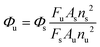Fluorescence probe for real-time malonaldehyde detection in epilepsy model†
Yongtao
Duan‡
 *a,
Zhenling
Liu‡
a,
Yi-Fan
Liao‡
ab,
Mingzhu
Wang
a,
Yongfang
Yao
*c and
Hai-Liang
Zhu
*a,
Zhenling
Liu‡
a,
Yi-Fan
Liao‡
ab,
Mingzhu
Wang
a,
Yongfang
Yao
*c and
Hai-Liang
Zhu
 *ab
*ab
aHenan Provincial Key Laboratory of Pediatric Hematology, Children's Hospital Affiliated to Zhengzhou University, Zhengzhou University, Zhengzhou 450018, China. E-mail: duanyongtao860409@163.com; zhuhl@nju.edu.cn
bState Key Laboratory of Pharmaceutical Biotechnology, School of Life Sciences, Nanjing University, Nanjing, 210023, China
cSchool of Pharmaceutical Science, Zhengzhou University, Zhengzhou, Henan 450001, China. E-mail: yongfangyao@zzu.edu.cn
First published on 28th November 2023
Abstract
Oxidative stress, a condition involving an imbalance between reactive oxygen species (ROS) and antioxidants, is closely linked to epilepsy, contributing to abnormal neuronal excitability. This study introduces a novel fluorescent probe, the MDP probe, designed for the efficient detection of malondialdehyde (MDA), a critical biomarker associated with oxidative stress. The MDP probe offers several key advantages, including high sensitivity with a low detection limit of 0.08 μM for MDA, excellent selectivity for MDA even in the presence of interfering substances, and biocompatibility, making it suitable for cell-based experiments. The probe allows for real-time monitoring of MDA levels, enabling dynamic studies of oxidative stress. In vivo experiments in mice demonstrate its potential for monitoring MDA levels, particularly in epilepsy models, which could have implications for disease research and diagnosis. Overall, the MDP probe represents a promising tool for studying oxidative stress, offering sensitivity and specificity in cellular and in vivo settings. Its development opens new avenues for exploring the role of oxidative stress in various biological processes and diseases, contributing to advancements in healthcare and biomedical research.
Introduction
Oxidative stress is a complex physiological process that typically occurs within organisms, involving the production of oxygen free radicals and other reactive oxygen species in excess of the cell's antioxidant defense capabilities.1–4 This phenomenon often poses a threat of oxidative damage to cells and tissues. In the context of epileptic seizures, oxidative stress becomes particularly important as it may serve as a key factor triggering abnormal neuronal excitability.5,6 This increased excitability can alter the electrical conductivity of neuronal membranes, making neurons more prone to overactivation and thereby leading to seizures.7–9 Fortunately, the human body possesses a range of antioxidants such as vitamin C and glutathione that can help regulate oxidative stress, potentially having a positive impact on certain epilepsy patients.10,11Among the biomarkers of oxidative stress, malondialdehyde (MDA) is a prominent representative.12–14 MDA is one of the products of lipid peroxidation, and its concentration is commonly measured to assess the extent of oxidative stress and to delve deeper into the close relationship between oxidative stress and epilepsy.15 During epileptic seizures, the abnormal activity of neurons can induce a significant increase in oxidative stress, further stimulating the generation of free radicals within cells, leading to the production of oxidative byproducts such as MDA.16–18 Therefore, the measurement of MDA concentration has become an important method for evaluating the internal oxidative stress levels in epilepsy patients.19 Importantly, MDA concentration can significantly increase during or after epileptic seizures, reinforcing its significance in research and diagnosis.20–22
Currently, there are several methods for detecting MDA, including the thiobarbituric acid (TBA) assay,23,24 high-performance liquid chromatography (HPLC),25,26 gas chromatography (GC),27,28 mass spectrometry, and ultraviolet-visible spectrophotometry.29,30 However, these methods have limitations in some aspects, such as not being suitable for complex sample analysis, high equipment costs, requiring specialized instruments and skills, long analysis times, and high costs. These limitations restrict the widespread detection and application of MDA. In this regard, fluorescence probe methods hold great potential due to their advantages of real-time monitoring, high specificity, and high sensitivity, making them particularly suitable for the analysis of cellular or biological samples.31–36 However, the currently available fluorescence probes for MDA are still relatively limited and require further research and development, especially in the context of epilepsy-related MDA probes,37–42 to comprehensively explore the deep connections between oxidative stress and epilepsy.
