Multifunctional AuPd-cluster nanotheranostic agents with a cascade self-regulating redox tumor-microenvironment for dual-photodynamic synergized enzyme catalytic therapy†
Shilei
Ren‡
a,
Rong
Dai‡
b,
Ziliang
Zheng‡
b,
Xuejiao
Chen
b,
Shutong
Wu
b,
Ruiping
Zhang
 *b and
Zhiguo
Gui
*a
*b and
Zhiguo
Gui
*a
aShanxi Provincial Key Laboratory for Biomedical Imaging and Big Data, College of Information and Communication Engineering, North University of China, Taiyuan, 030051, China. E-mail: zrp_7142@sxmu.edu.cn
bDepartment of Radiology, First hospital of Shanxi Medical University, Taiyuan, 030001, China. E-mail: gzgtg@163.com
First published on 26th November 2022
Abstract
Photodynamic therapy (PDT) has emerged as a promising strategy with higher selectivity and spatiotemporal control than conventional therapies. However, deep hypoxia in tumours has hampered the clinical use of PDT. In this study, a novel multifunctional cluster nanotheranostic agent (AuPd-BSA CN) was fabricated to generate a high amount of reactive oxygen species, regardless of oxygen dependence under 660 nm laser irradiation. The structure and properties of the AuPd-BSA CN were characterised using various technologies. The synthesised AuPd-BSA CN with high biocompatibility served as a superior photodynamic agent, showing prominent antitumour properties under laser irradiation. Additionally, the glucose oxidase-like activity of the AuPd-BSA CN synergistically enhanced the therapeutic performance. Notably, the intrinsic characteristics of the AuPd-BSA CN include dual-modal second near-infrared window fluorescence/photoacoustic imaging capabilities for monitoring and tracking the in vivo tumour therapeutic process. This work provides innovative insights into the AuPd-BSA CN as an “all-in-one” nanoplatform for cancer therapy.
1. Introduction
Tumour theranostics that uses molecular imaging techniques to study the heterogeneity of tumours before initiating therapy has received wide attention and can achieve higher therapeutic effects guided by imaging for more precise treatment.1,2 Compared to traditional imaging techniques, fluorescence (FL) imaging offers great advantages in terms of spatial resolution, sensitivity, and detection safety.3,4 FL imaging, particularly in the second near-infrared window (NIR-II, 1000–1700 nm), greatly lowers tissue autofluorescence and scattering, allowing for a much higher signal-to-noise ratio and deeper tissue penetration ability compared to the traditional NIR-I (780–900 nm) window.5–7 The unique merits of NIR-II FL imaging allow for precisely guided cancer treatment, which has the potential for future clinical therapy.Among various non-invasive therapies, photodynamic therapy (PDT) has emerged as a clinical modality owing to its non-invasive nature and rapid healing process.8–12 In PDT, photosensitisers can transfer light energy into highly toxic reactive oxygen species (ROS) to induce tumour cell apoptosis. At present, most developed PDT photosensitisers work through the oxygen (O2)-dependent PDT-II path. These photosensitisers can produce cytotoxic singlet oxygen (1O2) to kill tumour cells upon reaction with tissue oxygen; nevertheless, tumour hypoxia greatly reduces therapeutic efficiency.13–15 Therefore, multiple O2-replenishing strategies have been developed to alleviate tumour hypoxia and enhance PDT efficiency,16–18 but these strategies also increase the complexity of treatments. The recently developed type I PDT can directly transfer a proton or electron from biological substrates to form free radicals and react with water molecules to generate O2˙− and ˙OH, which perform well in a hypoxic tumour environment.19,20 To date, most photosensitisers capable of type I PDT are organic materials.13,21 For example, Chang et al.21 reported that 2,6-di-anisole substituted BODIPY with simultaneous types I and II photosensitisation showed a highly efficient anticancer effect via photodamage. However, organic photosensitisers easily undergo photobleaching, which leads to a reduction in the PDT efficacy. Fortunately, functionalised inorganic photosensitisers with excellent photostability and high molar extinction coefficients are expected to compensate for these deficiencies. However, owing to the biosafety of inorganic materials, the design of efficient and safe multifunctional photosensitisers remains a challenge.
Herein, we report a novel multifunctional cluster nanotheranostic agent (AuPd-BSA CN) for dual-modal NIR-II FL/Photoacoustic (PA) imaging-guided type I/II PDT (Scheme 1). First, ultrasmall AuPd-BSA CNs have shown excellent biocompatibility after BSA modification and exhibit good properties because of their intrinsic physics, such as a strong NIR-II FL intensity and superior catalytic activity. Second, alloying palladium (Pd) with gold (Au), with improved NIR absorption and FL intensity, grants the cluster nanotheranostic agent not only excellent NIR-II FL and PA contrast, but also an efficient combination of PDT-I and PDT-II upon laser irradiation, leading to an “all-in-one” theranostic agent with both diagnostic and therapeutic functions. Specifically, through type I PDT photochemical reactions with water and via type II, the AuPd-BSA CN exhibited excellent photosensitising properties under 660 nm laser irradiation, which produced ˙OH, O2˙−, and 1O2. The AuPd-BSA CN exhibited enhanced reaction activity compared with the Au-BSA CN owing to an increased electron and hole pair separation,22 which endowed the AuPd-BSA CN with strong light absorption and good type I/type II PDT capability in a hypoxic tumour environment. In addition, the intrinsic glucose oxidase (Gox)-like activity of the AuPd-BSA CN further enhanced the therapeutic effect. The AuPd-BSA CN combined with type I/type II PDT realised efficient hypoxic tumour killing under the guidance of dual-modal NIR-II FL/PA imaging, which provides a strategy for the practical design of multifunctional PDT photosensitisers.
