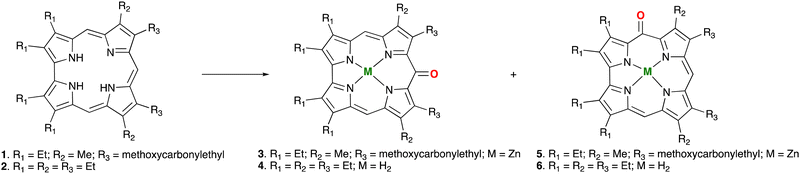
5- and 10-oxocorroles from β-octaalkylcorroles†
Lorena
Di Zazzo
 a,
Sara
Nardis
a,
Sara
Nardis
 *a,
Fabrizio
Caroleo
*a,
Fabrizio
Caroleo
 a,
Francesco
Pizzoli
a,
Francesco
Pizzoli
 a,
Frank R.
Fronczek
a,
Frank R.
Fronczek
 b,
Kevin M.
Smith
b,
Kevin M.
Smith
 b,
Beatrice Berionni
Berna
b,
Beatrice Berionni
Berna
 c and
Roberto
Paolesse
c and
Roberto
Paolesse
 a
a
aDepartment of Chemical Science and Technologies, University of Rome Tor Vergata, via della Ricerca Scientifica 1, Rome 00133, Italy. E-mail: nardis@scienze.uniroma2.it
bDepartment of Chemistry, Louisiana State University, Baton Rouge, LA 70803, USA
cInstitute of Organic Chemistry, University of Vienna, Währinger Straße 38, 1090, Vienna, Austria
First published on 20th November 2023
Abstract
The reaction of Zn ions with β-octaalkylcorroles leads to the air oxidation of the macrocycle, with the formation of a mixture of 5- and 10-oxocorroles. Spectroscopic characterization confirms the antiaromatic character of these macrocycles. A simple synthetic protocol opens the way for more detailed studies of oxocorrole chemistry.
A research bonanza driven by the interesting and peculiar properties shown by the corrole macrocycle has characterized corrole chemistry over the past two decades. As a result, the contracted porphyrinoid has been shown to be both different from the parent porphyrin and also interesting in diverse fields of applications.1 For example, the coordination chemistry of corrole is characterized by the so-called non-innocent character of this macrocycle, which makes it challenging from one side to elucidate the electronic structure of the complexes, and from the other side, interesting from both catalysis and clinical applications of these metal derivatives.2 The bright emissions of corroles have also been of interest for potential applications in solar cells or photodynamic therapy (PDT) of tumors.3
The increase of interest in corrole chemistry at the beginning of this century was possible due to the discovery of simple synthetic procedures for the preparation of 5,10,15-triarylcorroles, which made available this macrocycle for studies in several research groups.4 For this reason, the research has been focused on triarylcorrole derivatives, while β-octa-alkylcorroles, the first examples reported for this porphyrinoid,5 almost disappeared from the scene.6 Apart from the simple synthetic approach, the bigger popularity of triarylcorroles has been motivated by their high stability under oxidative conditions, due primarily to the protective action of the meso-phenyl groups.
However, reactivity at the meso-positions of corroles has been sparsely explored7 and not always devoted to macro ring opening. One interesting example is represented by the formation of 10-oxocorroles. These macrocycles were first described by Bröring as by-products of the oxidative ring closure of linear tetrapyrroles to give 5,15-diphenylcorroles,8 while Guilard obtained them from the air oxidation of Ni complexes of corrole dyads.9 More recently, Osuka and Tanaka reported their formation by oxidation of meso-unsubstituted 5,15-bis(pentafluorophenyl)corrole.10
The nomenclature adopted by these groups for their macrocycles has been different, starting from the 10-oxocorroles used by Bröring to the 10-isooxocorrole used by Osuka. To avoid confusion, and because the term isocorrole has been used to indicate a corrole wherein the conjugation is interrupted by a sp3 carbon, we prefer in this work to use the term oxocorrole.
Oxocorroles have been usually obtained by using strong oxidants, and we were surprised to obtain them upon attempting to coordinate Zn ion to a β-octaalkylcorrole, as indicated below.