We proposed an innovative fluorescent probe design for the efficient detection of MDA. By measuring the dynamic changes in fluorescent signals, we were able to gain a more comprehensive understanding of how MDA levels vary in different physiological and pathological states, especially in an epilepsy model. This research also enabled the real-time monitoring of MDA activity development. Real-time imaging with this fluorescent probe allowed for the immediate capture and assessment of epileptic seizures, providing precise insights for treatment strategies.
Experimental section
Synthesis of the compounds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (ethyl acetate) EA (v/v 15
(ethyl acetate) EA (v/v 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent, obtaining dark green solid (yield 84%). 1H NMR (600 MHz, DMSO-d6) δ 6.93–6.86 (m, 1H), 5.34 (s, 1H), 4.60 (s, 1H), 2.56 (s, 1H), 2.50 (s, 1H), 1.00 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 170.21, 158.03, 141.05, 139.73, 135.08, 125.18, 123.72, 121.52, 120.16, 115.14, 114.31, 114.17, 113.23, 72.69, 42.81, 38.73, 32.12, 27.95.
1) as eluent, obtaining dark green solid (yield 84%). 1H NMR (600 MHz, DMSO-d6) δ 6.93–6.86 (m, 1H), 5.34 (s, 1H), 4.60 (s, 1H), 2.56 (s, 1H), 2.50 (s, 1H), 1.00 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 170.21, 158.03, 141.05, 139.73, 135.08, 125.18, 123.72, 121.52, 120.16, 115.14, 114.31, 114.17, 113.23, 72.69, 42.81, 38.73, 32.12, 27.95.
Determination of spectrum
The MDP probe was solubilized in (dimethyl sulfoxide) DMSO to achieve a final concentration of 1 mM, a concentration employed for subsequent assessments. Additional analytes utilized to determine specificity encompassed benzaldehyde, formaldehyde, acetaldehyde, n-propanal, p-chlorobenzaldehyde, NaCl, KCl, CaCl2, ZnCl2, FeCl3, CH3COONa, Na2CO3, Na2SO3, NaHSO3, NaHSO4, Na2S, NaClO, and HClO4. Inclusion of biological compounds encompassed (Alanine) Ala, (Homocysteine) Hcy, (Glutathione) GSH, (Glycine) Gly, (Valine) Val, 30% H2O2. High-purity water was generated through a Milli-Q reference system (Millipore Elix 5). Minor pH adjustments of the PBS buffer were enacted with minimal volumes of 1 M HCl or NaOH, and pH levels were ascertained employing a pH meter (PHS-25). Spectroscopic evaluations of the MDP probe (10 μM, 2 μL) were executed within a PBS buffer (10 mM, pH 7.4) containing 1% DMSO and 2 mM (Cetrimonium Bromide) CTAB. Final volume was attuned to 200 μL with ultrapure water. All spectroscopic procedures were conducted at 37 °C. The Edinburgh-Steady state/transient fluorescence spectrometer FLS980 was utilized for all spectral measurements. Excitation occurred at 452 nm, with both excitation and emission slit widths configured to 10 nm and a step size of 1 nm. Emission spectrum ranging from 500 nm to 800 nm was scanned, unless otherwise specified.The detection limit (DL) of MDP
The emission spectrum of free MDP was obtained in a PBS buffer (10 mM, pH = 7.40, 2 mM CTAB) containing 1% DMSO. This spectrum was collected a total of 35 times to establish the background noise level (σ). A linear regression curve was subsequently fitted using the collected data, specifically within the concentration range of MDA spanning from 0 to 30 μM. This analysis allowed for the determination of the slope of the curve. The detection limit (DL) was calculated using the formula DL = 3σ/slope. This value was found to be 0.08 μM.Determination of the fluorescence quantum yield
To determine the fluorescence quantum yield (FQY) of MDP, rhodamine B was utilized as a standard with a known quantum yield (Φ = 0.69). The following equation was used to calculate FQY:where, Φu is the quantum yield of the unknown sample (MDP). As and Au are the absorbance values of the reference (rhodamine B) and unknown (MDP) samples, respectively. Fs and Fu are the area under the corrected fluorescence spectra of the reference and unknown samples, respectively. νu and νs are the solvent refractive indices of the unknown sample (MDP) and reference sample (rhodamine B), respectively. Φs is the known quantum yield of the reference (rhodamine B), which is 0.69.
| Φu = 0.18%. |
The fluorescence quantum yield (FQY) of the sample was determined using the Edinburgh-Steady state/transient fluorescence spectrometer FLS980. Rhodamine B was also utilized as a standard. The method was the same as above. The measured quantum yield was found to be Φ = 0.22%.
