN (50.1%). The high inorganic-N fraction of the coagulant-DAB samples showed that a high percentage of N fraction of the DAB will be readily available for plant nutrition.82
972 mg kg−1) and the relative percentage of inorganic-N to TN (50.1%). The high inorganic-N fraction of the coagulant-DAB samples showed that a high percentage of N fraction of the DAB will be readily available for plant nutrition.82
Simple organic nitrogen, such as amino acids, amides, and highly hydrolyzable protein nitrogen are classified as alkali-hydrolyzed organic nitrogen (alkali-ON), which can be directly and readily absorbed and utilized by plants, much like inorganic nitrogen. The percentage of alkali-ON in the TN fraction of the different DAB samples was 37.4% (MO-DAB), 36.1% (MDFE-DAB), 27.8% (CG-DAB), 26.3% (FeCl3-DAB), 25.3% (CGS-DAB), and 24.3% (AlCl3-DAB). Significantly (p ≤ 0.05) higher Alkali-ON contents were recorded for MDFE-DAB (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 302.0 ± 13.3 mg kg−1) and MO-DAB (36
302.0 ± 13.3 mg kg−1) and MO-DAB (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 155.0 ± 8.7 mg kg−1) (Table 5) due to the fact that they are plant-based coagulants, which have the capacity to incorporate more protein and organic nitrogen into the algae biomass.
155.0 ± 8.7 mg kg−1) (Table 5) due to the fact that they are plant-based coagulants, which have the capacity to incorporate more protein and organic nitrogen into the algae biomass.
Hot hydrochloric acid extracted nitrogen, such as hexosamine nitrogen and some heterocyclic nitrogen are referred to as acid-hydrolyzed organic nitrogen (acid-ON), while residual nitrogen (RN), exists in heterocyclic form and is bonded to heterocyclic or aromatic rings with a high degree of condensation. These nitrogen species are not readily available for plant uptake and are called slow-release nitrogen. The content of acid-ON (8573.0 ± 8.1 mg kg−1) and RN (9522.0 ± 9.8 mg kg−1) in the control (GC-DAB) were significantly higher than in all the other coagulant DABs (Table 5). Although the acid-ON of MO-DAB (4751.0 ± 6.9 mg kg−1) was significantly lower, when compared with the control, it was higher than the other coagulant-DABs. MO-DAB was complemented by a significantly low value of RN (1379.0 ± 6.4 mg kg−1). Significantly (p ≤ 0.05) low acid-ON (3916.0 ± 8.7 mg kg−1) and RN (1407.0 ± 3.5 mg kg−1) contents were obtained in MDFE-DAB. In summary, the percentage of the sum of accessible nitrogen (i.e., alkali-ON, nitrate N and ammonia N) in the TN content of MDFE-DAB (95.2%), MO-DAB (93.7%), FeCl3-DAB (91.7%), CGS-DAB (91.6%), and AlCl3-DAB (86.5%) were higher than for the non-coagulant approach (i.e., CG-DAB (77.8%)). The unavailable nitrogen (acid-ON and RN) in the TN fraction of MDFE-DAB (4.8%), MO-DAB (6.3%), FeCl3-DAB (8.3%), CGS-DAB (8.4%), and AlCl3-DAB (13.3%) were much lower than for CG-DAB (22.2%).