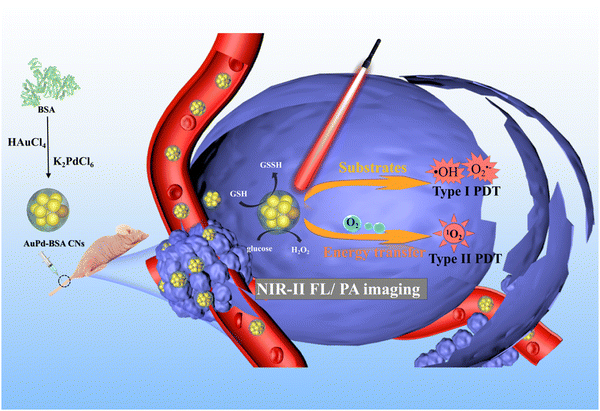 | ||
| Scheme 1 Schematic illustration of AuPd-BSA cluster nanotheranostic for NIR-II FL/PA imaging guided PDT hypoxia tumor therapy. | ||
2. Results and discussion
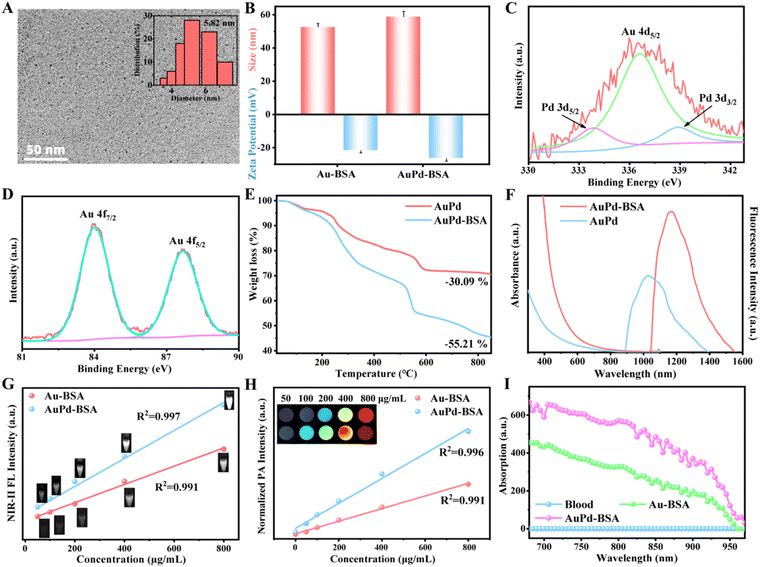
AuPd-BSA CNs were synthesised using BSA as a stabiliser via a facile one-pot synthesis. First, the Au and Pd precursors (i.e. HAuCl4 and K2PdCl6) were reduced by glutathione (GSH), and the colour of the solution changed to pale yellow. Second, the aforementioned solution was subjected to carbon monoxide reduction under alkaline conditions with BSA as a model protein. The structure and properties of the AuPd-BSA CNs were characterised using various technologies. Transmission electron microscopy (TEM) images (Fig. 1A) showed that the AuPd-BSA cluster nanotheranostics were well dispersed and had a spherical morphology with an average size of 5.82 nm. The hydrated size determined by dynamic light scattering (∼55 nm) (Fig. 1B) is larger than that observed by TEM, which is due to the presence of hydration layers. The zeta potential of the AuPd-BSA CN was found to be −26.5 ± 1.62 mV, which could be due to the large negative surface charge of BSA. Moreover, the surface composition and valence state of nanozymes were examined by X-ray photoelectron spectroscopy (XPS). As shown in Fig. 1C, the Au 4f spectra showed two peaks at 83.97 and 87.65 eV for Au 4f7/2 and Au 4f5/2 of metallic Au(0), respectively. In the high-resolution Pd XPS spectrum (Fig. 1D), the doublet peaks located at 333.66 and 338.86 eV corresponded to the Pd 3d5/2 and Pd 3d3/2 states of metallic Pt, respectively.23 Based on the XPS results, the peak (Au 4d5/2, 336.57 eV) would appear in the Pd 3d XPS spectra if one metal was doped with Au. The binding energy for Au shifted negatively with the introduction of Pd, which was indicative of Au and Pd in the alloy state in the AuPd-BSA CN.24 The Au and Pd content of the AuPd-BSA CN were measured by the ICP, and the ratio of Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd (23.9
Pd (23.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was near the initial metal precursor concentrations. The weight ratio of BSA in AuPd-BSA was determined to be 25.12% using thermogravimetric analysis (Fig. 1E). Furthermore, the size and zeta potential of the AuPd-BSA CN showed negligible change in different solutions (DI, PBS and FBS) at 37 °C within 3 days (Fig. S1, ESI†), suggesting that the AuPd-BSA CN had high physiological stability.
1) was near the initial metal precursor concentrations. The weight ratio of BSA in AuPd-BSA was determined to be 25.12% using thermogravimetric analysis (Fig. 1E). Furthermore, the size and zeta potential of the AuPd-BSA CN showed negligible change in different solutions (DI, PBS and FBS) at 37 °C within 3 days (Fig. S1, ESI†), suggesting that the AuPd-BSA CN had high physiological stability.