Detection of molar extinction coefficient
The molar extinction coefficient is measured according to the following formula:| A = ε × l × c |
| ε = 2.24 × 104 L mol−1 cm−1. |
Cell culture and imaging
(Pheochromocytoma cells-12) PC12 cells were procured from Sciencell (Shanghai, China). These cells were cultivated in Dulbecco's Modified Eagle's medium (DMEM, Sbjbio), supplemented with 10% fetal bovine serum (FBS, Gibco), and 1% antibiotics (streptomycin and penicillin, Sbjbio). Cell maintenance transpired in an atmosphere of 5% CO2 at 37 °C. For cell detachment, a solution of 0.25% Trypsin-EDTA (Sbjbio) was employed. Incubation of the cells took place within Tri-gas CO2 incubators from Thermo Fisher Scientific Inc., USA.PC12 cells were seeded in glass-bottom culture dishes (Corning) at a density of 1 × 105 cells per well. The specific experimental procedures were tailored to the objectives of the study, and detailed steps can be found in the respective protocol documents. For the acquisition of cellular images, Confocal Laser Scanning Microscopy (CLSM) was conducted using a Leica SP8 STED 3× confocal imaging system.
Cell viability
Cell viability was evaluated in vitro using the standard (Cell Counting Kit-8) CCK-8 assay. PC12 cells were initially seeded in a 96-well plate at a density of 5 × 103 cells per well and allowed to incubate overnight. Subsequently, the cells were treated with various concentrations of MDP (ranging from 0 to 50 μM) for a duration of 24 hours in fresh DMEM medium. Following the 24-hour incubation period, CCK-8 solution (10 μL per well) was added to each well, and the cells were further incubated for 4 hours. A microplate reader was employed to measure absorbance at 450 nm (ELx800, BioTek, USA). To ensure thorough mixing, the 96-well plate was shaken on the microplate reader for 5 minutes. The experiments were repeated six times to enhance the reliability and consistency of the results.Establishment of animal models and imaging
All BABL/c Nude mice were procured from the Institute of Zoology, Nanjing University. All procedures involving animals adhered to the Animal Management Rules of the Ministry of Health of the People's Republic of China and were granted approval by the Science and Technology Ethics Committee of Nanjing University.For the model mice, epileptic conditions were induced through intraperitoneal injection of kainic acid (KA) (6 mg kg−1). This compound was used to provoke an epilepsy model in the mice. In the model group, mice were intraperitoneally injected with KA, and this treatment was administered for a duration of 12 hours. Upon completion of the experimental period, mice were anesthetized using Isoflurane gas anesthesia.
Fluorescent imaging was conducted using the PE IVIS system. BAIB/c Nude mice were intraperitoneally injected with MDP (100 μM in saline, 200 μL). Following the initial imaging, MDP (500 μM in saline, 500 μL) was intraperitoneally injected into the mice. Subsequent fluorescent images of the mice were captured at an excitation–emission wavelength of 460/560 nm.
Mice in both control and model groups received a tail vein injection of MDP (100 μM in saline, 200 μL) and were allowed to incubate for 1 hour. Following fluorescent images of the mice were acquired at an excitation–emission wavelength of 460/560 nm.
