Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Isolable rubidium and caesium derivatives of common organic carbonyl compounds†‡
Jakoba
Wacker§
ab,
Jennifer R.
Lynch§
a,
Sumanta
Banerjee
a,
Peter A.
Macdonald
a,
Alan R.
Kennedy
 a,
Biprajit
Sarkar
a,
Biprajit
Sarkar
 b and
Robert E.
Mulvey
b and
Robert E.
Mulvey
 *a
*a
aDepartment of Pure and Applied Chemistry, University of Strathclyde, Glasgow, G1 1XL, UK. E-mail: r.e.mulvey@strath.ac.uk
bInstitut für Anorganische Chemie, Universität Stuttgart, Pfaffenwaldring 55, Stuttgart, 70569, Germany
First published on 22nd November 2023
Abstract
Light alkali metal (Li, Na, K) amides have a long history of synthetic utility, but heavier (Rb, Cs) congeners have barely been studied. This study reveals remarkable structurally complex outcomes of reacting AM(HMDS) (AM = Rb, Cs; HMDS = hexamethyldisilazide) with benzaldehyde and acetophenone. Though complicated, reactions give a diversity of eye-catching isolated products, an enolate with a hexagonal prismatic network, two dienolates with distinct extended ladder motifs, and two β-imino-alkoxides comprising zig-zag chains of metal–oxygen bonds in infinite cages.
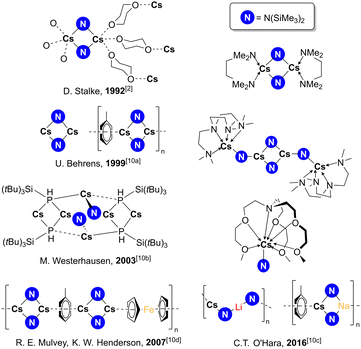
Whereas Li, Na, and K amides have long been key tools in chemists’ toolbox,1 Rb and Cs congeners have so far not merited space within it. First synthesised in 1992 by Stalke from Cs metal and free amine,2 CsHMDS [CsN(SiMe3)2] has been the one Cs amide of note albeit featuring in only a handful of applications, though recent studies hint at a more promising future.3–6 These include as a catalyst for selective hydrogen isotope exchange of benzylic C–H bonds with D2;3 as a pre-catalyst for imine to amine transfer hydrogenation,4a and as a mediator in magnesiate-catalysed transfer hydrogenation of alkenes to alkanes.4b Also reported are indirect applications where CsHMDS was either not added directly but suspected of forming in situ, as in catalytic alkynylations of polyfluoro-arenes,5 and in alkaline-metal-catalysed aminobenzylation of aldehydes with toluenes.6 Since HMDS is a common Brønsted base anion1a,7 one might intuitively expect CsHMDS to be a strong stoichiometric base, but though used to deprotonate indene and fluorene,8 its reactivity with organic substrates has not yet been studied in any detail. While organocaesium Brønsted bases are rare, inorganic bases such as CsOH and Cs2CO3 have made a significant impact in organic synthesis with acidic substrates, such that a “caesium effect” has been postulated, derived from observations that these salts and their derivatives sometimes outperform smaller alkali metal congeners in assorted organic transformations. However, this is not a unified effect since it is cited in different contexts and the origin/s of this superior performance remain uncertain.9 To gain insight into their chemistry, here we report benchmarking studies of RbHMDS and CsHMDS with the common carbonyls benzaldehyde and acetophenone, focusing on their reactivity, but tracking the remarkable structural outcomes of isolable metal products. Such structures are exceptionally rare, though a variety of crystallographically characterised monometallic and heterobimetallic CsHMDS complexes are known (Fig. 1 and Fig. S1, ESI†).2,10
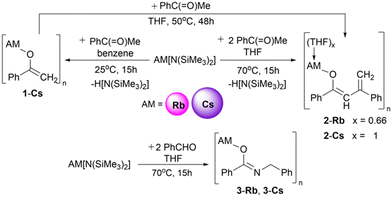