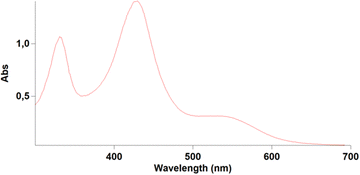
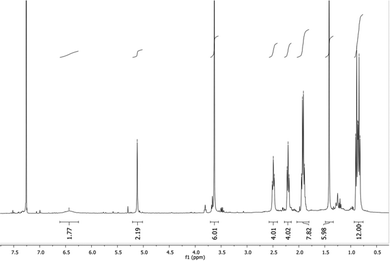
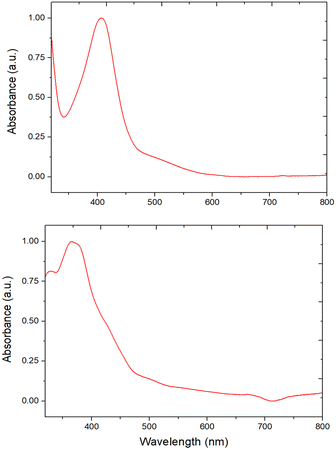
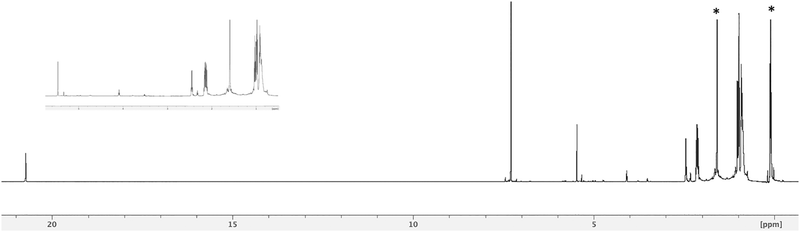
In the past we observed the formation of the Zn corrole anion in pyridine,11 but our recent reports on the formation of the neutral Zn complexes with the corrole radical species,12 led us to investigate the reaction of corrole 1 with Zn ions in boiling methanol (Fig. 1). The reaction was spontaneous with the disappearance of the corrole and the prompt formation of an orange product in good yield, which upon TLC analysis revealed the presence of two species. Preparative TLC allowed the complete separation of a first fraction, while the second band was incompletely separated. Our spectroscopic characterization of the first fraction gave interesting results; the UV-vis spectrum of the product showed a band centered at 420 nm, together with an absorption at 332 nm (Fig. 2). This spectrum was similar to what was reported for the corresponding oxo-vanadyl derivative.131H NMR characterization confirmed the probable formation of an oxocorrole, as demonstrated by both the diamagnetic nature of the compound coupled with the absence of resonances attributable to the meso protons of an aromatic corrole derivative (Fig. 3).
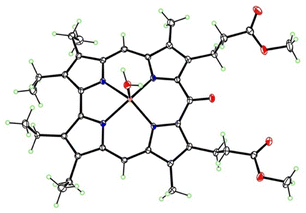
A singlet around 5 ppm was instead present; according to the anti-aromatic character of oxocorroles,10 this signal can be attributed to the 5,15-protons of the corrole macrocycle and the X-ray crystallographic characterization of a suitable crystal of this product revealed the formation of the Zn complex of the 10-oxocorrole 3, as a penta-coordinated aquocomplex (Fig. 4).
The corrole has a dome conformation, with the four N atoms lying an average of 0.21 Å out of the best plane of the 19 carbon atoms. But the ketone does not conform to the overall dome shape, having its oxygen atom tilted out of plane by 0.49 Å to the same side as the N atoms. The Zn atom has square pyramidal coordination geometry, lying 0.50 Å out of the N4 plane, with Zn–N distances 1.9879(11)–2.0233(12) Å and Zn–OH2 distance 2.0536(11) Å.
Although we failed to completely separate the second fraction, the 1HNMR spectrum (Fig. S2, ESI†) of the mixture showed the formation of a less symmetrical product, as indicated by the presence of two different singlets in the 5 ppm region, although the residual signals of 3 present as an impurity indicated the incomplete separation of the two products. X-ray analysis of a crystal obtained from the NMR tube indicated the formation of the 5-oxocorrole regioisomer 5 (Fig. S3, ESI†), although also in this case contaminated with 3. A crystal structure analysis (ESI†) showed partially occupied carbonyls at the 5, 10 and 15 positions.