Tissue processing
The entire brain, comprising both the control and model groups, was carefully removed and placed in a solution of 4% paraformaldehyde for preservation. The whole brain tissue, encompassing both the control and model groups, was sent to Wuhan Seville Biological Co., Ltd, for expert handling. The company was entrusted with the task of cutting the tissue into blank paraffin sections, each measuring 4 μm in thickness. Thin sections (4 μm) of the blank tissue were subjected to Hematoxylin and Eosin (H&E) staining. This staining procedure was employed to facilitate the observation of the hippocampal region. For the blank sections, images were captured using a Leica Two-photon fluorescence microscope equipped with a 20× objective lens. The excitation wavelength was set at 470 nm, and emission wavelengths were collected within the range of 520 nm to 600 nm.Results and discussion
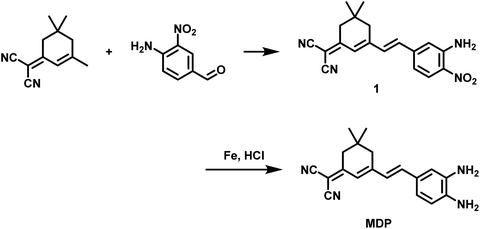
We successfully synthesized the MDP probe, and the detailed synthesis pathway was presented in Fig. 1. Initially, suitable raw materials were selected, and the synthesis process was completed through a series of chemical reactions. To confirm the structure of the MDP probe, we employed various techniques, including H-NMR, C-NMR, and mass spectrometry for a comprehensive characterization (Fig. S1, S2, S9–12†). Of particular significance, liquid chromatography experiments were conducted and the data was provided in Fig. S1 and S2.† These experimental data validated the reaction mechanism between the MDP probe and MDA, further establishing the structure and properties of the MDP probe.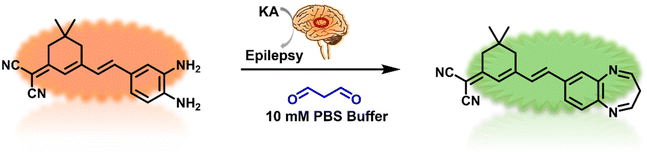 | ||
| Fig. 1 The illustration of the probe MDP for the detection of MDA in kainite-induced epileptic brains. | ||
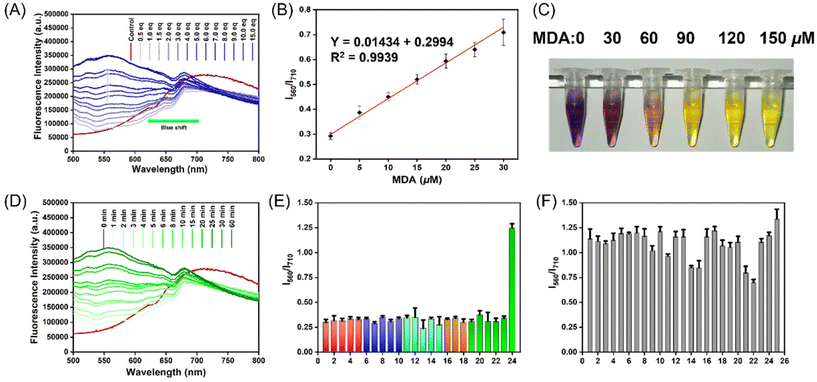
Firstly, we evaluated the spectral properties of the MDP probe after reacting with MDA in PBS buffer. By examining the UV absorption spectrum and fluorescence emission spectrum, it was clearly evident that the MDP (10 μM) effectively reacts with MDA (150 μM), resulting in significant changes in the spectral signals. These changes accurately reflect the outcomes of the MDA reaction (Fig. S3†). In the fluorescence emission spectrum, we observed an emission peak at 710 nm for the MDP (10 μM) probe. However, upon the addition of MDA, the emission peak shifted from 710 nm to 556 nm, and after 30 min of reaction, the fluorescence intensity increased significantly with increasing MDA concentration (0–150 μM) (Fig. 2A). This discovery indicates the sensitivity of the MDP to MDA and its suitability for quantitative detection at different concentrations. Additionally, we visually observed a color change in the solution to confirm the changes before and after the reaction with MDA (0–150 μM). Before the reaction, the solution appeared purple-red, but after reacting with MDA, the solution visibly lightened in color, transitioning from pale orange to yellow (Fig. 2C). This visual observation aligns with the fluorescence spectroscopy data and further confirms the reaction between the MDP probe and MDA.
We also assessed the kinetic properties of the MDP probe's reaction with MDA by studying the relationship between fluorescence intensity and emission spectra with MDA concentration and reaction time. Initially, we determined the quantum yield of the MDP probe to be 0.18, indicating efficient fluorescence generation upon excitation. Within the range of MDA concentrations from 0 to 30 μM, there was a strong linear relationship between MDA concentration and fluorescence intensity, with a correlation coefficient (R2) of 0.9939 (Fig. 2B). This linear relationship demonstrates the high precision of the MDP probe for quantitative detection, providing important feasibility for its use in biomedical research and clinical applications. Furthermore, we studied the reaction of the MDP (10 μM) with 100 μM of MDA in 10 mM PBS buffer at different time intervals. As reaction time increased, the emission peak shifted from 710 nm to 556 nm in the emission spectrum, and fluorescence intensity gradually increased, reaching its peak around 30 min (Fig. 2D). The stability of the MDP (10 μM) probe's reaction with MDA (100 μM) was analyzed dynamically over 48 hours, and the results showed that the fluorescence intensity remained stable within 48 hours without significant decay (Fig. S6†). This demonstrates the reliability and durability of the MDP probe's reaction with MDA, providing a solid foundation for its use in long-term monitoring and dynamic analysis.
