Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cross-metathesis of technical grade methyl oleate for the synthesis of bio-based polyesters and polyamides†
Paweł
Krzesiński
 ab,
Vincent
César
ab,
Vincent
César
 a,
Karol
Grela
a,
Karol
Grela
 b,
Sergio
Santos
b,
Sergio
Santos
 c and
Pablo
Ortiz
c and
Pablo
Ortiz
 *c
*c
aLCC-CNRS, Université de Toulouse, CNRS, 205 Route de Narbonne, 31077 Toulouse, France
bBiological and Chemical Research Centre, Faculty of Chemistry, University of Warsaw, Żwirki i Wigury Street 101, 02-089 Warsaw, Poland
cTecnalia, Basque Research and Technology Alliance (BRTA), Parque Tecnológico de Álava, LeonardoDa Vinci 1, E-01510 Miñano, Álava, Spain. E-mail: pablo.ortiz@tecnalia.com
First published on 28th September 2023
Abstract
Fatty acid methyl esters (FAMEs), despite being currently used mainly as a biodiesel, could be also a source of various building blocks. Their cross-metathesis allows the production of diesters—attractive bio-based monomers. However, most of the existing reports from academic research groups are mainly restricted to pure methyl oleate. With the goal of future industrial feasibility, a process for the self-cross metathesis of technical grade methyl oleate, which means containing a significant amount (20 wt%) of methyl linoleate, has been developed. The resulting dimethyl octadec-9-enedioate has been obtained with high selectivity and in good yield. Subsequent polycondensation with a co-monomer (diol or diamine) resulted in the formation of polymeric materials which were characterized using thermogravimetric analysis and differential scanning calorimetry.
Sustainability spotlightThe current situation demands an urgent transition towards sustainable and renewable sources of energy and materials to mitigate the environmental impact of the use of fossil fuels. Most polymers are currently made from fossil feedstocks, and they need to be replaced by renewable sources. Fatty acid methyl esters are derived from vegetable oil and currently used mainly as a biodiesel, but they hold promising use as a source of various building blocks. Their cross-metathesis yields diesters, monomers for the synthesis of polyesters and polyamides. However, pure fatty acid methyl esters are used for this process. The sustainable advancement of our work lies in the development of a process for the self-cross metathesis of technical grade methyl oleate, containing 20 wt% of methyl linoleate, derived from broadly available sunflower and rapeseed oil. Thus, it represents a real opportunity for making these polymers at large scale. Our work aligns with several United Nations Sustainable Development Goals: Industry, Innovation, and Infrastructure (SDG 9), Responsible Consumption and Production (SDG 12) and Climate Action (SDG 13). |
Introduction
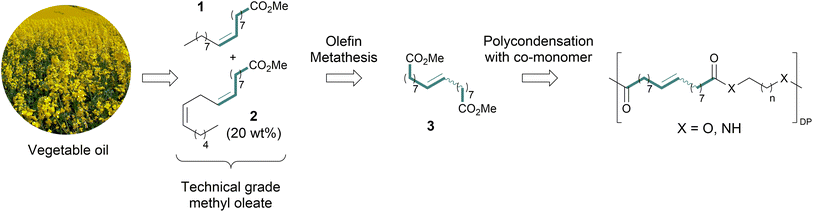
Fossil fuels are the main source of raw materials for the chemical industry.1 However, as we move towards a net zero scenario, renewable feedstocks will take the place of fossil fuels as the starting material for the synthesis of chemicals. This transition is especially urgent in the case of polymers, which account for the largest volume of chemical industry products.1 Biomass is currently the main source of renewable feedstocks, and among the different types of biomass, vegetable oils are the most widely used in the chemical industry, due to their availability, affordability, and functionality.2–5 Transesterification of vegetable oils with methanol yields fatty acid methyl esters (FAMEs) with concomitant production of glycerol. Both feedstocks have already found applications in the production of cosmetics, detergents, plasticizers, and other products.2,6,7Olefin metathesis (OM) has become a tool of prominent importance for C–C double bond formation, thus earning an outstanding position in the organic synthesis toolbox, and has already been proven useful in the synthetic modifications of FAMEs.8,9 Notably, the ethenolysis reaction (CM with ethylene) of FAMEs has gained significant interest, since it gives access to valuable terminal olefins.10–12 Moreover, the self-CM of pure methyl oleate (1, Scheme 1), which is the most abundant unsaturated FAME found in natural oil, affords dimethyl octadec-9-enedioate (3, Scheme 1) and internal alkene 5 (Scheme 2a)—both desirable products.9,13–16 Diester 3 is an excellent monomer for synthesizing bio-based and potentially biodegradable polyesters (Scheme 1). A saturated analog of diester 3 was used to obtain long-spaced aliphatic polyesters,17–19 although reports featuring direct use of 3 in polymer synthesis are scarce.20