It is significant to note that the formation of the oxocorroles does not require the use of strong oxidants, as observed in the case of the previous examples for monomeric species, the presence of molecular oxygen was sufficient.13
As previously reported for corrole dyads,9 the formation of the oxocorrole is probably due to the attack of molecular oxygen upon the Zn corrole complex, through the intermediate hydroperoxide isocorrole. To investigate the influence of the solvent, we repeated the same reaction in pyridine, observing again the formation of the Zn complex of the 5- and 10-oxocorroles, in similar yields.
With the aim to achieve the separation of the two regioisomers and to investigate the scope of the reaction, we tested octaethylcorrole in the reaction, obtaining again a mixture of 5- and 10-oxocorrole in an almost statistical ratio. However although the UV-visible monitoring of the reaction indicated the formation of the Zn complex, the complex was less stable and subsequent reaction work up led to demetalation of the product, affording the corresponding oxocorrole free bases. This demetalation was advantageous because we were able to separate the 5- and 10-oxocorroles, allowing characterization of the two regioisomers 4 and 6 (Fig. 1).
The UV-vis spectra indicated some important differences between the two regioisomers. While the regiosomer 4 shows a red shifted absorption, 6 possessed a blue shifted absorption centered below 400 nm (Fig. 5).
The 1H NMR characterization of both isomers confirmed the anti-aromatic character of the macrocycles, with the inner core NH protons resonating above 20 ppm (Fig. 6 and Fig. S6, ESI†).
It is interesting to note that the same complexes can be obtained by reaction of the corresponding a,c-octaethylbiladiene with Zn ions in refluxing methanol; following the progress of the reaction by UV-vis spectroscopy, we noted the initial formation of the corrole free base, followed by the formation of the oxocorrole products.
To explore a possible improvement in the reaction yields, we decided to test the reaction of the octaethylcorrole using an oxidant salt, such as Tl(III) acetate. Also in this case, the reaction afforded the same mixture of 5- and 10-oxocorrole, but it was possible also to isolate a further product of the reaction. Although this species was not stable, we were able to characterize it; the UV-vis spectrum (Fig. S7, ESI†) shows both a Soret-like band below 400 nm, together a red shifted band above 700 nm.
The 1H NMR (Fig. S8, ESI†) indicated the absence of an aromatic ring current effect and was in agreement with the formation of 7, which can be considered to be the ketal species of the 5-oxocorrole, which is an unprecedented example of an isocorrole (Fig. 7).
Unfortunately, the low stability of this intermediate did not allow us to obtain a crystal suitable for X-ray crystallographic characterization.
The electrochemical properties of 4 and 6 were investigated using cyclic voltammetry (CV) (Table 1 and Fig. S9, ESI†). Both derivatives exhibited electrochemical reversibility at all measured scan rates for their first oxidation and reduction processes (E1/2ox1, E1/2red1), indicating good chemical and thermal stability of the monoionic species. In comparison to 5-oxocorrole, 10-oxocorrole showed no significant changes in E1/2ox1 and E1/2ox2 values, whilst the E1/2red1 value is anodically shifted by 60 mV indicating the formation of a more electron-rich system, resulting in a widening of the ΔECV by 100 mV.
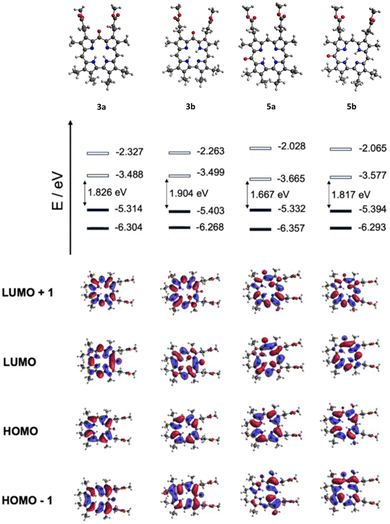
To provide further insight into the electronic transitions, density functional theory (DFT) calculations were performed at the b3lyp/6-311+g(d,p) level of theory. The Kohn–Sham representation of the frontier orbitals suggests a similar HOMO distribution between the free bases of 3 and 5 (Fig. 8 and Table 1), consistent with the experimental data, as well as a lowering of the LUMO energy level observed for the 5-oxocorrole, probably due to the participation of oxygen within the orbital distribution.