We used the 3σ/slope method to assess the detection limit of the MDP (10 μM) probe, and the result indicates a detection limit of 0.08 μM. This result highlights the MDP probe's high sensitivity and outstanding detection capability for detecting MDA as a substrate. Additionally, we studied the reactivity of the MDP probe with the substrate MDA at different pH values (pH 4–12). Through spectral analysis, we observed that the reaction exhibited optimal performance and stability at pH levels between 7 and 10 (Fig. S4†). Under different pH conditions, we observed that the kinetic properties of the MDP-MDA reaction remain consistent and are not influenced by the pH environment. This indicates that the probe's reactivity remains uniform across various pH values and is not significantly affected by external pH conditions (Fig. S5†). This suggests that the MDP (10 μM) can effectively detect MDA under physiological conditions. Furthermore, we examined the probe's reaction with different reactants, demonstrating its good selectivity in various reactions. We also investigated the probe's specificity for MDA in the presence of mixed reactants. We introduced multiple interfering substances and allowed them to coexist with MDA in the reaction system. The results were encouraging, as the probe was able to selectively detect MDA even in complex environments, demonstrating excellent interference resistance (Fig. 2E and F). The MDP probe not only exhibits high sensitivity and detection capability but also performs well under different pH conditions. It shows good reactivity and stability. Moreover, it demonstrates selectivity for various reactants and specific detection of MDA in complex environments, indicating its potential and reliability in practical applications.
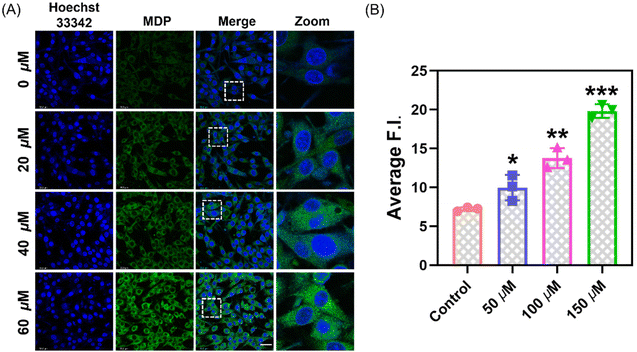
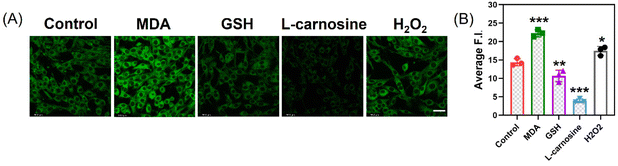
Based on the MTT assay results, the cell viability exceeds 85% under the conditions of 50 μM (Fig. S7†). This suggests that at this concentration, the probe has low toxicity and excellent biocompatibility, providing a reliable foundation for cell imaging experiments. Through confocal laser scanning microscopy (CLSM) experiments, we cultured PC12 cells in optical glass dishes. After pretreatment with different concentrations of MDA (0, 50, 100, 150 μM), PC12 cells co-incubated with MDP (10 μM) exhibited varying fluorescence intensities (Fig. 3). By observing the localization of nuclear dyes, we can see that the probe effectively penetrates the cell membrane and evenly distributes within the cytoplasm. We treated PC12 cells with different drug treatments to induce and inhibit the production of endogenous MDA. After pretreatment with GSH (1 mM, 12 h), L-carnitine (2 mM, 1 h), and H2O2 (0.5 mM, 10 min), cells were incubated with MDP probe for 30 minutes, and confocal imaging showed different fluorescence intensities (Fig. 4). GSH and hydrogen peroxide promote oxidative stress, leading to an increase in MDA content. Conversely, the addition of the MDA scavenger L-carnitine reduced MDA content, resulting in decreased fluorescence intensity.
These experimental results demonstrate the high sensitivity and specificity of the MDP probe to MDA inside cells, as well as its potential applications in the field of biological imaging. These findings also provide powerful tools and a basis for further research into the biological processes of MDA inside cells and potential drug screening.