3.5 Potassium speciation in biomass
The different forms of K present in the coagulant-DAB samples vary significantly, as presented in Table 6, such that MDFE-DAB (7895 ± 38.68 mg kg−1) > MO-DAB (7495 ± 34.1 mg kg−1) > CGS-DAB (5760.0 ± 44.5 mg kg−1) > FeCl3-DAB (5647.0 ± 30.0 mg kg−1) = AlCl3-DAB (5569.0 ± 33.5 mg kg−1) = CG-DAB (5535.0 ± 31.8 mg kg−1) (Table 5). The TK fractions of the DAB samples were within the range of the TK contained in algae grown in dairy effluent (6100–8800 mg kg−1)83 but higher than those recorded for Chlorella vulgaris (4500 mg kg−1) cultivated through inoculation of axenic microalgal cultures in appropriate growth media.81| Sample | Soluble K (SK) | Exchangeable K (EK) | Slow-release K (SRK) | Organic-bound K (OBK) | Residual K (RK) | Total K (TK) |
|---|---|---|---|---|---|---|
| AlCl3 | 1624.0 ± 5.2d | 1357.0 ± 7.5d | 512.0 ± 5.2a | 978.0 ± 8.1b | 1098.0 ± 7.5c | 5569.0 ± 33.5a |
| FeCl3 | 1128.0 ± 4.6a | 1092.0 ± 4.6c | 890.0 ± 6.9b | 1073.0 ± 6.4c | 1464.0 ± 7.5e | 5647.0 ± 30.0a |
| GS | 1513.0 ± 6.4c | 729.0 ± 2.9a | 1200.0 ± 11.0c | 1225.0 ± 13.3d | 1093.0 ± 11.0c | 5760.0 ± 44.5b |
| MD | 2018.0 ± 7.5e | 1557.0 ± 6.9e | 1673.0 ± 9.2f | 1882.0 ± 10.4f | 765.0 ± 4.6b | 7895.0 ± 38.7d |
| MO | 2191.0 ± 10.4f | 1581.0 ± 5.2f | 1445.0 ± 4.6d | 1839.0 ± 9.8e | 439.0 ± 4.0a | 7495.0 ± 34.0c |
| CG | 1256.0 ± 9.2b | 751.0 ± 4.0b | 1562.0 ± 9.8e | 699.0 ± 2.3a | 1267.0 ± 6.4d | 5535.0 ± 31.8a |
Both the soluble K (SK) and exchangeable K (EK) are referred to as available potassium, which can be directly absorbed and utilized for plant growth. The sums of the available K (SK plus EK) in the DAB samples were 2981 mg kg−1 (AlCl3-DAB), 2220 mg kg−1 (FeCl3-DAB), 2242 mg kg−1 (CGS-DAB), 3575 mg kg−1 (MDFE-DAB), 3772 mg kg−1 (MO-DAB), and 2007 mg kg−1 (CG), which accounted for 53.5% (AlCl3-DAB), 39.3% (FeCl3-DAB), 38.9% (CGS-DAB), 45.3% (MDFE-DAB), 50.3% (MO-DAB) and 36.3% (CG-DAB) of TK.
HNO3-extractable potassium, which is slow release K (SRK), and organic-bound K (OBK), the species of K that can be gradually converted to effective K for plant uptake, were also quantified. The percentage of the sum of these K species (SRK + OBK) in the TK fraction of the DAB samples are 26.8% (AlCl3-DAB), 34.8% (FeCl3-DAB), 42.1% (CGS-DAB), 45.0% (MDFE-DAB), 43.8% (MO-DAB) and 40.8% (CG-DAB).
The total percentage of available K species for plant uptake (SK + EK + SRK + OBK) were 80.3% (AlCl3-DAB), 74.1% (FeCl3-DAB), 81.0% (CGS-DAB), 90.3% (MDFE-DAB), 94.1% (MO-DAB) and 77.1% (CG-DAB). These available K species were much higher than the percentages of unavailable K (RK), which were 19.7% (AlCl3-DAB). 25.9% (FeCl3-DAB), 18.9% (CGS-DAB), 9.7% (MDFE-DAB), 5.9% (MO-DAB) and 22.9% (CG-DAB).