At the same time, a destabilization of LUMO+1 was observed in the case of 5-oxocorrole. This computational finding aligns well with the experimentally observed blue shift of 5-oxocorrole in its UV-vis spectrum, given that the Soret band involves the transition of four main orbitals, namely HOMO−1, HOMO, LUMO, and LUMO+1 (the latter to a greater extent) (Table S1, ESI†).
The financial support from MUR PRIN2020 project Astrali (SN, Grant 2020CBEYHC_004) is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- C. Di Natale, C. P. Gros and R. Paolesse, Chem. Soc. Rev., 2022, 51, 1277 RSC.
- A. Ghosh, Chem. Rev., 2017, 117, 3798 CrossRef CAS; Z. Gross and H. B. Gray, Adv. Synth. Catal., 2004, 346, 165 CrossRef; R. D. Teo, J. Y. Hwang, J. Termini, Z. Gross and H. B. Gray, Chem. Rev., 2017, 117, 2711 CrossRef PubMed.
- A. B. Alemayehu, N. U. Day, T. Mani, A. B. Rudine, K. E. Thomas, O. A. Gederaas, S. A. Vinogradov, C. C. Wamser and A. Ghosh, ACS Appl. Mater. Interfaces, 2016, 8, 18935 CrossRef CAS.
- Z. Gross, N. Galili and I. Saltsman, Angew. Chem., Int. Ed., 1999, 38, 1427 CrossRef CAS; R. Paolesse, L. Jaquinod, D. J. Nurco, S. Mini, F. Sagone, T. Boschi and K. M. Smith, Chem. Commun., 1999, 1307 RSC; B. Koszarna and D. T. Gryko, J. Org. Chem., 2006, 71, 3707 CrossRef.
- A. W. Johnson and I. T. Kay, J. Chem. Soc., 1965, 1620 RSC; R. Paolesse, Syntheses of Corroles, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, New York, 2000, ch. 11, vol. 2, pp. 201 Search PubMed.
- The search for octaethylcorrole reported 10 articles over 1276 on corrole in the period 2000–2023 (Scopus search, September 2023).
- R. Paolesse, L. Jaquinod, M. O. Senge and K. M. Smith, J. Org. Chem., 1997, 62, 6193 CrossRef CAS; M. Stefanelli, M. Mastroianni, S. Nardis, S. Licoccia, F. R. Fronczek, K. M. Smith, W. Zhu, Z. Ou, K. M. Kadish and R. Paolesse, Inorg. Chem., 2007, 46, 10791 CrossRef PubMed.
- M. Bröring, C. Hell, C. D. Brandt and E. C. Tejero, J. Porphyrins Phthalocyanines, 2003, 7, 214 CrossRef.
- F. Jérôme, J.-M. Barbe, C. P. Gros, R. Guilard, J. Fischer and R. Weiss, New J. Chem., 2001, 25, 93 RSC.
- K. Ueta, J. Kim, S. Ooi, J. Oh, J. Shin, A. Nakai, M. Lim, T. Tanaka, D. Kim and A. Osuka, J. Am. Chem. Soc., 2021, 143, 7958 CrossRef CAS PubMed.
- R. Paolesse, S. Licoccia and T. Boschi, Inorg. Chim. Acta, 1990, 178, 9 CrossRef CAS.
- M. L. Naitana, W. R. Osterloh, L. Di Zazzo, S. Nardis, F. Caroleo, P. Stipa, K.-N. Truong, K. Rissanen, Y. Fang, K. M. Kadish and R. Paolesse, Inorg. Chem., 2022, 61, 17790 CrossRef CAS PubMed.
- M. Bröring, C. Hell, F. Brégier, O. Burghaus and E. Cónsul Tejero, Inorg. Chem., 2007, 46, 5477 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic characterization, X-ray structure. CCDC 2302278 and 2302279. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc05204d |
| This journal is © The Royal Society of Chemistry 2024 |