CsHMDS was mixed with acetophenone in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in benzene (Scheme 1), to form a white suspension. 1H NMR analysis of the isolated solid in THF-d8 revealed two multiplet resonances in the olefinic region at 3.28 and 3.90 ppm and aromatic signals in the range 7.02 to 7.68 ppm indicated formation of a metal-enolate species (see ESI,‡ pg. 6). The 13C NMR shift of
1 ratio in benzene (Scheme 1), to form a white suspension. 1H NMR analysis of the isolated solid in THF-d8 revealed two multiplet resonances in the olefinic region at 3.28 and 3.90 ppm and aromatic signals in the range 7.02 to 7.68 ppm indicated formation of a metal-enolate species (see ESI,‡ pg. 6). The 13C NMR shift of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 at 72.3 ppm compares to that of potassium enolate [{KOC(Mes)
CH2 at 72.3 ppm compares to that of potassium enolate [{KOC(Mes)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2·toluene}4] (74.6 ppm, Mes = mesityl).11 The compound's identity was confirmed by an X-ray diffraction study on crystals grown from a THF/hexane mixture at −20 °C. Enolate [CsOC(Ph)
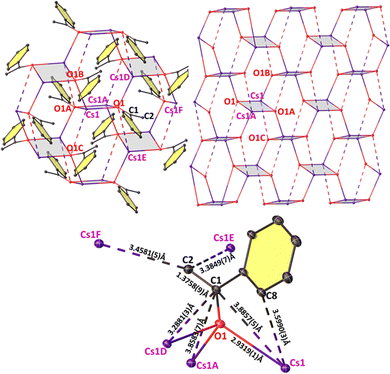
CH2·toluene}4] (74.6 ppm, Mes = mesityl).11 The compound's identity was confirmed by an X-ray diffraction study on crystals grown from a THF/hexane mixture at −20 °C. Enolate [CsOC(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2]∞ (1-Cs) was revealed having a remarkable infinite non-covalent network with ionic Cs–O bonds in a Pbca space group (Fig. 2). Two asymmetric Cs-enolate bridges form a four-atom ring (grey shaded) with Cs and O atoms occupying opposite corners. The edges [2.9319(1) Å and 2.9631(6) Å] and bond angles [91.1(2)° at O and 88.8(7)° at Cs] described by this Cs2O2 plane are close to that of a perfect square. These square planes stack in parallel in an alternating staggered arrangement. Four such planes from three adjacent stacks assemble together generating a distorted hexagonal prism with two parallel planes from the middle stack sharing their faces and two adjacent ones sharing one edge. These hexagonal prisms fuse together via all their quadrilateral faces in an infinite non-covalent network. Each Cs interacts with two other enolate O atoms [e.g., Cs1–O1B, 2.9582(1) Å; Cs1–O1C 4.2610(8) Å], respectively shorter and longer than the sum of the ionic radii of [Cs (1.67 Å) and O (1.35 Å)],12 of two adjacently stacked shaded square planes. The difference in contact lengths is the result of the shaded planes tilted at 27.0(9)° with respect to an adjacent stack. Each enolate ion engages with five Cs atoms (Fig. 2 – bottom) with O and benzylic C atoms (e.g., O1 and C1) bound to three centres (Cs1, Cs1A, Cs1D); whereas terminal C2 of the enolate interacts with two Cs atoms (Cs1E, Cs1F). The C1–C2 length [1.376(11) Å] indicates a C
CH2]∞ (1-Cs) was revealed having a remarkable infinite non-covalent network with ionic Cs–O bonds in a Pbca space group (Fig. 2). Two asymmetric Cs-enolate bridges form a four-atom ring (grey shaded) with Cs and O atoms occupying opposite corners. The edges [2.9319(1) Å and 2.9631(6) Å] and bond angles [91.1(2)° at O and 88.8(7)° at Cs] described by this Cs2O2 plane are close to that of a perfect square. These square planes stack in parallel in an alternating staggered arrangement. Four such planes from three adjacent stacks assemble together generating a distorted hexagonal prism with two parallel planes from the middle stack sharing their faces and two adjacent ones sharing one edge. These hexagonal prisms fuse together via all their quadrilateral faces in an infinite non-covalent network. Each Cs interacts with two other enolate O atoms [e.g., Cs1–O1B, 2.9582(1) Å; Cs1–O1C 4.2610(8) Å], respectively shorter and longer than the sum of the ionic radii of [Cs (1.67 Å) and O (1.35 Å)],12 of two adjacently stacked shaded square planes. The difference in contact lengths is the result of the shaded planes tilted at 27.0(9)° with respect to an adjacent stack. Each enolate ion engages with five Cs atoms (Fig. 2 – bottom) with O and benzylic C atoms (e.g., O1 and C1) bound to three centres (Cs1, Cs1A, Cs1D); whereas terminal C2 of the enolate interacts with two Cs atoms (Cs1E, Cs1F). The C1–C2 length [1.376(11) Å] indicates a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond fitting the description of an enolate (see Table S1 for selected bond angles, ESI†). Note that this hexagonal grid network is preferred over face-on cation–π interactions between Cs and the Ph π-surface, a common feature in heavy alkali metal compounds having aryl rings.13 Instead Cs interacts side-on to the π-electron cloud of the ring (Cs1⋯C8) in 1-Cs.
C double bond fitting the description of an enolate (see Table S1 for selected bond angles, ESI†). Note that this hexagonal grid network is preferred over face-on cation–π interactions between Cs and the Ph π-surface, a common feature in heavy alkali metal compounds having aryl rings.13 Instead Cs interacts side-on to the π-electron cloud of the ring (Cs1⋯C8) in 1-Cs.
While alkali metal amides, alkoxides, and enolates are known to exhibit ladder-like, cubic, or hexagonal prism motifs derived from folding of four-rung ladders,1c,d,14 to our knowledge, the special architecture of 1-Cs is the first such example of a Cs-organoelement compound manifested in a near hexagonal prism. Isolation of 1-Cs therefore confirms the Brønsted basicity of CsHMDS for deprotonation of an enolizable ketone.
Mimicking the above experiment with RbHMDS led to isolation of a highly reactive set of crystals (coated in oil) which decomposed during the XRD operation. After failed attempts to obtain a clean NMR spectrum of the product, the reaction solvent was altered to more polar THF. This reaction mixture gave a white suspension that gradually converted into a dark yellow solution, which gave X-ray quality crystals at −20 °C, albeit in poor yield. Surprisingly, the structure determined (space group: C2/c) was the C–C coupled product [{RbOC(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)C(Ph)
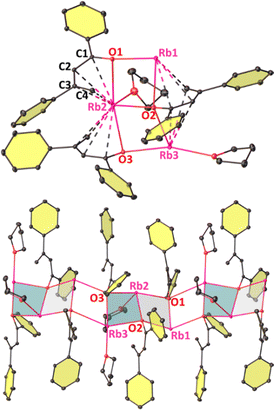
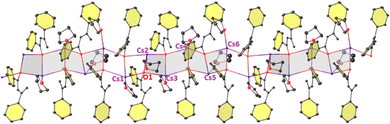
C(H)C(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2}3·(THF)2]∞ (2-Rb), presumably formed by a base mediated self-aldol-type reaction (Scheme S1, p12, ESI†). 2-Rb is composed of partly solvated trinuclear asymmetric units (with disordered THF ligands) with Rb–O interactions resembling a skewed three-rung (Rb3O3) ladder (Fig. 3). Alternating short and long C–C bonds indicate a dienolate intermediate (see Table S2, ESI†). The planes defined by Rb2O2 units lie at an angle of 14.5(2)° with respect to their plane normal. Two of the three Rb atoms (Rb2 and Rb3) in the asymmetric unit in one disordered model are solvated by THF, whereas THF-free Rb1 has the shortest Rb1–O1 bond distance of 2.685(3) Å [cf., Rb2–O2, 2.770(4) Å; Rb3–O3, 2.797(3) Å] due to the lower coordination. The trinuclear fused ladder propagates linearly in a slipped manner via interactions with Rb and the adjacent diene unit to form an infinite chain. The four sp2 hybridized C atoms sandwich between two Rb cations or vice versa and lie in the range 3.131(6)–3.770(6) Å from Rb. Reaction of RbHMDS and acetophenone in 1
CH2}3·(THF)2]∞ (2-Rb), presumably formed by a base mediated self-aldol-type reaction (Scheme S1, p12, ESI†). 2-Rb is composed of partly solvated trinuclear asymmetric units (with disordered THF ligands) with Rb–O interactions resembling a skewed three-rung (Rb3O3) ladder (Fig. 3). Alternating short and long C–C bonds indicate a dienolate intermediate (see Table S2, ESI†). The planes defined by Rb2O2 units lie at an angle of 14.5(2)° with respect to their plane normal. Two of the three Rb atoms (Rb2 and Rb3) in the asymmetric unit in one disordered model are solvated by THF, whereas THF-free Rb1 has the shortest Rb1–O1 bond distance of 2.685(3) Å [cf., Rb2–O2, 2.770(4) Å; Rb3–O3, 2.797(3) Å] due to the lower coordination. The trinuclear fused ladder propagates linearly in a slipped manner via interactions with Rb and the adjacent diene unit to form an infinite chain. The four sp2 hybridized C atoms sandwich between two Rb cations or vice versa and lie in the range 3.131(6)–3.770(6) Å from Rb. Reaction of RbHMDS and acetophenone in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio in THF-d8 reveals the appearance of a singlet CH resonance at 5.25 ppm of 2-Rb accompanied by doublets at 6.23 (J = 3.96 Hz) and 4.69 ppm (J = 3.80 Hz) for the terminal CH2 H atoms in the 1H NMR spectrum. Corresponding 13C NMR signals appear at 90.8 ppm (for CH) and 99.5 ppm (for CH2). Unreacted CsHMDS, acetophenone and free HMDS(H) signals can also be seen in the spectrum.
2 ratio in THF-d8 reveals the appearance of a singlet CH resonance at 5.25 ppm of 2-Rb accompanied by doublets at 6.23 (J = 3.96 Hz) and 4.69 ppm (J = 3.80 Hz) for the terminal CH2 H atoms in the 1H NMR spectrum. Corresponding 13C NMR signals appear at 90.8 ppm (for CH) and 99.5 ppm (for CH2). Unreacted CsHMDS, acetophenone and free HMDS(H) signals can also be seen in the spectrum.
Inspired by this unexpected result, reaction of CsHMDS and acetophenone was repeated in THF to yield a similar ladder-shaped dienolate [CsOC(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)C(Ph)
C(H)C(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2·THF]∞ (2-Cs), as confirmed by its XRD analysis (P
CH2·THF]∞ (2-Cs), as confirmed by its XRD analysis (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, Fig. 4). The structure of 2-Cs bears resemblance to that of 2-Rb as the asymmetric units [{CsOC(Ph)
space group, Fig. 4). The structure of 2-Cs bears resemblance to that of 2-Rb as the asymmetric units [{CsOC(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)C(Ph)
C(H)C(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2·THF}6·THF] propagate linearly into an infinite four-rung ladder of Cs2O2 rings. Rungs and edges have a mean bond length of 2.899 Å and 3.209 Å, respectively. The diene π-electrons interact with Cs akin to that in 2-Rb. 1H NMR spectrum of the crystals in THF-d8 confirmed the identity of 2-Cs in solution through a singlet CH resonance at 5.36 ppm and doublets at 6.35 (J = 3.92 Hz) and 4.72 ppm (J = 3.20 Hz) for the terminal CH2 atoms with aromatic signals in the range of 7.79–7.06 ppm (see ESI,‡ p. 16). Heating the CsHMDS and acetophenone mixture in THF-d8 to 50 °C also gave 2-Cs as evidenced by a 1H NMR spectrum (see ESI,‡ p. 8).
CH2·THF}6·THF] propagate linearly into an infinite four-rung ladder of Cs2O2 rings. Rungs and edges have a mean bond length of 2.899 Å and 3.209 Å, respectively. The diene π-electrons interact with Cs akin to that in 2-Rb. 1H NMR spectrum of the crystals in THF-d8 confirmed the identity of 2-Cs in solution through a singlet CH resonance at 5.36 ppm and doublets at 6.35 (J = 3.92 Hz) and 4.72 ppm (J = 3.20 Hz) for the terminal CH2 atoms with aromatic signals in the range of 7.79–7.06 ppm (see ESI,‡ p. 16). Heating the CsHMDS and acetophenone mixture in THF-d8 to 50 °C also gave 2-Cs as evidenced by a 1H NMR spectrum (see ESI,‡ p. 8).
Reports of alkali-metal dienolate structures are extraordinarily rare15 with the one exception of Seebach's N,N′-dimethylpropyleneurea (DMPU)-solvated lithium dienolate of pinacolone, made via the aldol condensation product of two pinacolones. It was postulated that aldol addition occurred while making the pinacolonenolate and after a reaction sequence of water splitting to enone and deprotonation to the dienolate, the final product [(DMPU)LiOC(H)(tBu)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)C(tBu)
C(H)C(tBu)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2]2 was isolated.15a Since a small amount of final product was isolated, this could imply a similar pathway in forming 2-Rb/Cs.
CH2]2 was isolated.15a Since a small amount of final product was isolated, this could imply a similar pathway in forming 2-Rb/Cs.
To test the reactivity of RbHMDS and CsHMDS in the absence of an acidic proton, stoichiometric reactions with benzaldehyde were performed in THF at 70 °C (Scheme 1). Colourless reaction mixtures turned purple for Cs and pink for Rb. Crystals grown from the supernatants at – 20 °C studied by XRD revealed them to be [RbOC(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCH2Ph]∞ (3-Rb) and [CsOC(Ph)
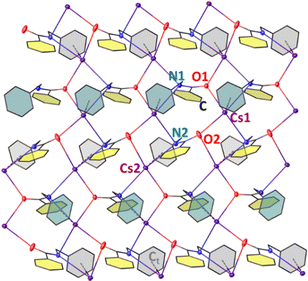
NCH2Ph]∞ (3-Rb) and [CsOC(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCH2Ph]∞ (3-Cs), which crystallised without any solvent coordination in P21/c and Pca21 space groups respectively (Fig. S5 for 3-Rb; Fig. 5 and Fig. S6 for 3-Cs, ESI†). These compounds may be viewed as alkali metal β-imino-alkoxides derived from the tautomer or iminol form of N-(benzyl)benzamide. While the asymmetric unit of 3-Rb contains one [RbOC(Ph)
NCH2Ph]∞ (3-Cs), which crystallised without any solvent coordination in P21/c and Pca21 space groups respectively (Fig. S5 for 3-Rb; Fig. 5 and Fig. S6 for 3-Cs, ESI†). These compounds may be viewed as alkali metal β-imino-alkoxides derived from the tautomer or iminol form of N-(benzyl)benzamide. While the asymmetric unit of 3-Rb contains one [RbOC(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCH2Ph] unit, 3-Cs has two [CsOC(Ph)
NCH2Ph] unit, 3-Cs has two [CsOC(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCH2Ph]2 units. One Cs atom in the asymmetric unit bridges two β-imino-alkoxide anions via their O atoms while the other Cs binds via the N atom and the π-cloud of Ph ring closest to N (Cs⋯Ct, 3.359 Å, Ct = centroid of Ph ring) of one anion. Analysing bond metrics of 3-Rb and 3-Cs revealed the existence of Rb/Cs–O σ-interactions [Rb1–O1, 2.877(9) Å; Cs1–O1, 2.9233(5) Å, Cs1–O2, 2.9837(3) Å] and of an unsaturated N environment [for 3-Rb C1
NCH2Ph]2 units. One Cs atom in the asymmetric unit bridges two β-imino-alkoxide anions via their O atoms while the other Cs binds via the N atom and the π-cloud of Ph ring closest to N (Cs⋯Ct, 3.359 Å, Ct = centroid of Ph ring) of one anion. Analysing bond metrics of 3-Rb and 3-Cs revealed the existence of Rb/Cs–O σ-interactions [Rb1–O1, 2.877(9) Å; Cs1–O1, 2.9233(5) Å, Cs1–O2, 2.9837(3) Å] and of an unsaturated N environment [for 3-Rb C1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N1 1.3152(9) Å; for 3-Cs C1
N1 1.3152(9) Å; for 3-Cs C1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N1 1.2833(7) Å, C16
N1 1.2833(7) Å, C16![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N2 1.303(1) Å] (see Tables S4 and S5, ESI†).
N2 1.303(1) Å] (see Tables S4 and S5, ESI†).
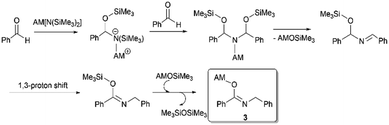
3-Rb forms a cage-like network comprising parallel zig-zag Rb–O–Rb chains which are intercalated by Rb–N and Rb–π (η6 to the Ph ring shaded grey, η2 to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, and η1 to the ipso C of the Ph ring shaded yellow) interactions from neighbouring β-imino-alkoxide moieties. Rb coordinates to two O and N atoms forming two different cyclic rings, a Rb–O–Rb–N 4-atom and a highly distorted Rb–O–C–N–Rb–O–C–N 8-atom ring. Each former ring is fused with the latter rings on all sides. This allows the 4-atom grids to propagate linearly in one direction (vertically in Fig. S5, ESI†), via the Rb corners giving it a unique cage shape. The cage motif is preserved in 3-Cs with interlocking zig-zag Cs–O–Cs chains containing an excess of Cs–π interactions on account of the larger radius of Cs versus Rb. Interestingly, this is a marked deviation from the usual aza-Peterson olefination mechanism with NaHMDS and benzaldehyde affording N-(trimethylsilyl)imine, an intermediate in the reaction pathway for aminobenzylation of aldehyde with NaHMDS/caesium trifluoroacetate co-operation.6a Though the mechanism of formation of 3 is unknown, germane literature by Toru et al.16 reported heating 4-pyridinecarboxaldehyde and HMDS(H) in a 2
N bond, and η1 to the ipso C of the Ph ring shaded yellow) interactions from neighbouring β-imino-alkoxide moieties. Rb coordinates to two O and N atoms forming two different cyclic rings, a Rb–O–Rb–N 4-atom and a highly distorted Rb–O–C–N–Rb–O–C–N 8-atom ring. Each former ring is fused with the latter rings on all sides. This allows the 4-atom grids to propagate linearly in one direction (vertically in Fig. S5, ESI†), via the Rb corners giving it a unique cage shape. The cage motif is preserved in 3-Cs with interlocking zig-zag Cs–O–Cs chains containing an excess of Cs–π interactions on account of the larger radius of Cs versus Rb. Interestingly, this is a marked deviation from the usual aza-Peterson olefination mechanism with NaHMDS and benzaldehyde affording N-(trimethylsilyl)imine, an intermediate in the reaction pathway for aminobenzylation of aldehyde with NaHMDS/caesium trifluoroacetate co-operation.6a Though the mechanism of formation of 3 is unknown, germane literature by Toru et al.16 reported heating 4-pyridinecarboxaldehyde and HMDS(H) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at 70 °C to afford N-(pyridin-4-ylmethyl)isonicotinamide. Reaction steps involve breaking a Si–N bond, forming a strong Si–O bond, then condensation to the imine which tautomerizes to the amide via a 1,3-proton shift. We have postulated a mechanism for formation of 3 (Scheme 2) along similar lines.
1 ratio at 70 °C to afford N-(pyridin-4-ylmethyl)isonicotinamide. Reaction steps involve breaking a Si–N bond, forming a strong Si–O bond, then condensation to the imine which tautomerizes to the amide via a 1,3-proton shift. We have postulated a mechanism for formation of 3 (Scheme 2) along similar lines.
In conclusion, this study reveals that both CsHMDS and RbHMDS display interesting reactivities with organic carbonyl substrates. With acetophenone, CsHMDS behaves predictably as a Brønsted base deprotonating the α-CH3 group, but the reaction is still novel through the remarkable hexagonal prism shaped network of the enolate product (1-Cs). Adding more novelty, in donor THF, the alkali metal enolates formed in situ ultimately produces dienolates 2-Rb and 2-Cs, which have rarely been isolated for any alkali metal. Further diversity is seen with benzaldehyde in producing β-imino-alkoxides 3-Rb and 3-Cs, which both adopt eye-catching cage networks that interconnect zig-zag M–O–M chains but in distinct ways. Finally, while this study shows that RbHMDS and CsHMDS can exhibit Brønsted basicity, some reactions and all the new structures have unique features that demand these heavier alkali metal amides should be studied with a much wider range of organic substrates.
This research was supported by the Leverhulme Trust (grant award no: RPG-2019-264).
Conflicts of interest
The authors have no conflicts of interest to declare.Notes and references
- (a) M. Lappert, P. Power, A. Protchenko and A. Seeber, Metal Amide Chemistry, Wiley, Chichester, 2009, pp. 7–38 Search PubMed; (b) P. C. Andrews, K. W. Henderson and K. Ruhlandt-Senge, Comprehensive organometallic chemistry III, ed. R. H. Crabtree and D. M. P. Mingos, Elsevier, Oxford, 2006, pp. 1–66 Search PubMed; (c) R. E. Mulvey, Chem. Soc. Rev., 1991, 20, 167–209 RSC; (d) R. E. Mulvey, Chem. Soc. Rev., 1998, 27, 339–346 RSC; (e) S. D. Robertson, M. Uzelac and R. E. Mulvey, Chem. Rev., 2019, 119, 8332–8405 CrossRef CAS PubMed; (f) T. X. Gentner and R. E. Mulvey, Angew. Chem., Int. Ed., 2021, 60, 9247–9262 CrossRef CAS PubMed.
- F. T. Edelmann, F. Pauer, M. Wedler and D. Stalke, Inorg. Chem., 1992, 31, 4143–4146 CrossRef CAS.
- H.-Z. Du, J.-Z. Fan, Z.-Z. Wang, N. A. Strotman, H. Yang and B.-T. Guan, Angew. Chem., Int. Ed., 2023, 62, e202214461 CrossRef CAS PubMed.
- (a) P. A. Macdonald, S. Banerjee, A. R. Kennedy, A. van Teijlingen, S. D. Robertson, T. Tuttle and R. E. Mulvey, Angew. Chem., Int. Ed., 2023, 62, e202304966 CrossRef CAS; (b) T. X. Gentner, A. R. Kennedy, E. Hevia and R. E. Mulvey, ChemCatChem, 2021, 13, 2371–2378 CrossRef CAS.
- M. Shigeno, T. Okawa, M. Imamatsu, K. Nozawa-Kumada and Y. Kondo, Chem. – Eur. J., 2019, 25, 10294–10297 CrossRef CAS PubMed.
- (a) Z. Wang, Z. Zheng, X. Xu, J. Mao and P. J. Walsh, Nat. Commun., 2018, 9, 3365 CrossRef; (b) Y. Gu, Z. Zhang, Y.-E. Wang, Z. Dai, Y. Yuan, D. Xiong, J. Li, P. J. Walsh and J. Mao, J. Org. Chem., 2022, 87, 406–418 CrossRef CAS PubMed.
- (a) R. E. Mulvey and S. D. Robertson, Angew. Chem., Int. Ed., 2013, 52, 11470–11487 CrossRef CAS; (b) R. A. Woltornist and D. B. Collum, J. Am. Chem. Soc., 2021, 143, 17452–17464 CrossRef CAS PubMed; (c) M. P. Coles, Coord. Chem. Rev., 2015, 297–298, 2–23 CrossRef CAS.
- S. Neander, U. Behrens and F. Olbrich, J. Organomet. Chem., 2000, 604, 59–67 CrossRef CAS.
- (a) H. Chen, W. Dai, Y. Chen, Q. Xu, J. Chen, L. Yu, Y. Zhao, M. Yea and Y. Pan, Green Chem., 2014, 16, 2136–2141 RSC; (b) R. Rabie, M. M. Hammouda and K. M. Elattar, Res. Chem. Intermed., 2017, 43, 1979–2015 CrossRef CAS.
- (a) S. Neander and U. Behrens, Z. Anorg. Allg. Chem., 1999, 625, 1429–1434 CrossRef CAS; (b) M. Westerhausen, S. Weinrich, B. Schmid, S. Schneiderbauer, M. Suter, H. Nöth and H. Piotrowski, Z. Anorg. Allg. Chem., 2003, 629, 625–633 CrossRef CAS; (c) A. I. Ojeda-Amador, A. J. Martínez-Martínez, A. R. Kennedy and C. T. O’Hara, Inorg. Chem., 2016, 55, 5719–5728 CrossRef CAS; (d) J. J. Morris, B. C. Noll, G. W. Honeyman, C. T. O'Hara, A. R. Kennedy, R. E. Mulvey and K. W. Henderson, Chem. – Eur. J., 2007, 13, 4418–4432 CrossRef CAS.
- X. He, B. C. Noll, A. Beatty, R. E. Mulvey and K. W. Henderson, J. Am. Chem. Soc., 2004, 126, 7444–7445 CrossRef CAS.
- (a) L. H. Ahrens, Geochim. Cosmochim. Acta, 1952, 2, 155–169 CrossRef CAS; (b) Database of Ionic Radii, https://abulafia.mt.ic.ac.uk/shannon/ptable.php, (accessed July 2023).
- (a) M. G. Davidson, D. Garcia-Vivo, A. R. Kennedy, R. E. Mulvey and S. D. Robertson, Chem. – Eur. J., 2011, 17, 3364–3369 CrossRef CAS; (b) A. Rae, K. M. Byrne, S. A. Brown, A. R. Kennedy, T. Krämer, R. E. Mulvey and S. D. Robertson, Chem. – Eur. J., 2022, 28, e202104260 CrossRef CAS.
- (a) J. R. Lynch, A. R. Kennedy, J. Barker, J. Reid and R. E. Mulvey, Helv. Chim. Acta, 2022, 105, e202200082 CrossRef CAS; (b) P. A. Macdonald, S. Banerjee, A. R. Kennedy, R. E. Mulvey and S. D. Robertson, Polyhedron, 2023, 234, 116302 CrossRef CAS.
- (a) R. Amstutz, J. D. Dunitz, T. Laube, W. B. Schweizer and D. Seebach, Chem. Ber., 1986, 119, 434–443 CrossRef CAS; (b) M. Oiarbide and C. Palomo, Chem. – Eur. J., 2021, 27, 10226–10246 CrossRef CAS PubMed; (c) T. Murahashi and H. Kurosawa, J. Organomet. Chem., 1999, 574, 142–147 CrossRef CAS.
- H. Uchida, T. Shimizu, P. Y. Reddy, S. Nakamura and T. Toru, Synthesis, 2003, 1236–1240 CAS.
Footnotes |
| † Open data available at https://doi.org/10.15129/5e6a35e0-1601-4b22-8bf2-2046d17b6281. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2293054–2293058. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc05527b |
| § JW and JRL contributed equally to this research. |
| This journal is © The Royal Society of Chemistry 2024 |