4 Conclusions
The physicochemical characteristics of microalgae biomass harvested by the coagulation–flocculation approach were differently impacted by the coagulant used. Relative to non-coagulant harvested DAB, the coagulation process enhanced (i.e., low % transmittance) the intensities of the diagnostic peaks (i.e., peaks synonymous with polysaccharide and protein). The difference in the coagulant for harvesting had no influence on the TP fraction of the DAB. Metal based-coagulants (i.e., FeCl3, AlCl3, and CGS) reduced the concentration of soluble P in the DAB. The concentrations of the soluble-P in the biomass harvested with plant-based coagulants were comparable with the biomass harvested with the CG method. Considering the P speciation in the biomass harvested with metal-based coagulants, they are capable of functioning as slow-release fertilizers. The slow nutrient release potentials of FeCl3-DAB and AlCl3-DAB were attributed to the appreciable amount of Fe/Al–P fraction in them. The presence of Ca10-P was responsible for the slow nutrient release potential of CGS-DAB. Relative to the magnitude of TN present, the metal-based coagulants produced biomass with a higher fraction of available N than the plant-based coagulants. The CG approach produced biomass with the lowest available N. Biomass harvesting using synthetic coagulant had no effect on K speciation.Conflicts of interest
There are no conflicts to declare.References
- J. A. Gimondo and C. J. Currey, Wastewater-grown Algae Pellets and Paste as Fertilizers for Containerized Crops, HortScience, 2019, 54(3), 528–536 CAS.
- E. Alobwede, J. R. Leake and J. Pandhal, Circular economy fertilization: Testing micro and macro algal species as soil improvers and nutrient sources for crop production in greenhouse and field conditions, Geoderma, 2019, 334, 113–123 CrossRef CAS.
- R. A. Hamouda, M. A. Shehawy, S. M. Mohy El Din, F. M. Albalwe, H. M. R. Albalawi and M. H. Hussein, Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L, Green Process. Synth., 2022, 11, 648–658 CrossRef CAS.
- R. Jimenez, G. Markou, S. Tayibi, A. Barakat, C. Chapsal and F. Monlau, Production of Microalgal Slow-Release Fertilizer by Valorizing Liquid Agricultural Digestate: Growth Experiments with Tomatoes, Appl. Sci., 2020, 10, 3890, DOI:10.3390/app10113890.
- M. F. Salcedo, S. L. Colman, A. Y. Mansilla, M. A. Martínez, D. F. Fiola, V. A. Alvarez and C. A. Casalongué, Amelioration of tomato plants cultivated in organic-matter impoverished soil by supplementation with Undaria pinnatifida, Algal Res., 2020, 46, 101785 CrossRef.
- J. Coppens, O. Grunert, S. Van Den Hende, I. Vanhoutte, N. Boon, G. Haesaert and L. De Gelder, The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels, J. Appl. Phycol., 2015, 28, 2367–2377 CrossRef.
- C. H. Ahn, S. Lee, J. R. Park, H. K. Ahn, S. Yoon, K. Nam and J. C. Joo, Physicochemical and fertility characteristics of microalgal soil ameliorants using harvested Cyanobacterial microalgal sludge from a freshwater ecosystem, Republic of Korea, Heliyon, 2022, 8, e09700 CrossRef CAS PubMed.
- I. Michalak and K. Chojnacka, Algae as production systems of bioactive compounds, Eng. Life Sci., 2014, 15, 191, DOI:10.1002/elsc.201400191.
- V. G. Checker, H. R. Kushwaha, P. Kumari and S. Yadav, Role of phytohormones in plant defense: signaling and cross talk, in Molecular Aspects of Plant-Pathogen Interaction, ed. A. Singh and I. Singh, Springer, Singapore, 2018, pp. 159–184, DOI:10.1007/978-981-10-7371-7_7.
- S. Guo, P. Wang, X. Wang, M. Zou, C. Liu and J. Hao, Microalgae as biofertilizer in modern agriculture, in Microalgae Biotechnology for Food, Health and High Value Products, ed. M Alam, J. L. Xu and Z. Wang, Springer, Singapore, 2020, pp. 397–411, DOI:10.1007/978-981-15-0169-2-12.