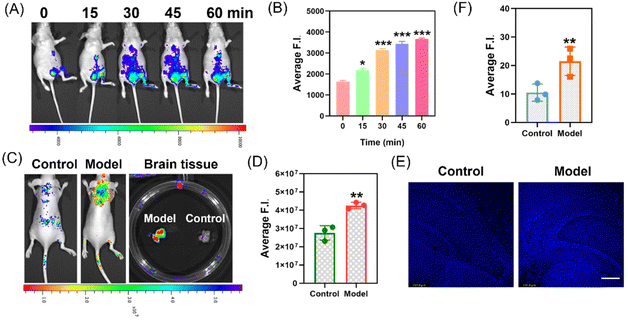
After successfully obtaining cell imaging results, we further applied the MDP probe to in vivo experiments in live mice. Initially, we selected 4-week-old nude mice and intraperitoneally injected them with 500 μM of MDA, followed by injection of the MDP (100 μM) probe at the same location, and collected fluorescent images (Fig. 5A and B). The results showed that over time, the fluorescence intensity in the abdomen of the mice gradually increased. This indicates that the MDP probe can be used to monitor dynamic changes in MDA levels within the mice. We also performed imaging in a mouse model of epilepsy induced by intraperitoneal injection of kainic acid (KA) and subsequent tail vein injection of the MDP (100 μM) 6 hours later. Initially, we administered the probe via tail vein injection into the epileptic model mice, and after 30 minutes, we retrieved brain tissue. Through mass spectrometry analysis (Fig. S8†), we detected the presence of the probe in the brain tissue. The probe effectively crosses the blood–brain barrier to reach the brain tissue, providing the necessary conditions for imaging. Next, the brain fluorescence of the model mice displayed significant differences compared to normal mice (Fig. 5C and D). Post-mortem brain tissues also exhibited statistically significant differences. We used fluorescence confocal microscopy to capture fluorescent images of frozen brain tissue slices, which showed a significant increase in fluorescence intensity in the epilepsy model mice (Fig. 5E and F).
These experimental results suggest that the MDP probe has potential applications for monitoring MDA in live mice, especially in epilepsy models where its fluorescence signals differ significantly from normal conditions, providing a powerful tool for epilepsy research. This technology can be used not only to monitor oxidative stress in living organisms but also to provide crucial information for drug development and disease diagnosis.
Conclusion
In summary, we introduced a novel fluorescent probe, the MDP probe, designed for the efficient detection of malondialdehyde (MDA), a critical biomarker of oxidative stress. Oxidative stress, characterized by an imbalance between reactive oxygen species and antioxidants, was implicated in various physiological and pathological processes, including epilepsy. The MDP probe offers several key advantages: (1) high sensitivity, with a low detection limit of 0.08 μM for MDA; (2) selectivity and specificity, even in complex environments with other substances, ensuring accurate and specific MDA detection; (3) biocompatibility that the MDP probe is well-tolerated by cells; (4) real-time monitoring with the MDP probe allows for real-time monitoring of MDA levels; (5) in vivo applications with the MDP probe effectively monitors MDA levels, particularly in an epilepsy model, offering valuable insights into disease research and diagnosis. Overall, the MDP probe represents a significant advancement in the field of oxidative stress research. Its sensitivity, specificity, and applicability in both cellular and in vivo contexts make it a versatile tool for scientists and clinicians in various fields, including biology, medicine, and drug development. This innovative probe opens doors for deeper exploration of oxidative stress's role in diverse biological processes and diseases, promising to contribute to advancements in healthcare and biomedical research.Conflicts of interest
There is no conflict of interest to declare.Acknowledgements
This work was supported by Natural Science Foundation of Henan (No. 232300421086), Henan Medical Science and Technology Program (No. SBGJ202302102) and Joint Fund of Henan Provincial Science and Technology Research (No. 225200810114 and 232301420055).References
- H. Sies and D. P. Jones, Reactive oxygen species (ROS) as pleiotropic physiological signalling agents, Nat. Rev. Mol. Cell Biol., 2020, 21(7), 363–383 CrossRef CAS.
- M. Valko, D. Leibfritz, J. Moncol, M. T. Cronin, M. Mazur and J. Telser, Free radicals and antioxidants in normal physiological functions and human disease, Int. J. Biochem. Cell Biol., 2007, 39(1), 44–84 CrossRef CAS PubMed.
- T. Finkel and N. J. Holbrook, Oxidants, oxidative stress and the biology of ageing, Nature, 2000, 408(6809), 239–247 CrossRef CAS.
- B. Halliwell, Biochemistry of oxidative stress, Biochem. Soc. Trans., 2007, 35(5), 1147–1150 CrossRef CAS PubMed.
- A. Vezzani and A. Friedman, Brain inflammation as a biomarker in epilepsy, Biomarkers Med., 2011, 5(5), 607–614 CrossRef CAS PubMed.
- Z. Z. Chong, Y. C. Shang and K. Maiese, Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling, Curr. Neurovasc. Res., 2007, 4(3), 194–204 CrossRef CAS.