The optical absorption and FL spectra of the AuPd-BSA CN were evaluated. As shown in Fig. 1F, Au-BSA and AuPd-BSA showed broad absorption in the ultraviolet (UV)-NIR wavelength range. Notably, AuPd-BSA exhibited a higher absorption intensity than Au-BSA, which could be due to the alloying of Pd with Au, which increased electron and hole pair separation. Moreover, the FL intensity of AuPd-BSA was higher than that of Au-BSA. The FL intensity at 1064 nm gradually increased with the concentration of AuPd-BSA and exhibited a desirable linear relationship (R2 = 0.997) with the concentration of the sample in the wide range of 0–800 μg mL−1 (Fig. 1G).
Encouraged by its superior absorption ability, we presented the PA intensity and the corresponding images at different concentrations of AuPd-BSA and Au-BSA CNs in Fig. 1H. The PA signal intensity was linearly enhanced with increasing AuPd-BSA concentrations (0–800 μg mL−1) under 808 nm laser irradiation. Moreover, Au-BSA and AuPd-BSA upon 808 nm laser irradiation exhibited ∼10-fold and ∼15-fold higher PA signals, respectively, than mouse blood (Fig. 1I). The superior absorption and FL emission of the AuPd-BSA CN were expected to guarantee highly sensitive PA and NIR-II FL imaging.
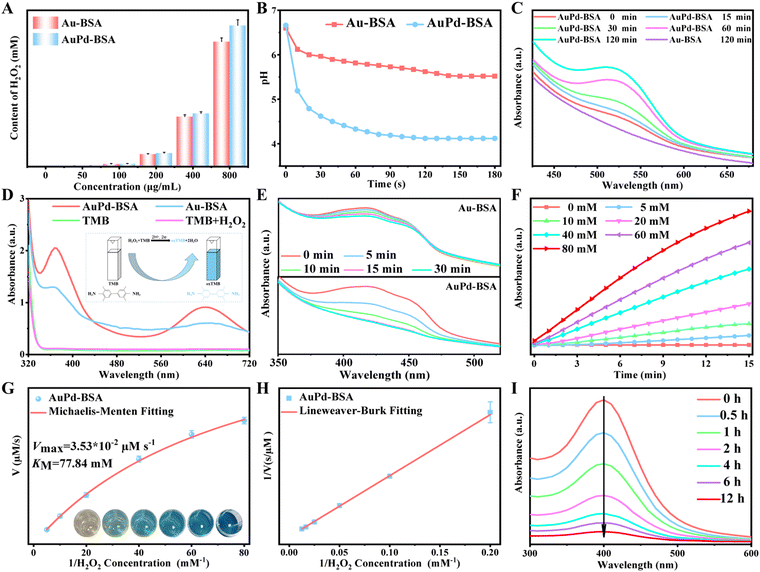
The GOx-like activities of the Au-BSA and AuPd-BSA CNs were first evaluated for cluster nanotheranostics. Titanium oxysulfate was used as an indicator to test the hydrogen peroxide (H2O2) production efficiency of the Au-BSA and AuPd-BSA CNs in the presence of glucose. Because titanium peroxide composites present a strong absorption peak at 415 nm, Fig. 2A quantitatively reveals that AuPd-BSA can induce higher H2O2 generation than Au-BSA, and the absorption intensity is positively correlated with the concentration of AuPd-BSA. Simultaneously, an obvious pH decline occurred in the AuPd-BSA group over time (Fig. 2B), suggesting the effective catalysis of glucose by AuPd-BSA. Moreover, the trends of dissolved O2 in the various groups were consistent with the pH value changes (Fig. S2, ESI†), further demonstrating that AuPd-BSA possesses superior GOx-like activity.
Alloying Pd with Au was found to have a positive effect on the electron transfer process involving Type I PDT.22 The production of O2˙− was investigated using hydroxylamine hydrochloride (NH2OH·HCl). NH2OH can be oxidised by O2˙− to NO2−, then diazotised with sulfanilic acid and coupled with α-naphthylamine to produce a red azo dye, accompanied by an increase in absorbance at 530 nm. As shown in Fig. 2C, the production of O2˙− in the AuPd-BSA group was higher than that in the Au-BSA group with 660 nm laser irradiation, suggesting that alloying Pd with Au promotes the generation of O2˙−. Moreover, the O2˙− generation efficiency was positively correlated with the increase in the illumination time.
Next, the generation of ˙OH during Type I PDT was assessed by 3,3′,5,5′-tetramethylbenzidine (TMB), which could be oxidised to the blue-coloured oxidised (ox)-TMB with the UV-visible absorbance peak at 652 nm. As shown in Fig. 2D, only a slight enhancement at 652 nm was observed in the Au-BSA + H2O2 group. Furthermore, the absorption spectrum peaks of oxTMB increased over time with the addition of H2O2 and TMB (Fig. S3, ESI†). The introduction of Pd into Au could obviously increase electron/hole separation, thus enhancing the optical absorption efficiency at 660 nm, which would lead to a high level of 1O2 generation via type II PDT. Therefore, 1,3-diphenylisobenzofuran (DPBF) was used to generate 1O2. As revealed by the DPBF decay curves (Fig. 2E), AuPd-BSA produced more 1O2 than Au-BSA did upon laser irradiation.
The produced 1O2 and ˙OH radicals were further verified by electron spin resonance (ESR) spectroscopy. 2,2,6,6-Tetramethylpiperidine (TEMP) was a specific target molecule of 1O2. Meanwhile, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) was used as the spin trap for ˙OH. As shown in Fig. S4 (ESI†), an obvious 1O2 and ˙OH signal were observed in the spectra of the AuPd-BSA CN upon laser irradiation (660 nm), whereas a negligible ESR signal was observed in the absence of laser irradiation, which indicated that the AuPd-BSA CN could lead to a high level of ˙OH and 1O2 generation via type I and type II PDT, respectively.