- J. S. Singh, A. Kumar, A. N. Rai and D. P. Singh, Cyanobacteria: a precious bioresource in agriculture, ecosystem, and environmental sustainability, Front. Microbiol., 2016, 7, 529 Search PubMed.
- A. Kumar and J. S. Singh, Microalgal bio-fertilizers, Handbook of Microalgae-Based Processes and Products, 2020, pp. 445–463, DOI:10.1016/b978-0-12-818536-0.00017-8.
- L. G. Gemin, Á. F. Mógor, J. De Oliveira Amatussi and G. Mógor, Microalgae associated to humic acid as a novel biostimulant improving onion growth and yield, Sci. Hortic., 2019, 256, 108560, DOI:10.1016/j.scienta.2019.108560.
- R. Dineshkumar, J. Subramanian, A. Arumugam, A. Ahamed Rasheeq and P. Sampathkumar, Exploring the microalgae biofertilizer effect on onion cultivation by field experiment, Waste Biomass Valorization, 2020, 11, 77–87, DOI:10.1007/s12649-018-0466-8.
- O. Bumandalai, Effect of Chlorella vulgaris as a biofertilizer on germination of tomato and cucumber seeds, Int. J. Aquat. Biol., 2019, 7, 95–99, DOI:10.22034/ijab.v7i2.582.
- R. Farid, C. Mutale-Joan, B. Redouane, E. L. Mernissi Najib, A. Abderahime and S. Laila, et al.). Effect of microalgae polysaccharides on biochemical and metabolomics pathways related to plant defense in Solanum lycopersicum, Appl. Biochem. Biotechnol., 2019, 188, 225–240, DOI:10.1007/s12010-018-2916-y.
- F. Rachidi, R. Benhima, L. Sbabou and H. El Arroussi, Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution, Biotechnol. Rep., 2020, 25, 00426 Search PubMed.
- Z. Shariatmadari, H. Riahi, S. Mehri, M. Seyed Hashtroudi, A. Ghassempour and Z. Aghashariatmadary, Plant growth promoting cyanobacteria and their distribution in terrestrial habitats of Iran, Soil Sci. Plant Nutr., 2013, 59, 535–547, DOI:10.1080/00380768.2013.782253.
- G. A. Dias, R. H. C. Rocha, J. L. Araújo, J. F. Lima and W. A. Guedes, Growth, yield, and postharvest quality in eggplant produced under different foliar fertilizer (Spirulina platensis) treatments, Semina: Cienc. Agrar., 2016, 37, 3893–3902, DOI:10.5433/1679-0359.2016v37n6p3893.
- M. A. Guzmán-Murillo, F. Ascencio and J. A. Larrinaga-Mayoral, Germination and ROS detoxification in bell pepper (Capsicum annuum L.) under NaCl stress and treatment with microalgae extracts, Protoplasma, 2013, 250, 33–42, DOI:10.1007/s00709-011-0369-z.
- O. Ergun, H. Dasgan and O. Isık, Effects of microalgae Chlorella vulgaris on hydroponically grown lettuce, Acta Hortic., 2020, 2, 169–176, DOI:10.17660/ActaHortic.2020.1273.23.
- I. Puglisi, E. La Bella, E. I. Rovetto, A. R. Lo Piero and A. Baglieri, Biostimulant effect and biochemical response in lettuce seedlings treated with A Scenedesmus quadricauda extract, Plants, 2020, 9, 123, DOI:10.3390/plants9010123.
- G. K. Morse, S. W. Brett, J. A. Guy and J. N. Lester, Review: phosphorus removal and recovery technologies, Sci. Total Environ., 1998, 212, 69–81 CrossRef CAS.
- A. E. Johnston and I. R. Richards, Effectiveness of different precipitated phosphates as phosphorus sources for plants, Soil Manage., 2003, 19, 45–49 Search PubMed.
- L. E. de-Bashan and Y. Bashan, Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003), Water Res., 2004, 38, 4222–4246, DOI:10.1016/j.watres.2004.07.014.