- S. N. Rakhade and F. E. Jensen, Epileptogenesis in the immature brain: emerging mechanisms, Nat. Rev. Neurol., 2009, 5(7), 380–391 CrossRef CAS.
- W. Löscher, Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs, Seizure, 2011, 20(5), 359–368 CrossRef.
- O. Devinsky, A. Vezzani, S. Najjar, N. C. De Lanerolle and M. A. Rogawski, Glia and epilepsy: excitability and inflammation, Trends Neurosci., 2013, 36(3), 74–184 CrossRef.
- Y. Gupta, S. Briyal and G. Chaudhary, Protective effect of trans-resveratrol against kainic acid-induced seizures and oxidative stress in rats, Pharmacol., Biochem. Behav., 2002, 71(1–2), 245–249 CrossRef CAS PubMed.
- S. Koppula, H. Kumar, S. V. More, H.-W. Lim, S.-M. Hong and D.-K. Choi, Recent updates in redox regulation and free radical scavenging effects by herbal products in experimental models of Parkinson's disease, Molecules, 2012, 17(10), 11391–11420 CrossRef CAS.
- O. Erel, A novel automated method to measure total antioxidant response against potent free radical reactions, Clin. Biochem., 2004, 37(2), 112–119 CrossRef CAS.
- B. Halliwell and J. M. Gutteridge, Free radicals in biology and medicine, Oxford University Press, USA, 2015 Search PubMed.
- A. Ayala, M. F. Muñoz and S. Argüelles, Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal, Oxid. Med. Cell. Longevity, 2014, 2014, 360438 Search PubMed.
- S. Sifuentes-Franco, D. E. Padilla-Tejeda, S. Carrillo-Ibarra and A. G. Miranda-Díaz, Oxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy, Int. J. Endocrinol., 2018, 2018, 1875870 Search PubMed.
- G. F. Oxenkrug, The extended life span of Drosophila melanogaster eye-color (white and vermilion) mutants with impaired formation of kynurenine, J. Neural Transm., 2010, 117, 23–26 CrossRef CAS.
- M. Mazzuferi, G. Kumar, C. Rospo and R. M. Kaminski, Rapid epileptogenesis in the mouse pilocarpine model: video-EEG, pharmacokinetic and histopathological characterization, Exp. Neurol., 2012, 238(2), 156–167 CrossRef CAS PubMed.
- M. Milh, N. Villeneuve, M. Chouchane, A. Kaminska, C. Laroche, M. A. Barthez, C. Gitiaux, C. Bartoli, A. Borges-Correia and P. Cacciagli, Epileptic and nonepileptic features in patients with early onset epileptic encephalopathy and STXBP1 mutations, Epilepsia, 2011, 52(10), 1828–1834 CrossRef.
- S. A. Hamed, M. M. Abdellah and N. El-Melegy, Blood levels of trace elements, electrolytes, and oxidative stress/antioxidant systems in epileptic patients, J. Pharmacol. Sci., 2004, 96(4), 465–473 CrossRef CAS.
- E. B. Kurutas, O. Arican and S. Sasmaz, Superoxide dismutase and myeloperoxidase activities in polymorphonuclear leukocytes in acne vulgaris, Acta Dermatovenerol. Alp. Panonica Adriat., 2005, 14(2), 39–42 Search PubMed.
- R. Shi, D. Gao, R. Stoika, K. Liu, A. Sik and M. Jin, Potential implications of polyphenolic compounds in neurodegenerative diseases, Crit. Rev. Food Sci. Nutr., 2022, 1–24 CAS.
- C. Bennett, F. Mohammed, A. Álvarez-Ciara, M. A. Nguyen, W. D. Dietrich, S. M. Rajguru, W. J. Streit and A. Prasad, Neuroinflammation, oxidative stress, and blood-brain barrier (BBB) disruption in acute Utah electrode array implants and the effect of deferoxamine as an iron chelator on acute foreign body response, Biomaterials, 2019, 188, 144–159 CrossRef CAS PubMed.
- R. Guillen-Sans and M. Guzman-Chozas, The thiobarbituric acid (TBA) reaction in foods: a review, Crit. Rev. Food Sci. Nutr., 1998, 38(4), 315–350 CrossRef CAS.
- M. A. Ghani, C. Barril, D. R. Bedgood Jr. and P. D. Prenzler, Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay, Food Chem., 2017, 230, 195–207 CrossRef CAS.
- V. R. Meyer, Practical high-performance liquid chromatography, John Wiley & Sons, 2013 Search PubMed.