To systematically evaluate the catalytic performance of the AuPd-BSA CN, a steady-state kinetic assay was performed at 25 °C using TMB and H2O2 as substrates in acid phosphate buffer saline. The AuPd-BSA CNs (800 μg mL−1) with different concentrations of H2O2 (0, 5, 10, 20, 40, 60, and 80 mM) were included in the presence of TMB (0.5 mM), and the absorbance was recorded (Fig. 2F). The initial reaction rates (v0) of ˙OH generation were calculated according to the Beer–Lambert law (eqn (1)). Then, the reaction rates were plotted against the corresponding H2O2 concentrations and fitted using the Michaelis–Menten saturation curve in Fig. 2G. Vmax represents the maximal reaction velocity, [S] represents the initial concentration of the substrates, and KM represents the Michaelis–Menten constant.
| V0 = Vmax[S]/(KM + [S]) | (1) |
To obtain KM and Vmax, the double reciprocal plot of the Michaelis–Menten equation was converted (eqn (2)), as shown in Fig. 2H. KM revealed the affinity of the nanozyme for the substrate. Based on the following equations, the Vmax and KM of the AuPd-BSA CN at 25 °C were calculated to be 3.53 × 10−8 M s−1 and 77.84 mM, respectively, which are smaller than those of the other catalysts (Table S1, ESI†).25–30
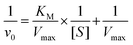 | (2) |
Although the AuPd-BSA CN exhibited excellent type I and type II PDT activities, the overproduction of GSH in tumour cells depleted the generated ROS. Thus, the GSH-depletion capability of AuPd-BSA was further investigated using 5,5′-dithiobis-(2-nitrobenzoic acid), which reacts with the remnant GSH to produce TNB with a typical absorbance at 412 nm. Fig. 2I quantitatively shows that the absorption intensity at 412 nm decreased with interaction time, indicating that a certain amount of GSH was depleted by the AuPd-BSA CN. Moreover, the consumed GSH was gradually converted to glutathione disulfide (Fig. S5, ESI†). Based on the aforementioned analysis, the AuPd-BSA CN can serve as effective PDT photosensitiser through type I and II mechanisms.
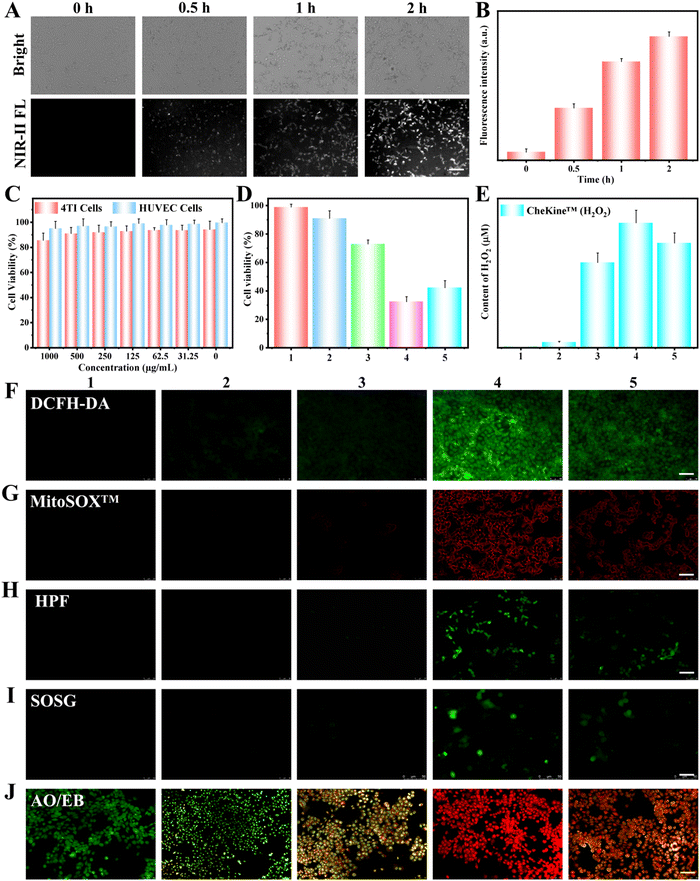
The cellular uptake of AuPd-BSA was investigated in 4T1 cells. As shown in Fig. 3A and B, the intensity of the intracellular NIR-II FL signal emitted by AuPd-BSA gradually increased over incubation time, revealing that AuPd-BSA can be largely taken up by 4T1 cells, which is beneficial for the AuPd-BSA-catalysed killing of 4T1 cells. The biocompatibility of the AuPd-BSA CN is a key element in clinical applications, and their cytotoxicity to 4T1 and human umbilical vein endothelial (HUVEC) cells was measured via the Cell Counting Kit-8 assay. AuPd-BSA displayed a negligible cytotoxic effect on 4T1 and HUVEC cells (Fig. 3C), even when the concentration was as high as 1000 μg mL−1, suggesting its good biocompatibility in vitro. Encouraged by the superior performance of AuPd-BSA, we evaluated its photodynamic therapeutic effect at the cellular level (Fig. 3D). AuPd-BSA with extra glucose exhibited 71% cell survival, which was attributed to the GOx-like properties of AuPd-BSA. Upon laser irradiation, AuPd-BSA with extra glucose presented higher cell killing efficiency under both hypoxic (42.1%) and normoxic (32.3%) conditions, which indicated that AuPd-BSA could realise PDT regardless of oxygen dependence.
To evaluate the different types of ROS produced during PDT, 4T1 cells treated with different groups were stained with various FL probes. MitoSOXTM, HPF, SOSG, and DCFH-DA were selected as probes to assess the generation of intracellular O2˙−, ˙OH, 1O2, and total ROS, respectively (Fig. 3F–I). In addition, we used the CheKine™ H2O2 colorimetric assay kit to monitor the intracellular generation of H2O2 in 4T1 cells in different groups (Fig. 3E). Without laser irradiation, a certain amount of H2O2 with only slight HPF FL was observed in the AuPd-BSA + glucose group, indicating that oxidative stress was induced by the GOx-like catalytic activity of AuPd-BSA. Notably, upon laser irradiation, all four probes exhibited bright FL after treatment with AuPd-BSA and glucose under normoxic conditions, suggesting that 1O2, O2˙−, and ˙OH were generated, effectively inducing cell apoptosis. Under hypoxic conditions, MitoSOXTM, HPF, and DCFH-DA showed clear “turn-on” FL. In contrast, the SOSG FL signal was significantly reduced compared with that under normal conditions. These results demonstrate that the therapeutic efficacy of oxygen-dependent type II PDT is limited by hypoxic conditions; however, under hypoxic conditions, O2˙− and ˙OH were produced by AuPd-BSA CNs through the type I mechanism, thus effectively killing cancer cells. Thus, the AuPd-BSA CN could realise a combinational PDT through types I and II mechanisms which was expected to improve the in vivo anti-tumour activity.
Furthermore, apoptosis was visually observed by acridine orange/ethidium bromide staining. Early and late apoptotic cells are highlighted in green and orange, respectively, while dead cells are highlighted in red. As shown in Fig. 3J, upon laser irradiation, AuPd-BSA with extra glucose exhibited greater suppression of cancer cells under normoxia than under hypoxic conditions. Meanwhile, in order to further demonstrate the apoptosis of cells the flow cytometer analysis of annexin V-FITC/PI-stained cells after different treatments were explored (Fig. S6, ESI†). The AuPd-BSA + glucose + Laser group exhibited the highest suppression of cancer cells than other groups, which was consistent with CCK-8 and AO/EB staining results. This phenomenon revealed that the PDT process of AuPd-BSA could undergo both the O2-insensitive type I PDT pathway and O2-dependent type II pathway under normoxia, revealing that AuPd-BSA could serve as a promising photosensitiser in hypoxic tumours.
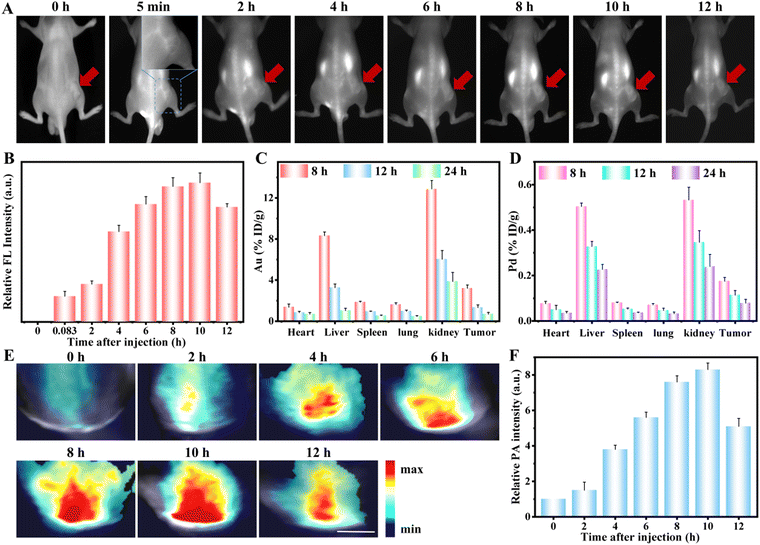
Encouraged by the excellent NIR-II FL and PA signals of the AuPd-BSA aqueous solution, we further evaluated the in vivo NIR-II FL and PA imaging. We intravenously administered AuPd-BSA into tumour-bearing mice. As shown in Fig. 4A, during the entire monitoring process, the FL signals in the tumour region increased gradually over time and peaked at 10 hours post-injection. Quantitative analysis revealed a 4-fold increase in the NIR-II FL signal at 10 hours in the AuPd-BSA group, suggesting high tumour accumulation of AuPd-BSA through the enhanced permeability and retention effect owing to its excellent biocompatibility and tumour accumulation (Fig. 4B).31 Then, we lysed the major organs and tumours from the AuPd-BSA group for quantitative measurement of the Au and Pd contents via inductively coupled plasma analysis (Fig. 4C and D). The highest accumulation of AuPd-BSA was at 10 hours post-injection. Moreover, AuPd-BSA was mainly distributed in the liver and kidneys, a bio-distribution pattern typical of nanoparticles, which could then be properly cleared from the body 12 hours post-injection. Similarly, the PA signals gradually increased in the tumour region and peaked at 10 hours post-injection with the AuPd-BSA CN (Fig. 4E and F). Based on the aforementioned analysis, 10 hours was selected as the optimal time for subsequent PDT, owing to the maximal accumulation of AuPd-BSA CNs in the tumour region.
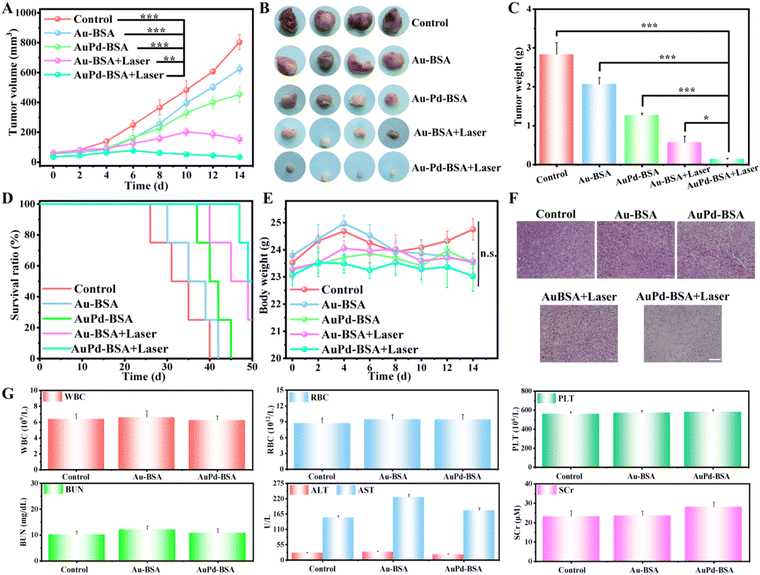
To evaluate tumour therapy in vivo, when the tumour size reached 80–100 mm3, the 4T1 tumour-bearing mice were randomly divided into five groups: (1) PBS + Laser, (2) Au-BSA, (3) AuPd-BSA, (4) Au-BSA + Laser, and (5) AuPd-BSA + Laser. After 10 hours of intravenous systemic administration of the samples, the tumour region was irradiated using a 660 nm laser. The volume of the tumour was recorded every other day. As shown in Fig. 5A, Au-BSA- and AuPd-BSA-treated groups without laser irradiation showed little tumour inhibition, presumably due to the GOx-like catalytic activity originating from the intrinsic characteristics of the cluster nanotheranostic agent. Comparatively, obvious tumour inhibition was observed in the Au-BSA + Laser and AuPd-BSA + Laser groups, which could be due to the combinational type I/type II PDT performance of AuPd-BSA, as well as the intrinsic enzyme catalytic properties of AuPd-BSA CN. Notably, the AuPd-BSA + Laser group exhibited a much better therapeutic effect than the Au-BSA + Laser group, suggesting that alloying Pd with Au had a positive effect on ROS generation. Digital photographs (Fig. 5B) and tumour weights (Fig. 5C) confirmed this trend. Moreover, mice in the AuPd-BSA + Laser group showed significantly prolonged survival, with approximately 48% of the mice surviving more than 40 days (Fig. 5D). During the treatment, the body weights of mice in all groups exhibited no obvious differences (Fig. 5E), which verified that the cluster nanotheranostic agents showed negligible toxicity for in vivo applications. Haematoxylin and eosin staining of tumour tissues showed that most tumour cells were completely destroyed in the AuPd-BSA + Laser group (Fig. 5F) compared with the other groups, further demonstrating the efficacy of AuPd-BSA for PDT ablation of tumour cells. Furthermore, no significant pathological changes were observed in the major organs (heart, liver, spleen, lungs, and kidneys) of the different groups (Fig. S7, ESI†).
The biosafety of the administration was further evaluated after 15 days of therapy using routine blood parameters. As shown in Fig. 5G, routine blood parameters (white blood cell count, red blood cell count, haemoglobin level, and platelet count) and serum enzyme levels (Alanine transaminase, aspartate transaminase, and blood urea nitrogen) were slightly elevated between the groups, suggesting that the blood toxicity of the AuPd-BSA CN was negligible. In general, AuPd-BSA exhibited prominent tumour growth suppression efficacy without obvious toxicity, which will inspire further development of NIR-II-emissive and type I/II combined PDT photosensitisers for hypoxic tumour theranostics.
3. Conclusions
In summary, we successfully prepared a multifunctional biocompatibility cluster nanotheranostic agent (AuPd-BSA CN) for dual-modal NIR-II FL/PA imaging-guided hypoxic cancer PDT. The remarkable performance of the AuPd-BSA CN can be attributed to the following: (1) in vitro and in vivo studies have confirmed that the AuPd-BSA CN with good biocompatibility can not only enhance dual-modal NIR-II FL/PA imaging capabilities but also promote PDT efficacy. (2) Alloying Pd with Au (AuPd-BSA CN) could lead to a high level of ROS generation regardless of oxygen dependence under 660 nm laser irradiation, thus realising an efficient combination of PDT-I and PDT-II. (3) The intrinsic characteristic of Au-Pd-BSA CN endows them with GOx-like activity and GSH elimination ability, thus synergistically improving the therapy efficiency. This work opens new avenues for the development of “all-in-one” nanoplatforms for deep and hypoxic cancer therapy.Author contributions
All authors have participated in conceptualization and discussion steps. S. L. Ren have worked on the conceptualization, investigation, writing – original draft. Ziliang Zheng and Rong Dai contributed to the study conception and design writing – review & editing. X. J. Chen and S. T. Wu were responsible for writing – original draft. R. P. Zhang, and Z. G. Gui contributed to supervision, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (82102124, 82071987, 81771907, 81802440, 8210072688, and 82202238); the Science and Technology Innovation Team Project of Shanxi Province (201705D131026); the Engineering Technology Research Center of Shanxi Province (201805D121008); the Scientific and Technological Achievements Transformation Project of Shanxi Province (201704D131006); the Laboratory Construction Project of Shanxi Province, the Projects for Local Science and Technology Development Guided by the Central Committee (YDZX20191400002537); the Research Project Supported by Shanxi Scholarship Council of China (2020-177); th eFund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (20200006); the Four Batches of Scientific Research Projects of Shanxi Provincial Health Commission (2020TD11, 2020SYS15, and 2020XM10); the Key Laboratory of Nano-imaging and Drug-loaded Preparation of Shanxi Province (202104010910010); the Shanxi Nanobiomedical Engineering Research Center, Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2019L0415); and the Shanxi Province Science Foundation for Youths (201801D221400 and 201901D211343); The authors also would like to thank the shiyanjia lab (https://www.shiyanjia.com) for the XPS analysis.References
- Z. Zheng, X. Chen, Y. Ma, R. Dai, S. Wu, T. Wang, J. Xing, J. Gao and R. Zhang, Dual H2O2-Amplified Nanofactory for Simultaneous Self-Enhanced NIR-II Fluorescence Activation Imaging and Synergistic Tumor Therapy, Small, 2022, 2203531 CrossRef CAS.
- L. Liu, X. Wang, L. J. Wang, L. Guo, Y. Li, B. Bai, F. Fu, H. Lu and X. Zhao, One-for-All Phototheranostic Agent Based on Aggregation-Induced Emission Characteristics for Multimodal Imaging-Guided Synergistic Photodynamic/Photothermal Cancer Therapy, ACS Appl. Mater. Interfaces, 2021, 13(17), 19668–19678 CrossRef CAS.
- C. Li, G. Chen, Y. Zhang, F. Wu and Q. Wang, Advanced Fluorescence Imaging Technology in the Near-Infrared-II Window for Biomedical Applications, J. Am. Chem. Soc., 2020, 142(35), 14789–14804 CrossRef CAS PubMed.
- S. Wang, W. X. Ren, J. T. Hou, M. Won, J. An, X. Chen, J. Shu and J. S. Kim, Fluorescence Imaging of Pathophysiological Microenvironments, Chem. Soc. Rev., 2021, 50(16), 8887–8902 RSC.
- T. Wei and O. C. Farokhzad, Theranostic nanomedicine in the NIR-II window: Classification, fabrication, and biomedical applications, Chem. Rev., 2022, 122(6), 5405–5407 CrossRef.
- D. Li, X. Chen, D. Wang, H. Wu, H. Wen, L. Wang, Q. Jin, D. Wang, J. Ji and B. Z. Tang, Synchronously Boosting Type-I Photodynamic and Photothermal Efficacies via Molecular Manipulation for Pancreatic Cancer Theranostics in the NIR-II Window, Biomaterials, 2022, 283, 121476 CrossRef CAS PubMed.
- C. Li and Q. Wang, Challenges and Opportunities for Intravital Near-Infrared Fluorescence Imaging Technology in the Second Transparency Window, ACS Nano, 2018, 12(10), 9654–9659 CrossRef CAS PubMed.
- K. Wang, B. Yu and J. L. Pathak, An update in clinical utilization of photodynamic therapy for lung cancer, J. Cancer, 2021, 12(4), 1154–1160 CrossRef CAS PubMed.
- F. Xu, H. Ge, N. Xu, C. Yang, Q. Yao, S. Long, W. Sun, J. Fan, X. Xu and X. Peng, Radical induced quartet photosensitizers with high 1O2 production for in vivo cancer photodynamic therapy. Science. China, Chemistry, 2021, 64(3), 488–498 CAS.
- Y. Li, L. Du, F. Li, Z. Deng and S. Zeng, Intelligent Nanotransducer for Deep-Tumor Hypoxia Modulation and Enhanced Dual-Photosensitizer Photodynamic Therapy, ACS Appl. Mater. Interfaces, 2022, 14(13), 14944–14952 CrossRef CAS PubMed.
- J. Karges, Clinical Development of Metal Complexes as Photosensitizers for Photodynamic Therapy of Cancer, Angew. Chem., Int. Ed., 2022, 61(5), e202112236 CrossRef CAS PubMed.
- T. C. Pham, V. N. Nguyen, Y. Choi, S. Lee and J. Yoon, Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy, Chem. Rev., 2021, 121(21), 13454–13619 CrossRef CAS PubMed.
- J. An, S. Tang, G. Hong, W. Chen, M. Chen, J. Song, Z. Li, X. Peng, F. Song and W. H. Zheng, An unexpected strategy to alleviate hypoxia limitation of photodynamic therapy by biotinylation of photosensitizers, Nat. Commun., 2022, 13(1), 2225 CrossRef CAS.
- I. S. Turan, D. Yildiz, A. Turksoy, G. Gunaydin and E. U. Akkaya, A Bifunctional Photosensitizer for Enhanced Fractional Photodynamic Therapy: Singlet Oxygen Generation in the Presence and Absence of Light, Angew. Chem., Int. Ed., 2016, 55(8), 2875–2878 CrossRef CAS.
- Y. Liu, Y. Liu, W. Bu, C. Cheng, C. Zuo, Q. Xiao, Y. Sun, D. Ni, C. Zhang, J. Liu and J. Shi, Hypoxia Induced by Upconversion-Based Photodynamic Therapy: Towards Highly Effective Synergistic Bioreductive Therapy in Tumors, Angew. Chem., Int. Ed., 2015, 54(28), 8105–8109 CrossRef CAS PubMed.
- G. Yang, L. Xu, Y. Chao, J. Xu, X. Sun, Y. Wu, R. Peng and Z. Liu, Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses, Nat. Commun., 2017, 8(1), 902 CrossRef PubMed.
- T. Lin, X. Zhao, S. Zhao, H. Yu, W. Cao, W. Chen, H. Wei and H. Guo, O2-generating MnO2 nanoparticles for enhanced photodynamic therapy of bladder cancer by ameliorating hypoxia, Theranostics, 2018, 8(4), 990–1004 CrossRef CAS PubMed.
- H. S. Jung, J. Han, H. Shi, S. Koo, H. Singh, H. J. Kim, J. L. Sessler, J. Y. Lee, J. H. Kim and J. S. Kim, Overcoming the Limits of Hypoxia in Photodynamic Therapy: A Carbonic Anhydrase IX-Targeted Approach, J. Am. Chem. Soc., 2017, 139(22), 7595–7602 CrossRef CAS.
- L. Li, C. Shao, T. Liu, Z. Chao, H. Chen, F. Xiao, H. He, Z. Wei, Y. Zhu, H. Wang, X. Zhang, Y. Wen, B. Yang, F. He and L. Tian, An NIR-II-Emissive Photosensitizer for Hypoxia-Tolerant Photodynamic Theranostics, Adv. Mater., 2020, 32(45), 2003471 CrossRef CAS PubMed.
- D. Chen, Q. Xu, W. Wang, J. Shao, W. Huang and X. Dong, Type I Photosensitizers Revitalizing Photodynamic Oncotherapy, Small, 2021, 17(31), 2006742 CrossRef CAS PubMed.
- C. Yao, Y. Li, Z. Wang, C. Song, X. Hu and S. Liu, Cytosolic NQO1 Enzyme-Activated Near-Infrared Fluorescence Imaging and Photodynamic Therapy with Polymeric Vesicles, ACS Nano, 2020, 14(2), 1919–1935 CrossRef CAS PubMed.
- Y. Yin, Y. Yang, L. Zhang, Y. Li, Z. Li, W. Lei, Y. Ma and Z. Huang, Facile synthesis of Au/Pd nano-dogbones and their plasmon-enhanced visible-to-NIR light photocatalytic performance, RSC Adv., 2017, 7(59), 36923–36928 RSC.
- P. Das, S. V. Mudigunda, G. Darabdhara, P. K. Boruah, S. Ghar, A. K. Rengan and M. R. Das, Biocompatible functionalized AuPd bimetallic nanoparticles decorated on reduced graphene oxide sheets for photothermal therapy of targeted cancer cells, J. Photochem. Photobiol., B, 2020, 212, 112028 CrossRef CAS PubMed.
- C. Hsu, C. Huang, Y. Hao and F. Liu, Au/Pd core–shell nanoparticles for enhanced electrocatalytic activity and durability, Electrochem. Commun., 2012, 23, 133–136 CrossRef CAS.
- S. Tanaka, Y. V. Kaneti, R. Bhattacharjee, M. N. Islam, R. Nakahata, N. Abdullah, S. I. Yusa, N. T. Nguyen, M. J. A. Shiddiky, Y. Yamauchi and M. S. A. Hossain, Mesoporous Iron Oxide Synthesized Using Poly(styrene-b-acrylic acid-b-ethylene glycol) Block Copolymer Micelles as Templates for Colorimetric and Electrochemical Detection of Glucose, ACS Appl. Mater. Interfaces, 2018, 10(1), 1039–1049 CrossRef CAS PubMed.
- C. W. Lien, C. C. Huang and H. T. Chang, Peroxidase-mimic bismuth-gold nanoparticles for determining the activity of thrombin and drug screening, Chem. Commun., 2012, 48(64), 7952–7954 RSC.
- J. M. Park, H. W. Jung, Y. W. Chang, H. S. Kim, M. J. Kang and J. C. Pyun, Chemiluminescence lateral flow immunoassay based on Pt nanoparticle with peroxidase activity, Anal. Chim. Acta, 2015, 853, 360–367 CrossRef CAS PubMed.
- S. Li, L. Shang, B. Xu, S. Wang, K. Gu, Q. Wu, Y. Sun, Q. Zhang, H. Yang, F. Zhang, L. Gu, T. Zhang and H. Liu, A Nanozyme with Photo-Enhanced Dual Enzyme-Like Activities for Deep Pancreatic Cancer Therapy, Angew. Chem., Int. Ed., 2019, 58(36), 12624–12631 CrossRef CAS PubMed.
- Y. Wu, J. Wu, L. Jiao, W. Xu, H. Wang, X. Wei, W. Gu, G. Ren, N. Zhang, Q. Zhang, L. Huang, L. Gu and C. Zhu, Cascade Reaction System Integrating Single-Atom Nanozymes with Abundant Cu Sites for Enhanced Biosensing, Anal. Chem., 2020, 92(4), 3373–3379 CrossRef CAS.
- A. P. Nagvenkar and A. Gedanken, Cu0.89Zn0.11O, A New Peroxidase-Mimicking Nanozyme with High Sensitivity for Glucose and Antioxidant Detection, ACS Appl. Mater. Interfaces, 2016, 8(34), 22301–22308 CrossRef CAS PubMed.
- G. Zhou and M. Li, Near-Infrared-II Plasmonic Trienzyme-Integrated Metal-Organic Frameworks with High-Efficiency Enzyme Cascades for Synergistic Trimodal Oncotherapy, Adv. Mater., 2022, 34(24), 2200871 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Chemicals and materials, characterization, synthetic methods, the detection of ROS in vitro, the Michaelis–Menten constant (Km) and maximum reaction rate (Vmax), GSH consumption in vitro, cell culture, cell experiment, cytotoxicity assessment and spoptosis assay, in vitro intracellular ROS detection, live/dead cell staining assay, biodistribution analysis, in vitro and in vivo dual-modal imaging, in vivo antitumor treatment study, in vivo biocompatibility assay, representative photographs of tumor on different groups after treatments, and H&E-staining images of major organs. Supplementary data associated with this article can be found in the online version. See DOI: https://doi.org/10.1039/d2tb02096c |
| ‡ These authors contributed equally to this work and should be considered cofirst authors. |
| This journal is © The Royal Society of Chemistry 2023 |