- X.-N. Huang, Y. Chen and M. Chenker, Solid phosphorus phase in aluminum- and iron treated biosolids, J. Environ. Qual., 2007, 36, 549–556, DOI:10.2314/jeq2006.0155.
- A. I. Barros, A. L. Gonçalves, M. Simões and J. C. M. Pires, Harvesting techniques applied to microalgae: A review, Renewable Sustainable Energy Rev., 2015, 41, 1489–1500 CrossRef.
- C. A. Laamanen, G. M. Ross and J. A. Scott, Flotation harvesting of microalgae, Renewable Sustainable Energy Rev., 2016, 58, 75–86, DOI:10.1016/j.rser.2015.12.293.
- H. Al-Jabri, P. Das, S. Khan, M. Thaher and M. Abdul Quadir, Treatment of Wastewaters by Microalgae and the Potential Applications of the Produced Biomass—A Review, Water, 2021, 13(1), 27, DOI:10.3390/w13010027.
- V. Ananthi, P. Balaji, R. Sindhu, S. H. Kim, A. Pugazhendhi and A. Arun, A critical review on different harvesting techniques for algal based biodiesel production, Sci. Total Environ., 2021, 780, 146467, DOI:10.1016/j.scitotenv.2021.146467.
- I. Matter, V. Bui, S. C. Jung, J. Y. Seo, Y. E. Kim, Y. C. Lee and Y. K. Oh, Flocculation Harvesting Techniques for Microalgae: A Review, Appl. Sci., 2019, 9(15), 3069 CrossRef CAS.
- S. Li, T. Hu, Y. Xu, J. Wang, R. Chu, Z. Yin, F. Mo and L. Zhu, A review on flocculation as an efficient method to harvest energy microalgae: Mechanisms, performances, influencing factors and perspectives, Renewable Sustainable Energy Rev., 2020, 131, 110005, DOI:10.1016/j.rser.2020.110005.
- F. Sun, H.-Y. Pei, W.-R. Hu and C.-X. Ma, The lysis of Microcystis aeruginosa in AlCl3 coagulation and sedimentation processes, Chem. Eng. J., 2012, 193–194, 196–202, DOI:10.1016/j.cej.2012.04.043.
- S. Wang, M. Yerkebulan, A. E. Abomohra, S. El-khodary and Q. Wang, Microalgae harvest influences the energy recovery: a case study on chemical flocculation of Scenedesmus obliquus for biodiesel and crude bio-oil production, Bioresour. Technol., 2019, 286, 121371, DOI:10.1016/j.biortech.2019.121371.
- R. J. Anthony, J. T. Ellis, A. Sathish, A. Rahman, C. D. Miller and R. C. Sims, Effect of coagulant/flocculants on bioproducts from microalgae, Bioresour. Technol., 2013, 149, 65–70, DOI:10.1016/j.biortech.2013.09.028.
- C. Wan, M. A. Alam, X. Q. Zhao, X. Y. Zhang, S. L. Guo, S. H. Ho, J. S. Chang and F. W. Bai, Current progress and future prospect of microalgal biomass harvest using various flocculation technologies, Bioresour. Technol., 2015, 184, 251–257 CrossRef CAS PubMed.
- N. Abdoulmoumine, S. Adhikari, A. Kulkarni and S. Chattanathan, A review on biomass gasification syngas cleanup, Appl. Energy, 2015, 155, 294–307, DOI:10.1016/j.apenergy.2015.05.095.
- F. Sotoudehniakarani, A. Alayat and A. G. Mcdonald, Journal of analytical and applied pyrolysis characterization and comparison of pyrolysis products from fast pyrolysis of commercial Chlorella vulgaris and cultivated microalgae, J. Anal. Appl. Pyrolysis, 2019, 139, 258–273, DOI:10.1016/j.jaap.2019.02.014.
- D. López-González, M. Fernandez-Lopez, J. L. Valverde and L. Sanchez-Silva, Pyrolysis of three different types of microalgae: kinetic and evolved gas analysis pez-Gonz a, Energy, 2014, 73, 33–43, DOI:10.1016/j.energy.2014.05.008.
- T. D. Saliu, I. A. Lawal, O. J. Akinyeye, Y. I. Bulu, M. Klink, I. A. Ololade and N. A. Oladoja, Biocoagulant with Frother Properties for Harvesting Invasive Microalgae Colonies from the Eutrophicated System, ACS Sustainable Chem. Eng., 2022, 10(15), 5024–5034, DOI:10.1021/acssuschemeng.2c00365.
- T. Okuda, A. U. Baes, W. Nishijima and M. Okada, Improvement of extraction method of coagulation active components from Moringa oleifera seed, Water Res., 1999, 33, 3373–3378 CrossRef CAS.
- N. A. Pan G. Oladoja, Modification of local soil/sand with Moringa oleifera extracts for effective removal of cyanobacterial blooms, Sustainable Chem. Pharm., 2015, 2, 37–43 CrossRef CAS.
- N. A. Oladoja, J. Ali, W. Lei, N. Yudong and G. Pan, Coagulant derived from waste biogenic material for sustainable algae biomass harvesting, Algal Res., 2020, 50, 101982 CrossRef.
- N. A. Oladoja, T. D. Saliu, I. A. Ololade and E. Anthony, A new indigenous green option for turbidity removal from aqueous system, Sep. Purif. Technol., 2017, 186, 166–174 CrossRef CAS.
- T. Li, Z. Tong, S. Meng, Y. C. Li, B. Gao and H. K. Bayabil, Characterization of residues from non-woody pulping process and its function as fertilizer, Chemosphere, 2021, 262, 127906 CrossRef CAS PubMed.
- M. García-Albacete, A. Martín and M. C. Cartagena, Fractionation of Phosphorus Biowastes: Characterisation and Environmental Risk, Waste Manage., 2012, 32, 1061–1068 CrossRef PubMed.
- J. Xiong, S. Chen, J. Wang, Y. Wang, X. Fang and H. Huang, Speciation of Main Nutrients (N/P/K) in Hydrochars Produced from the Hydrothermal Carbonization of Swine Manure under Different Reaction Temperatures, Materials, 2021, 14, 4114, DOI:10.3390/ma14154114.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington, DC, 20th edn, 1999 Search PubMed.
- S. C. Chang and M. L. Jackson, Fractionation of soil phosphorus, Soil Sci., 1957, 84, 133–144 CrossRef CAS.
- Y. C. Gu and B. F. Jiang, A method for fractionation of inorganic phosphorus of calcareous soils, Soils, 1990, 22, 101–102 CAS.
- J. B. Xiong, Z. Q. Pan, X. F. Xiao, H. J. Huang, F. Y. Lai, J. X. Wang and S. W. Chen, Study on the hydrothermal carbonization of swine manure: The effect of process parameters on the yield/properties of hydrochar and process water, J. Anal. Appl. Pyrolysis, 2019, 144, 104692, DOI:10.1016/j.jaap.2019.104692.
- M. Pansu and J. Gautheyrou, Organic Forms of Nitrogen, Mineralizable Nitrogen (and Carbon), in Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods, Springer: Berlin/Heidelberg, Germany, 2006, pp. 497–547, DOI:10.1007/978-3-540-31211-6_14.
- S. Zhang, X. Zhang, X. Liu, W. Liu and Z. Liu, Spatial distribution of soil nutrient at depth in black soil of Northeast China: A case study of soil available potassium, Nutr. Cycling Agroecosyst., 2013, 95, 319–331, DOI:10.1007/s11368-014-0935-z.
- J. Li, L. Niu, Q. Zhang, H. Di and J. Hao, Impacts of long-term lack of potassium fertilization on different forms of soil potassium and crop yields on the North China Plains, J. Soils Sediments, 2017, 17, 1607–1617, DOI:10.1007/s11368-017-1658-8.
- Q. Wang, K. Oshita and M. Takaoka, Flocculation properties of eight microalgae induced by aluminum chloride, chitosan, amphoteric polyacrylamide, and alkaline: Life-cycle assessment for screening species and harvesting methods, Algal Res., 2021, 54, 102226 CrossRef.
- N. B. Wyatt, L. M. Gloe, P. V. Brady, J. C. Hewson, A. M. Grillet, M. G. Hankins and P. I. Pohl, Critical conditions for ferric chloride-induced flocculation of freshwater algae, Biotechnol. Bioeng., 2012, 109(2), 493–501, DOI:10.1002/bit.23319.
- M. Yan, D. Wang, J. Qu, J. Ni and C. W. Chow, Enhanced coagulation for high alkalinity and micro-polluted water: The third way through coagulant optimization, Water Res., 2008, 42(8–9), 2278–2286 CrossRef CAS PubMed.
- S. Sirin, R. Trobajo, C. Ibanez and J. Salvado, Harvesting the microalgae Phaeodactylum tricornutum with polyaluminum chloride, aluminium sulphate, chitosan and alkalinity-induced flocculation, J. Appl. Phycol., 2012, 24(5), 1067–1080 CrossRef CAS.
- L. Chen, C. Wang, W. Wang and J. Wei, Optimal conditions of different flocculation methods for harvesting Scenedesmus sp. cultivated in an open-pond system, Bioresour. Technol., 2013, 133, 9–15 CrossRef CAS PubMed.
- M. Maaitah, G. Hodaifa, A. Malvis and S. Sanchez, Kinetic growth and biochemical composition variability of Chlorella pyrenoidosa in olive oil washing wastewater cultures enriched with urban wastewater, J. Water Process Eng., 2020, 35, 101197 CrossRef.
- H. Li, Y. Zhang, J. Liu, Z. Shen, A. Li, T. Ma, Q. Feng and Y. Sun, Treatment of high-nitrate wastewater mixtures from MnO2 industry by Chlorella vulgaris, Bioresour. Technol., 2019, 291, 121836 CrossRef CAS PubMed.
- R. B. Soares, M. F. Martins and R. F. Gonçalves, Thermochemical conversion of wastewater microalgae: The effects of coagulants used in the harvest process, Algal Res., 2020, 47, 101864, DOI:10.1016/j.algal.2020.101864.
- D. Meier, V. Zunigapartida, F. Ramirezcano, N. C. Hahn and O. Faix, Conversion of technical lignins into slow-release nitrogenous fertilizers by ammoxidation in liquid-phase, Bioresour. Technol., 1994, 49, 121e128, DOI:10.1016/0960-8524(94)90075-2.
- F. Ramirez, V. Gonz_alez, M. Crespo, D. Meier, O. Faix and V. Zúniga, Ammoxidized kraft lignin as a slow-release fertilizer tested on Sorghum vulgare, Bioresour. Technol., 1997, 61(1), 43e46, DOI:10.1016/S0960-8524(97)84697-4.
- J. J. Mortvedt, Calculating salt index, Fluid J., 2001, 9(2), 8–11 Search PubMed.
- J. L. Havlin, J. D. Beaton, S. L. Tisdale and W. L. Nelson, Soil Fertility and Fertilizers, Prentice Hall, Upper Saddle River, NJ, 6th edn, 1999 Search PubMed.
- C. A. Laboski, Understanding salt index of fertilizers, Proc. Wisconsin Fertilizer, Aglime and Pest Management Conference, 2008, vol. 47, p. 37e41 Search PubMed.
- A. A. Helal, G. A. Murad and A. A. Helal, Characterization of different humic materials by various analytical techniques, Arabian J. Chem., 2011, 4, 51–54, DOI:10.1016/j.arabjc.2010.06.018.
- P. D. Dzung, D. V. Phu, B. D. Du, L. S. Ngoc, N. N. Duy, H. D. Hiet, D. H. Nghia, N. T. Thang, B. V. Le and N. Q. Hien, Effect of foliar application of oligochitosan with different molecular weight on growth promotion and fruit yield enhancement of chili plant, Plant Prod. Sci., 2017, 20, 389e395 Search PubMed.
- E. Pretsch, P. Buehlmann, C. Affolter, E. Pretsch, P. Bhuhlmann and C. Affolter, Structure Determination of Organic Compounds, Springer, 2000 Search PubMed.
- Y. Shi, J. Sheng, F. Yang and Q. Hu, Purification and identification of polysaccharide derived from Chlorella pyrenoidosa, Food Chem., 2007, 103, 101–105 CrossRef CAS.
- A. Mishra and B. Jha, Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress, Bioresour. Technol., 2009, 100, 3382–3386 CrossRef CAS PubMed.
- I. T. D. Cabanelas, J. Ruiz, Z. Arbib, F. A. Chinalia, C. Garrido-Pérez, F. Rogalla, I. A. Nascimento and J. A. Perales, Comparing the use of different domestic wastewaters for coupling microalgal production and nutrient removal, Bioresour. Technol., 2013, 131, 429–436 CrossRef CAS PubMed.
- R. Whitton, A. Le Mével, M. Pidou, F. Ometto, R. Villa and B. Jefferson, Influence of microalgal N and P composition on wastewater nutrient remediation, Water Res., 2016, 91, 371–378 CrossRef CAS PubMed.
- A. Beuckels, E. Smolders and K. Muylaert, Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment, Water Res., 2015, 501(77), 98–106 CrossRef PubMed.
- H. J. Choi and S. M. Lee, Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater, Bioprocess Biosyst. Eng., 2015, 38(4), 761–766 CrossRef CAS PubMed.
- J. Phasey, D. Vandamme and H. J. Fallowfield, Harvesting of algae in municipal wastewater treatment by calcium phosphate precipitation mediated by photosynthesis, sodium hydroxide and lime, Algal Res., 2017, 27, 115–120, DOI:10.1016/j.algal.2017.06.015.
- W. J. Emsens, C. J. S. Aggenbach, A. J. P. Smolders, D. Zak and R. van Diggelen, Restoration of endangered fen communities: the ambiguity of iron–phosphorus binding and phosphorus limitation, J. Appl. Ecol., 2017, 54, 1755–1764 CrossRef CAS.
- A. M. Kooijman, C. Cusell and L. Hedenäs, et al., Re-assessment of phosphorus availability in fens with varying contents of iron and calcium, Plant Soil, 2020, 447, 219–239, DOI:10.1007/s11104-019-04241-4.
- J. Liu, B. Danneels, P. Vanormelingen and W. Vyverman, Nutrient removal from horticultural wastewater by benthic filamentous algae Klebsormidium sp., Stigeoclonium spp. and their communities: From laboratory flask to outdoor Algal Turf Scrubber (ATS), Water Res., 2016, 92(1), 61–68 CrossRef CAS PubMed.
- A. P. Batista, L. Gouveia, N. M. Bandarra, J. M. Franco and A. Raymundo, Comparison of microalgal biomass profiles as novel functional ingredient for food products, Algal Res., 2013, 2(2), 164–173, DOI:10.1016/j.algal.2013.01.004.
- C. Song, W. Yuan, S. Shan, Q. Ma, H. Zhang, X. Wang, N. K. Niazi and H. Wang, Changes of nutrients and potentially toxic elements during hydrothermal carbonization of pig manure, Chemosphere, 2020, 243, 125331, DOI:10.1016/j.chemosphere.2019.125331.
- W. Mulbry, S. Kondrad and C. Pizarro, Biofertilizers from Algal Treatment of Dairy and Swine Manure Effluents, J. Veg. Sci., 2007, 12(4), 107–125, DOI:10.1300/J484v12n04_08.
| This journal is © The Royal Society of Chemistry 2023 |