However, procuring pure methyl oleate is not a straightforward task.‡ Vegetable oils are triglycerides commonly composed of different fatty acids which lead to mixtures of FAMEs after transesterification. Concerning OM transformation of FAMEs, this can be particularly problematic when a mixture of unsaturated FAMEs is formed. Historically, methyl oleate with a low content of polyunsaturated FAMEs could be procured by selective hydrogenation of polyunsaturated fatty acids.21 Nowadays methyl oleate with low content of polyunsaturated FAMEs is produced from high oleic safflower oil (HOSO) raw material. HOSO is obtained from genetically modified crops, which are safflower plants into which one or several genes coding have been inserted through the process of genetic engineering.22,23 So far, reports concerning self-CM of methyl oleate mainly focused on the use of pure, refined 1, but this cannot be a viable raw material for industrial scale due to high purification costs.13,24 Moreover, to develop a process susceptible of potential future industrialization, raw material availability must be considered. Consequently, it is desirable to move from HOSO towards significantly more broadly available vegetable oil sources such as sunflower or rapeseed oils. This area has been largely unexplored and led us to embark on a search for a process utilizing technical grade methyl oleate, which means containing a substantial amount (20 wt%) of methyl linoleate (2, Scheme 1), as starting material.§,25,26
Results
Owing to their relatively high activity and functional group tolerance, ruthenium alkylidene complexes are widely used, of which Grubbs 1st generation catalyst (Ru1, Scheme 2b) is a notable example. Exchanging the phosphine ligand to an N-heterocyclic carbene (NHC) ligand resulted in a more efficient second generation of the catalyst (Ru2, Scheme 2b). Furthermore, the installation of o-isopropoxybenzylidene ligand led to an even more stable and productive, phosphine-free second generation of Hoveyda–Grubbs catalysts (Ru3–Ru6, Scheme 2b).27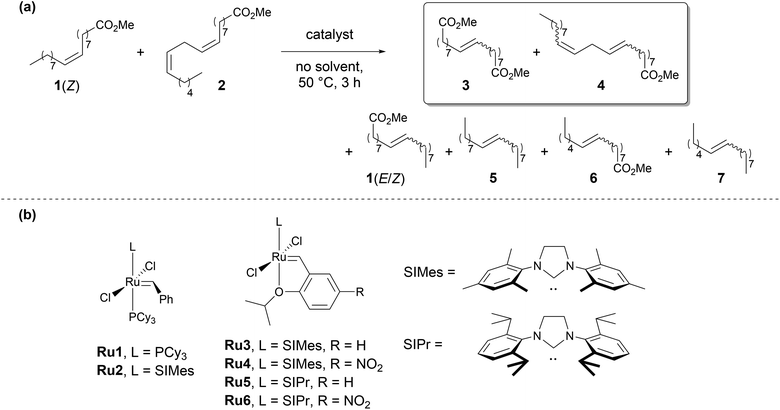 | ||
| Scheme 2 (a) Self-CM of technical methyl oleate (1) containing methyl linoleate (2). (b) Ruthenium catalysts used in OM. | ||
When anticipating a large-scale application of a homogeneous catalyst, it is paramount to have a high turnover number (TON) to render the process profitable. It was estimated that OM catalyst should give TON exceeding 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to achieve an economically viable process10,28 and this level of efficiency is achievable for contemporary catalysts.16,29,30 However, TON is significantly dependent on the substrate quality which is connected to the content of catalyst poisons such as peroxides, phosphates, sulfates, soap, etc. Thus, several protocols to remove those poisons from methyl oleate have been developed and their impact on the overall economic viability of the process has been discussed in the literature.¶ Most commonly utilized method is 1 pretreatment with activated alumina combined with degassing.||
000 to achieve an economically viable process10,28 and this level of efficiency is achievable for contemporary catalysts.16,29,30 However, TON is significantly dependent on the substrate quality which is connected to the content of catalyst poisons such as peroxides, phosphates, sulfates, soap, etc. Thus, several protocols to remove those poisons from methyl oleate have been developed and their impact on the overall economic viability of the process has been discussed in the literature.¶ Most commonly utilized method is 1 pretreatment with activated alumina combined with degassing.||
We started off with the self-CM of technical grade 1 after its prior simple distillation from activated alumina to decrease the content of catalyst poisons. 100 ppm of Ru3 allowed to reach equilibrium in 3 h (50 °C, no solvent, Table 1, entry 2). Even though the reaction mixture contained a variety of products including several internal alkenes and monoesters (1(E/Z), 4, 5, 6, 7, Scheme 2a), we were pleased to find that the equilibrium mixture contained predominantly 3 as a diester species. Remarkably, when non-pretreated, technical grade 1 was used for the reaction (Table 1, entry 1) no diester 3 was formed with 100 ppm of Ru3. This highlights how substrate pretreatment can significantly impact catalyst efficiency.
| Entry | Catalyst | Loading (ppm) | Conversion of 1(Z) + 2 (%) | Yield of 3 (%) | 3/4 wt. ratio | E/Z ratio of 3 |
|---|---|---|---|---|---|---|
| a Reaction conditions: no solvent, pretreated technical grade methyl oleate, 50 °C, 3 h. b Non-pretreated, technical grade 1. | ||||||
| 1b | Ru3 | 100b | <5 | 0 | — | — |
| 2 | 100 | 95 | 54 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
|
| 3 | 50 | 94 | 51 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
|
| 4 | 25 | 80 | 26 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
|
| 5 | Ru4 | 100 | 94 | 45 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 6 | Ru5 | 94 | 54 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
|
| 7 | Ru6 | 95 | 49 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
|
Notably, although isomerization side reactions can be problematic for OM catalysts,31 no significant formation of isomerization byproducts was observed. Such side reactions can be circumvented by a careful choice of reaction conditions which proved a good strategy in this case. Prolonged reaction time at higher temperatures promotes the undesired isomerization reactions,16 presumably due to the formation of hydride species31 and metal nanoparticles32 during catalyst decomposition. Additionally, certain impurities derived from substrate or catalyst, such as alcohols,33 metal nanoparticles,32 can also contribute to lower selectivity due to increased formation of isomerized byproducts. To suppress those side reactions use of quinone additives was studied in the literature.34 Moreover, catalysts bearing unsymmetrical NHC ligands do not promote isomerization, however, at the cost of lower activity in OM.35,36
Upon decreasing the catalyst loading, we noticed that monoester 4 was also present in the reaction mixture and that the relation was inversely proportional (Table 1, entries 3–4). This suggests that at loadings lower than 100 ppm of Ru3 the reaction equilibrium is not reached and 4 might only be a kinetically favored product. We wanted to avoid any monofunctional species such as 1, 2, 4, or 6 in the isolated product since they would lead to a lower molecular mass of the resulting polyester, as monoesters would terminate a polymer chain. From gas chromatography, we deduced that boiling temperatures of 3 and 4 would be very similar (Fig. S4, ESI†), which would make the purification of 3 challenging. Hence, we decided to focus on minimizing the formation of 4 as the primary objective of catalyst screening, rather than maximizing catalyst efficiency. All of the complexes gave reasonably similar conversions of substrates 1(Z) and 2, around 95%. All catalysts gave a ratio of 3 to 4 above 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, with Ru3 and Ru6**,37 exceeding 95
10, with Ru3 and Ru6**,37 exceeding 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Ru3 gave a slightly higher yield of 3 than catalyst Ru5 (54% versus 49% respectively, the maximal yield being approximately 50% resulting from the reaction equilibrium) and therefore it was chosen to continue with the development.
5. Ru3 gave a slightly higher yield of 3 than catalyst Ru5 (54% versus 49% respectively, the maximal yield being approximately 50% resulting from the reaction equilibrium) and therefore it was chosen to continue with the development.
With the selected catalyst, self-CM of pretreated, technical grade methyl oleate was conducted with 300 g of substrate. After the reaction, product 3 was isolated by fractional distillation and subsequently purified by crystallization from methanol at −30 °C. Interestingly, this protocol gave pure 3 containing almost only E isomer (E/Z ratio of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) in 36% yield.
3) in 36% yield.
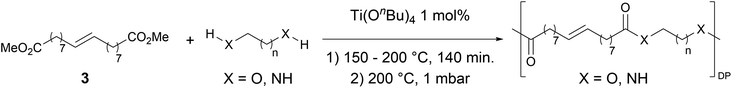
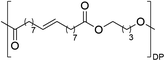
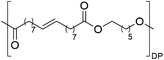
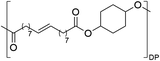
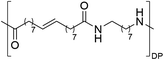
Having monomer 3 in hand, we attempted the melt polycondensation reaction with different co-monomers (Scheme 3). Titanium (IV) n-butoxide was used as the catalyst for this reaction.20 This resulted in a red-brown solid insoluble in all the most common solvents including trifluoroacetic acid. This stood in contradiction to the findings reported by Warwel et al.,20 where authors claimed to characterize the same polyesters using gel permeation chromatography (GPC), although no experimental data was provided therein. To confirm the formation of macromolecules, the reaction was stopped at an earlier stage and the formed oligomers (soluble in conventional organic solvents) were characterized by NMR spectroscopy. The obtained oligomers and polymers were characterized by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The obtained polyesters have relatively low melting temperatures between 9 and 42 °C (Table 2, entries 1–7), which is characteristic of linear aliphatic polyesters. Significantly higher was the melting temperature for polyamide (152 °C, Table 2, entry 8), which is due to the formation of hydrogen bonds in comparison to polyesters. Heats of fusion varied from 25 to 73 J g−1. Aliphatic polyesters exhibited degradation temperatures around 400 °C, however, the polyester containing 1,4-cyclohexanediol block unit showed an increased degradation temperature of 446 °C due to the higher rigidity of the polymer that such a monomer provides.
| Entry | Polyester | Vacuum time | Yield | DPb | M n (g mol−1)b | T m (°C)c | ΔH (J g−1)c | T d (°C)d | Mass lossd |
|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: Ti(OnBu)4 1 mol%, no solvent, 140 min melt time with temperature increase from 150 to 200 °C. b Degree of polymerization (DP) and number average molecular weight (Mn) were calculated from 1H NMR by end group analysis. c Determined by DSC. d T d (decomposition temperature) and mass loss of the samples was determined by TGA. e Evident mass loss of 50% was observed at temperature 353 °C. n.d. – not determined due to polymer insolubility. | |||||||||
| 1 |
|
30 min | 51% | 1.7 | 592 | 42 | 64 | 403 | 97% |
| 2 | 19 h | 52% | n.d | n.d | 14 | 25 | 413 | 96% | |
| 3 |
|
225 min | 76% | 6.6 | 2238 | 34 | 72 | 395 | 98% |
| 4 | 21 h | 81% | n.d | n.d | 11 | 55 | 398 | 97% | |
| 5 |
|
180 min | 74% | 7.5 | 2539 | 38 | 73 | 397 | 98% |
| 6 | 21 h | 88% | n.d | n.d | 10 | 60 | 398 | 96% | |
| 7 |
|
19 h | 24% | n.d | n.d | 9 | 35 | 446e | 78%e |
| 8 |
|
19 h | 51% | n.d | n.d | 152 | 55 | 443 | 82% |
Conclusions
In summary, a novel process of obtaining functional polymer materials from methyl oleate was developed. Self-cross metathesis of methyl oleate in the presence of substantial amounts of methyl linoleate was investigated and the reaction was optimized to obtain diester 2 with high selectivity and yield. Subsequent polycondensation with a co-monomer (diol or diamine) resulted in the formation of polymeric materials which were characterized using TGA and DSC.Data Availability Statement
Data for this paper, including gas chromatograms of catalytic reactions, NMR, TGA, and DCS characterization data, are available at the HAL repository at https://hal.science.Author contributions
Paweł Krzesiński: investigation, data curation, formal analysis, visualization, writing – original draft. Vincent César: funding acquisition, writing – review & editing. Karol Grela: funding acquisition, writing – review & editing. Sergio Santos: investigation, data curation, formal analysis, writing – original draft. Pablo Ortiz: conceptualization, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 860322 (Coordination Chemistry Inspires Molecular Catalysis, CCIMC).Notes and references
- F. Rodriguez, C. Cohen, C. K. Ober and L. Archer, Principles of Polymer Systems, CRC Press, 2014 Search PubMed.
- M. A. R. Meier, J. O. Metzger and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 1788–1802 RSC.
- H. Mutlu and M. A. R. Meier, Eur. J. Lipid Sci. Technol., 2010, 112, 10–30 CrossRef CAS.
- Y. Xia and R. C. Larock, Green Chem., 2010, 12, 1893–1909 RSC.
- U. Biermann, U. Bornscheuer, M. A. R. Meier, J. O. Metzger and H. J. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 3854–3871 CrossRef CAS PubMed.
- F. D. Gunstone, Eur. J. Lipid Sci. Technol., 2001, 103, 307–314 CrossRef CAS.
- Y.-M. Choo, K.-E. Ooi, I.-H. Ooi and D. D. H. Tan, J. Am. Oil Chem. Soc., 1996, 73, 333–336 CrossRef CAS.
- J. C. Mol, Green Chem., 2002, 4, 5–13 RSC.
- J. C. Mol, Top. Catal., 2004, 27, 97–104 CrossRef CAS.
- J. Spekreijse, J. P. M. Sanders, J. H. Bitter and E. L. Scott, ChemSusChem, 2017, 10, 470–482 CrossRef CAS PubMed.
- VERBIO Vereinigte BioEnergie, https://www.marketscreener.com/quote/stock/VERBIO-VEREINIGTE-BIOENER-516720/news/PRESS-RELEASE-VERBIO-Vereinigte-BioEnergie-AG-2-36482898/, (accessed January 29, 2023) Search PubMed.
- A. Sytniczuk, A. Kajetanowicz and K. Grela, Chem Catal., 2023, 9, 100713 CrossRef.
- H. L. Ngo and T. A. Foglia, J. Am. Oil Chem. Soc., 2007, 84, 777–784 CrossRef CAS.
- G. S. Forman, R. M. Bellabarba, R. P. Tooze, A. M. Z. Slawin, R. Karch and R. Winde, J. Organomet. Chem., 2006, 691, 5513–5516 CrossRef CAS.
- M. K. Elmkaddem, P. de Caro, S. Thiébaud-Roux, Z. Mouloungui and E. Vedrenne, OCL: Oilseeds Fats, Crops Lipids, 2016, 23, D507 CrossRef.
- R. Kadyrov, C. Azap, S. Weidlich and D. Wolf, Top. Catal., 2012, 55, 538–542 CrossRef CAS.
- D. Quinzler and S. Mecking, Angew. Chem., Int. Ed., 2010, 49, 4306–4308 CrossRef CAS PubMed.
- P. Ortmann and S. Mecking, Macromolecules, 2013, 46, 7213–7218 CrossRef CAS.
- F. Stempfle, B. S. Ritter, R. Mülhaupt and S. Mecking, Green Chem., 2014, 16, 2008–2014 RSC.
- S. Warwel, F. Brüse, C. Demes, M. Kunz and M. R. Gen Klaas, Chemosphere, 2001, 43, 39–48 CrossRef CAS PubMed.
- A. Behr, N. Döring, S. Durowicz-Heil, B. Ellenberg, C. Kozik, Ch. Lohr and H. Schmidke, Lipid. Fett., 1993, 95, 2–12 CrossRef CAS.
- S. Nogales-Delgado, J. M. Encinar and Á. G. Cortés, Ind. Crops Prod., 2021, 170, 113701 CrossRef CAS.
- M. Qaim, in Encyclopedia of Food Security and Sustainability, ed. P. Ferranti, E. M. Berry and J. R. Anderson, Elsevier, 2019, vol. 3, pp. 159–164 Search PubMed.
- G. D. Lawrence, in The Fats of Life: Essential Fatty Acids in Health and Disease, Rutgers University Press, 2010, pp. 219–221 Search PubMed.
- J. Zimmerer, L. Williams, D. Pingen and S. Mecking, Green Chem., 2017, 19, 4865–4870 RSC.
- Á. Balla, M. Al-Hashimi, A. Hlil, H. S. Bazzi and R. Tuba, ChemCatChem, 2016, 8, 2865–2875 CrossRef.
- D. Astruc, in Olefin Metathesis: Theory and Practice, ed. K. Grela, John Wiley & Sons, 2014, pp. 1–36 Search PubMed.
- K. A. Burdett, L. D. Harris, P. Margl, B. R. Maughon, T. Mokhtar-Zadeh, P. C. Saucier and E. P. Wasserman, Organometallics, 2004, 23, 2027–2047 CrossRef CAS.
- M. B. Dinger and J. C. Mol, Adv. Synth. Catal., 2002, 344, 671 CrossRef CAS.
- J. Allard, I. Curbet, G. Chollet, F. Tripoteau, S. Sambou, F. Caijo, Y. Raoul, C. Crévisy, O. Baslé and M. Mauduit, Chem.–Eur. J., 2017, 23, 12729–12734 CrossRef CAS PubMed.
- C. S. Higman, L. Plais and D. E. Fogg, ChemCatChem, 2013, 5, 3548–3551 CrossRef CAS.
- C. S. Higman, A. E. Lanterna, M. L. Marin, J. C. Scaiano and D. E. Fogg, ChemCatChem, 2016, 8, 2446–2449 CrossRef CAS.
- M. Rouen, P. Queval, E. Borré, L. Falivene, A. Poater, M. Berthod, F. Hugues, L. Cavallo, O. Baslé, H. Olivier-Bourbigou and M. Mauduit, ACS Catal., 2016, 6, 7970–7976 CrossRef CAS.
- S. H. Hong, D. P. Sanders, C. W. Lee and R. H. Grubbs, J. Am. Chem. Soc., 2005, 127, 17160–17161 CrossRef CAS PubMed.
- L. Monsigny, A. Kajetanowicz and K. Grela, Chem. Rec., 2021, 21, 3648–3661 CrossRef CAS PubMed.
- P. Małecki, K. Gajda, R. Gajda, K. Woźniak, B. Trzaskowski, A. Kajetanowicz and K. Grela, ACS Catal., 2019, 9, 587–598 CrossRef.
- A. Kajetanowicz and K. Grela, Angew. Chem., Int. Ed., 2021, 60, 13738–13756 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00305a |
| ‡ Extra-pure methyl oleate (>99%) is commercialized by Aldrich (79 €/5 g). Such reagent has been used in many academic research, for example in ref. 29. |
| § OM of conjugated polyolefins (such as methyl lineolate) can cause issues of reaction selectivity (ref. 25) and catalyst efficiency (ref. 26). |
| ¶ For methods of methyl oleate pre-treatment to maximize catalyst efficiency consult ref. 30 and references cited therein. |
| || For example, such pre-treatment protocol was utilized in ref. 29. |
| ** For effects of nitro and other electron withdrawing groups on catalyst activity and application see ref. 37. |
| This journal is © The Royal Society of Chemistry 2023 |