- B. L. Reuhs, High-performance liquid chromatography, Springer, 2017 Search PubMed.
- K. D. Bartle and P. Myers, History of gas chromatography, TrAC, Trends Anal. Chem., 2002, 21(9–10), 547–557 CrossRef CAS.
- R. L. Grob and E. F. Barry, Modern practice of gas chromatography, John Wiley & Sons, 2004 Search PubMed.
- A. Power, J. Chapman, S. Chandra and D. Cozzolino, Ultraviolet-visible spectroscopy for food quality analysis, in Evaluation Technologies for Food Quality, 2019, pp. 91–104 Search PubMed.
- A. G. Shard, R. C. Schofield and C. Minelli, Ultraviolet–visible spectrophotometry, Characterization of Nanoparticles, Elsevier, 2020, pp. 185–196 Search PubMed.
- H. Zhu, J. Fan, J. Du and X. Peng, Fluorescent probes for sensing and imaging within specific cellular organelles, Acc. Chem. Res., 2016, 49(10), 2115–2126 CrossRef CAS.
- D. Srikun, A. E. Albers, C. I. Nam, A. T. Iavarone and C. J. Chang, Organelle-targetable fluorescent probes for imaging hydrogen peroxide in living cells via SNAP-Tag protein labeling, J. Am. Chem. Soc., 2010, 132(12), 4455–4465 CrossRef CAS PubMed.
- Y. Wang, M. Qiu, M. Won, E. Jung, T. Fan, N. Xie, S.-G. Chi, H. Zhang and J. S. Kim, Emerging 2D material-based nanocarrier for cancer therapy beyond graphene, Coord. Chem. Rev., 2019, 400, 213041 CrossRef CAS.
- X. Wu, R. Wang, N. Kwon, H. Ma and J. Yoon, Activatable fluorescent probes for in situ imaging of enzymes, Chem. Soc. Rev., 2022, 51(2), 450–463 RSC.
- H. Li, H. Kim, F. Xu, J. Han, Q. Yao, J. Wang, K. Pu, X. Peng and J. Yoon, Activity-based NIR fluorescent probes based on the versatile hemicyanine scaffold: design strategy, biomedical applications, and outlook, Chem. Soc. Rev., 2022, 51(5), 1795–1835 RSC.
- H. Niu, J. Liu, H. M. O’Connor, T. Gunnlaugsson, T. D. James and H. Zhang, Photoinduced electron transfer (PeT) based fluorescent probes for cellular imaging and disease therapy, Chem. Soc. Rev., 2023, 52(7), 2322–2357 RSC.
- M. Qiang, Y. Xu, Y. Lu, Y. He, C. Han, Y. Liu and R. He, Autofluorescence of MDA-modified proteins as an in vitro and in vivo probe in oxidative stress analysis, Protein Cell, 2014, 5(6), 484–487 CrossRef.
- L. Xiao, S. Liang, L. Ge, H. Wan, W. Wu, J. Fei, S. Wu, B. Zhou and X. Zeng, 4, 5-di-O-caffeoylquinic acid methyl ester isolated from Lonicera japonica Thunb. targets the Keap1/Nrf2 pathway to attenuate H2O2-induced liver oxidative damage in HepG2 cells, Phytomedicine, 2020, 70, 153219 CrossRef CAS.
- J. Chen, L. Zeng, T. Xia, S. Li, T. Yan, S. Wu, G. Qiu and Z. Liu, Toward a biomarker of oxidative stress: a fluorescent probe for exogenous and endogenous malondialdehyde in living cells, Anal. Chem., 2015, 87(16), 8052–8056 CrossRef CAS PubMed.
- X. Wang, D. Su, C. Liu, P. Li, R. Zhang, W. Zhang, W. Zhang and B. Tang, Janus-Faced Fluorescence Imaging Agent for Malondialdehyde and Formaldehyde in Brains, Anal. Chem., 2022, 94(43), 14965–14973 CrossRef CAS.
- K.-C. Hsu, P.-F. Hsu, Y.-C. Chen, H.-C. Lin, C.-C. Hung, P.-C. Chen and Y.-L. Huang, Oxidative stress during bacterial growth characterized through microdialysis sampling coupled with HPLC/fluorescence detection of malondialdehyde, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1019, 112–116 CrossRef CAS.
- M. Lin and S. Liu, Naphthalimide-Based Fluorescent Probe for Profiling of Aldehydes during Oxidation of Unsaturated Lipids, J. Agric. Food Chem., 2022, 70(44), 14304–14311 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3an01583a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |