Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nano-rods in Ni-rich layered cathodes for practical batteries
Geon-Tae
Park†
 a,
Nam-Yung
Park†
a,
Hoon-Hee
Ryu†‡
a,
Nam-Yung
Park†
a,
Hoon-Hee
Ryu†‡
 a,
H. Hohyun
Sun
b,
Jang-Yeon
Hwang
a,
H. Hohyun
Sun
b,
Jang-Yeon
Hwang
 ac and
Yang-Kook
Sun
ac and
Yang-Kook
Sun
 *ac
*ac
aDepartment of Energy Engineering, Hanyang University, Seoul, 04763, South Korea. E-mail: yksun@hanyang.ac.kr
bDepartment of Chemical and Biological Engineering, The University of Alabama, Alabama 35487, USA
cDepartment of Battery Engineering, Hanyang University, Seoul, 04763, South Korea
First published on 9th October 2024
Abstract
Lithium transition metal oxide layers, Li[Ni1−x−yCox(Mn and/or Al)y]O2, are widely used and mass-produced for current rechargeable battery cathodes. Development of cathode materials has focused on increasing the Ni content by simply controlling the chemical composition, but as the Ni content has almost reached its limit, a new breakthrough is required. In this regard, microstructural modification is rapidly emerging as a prospective approach, namely in the production of nano-rod layered cathode materials. A comprehensive review of the physicochemical properties and electrochemical performances of cathodes bearing the nano-rod microstructure is provided herein. A detailed discussion is regarding the structural stability of the cathode, which should be maximized to suppress microcrack formation, the main cause of capacity fading in Ni-rich cathode materials. In addition, the morphological features required to achieve optimal performance are examined. Following a discussion of the initial nano-rod cathodes, which were based on compositional concentration gradients, the preparation of nano-rod cathodes without the inclusion of a concentration gradient is reviewed, highlighting the importance of the precursor. Subsequently, the challenges and advances associated with the nano-rod structure are discussed, including considerations for synthesizing nano-rod cathodes and surface shielding of the nano-rod structure. It goes on to cover nano-rod cathode materials for next-generation batteries (e.g., all-solid-state, lithium-metal, and sodium-ion batteries), inspiring the battery community and other materials scientists looking for clues to the solution of the challenges that they encounter.
1. Introduction
1.1. Developing trends for cathodes in industry
Since their debut in the consumer market by Sony in 1991, lithium-ion batteries (LIBs) have become one of the primary choices for energy storage, evolving from powering small portable electronics to fueling electric vehicles (EVs).1–4 Of the four major components present in LIBs, the cathode stands out as the costliest and heaviest component, exerting a significant impact on both the cost and performance aspects of LIBs.5–7 Consequently, the development of superior cathode materials has consistently been pivotal for the advancement of LIBs since their introduction.In the academic field, various types of cathode materials have been proposed over recent decades, although only a few have been commercially adopted since the initial commercialization of the LiCoO2 (LCO) cathode material.8–19 Even among these, the spinel-structured LiMn2O4, which does not offer significant performance advantages, is rarely used, and the layered Li[Ni1−x−y−zCox(Mny or Alz)]O2 (NCM, NCA, and/or NCMA) cathode materials and olivine-structured LiFePO4 cathode materials dominate the current cathode market share.20–22 New materials are constantly being researched and developed as candidates for next-generation cathode materials, with the ultimate goals of commercialization and deployment. Examples include Li-rich cathode materials and disordered rock-salt (DRX) cathode materials, which are considered to have significant potential for complementing the drawbacks associated with current NCM/NCA and LFP cathode materials while reducing their dependence on specific resources.23,24 However, the ability of these materials to reach the commercialization stage remains uncertain.
Layered NCM/NCA cathode materials have significant advantages in that their performances and stabilities can be easily modified by simply changing their compositional chemistries.6,25,26 The NCM333 cathode material was one of the first to appear in early applications, followed by the LCO cathode material, wherein the equal concentrations of the constituent elements ensured a balance between energy density, conductivity, stability, and cost.27,28 This material was initially employed in hybrid EV batteries in 2009.29 However, due to the rapidly increasing demand for higher energy densities, the NCM622 cathode material (containing 60% Ni) was commercialized in 2016.30 Currently, the Ni contents of such materials have been increased to reach even 100% in some cases.6,31,32 Although increasing Ni content is an effective strategy for increasing the energy density, inherent trade-off between capacity and stability leads to challenges in handling such materials owing to the high reactivity of Ni4+ (Fig. 1a).31–35
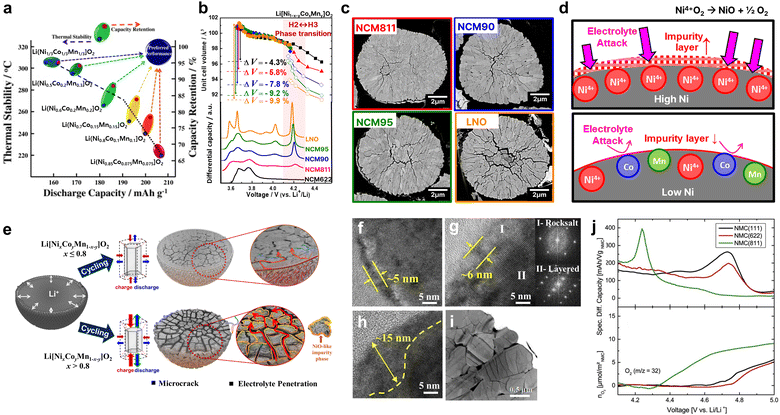 | ||
| Fig. 1 (a) Map showing the relationship between the discharge capacity, thermal stability, and capacity retention in Li/Li[NixCoyMn1−x−y]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8, and 0.85). (b) Unit cell volume changes and dQ dV−1 curves for NCM622, NCM811, NCM90, NCM95 and LNO cathodes upon charging. (c) Cross-sectional SEM images of NCM811, NCM90, NCM95 and LNO cathodes charged to 4.3 V. (d) Schematic illustration of surface reaction between cathode and electrolyte for cathodes with high and low Ni content. (e) Capacity fading scheme of Ni-rich Li[NixCoyMn1−x−y]O2 cathodes. High-resolution TEM image of the discharged Li[NixCoyMn1−x−y]O2 cathode after 100 cycles, (f) x = 0.6 and (g) x = 0.8, with Fourier transforms from the marked regions of I and II, and (h) x = 0.95. (i) TEM image of the primary particles showing numerous intragranular cracks in the cycled Li[Ni0.9Co0.05Mn0.05]O2 cathode. (j) Specific differential capacity vs. cell voltage and evolution of O2 as a function of the cell voltage for the NMC-Li cells. (a) is reproduced with permission,31 Copyright 2013, Elsevier. (e)–(i) are reproduced with permission,36 Copyright 2018, American Chemical Society. (j) is reproduced under Creative Commons CC-BY license,37 Copyright 2017, IOP Publishing. | ||
Particularly in Ni-rich cathodes above 80% Ni, the abrupt anisotropic lattice volume change that occurs at high state of charge (Fig. 1b) induces strain within the particles and leads to the formation of microcracks (Fig. 1c).36,38,39 This can be attributed to mechanical stress generated during the charge/discharge process exceeding the tolerance of materials, leading to the development of physical fractures throughout individual particles. The fractures exponentially increase the cathode surface area exposed to the electrolyte to induce undesirable side reactions and associated rapid capacity fading.40–44 At the cathode-electrolyte interface exposed to electrolyte, unstable Ni4+ ions undergo side reactions to degrade the layered cathode surface into a NiO-like rocksalt phase resulting in increased resistance and capacity fading (Fig. 1d). This degradation mechanism is limited to the surface in NCM/NCA cathodes with less than 80% Ni but amplifies to the bulk along interparticle boundaries in those with greater than 80% Ni (Fig. 1e). Note that the thickness of the impurity increases with increasing Ni content (Fig. 1f–h) and further extends. In addition to the microcracks observed along the interparticle boundaries, numerous intraparticle cracks generated by internal stress arising from the anisotropic volume change (Fig. 1i). This chemo-mechanical degradation not only impedes the facile (de)intercalation of Li+ ions, but also promotes gas generation to jeopardize battery safety (Fig. 1j).37 Naturally, both the scientific community and industry have devoted enormous efforts to overcoming this challenge, with doping and coating post-treatment methods being the most prevalent.
Among the various treatments reported to date, concentration gradient (CG) cathode materials are examples that have been successfully commercialized and applied in EVs.45 It has been estimated that these materials will be produced by >45 kton per year after 2026, which could support the production of up to 225![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 EVs annually. The original concept of concentration gradient cathodes aimed to achieve a high-energy density from the Ni-enriched core simultaneously and an exceptional safety originating from the Mn-rich shell.46,47 Besides compositional protection, surprisingly, concentration gradient cathode materials possess a characteristic microstructure comprising elongated primary particles that are radially aligned outward.46,48 This unique spatial configuration has been proven to be particularly effective in relieving the mechanical stress accumulated within the cathode particles, thereby effectively suppressing the formation of microcracks.49 The synergistic effects of these features ultimately result in an excellent cycling ability and superior thermal stability, even in cathodes containing ≥90% Ni.50 Consequently, this approach can be considered an optimal design strategy for extremely high-Ni cathode materials.
000 EVs annually. The original concept of concentration gradient cathodes aimed to achieve a high-energy density from the Ni-enriched core simultaneously and an exceptional safety originating from the Mn-rich shell.46,47 Besides compositional protection, surprisingly, concentration gradient cathode materials possess a characteristic microstructure comprising elongated primary particles that are radially aligned outward.46,48 This unique spatial configuration has been proven to be particularly effective in relieving the mechanical stress accumulated within the cathode particles, thereby effectively suppressing the formation of microcracks.49 The synergistic effects of these features ultimately result in an excellent cycling ability and superior thermal stability, even in cathodes containing ≥90% Ni.50 Consequently, this approach can be considered an optimal design strategy for extremely high-Ni cathode materials.
1.2. History of nano-rod cathodes
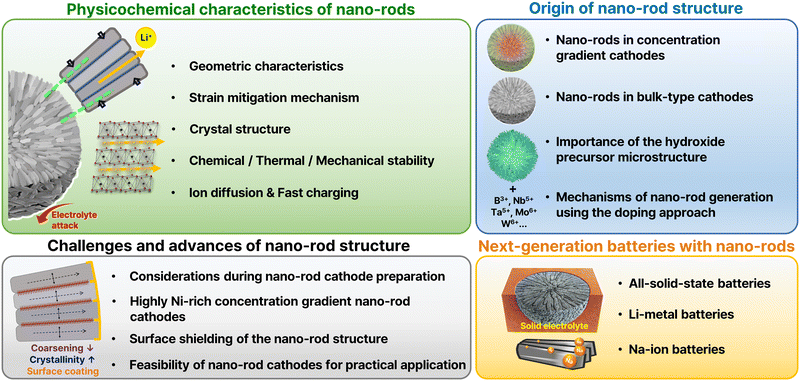
Unique cathode microsphere particles, whose microstructure consists of rod-shaped primary particles with radial alignment (hereafter referred to as nano-rods), were first observed in the CG Li[Ni0.75Co0.10Mn0.15]O2 cathode material by Sun et al. in 2012.46 A follow-up study by Noh et al. in 201348 placed significant emphasis on the nano-rod structure present in a full-concentration gradient Li[Ni0.60Co0.15Mn0.25]O2 material, highlighting its contribution to several key performance aspects. Inspired by these characteristics, research has intensified in the context of microstructure engineering aimed at achieving elongated rod-shaped primary particles exhibiting radial alignment, even in the absence of concentration gradients. The first such report was published by Park et al.,51 who demonstrated that boron doping can alter the shape of the primary particles. Following this study, various dopants were investigated for the development of nano-rod structures, and the number of related studies has rapidly increased in line with strong interest from both the academic and industrial fields.Thus, this review evaluates and discusses the nano-rod microstructure's physicochemical properties and related electrochemical features (Fig. 2). In addition, this review introduces various concepts developed to overcome the challenges encountered in implementing nano-rods. It also explores the potential application of nano-rod cathode materials in next-generation secondary batteries, such as all-solid-state, Li-metal, and Na-ion batteries.
2. Physicochemical characteristics and electrochemical performances
2.1. Geometric characteristics
Traditionally, the microstructural features of layered-oxide cathode grains have been broadly classified into two categories, namely polygonal- and rod-shaped primary particles. Due to the absence of advanced characterization technologies in the early stages of the cathode development, specification of the microstructural features of nano-rod cathodes was limited. With the recent development of novel and superior analytical methods, various parameters have been proposed for distinguishing the morphological features of nano-rod particles, including the dimensions, population density, and degree of orientation. Representative morphologic parameters reported to date include the width, length, area, square root size, aspect ratio, number density, alignment angle, and surface faceting. This section introduces the various distinguishing microstructural characteristics of nano-rod cathodes and provides the respective numerical distribution ranges required to obtain outstanding electrochemical performances. In addition, the conditions responsible for altering these morphology determinants are briefly introduced, and the exceptional relationships between the microstructural parameters and the electrochemical performance are discussed.Conventional layered-oxide cathodes consist of relatively thick, bulky, and irregular-shaped primary particles typical of ceramic materials.63 In contrast, nano-rod cathodes feature a rod-like morphology characterized by a lengthy longitudinal axis and a narrow width.46,64–66 In one study, Ryu et al.66 measured the widths and lengths of the primary particles of the bulk-structured Li[Ni0.901Co0.044Mn0.045Al0.01]O2 (BS-NCMA89) and the hybrid-structured Li[Ni0.899Co0.045Mn0.046Al0.01]O2 (HS-NCMA89) (Fig. 3a). They reported average widths of 371 and 158 nm for the BS-NCMA89 and HS-NCMA89 materials, respectively, and average lengths of 587 and 630 nm, respectively. The adoption of compositional partitioning in the NCMA90 cathode produced tightly packed nano-rod primary particles in HS-NCMA89, which are both long and narrow. Heteroelemental doping can also be used to tune the widths and lengths of the primary particles, wherein the introduction of B, Al, Ti, Mn, Sn, Zr, Te, Nb, W, Ta, Mo, and Sb provides narrower and longer primary particles.53,67–96 It should be noted here that the chemical composition of the cathode does not limit such size modification effects, with size engineering effects also being confirmed in NCA85, NCA88, NCM90, NM90, and LNO materials. Studies have also demonstrated that the width of the primary particles is unequivocally correlated with the electrochemical cycling stability of the cathode material. For example, Park et al.79 demonstrated an approximately linear relationship between the primary particle width and the capacity retention of Li[Ni0.95Co0.04X0.01]O2 (X = dopant) cathode (Fig. 3b). More specifically, the capacity retention increased with a reduced particle width, thereby suggesting that the chemistry of the dopant has a relatively small effect on the cycling performance, which appears to be primarily governed by the mechanical properties of the cathode. A notable feature of the grain size is that the distribution range of the particle width in conventional cathodes is broader than in nano-rod cathodes. For example, the width of the NCA89 particles ranges from 150 to 300 nm, whereas that of the W-NCA89 particles ranges from 50 to 100 nm.97 The same trend has also been observed in other studies.66,68,76 As will be discussed in the following section, the microstructure of the cathode active material is principally determined by its hydroxide precursor and the subsequent calcination conditions. More specifically, during high-temperature calcination, the high thermal energy input leads to coarsening of the primary particles, ultimately determining the cathode's microstructure. Since grain agglomeration mainly occurs between the lateral sides of adjacent primary particles, where the contact area between grains is large, the random coarsening of conventional primary particles yields an irregular width distribution.98 In contrast, introducing a concentration gradient or hetero-elemental doping mitigates vigorous sintering of the primary particles, thereby producing tightly packed nano-rod grains with a uniform width distribution. This difference can ultimately affect the microstructural stability of the cathode, thereby defining its mechanical and electrochemical properties, as discussed later.
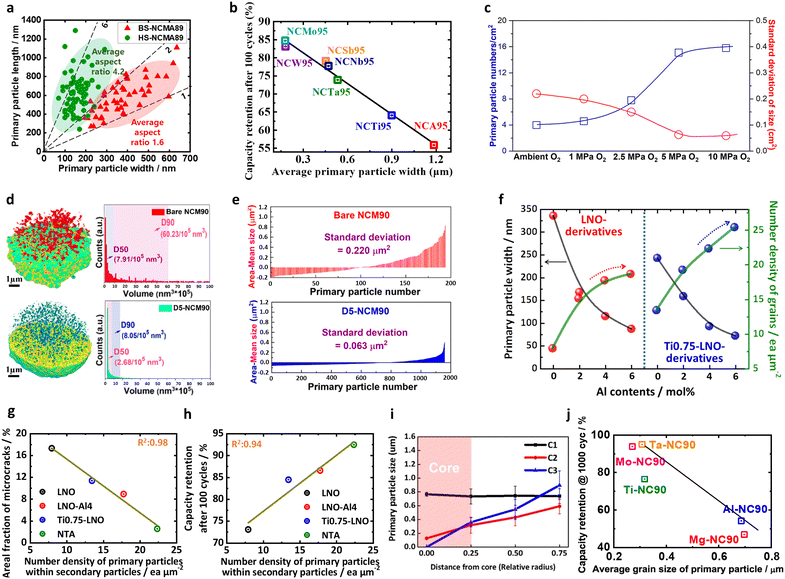 | ||
| Fig. 3 (a) Quantitative comparisons of the microstructures of the BS-NCMA89 and HS-NCMA89 cathodes: primary particle widths and lengths. (b) Capacity retention after 100 cycles for cells featuring various cathodes lithiated at 800 °C, as a function of the average primary particle width, reflecting the relationship between the primary particle width and the cycle life. (c) Number of primary particles per μm2 and standard deviation of the primary particle size for the bare NCM90 cathode materials sintered under O2 at ambient pressure and 1–10 MPa. (d) 3D distributions and statistical analyses of the pore volumes of the bare NCM90 and D5-NCM90 cathode particles. (e) Standard deviations of the sizes of the bare NCM90 and D5-NCM90 cathode materials. (f) Plots of the primary particle widths and the grain number densities of the LNO- and Ti0.75-LNO-derivative cathodes as functions of the Al doping contents. Linear regression plots between the number density of the primary particles and (g) the areal fraction of microcracks after 100 cycles and (h) the capacity retention after 100 cycles. (i) Plot showing the change in the primary particle size measured as a function of the relative radius [from the center (0) to the radius (R)]. (j) Summaries of the capacity retention values after 1000 cycles as a function of the average grain size of the primary particles. (a) is reproduced with permission,99 Copyright 2020, American Chemical Society. (b) is reproduced with permission,79 Copyright 2021, Royal Society of Chemistry. (c)–(e) are reproduced with permission,55 Copyright 2023, American Chemical Society. (f)–(h) are reproduced with permission,58 Copyright 2023, Elsevier. (i) is reproduced with permission,56 Copyright 2019, American Chemical Society. (j) is reproduced under Creative Commons CC-BY license,57 Copyright 2021, The Authors, Springer Nature. | ||
Although the area, square root size, and number density are different ways the grain size can be expressed, their intrinsic meanings are similar. As described above, the square root size corresponds to the square root of the grain area. Therefore, the two factors have the same physical meaning. In addition, since the number density is calculated by the number of grains per unit area, a decrease in the square root size indicates an increase in the number density. In this context, Ju et al.55 reported that increasing the oxygen partial pressure stimulated the formation of nano-rod primary particles, decreasing the average grain area and increasing the number density. More specifically, they reported the average grain areas of NCM90 and DS-NCM90 to be 0.243 and 0.065 μm2, respectively, whereas their corresponding number densities were 4 and 16 ea μm−2, respectively (Fig. 3c). This increase in the grain number density concomitantly led to an increase the density of the cathode active material due to a reduction in the pore volume. As shown in Fig. 3d, the total pore percentages in NCM90 and D5-NCM90 were 1.91 and 0.63%, respectively. As in the case of the grain size, the nano-rod grains exhibited a relatively uniform grain area distribution, with standard deviations of 0.220 and 0.063 μm2 for the NCM90 and DS-NCM90 cathodes, respectively (Fig. 3e). The number density of the primary particle can be further refined by adopting a multi-stage engineering approach. For example, Park et al.58 reported that the introduction of Ti during the synthesis of Ni(OH)2, followed by the addition of excess Al doping during the calcination step produced nanosized primary particles with exceptionally high number densities, i.e., 7.9, 13.4, 17.8, and 22.4 ea μm−2 for the LNO, Ti0.75-LNO, LNO-Al4, and NTA cathodes, respectively (Fig. 3f). Interestingly, correlations were found between the number density of the primary particles and several performance indicators. More specifically, an approximately linear relationship was observed between the areal fraction of microcracks and the number density of the primary particles, demonstrating the effectiveness of particle size refinement in suppressing microcrack formation and propagation (Fig. 3g). As a result, the cycling stability and rate characteristics were improved, and the cathode performance exhibited an approximate linear relationship with the particle number density (Fig. 3h). In the context of layered-oxide cathodes, the preparation of such an ultrafine-grained cathode is not a trivial matter. Because the conventional method for synthesizing cathodes involves the preparation of a hydroxide precursor and subsequent lithiation at high temperatures (>700 °C), the primary particles of the hydroxide precursor invariably coarsen such that the grain size of the final cathode active material routinely exceeds 500 nm. In addition, Shim et al.56 measured the locally varying square root size of the hollow-structured nano-rod NCM622 and reported that the size increases from the core to the shell of the cathode (i.e., 0.25 μm near the center and 0.8 μm at the surface; Fig. 3i). This remarkable decrease in the square root size of the nano-rods was also observed by Sun et al.57 More specifically, by doping high-valence elements, the square root size of the peripheral grains decreased to the ultrafine scale (<500 nm), i.e., 308 and 271 nm for Ta-NC90 and Mo-NC90, respectively, thereby highlighting the effectiveness of high-valence ion doping in reducing the grain size (Fig. 3j).
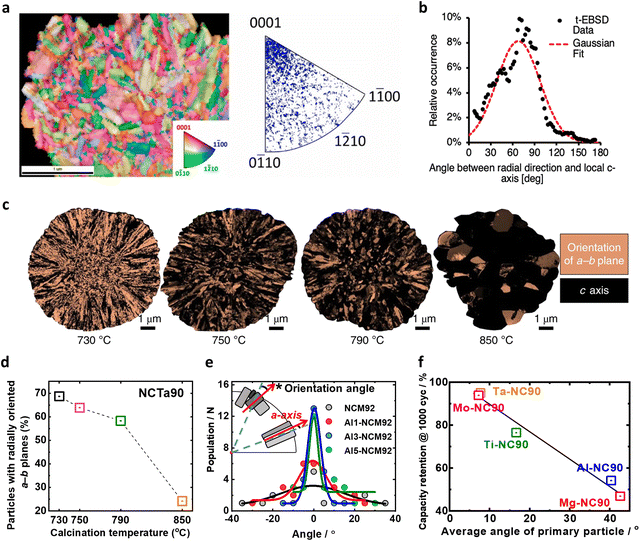 | ||
| Fig. 4 (a) Surface normal-projected orientation t-EBSD maps of the CP_2B_5ex species. Lamellas with the corresponding color-coded inverse pole legends are shown, which correspond to a hexagonal unit cell, and indicate the specific crystallographic orientation for each grain color. The folded inverse pole figure discrete maps show the orientation of the surface normal for all measured grains in each sample. (b) Distribution of angles between the c-axis of the hexagonal primary particle lattice in the particle shell and the particle radial direction. Red: Gaussian fit. (c) ASTAR images of the NCTa90 cathodes highlighting the tendency of the a–b planes of the primary particles to point to the radial direction as the lithiation temperature is lowered. (d) Estimated percentage of primary particles with a–b planes pointing in the radial direction for NCTa90. (e) Orientation angles of the primary particles of the NCM92 (grey), Al1-NCM92 (red), Al3-NCM92 (blue), and Al5-NCM92 (green) particles. (f) Summary of the capacity retention values after 1000 cycles as a function of the average angle of the primary particles. (a) is reproduced with permission,100 Copyright 2020, Royal Society of Chemistry. (b) is reproduced with permission,101 Copyright 2023, Elsevier. (c) and (d) are reproduced with permission,53 Copyright 2020, The Authors, Springer Nature. (e) is reproduced with permission,106 Copyright 2023, Elsevier. (f) is reproduced under Creative Commons CC-BY license,57 Copyright 2021, The Authors, Springer Nature. | ||
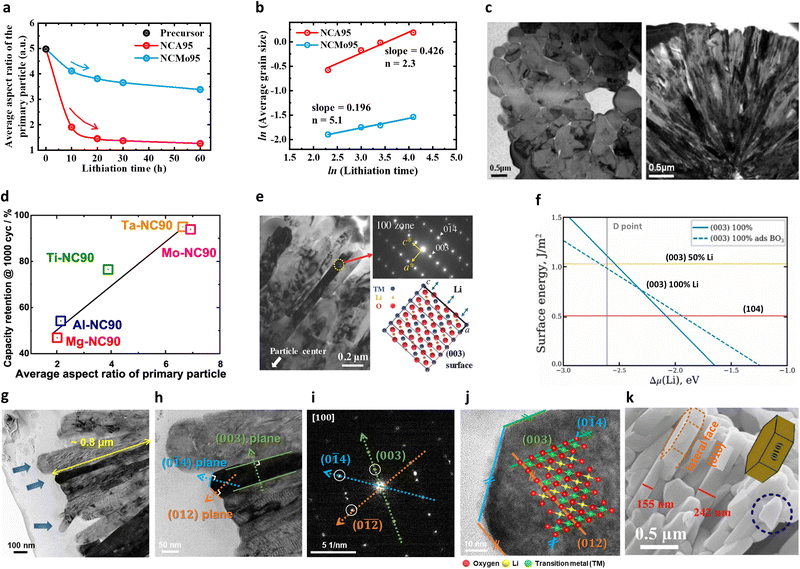 | ||
| Fig. 5 (a) Average primary particle aspect ratios (expressed as length/width) of the hydroxide precursor, NCA95 cathode, and NCMo95 cathode particles as a function of the lithiation time. (b) Grain growth exponents for the NCA95 and NCMo95 cathodes calculated using the grain growth equation. (c) Cross-sectional TEM images of the cathode particles: (left) fixed composition of Li[Ni0.5Co0.2Mn0.3]O2 without the compositional gradient, and (right) FCG cathode wherein the core composition of Li[Ni0.86Co0.07Mn0.07]O2 is continuously varied to Li[Ni0.67Co0.09Mn0.24]O2 at the surface. (d) Summary of the capacity retention values after 1000 cycles as a function of the average primary particle aspect ratio. (e) Magnified TEM image of the B1.0-NCM90 primary particles, electron diffraction pattern of the region marked by a yellow circle, and schematic drawing of the crystal structure of the oriented primary particle. (f) Surface energy as a function of the Li chemical potential Δμ(Li) at a fixed Δμ(O) = −1.06 eV. The (104) and (003) surfaces with 50/100% Li and the (003) surface with the adsorption of a single BO3 group are shown. The vertical gray line corresponds to the Li chemical potential at point D. (g) Bright-field cross-sectional scanning STEM images of the 4% Mn-doped CG NCA92 cathode primary particles. Blue arrows highlight the surface shapes of the primary particles. (h) High-magnification TEM images of the 4% Mn-doped CG NCA92 cathode primary particles. (i) SAED and (j) HRTEM images of the 4% Mn-doped CG NCA92 cathode obtained from the grains identified in part (h). (k) SEM image of the NCA-Urea-NMP specimen. (a) and (b) are reproduced under Creative Commons CC-BY license,79 Copyright 2021, Royal Society of Chemistry. (c) is reproduced with permission,48 Copyright 2013, American Chemical Society. (d) is reproduced under Creative Commons CC-BY license,57 Copyright 2021, Springer Nature. (e) is reproduced with permission,51 Copyright 2018, John Wiley and Sons. (f) is reproduced with permission,100 Copyright 2023, Elsevier. (g)–(j) are reproduced with permission,72 Copyright 2024, John Wiley and Sons. (k) is reproduced with permission,115 Copyright 2020, Elsevier. | ||
In some cases, the addition of certain dopants can promote crystallographic facets on the surfaces of the nano-rod particles. For example, B is a well-known element that produces angular and highly faceted nano-rod primary particles. In this context, Park et al.51 suggested that introducing B doping into the NCM90 cathode could potentially construct flat hexagonal-like primary particles, wherein most of the surface was enclosed at the sides by the (003) plane (Fig. 5e). Boron significantly decreases the energy of the (003) surface, thereby leading to the anisotropic plate-like shape observed for the primary particles of the NCM cathode (Fig. 5f).100 In the B-doped NCA89 cathode, such surface faceting was more pronounced. In another study, Ryu et al.52 demonstrated that B doping not only attenuated the surface energy of the (003) plane but also those of the (01![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ) and (014) planes. The sides of the B-NCA89 nano-rod particles developed zigzag surfaces so that the side surfaces were terminated with (003) and (01
) and (014) planes. The sides of the B-NCA89 nano-rod particles developed zigzag surfaces so that the side surfaces were terminated with (003) and (01![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ) planes. Such zigzag-like surfaces induced by crystallographic faceting was also confirmed in the B-NCA95 cathode.116 Recently, Park et al.72 demonstrated that adding Mn during calcination could stimulate nano-scale surface faceting in the NCA cathode. This was accounted for by considering that the near-surface introduction of Mn spatially refined the microstructure by capping the nano-rod particles with the (003), (0
) planes. Such zigzag-like surfaces induced by crystallographic faceting was also confirmed in the B-NCA95 cathode.116 Recently, Park et al.72 demonstrated that adding Mn during calcination could stimulate nano-scale surface faceting in the NCA cathode. This was accounted for by considering that the near-surface introduction of Mn spatially refined the microstructure by capping the nano-rod particles with the (003), (0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4), and (012) crystal planes (Fig. 5g–j). Notably, such nano-scale surface faceting is not observed in general NCM cathodes that use a hydroxide precursor to which Mn is added during the co-precipitation step, thereby confirming that subsurface Mn doping is key to attenuating the cathode surface energy. Although the degree of surface faceting is not as pronounced as that observed upon B or Mn doping, the cathodes prepared using NMP or PVP as a capping agent also demonstrated enhance exposure of the (010) planes (Fig. 5k).115,117 Since the (003) facets parallel to the Li+ and TM layers are more resistant to irreversible structural degradation than the non-(003) facets, highly faceted nano-rod primary particles contribute to improving the electrochemical cycling stability of the cathode.66
4), and (012) crystal planes (Fig. 5g–j). Notably, such nano-scale surface faceting is not observed in general NCM cathodes that use a hydroxide precursor to which Mn is added during the co-precipitation step, thereby confirming that subsurface Mn doping is key to attenuating the cathode surface energy. Although the degree of surface faceting is not as pronounced as that observed upon B or Mn doping, the cathodes prepared using NMP or PVP as a capping agent also demonstrated enhance exposure of the (010) planes (Fig. 5k).115,117 Since the (003) facets parallel to the Li+ and TM layers are more resistant to irreversible structural degradation than the non-(003) facets, highly faceted nano-rod primary particles contribute to improving the electrochemical cycling stability of the cathode.66
2.2. Strain mitigation mechanism
The degradation of Ni-rich cathodes is largely caused by the high proportion of labile Ni4+ species combined with the mechanical instability originating from intergranular crack formation.36,118,119 One of the beneficial effects of constructing nano-rod primary particles is that the cathode can mitigate any degradation arising from the microcracks. The following subsections discuss the various microcrack mitigation mechanisms associated with nano-rod primary particles. In addition to theoretical calculations, experimental demonstrations of the mechanical stabilities of the nano-rod particles under different states of charge (SoC) are also presented.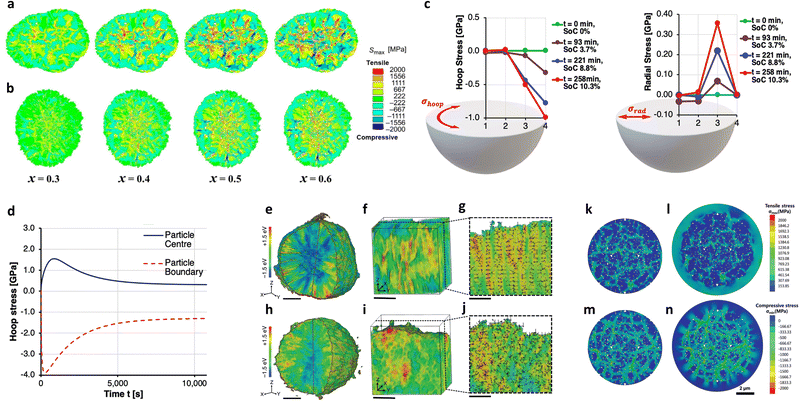 | ||
| Fig. 6 Calculated stress distribution (maximum principal stress) as a function of the state of charge inside the (a) P-NCA89 and (b) B-NCA88 particles. (c) Reconstructed stress state along the particle radius vs. time and SoC; hoop stress and radial stress. (d) Simulated hoop stress evolution versus time during charging in the core and shell of a secondary particle. (e) 3D Ni valence state distribution, (f) representative region of the 3D Ni valence state distribution, and (g) 2D nanodomain valence gradient of the rod-NMC. (h) 3D Ni valence state distribution, (i) representative region of the 3D Ni valence state distribution, and (j) 2D nanodomain valence gradient of the gravel-NMC. The nanodomain valence gradient vectors are represented by black arrows, wherein the vector direction and magnitude are represented by the arrow direction and arrow length, respectively. The scale bars in parts (e) and (h) are 3 μm, while the scale bars in parts (f,g) and (i,j) are 1 μm. The Ni K-edge absorption energies are color-coded, in which blue stands for a lower edge energy and red reflects a higher edge energy. Tensile stress distributions (maximum principal stress) for the (k) CC90 and (l) CSG90 particles, along with the compressive stress distributions (minimum principal stress) for the (m) CC90 and (n) CSG90 particles in the fully charged state. (a) and (b) are reproduced with permission,52 Copyright 2020, Elsevier. (c) and (d) are reproduced under Creative Commons CC-BY license,101 Copyright 2020, Royal Society of Chemistry. (e)–(j) are reproduced under Creative Commons CC-BY license,122 Copyright 2020, The Authors, Springer Nature. (k)–(n) are reproduced with permission,49 Copyright 2019, John Wiley and Sons. | ||
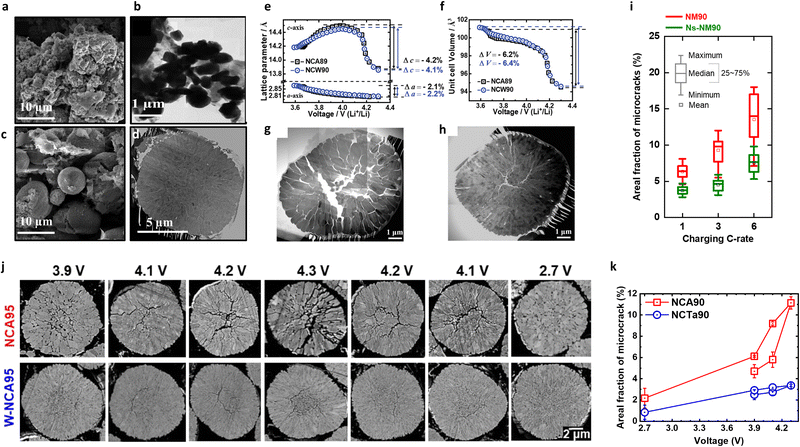 | ||
| Fig. 7 (a) SEM image and (b) TEM image of the Li[Ni0.85Co0.11Al0.04]O2 NCA electrode after 100 cycles with an upper cut-off voltage of 4.5 V. (c) SEM image and (d) TEM images in the [100] zone of the Li[Ni0.84Co0.06Mn0.09Al0.01]O2 TSFCG-Al electrode after 100 cycles with an upper cut-off voltage of 4.5 V. Changes in (e) the a- and c-axes lattice parameters and (f) the unit cell volumes for NCA89 and NCW90 as a function of the cell voltage. Bright-field STEM mosaic image of cross-sections of the (g) NCA89 and (h) NCW90 cathodes charged to 4.3 V. (i) Areal fractions of microcracks in the cathode secondary particles as a function of the charge C-rate determined from cross-sectional SEM images. The bars represent the distribution of results. (j) Comparison of the cross-sectional SEM images of the NCA95 and W-NCA95 cathodes in various charged and discharged states (charged to 3.9, 4.1, 4.2, and 4.3 V; discharged to 4.2, 4.1, and 2.7 V in the 1st cycle). (k) Areal fractions of the microcracks in the NCA90 and NCTa90 cathodes as a function of the cut-off voltage. (a)–(d) are reproduced with permission,124 Copyright 2016, American Chemical Society. (e)–(h) are reproduced under Creative Commons CC-BY license,129 Copyright 2019, The Authors, John Wiley and Sons. (i) is reproduced with permission,132 Copyright 2022, John Wiley and Sons. (j) is reproduced with permission,97 Copyright 2020, Elsevier. (k) is reproduced with permission,53 Copyright 2020, Springer Nature. | ||
2.3. Crystal structure & ordering
It is generally considered ideal that a layered oxide cathode consists of a rhombohedral crystal structure with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group and without impurity phases. However, in some cases, nano-rod primary particles contain crystal structural characteristics different from the layered structures that are typical of equiaxed grains. The disordered rock-salt, spinel, cation-ordered, and twinned crystals are representative crystal structures in the nano-rod cathodes. These crystal structures are different from the impurity phases observed in cycled or damaged Ni-rich cathodes. The following subsections will address the origins of these unexpected nano-scale crystal structures and their roles in determining the electrochemical performances of the nano-rod cathodes.
m space group and without impurity phases. However, in some cases, nano-rod primary particles contain crystal structural characteristics different from the layered structures that are typical of equiaxed grains. The disordered rock-salt, spinel, cation-ordered, and twinned crystals are representative crystal structures in the nano-rod cathodes. These crystal structures are different from the impurity phases observed in cycled or damaged Ni-rich cathodes. The following subsections will address the origins of these unexpected nano-scale crystal structures and their roles in determining the electrochemical performances of the nano-rod cathodes.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) and a minor rock-salt phase (Fm
m) and a minor rock-salt phase (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) (Fig. 8a). Structural analyses confirmed that the percentage of the rock-salt phase increased with the amount of W. Theoretical calculations suggested that these W-doped rocksalt surfaces are energetically favored over the bare rocksalt surfaces and the layered surfaces. It is generally accepted that the rock-salt phase is typically observed in cycled or damaged Ni-rich cathodes, wherein the electrochemical performance is extremely poor.139–145 However, the cycling performance of the rock-salt-containing W-doped LNO was unexpectedly superior to that of the undoped LNO cathode (Fig. 8b). Although W doping slightly decreased the initial discharge capacity, the W-doped LNO cathode exhibited remarkable stabilization, reflected by impressive cycling stability. It was therefore considered that the rocksalt phase segregated at the cathode surface protected the cathode active material from detrimental reactions with the electrolyte species and enhanced the cycling stability of the cathode.67,138 In contrast, the introduction of W doping in NCM cathodes led to a disordered spinel phase formation. More specifically, Park et al.146 demonstrated that the addition of W into the NCM90 cathode produced a 20 nm-thick spinel phase on the surfaces of the nano-rod grains (Fig. 8c). Similarly, the introduction of 0.5 mol% W doping in NCM940501 produced Fd
m) (Fig. 8a). Structural analyses confirmed that the percentage of the rock-salt phase increased with the amount of W. Theoretical calculations suggested that these W-doped rocksalt surfaces are energetically favored over the bare rocksalt surfaces and the layered surfaces. It is generally accepted that the rock-salt phase is typically observed in cycled or damaged Ni-rich cathodes, wherein the electrochemical performance is extremely poor.139–145 However, the cycling performance of the rock-salt-containing W-doped LNO was unexpectedly superior to that of the undoped LNO cathode (Fig. 8b). Although W doping slightly decreased the initial discharge capacity, the W-doped LNO cathode exhibited remarkable stabilization, reflected by impressive cycling stability. It was therefore considered that the rocksalt phase segregated at the cathode surface protected the cathode active material from detrimental reactions with the electrolyte species and enhanced the cycling stability of the cathode.67,138 In contrast, the introduction of W doping in NCM cathodes led to a disordered spinel phase formation. More specifically, Park et al.146 demonstrated that the addition of W into the NCM90 cathode produced a 20 nm-thick spinel phase on the surfaces of the nano-rod grains (Fig. 8c). Similarly, the introduction of 0.5 mol% W doping in NCM940501 produced Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m spinel structures.71 Such disordered spinel phases are considered to be an intermediate state between the layered and rock-salt structures.64 Owing to the high oxidation state of W6+, the Ni3+ species present in the TM layer are likely to be reduced to Ni2+ for maintenance of the charge neutrality, thereby promoting the migration of Ni2+ into the Li slabs to generate a cation-disordered spinel structure. The spinel structure formed at the surface of the nano-rod cathode protects the particle from electrolyte attack, thereby minimizing the formation of the NiO-like impurity phase observed after electrochemical cycling of the unmodified cathode.147 The fraction of the resulting cation-intermixed structure depends on the Li stoichiometry and the calcination temperature. In this context, Chen et al.148 determined the relative fractions of the rock-salt and layered structures by systematically varying the nominal Li ratio during calcination. Consequently, small primary particles consisting of intergrown rock-salt and layered phases were formed in the Li-deficient cathode active materials. Increasing the Li ratio led to a larger grain size but a decrease in the relative fraction of the rock-salt structure (Fig. 8d). It was found that the electrochemical performance of the 5% Li-deficient NM95 cathode featuring the layered-rock-salt composite structure was among the best in a series of Li-varying NM95 cathodes. Increasing the Li content and maximizing the calcination temperature increased the average crystallite size, signifying that mitigating crystal growth corresponds to a more disordered cation structure.149 Similar findings were observed by Yu et al.,150 who found that increasing the calcination temperature (i.e., promoting crystallite growth) led to a reduction in the number of stacking faults (lattice bending and distortion) and in the rock-salt structure fraction while increasing the relative fraction of the layered structure (Fig. 8e). Considering the structural variation owing to hetero-elemental doping, the Li stoichiometry, and the calcination temperature, it is possible that methods to alleviate grain coarsening suppress crystallite growth, thereby promoting the formation of cation intermixed intermediate structures (e.g., disordered spinel and rock-salt structures) prior to generating a perfect layered crystal structure through the formation of equiaxed primary particles.
m spinel structures.71 Such disordered spinel phases are considered to be an intermediate state between the layered and rock-salt structures.64 Owing to the high oxidation state of W6+, the Ni3+ species present in the TM layer are likely to be reduced to Ni2+ for maintenance of the charge neutrality, thereby promoting the migration of Ni2+ into the Li slabs to generate a cation-disordered spinel structure. The spinel structure formed at the surface of the nano-rod cathode protects the particle from electrolyte attack, thereby minimizing the formation of the NiO-like impurity phase observed after electrochemical cycling of the unmodified cathode.147 The fraction of the resulting cation-intermixed structure depends on the Li stoichiometry and the calcination temperature. In this context, Chen et al.148 determined the relative fractions of the rock-salt and layered structures by systematically varying the nominal Li ratio during calcination. Consequently, small primary particles consisting of intergrown rock-salt and layered phases were formed in the Li-deficient cathode active materials. Increasing the Li ratio led to a larger grain size but a decrease in the relative fraction of the rock-salt structure (Fig. 8d). It was found that the electrochemical performance of the 5% Li-deficient NM95 cathode featuring the layered-rock-salt composite structure was among the best in a series of Li-varying NM95 cathodes. Increasing the Li content and maximizing the calcination temperature increased the average crystallite size, signifying that mitigating crystal growth corresponds to a more disordered cation structure.149 Similar findings were observed by Yu et al.,150 who found that increasing the calcination temperature (i.e., promoting crystallite growth) led to a reduction in the number of stacking faults (lattice bending and distortion) and in the rock-salt structure fraction while increasing the relative fraction of the layered structure (Fig. 8e). Considering the structural variation owing to hetero-elemental doping, the Li stoichiometry, and the calcination temperature, it is possible that methods to alleviate grain coarsening suppress crystallite growth, thereby promoting the formation of cation intermixed intermediate structures (e.g., disordered spinel and rock-salt structures) prior to generating a perfect layered crystal structure through the formation of equiaxed primary particles.
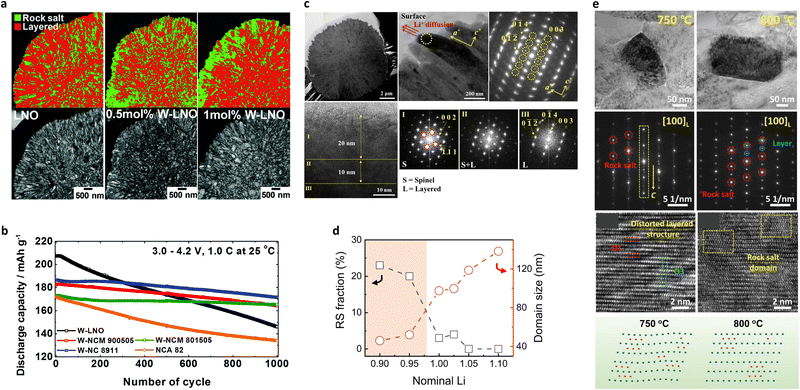 | ||
| Fig. 8 (a) ASTAR TEM phase mapping and corresponding bright field images of the LNO, 0.5 mol% W-LNO, and 1 mol% W-LNO species, showing distribution of the rocksalt phases in the respective cathodes. (b) Long term cycling of the 1 mol% W-doped Li[NixCoyMn1−x−y]O2 cathodes (x = 0.8, 0.89, 0.9, and 1.0; y = 0.15, 0.11, 0.05, and 0) and the Li[Ni0.82Co0.14Al0.04]O2 cathode (NCA 82, commercial Ni rich benchmark cathode), tested using pouch cells at 1.0C (180 mA g−1) and 25 °C within a voltage range of 3.0–4.2 V against graphite anodes. (c) Bright-field STEM, magnified TEM, SAED, HRTEM, and corresponding FT images of the W1.0-NCM90 cathode. (d) Calculated rock-salt phase fractions and domain sizes of the layered phase as a function of the nominal Li amount. (e) Low-magnification TEM, corresponding SAED, and HRTEM images, along with a schematic diagram of the microstructures of the NM85 cathodes synthesized at different temperatures. (a) and (b) are reproduced with permission,138 Copyright 2018, Royal Society of Chemistry. (c) is reproduced with permission,146 Copyright 2019, Elsevier. (d) is reproduced under Creative Commons CC-BY license,148 Copyright 2024, Springer Nature. (e) is reproduced with permission,150 Copyright 2022, John Wiley and Sons. | ||
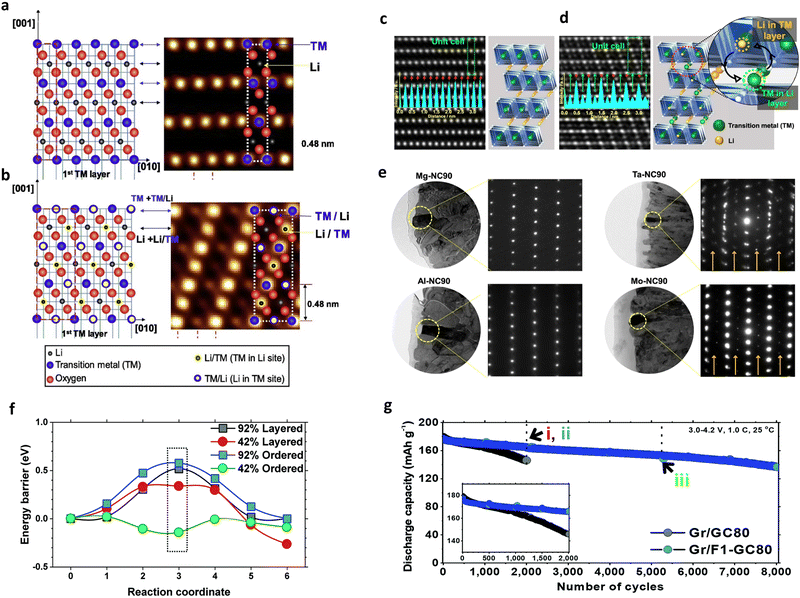 | ||
| Fig. 9 Schematic diagrams and HAADF TEM images of the (a) normal and (b) cation-ordered (Li/TM) structures. HAADF TEM images and derived structural models of the (c) Al-NC90 and (d) Ta-NC90 cathodes. (e) Bright-field images and corresponding SAED patterns of single primary particles of the Mg-NC90, Al-NC90, Ta-NC90, and Mo-NC90 cathodes. (f) Li-ion diffusion between adjacent octahedral sites (reaction coordinates 0 and 6) in the layered and ordered polymorphs of LixNiO2. The intermediate position (reaction coordinate 3) is the tetrahedral site representing the energetic barrier in all compositions except the delithiated ordered composition. (g) Long term cycling performances of the GC80 and F1-GC80 cathodes using pouch-type full cells at 25 °C. (a) and (b) are reproduced with permission,151 Copyright 2019, Elsevier. (c)–(e) are reproduced under Creative Commons CC-BY license,57 Copyright 2021, Springer Nature. (f) and (g) are reproduced under Creative Commons CC-BY license,154 Copyright 2021, Royal Society of Chemistry. | ||
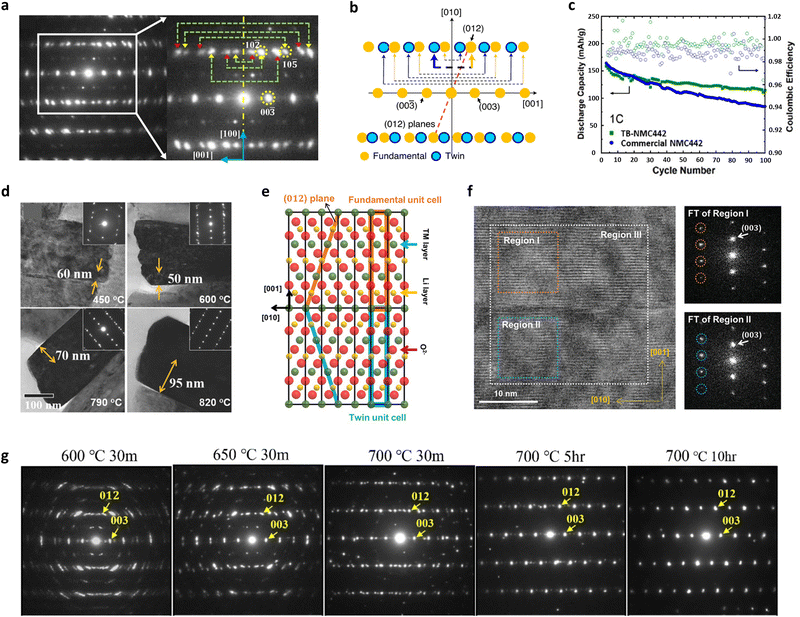 | ||
Fig. 10 (a) Electron diffraction pattern recorded for a twinned primary particle from the Zr-doped LiNiO2. The magnified pattern is also shown to indicate the paired diffraction spots caused by the mirror reflection of the [010] zone pattern around the [100] twin plane. (b) Schematic representation of the [100] zone electron diffraction pattern of the layered R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure corresponding to the fundamental and twin regions. (c) Electrochemical performances of TB-NMC442 and commercial NMC442 cathodes in the voltage range of 2.5–4.7 V. (d) Bright-field TEM images of the FCG cathode, Li[Ni0.75Co0.10Mn0.15]O2, lithiated at different temperatures. Thickness measurements for the respective twins are also shown. The corresponding SAED patterns showing the twin relationships are presented in the insets. (e) Schematic illustration showing an atomic arrangement of the twinned crystal structure. (f) HR-TEM image and corresponding Fourier transforms of the marked regions (regions I and II) of the 4% Mn-doped CG NCA92 cathode. (g) [100]-zone SAED pattern of the 1.5% B-doped NCA95 cathode lithiated at different temperatures and durations. (a) is reproduced with permission,77 Copyright 2018, American Chemical Society. (b) is reproduced with permission,53 Copyright 2020, Springer Nature. (c) is reproduced under Creative Commons CC-BY license,159 Copyright 2022, American Chemical Society. (d) is reproduced with permission,160 Copyright 2018, John Wiley and Sons. (e) and (f) are reproduced with permission,72 Copyright 2024, John Wiley and Sons. (g) is reproduced with permission,116 Copyright 2022, American Chemical Society. m structure corresponding to the fundamental and twin regions. (c) Electrochemical performances of TB-NMC442 and commercial NMC442 cathodes in the voltage range of 2.5–4.7 V. (d) Bright-field TEM images of the FCG cathode, Li[Ni0.75Co0.10Mn0.15]O2, lithiated at different temperatures. Thickness measurements for the respective twins are also shown. The corresponding SAED patterns showing the twin relationships are presented in the insets. (e) Schematic illustration showing an atomic arrangement of the twinned crystal structure. (f) HR-TEM image and corresponding Fourier transforms of the marked regions (regions I and II) of the 4% Mn-doped CG NCA92 cathode. (g) [100]-zone SAED pattern of the 1.5% B-doped NCA95 cathode lithiated at different temperatures and durations. (a) is reproduced with permission,77 Copyright 2018, American Chemical Society. (b) is reproduced with permission,53 Copyright 2020, Springer Nature. (c) is reproduced under Creative Commons CC-BY license,159 Copyright 2022, American Chemical Society. (d) is reproduced with permission,160 Copyright 2018, John Wiley and Sons. (e) and (f) are reproduced with permission,72 Copyright 2024, John Wiley and Sons. (g) is reproduced with permission,116 Copyright 2022, American Chemical Society. | ||
2.4. Chemical and structural stability
The degradation of NCM and NCA cathodes is largely caused by the presence of unstable Ni4+ in their highly delithiated states. During cell operation, Ni4+ ions can readily react with the electrolyte solution to produce Ni2+, thereby forming the NiO-like rock-salt structure, which increases the resistance and deteriorates the electrochemical performance of the cathode.162–165 Moreover, such side reactions can cause gas evolution, which leads to pressure buildup, potentially causing leaks or explosions in extreme cases, ultimately jeopardizing the safety of the battery.37,166–169 Such degradation becomes more severe as the Ni fraction of the cathode increases.31,36,37 As the degradation primarily occurs at the surface of the cathode that is in contact with the electrolyte, the rate of degradation is largely determined by the area exposed to the electrolyte. In the following subsections, the chemical stability and the extent of chemical degradation of the nano-rod cathodes will be addressed, both on the outer surfaces of the particles and on the interior surfaces of the cathode particles.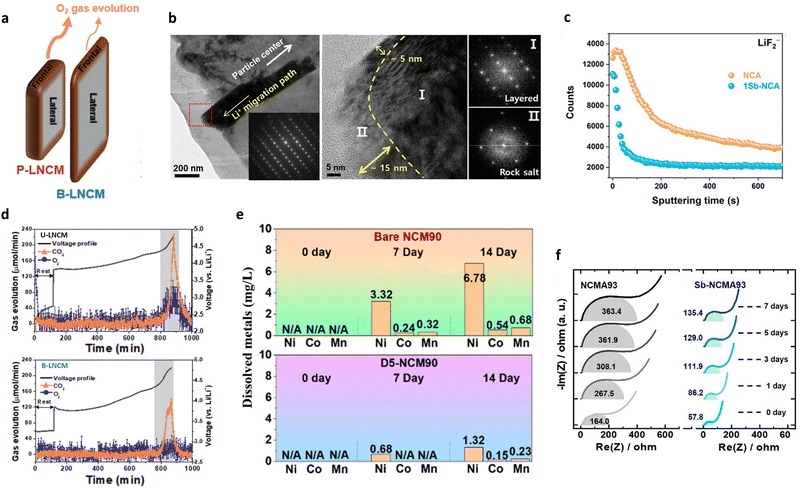 | ||
| Fig. 11 (a) Schematic illustration of the structural stability differences between the P-LNCM and B-LNCM cathodes. (b) Magnified TEM image, SAED pattern (inset), HR-TEM image, and corresponding Fourier transforms for the CSG-NCMA90 primary particle after 500 cycles at 100% depth of discharge (DoD). (c) ToF-SIMS depth profiles of LiF2− obtained from the NCA and 1Sb-NCA cathodes after 200 cycles. (d) Differential electrochemical mass spectrometry (DEMS) spectra of U-LNCM and B-LNCM when charged to 4.8 V at 0.1C (20 mA g−1). (e) ICP analysis results of the dissolved Ni, Co, and Mn ions for bare NCM90 and D5-NCM90 cathodes after storing the cathodes in the electrolyte solution for 14 d at 55 °C. (f) Changes in the charge-transfer resistances of the NCMA93 and Sb-NCMA93 cathodes during a storage test. (a) is reproduced with permission,69 Copyright 2021, John Wiley and Sons. (b) is reproduced with permission,66 Copyright 2021, John Wiley and Sons. (c) is reproduced with permission,170 Copyright 2023, American Chemical Society. (d) is reproduced with permission,69 Copyright 2021, John Wiley and Sons. (e) is reproduced with permission,55 Copyright 2023, American Chemical Society. (f) is reproduced with permission,172 Copyright 2023, American Chemical Society. | ||
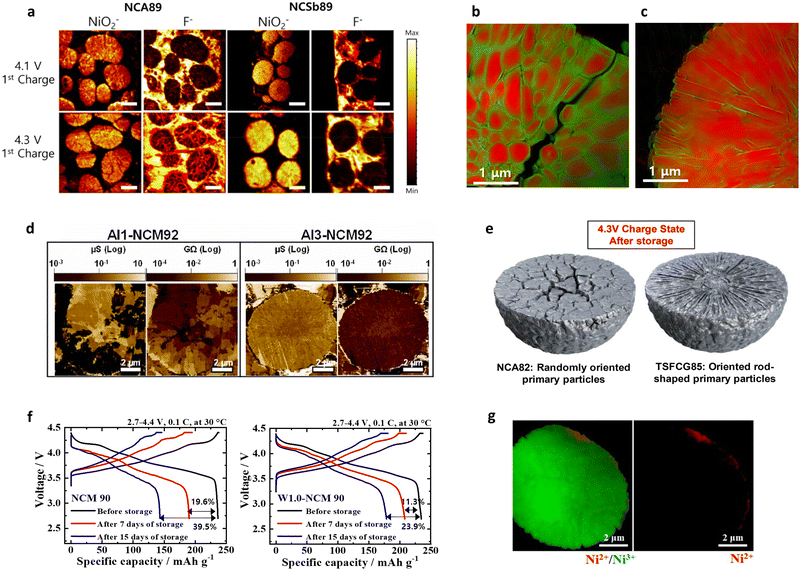 | ||
| Fig. 12 (a) Cross-sectional TOF-SIMS high-resolution maps showing the NiO2− and F− secondary ion spatial distributions acquired on the NCA89 and NCSb89 cathodes charged to 4.1 and 4.3 V after the first 0.1C (18 mA g−1) cycle. Scale bar = 5 μm. (b) Cross-sectional chemical maps of the P-NCA89 and B-NCA88 cathodes after 100 cycles. The fitting results (chemical information) and averaged OD (morphological information) are indicated based on the color and transparency, respectively. Red and green represent Ni3+ and Ni2+, respectively. (d) SSRM images of the Al1-NCM92 and Al3-NCM92 cathodes after 500 cycles mapping their conductance and resistance. (e) Schematic description of the high-temperature (55 °C) aged NCA82 and TSFCG85 cathodes showing the internal morphological differences and the sustained damage. (f) Charge–discharge curves at 0.1C (18 mA g−1) after n days of thermal aging, compared to those before storage (n = 7, 15), for the NCM90 and W1.0-NCM90 cathodes. (g) Cross-sectional chemical phase maps of the FCG NCM78_790 °C cathode particles after 1000 cycles. (a) is reproduced with permission,75 Copyright 2020, American Chemical Society. (b) and (c) are reproduced with permission,52 Copyright 2020, Elsevier. (d) is reproduced with permission,106 Copyright 2023, Elsevier. (e) is reproduced with permission,50 Copyright 2017, American Chemical Society. (f) is reproduced with permission,146 Copyright 2019, Elsevier. (g) is reproduced with permission,98 Copyright 2022, Elsevier. | ||
It has also been demonstrated that the formation of nano-rod grains mitigates the cycle-induced internal surface degradation of the cathodes and has a similar effect during thermal aging. More specifically, the storage of highly delithiated cathodes in an electrolyte solution at elevated temperatures (e.g., 45 and 60 °C) was found to undermine the structural stability of the equiaxed cathode, eventually leading to mechanical failure and capacity loss during aging (Fig. 12e and f).50,146 In contrast, the tighter packing of the cathode secondary particle surfaces imparted by the nano-rod grains effectively protected the particle interior from chemical attack during prolonged exposure to the electrolyte, thereby preserving the reversibility of Li+.52 By combining crystallographic textures and compositional partitioning of the particles of the cathode prepared using a concentration gradient, both chemical and structural degradation were noticeably circumvented, confining Ni2+ at the very edge of the secondary particle surface (Fig. 12g).98 When the electrolyte penetrates into the secondary particle of this cathode, the deterioration at the particle center is likely accelerated because the cathode center consists of capacity-maximizing but chemically unstable Ni-enriched compositions. Thus, suppressing the proliferation of intergranular cracks that extend from the secondary particle center to the surface is key to delaying the degradation of inner primary particles.
2.5. Thermal stability
Recently, the growing importance of battery safety has highlighted thermal propagation as a major issue. Excessive heat generation within a battery cell can lead to a chain reaction, wherein the neighboring cells also become overheated and contribute to thermal propagation, resulting in fires or even explosions.174–178 One of the primary causes of thermal instability in a battery is the cathode, which can continually supply O2 during thermal reactions, thereby intensifying the risk of thermal runaway.179–181 As has been well established, the thermal safety of conventional NCM or NCA cathodes depend on their relative fractions of Ni. More specifically, the higher Ni concentration required to achieve a greater energy density can adversely affect the thermal safety of a cathode active material (Fig. 13a).31,39 Indeed, during cell operation, delithiated NCM or NCA cathodes contain unstable Ni4+ species, which can readily react with the electrolyte solution to generate heat and gaseous species.182 To address this issue, Sun et al.46 suggested that the use of an NCM cathode possessing nano-rod grains with a concentration gradient should effectively promote the thermal safety of a cathode (Fig. 13b). DSC analysis of an FCG cathode material containing an inner composition (IC) of Li[Ni0.86Co0.10Mn0.04]O2 and an outer composition (OC) of Li[Ni0.70Co0.10Mn0.20]O2 revealed that the thermal properties resembled that of an OC cathode. More specifically, the exothermic reaction temperatures of the IC, FCG, and OC cathodes were 221.3, 257.3, and 259.7 °C, respectively. Relatively lower content of Ni at the outer surface of the nano-rod FCG material was found to be key to mitigating the thermal reaction at the cathode.183,184 In addition, a relatively higher fraction of Mn has been demonstrated to help mitigate oxygen release from the layered structure, reducing the risk of thermal reactions.185–187 Notably, the thermal safety of a nano-rod material with a concentration gradient is not confined to the particle level but is related to the cell level. In this context, Noh et al.48 reported overcharging and nail penetration test results for laminated-type Al-pouch cells (280 mA h) featuring a nano-rod concentration gradient cathode and found that no thermal runaway, black smoke or explosions occurred during the experiments (Fig. 13c).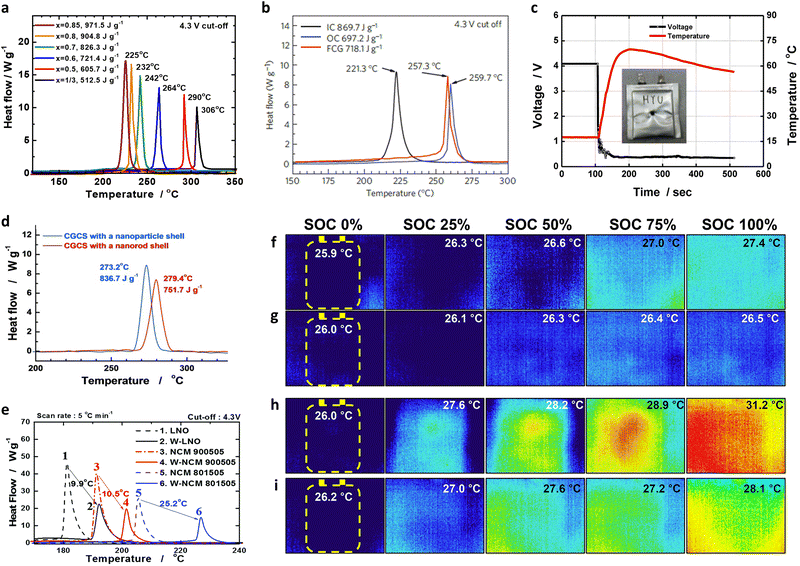 | ||
Fig. 13 (a) DSC profiles of the delithiated Li1−δ[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8, and 0.85) cathodes. (b) DSC profiles of the delithiated FCG material, the delithiated IC, and the delithiated OC (Li1−xNi0.70Co0.10Mn0.20O2) recorded at a scan rate of 1 °C min−1. The cells were charged in the constant-voltage mote to 4.3 V versus Li+/Li before disassembly. (c) Plots of the voltage and cell temperature against time for a laminated-type Al-pouch MCMB/FCG–Mn-F cell (280 mA h) during a penetration test at 100% SoC (4.2 V). (d) DSC profiles of the delithiated CGCS Li0.18[Ni0.60Co0.15Mn0.25]O2 particles with a nanoparticle shell and the delithiated CGCS Li0.16[Ni0.60Co0.15Mn0.25]O2 with a nano-rod shell. (e) DSC results of the pristine, W-doped Li[NixCoyMn1−x−y]O2 (x = 0.8, 0.9, and 1.0; y = 0.15, 0.05, and 0) combined with a 1.2 M LiPF6 (EC/EMC, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) + 2 wt% VC electrolyte solution. Distribution of the surface temperature in pouch-type full-cells measured using an IR camera during charge to 4.2 V vs. graphite. Thermal images of the (f) NCA93- and (g) Nb-NCA93-based cells charged at 0.1C (18 mA g−1), and of the (h) NCA93- and (i) Nb-NCA93-based cells charged at 3C (540 mA g−1). (a) is reproduced with permission,31 Copyright 2013, Elsevier. (b) is reproduced with permission,46 Copyright 2012, Springer Nature. (c) is reproduced with permission,48 Copyright 2013, American Chemical Society. (d) is reproduced with permission,188 Copyright 2014, John Wiley and Sons. (e) is reproduced with permission,138 Copyright 2018, Royal Society of Chemistry. (f)–(i) are reproduced under Creative Commons CC-BY license.189 Copyright 2022, American Chemical Society. 7 v/v) + 2 wt% VC electrolyte solution. Distribution of the surface temperature in pouch-type full-cells measured using an IR camera during charge to 4.2 V vs. graphite. Thermal images of the (f) NCA93- and (g) Nb-NCA93-based cells charged at 0.1C (18 mA g−1), and of the (h) NCA93- and (i) Nb-NCA93-based cells charged at 3C (540 mA g−1). (a) is reproduced with permission,31 Copyright 2013, Elsevier. (b) is reproduced with permission,46 Copyright 2012, Springer Nature. (c) is reproduced with permission,48 Copyright 2013, American Chemical Society. (d) is reproduced with permission,188 Copyright 2014, John Wiley and Sons. (e) is reproduced with permission,138 Copyright 2018, Royal Society of Chemistry. (f)–(i) are reproduced under Creative Commons CC-BY license.189 Copyright 2022, American Chemical Society. | ||
The primary particle morphology close to the secondary particle surface also plays a critical role in defining the thermal stability of a cathode. For example, concentration gradient core–shell (CGCS) cathodes bearing nano-particle and nano-rod shells exhibited exothermic peak temperatures of 273.2 and 279.4 °C while releasing 836.7 and 751.7 J g−1 of heat, respectively. These results demonstrate the superior thermal runaway resistance of the nano-rod-grained cathode (Fig. 13d).188 Recently, nano-rod grains without a concentration gradient were proven to improve the thermal safety profiles of cathodes effectively.73,109,138,190 More specifically, the W doping of Ni-rich cathodes substantially reduced the cathodic thermal activity, thereby shifting the onset of the exothermic reactions by >10 °C and reducing the amount of overall heat released during the thermal reactions (Fig. 13e).138 These outstanding thermal properties observed for nano-rod cathodes can be attributed to the chemo-mechanical protection offered by the tightly packed radially oriented grains, which mitigate microcrack propagation and protect internal particle surfaces from direct contact with the electrolyte.68,97 In this context, using an infrared camera, Kim et al.189 directly demonstrated the thermal response of a pouch-type full cell (800 mA h) during charging. During charging of the full cells at 0.1C, the NCA93- and Nb-NCA93-based full cells showed temperature increments of 1.5 and 0.5 °C, respectively (Fig. 13f and g). However, upon charging the full cells at 3C, the equiaxed-grained NCA93 revealed high levels of heat localization, whereas the nano-rod-grained Nb-NCA93 exhibited a relatively lower surface temperature and no localized heat generation, even at a high current density (Fig. 13h and i). These results indicate the highly textured nano-rod grains can effectively protect the internal surface of the cathode active material, thereby delaying the onset of exothermic reactions, and improving the cathode and battery safety.
2.6. Mechanical strength
The fundamental mechanical strength of a single cathode active material largely affects the electrochemical cycling stability of a cathode. However, the high-pressure rolling process employed during electrode manufacture hampers the mechanical integrity of the cathode.191–194 To determine the mechanical strength of a cathode active material, the micro-indentation approach is commonly employed.195–198 This involves loading a flat indenter on a single cathode particle, applying an external force, and recording the mechanical response until particle fracture occurs (Fig. 14a).55 Compression tests can be accelerated tests to determine whether a cathode can dissipate the internal strain under electrochemical effects. In particular, the test provides valuable data when adopted in Ni-rich cathodes, which generally suffer from microcrack formation and subsequent mechanical failure during electrochemical charging and discharging.36,79,199 In this context, Ju et al.55 compared the mechanical strengths of the D5-NCM90 and NCM90 cathodes, which possess identical chemical compositions but different grain morphologies (i.e., randomly oriented equiaxed grains and radially aligned nano-rod grains, respectively). They found that the D5-NCM90 cathode exhibited a higher mechanical strength than the NCM90 cathode, with the maximum applied forces reaching 30.5 and 10.2 mN, respectively. These results demonstrate that nano-rod grains effectively enhance the fracture toughness of a cathode. Similarly, it was found that an ultrafine-grained Mo-doped NCMA94 cathode showed a higher mechanical strength than the corresponding undoped cathode. Moreover, increasing the Mo fraction refined the nano-rod grains to further increase the mechanical robustness of the cathode particles (Fig. 14b).80 This increased mechanical strength was attributed to the presence of abundant triple junctions at the interparticle boundaries, which can serve as barriers to relaxing the mechanical strain effectively.200,201 Recent studies on nano-rod-grained cathodes have revealed that the cathodes exhibit a peculiar ductile mechanical behavior. More specifically, the force-displacement curves recorded for conventional cathode materials displayed relatively straight load curves before particle breakage, indicating their brittle characteristics typical in ceramic materials (Fig. 14c).79 In contrast, ultrafine-scaled nano-rod grains showed nonlinear and serrated flow load curves, indicating their ductile properties. Such a ductile response against external forces can improve the fracture toughness of a cathode particle by effectively dissipating the strain accumulation into the cathode material.202,203 Scanning electron microscopy (SEM) images of the fractured particles demonstrated the different mechanical responses between the equiaxial-grained and nano-rod-grained cathodes; the former catastrophically shattered into numerous grain fragments, whereas the latter fractured into larger aggregates (Fig. 14d). The use of cyclic loading/unloading tests to simulate lattice contraction and expansion in a cathode during electrochemical cycling substantiated the mechanical durability of the nano-rod-grained particles. More specifically, during the cyclic test, the equiaxial-grained FCG NCM78 was steadily but increasingly compressed to 165% compared to its initial displacement (L1) (Fig. 14e).204 In comparison, the compression ratio of the rod-shaped W0.5-FCG NCM78 was limited to 125% after the same number of cycles. Furthermore, the equiaxial-grained particles collapsed before reaching 150 cycles, whereas most nano-rod-grained particles remained intact after 250 cycles (Fig. 14f).205 Moreover, the indentation results suggested that the radial orientation of the nano-rod grains helped dissipate the inherent strain buildup of the layered oxide cathode, mitigating microcrack formation and enhancing the electrochemical performance. These characteristics of nano-rod cathodes are also advantageous during manufacture and application. Indeed, the electrode manufacturing process involves the implementation of high compression forces perpendicular to the particle surface to increase the packing and energy density of the electrode. By securing a high fracture toughness, the nano-rod-grained cathodes can preserve their original particle morphologies in high-density electrodes, avoiding the particle breakage observed for conventional equiaxial-grained layered oxide cathodes.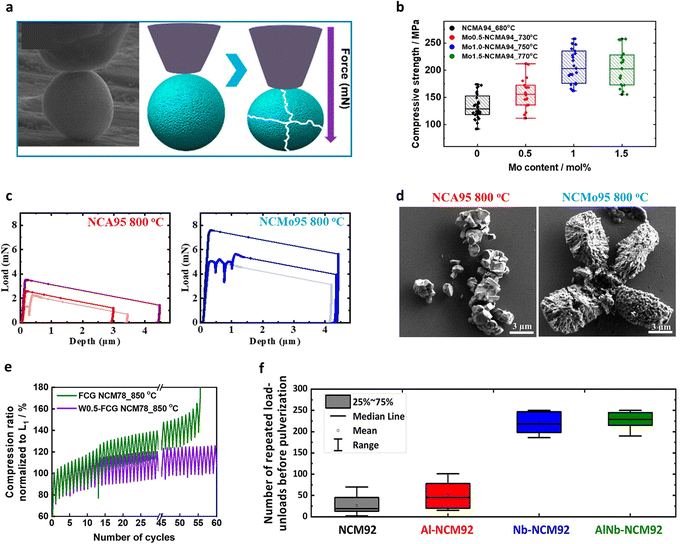 | ||
| Fig. 14 (a) Schematic representation of nanoindentation analysis carried out before and after mechanical failure of the cathode particle. (b) Distribution of the particle strength for the optimized NCMA94 and Mo-doped NCMA94 cathodes. (c) Representative load-displacement curves for the NCA95 and NCMo95 cathode particles lithiated at 800 °C for 10 h. (d) Post-compression SEM images of the NCA95 and NCMo95 cathode particles lithiated at 800 °C for 10 h. (e) Comparison of the mechanical durability during cyclic loading/unloading tests of the FCG NCM78 and W0.5-FCG NCM78 cathodes calcined at 850 °C. (f) Number of repeated loading/unloading cycles before pulverization was observed for the NCM92, Al-NCM92, Nb-NCM92, and AlNb-NCM92 cathodes, as obtained via micro-compression analysis. (a) is reproduced with permission,55 Copyright 2023, American Chemical Society. (b) is reproduced with permission,80 Copyright 2023, Elsevier. (c) and (d) are reproduced under Creative Commons CC-BY license,79 Copyright 2021, Royal Society of Chemistry. (e) is reproduced with permission,204 Copyright 2023, American Chemical Society. (f) is reproduced with permission,205 Copyright 2024, American Chemical Society. | ||
2.7. Ion diffusion & fast charging
The sizes, morphologies, and grain orientations of primary particles largely affect the Li+ ion diffusivity of the material. One of the benefits of constructing radially oriented nano-rod grains is the enhancement in the Li+ ion diffusivity. In the following subsections, the Li+ diffusivity and uniformity in a single cathode material are discussed in the context of the cathode microstructure, as is the resulting structural stability of the final cathode material.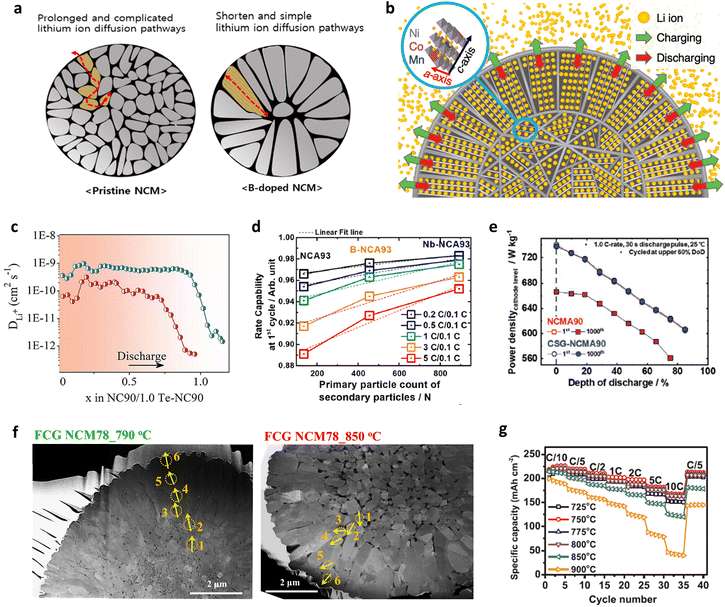 | ||
| Fig. 15 (a) Schematic illustration of the Li+ diffusion pathways in pristine and B-doped NCM cathode particles. (b) Rendered image of a core–shell particle based on t-EBSD data. Radial alignment of primary particle Li layers allows faster diffusion from the electrolyte towards the randomly oriented core during discharging (red arrows), and in the inverse direction during charging (green arrows). (c) Calculated DLi+ values of the NC90 and 1.0 Te-NC90 cathodes during charging. (d) Plots of the 0.2/0.1, 0.5/0.1, 1/0.1, 3/0.1, and 5/0.1C (0.1C corresponds to 18 mA g−1) capacity ratios for the half-cells based on NCA93, B-NCA93, and Nb-NCA93 cathodes as a function of the primary particle count in the secondary particles. (e) Power densities of the NCMA90 and CSG-NCMA90 cathodes as a function of the DoD before and after 1000 cycles at upper 60% DoD (blue dashed lines indicate the fully charged state). (f) Cross-sectional dark-field TEM images showing the morphologies and crystal orientations of FCG NCM78_790 °C and FCG NCM78_850 °C cathodes. The yellow arrows indicate alignment of the Li+ paths of the numbered primary particles from the secondary particle core to its surface, determined by SAED analysis. (g) Rate performances of LiNi0.76Mn0.14Co0.10O2 cathodes calcined at different temperatures. (a) is reproduced under Creative Commons CC-BY license,206 Copyright 2019, Springer Nature. (b) is reproduced under Creative Commons CC-BY license,101 Copyright 2020, Royal Society of Chemistry. (c) is reproduced with permission,73 Copyright 2024, Elsevier. (d) is reproduced under Creative Commons CC-BY license,189 Copyright 2022, American Chemical Society. (e) is reproduced with permission,66 Copyright 2021, John Wiley and Sons. (f) is reproduced with permission,98 Copyright 2022, Elsevier. (g) is reproduced with permission,211 Copyright 2018, Elsevier. | ||
Another possible reason for the improved rate performance in nano-rod-grained cathodes is the large fraction of interparticle boundaries. It is often reported that grain boundary diffusion is 3–10 times faster than bulk diffusion.212,213 With this in mind, Fig. 15d compares the rate capabilities of the NCA93, B-NCA93, and Nb-NCA93 cathodes as a function of the primary particle count in a respective secondary particle. Notably, an increment in the number of grains in a single secondary particle indicates augmentation in the relative fraction of interparticle boundaries. The rate performance of the nano-rod Nb-NCA93 cathode containing the highest number of grains was found to be superior to that of the B-NCA93 and NCA93 cathodes, featuring elongated rod-like grains and equiaxed grains, respectively. These results confirm the positive impact of numerous grain boundaries in promoting Li+ migration. The construction of nano-rod grains with a concentration gradient offers outstanding power density retention compared to the cathode without any microstructural modification or concentration gradient (Fig. 15e).66 However, the Li+ kinetics of a cathode containing a concentration gradient largely depend on its calcination temperature. The corresponding selected area electron diffraction (SAED) patterns confirmed that FCG NCM78 calcined at 790 °C possessed a continuous Li+ pathway across the adjacent primary particles (Fig. 15f).160 However, the cathode calcined at 850 °C, wherein a unique texture was barely discernible owing to grain coarsening, possessed a discontinuous migration pathway for Li+ (de)intercalation. The calcination temperature-dependent rate performance of the FCG NCM761410 cathode system is presented in Fig. 15g,211 wherein it can be seen that the cathode calcined at 750 °C exhibited a superior rate capability to the cathodes calcined at other calcination temperatures. These results indicate that the selection of an optimal calcination temperature and microstructure is essential to achieving an outstanding Li+ diffusivity and rate performance.
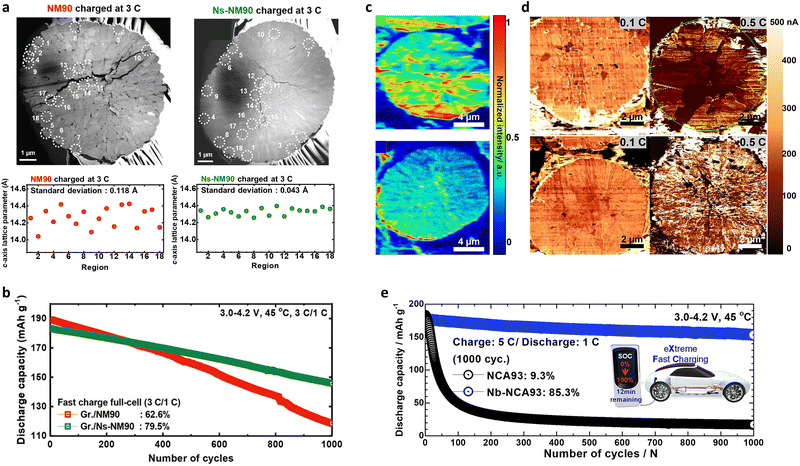 | ||
| Fig. 16 (a) Cross-sectional dark-field TEM images of the NM90 and Ns-NM90 secondary cathode particles charged to 4.4 V at 3C (540 mA g−1). Variation in the c-axis lattice parameters (corresponding to the encircled regions in part (a)) for the NM90 and Ns-NM90 cathodes charged to 4.4 V at 3C (540 mA g−1). (b) Long-term cycling performances of the NM90 and Ns-NM90 cathodes in full cells containing graphite anodes and cycled at a charge C-rate of 3C (540 mA g−1) at 45 °C. (c) TOF-SIMS maps of the normalized elemental distributions of 7Li in the NCA93 (upper) and Nb-NCA93 (lower) cathodes charged to 4.3 V at 3C (540 mA g−1). (d) SSRM images of the NCA93 (upper) and Nb-NCA93 (lower) cathodes charged to 4.3 V at various C-rates. (e) Comparison of the long-term cycling performances of full cells based on NCA93 and Nb-NCA93 cathodes and an EF31 + DFOB electrolyte at 5C (900 mA g−1)/1C (180 mA g−1). (a) and (b) are reproduced with permission,132 Copyright 2022, John Wiley and Sons. (c)–(e) are reproduced under Creative Commons CC-BY license,189 Copyright 2022, The Authors, American Chemical Society. | ||
3. Concentration gradient cathodes
3.1. Origin of the nano-rod structure in concentration gradient cathodes
Nano-rod structures were first observed in layered oxide concentration gradient cathode materials.46 These materials have two defining characteristics, namely the varying TM concentrations (Ni, Co, Mn, etc.) throughout the secondary particles and the nano-rod-shaped grain morphology found near the particle surface (Fig. 17a and b).46,47,224–228 Concentration gradient-type cathode materials originally stemmed from the idea of placing the low-capacity but chemically stable Mn at the particle surface and the capacity contributing but reactive Ni in the core to protect the core and develop a cathode material capable of delivering a high capacity and stability.31,47 As intended, the concentration gradient design has significantly contributed to enhancing the performances of cathode materials. However, a common finding has been the presence of the nano-rod morphology as another unique characteristic of concentration gradient materials. This spatial configuration also has played a significant role in enhancing the performances of cathode materials, as has the compositional design, with its importance becoming more pronounced as the Ni content in cathode materials increases.50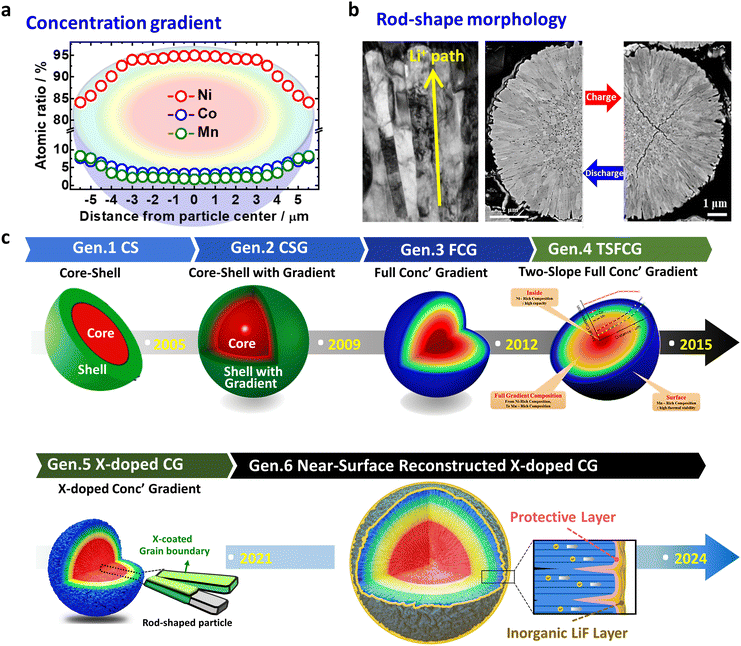 | ||
| Fig. 17 Concentration gradient cathode materials defined by: (a) Varying TM concentrations throughout the secondary particles, and (b) the rod-shaped morphology of the primary particles. (c) Schematic representation outlining the six generations of concentration gradient-type materials: (i) CS, (ii) CS with gradient, (iii) FCG, (iv) two-slope FCG, (v) X-doped concentration gradient, and (vi) surface-modified concentration gradient. (b) is reproduced with permission,48,98 Copyright 2013, American Chemical Society and Copyright 2022, Elsevier. | ||
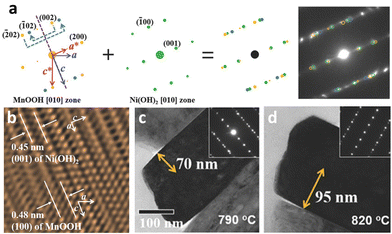
In concentration gradient-type materials, the characteristic features are formed at the precursor synthesis stage during co-precipitation, and these features are known to impact the final microstructures of the lithiated gradient cathode materials.68 At a glance, the precursor primary particles appear as fiber-like bundles with fine boundaries between the subgrains and are oriented in the radial direction of the secondary particle.160 Structurally, the Ni(OH)2 unit cell exhibits a strong crystallographic texture, where the (001) TM planes are aligned parallel to the direction of the elongated primary particles (Fig. 18a). Upon closer examination, the subgrains contain stacking faults that are indicated by discontinuous lattice fringes in the high-resolution transmission electron microscopy (HRTEM) image and horizontal streaks in the diffraction pattern. This diffraction pattern consists of three sets of overlapping patterns of two mirror images of the [010] zone diffraction pattern from the twinned MnOOH crystals (space group: P21/c) and a single [010] zone pattern from Ni(OH)2 (space group: P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1). The additional MnOOH phase co-exists with the Ni(OH)2 phase within the primary particles and stems from Mn-enrichment at the surface and the oxidation of Mn2+ to Mn3+ under a relatively O-rich atmosphere (Fig. 18b).229,230 This is corroborated by the gradual disappearance of the twinned crystal SAED patterns while approaching the particle center where the Mn fraction progressively decreases in the FCG Li[Ni0.75Co0.10Mn0.15](OH)2. However, the twin phases do not disrupt the layered structure nor the crystallographic texture of the gradient precursors, as the (100) planes of the MnOOH phase are parallel to the (001) planes of the Ni(OH)2 phase, with the difference in the interplanar distance of the two phases being approximately 0.03 nm. They contribute to preserving the nano-rod microstructures of gradient cathodes during the lithiation process. More specifically, during high-temperature lithiation, the precursor microstructure and crystallinity become distorted from adjacent precursor particles to coalesce into randomly oriented, polygonal particles. At the same time, Ni migrates to the vacant Li sites (cation disordering).79,211 The presence of the twin MnOOH and Ni(OH)2 phases at the precursor stage mitigates this degradation by maintaining the layered planes parallel along the radial direction, while also retarding sintering of the adjacent primary particles.160 Furthermore, the twins persist in the lithiated cathode (Fig. 18c); however, their density decreases with increasing lithiation temperature (Fig. 18d). This effect is further strengthened by concentration gradients within individual partitioned crystal structures present along the Li-containing (003) lattice in the direction of the columnar primary particles. It is also strengthened by preferential migration of the X-dopants to the grain boundary regions to act as barriers to sintering, as discussed above.153,204,231
m1). The additional MnOOH phase co-exists with the Ni(OH)2 phase within the primary particles and stems from Mn-enrichment at the surface and the oxidation of Mn2+ to Mn3+ under a relatively O-rich atmosphere (Fig. 18b).229,230 This is corroborated by the gradual disappearance of the twinned crystal SAED patterns while approaching the particle center where the Mn fraction progressively decreases in the FCG Li[Ni0.75Co0.10Mn0.15](OH)2. However, the twin phases do not disrupt the layered structure nor the crystallographic texture of the gradient precursors, as the (100) planes of the MnOOH phase are parallel to the (001) planes of the Ni(OH)2 phase, with the difference in the interplanar distance of the two phases being approximately 0.03 nm. They contribute to preserving the nano-rod microstructures of gradient cathodes during the lithiation process. More specifically, during high-temperature lithiation, the precursor microstructure and crystallinity become distorted from adjacent precursor particles to coalesce into randomly oriented, polygonal particles. At the same time, Ni migrates to the vacant Li sites (cation disordering).79,211 The presence of the twin MnOOH and Ni(OH)2 phases at the precursor stage mitigates this degradation by maintaining the layered planes parallel along the radial direction, while also retarding sintering of the adjacent primary particles.160 Furthermore, the twins persist in the lithiated cathode (Fig. 18c); however, their density decreases with increasing lithiation temperature (Fig. 18d). This effect is further strengthened by concentration gradients within individual partitioned crystal structures present along the Li-containing (003) lattice in the direction of the columnar primary particles. It is also strengthened by preferential migration of the X-dopants to the grain boundary regions to act as barriers to sintering, as discussed above.153,204,231
 | ||
| Fig. 18 (a) Combination of the calculated electron diffraction patterns of the MnOOH and Ni(OH)2 phases indexed to reproduce the SAED pattern observed for FCG Li[Ni0.75Co0.10Mn0.15](OH)2. (b) Fourier-filtered image exemplifying the coexistence of the Ni(OH)2 and MnOOH phases with an interplanar distance difference of approximately 0.03 nm. (c) and (d) Bright-field TEM images for the FCG Li[Ni0.75Co0.10Mn0.15]O2 lithiated at 790 and 820 °C, and the corresponding SAED patterns in the insets, showing the twin phase structures. (a)–(d) are reproduced with permission,160 Copyright 2018, John Wiley and Sons. | ||
3.2. Nano-rods in concentration gradient cathodes
So far, six generations of gradient-type materials exist, namely the CS, CS with gradient, FCG, TSFCG, X-doped concentration gradient, and surface-modified concentration gradient materials, as shown in Fig. 17c. Notably, the nano-rod structure has been observed consistently in the particle morphologies of all six generations of material.The CS cathode material was the first concentration gradient material introduced in 2005. As a precursor to subsequent generations, the CS Li[(Ni0.8Co0.1Mn0.1)0.8(Ni0.5Mn0.5)0.2]O2 material consisted of a Mn-rich Li[Ni0.5Mn0.5]O2 shell and a Ni-rich Li[Ni0.8Co0.1Mn0.1]O2 core.232,233 This system exhibited improved cell performance and thermal stability due to the Mn-rich shell shielding the Ni-rich core to minimize the Ni3+/4+-electrolyte parasitic side reactions akin to a coating layer. However, the sudden change in chemical composition and the crystal lattice mismatch at the CS interface resulted in physicochemical separation between the two sections. This was attributed to the volume changes occurring during cycling, eventually leading to rapid electrochemical degradation. To address the shortcomings of the CS material, a concentration gradient connecting the core and the shell was added at their interface to give a CS-with-gradient (CSG) cathode material.47,184,234 By resolving the shortcomings of the CS cathode, the CSG cathode demonstrated a greatly improved cycle life, with approximately 90% retention being achieved over 1000 cycles in some cases.49,66 When the concentration gradient and CSG materials were first reported, their morphologies were not discussed in depth, and much of the enhanced performance was attributed to the concentration gradient. It is important to note that the characteristic rod-shaped grain structures and their radial alignment can still be clearly observed in the shell region, as shown in Fig. 19a and b. The efficacy of the nano-rod structure was confirmed by comparing two different CS cathode materials having the same concentration gradient profile but different primary particle morphologies in the shell region, one being the nano-rod structure and the other simply being nano-scale particles.188 These two different morphologies originated from the disorder of primary particles in a hydroxide precursor, a result of alterations in the synthetic conditions during the co-precipitation reactions. Compared to the nano-scale particles in the shell, the nano-rods more effectively protected the core region due to their dense packing and reduced surface area, even demonstrating improved thermal stability. Subsequent studies into these nano-rod materials have also attributed the performance enhancement to the compactly packed nano-rod structure in the shell, alleviating the stress associated with the taxing H2 → H3 phase transition. Two-dimensional finite element analysis calculations demonstrated that the nano-rod grains in the shell region homogeneously lower the tensile stress in the core region to prevent microcrack nucleation and propagation to the surface, as illustrated in Fig. 19c.49,66,151 As a result, the capacity fading and impedance increase caused by intergranular boundary surface degradation from direct contact with the infiltrated electrolyte was prevented to enable the CSG cathode to continuously deliver high specific discharge capacities during long-term cycling (see Fig. 19c), along with the added effect of reducing TM dissolution. More recently, Park et al. reported a Co-free CSG Li[Ni0.90Mn0.10]O2 delivering a 79.5% retention rate after 1000 cycles under a fast 3C charge rate. These characteristics were attributed to the nano-rod primary particles preserving the strong crystallographic texture by inducing a homogenous Li distribution, i.e., homogeneity in the SoC, leading to uniform expansion and contraction of the unit cell (Fig. 16a).132
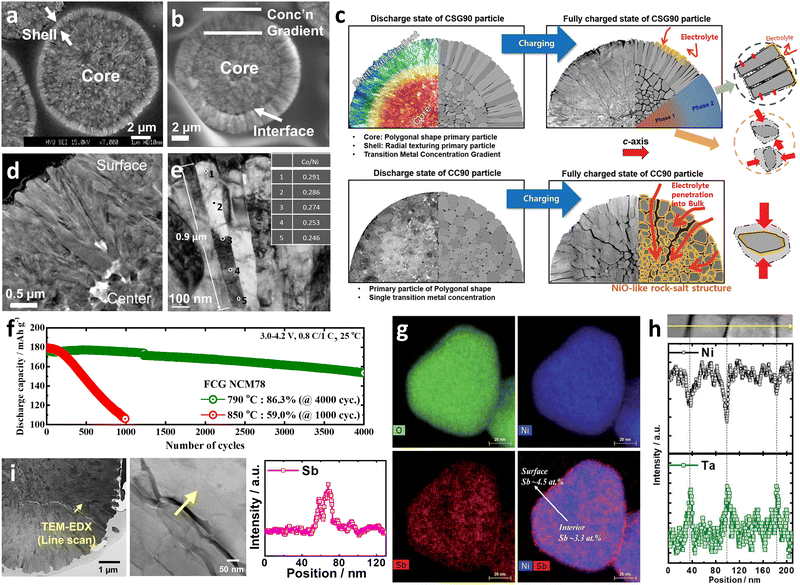 | ||
| Fig. 19 Cross-sectional SEM images of the (a) CS and (b) CSG materials showing the rod-shaped primary particle morphology in the shell region. (c) Schematic illustration showing the CSG Li[Ni0.90Co0.05Mn0.05]O2 (CSG90, top) and conventional Li[Ni0.90Co0.05Mn0.05]O2 (CC90, bottom) materials in the charged and discharged states. In CSG90, the radially textured nano-rods in the shell prevent microcrack nucleation and propagation, while in CC90, microcracks propagate and allow the electrolyte to penetrate. (d) Cross-sectional TEM image of a FCG Li[Ni0.60Co0.15Mn0.25]O2 particle showing the nano-rod grains at the particle surface. (e) TEM image and EDS data along a single elongated grain of an FCG Li[Ni0.60Co0.15Mn0.25]O2 nano-rod particle, illustrating the concentration gradient within individual grains. (f) Long-term cycling performances of the nano-rod microstructure optimized FCG Li[Ni0.78Co0.10Mn0.12]O2 (calcined at 790 and 850 °C) in pouch-type full cells versus the graphite anode. (g) EDS maps of O, Ni, and Sb, along with the overlapped Sb and Ni maps for the Sb-doped LiNiO2 system. (h) TEM-EDS line scan of Ta-doped CS Li[Ni0.94Co0.04Mn0.02]O2 cathode across the grain boundary, showing the concentration spikes of Ta at the grain boundary regions. (i) STEM image of the Sb-doped CSG90 after 1000 cycles, and the corresponding line scan across the microcrack, as indicated by the marked line. (a) and (b) are reproduced with permission,47 Copyright 2009, Springer Nature. (c) is reproduced with permission,49 Copyright 2019, John Wiley and Sons. (d) and (e) are reproduced with permission,48 Copyright 2013, American Chemical Society. (f) is reproduced with permission,98 Copyright 2022, Elsevier. (g) is reproduced under Creative Commons CC-BY license,93 Copyright 2020, Royal Society of Chemistry. (h) is reproduced with permission,153 Copyright 2023, John Wiley and Sons. (i) is reproduced under Creative Commons CC-BY license,231 Copyright 2021, The Authors, American Chemical Society. | ||
The FCG material is the third generation of the CG-type materials. As the name implies, it displays a concentration gradient that extends fully from the particle center to the surface. The first FCG material possessed a chemical composition of Li[Ni0.86Co0.10Mn0.04]O2 at the particle center that graded to a surface composition of Li[Ni0.70Co0.12Mn0.20]O2, thereby representing an average composition of Li[Ni0.75Co0.10Mn0.15]O2.46,48,98,185 The FCG material is also interesting in that it demonstrated the generation of elongated rod-like primary particles in a radial arrangement with well-developed crystallographic textures near the particle surface for the first time. The aspect ratios of the grains were also maximized because the primary nano-rod particles were crystallographically aligned in the radial direction and had their own concentration gradients within individual grains (Fig. 19d and e).46,48 Due to the fully extended gradient, the nano-rod region within the secondary particle extended well beyond the shell region seen in the CSG system. To the best of our knowledge, this was the first report of the nano-rod grain structure in layered oxide cathode materials. The combination of these two features in FCG materials greatly reduced the strain and stress to minimize microcrack generation during cycling among the gradient types to deliver enhanced cycling, especially during extended cycles. For example, the nano-rod microstructure of the optimized FCG Li[Ni0.78Co0.10Mn0.12]O2 was found to retain 86.3% of its original discharge capacity over 4000 cycles at a 0.8C/1C charge/discharge rate as shown in Fig. 19f.98 In addition to the radially parallel nano-rod alignment, the crystal lattice was oriented such that the Li slabs point outward from the center of the particle along the ab plane to minimize the tortuosity of inbound or outbound Li+. Accordingly, a nearly straight diffusion path for Li+ transport across adjacent primary particles was provided for rapid charge/discharge. This aligned diffusion path was also advantageous during operation at high and low temperatures.48 The presence of concentration gradients within the elongated rod-shaped primary particles also brings another beneficial effect. Since more Li-ions are (de)intercalated in Ni-rich regions, expansion and contraction of the crystal structure take place, allowing different structural behaviors to be observed within the rod-shaped primary particles depending on the Ni concentrations in the local regions.36,235,236 The inner region with a relatively higher Ni content contracts to a greater extent than the outer surface region, resulting in the generation of a relative compressive stress on the inner side and tensile stress on the outer side. This differential state of internal stress within the nano-rod particles enhances the mechanical strength against microcracking, much like tempered glass.99 However, the fully extended concentration gradient limits the Ni content, achieving a Ni content of ≥80% difficult. A steeper second gradient was added to the surface to circumvent this limitation and generate a TSFCG material.50,237,238 The TSFCG displayed similarly distinct nano-rod grain morphologies with large aspect ratios in a radial alignment and delivered excellent electrochemical and thermal properties despite the increased Ni content. It should also be noted that the synthesis process for gradient materials is simple and can easily be substituted for existing manufacturing lines. This is possible since it is only necessary to add a secondary tank that feeds into the original feed tank to generate the chemical concentration gradient.
Despite the numerous advantages of CG-type materials and their revisions through multiple generations, they are not without their challenges. One major challenge is the deterioration occurring during the high-temperature lithiation process, wherein the slope of the pre-designed concentration gradient flattens due to the interdiffusion of TMs. More concerningly, the well-developed, needle-shaped primary particles in the precursors sinter to form the irregular, polygonal primary particles often seen in conventional layered cathode materials in the absence of meticulous and optimized temperature control.98 However, the addition of high valence state dopants (e.g., Ta5+, Sb5+, W5+, and Nb5+) has effectively reduced the above deterioration during synthesis. Studies have revealed that high valence dopants (denoted as X-dopants) show a strong tendency to segregate to the surface during lithiation, as shown in Fig. 19g and h.50,153,204,231,239 These X-dopants present at the grain boundary regions act as sintering inhibitors to maintain the characteristic nano-rod shape. They also reduce TM (i.e., Ni, Co, Mn, and Al) interdiffusion to maintain the pre-designed gradient slope more effectively. Consequently, the X-doped concentration gradient materials, as the 5th generation gradient-type materials, resist deterioration during high-temperature calcination and preserve the original physicochemical features.153,204,231,239 This directly translates into an improved suitability for large-scale industrial synthesis where precise temperature control is difficult. This is possible due to the lower sensitivity of X-doped CG materials to temperature fluctuations. The X-dopants also act as a protective layer to minimize the NiO-like impurity formations when microcracks eventually occur, and consequently, they further increase the electrochemical durability of the concentration gradient material (Fig. 19i). This can be seen in the case of the W-doped FCG Li[Ni0.78Co0.10Mn0.12]O2, which continues to provide 72% of its 0.2C capacity at 5C even after 1000 cycles, leading to preservation of the fast-charge capability.204,231
The most recent development in concentration gradient-type materials is the near-surface reconstructed concentration gradient.240 This material is treated with a Co-dissolved aqueous solution and coated with fluorine to concurrently eliminate any residual Li found in the Ni-enriched layered oxides, ultimately establishing a fluorinated protective layer. As a result, the undesirable gas evolution that leads to cell swelling is suppressed. Coupled with these two features of concentration gradient-type material, the surface-modified concentration gradient Li[Ni0.90Co0.05Mn0.05]O2 material delivered an unprecedented 65% retention over 6000 cycles at 0.8/1C (charge/discharge) in a full-cell versus the graphite configuration. This result hints at the possibility of a material that can satisfy the energy density, durability, and safety requirements of LIBs in practical applications.
4. Nano-rod cathodes without a concentration gradient
4.1. Nano-rod structure in bulk-type cathodes
As it has been recognized that the presence of the nano-rod structure in concentration gradient materials is a key factor in improving the cathode performance, research has also focused on the development of this nano-rod structure in conventional cathode materials without concentration gradients. As summarized in Table 1, the nano-rod structure is found in various bulk-type cathode materials, such as LiNiO2 (LNO), Li[NixCoyAl1−x−y]O2 (NCA), Li[NixCoyMn1−x−y]O2 (NCM), Li[NixCoyMnzAl1−x−y−z]O2 (NCMA), Li[NixCo1−x]O2 (NC), and Li[NixMn1−x]O2 (NM), regardless of the composition. In particular, many studies have been conducted to develop nano-rod structures in cathode materials with Ni contents >80% since the main degradation mechanism of Ni-rich cathodes is microcrack formation. As discussed above, the nano-rod structure can effectively suppress these microcracks.52,53,57,58,71,76,78–80,97,106,108,130,207,241–243| Cathode | Composition | Method for developing the nano-rod morphology | Ref. |
|---|---|---|---|
| LNO | LiNiO2 | Doping (Al,Ti) | 58 |
| LNO | LiNiO2 | Doping (Nb/Ta/Mo) | 241 |
| LNO | LiNiO2 | Doping (W) | 242 |
| NCA | Li[Ni0.885Co0.1Al0.015]O2 | Doping (B) | 52 |
| NCA | Li[Ni0.855Co0.13Al0.015]O2 | Doping (Nb) | 76 |
| NCA | Li[Ni0.95Co0.04Al0.01]O2 | Doping (W) | 97 |
| NCM | Li[Ni0.92Co0.04Mn0.04]O2 | Doping (Al) | 106 |
| NCM | Li[Ni0.92Co0.05Mn0.03]O2 | Doping (B,Na/Mg/Al/Zr) | 108 |
| NCM | Li[Ni0.83Co0.06Mn0.11]O2 | Doping (Si) | 243 |
| NCM | Li[Ni0.94Co0.05Mn0.01]O2 | Doping (W) | 71 |
| NCM | Li[Ni0.95Co0.025Mn0.025]O2 | Doping (W,F) | 207 |
| NCM | Li[Ni0.8Co0.1Mn0.1]O2 | Controlled co-precipitation synthetic conditions | 130 |
| NCM | Li[Ni0.83Co0.05Mn0.12]O2 | Controlled co-precipitation synthetic conditions | 244 |
| NCM | Li[Ni0.6Co0.2Mn0.2]O2 | Controlled co-precipitation synthetic conditions | 245 |
| NCM | Li[Ni0.6Co0.2Mn0.2]O2 | Controlled co-precipitation synthetic conditions | 246 |
| NCM | Li[Ni0.8Co0.1Mn0.1]O2 | Controlled co-precipitation synthetic conditions | 247 |
| NCMA | Li[Ni0.94Co0.03Mn0.02Al0.01]O2 | Doping (Mo) | 80 |
| NC | Li[Ni0.96Co0.04]O2 | Doping (Mo) | 79 |
| NC | Li[Ni0.9Co0.1]O2 | Doping (B,Ta) | 53 |
| NC | Li[Ni0.9Co0.1]O2 | Doping (Ti/Ta/Mo) | 57 |
| NM | Li[Ni0.9Mn0.1]O2 | Doping (Mo) | 78 |
Despite the absence of a TM concentration gradient as a driving force to develop nano-rods, it was possible to develop the nano-rod structure in cathode materials without a concentration gradient by controlling the co-precipitation reaction conditions during precursor synthesis or by doping heterogeneous elements into the cathode (Table 1). For this purpose, the precursor should be preferentially grown in the radial direction, and the nano-rod microstructure of the precursor should be passed down to the cathode material during the lithiation process. Spherical particles are synthesized during the preparation of the precursor through the nucleation step and the subsequent particle growth step (Fig. 20a). In the initial stage of precursor synthesis, the TM ions inside the reactor form a complex with ammonia molecules and react with OH− to generate TM(OH)2 nuclei, wherein nanosized crystal nuclei spontaneously agglomerate into porous microspheres to reduce their surface energies. Subsequently, these microspheres spontaneously aggregate into irregular quasi-spheres during continuous stirring. Ultimately, the nanosheets on the surface of the quasi-sphere grow radially until the interface gradually disappears and the surface becomes compact. During this particle growth stage, the concentration of ammonium, pH conditions, stirring environment, and other synthetic conditions affect particles' continuous and preferential radial growth.130,244–247 However, during the particle growth process, the newly generated nucleus or microspheres can be adsorbed on the surfaces of the existing quasi-spheres, and they subsequently tightly adhere to one another and grow together. This new microsphere generation and re-agglomeration processes with the existing quasi-spheres play dominant roles in forming the randomly distributed primary particles and the irregular morphology found in the precursor. In contrast, when this re-agglomeration of new nuclei is inhibited and when the preferential radial growth of particles is promoted, a radially oriented microstructure is generated in the precursor. When this precursor is composed of rod-shaped grains and coarsening of the grains is minimized during calcination, the precursor microstructure is passed down to the lithiated cathode, resulting in a nano-rod structure.79,241
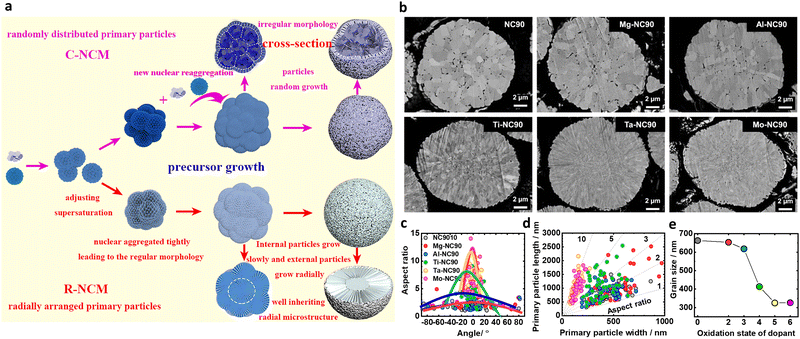 | ||
| Fig. 20 (a) Schematic diagram illustrating the growth mechanism of precursors with and without the nano-rod structure. (b) Cross-sectional SEM images of cathode particles that show the morphological and orientation differences between the polygonal grains of NC90, Mg-NC90, and Al-NC90, and the elongated grains of Ti-NC90, Ta-NC90, and Mo-NC90. Quantitative analytical results for the cathode grains and their orientations: (c) relationship between the aspect ratio and the relative grain orientation, (d) the aspect ratio (length/width), and (e) the grain size as a function of the oxidation state of dopant. (a) is reproduced with permission,247 Copyright 2020, American Chemical Society. (b)–(e) are reproduced under Creative Commons CC-BY license,57 Copyright 2021, Springer Nature. | ||
During high-temperature heat treatment (i.e., calcination) of a mixture of the precursor and the Li source, the morphology and orientation of the primary particles comprising the cathode material can be precisely controlled by doping heterogeneous elements. As shown in Fig. 20b, the shape of the primary particles could be modified depending on the doping element employed (e.g., Mg, Al, Ti, Ta, or Mo). In particular, doping elements with higher oxidation states led to higher aspect ratios in the primary grains (narrower and longer). In addition, the smaller the grain size, the more radially aligned the orientation of the primary grains tended to be (Fig. 20c–e). Since high-valence elements are segregated at the grain boundaries rather than being incorporated into the crystal structure, they inhibit grain coarsening during calcination and ultimately reduce the size of the primary particles to form nano-rods.76,79,241,242 Therefore, as the doping amount was increased, the primary grains became increasingly nano-sized in nature.80,242 Exceptionally, in the case of B doping into the cathode with a layered structure, the (003) facet was dominantly developed, resulting in a rod-shaped primary particle with a particularly high aspect ratio.52,53 As the content of B was increased, the primary grains became thinner and longer, resulting in rod-shaped primary grains with an aspect ratio of >10, wherein crack growth was further suppressed.54 Likewise, the primary particle morphology modification has been achieved by controlling the types and contents of the doping elements. Since the aspect ratio and size of the primary particles can be effectively tuned by doping, the cathode microstructure has been successfully optimized to effectively distribute the strain induced by anisotropic lattice volume changes, thereby suppressing microcrack formation and enhancing the electrochemical performance.52,248
4.2. Importance of the hydroxide precursor microstructure
All cathode materials with a layered structure that are currently used in LIBs are synthesized by mixing a hydroxide precursor with LiOH and calcinating the mixture at high temperatures. During calcination, the hydroxide precursor undergoes crystal structural changes (from the P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1 to the R
m1 to the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure) as it transforms into a lithiated oxide.249 Simultaneously, the fine grains of the precursor undergo a morphological change through coarsening to become the primary particles of the cathode material.79,211,241 Therefore, as shown in Fig. 21a, if the grains comprising the precursor are randomly oriented in a polygonal shape, the primary particles of the cathode material prepared from such a precursor will also be randomly oriented in a polygonal shape. Conversely, if the grains comprising the precursor are radially oriented in a rod-shaped form, the primary particles of the cathode material prepared from such a precursor will also have a radially oriented rod-shaped morphology. Therefore, the precursor's microstructure plays a crucial role in determining whether or not the microstructure of the cathode material contains a TM concentration gradient. To investigate the inheritance of the precursor microstructure by the cathode, a precursor with randomly oriented grains was synthesized by suppressing the uniform growth of the precursor through the intermittent pumping of solutions during the co-precipitation reaction (Fig. 21b).68 When the cathode material was prepared using such a precursor, the grains were randomly coarsened during the calcination process, resulting in a cathode material composed of randomly oriented polygonal primary grains, as shown in Fig. 21c. However, when the cathode material was prepared using a precursor whose grains were rod-shaped and radially oriented (Fig. 21d), the resulting cathode material also exhibited a well-developed radially oriented nano-rod structure (Fig. 21e). Even when various methods are used to induce the nano-rod structure, the development of this structure in the precursor is a prerequisite for the eventual development of a nano-rod structure in the cathode material. Zhou et al.246 also reported that the development of the nano-rod structure in a cathode material is dependent on the microstructure of the precursor (Fig. 21f–i). Since the microstructure of the cathode material is inherited from the precursor, this structure must be developed in the precursor via the co-precipitation approach. In other words, the presence of a well-developed nano-rod structure in the cathode material indicates that the rod-like microstructure of the precursor was maintained during the calcination process. In addition to the hydroxide precursor method, cathode materials can also be synthesized without the requirement for a precursor, such as via the sol–gel and solid-state reaction approaches. More specifically, LiMO2 cathodes can be prepared using the sol–gel method, wherein acetate and nitrate sources, such as Li(CH3COO), Mn(CH3COO)2·4H2O, Ni(CH3COO)2·4H2O, and Co(NO3)2·6H2O, are dissolved in water, and citric acid is added to form a gel. After subsequent drying and grinding of the gel, it is calcined to give the desired cathode material.250 Regarding the solid-state reaction approach, LiMO2 cathodes can be prepared by mixing oxide sources, such as NiO, Co3O4, and MnO2, with LiOH and subsequent calcination at high temperatures.251 Although these methods can yield cathode materials without the requirement for a precursor, they produce microstructures consisting of several large agglomerated grains, which cannot develop the nano-rod structure (Fig. 21j and k).250,251 Furthermore, the cathode particles synthesized using these methods are sub-micron in size, resulting in a lower tap density and volumetric energy density compared to cathodes with spherical secondary particles measuring >10 μm. Therefore, the nano-rod structure can only be developed through the lithiation of precursors synthesized via a co-precipitation reaction, which is currently a standard method in this field. These studies clearly emphasize the importance of the precursor microstructure in the development of cathode materials with nano-rod structures.
m structure) as it transforms into a lithiated oxide.249 Simultaneously, the fine grains of the precursor undergo a morphological change through coarsening to become the primary particles of the cathode material.79,211,241 Therefore, as shown in Fig. 21a, if the grains comprising the precursor are randomly oriented in a polygonal shape, the primary particles of the cathode material prepared from such a precursor will also be randomly oriented in a polygonal shape. Conversely, if the grains comprising the precursor are radially oriented in a rod-shaped form, the primary particles of the cathode material prepared from such a precursor will also have a radially oriented rod-shaped morphology. Therefore, the precursor's microstructure plays a crucial role in determining whether or not the microstructure of the cathode material contains a TM concentration gradient. To investigate the inheritance of the precursor microstructure by the cathode, a precursor with randomly oriented grains was synthesized by suppressing the uniform growth of the precursor through the intermittent pumping of solutions during the co-precipitation reaction (Fig. 21b).68 When the cathode material was prepared using such a precursor, the grains were randomly coarsened during the calcination process, resulting in a cathode material composed of randomly oriented polygonal primary grains, as shown in Fig. 21c. However, when the cathode material was prepared using a precursor whose grains were rod-shaped and radially oriented (Fig. 21d), the resulting cathode material also exhibited a well-developed radially oriented nano-rod structure (Fig. 21e). Even when various methods are used to induce the nano-rod structure, the development of this structure in the precursor is a prerequisite for the eventual development of a nano-rod structure in the cathode material. Zhou et al.246 also reported that the development of the nano-rod structure in a cathode material is dependent on the microstructure of the precursor (Fig. 21f–i). Since the microstructure of the cathode material is inherited from the precursor, this structure must be developed in the precursor via the co-precipitation approach. In other words, the presence of a well-developed nano-rod structure in the cathode material indicates that the rod-like microstructure of the precursor was maintained during the calcination process. In addition to the hydroxide precursor method, cathode materials can also be synthesized without the requirement for a precursor, such as via the sol–gel and solid-state reaction approaches. More specifically, LiMO2 cathodes can be prepared using the sol–gel method, wherein acetate and nitrate sources, such as Li(CH3COO), Mn(CH3COO)2·4H2O, Ni(CH3COO)2·4H2O, and Co(NO3)2·6H2O, are dissolved in water, and citric acid is added to form a gel. After subsequent drying and grinding of the gel, it is calcined to give the desired cathode material.250 Regarding the solid-state reaction approach, LiMO2 cathodes can be prepared by mixing oxide sources, such as NiO, Co3O4, and MnO2, with LiOH and subsequent calcination at high temperatures.251 Although these methods can yield cathode materials without the requirement for a precursor, they produce microstructures consisting of several large agglomerated grains, which cannot develop the nano-rod structure (Fig. 21j and k).250,251 Furthermore, the cathode particles synthesized using these methods are sub-micron in size, resulting in a lower tap density and volumetric energy density compared to cathodes with spherical secondary particles measuring >10 μm. Therefore, the nano-rod structure can only be developed through the lithiation of precursors synthesized via a co-precipitation reaction, which is currently a standard method in this field. These studies clearly emphasize the importance of the precursor microstructure in the development of cathode materials with nano-rod structures.
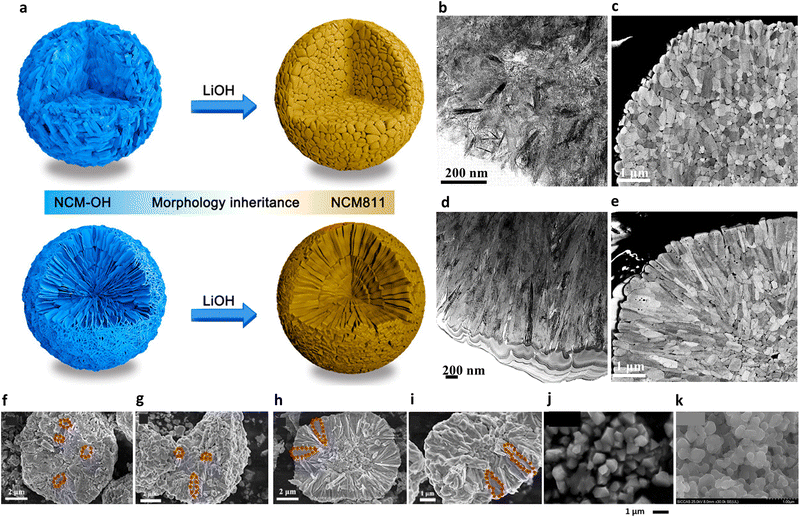 | ||
| Fig. 21 (a) Schematic representation showing inheritance of the precursor microstructure by the cathode material. (b) TEM image of a precursor with randomly oriented grains and (c) cross-sectional SEM image of the cathode microstructure synthesized using this precursor. (d) TEM image of a precursor with radially oriented grains and (e) cross-sectional SEM image of the cathode microstructure synthesized using this precursor. Cross-sectional SEM images of (f) a precursor without the nano-rod structure and (g) the cathode synthesized using this precursor. Cross-sectional SEM images of (h) a precursor with the nano-rod structure and (i) the cathode synthesized using this precursor. SEM images of the cathodes synthesized using (j) the sol–gel method and (k) the solid state reaction method. (a) is reproduced with permission,109 Copyright 2023 American Chemical Society. (b)–(e) are reproduced with permission,68 Copyright 2021, Royal Society of Chemistry. (f)–(i) are reproduced with permission,246 Copyright 2016, Elsevier. (j) is reproduced with permission,250 Copyright 2022, American Chemical Society. (k) is reproduced under Creative Commons CC-BY license,251 Copyright 2017, Royal Society of Chemistry. | ||
4.3. Mechanisms of nano-rod generation using the doping approach
The primary particle morphology of a cathode material can be further engineered by introducing dopants during the lithiation of the nano-rod precursors. The morphology of the primary particles differs depending on the dopant type, and the resulting size and shape can vary depending on the type and amount of dopant. In this context, Sun et al.51,52 reported that B doping on NCA or NCM cathodes lowers the surface energy of certain facets during lithiation, forcing the primary particles to grow into thin elongated shapes enclosed on the sides by the (003) planes (Fig. 22a–c). To verify the surface energy modification caused by boron doping, the (003), (012), (014), and (100) surface energies of pristine LiNi0.92Co0.04Al0.04O2 and B-doped LiB0.02Ni0.90Co0.04Al0.04O2 cathodes were calculated using the density functional theory approach.52Fig. 22d shows the surface ene rgy plots γ(ΔμLi) of the pristine LiNi0.92Co0.04Al0.04O2 and B-doped LiNi0.90Co0.04Al0.04 B0.02O2 surfaces. ΔμLi was referenced to the total energy per atom of a metal Li reservoir, and the low and high values of ΔμLi represent Li-poor and -rich environments, respectively. For low Li chemical potential values of ΔμLi ≤ −3.67 eV, the pristine LiNi0.92Co0.04Al0.04O2 (003) surface was found to be the most favorable, while for values of −3.67 eV ≤ ΔμLi ≤ −2.85 eV, the B-doped LiB0.02Ni0.90Co0.04Al0.04O2 (104) surface was stabilized. However, for cases where −2.85 eV ≤ ΔμLi, the B-doped LiNi0.90Co0.04Al0.04 B0.02O2 (003) surface was the most favorable. As lithiation of the hydroxide precursor was performed using excess Li, it is likely that the surface of the B-doped cathode was enriched in Li and that the B atoms preferentially lowered the energy of the (003) surface. Therefore, through B doping, the surface energy was engineered to maximize the (003) facet, and consequently, rod-shaped primary particles with high aspect ratios were achieved.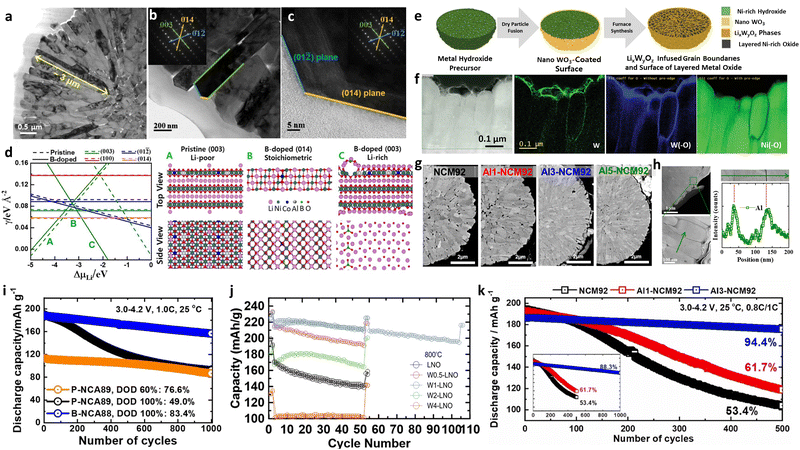 | ||
| Fig. 22 (a) and (b) Bright-field TEM images of the as-synthesized particles showing the B-NCA88 microstructure. (c) HRTEM images of the magnified region in part (b). (d) Surface energies of the pristine and B-doped LiNi0.9Co0.04Al0.04O2 (003), (012), (100), and (104) surfaces as a function of the chemical potential of Li (ΔμLi) referenced to bulk metal Li (left). The solid and dashed lines represent the B-doped and pristine surfaces, respectively. The most favorable surface structures at different values of ΔμLi (right), labeled as A: pristine (003)-Li poor, B: B-doped (014)-stoichiometric, and C: B-doped (003)-Li rich in the surface energy plot. (e) Schematic illustrations of the secondary particle cross-sections at various stages of the W-modification procedure. (f) TEM image, EELS mapping of the W signal, MLLS fitting of oxygen without pre-edge spectra, and MLLS fitting of oxygen with pre-edge spectra for the W1-LNO sample where the precursor was coated with WO3via the dry particle fusion approach. (g) Cross-sectional SEM images of the NCM92, Al1-NCM92, Al3-NCM92, and Al5-NCM92 particles. (h) TEM–EDX elemental line scan results for the 4 mol% Al-doped NCA cathode analyzed across the grain boundaries. (i) Long-term cycling performances of the P-NCA89 cathodes cycled at DoD 60 and 100%, and the B-NCA88 cathode cycled at DoD 100%. (j) Electrochemical cycling performances of LNO, W0.5-LNO, W1-LNO, W2-LNO, and W4-LNO synthesized at 800 °C (2 cycles at C/20, 50 cycles at C/5, and 2 cycles at C/20). (k) Long-term cycling performances of the NCM92, Al1-NCM92, and Al3-NCM92 cathodes in pouch-type full cells containing graphite anodes. (a)–(d) and (i) are reproduced with permission,52 Copyright 2020, Elsevier. (e) is reproduced under Creative Commons CC-BY license,252 Copyright 2021, IOP Publishing. (f) and (j) are reproduced with permission,242 Copyright 2021, John Wiley and Sons. (g) and (k) are reproduced with permission,106 Copyright 2023, Elsevier. (h) is reproduced with permission,68 Copyright 2021, Royal Society of Chemistry. | ||
If the precursor with a nano-rod structure is lithiated at a low calcination temperature, the nano-rod structure is well preserved in the cathode material by minimizing grain coarsening. However, the electrochemical performance of the cathode is poor due to its low crystallinity.211 However, during calcination at higher temperatures or for longer durations, the crystallinity is enhanced, and the precursor grains are coarsened in a random direction, resulting in larger primary grains and random polygonal shapes.79,211,241 High-valence elements such as Sb, Nb, Ta, Mo, and W impede the coarsening of primary particles by segregating at the particle boundaries, thereby preserving the microstructure until the cathode attains an optimal crystal structure.79,241,242 These high valence elements are likely insoluble in the crystal structure, resulting in accumulation along the interparticle boundaries. In this context, Dahn et al.242,252 reported that the W introduced during lithiation of the precursor is concentrated in the form of LixWyOz phases on the surfaces of the secondary particles and in the grain boundaries between the primary particles after calcination (Fig. 22e and f). The presence of these phases limits grain coarsening in polycrystalline materials, and the resulting smaller primary particles lead to an enhanced resistance to the microcrack formation induced by the anisotropic stress during the (de)intercalation of Li+ ions. Furthermore, the LixWyOz phases can act as a glue between the primary particles. Hence, a lesser degree of microcracking was observed in the W-doped cathode materials.242,252 Similarly, Nb, Ta, and Mo form LiNbO3, LiTaO3, Li2MoO4 compounds during lithiation, which retard coarsening by segregating at the grain boundaries.241 Therefore, by doping with high-valence elements, the crystallinity can be maximized without sacrificing the integrity of the microstructure, resulting in a wide range of possible calcination temperatures and enhanced electrochemical performances compared to those of the pristine cathodes. Furthermore, since solubility differences also exist in the crystal structure depending on the type of dopant, elements such as Mo (which remain as lithiated oxides at high temperatures) maintain the microstructure and electrochemical performance of the cathode at higher temperatures than is possible for Ta or Nb.79,241 Since these high valence elements develop a nano-rod structure through a mechanism that maintains the microstructure of the precursor during lithiation, the development of a nano-rod structure in the precursor is clearly a prerequisite.
Elements such as Al, which are soluble in the crystal structure due to their low atomic weights, can be sufficiently incorporated into the lattice structure at concentrations of up to 2 mol%. However, introducing excessive doping beyond the dopant solubility causes segregation at the grain boundaries (Fig. 22g and h).68,106 As in the case of high-valence element doping, segregation by such excessive doping suppresses the coarsening of primary particles by pinning the grain boundary, thereby maintaining the microstructure of the precursor after lithiation. Therefore, developing a nano-rod structure in the precursor is also a prerequisite for inducing the nano-rod structure through such excess doping. As excess doping increases, a greater degree of segregation occurs, and the precursor microstructure can be more effectively maintained, generating nano-sized primary particles.68,106 However, the reversible capacity decreases as the content of electrochemically inactive elements increases, and the excessive segregation of dopants can act as an impurity. Therefore, the optimum excess doping level in the case of Al has been defined as 3 mol%.106 Conventionally, the doping strategy considers only incorporating additional elements into the bulk structure of the cathode in terms of fortifying the crystal structure. However, the ability to engineer a cathode material's crystal structure and microstructure through methods such as excessive doping has made it possible to maximize long-term cycling stabilities, especially for Ni-rich cathodes that suffer from microcrack-induced capacity fading.
The above results indicate that doping is a simple and effective method to induce nano-rod formation via various mechanisms (e.g., surface energy modification and suppression of coarsening via the segregation of doping elements). In addition, it is necessary to develop a nano-rod structure in the precursor stage to achieve the nano-rod structure using these doping methods. As the radial alignment of thin and long particles is effective in preventing microcracks, which are the main culprit of the degradation of Ni-rich cathodes, cathodes with a nano-rod structure developed through doping tend to exhibit enhanced cycling stabilities compared to those without doping (Fig. 22i–k). Therefore, multi-stage engineering, from precursor synthesis to morphological control through doping, is important for developing the nano-rod structure in cathode materials and enhancing their respective electrochemical performances.
5. Challenges and advances of the nano-rod structure
5.1. Considerations during nano-rod cathode preparation
The performances of Ni-rich layered cathode materials greatly depend on various fundamental properties, such as crystallinity, grain morphology, and surface conditions, which are determined during the synthetic process. Hence, it is crucial to understand the relationship between the synthetic conditions and the cathode material structure to optimize the performance of the nano-rod cathode material. In this context, it is known that Ni-rich cathode materials are produced in a two-step process involving the synthesis of the hydroxide precursor and subsequent calcination of this precursor in the presence of a Li source. While various reaction systems could be considered, a hydroxide-type precursor is favored due to its ease of mass production and ability to provide template structures for the final cathode product. Ultimately, this facilitates the development of a desirable crystallinity, Li pathway, and grain alignment.253 The calcination process is also important since it should preserve the desired characteristics of the hydroxide precursors even after heat treatment at high temperatures (Fig. 23a).79,231,241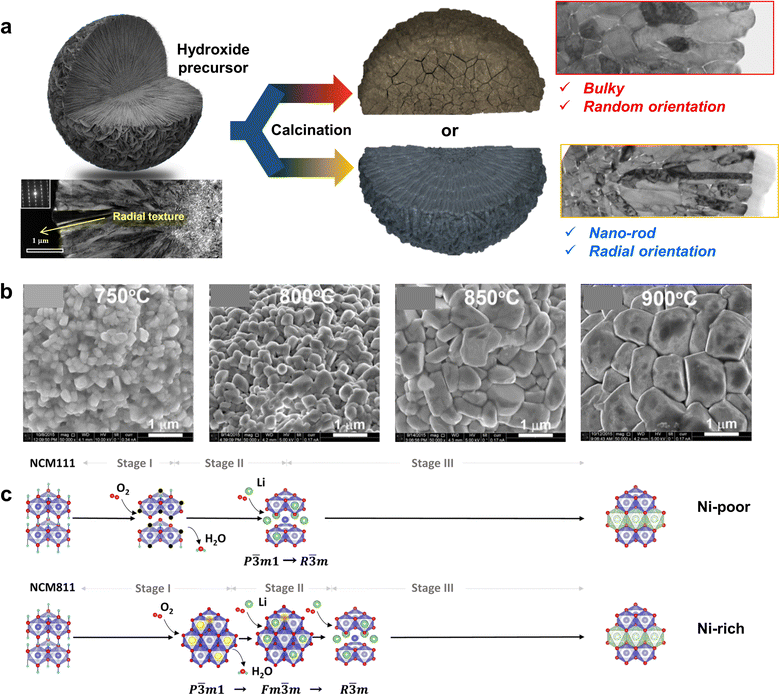 | ||
| Fig. 23 (a) Schematic representation of the microstructure of the cathode material, which varies depending on the sintering conditions. (b) Variation in the evolution of the primary particle morphology under different heat treatment temperatures. (c) Variation in the crystal structural evolution depending on the chemical composition during the sintering process. (b) is reproduced with permission,211 Copyright 2018, Elsevier. (c) Reproduced under Creative Commons CC-BY license,249 Copyright 2023, American Chemical Society. | ||
As the temperature rises during calcination, Li ions are inserted into the hydroxide precursor with a P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1 space group structure, forming an intermediate state where Li and the TMs are randomly arranged.249 At higher temperatures, Li and the TMs form alternating layers, accompanied by changes in the chemical states of the TMs. This process forms a layered structure with an R
m1 space group structure, forming an intermediate state where Li and the TMs are randomly arranged.249 At higher temperatures, Li and the TMs form alternating layers, accompanied by changes in the chemical states of the TMs. This process forms a layered structure with an R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. In addition, various crystal structural features, such as Li/TM intermixing and the Li–slab distance, are known to significantly influence the mobility of the Li ions, affecting overall performance. Furthermore, it has been reported that the skeletal structure and crystallinity of a cathode material are predominantly influenced by both the peak temperature during the calcination step and the duration of this elevated temperature.80,211 An insufficient thermal energy cannot induce adequate Li/TM ordering, while excessive heat and prolonged exposure to such heat can lead to the loss of Li and O from the crystal structure, resulting in increased disordering.211 Therefore, it is crucial to establish an optimal sintering temperature to ensure a sufficiently high crystallinity for any specific composition.
m space group. In addition, various crystal structural features, such as Li/TM intermixing and the Li–slab distance, are known to significantly influence the mobility of the Li ions, affecting overall performance. Furthermore, it has been reported that the skeletal structure and crystallinity of a cathode material are predominantly influenced by both the peak temperature during the calcination step and the duration of this elevated temperature.80,211 An insufficient thermal energy cannot induce adequate Li/TM ordering, while excessive heat and prolonged exposure to such heat can lead to the loss of Li and O from the crystal structure, resulting in increased disordering.211 Therefore, it is crucial to establish an optimal sintering temperature to ensure a sufficiently high crystallinity for any specific composition.
The electrochemical properties of cathode materials are significantly influenced by their microstructures, surface areas, pore distributions, grain sizes, and Li-ion pathways. The hydroxide precursors, typically synthesized via the co-precipitation approach, generate secondary particles through the aggregation of nano-scale primary particles. During calcination, the insertion of Li ions into the structure induces volume expansion of the primary particles.204 While the size of the secondary particles is relatively less sensitive to the calcination temperature, the primary particles coarsen through the fusion of neighboring particles, resulting in grain growth at certain calcination temperatures. In this context, Zheng et al.211 investigated the effect of the calcination temperature on Ni-rich cathode materials. They found that the primary particle size increased with an increasing calcination temperature, and the resulting cathode performance was directly related to the primary particle size (Fig. 23b). Notably, changes in the shape, size, and orientation of the primary particles during calcination play a crucial role in determining the morphology of the cathode material. More specifically, the size and shape of the primary particles not only determine the exposed surface area on the outside of the secondary particles but also influence the reconstruction of the surface structure and the degree of residual Li formation depending on the calcination conditions (i.e., O2 flow, pressure, and cooling rate).254
A Ni-rich cathode material can exhibit its peak performance when synthesized under optimal conditions. However, one of the challenges associated with optimizing the performances of such materials is the complex synthetic conditions that become more intricate as the Ni content rises. For example, the optimal calcination temperature decreases and its range narrows as the degree of O loss becomes more prevalent with the enrichment of Ni. Kleiner et al.249 reported that Ni-rich and Ni-poor cathode materials underwent different structural reaction mechanisms (Fig. 23c). In contrast to Ni-poor hydroxide precursors, which maintain a stable layered scaffold upon calcination, Ni-rich precursors transition through a rock-salt structure to produce an R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m layered structure. This transformation necessitates precise temperature control to avoid incomplete phase transformation and structural disorder. Additionally, compositional changes in the Co or Mn ions influence structural evolution.255,256 Each TM ion exhibits a different preference for oxidation during the calcination process, thereby affecting the breakage or reconstruction of NiO6 octahedra. Different magnetic and electronic interactions among the constituent cations in the framework, which can be imparted by tuning the extent of Li/Ni exchange, further determine the order of the local structure.255 Many comprehensive studies based on the use of advanced analytical tools have provided insights into the crystalline and morphological changes taking place during the calcination of conventional layered cathode materials. However, intriguingly, adding a small amount of dopant leads to entirely different transformations in the cathode material during sintering, posing a challenge in performance optimization in nano-rod cathode materials.
m layered structure. This transformation necessitates precise temperature control to avoid incomplete phase transformation and structural disorder. Additionally, compositional changes in the Co or Mn ions influence structural evolution.255,256 Each TM ion exhibits a different preference for oxidation during the calcination process, thereby affecting the breakage or reconstruction of NiO6 octahedra. Different magnetic and electronic interactions among the constituent cations in the framework, which can be imparted by tuning the extent of Li/Ni exchange, further determine the order of the local structure.255 Many comprehensive studies based on the use of advanced analytical tools have provided insights into the crystalline and morphological changes taking place during the calcination of conventional layered cathode materials. However, intriguingly, adding a small amount of dopant leads to entirely different transformations in the cathode material during sintering, posing a challenge in performance optimization in nano-rod cathode materials.
As discussed above, the addition of dopants can alter the optimal calcination temperature. For example, it has been reported that when a small amount of W is doped into the LNO material with an optimal calcination temperature of 650 °C, the optimal calcination temperature increases by >100 °C.257 Notably, at temperatures far beyond the optimal range, numerous defects are generated in the pure LiNiO2 cathode material due to O loss, and the layered structure is also collapsed. However, adding small amounts of W during the sintering process maintained the layered structure even at high temperatures and enhanced the structural stability for long-term cycling. In this context, Bianchini et al.81 observed that the incorporation of W into an LNO cathode material suppressed the occupancy of Ni on the Li sites, indicating a higher structural stability at elevated calcination temperatures. They suggested that the doping-induced increase in the calcination temperature may be attributed to the formation of trace amounts of rock-salt-like impurity structures. This, in turn, was potentially caused by the high valence state of W6+, leading to the migration of Ni2+ to the Li sites.
A similar behavior was observed in Mo-doped NCMA94 materials during variable-temperature X-ray diffraction (XRD) measurements (Fig. 24a).80 Upon increasing the temperature during calcination, the (003)/(104) peak ratio of the pristine NCMA94 cathode rapidly increased, the degree of cation mixing decreased, and both parameters were found to level off at 680 °C. However, when the temperature was further elevated to 770 °C after a 10 h holding period at 680 °C, the crystalline parameters of the cathode deteriorated, resulting in the collapse of its R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m layered structure in the presence of such an excessive thermal energy. In contrast, for the NCMA94 cathode containing 1.5 mol% MoO3 doping, the optimal crystal structure formed at 770 °C rather than 680 °C, displaying an even higher (003)/(104) peak ratio than that of the pristine cathode at 680 °C. These results confirm that even trace amounts of MoO3 significantly influence the crystallinity of NCMA94 and increase the optimal lithiation temperature. Interestingly, the magnitude of the shift depends on the amount of Mo, such that four cathode materials with varying doping levels exhibit their optimal crystallinities when calcined at different temperatures. The impact of the Mo doping content also includes morphological refinements, which become increasingly more pronounced at higher Mo concentrations (Fig. 24b).80 Moreover, a higher degree of Mo doping suppresses primary particle growth over a wider temperature range, likely due to kinetic or thermodynamic effects hindering grain boundary diffusion by solute accumulation.79,242,252 These variations in the material crystallinity and morphology distinctly affect its electrochemical performance. The left panel in Fig. 24c shows the relationship between the crystallinity and discharge capacity. Notably, the discharge capacities of the cathodes calcined at different temperatures show a roughly similar tendency to the variations in their c/3a ratio, with the exception of the pristine cathode. On the other hand, the right panel in Fig. 24c shows the relationship between the primary particle size and capacity retention. While the capacity retention of the pristine NCMA94 cathode decreases with an increasing lithiation temperature, that of the Mo-doped NCMA94 cathode remained stable over a wide temperature range. This trend suggests that capacity retention is inversely related to the average primary particle size of the cathode materials. However, an excess Mo doping of 2 mol% compromises the initial capacity while providing limited benefits in terms of capacity retention. It was therefore suggested that the optimized Mo-doped NCMA94 cathode could be synthesized using 1 mol% Mo doping and calcination at 750 °C. The results showed that determining the optimal synthetic conditions for tuning the electrochemical performance (i.e., doping amount, calcination temperature, and calcination time) requires a trial-and-error approach. This process depends on the compositional and morphological features of precursors/cathodes and the characteristics of the dopants, thereby rendering the development of high-quality nano-rod cathode materials a significant challenge.
m layered structure in the presence of such an excessive thermal energy. In contrast, for the NCMA94 cathode containing 1.5 mol% MoO3 doping, the optimal crystal structure formed at 770 °C rather than 680 °C, displaying an even higher (003)/(104) peak ratio than that of the pristine cathode at 680 °C. These results confirm that even trace amounts of MoO3 significantly influence the crystallinity of NCMA94 and increase the optimal lithiation temperature. Interestingly, the magnitude of the shift depends on the amount of Mo, such that four cathode materials with varying doping levels exhibit their optimal crystallinities when calcined at different temperatures. The impact of the Mo doping content also includes morphological refinements, which become increasingly more pronounced at higher Mo concentrations (Fig. 24b).80 Moreover, a higher degree of Mo doping suppresses primary particle growth over a wider temperature range, likely due to kinetic or thermodynamic effects hindering grain boundary diffusion by solute accumulation.79,242,252 These variations in the material crystallinity and morphology distinctly affect its electrochemical performance. The left panel in Fig. 24c shows the relationship between the crystallinity and discharge capacity. Notably, the discharge capacities of the cathodes calcined at different temperatures show a roughly similar tendency to the variations in their c/3a ratio, with the exception of the pristine cathode. On the other hand, the right panel in Fig. 24c shows the relationship between the primary particle size and capacity retention. While the capacity retention of the pristine NCMA94 cathode decreases with an increasing lithiation temperature, that of the Mo-doped NCMA94 cathode remained stable over a wide temperature range. This trend suggests that capacity retention is inversely related to the average primary particle size of the cathode materials. However, an excess Mo doping of 2 mol% compromises the initial capacity while providing limited benefits in terms of capacity retention. It was therefore suggested that the optimized Mo-doped NCMA94 cathode could be synthesized using 1 mol% Mo doping and calcination at 750 °C. The results showed that determining the optimal synthetic conditions for tuning the electrochemical performance (i.e., doping amount, calcination temperature, and calcination time) requires a trial-and-error approach. This process depends on the compositional and morphological features of precursors/cathodes and the characteristics of the dopants, thereby rendering the development of high-quality nano-rod cathode materials a significant challenge.
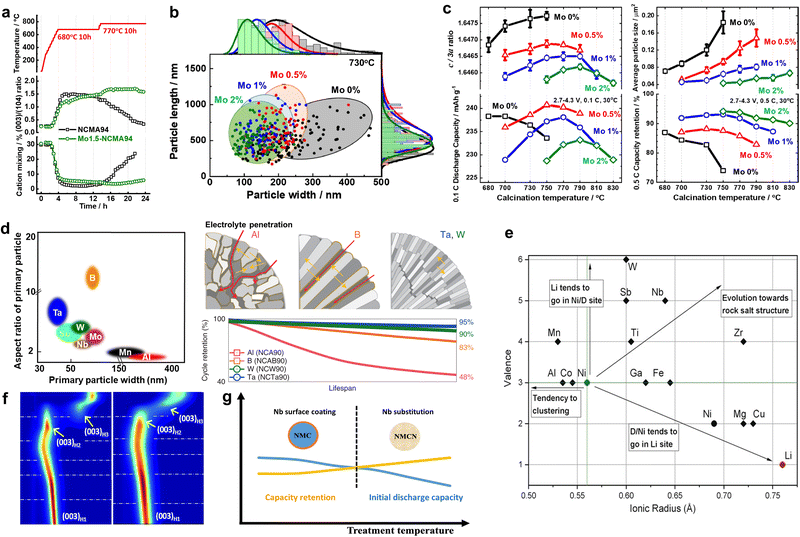 | ||
| Fig. 24 Comparison of (a) the crystallinity changes during the sintering process, and (b) the primary particle size depending on the doping content. (c) Correlation between the physicochemical properties and the electrochemical performances of cathode materials with different doping amount. (d) Map of the primary particle morphology and the electrochemical performance with variation in the oxidation state of the dopant. (e) Dopant categorization as a function of the ionic size and valence. (f) Contour plots of the (003) reflections of the LNO (left) and W-1 LNO (right) materials analysed by operando XRD. (g) Controversy regarding the presence of dopants, i.e., whether they exist only at grain boundaries, substitute within the crystal structure, or coexist on both sides. (a)–(c) are reproduced with permission,80 Copyright 2023, Elsevier. (d) is reproduced with permission,53 Copyright 2020, Springer Nature. (e) is reproduced with permission,258 Copyright 2018, John Wiley and Sons. (f) is reproduced with permission,257 Copyright 2019, Royal Society of Chemistry. (g) is reproduced with permission,259 Copyright 2021, American Chemical Society. | ||
Various dopants have been applied and tested in Ni-rich cathode materials; however, their properties and roles require further clarification. Significant efforts have been aimed at categorizing and understanding the characteristics of various types of dopants when introduced into Ni-rich layered cathode materials. For example, Sun et al.53,57 experimentally evaluated the electrochemical, morphological, and structural characteristics of dopants and categorized them according to the extent to which doping altered the physical properties of the material and increased the cathode cycle life (Fig. 24d). They determined that dopants with higher oxidation states are more effective at increasing the stability of the cathode material, as a higher oxidation state tends to induce a highly oriented elongated grain microstructure and generates a cation-ordered superlattice atomic arrangement. In addition, their results highlighted the close relationship between the resulting electrochemical performance and the micro- and atomic-level structures formed by the dopants. In another study, Janek et al.258 summarized previous research carried out on LNO cathode materials, categorizing the characteristics of the dopants based on their oxidation states and ion sizes (Fig. 24e). Their summary indicated that dopants with smaller cation sizes tend to lead to non-uniform doping due to their clustering inside the structure, resulting in a low tolerance of the doping concentration. In contrast, larger dopants can be easily accommodated into the structure. They also concluded that large dopants with low oxidation states tend to occupy Li sites, while those with high oxidation states induce the formation of a rock-salt structure. Furthermore, Cho et al.260 used first-principles calculations to predict the trends induced by dopants, such as phase instability, Li–Ni exchange, Ni segregation, lattice distortion, and oxygen evolution. They noted a strong site preference depending on the Mn and Co concentration ratio, suggesting multicomponent doping strategies are required.
The mechanisms by which dopants are incorporated into cathode materials during synthesis have yet to be precisely elucidated. This leads to debates over whether dopants diffuse into the structure, substitute Li or TM sites, or segregate at the grain boundaries as solutes. Indeed, some dopants have been shown to refine the microstructure by segregating at the grain boundaries, thereby delaying the coalescence of the primary particles. This raises the possibility that the added dopants may not substitute Ni or Li but that they instead exist only at the grain boundaries and surfaces (Fig. 22e and f).242 To date, numerous cases of dopants distributed at grain boundaries have been captured by electron energy loss spectroscopy (EELS) and energy-dispersive X-ray spectroscopy (EDS).90,241,242,261,262 On the other hand, tracking changes in the lattice parameters or the Li–Ni exchange degree via XRD is a standard method for determining whether dopants have diffused into a crystal structure or not. As shown in Fig. 24f, the phase transition behavior clearly changed during W doping, wherein the wholly separated H2 and H3 phases of the pristine LNO cathode transformed to exhibit a continuous transition, as indicated by the (003) shift. However, obtaining clear evidence using solely XRD for NCM/NCA cathodes with a Ni content <95% is challenging in most cases. This can be attributed to the fact that their phase transitions are not as abrupt as those of LNO, owing to the stabilization effects imparted by Co, Mn, and Al. In addition, the effective doping amounts are mostly restricted to <2 mol%, which leads to extremely subtle changes. It has also been reported that the crystal structural information varies depending on the synthetic conditions and doping amount employed.80,261 Currently, the most plausible explanation is that some dopants are inserted into the crystal structure while the rest remain at the grain boundaries (Fig. 24g).259 However, due to the complexity of the system, further multidimensional research into the doping mechanisms is required to promote the development of high-performance cathode materials.
5.2. Highly Ni-rich concentration gradient nano-rod cathodes
Doping is generally considered the most effective method for enhancing the performances of Ni-rich cathode materials. However, it is not a panacea, necessitating careful consideration of various combinations, doping methods, and dopant types/amounts. This section introduces recent advancements in doping strategies.Most degradation in Ni-rich cathode materials originates from the surface, indicating the necessity to protect the cathode material from microcracks and minimize the exposed surface area that comes into contact with the electrolyte. Ideally, if microcracks do not form, the electrolyte only attacks the outer surface of the secondary particles. In this regard, concentration gradient cathode materials possess significant advantages by minimizing the Ni content on the surface to reduce side reactions with the electrolyte.46,47,99,224–228 More specifically, concentration gradient materials composed of a hybrid structure (i.e., a high Ni content in the core and a low Ni content on the surface) suppress the formation of microcracks by creating a non-uniform spatial distribution of microstrain within the cathode particle. This structure resembles tempered glass, enhancing resistance to microcracking during electrochemical operations.99 In the absence of microcracking, the lower Ni content on the surface of a concentration gradient cathode delays parasitic attack by the electrolyte, leading to exceptional long-term stability. However, despite increasing the demand for concentration gradient cathode materials with higher Ni contents and energy densities, limited spatial flexibility exists for implementing concentration gradients within the precursor.153 Consequently, the thickness of the shell gradually decreases, and the difference in concentration between the interior and the exterior is reduced, leading to issues resembling those of materials without concentration gradients (Fig. 25a). Furthermore, challenges are also associated with the use of thin concentration gradient layers that are unable to withstand high sintering temperatures due to interdiffusion between TM ions.98,153,231 Therefore, the development of concentration gradient cathode materials containing >90% Ni poses significant challenges. Drawing inspiration from high-entropy alloys, some of the challenges found the intermixing of TMs during calcination were overcome by the addition of high-valence dopants. For examples, a Sb-doped concentration gradient cathode material containing 90% Ni was found to possess Sb compounds within its grain boundaries after calcination, which enveloped the primary particles, and segregated at their boundaries (Fig. 25b).231 This layer slowed down the interdiffusion of TMs (e.g., Ni, Co, and Mn) between the neighboring primary particles. It was therefore considered that the incorporation of Sb ions into the crystal structure delays cation diffusion by altering the adjacent atomic distance. This alteration increases the bulk diffusion barrier energy, thereby impeding the bulk migration of Ni ions. Indeed, concentration gradient cathode materials doped with antimony maintained at least double the concentration difference between the core and surface compared to the corresponding undoped materials. Another benefit of doping concentration gradient cathode materials is the inhibition of coarsening due to the presence of a dopant solute at the grain boundaries.153,231 While concentration gradient materials without dopants also possess rod-shaped primary particles with a radial alignment, any deviation from the optimal calcination temperature range results in shorter and thicker primary particles, which possess less desirable properties. However, such grain growth can be hindered by solute segregation, which pins the grain boundaries kinetically or thermodynamically. Therefore, this microstructure resulting from high-valence ion doping can be maintained over a wide range of calcination temperatures (i.e., approximately 100 °C), providing a competitive advantage in mass production. Unlike in the laboratory, where the synthetic temperatures can be precisely controlled, industrial-scale cathode synthesis operates over a wide range of temperatures, which may lead to the sintering of the nano-rod primary particles. High-valence ion doping can address this challenge by producing a strong crystallographic texture that ensures exceptional product quality over a wide range of temperatures.
 | ||
| Fig. 25 (a) Concentration gradient profiles demonstrating the limitations of the concentration gradient design as the average Ni content increases. (b) Schematic representation of a nano-rod concentration gradient cathode with a grain boundary coating. (c) and (d) Advanced cathode materials containing dopant concentration gradients. (a) is reproduced with permission,153 Copyright 2023, John Wiley and Sons. (b) is reproduced with permission,99 Copyright 2021, American Chemical Society. (c) is reproduced with permission,262 Copyright 2019, American Chemical Society. (d) is reproduced with permission,263 Copyright 2022, Elsevier. | ||
Unlike concentration gradient cathode materials, wherein the concentrations of the major elements (i.e., Ni, Co, and Mn) change gradually, varying the dopant concentration in a cathode material has been demonstrated to enhance cyclability effectively. In this context, Lin et al.262 aimed to enhance the stability of LiNiO2 using dual dopants, namely Mg and Ti (Fig. 25c). They found that both dopants were present at the Ni sites, although Ti was concentrated on the surface, leading to a gradient distribution; in contrast, Mg was uniformly distributed throughout the particles. Similarly, Chen et al.263 synthesized Mg- and Al-gradient Ni-rich cathode materials via a co-precipitation approach (Fig. 25d). They found that the high concentrations of Al and Mg on the outer surface induced gentle phase transitions in the highly charged state, while the local segregation of Mg formed a MgO coating layer, which provided a stabilization effect at the electrode–electrolyte interface. Furthermore, Gao et al.264 implemented surface gradient doping by exploiting the characteristic surface segregation caused by the high formation energies associated with yttrium oxides. Importantly, these gradient doping methods have been shown to maintain or enhance Li diffusion while suppressing irreversible phase transitions and TM leaching on the surface. In another study, Whittingham et al.259 controlled the role of the Nb dopant in an NCM811 cathode material by varying the calcination temperatures. The presence of Nb in the coating layer was found to play a role in reducing initial cycle losses and improving the cycling performance, while Nb substitution stabilized the crystal structure to enhance the long-term performance characteristics. It was therefore considered that an optimal combination of coating and doping is ideal, considering the different roles adopted by the dopant depending on its form. Although no extensive studies have focused on engineering the dopant distributions in battery materials, there is ample room to manipulate the three-dimensional gradient distributions of dopants to enhance the stabilities of Ni-rich cathode materials.
The impact of such doping approaches has also been demonstrated in Co-free cathode materials. This is relevant because it is crucial to devise strategies to tackle the expected Co shortage as the battery demand rises.265 It has been noted that Co3+ helps mitigate cation mixing and stabilizes the layered structure by easing magnetic frustration.266 Additionally, an absence of Co in a layered cathode diminishes the electronic conductivity, leading to a decline in both the rate capability and the performance at low temperatures.267 Despite numerous efforts to find alternative dopants to substitute the role of Co, these endeavors have failed to yield success. Nevertheless, it has been reported that the design of nanostructured architectures and the alignment of diffusion pathways lead to rapid charging and enhanced durability characteristics.
Although the intrinsic properties of Co cannot be replaced by doping, a strategy has been employed to complement them by forming nano-rod primary particles. Park et al.78 proposed a Co-free NM90 cathode material consisting of ultrafine grains. High-quality doping achieved this, and the resulting grain size refinement dissipated the deleterious strain through fracture toughening, ultimately suppressing microcrack formation during long-term cycling.268 In addition, the enhanced cation ordering attributed to the presence of high-valence dopants has also been found to stabilize the delithiated structure when the structure is most vulnerable due to the non-uniform extraction of Li+ ions. Furthermore, Yang et al.252 also enhanced the performance of co-free cathode materials using W6+. More specifically, the cathode material was obtained by coating WO3 onto the hydroxide precursor and then calcining it. The presence of LixWyOz on the surface reduced the primary particle size, which enhanced the material's mechanical strength against the anisotropic stress experienced during Li+ insertion/extraction. It was therefore suggested that the LixWyOz phases form strong bonds between the primary particles, resulting in fewer microcracks in the W-modified materials.
5.3. Surface shielding of the nano-rod structure
One of the key advantages of microstructural refinement through nano-rod formation is the effective relief of the structural stress generated during charge/discharge, which allows the cathode material to resist microcracking.269,270 Indeed, many studies have demonstrated that this method suppresses microcrack formation and enhances the cathode performance.52,120–122,124,129–132 However, as high Ni contents are required to increase the energy density, it is necessary to develop methods that suppress the resulting material.36 Although the preparation of crack-resistant particles has been reported, coating the outer surfaces of the particles may also constitute an effective approach to protecting against high Ni contents on the exposed surfaces.271One of the methods proposed for controlling the surfaces of nano-rod cathode materials is F coating.154,172,240,272 More specifically, F coating has been extensively researched to react with any residual Li present on the surface to form stable LiF compounds. One of the key features of F coating is that, during heat treatment, F is infused into the particles and forms a coating layer along the grain boundaries. When applying such a coating, it is crucial to control the thickness of the coating layer; otherwise, it may simply add to the electrochemically resistive layer, diminishing its effectiveness. In this context, Ryu et al.172 synthesized nano-rod cathode materials by doping Sb into a 93% Ni NCMA material, and subsequently, they further enhanced the material performance by coating with F. Despite controlling the thickness of the F coating layer within tens of micrometers, an abnormal behavior was observed during the initial cycles in the half-cell tests. More specifically, a gradual increase in the delivered capacity was recorded, suggesting an “activation” process was necessary over several cycles. After this process, the rate of capacity degradation decreased, and this effect was more pronounced in the full-cell evaluations. It was therefore considered that the LiF layer in the F-coated nano-rod cathode protects the cathode surface from parasitic side reactions with the electrolyte, attack by HF, and TM dissolution, as evidenced by the reduced intensities of the decomposed electrolyte fragment peaks in the ToF-SIMS depth profile. Interestingly, the F coating significantly improved the lifespans of nano-rod cathode materials, whereas the protective effect of the F coating was insignificant on conventional cathode materials (Fig. 26a). This difference was attributed to the suboptimal microstructure of the conventional cathode, which suffered severe microcracking during cycling to expose the new surface (Fig. 26b). These results, therefore, suggested that the surface protection effect can be maximized when coupled with appropriate microstructure modification.
 | ||
| Fig. 26 (a) Comparison of long-term cycling between conventional and nano-rod cathode materials with and without F coating, and (b) cross-section SEM images of the (left) F-NCMA93 and (right) F-Sb-NCMA93 cathodes. (c) TEM-EDS maps, and (d) near-surface structures of the Co-coated cathode surface after washing. (e) Long-term cycling performances of the surface-treated cathode materials. (a) and (b) are reproduced with permission,172 Copyright 2023, American Chemical Society. (c)–(e) are reproduced with permission,240 Copyright 2024, Springer Nature. | ||
Another significant issue related to the surfaces of Ni-rich cathodes is the presence of residual Li,273–276 which forms when the cathode is exposed to air (containing moisture and CO2) during calcination. The primary cause of the increased residual Li content, particularly in compositions with higher nickel content, is the instability of Ni3+ rather than the addition of excessive lithium during synthesis. It is known that the highly reactive Ni3+ species present on the surface are reduced to Ni2+, releasing O from the lattice and causing Li to leach out to the surface.31,34 As a result, the amount of residual Li on the cathode surface increases with the Ni content, thereby rendering residual Li treatment a necessary process.
When a slurry is exposed to air during the electrode coating process, residual Li can gel the slurry, thereby leading to significant manufacturing difficulties. Consequently, strict process control and storage conditions are required to minimize exposure to external air, ultimately leading to increased processing costs.34,277 Additionally, the residual Li can impede Li movement on the surface during the electrochemical reactions, and it can decompose to generate a gas, thereby causing cell swelling and localized heating, which poses potential safety risks during practical applications.167 Thus, the presence of residual Li must be carefully considered in the context of commercialization since it increases battery manufacturing costs and can lead to safety and reliability issues. In this regard, nano-rod cathode materials, composed of smaller primary particles than conventional ones, have been considered due to their potential to exhibit different surface conditions and properties. Although the overall fracture toughening effect of nano-sizing has been investigated, the surface issues arising from smaller primary particles have yet to be addressed accurately. Two approaches can be used to prepare such structures: a dopant can be employed, or the calcination temperature can be lowered. However, both methods are likely to increase the amount of residual Li on the surface. More specifically, at low calcination temperatures, there is a high possibility that the Li may not sufficiently penetrate the crystal structure, leaving residual Li on the surface.278,279 Additionally, when using a dopant, a residue, which is suspected to be a Li–dopant–oxide composite, can remain on the surface.272 Therefore, controlling and protecting the reactivity of the surface is important in both cases.
When the doping approach is used to form nano-rods, the dopant compounds are passivated on the surface of the cathode material. Since only a very small amount of the dopant compound is present on the surface, it is difficult to determine exactly what structure and composition is formed; however, these are widely speculated to be the abovementioned Li–dopant–oxides. For example, when B doping is used, the possibility of LixByOz generation has been studied, while the possibility of LixWyOz formation during W doping has been explored.81,242,252,257 The presence of such passivation layers reportedly delays material degradation by reducing the amount of direct contact between the electrolyte and the cathode material surface.231 However, recent studies have shown that materials doped with Mo produce greater amounts of gas than non-doped materials when left in a charged state for an extended period of time.272 The gas bubbles formed on the surface disrupt the movement of Li ions and hinder proper contact, thereby preventing the cell from operating normally.278,280 Moreover, this can lead to battery swelling, posing safety risks. These issues could be the largest obstacles associated with the commercialization process. This increased gas production may be attributed to the inherent characteristics of Mo doping and the presence of various Li compounds on the surface, which originate from the high-valence doping. More specifically, these gas byproducts could be formed from self-decomposition of the Li–dopant compounds, or from the ability of these compounds to act as catalysts and accelerate electrolyte decomposition.
To avoid the detrimental effects associated with residual Li, the current industrial process implemented by battery manufacturers involves washing the Ni-rich cathodes.281–284 However, washing often leads to performance degradations because the air- and moisture-sensitive cathode materials are immersed in water.282–284 Therefore, additional coating and heat treatment processes are required to minimize such performance degradations. Notably, the washing procedure removes residual Li from the cathode surface and any Li ions located within the crystal structure through the proton-exchange reaction.285–288 This extraction may result in surface reconstruction to form rock-salt structures and promote further performance degradation. Heat treatment at significantly high temperatures is necessary to restore the damaged surface structure. For example, Hou et al.289 conducted a systematic investigation into the effect of recalcination on temperature after washing. They varied the holding temperature between 500 and 740 °C and determined that temperatures >600 °C were necessary to restore the layered structure from the rock-salt structure generated during washing.
Dry coating is a simple method to protect the cathode surface from degradation during washing or convert the residual Li into a desirable coating.240,290–296 However, given that the washing solution comes into contact with all exposed surfaces of the cathode material, conventional dry coating, which results in an island-type coating, generally cannot cover every degraded surface unless the coating agent can diffuse inside the particles and along the grain boundaries. In addition, dry coating has the disadvantage of adding an additional step between the washing and heat treatment stages. In contrast, the wet coating has several advantages despite is associated handling difficulties. More specifically, since a liquid solution is used in the washing process, a coating process could be included in the washing step to achieve the simultaneous removal of residual Li while forming a protective layer.240,293,296 In this case, the residual Li is employed as a reactant to form the coating layer, and the coating layer formed at the liquid–solid interphase can be uniformly deposited to cover the exposed surface completely. As one literature example, Chen et al.296 carried out the wet coating of LiNi0.83Mn0.1Co0.07O2 by soaking in a diluted Al(H2PO4)3 solution and mixing under ultra-sonication. The slightly acidic solution readily reacted with Li2CO3 to form Li3PO4 and AlPO4 after heat treatment at 550 °C. The same group further investigated the wet impregnation process using several dihydro-phosphate solutions in which the coating proceeded through a similar reaction mechanism with Al(H2PO4)3.297 They paid particular attention to the acidity of the coating solution and argued that a weakly acid solution with a pKa of 7.2 imparted the best protection to Ni-rich NMC cathode materials. These protective phosphate layers block chemical attacks by the electrolyte and prevent exposure of the TM atoms, thereby suppressing CO2 evolution and structural degradation to the rock-salt structure. In another example, Ryu et al.240 combined the idea from co-precipitation process with the wet-coating of a 90% Ni cathode material. They considered that, during co-precipitation of the cathode precursor, dissolved TM ions precipitate as metal hydroxides in the high-pH aqueous solution. In addition, when the Ni-rich cathode material is immersed in water, the pH rapidly rises to >11 due to the high solubility of the residual Li compounds. Thus, to harness these two reactions during the washing step, a Co source was added to the washing solution to promote the direct precipitation reaction on the cathode surface. Since this coating reaction consumed the residual Li present on the cathode surface, it combines the washing and coating steps into a single process. It was demonstrated that the resulting Co coating layer uniformly covered the outer surface with a thickness of a few nanometers (Fig. 26c). Importantly, this precipitate layer was epitaxially grown on the cathode surface through the formation of a crystal structure between the layered and spinel-like phases (Fig. 26d). The Co coating layer remained even after 4000 cycles, which prevented structural degradation by suppressing direct contact between the Ni-enriched cathode surface and the electrolyte. Additionally, a LiF coating layer was added to the Co-coated cathode surface to further enhance the surface stability. While the Co coating process itself removed approximately 40% of the residual Li compared to the unwashed state, the additional F coating further reduced the Li level, resulting in <50% of the residual Li being retained compared to the unwashed cathode material. The synergistic effect of the F and Co coatings significantly reduced the amount of gas released from the charged cathode materials compared to the unwashed and Co-coated cathodes. Interestingly, the F-coated cathode without Co-washing produced similarly small amounts of gas, suggesting that the suppression of gas evolution from the cathode materials is mostly attributed to the F-coating effect. However, the F coating alone did not sufficiently protect the surface structure from accumulation of the rock-salt phase during cycling, although the LiF layer effectively suppresses the autocatalytic side reactions triggered by electrolyte and impurities decomposition. Therefore, combining a LiF coating and Co-washing effectively protects the near-surface structures of Ni-rich cathodes and removes residual Li impurities, resulting in excellent cycling stabilities without problematic gas release (Fig. 26e).
5.4. Feasibility of nano-rod cathodes for practical application
The cathode material significantly impacts the performance, safety, lifespan, and cost of EV batteries. Cathode materials with higher energy density increase the driving range of EVs to deliver longer driving distances on a single charge and reduce frequency of charging to allow manufacturers to offer longer warranty periods. Cathode materials with higher durability and thermal stability also increase the lifespan of the battery application and reduce the chances of battery swelling or thermal runaway, as safety is an increasingly emphasized aspect of batteries in electric transport applications. However, it is difficult for conventional cathode materials to satisfy these key characteristics, as materials with high energy tend to be highly reactive.31 Cathode materials that are stable, on the other hand, exhibit low energy density and require a more materials to achieve the desired energy level within a battery cell. This translates to decreased cost-effectiveness and resource inefficiency. As such, determining the performance of electrode materials solely through chemical composition creates a dilemma of balancing energy density, stability, and cost, which makes satisfying all aspects challenging.Considering these requirements and features, the use of nano-rod cathode materials in EV batteries has become a feasible choice due to their practical advantages in high-performance and long-term applications. Nano-rod cathode materials with microstructural engineering, in addition to chemical composition engineering, can minimize the compromises derived from the trade-offs between energy density, safety, and durability.50,153 Owing to the systematically structured, spatial configurations, nano-rod cathode materials exhibit excellent durability that preserve the particles coherency and minimize surface degradation even after long-term use. For these reasons, nano-rod cathode materials are adopted in EV batteries such as the KIA Niro, Hyundai Kona EU and Ford F-150 lightning (Fig. 27a).298–301 The long-term cycling stability of the nano-rod cathodes has been further improved through modifications such as surface coating and additional doping. As shown in Fig. 26a and b, particles with radially aligned nano-rod structures not only retain their structural integrity after cycling but also demonstrate enhanced synergy with surface coating effects. Further strengthening the crystal structure by doping the nano-rod cathode can inhibit the occurrence of microcracks during the cycling process, resulting in enhanced long-term cycling stability; the Al-doped concentration gradient cathode with nano-rods retained 84.5% of its initial capacity after 3000 cycles (Fig. 27b) and inhibited particle pulverization after long-term cycling (Fig. 27c and d).302 More recently, the fluorine-doped concentration gradient cathode exhibited an unprecedented 8000 cycles operational capability (Fig. 27e),154 where the cathode material minimized the formation of NiO-like impurity phase on the inner particle surface to bypass the degradation pathway during long-term cycling (Fig. 27f and g). This is significant because impurity phase generated throughout the particles by electrolyte infiltration increases the resistance during Li (de)intercalation, resulting in a significant kinetic capacity loss especially at high C-rates.119 When these nano-rod cathodes used a carbon nanotube-Si composite anode instead of a graphite anode, which allows higher energy density, the full cell produced an energy density of 350 W h kg−1 with excellent capacity retention for 500 cycles at 1C rate, satisfying the energy density and driving range requirement for EVs (Fig. 27h).238
 | ||
| Fig. 27 (a) Kia Niro, Hyundai Kona EU and Ford F-150 Lightning EVs adopting Sk on's cells with nano-rod cathodes. (b) Cycling performance of FCG61 and Al-FCG61 cathodes in a full-cell versus graphite anode. SEM images of the cycled (c) FCG61 cathode and (d) Al-FCG61 cathode after 3000 cycles. (e) Long term cycling performance of the GC80 and F1-GC80 cathodes using pouch-type full cells at 25 °C. Mapping of Ni oxidation states for the cycled (f) GC80 (2000 cycles) and (g) F1-GC80 (5000 cycles) cathodes analyzed by STXM. The presence of pure Ni3+ and Ni2+ are assigned colors green and red, respectively. (h) Energy density of different LIBs. Comparison of the CNT–Si/TSFCG against the currently developed LIBs and schematic illustration of Li-rechargeable battery system based on CNT–Si anode and TSFCG cathode for vehicle application (inset). (i) Long-term cycling performance of NCMA90 and CSG-NCMA90 cathodes cycled at 100% DoD and upper 60% DoD using pouch-type full cells. (j) Long-term cycling performances of the cathodes in full cells under normal charging (0.8C) and 4N3F cycling protocols (4N: 4 times of normal charging at 0.8C, 3F: 3 times of fast charging at 2.0C), with a fixed discharging current of 1.0C (180 mA g−1). (k) Voltage and cell temperature plots with time for laminated-type Al-pouch MCMB/FCG–Mn-F cell (280 mA h), as a function of time during overcharge test. (b)–(d) are reproduced with permission,302 Copyright 2016, John Wiley and Sons. (e)–(g) are reproduced with permission,154 Copyright 2021, Royal Society of Chemistry. (h) is reproduced with permission,238 Copyright 2016, Royal Society of Chemistry. (i) is reproduced with permission,66 Copyright 2021, John Wiley and Sons. (j) is reproduced with permission,303 Copyright 2024, John Wiley and Sons. (k) is reproduced with permission,48 Copyright 2013, American Chemical Society. | ||
By suppressing electrolyte infiltration into the particle interior at the charged state, nano-rod cathode materials exhibit high storage stability and remain stable over prolonged exposure times. When the cathode material is at the charged state, high oxidation states of nickel and structural instability lead to rampant side reactions. Problem is that devices are often stored in a charged state in practical applications. In a study simulating such a scenario by Namkoong et al., cells that remained in the charged state exhibited shortened lifespan. However, in cells with the nano-rod structure cathode, the performance remained largely unaffected even with prolonged storage in a charged state.173 Similarly, the range and limit of the depth of discharge (DoD), which determine the extent of microcracking, critically affect the cycling stability as persistent microcracks in the upper DoD range allowed electrolyte to penetrate the particle interior.66,304 Nano-rod cathode materials not only can be utilized across the entire DoD range, but also they show superior characteristics even at the commonly used upper DoD range compared to the conventional cathodes (Fig. 27i).
The unique microstructural features of nano-rod cathodes also offer additional advantages for fast charging, making them highly suitable for practical applications. The extended primary particle morphology in a radial configuration facilitates Li+ diffusion by providing a direct pathway from the core to the surface. This enables the battery to deliver high performance even at high C-rates and reduces charging time. Unlike the conventional cathode, which does not sufficiently dissipate the stress induced by the H2–H3 phase transition under fast charging conditions, the exceptional durability of the nano-rod cathode helps maintain superior rate capabilities even after long-term cycling.189,303 Kim et al. applied extreme-fast-charging protocol to test the effect of fast charging on cathode materials.189 In a full cell evaluation driven at 4C over 500 cycles at elevated temperatures, the nano-rod-structured cathode material not only maintained a more intact form and performance but also exhibited a more uniform lithium distribution throughout the particles. This indicates that the migration of Li ions is expedited through short-distance diffusion facilitated by numerous interparticle boundaries. Considering that EV users employ a mix of normal and fast charging, a cycling protocol of four normal charges (0.8C) followed by three fast charges (2.0C), denoted as 4N3F, was applied (Fig. 27j).303 Unlike NCM90 which shows sharply deteriorated capacity after 200 cycles under 4N3F condition, Ta-NCM90 with nano-rods exhibited a minimal capacity difference between fast and normal charging conditions, demonstrating that the latter effectively maintain fast charging capabilities during long-term cycling. These properties enhance user convenience and maximize feasibility as cathode materials for EV batteries.
In addition, nano-rod cathode materials demonstrate superior thermal stability even after long-term cycling, as confirmed by DSC analysis, indicating reduced risk of thermal propagation or explosion during device usage.48Fig. 27k shows the variation of cell voltage and temperature with time after overcharge. After overcharge (at 250% state of charge), the cell featuring nano-rod cathode shows a voltage increase to only 5.5 V, with the temperature remaining below 20 °C. The nano-rod cathode also shows stable characteristics in nail penetration test, indicating robust resistance to physical impact as shown in Fig. 13c. The superior thermal stability is attributed to the material not breaking apart to minimize surface exposure to electrolyte even after cycling. These tests clearly show the significant improvements in the safety aspect, ensuring the reliability even in the event of accidents and providing greater relief to battery consumers. As demonstrated by their adoption in commercial EV batteries, various enhanced properties of nano-rod cathodes compared to conventional cathodes make nano-rod cathodes feasible for practical applications.
6. Next-generation batteries
In addition to their application in LIBs, cathode materials with nano-rod primary particles are also employed in next-generation energy storage devices, including all-solid-state batteries (ASSBs), Li-metal batteries (LMBs), and Na-ion batteries (SIBs).6.1. All-solid-state batteries
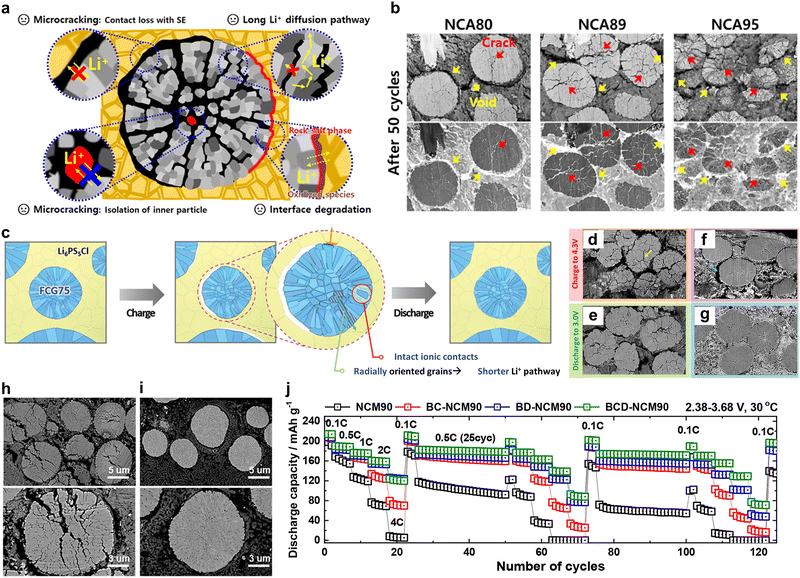 | ||
| Fig. 28 (a) Schematic illustration of the capacity fading mechanism of a Ni-rich cathode in an ASSB. (b) Cross-sectional SEM images of the NCA80, NCA89, and NCA95 electrodes recovered from ASSBs after 50 cycles. (c) Schematic diagram of the impact of cathode materials with customized microstructures on the performance of ASSBs (related to interface stability). Cross-sectional SEM images of (d), (e) NCA80 and (f), (g) FCG75 electrodes after (d), (f) first charge to 4.3 V (vs. Li/Li+) and (e),(g) the subsequent discharge to 3.0 V (vs. Li/Li+). Cross-sectional SEM images of (h) NCM90 and (i) BCD-NCM90 cathodes after cycling recovered from ASSBs. (j) Electrochemical test combining cycle and rate capability tests for NCM90, BC-NCM90, BD-NCM90 and BCD-NCM90 cathodes. (a) and (b) are reproduced with permission,308 Copyright 2023, Royal Society of Chemistry. (c)–(g) are reproduced with permission,314 Copyright 2019, John Wiley and Sons. (h)–(j) are reproduced with permission,315 Copyright 2023, American Chemical Society. | ||
6.2. Li-metal batteries
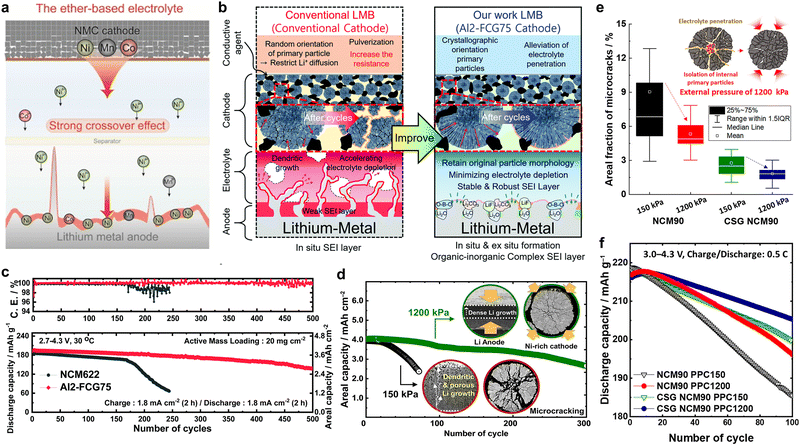 | ||
| Fig. 29 (a) Schematic illustration of crossover effect resulted from TM dissolution in LMBs. (b) Summary of conventional LMBs and the LMB concept using Al2-FCG75 cathode with nano-rods. (c) Comparison of the cycling performance of Li/NCM622 and Li/Al2-FCG75 cells with high capacity loading of 4.1 mA h cm−2 cycling at 1.8 mA cm−2. (d) Comparison of the cycling performances of Li/FCG NCM78 (loading level of 20 mg cm−2) PPC150 with the coated separator and PPC1200 with the coated separator. (e) Box-and-whisker plot demonstrating the areal fraction of microcracks in the NCM90 and CSG NCM90 particles after 100 cycles in PPC150 and PPC1200. (f) Comparison of cycling performances of Li/NCM90 (loading level of 10 mg cm−2) PPC150 and PPC1200 and Li/CSG NCM90 (loading level of 10 mg cm−2) PPC150 and PPC1200. (a) is reproduced with permission,323 Copyright 2023, Springer Nature. (b) and (c) are reproduced with permission,327 Copyright 2019, Royal Society of Chemistry. (d)–(f) are reproduced with permission,328 Copyright 2023, American Chemical Society. | ||
In addition to modifying the electrolyte solution or the surface of the lithium metal, external pressure application has been another successful strategy in suppressing Li dendrite growth and controlling Li metal deposition.328,335 Kim et al.328 induced uniform Li metal deposition in a LMB using a carbonate-based electrolyte and a boehmite-coated separator under an external pressure of 1200 kPa. As shown in Fig. 29d, pressure-controlled pouch-type cells (PPCs) at a high pressure (1200 kPa) level drives Li metal growth as a dense uniform layer, whereas at a low pressure (150 kPa) level, the Li metal growth was dendritic and porous. Furthermore, the analysis of the cycled NCM90 cathode revealed that compressive stress mitigates crack nucleation and propagation, thus providing a so-far-unexplored option for suppressing inherent microcracking and improving the cycling stability of Ni-rich layered cathodes. As shown in Fig. 29e, the effectiveness of this external pressure in suppressing microcracks in the cathode was maximized when using a nano-rod cathode such as the CSG NCM90, which inhibits the formation of microcracks during charge and discharge and maintains the electrical pathways throughout the particle without any discontinuity. Therefore, as shown in Fig. 29f, the CSG NCM90 cathode with nano-rods exhibited the most stable cycling when evaluated at 1200 kPa. Furthermore, the nano-rod cathode materials were able to effectively withstand the external pressure, emphasizing the mechanical stability of this structure and aptitude for high pressure conditions. When the surface of CSG NCM90 cathode was further coated with F, the PPC featuring the coated cathode cycled at 1200 kPa retained 82.0% of the initial capacity after 500 cycles with a loading level of 22 mg cm−2, an E/C ratio of 2.8 μL mA h−1, and an anode to cathode capacity ratio (N/P ratio) of 2.7 using a 70 μm thick Li foil. The capacity retention at high loading levels of >4 mA h cm−2 is markedly superior to that observed in LMBs utilizing carbonate-based electrolytes, and is comparable to that observed in LMBs utilizing ether-based electrolytes. These studies demonstrate the importance of cathode material properties, particularly mechanical stability and resistance to microcracks, for future LMBs, as the performance of an LMB are significantly impacted by the cathode.
6.3. Sodium-ion batteries
 | ||
| Fig. 30 (a) Comparison of ionic radius and standard hydrogen electrode potential of Li/Li+ and Na/Na+. (b) Schematic illustration of lithium- and sodium-ion layered crystal structure. (c) Differential capacity (dQ dV−1) profile of the LiNi0.5Mn0.5O2 (Li-NM5050) and NaNi0.5Mn0.5O2 (Na-NM5050) cathodes. (d) Phase transition scheme of the NaNi0.5Mn0.5O2 cathode during the charge and discharge process. (e) Cross-sectional SEM images of NaxNi0.5Mn0.5O2 cathode in different charged states during the 1st cycle and corresponded unit cell volume variations combined with dQ dV−1 profile. (f) Rate capability comparison of CC Na[Ni0.61Co0.12Mn0.27]O2 (CC 611227) and SNA Na[Ni0.61Co0.12Mn0.27]O2 (SNA 611227) at various discharge C-rates from 0.1C (15 mA g−1) to 10C (1500 mA g−1). (g) Micro-compression test results for CC Na[Ni0.61Co0.12Mn0.27]O2 and SNA Na[Ni0.61Co0.12Mn0.27]O2 single cathode particles. (h) DSC tests results of desodiated CC and SNA Na[Ni0.61Co0.12Mn0.27]O2. (i) TEM image and the corresponding electron diffraction pattern from a RAHC primary particle, illustrating the crystallographic alignment of the primary particle along the radial direction. (j) Long-term cycling performance of Na[Ni0.60Co0.05Mn0.35]O2-RAHC/hard carbon and Na[Ni0.60Co0.05Mn0.35]O2-bulk/hard carbon full cells within voltage range of 1.5–3.9 V. (c) and (e) are reproduced with permission,342 Copyright 2020, John Wiley and Sons. (d) is reproduced with permission,344 Copyright 2019, American Chemical Society. (f), (g), and (h) are reproduced with permission,346 Copyright 2016, John Wiley and Sons. (i) and (j) are reproduced with permission,347 Copyright 2015, Spring Nature. | ||
A radially aligned hierarchical columnar (RAHC) structure, which is similar to SNAs, in spherical particles with varied chemical composition from the inner core (Na[Ni0.75Co0.02Mn0.23]O2) to the outer shell (Na[Ni0.58Co0.06Mn0.36]O2) of the structure has been developed.347 The TEM image of the RAHC particle shows that the primary particles are radially oriented (Fig. 30i). The SAED pattern from the marked region confirms that the layer planes (i.e., a–b planes) in the hexagonal structure, perpendicular to the c-axis, are nearly parallel to the radial direction emanating from the particle center, demonstrating that the layer planes of the marked primary particle are radially oriented, confirming an apparent crystallographic texture in the primary particles of the RAHC cathode. The cathode with RAHC structure reduced side reactions during long-term electrochemical cycling (Fig. 30j). This cathode material facilitates an electrochemical reaction based on Ni2+/3+/4+, delivering a discharge capacity of 157 mA h (g-oxide)−1 at 15 mA g−1, a capacity retention of 80% (125 mA h g−1) over 300 cycles when paired with a hard carbon anode, and a rate capability of 132.6 mA h g−1 at 1500 mA g−1 (10C-rate). Additionally, the cathode performs well at low temperatures, even at −20 °C. These results are attributed to the unique chemistry of the cathode material, which enables efficient Ni redox reactions and minimizes the surface area in contact with the corrosive electrolyte. Furthermore, the nano-rods uniformly expanded and contracted during Na ion insertion and extraction, allowing the whole secondary spherical particles to reduce or absorb the cycling stresses.49 Therefore, the nano-rod-based cathode was able to withstand high currents without any morphological damage, even at extremely low operation temperatures (e.g., −10 °C).347 In addition, the nano-rods enhanced the electrode energy density due to the high tap density of the densely packed spherical micron-sized particles, which reduced the exposure of the active material to the electrolyte solution and to HF attack. Owing to the excellent integrated structure and mechanical strength, the nano-rod-based cathode exhibited a significant improvement in its thermal reactions (with a high onset temperature and a lower level of heat generation) at the deeply charged state.352,353 The interfacial stability (i.e., moisture resistance, delayed active material dissolution, and prevention of electrolyte side reactions/decomposition) of the cathode was further stabilized by utilizing aluminum fluoride (AlF3) coating, improving the cycling performances of practical cells.188,354–356 This combination of particle engineering and surface modification strategies to develop advanced cathode materials is therefore crucial for achieving the large-scale applications of SIBs, which requires further research efforts in the coming years.355,357
7. Conclusions and perspectives
In this paper, we comprehensively reviewed the origin, physicochemical properties, challenges, and advances of nano-rods in layered oxide cathodes and as well as their application in lithium-ion and next-generation batteries (ASSBs, LMBs, SIBs). The microstructure of the cathode active material is principally determined by its hydroxide precursor.68,109,160,247 For this reason, the development of radially aligned rod-shaped primary particles in the precursor is a prerequisite for the eventual development of a nano-rod structure in the cathode material (Fig. 31a). However, the conventional method for synthesizing cathodes, in which materials are subject to high calcining temperatures (>700 °C), invariably leads primary particles to coarsen and form the randomly oriented and equiaxed primary particles often observed in conventional layered cathode materials.79,211 Therefore, multi-stage engineering, from precursor synthesis to morphological control through doping, is important for developing the nano-rod structure in cathode materials and enhancing their respective electrochemical performances. The representative methods for developing nano-rod in cathode materials are summarized in below: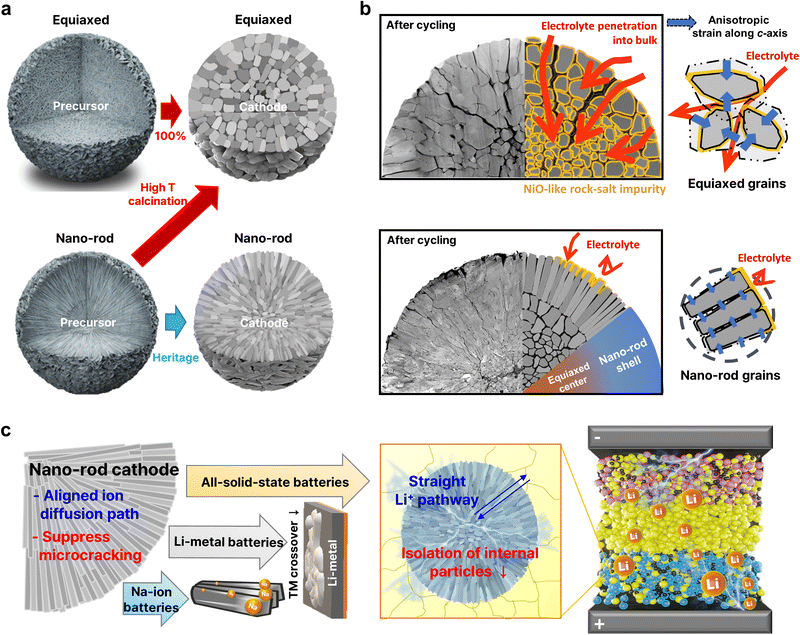 | ||
| Fig. 31 (a) Schematic illustration showing inheritance of the precursor microstructure by the cathode material. (b) Schematic illustration showing the cycled cathode particles featuring equiaxed and nano-rod grains, respectively. In a nano-rod cathode, the radially textured columnar grains in the shell prevent microcrack nucleation and propagation, while in conventional cathode, microcracks propagate and allow the electrolyte to penetrate. (c) Schematic illustration of the application of nano-rod cathodes in various next-generation batteries (ASSBs, LMBs and SIBs). (a) is reproduced with permission,68 Copyright 2021, Royal Society of Chemistry. (b) is reproduced with permission,49 Copyright 2019, John Wiley and Sons. | ||
(1) Nano-rod structures can be obtained by compositional partitioning of the cathodes, in which nano-rods are firstly recognized.46 In a concentration gradient precursor, the presence of the twin MnOOH and Ni(OH)2 phases mitigates the thermally-induced crystallographic distortion by maintaining the layered planes parallel along the radial direction, while also retarding sintering of the adjacent primary particles.160 The nano-rod structures are mostly observed in Ni-rich (Ni ≥ 60%) cathodes.46,55,58,78,122,188 However, because the twin MnOOH plays a role in mitigating grain coarsening, cathodes featuring high Mn concentration favor the formation of nano-rod grains.
(2) For conventional cathodes without a concentration gradient, nano-rod structure can be achieved through controlled precursor synthesis and doping processes. Doping with elements such as B facilitates the formation of nano-rods by reducing the formation energy of the (003) facet during lithiation.51,52,69,100
(3) Doping with high-valent elements, such as Nb, Ta, W, Sb, and Mo, tends to lead to their segregation at the grain boundaries, which prevents the coarsening of the primary particles and maintains the nano-rod microstructure of the precursor.53,57,76,79,80,129,153
(4) Similarly, even when easily incorporated elements, e.g., Al and Mn, are introduced into the lattice in excess doping amounts, segregation at the grain boundaries was still observed, and a rod-shaped cathode material was obtained.68,72,106
Although various dopants have been investigated, it is essential to comprehend the principles behind their mechanisms of action and categorize the dopants accordingly. This understanding will ultimately facilitate the rational design of cathode materials.
The unique geometric characteristics of nano-rod-grained cathodes greatly influence layered oxide cathodes' physicochemical and electrochemical properties. Recently, the use of nano-rod-grained cathodes in EV batteries has become a feasible choice due to their practical advantages in high-performance and long-term applications.298–301 The characteristics arising from constructing radially aligned nano-rod grains are summarized as follow:
(1) One of the main benefits of constructing radially aligned nano-rod grains is the resulting enhanced mechanical durability. The nano-rod grains effectively suppress microcrack formation and propagation by mitigating stress inside the cathode particle, which is induced by lattice volume contraction upon charging.36,51,52,122 Protection of the cathode particle interior from direct contact with the electrolyte offers additional sturdiness in terms of both the chemical and structural stabilities, thereby enhancing the electrochemical performance of the cathode (Fig. 31b).50,66,75
(2) The construction of a strong crystallographic texture in the nano-rod-grained cathode provides direct Li+ diffusion paths, thereby improving the rate performance of the cathode.98,101,206
(3) The generated nano-rod grains sometimes contain different local domains, such as rock-salt, disordered spinel, cation-ordered, and twinned crystal structures.57,116,138,146,148,150 These modified crystal structures contribute to delaying the electrochemical cycle-induced irreversible structural degradation, thereby improving the cycle life of the cathode.
(4) Nano-rod grains feature more abundant lateral facets and fewer frontal facets than equiaxed-shaped grains.69,101 Because the structural degradation along the lateral side of the grains is insignificant, nano-rod grains can attenuate parasitic chemical reactions along the surfaces in contact with the electrolyte solution.66,231
(5) Cathode particles constructed of nano-rod grains feature outstanding thermal stability. This is attributed to the chemo-mechanical protection offered by the tightly packed radially oriented grains, which mitigate microcrack propagation and protect internal particle surfaces from direct contact with the electrolyte.129,188
Though rarely reported, formation of nano-rod can adversely accelerate oxidative reactions between the highly reactive Ni4+ and electrolytes.120,272 Nano-rod grains can increase specific surface area of the cathode, thereby providing more risks for cathode-electrolyte reactions and gas evolutions along the secondary particle surfaces.54 Thus, it is important to understand the altered surface properties resulting from these nano-rod structures. Viable solutions to resolve such chemical degradation issue in nano-rod cathode are shown below:
(1) Formation of concentration gradient in nano-rod cathode can significantly attenuate surface reactivity of the cathode active material.36,49,98 The compositional and spatial configurations offered by concentration gradient provide the greatest benefit when the Ni content of the gradient cathode increases dramatically toward 100%. By reducing the Ni contents of the nano-rod primary particles on the outer surfaces, gradient cathode materials can delay surface degradation.
(2) Optimization of the washing and coating processes can enhance the surface chemical stability of the cathode. For instance, F-coating has been demonstrated to inhibit gas generation and electrolyte decomposition, while Co-washing prevents degradation of the surface structure.172,240,272
(3) Dopant-gradient approaches have demonstrated successful cathode surface shielding, with dopants preferentially locating to the surface and interparticle boundaries to protect active material from irreversible structural degradation and oxidative decomposition of the electrolyte.93,252,262,263
(4) Careful selection of electrolyte solution is also an option to greatly mitigate deleterious side reactions between electrolyte solution and cathodes.42,358,359 Since the surface degradation arises from the existence of highly-reactive Ni4+, which is a dominant ionic species comprising the delithiated Ni-rich cathodes, the aforementioned methods, e.g., coating and doping, etc., does not solely overcome the intrinsic surface degradation issue of the Ni-rich cathode.139,142 One example of mitigating surface reactivity via modification of electrolyte is the use of fluoroethlyene carbonate (FEC). FEC is a well-known electrolyte additive to improve the performance of anodes (Si, MoS2, Li metal).360–363 However, recent studies suggested that use of FEC can significantly improve the long-term cycling performance of the nano-rod cathodes.58,78 FEC promotes formation of a stable LiF-rich layer on the cathode surface, thereby protecting cathode active material from side reactions.328,364,365 Additionally, various additives, such as Li3PO4, LiDFOB, and etc. those are compatible with Ni-rich cathodes are also suggested.366–369 Nevertheless, the feasibility of adopting new electrolyte solutions in nano-rod cathodes for practical batteries needs further investigation.370 Comprehensive studies regarding the formation mechanism of CEI, degree of TM dissolution, gas evolution extent, aging characteristics, and etc. should be verified before adoption in practical batteries.371 The use of advanced electrolytes that are stable at high potentials and long-term cycling will allow high-energy-density and long range batteries. Therefore, both the nano-rod cathode and advanced electrolyte should be considered in tandem for developing high-performance LIBs.
Next-generation batteries such as all-solid-state batteries (ASSBs), Li-metal batteries (LMBs), and Na-ion batteries (SIBs) are at the forefront of current research and development to address key energy storage, safety, and performance challenges. Among the critical components of these advanced battery systems, cathode materials with nano-rod structures have emerged as pivotal elements contributing to their advancement. For example, nano-rod cathode structures have been demonstrated to facilitate ion diffusion and suppress the mechanical failures induced by volume changes; these characteristics are crucial for achieving high energy densities and long cycle lives in ASSBs and LMBs.314,315,327,328 Similarly, nano-rod cathode materials exhibit superior mechanical stabilities, suppressing microcrack formation and improving the electrochemical activities of SIBs.346,347 Overall, it is clear that cathode materials possessing the nano-rod structure will play a pivotal role in advancing next-generation battery technologies, offering solutions to key challenges and paving the way for safer, more efficient, and longer-lasting energy storage systems (Fig. 31c).
Nano-rod-based cathode materials, therefore, hold immense promise in revolutionizing batteries across various applications, from LIBs to next-generation systems (ASSBs, LMBs, and SIBs). These cathodes exhibit remarkable physicochemical and electrochemical properties, enhancing the battery's performances in multiple aspects. Continued research and development are therefore crucial to unlocking the full potential of nano-rod cathode materials and accelerating the adoption of advanced battery technologies in various applications, from portable electronics to electric vehicles and grid-scale energy storage.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Tae-Yeon Yu, Hun Kim for help in discussions, and Jae-Ho Kim, Min-Soo Kim for preparation of parts of the figures. This work was supported by the Human Resources Development Program (No. 20214000000320) and ESS Big Data-Based O&M and Asset Management Technical Manpower Training (RS-2024-00398346) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), funded by the Ministry of Trade, Industry, and Energy of the Korean government.Notes and references
- A. Yoshino, Angew. Chem., Int. Ed., 2012, 51, 5798 Search PubMed.
- M. Li, J. Lu, Z. Chen and K. Amine, Adv. Mater., 2018, 30, 1800561 Search PubMed.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587 Search PubMed.
- J. B. Goodenough and K.-S. Park, J. Am. Chem. Soc., 2013, 135, 1167 Search PubMed.
- R. Schmuch, R. Wagner, G. Hörpel, T. Placke and M. Winter, Nat. Energy, 2018, 3, 267 Search PubMed.
- S.-T. Myung, F. Maglia, K.-J. Park, C. S. Yoon, P. Lamp, S.-J. Kim and Y.-K. Sun, ACS Energy Lett., 2016, 2, 196 Search PubMed.
- M. Wentker, M. Greenwood and J. Leker, Energies, 2019, 12, 504 Search PubMed.
- K. Mizushima, P. C. Jones, P. J. Wiseman and J. B. Goodenough, Mater. Res. Bull., 1980, 15, 783 Search PubMed.
- M. S. Whittingham, Chem. Rev., 2004, 104, 4271 Search PubMed.
- Q. Zhong, A. Bonakdarpour, M. Zhang, Y. Gao and J. R. Dahn, J. Electrochem. Soc., 1997, 144, 205 Search PubMed.
- Z. Lu, D. D. MacNeil and J. R. Dahn, Electrochem. Solid-State Lett., 2001, 4, A191 Search PubMed.
- J. R. Dahn, U. von Sacken and C. A. Michal, Solid State Ionics, 1990, 44, 87 Search PubMed.
- D. Aurbach, Z. Lu, A. Schechter, Y. Gofer, H. Gizbar, R. Turgeman, Y. Cohen, M. Moshkovich and E. Levi, Nature, 2000, 407, 724 Search PubMed.
- Z. Lu, D. D. MacNeil and J. R. Dahn, Electrochem. Solid-State Lett., 2001, 4, A200 Search PubMed.
- A. Nytén, A. Abouimrane, M. Armand, T. Gustafsson and J. O. Thomas, Electrochem. Commun., 2005, 7, 156 Search PubMed.
- R. Dominko, M. Bele, A. Kokalj, M. Gaberscek and J. Jamnik, J. Power Sources, 2007, 174, 457 Search PubMed.
- C. Delmas, J.-J. Braconnier, C. Fouassier and P. Hagenmuller, Solid State Ionics, 1981, 3–4, 165 Search PubMed.
- A. Manthiram, Nat. Commun., 2020, 11, 1550 Search PubMed.
- T. Ohzuku, A. Ueda, M. Nagayama, Y. Iwakoshi and H. Komori, Electrochim. Acta, 1993, 38, 1159–1167 Search PubMed.
- D. Andre, S.-J. Kim, P. Lamp, S. F. Lux, F. Maglia, O. Paschos and B. Stiaszny, J. Mater. Chem. A, 2015, 3, 6709 Search PubMed.
- W. Li, E. M. Erickson and A. Manthiram, Nat. Energy, 2020, 5, 26 Search PubMed.
- C. Daniel, D. Mohanty, J. Li and D. L. Wood, AIP Conf. Proc., 2014, 1597, 26 Search PubMed.
- P. Rozier and J. M. Tarascon, J. Electrochem. Soc., 2015, 162, A2490 Search PubMed.
- R. J. Clément, Z. Lun and G. Ceder, Energy Environ. Sci., 2020, 13, 345 Search PubMed.
- A. O. Kondrakov, H. Geßwein, K. Galdina, L. de Biasi, V. Meded, E. O. Filatova, G. Schumacher, W. Wenzel, P. Hartmann, T. Brezesinski and J. Janek, J. Phys. Chem. C, 2017, 121, 24381 Search PubMed.
- T. Liu, L. Yu, J. Liu, J. Lu, X. Bi, A. Dai, M. Li, M. Li, Z. Hu, L. Ma, D. Luo, J. Zheng, T. Wu, Y. Ren, J. Wen, F. Pan and K. Amine, Nat. Energy, 2021, 6, 277 Search PubMed.
- K. M. Shaju, G. V. Subba Rao and B. V. R. Chowdari, Electrochim. Acta, 2002, 48, 145 Search PubMed.
- M. H. Lee, Y. J. Kang, S. T. Myung and Y. K. Sun, Electrochim. Acta, 2004, 50, 939 Search PubMed.
- LG Energy Solution's Automotive Batteries for Commercial Vehicles, https://www.lgensol.com/assets/file/202312_LGES_Commercial_EV_Catalog_en.pdf, (accessed June 2024).
- LG Energy Solution expanding R&D for battery leadership, https://www.koreatimes.co.kr/www/tech/2024/05/129_306276.html, (accessed June 2024).
- H.-J. Noh, S. Youn, C. S. Yoon and Y.-K. Sun, J. Power Sources, 2013, 233, 121 Search PubMed.
- Y.-K. Sun, D.-J. Lee, Y. J. Lee, Z. Chen and S.-T. Myung, ACS Appl. Mater. Interfaces, 2013, 5, 11434 Search PubMed.
- J. Jiang, K. W. Eberman, L. J. Krause and J. R. Dahn, J. Electrochem. Soc., 2005, 152, A566 Search PubMed.
- W. Zhang, C. Yuan, J. Zhu, T. Jin, C. Shen and K. Xie, Adv. Energy Mater., 2023, 13, 2202993 Search PubMed.
- J. Kim, H. Lee, H. Cha, M. Yoon, M. Park and J. Cho, Adv. Energy Mater., 2018, 8, 1702028 Search PubMed.
- H.-H. Ryu, K.-J. Park, C. S. Yoon and Y.-K. Sun, Chem. Mater., 2018, 30, 1155 Search PubMed.
- R. Jung, M. Metzger, F. Maglia, C. Stinner and H. A. Gasteiger, J. Electrochem. Soc., 2017, 164, A1361 Search PubMed.
- H. Li, A. Liu, N. Zhang, Y. Wang, S. Yin, H. Wu and J. R. Dahn, Chem. Mater., 2019, 31, 7574 Search PubMed.
- G. W. Nam, N.-Y. Park, K.-J. Park, J. Yang, J. Liu, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2019, 4, 2995 Search PubMed.
- E. Trevisanello, R. Ruess, G. Conforto, F. H. Richter and J. Janek, Adv. Energy Mater., 2021, 11, 2003400 Search PubMed.
- H.-H. Ryu, B. Namkoong, J.-H. Kim, I. Belharouak, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2021, 6, 2726 Search PubMed.
- D. Aurbach, B. Markovsky, G. Salitra, E. Markevich, Y. Talyossef, M. Koltypin, L. Nazar, B. Ellis and D. Kovacheva, J. Power Sources, 2007, 165, 491 Search PubMed.
- B. L. D. Rinkel, J. P. Vivek, N. Garcia-Araez and C. P. Grey, Energy Environ. Sci., 2022, 15, 3416 Search PubMed.
- J. Li, L. E. Downie, L. Ma, W. Qiu and J. R. Dahn, J. Electrochem. Soc., 2015, 162, A1401 Search PubMed.
- EcoProBM, SK On and Ford to invest C$1.2B to build NCM cathode manufacturing facility in Bécancour, Québec, https://www.greencarcongress.com/2023/08/20230818-ford.html, (accessed June 2024).
- Y.-K. Sun, Z. Chen, H.-J. Noh, D.-J. Lee, H.-G. Jung, Y. Ren, S. Wang, C. S. Yoon, S.-T. Myung and K. Amine, Nat. Mater., 2012, 11, 942 Search PubMed.
- Y.-K. Sun, S.-T. Myung, B.-C. Park, J. Prakash, I. Belharouak and K. Amine, Nat. Mater., 2009, 8, 320 Search PubMed.
- H.-J. Noh, Z. Chen, C. S. Yoon, J. Lu, K. Amine and Y.-K. Sun, Chem. Mater., 2013, 25, 2109 Search PubMed.
- U.-H. Kim, H.-H. Ryu, J.-H. Kim, R. Mücke, P. Kaghazchi, C. S. Yoon and Y.-K. Sun, Adv. Energy Mater., 2019, 9, 1803902 Search PubMed.
- C. S. Yoon, K.-J. Park, U.-H. Kim, K. H. Kang, H.-H. Ryu and Y.-K. Sun, Chem. Mater., 2017, 29, 10436 Search PubMed.
- K.-J. Park, H.-G. Jung, L.-Y. Kuo, P. Kaghazchi, C. S. Yoon and Y.-K. Sun, Adv. Energy Mater., 2018, 8, 1801202 Search PubMed.
- H.-H. Ryu, N.-Y. Park, J. H. Seo, Y.-S. Yu, M. Sharma, R. Mücke, P. Kaghazchi, C. S. Yoon and Y.-K. Sun, Mater. Today, 2020, 36, 73 Search PubMed.
- U.-H. Kim, G.-T. Park, B.-K. Son, G. W. Nam, J. Liu, L.-Y. Kuo, P. Kaghazchi, C. S. Yoon and Y.-K. Sun, Nat. Energy, 2020, 5, 860 Search PubMed.
- H.-H. Ryu, N.-Y. Park, D. R. Yoon, U.-H. Kim, C. S. Yoon and Y.-K. Sun, Adv. Energy Mater., 2020, 10, 2000495 Search PubMed.
- P. Ju, L. Ben, Y. Li, H. Yu, W. Zhao, Y. Chen, Y. Zhu and X. Huang, ACS Energy Lett., 2023, 8, 3800 Search PubMed.
- J.-H. Shim, Y.-H. Kim, H.-S. Yoon, H.-A. Kim, J.-S. Kim, J. Kim, N.-H. Cho, Y.-M. Kim and S. Lee, ACS Appl. Mater. Interfaces, 2019, 11, 4017 Search PubMed.
- H. H. Sun, U.-H. Kim, J.-H. Park, S.-W. Park, D.-H. Seo, A. Heller, C. B. Mullins, C. S. Yoon and Y.-K. Sun, Nat. Commun., 2021, 12, 6552 Search PubMed.
- G.-T. Park, S.-B. Kim, B. Namkoong, N.-Y. Park, H. Kim, C. S. Yoon and Y.-K. Sun, Mater. Today, 2023, 71, 38 Search PubMed.
- M. Dalkilic, A. Schmidt, T. D. Schladt, P. Axmann, M. Wohlfahrt-Mehrens and M. Lindén, J. Electrochem. Soc., 2022, 169, 120501 Search PubMed.
- C. T. Rueden, J. Schindelin, M. C. Hiner, B. E. DeZonia, A. E. Walter, E. T. Arena and K. W. Eliceiri, BMC Bioinf., 2017, 18, 529 Search PubMed.
- T. R. Jones, I. H. Kang, D. B. Wheeler, R. A. Lindquist, A. Papallo, D. M. Sabatini, P. Golland and A. E. Carpenter, BMC Bioinf., 2008, 9, 482 Search PubMed.
- J. M. Sosa, D. E. Huber, B. Welk and H. L. Fraser, Integr. Mater. Manuf. Innov., 2014, 3, 123 Search PubMed.
- D. Hou, Z. Xu, Z. Yang, C. Kuai, Z. Du, C.-J. Sun, Y. Ren, J. Liu, X. Xiao and F. Lin, Nat. Commun., 2022, 13, 3437 Search PubMed.
- W. Li, J. Zhang, Y. Zhou, W. Huang, X. Liu, Z. Li, M. Gao, Z. Chang, N. Li, J. Wang, S. Lu, X. Li, W. Wen, D. Zhu, Y. Lu and W. Zhuang, ACS Appl. Mater. Interfaces, 2020, 12, 47513 Search PubMed.
- C. Zhu, M. Cao, H. Zhang, G. Lv, J. Zhang, Y. Meng, C. Shu, W. Fan, M. Zuo, W. Xiang and X. Guo, ACS Appl. Mater. Interfaces, 2021, 13, 48720 Search PubMed.
- N.-Y. Park, H.-H. Ryu, G.-T. Park, T.-C. Noh and Y.-K. Sun, Adv. Energy Mater., 2021, 11, 2003767 Search PubMed.
- W. Huang, W. Li, L. Wang, H. Zhu, M. Gao, H. Zhao, J. Zhao, X. Shen, X. Wang, Z. Wang, C. Qi, W. Xiao, L. Yao, J. Wang, W. Zhuang and X. Sun, Small, 2021, 17, 2104282 Search PubMed.
- G.-T. Park, N.-Y. Park, T.-C. Noh, B. Namkoong, H.-H. Ryu, J.-Y. Shin, T. Beierling, C. S. Yoon and Y.-K. Sun, Energy Environ. Sci., 2021, 14, 5084 Search PubMed.
- C.-H. Jung, D.-H. Kim, D. Eum, K.-H. Kim, J. Choi, J. Lee, H.-H. Kim, K. Kang and S.-H. Hong, Adv. Funct. Mater., 2021, 31, 2010095 Search PubMed.
- W. Yang, H. Li, D. Wang, C. Xu, W. Xiang, Y. Song, F. He, J. Zhang, B. Zheng, B. Zhong, Z. Wu and X. Guo, Nano Energy, 2022, 104, 107880 Search PubMed.
- L. Wang, B. Zhu, D. Xiao, X. Zhang, B. Wang, H. Li, T. Wu, S. Liu and H. Yu, Adv. Funct. Mater., 2023, 33, 2212849 Search PubMed.
- G.-T. Park, S.-B. Kim, J.-I. Yoon, N.-Y. Park, M.-C. Kim, S.-M. Han, D.-H. Kim, M.-S. Kim and Y.-K. Sun, Adv. Energy Mater., 2024, 14, 2400130 Search PubMed.
- R. Wang, J. Chen, Y. Zhang, Z. Li, S. Cao, X. Liu, H. Hu, L. Wu, Y. Shen and X. Wang, Chem. Eng. J., 2024, 485, 149827 Search PubMed.
- T. Thien Nguyen, U.-H. Kim, C. S. Yoon and Y.-K. Sun, Chem. Eng. J., 2021, 405, 126887 Search PubMed.
- H. H. Sun, A. Dolocan, J. A. Weeks, A. Heller and C. B. Mullins, ACS Nano, 2020, 14, 17142 Search PubMed.
- U.-H. Kim, J.-H. Park, A. Aishova, R. M. Ribas, R. S. Monteiro, K. J. Griffith, C. S. Yoon and Y.-K. Sun, Adv. Energy Mater., 2021, 11, 2100884 Search PubMed.
- C. S. Yoon, M.-J. Choi, D.-W. Jun, Q. Zhang, P. Kaghazchi, K.-H. Kim and Y.-K. Sun, Chem. Mater., 2018, 30, 1808 Search PubMed.
- G.-T. Park, B. Namkoong, S.-B. Kim, J. Liu, C. S. Yoon and Y.-K. Sun, Nat. Energy, 2022, 7, 946 Search PubMed.
- G.-T. Park, D. R. Yoon, U.-H. Kim, B. Namkoong, J. Lee, M. M. Wang, A. C. Lee, X. W. Gu, W. C. Chueh, C. S. Yoon and Y.-K. Sun, Energy Environ. Sci., 2021, 14, 6616 Search PubMed.
- H.-H. Ryu, H.-W. Lim, S. G. Lee and Y.-K. Sun, Energy Storage Mater., 2023, 59, 102771 Search PubMed.
- D. Goonetilleke, A. Mazilkin, D. Weber, Y. Ma, F. Fauth, J. Janek, T. Brezesinski and M. Bianchini, J. Mater. Chem. A, 2022, 10, 7841 Search PubMed.
- X. Li, W. Ge, K. Zhang, G. Peng, Y. Fu and X. Ma, Electrochim. Acta, 2022, 403, 139653 Search PubMed.
- Y. Kim, H. Kim, W. Shin, E. Jo and A. Manthiram, Adv. Energy Mater., 2023, 13, 2204054 Search PubMed.
- L. Yao, Y. Li, X. Gao, M. Cai, J. Jin, J. Yang, T. Xiu, Z. Song, M. E. Badding and Z. Wen, Energy Storage Mater., 2021, 36, 179 Search PubMed.
- J. Wang, C. Liu, G. Xu, C. Miao, M. Wen, M. Xu, C. Wang and W. Xiao, Chem. Eng. J., 2022, 438, 135537 Search PubMed.
- S. Hashigami, Y. Kato, K. Yoshimi, A. Fukumoto, Z. Cao, H. Yoshida, T. Inagaki, M. Hashinokuchi, M. Haruta, T. Doi and M. Inaba, ACS Appl. Mater. Interfaces, 2019, 11, 39910 Search PubMed.
- Y. Mo, L. Guo, H. Jin, B. Du, B. Cao, Y. Chen, D. Li and Y. Chen, J. Power Sources, 2020, 448, 227439 Search PubMed.
- L. Li, L. Fu, M. Li, C. Wang, Z. Zhao, S. Xie, H. Lin, X. Wu, H. Liu, L. Zhang, Q. Zhang and L. Tan, J. Energy Chem., 2022, 71, 588 Search PubMed.
- S. F. Amalraj, R. Raman, A. Chakraborty, N. Leifer, R. Nanda, S. Kunnikuruvan, T. Kravchuk, J. Grinblat, V. Ezersky, R. Sun, F. L. Deepak, C. Erk, X. Wu, S. Maiti, H. Sclar, G. Goobes, D. T. Major, M. Talianker, B. Markovsky and D. Aurbach, Energy Storage Mater., 2021, 42, 594 Search PubMed.
- L. Cai, Q. Han, H. Zhu, H. Yu, Y. Hu and H. Jiang, J. Mater. Chem. A, 2023, 11, 8352 Search PubMed.
- Y. B. Hendri, L.-Y. Kuo, M. Seenivasan, Y.-S. Wu, S.-H. Wu, J.-K. Chang, R. Jose, M. Ihrig, P. Kaghazchi and C.-C. Yang, J. Colloid Interface Sci., 2024, 661, 289 Search PubMed.
- J. Wang, Z. Yi, C. Liu, M. He, C. Miao, J. Li, G. Xu and W. Xiao, J. Colloid Interface Sci., 2023, 635, 295 Search PubMed.
- J. Cheng, L. Mu, C. Wang, Z. Yang, H. L. Xin, F. Lin and K. A. Persson, J. Mater. Chem. A, 2020, 8, 23293 Search PubMed.
- Y. Lv, S. Huang, J. Zhang, G. Kang, Y. Liu, N. Li, Y. Liang, X. Zhong, T. Jia, Y. Ouyang, P. Qin, F. Kang, J. Zhang and Y. Cao, Adv. Funct. Mater., 2024, 34, 2312284 Search PubMed.
- Z.-C. Liu, S. Hao and X.-P. Gao, ACS Appl. Mater. Interfaces, 2023, 15, 52552 Search PubMed.
- K. Huang, X. Mu, Y. Ding, J. Li, M. Sui and P. Yan, ACS Appl. Energy Mater., 2024, 7, 4856–4865 Search PubMed.
- U.-H. Kim, N.-Y. Park, G.-T. Park, H. Kim, C. S. Yoon and Y.-K. Sun, Energy Storage Mater., 2020, 33, 399 Search PubMed.
- G.-T. Park, H.-H. Ryu, T.-C. Noh, G.-C. Kang and Y.-K. Sun, Mater. Today, 2022, 52, 9 Search PubMed.
- H.-H. Ryu, N.-Y. Park, T.-C. Noh, G.-C. Kang, F. Maglia, S.-J. Kim, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2021, 6, 216 Search PubMed.
- I. A. Skvortsova, E. D. Orlova, A. O. Boev, D. A. Aksyonov, I. Moiseev, E. M. Pazhetnov, A. A. Savina and A. M. Abakumov, J. Power Sources, 2023, 583, 233571 Search PubMed.
- L. Romano Brandt, J.-J. Marie, T. Moxham, D. P. Förstermann, E. Salvati, C. Besnard, C. Papadaki, Z. Wang, P. G. Bruce and A. M. Korsunsky, Energy Environ. Sci., 2020, 13, 3556 Search PubMed.
- T. Chen, C. Wen, C. Wu, L. Qiu, Z. Wu, J. Li, Y. Zhu, H. Li, Q. Kong, Y. Song, F. Wan, M. Chen, I. Saadoune, B. Zhong, S. Dou, Y. Xiao and X. Guo, Chem. Sci., 2023, 14, 13924 Search PubMed.
- D. Ren, E. Padgett, Y. Yang, L. Shen, Y. Shen, B. D. A. Levin, Y. Yu, F. J. DiSalvo, D. A. Muller and H. D. Abruña, ACS Appl. Mater. Interfaces, 2019, 11, 41178 Search PubMed.
- J. M. Dake, J. Oddershede, H. O. Sørensen, T. Werz, J. C. Shatto, K. Uesugi, S. Schmidt and C. E. Krill, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E5998 Search PubMed.
- H. Sharma, R. M. Huizenga, A. Bytchkov, J. Sietsma and S. E. Offerman, Acta Mater., 2012, 60, 229 Search PubMed.
- U.-H. Kim, S.-B. Lee, J.-H. Ryu, C. S. Yoon and Y.-K. Sun, J. Power Sources, 2023, 564, 232850 Search PubMed.
- X. He, S. Su, B. Zhang, Z. Xiao, Z. Zhang and X. Ou, J. Energy Chem., 2024, 88, 213 Search PubMed.
- X. Wang, C. Zhu, L. Ren, S. Wang, M. Zuo, W. Xing, W. Fan, B. Zhang, Z. Zheng, C. Shu and W. Xiang, Energy Technol., 2023, 11, 2300133 Search PubMed.
- Z. Wang, W. Wei, Q. Han, H. Zhu, L. Chen, Y. Hu, H. Jiang and C. Li, ACS Nano, 2023, 17, 17095 Search PubMed.
- J. E. Burke and D. Turnbull, Prog. Met. Phys., 1952, 3, 220 Search PubMed.
- J. E. Burke, J. Am. Chem. Soc., 1957, 40, 80 Search PubMed.
- S. L. Dai, J. P. Delplanque and E. J. Lavernia, J. Mater. Res., 1999, 14, 2814 Search PubMed.
- S. H. Brodhag and M. Herwegh, Contrib. Mineral. Petrol., 2010, 160, 219 Search PubMed.
- Y. Lv, S. Huang, S. Lu, T. Jia, Y. Liu, W. Ding, X. Yu, F. Kang, J. Zhang and Y. Cao, Chem. Eng. J., 2023, 455, 140652 Search PubMed.
- T.-P. Gao, K. W. Wong and K. M. Ng, Nano Energy, 2020, 73, 104798 Search PubMed.
- Y. S. Kim, J. H. Kim, Y.-K. Sun and C. S. Yoon, ACS Appl. Mater. Interfaces, 2022, 14, 17500 Search PubMed.
- F. Fu, G.-L. Xu, Q. Wang, Y.-P. Deng, X. Li, J.-T. Li, L. Huang and S.-G. Sun, J. Mater. Chem. A, 2013, 1, 3860 Search PubMed.
- A. O. Kondrakov, A. Schmidt, J. Xu, H. Geßwein, R. Mönig, P. Hartmann, H. Sommer, T. Brezesinski and J. Janek, J. Phys. Chem. C, 2017, 121, 3286 Search PubMed.
- N.-Y. Park, G.-T. Park, S.-B. Kim, W. Jung, B.-C. Park and Y.-K. Sun, ACS Energy Lett., 2022, 7, 2362 Search PubMed.
- Y.-K. Hou, C. Li, D. Ren, F. He, K. Zhuang, S. Yin, G. Yuan, Y. Wang, Y. Guo, S. Liu, P. Sun, Z. Zhang, T. Tan, G. Zhu, L. Lu, X. Liu and M. Ouyang, Adv. Sci., 2023, 10, 2306347 Search PubMed.
- Z. Gao, C. Zhao, K. Zhou, J. Wu, Y. Tian, X. Deng, L. Zhang, K. Lin, F. Kang, L. Peng, M. Wagemaker and B. Li, Nat. Commun., 2024, 15, 1503 Search PubMed.
- Z. Xu, Z. Jiang, C. Kuai, R. Xu, C. Qin, Y. Zhang, M. M. Rahman, C. Wei, D. Nordlund, C.-J. Sun, X. Xiao, X.-W. Du, K. Zhao, P. Yan, Y. Liu and F. Lin, Nat. Commun., 2020, 11, 83 Search PubMed.
- S. Daubner, M. Weichel, P. W. Hoffrogge, D. Schneider and B. Nestler, Batteries, 2023, 9, 310 Search PubMed.
- B.-B. Lim, S.-T. Myung, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2016, 1, 283 Search PubMed.
- J. Hu, L. Li, E. Hu, S. Chae, H. Jia, T. Liu, B. Wu, Y. Bi, K. Amine, C. Wang, J. Zhang, J. Tao and J. Xiao, Nano Energy, 2021, 79, 105420 Search PubMed.
- J. Kim, I. Lee, Y.-H. Kim, J. Ho Bae, K. Hwang, H. Kang, J.-H. Shim, J.-S. Kim, C. Wan Park, Y.-M. Kim and S. Yoon, Chem. Eng. J., 2023, 455, 140578 Search PubMed.
- H.-H. Sun, J. A. Weeks, A. Heller and C. B. Mullins, ACS Appl. Energy Mater., 2019, 2, 6002 Search PubMed.
- F. Tian, L. Ben, H. Yu, H. Ji, W. Zhao, Z. liu, R. Monteiro, R. M. Ribas, Y. Zhu and X. Huang, Nano Energy, 2022, 98, 107222 Search PubMed.
- H.-H. Ryu, K.-J. Park, D. R. Yoon, A. Aishova, C. S. Yoon and Y.-K. Sun, Adv. Energy Mater., 2019, 9, 1902698 Search PubMed.
- X. Xu, H. Huo, J. Jian, L. Wang, H. Zhu, S. Xu, X. He, G. Yin, C. Du and X. Sun, Adv. Energy Mater., 2019, 9, 1803963 Search PubMed.
- S. Xia, L. Mu, Z. Xu, J. Wang, C. Wei, L. Liu, P. Pianetta, K. Zhao, X. Yu, F. Lin and Y. Liu, Nano Energy, 2018, 53, 753 Search PubMed.
- G.-T. Park, H. H. Sun, T.-C. Noh, F. Maglia, S.-J. Kim, P. Lamp and Y.-K. Sun, Adv. Energy Mater., 2022, 12, 2202719 Search PubMed.
- C. Tian, Y. Xu, D. Nordlund, F. Lin, J. Liu, Z. Sun, Y. Liu and M. Doeff, Joule, 2018, 2, 464 Search PubMed.
- J. Li, J. Huang, H. Li, X. Kong, X. Li and J. Zhao, ACS Appl. Mater. Interfaces, 2021, 13, 27074 Search PubMed.
- L. Mu, J. Zhang, Y. Xu, C. Wei, M. M. Rahman, D. Nordlund, Y. Liu and F. Lin, Nano Lett., 2022, 22, 1278 Search PubMed.
- H. Liu, M. Wolfman, K. Karki, Y.-S. Yu, E. A. Stach, J. Cabana, K. W. Chapman and P. J. Chupas, Nano Lett., 2017, 17, 3452 Search PubMed.
- D.-S. Ko, J.-H. Park, S. Park, Y. N. Ham, S. J. Ahn, J.-H. Park, H. N. Han, E. Lee, W. S. Jeon and C. Jung, Nano Energy, 2019, 56, 434 Search PubMed.
- U. H. Kim, D. W. Jun, K. J. Park, Q. Zhang, P. Kaghazchi, D. Aurbach, D. T. Major, G. Goobes, M. Dixit, N. Leifer, C. M. Wang, P. Yan, D. Ahn, K. H. Kim, C. S. Yoon and Y. K. Sun, Energy Environ. Sci., 2018, 11, 1271 Search PubMed.
- S. Watanabe, M. Kinoshita, T. Hosokawa, K. Morigaki and K. Nakura, J. Power Sources, 2014, 258, 210 Search PubMed.
- S. S. Zhang, Energy Storage Mater., 2020, 24, 247 Search PubMed.
- M. Jiang, D. L. Danilov, R.-A. Eichel and P. H. L. Notten, Adv. Energy Mater., 2021, 11, 2103005 Search PubMed.
- S.-K. Jung, H. Gwon, J. Hong, K.-Y. Park, D.-H. Seo, H. Kim, J. Hyun, W. Yang and K. Kang, Adv. Energy Mater., 2014, 4, 1300787 Search PubMed.
- C. Xu, K. Märker, J. Lee, A. Mahadevegowda, P. J. Reeves, S. J. Day, M. F. Groh, S. P. Emge, C. Ducati, B. Layla Mehdi, C. C. Tang and C. P. Grey, Nat. Mater., 2021, 20, 84 Search PubMed.
- P. Yan, J. Zheng, Z.-K. Tang, A. Devaraj, G. Chen, K. Amine, J.-G. Zhang, L.-M. Liu and C. Wang, Nat. Nanotechnol., 2019, 14, 602 Search PubMed.
- P. Yan, J. Zheng, T. Chen, L. Luo, Y. Jiang, K. Wang, M. Sui, J.-G. Zhang, S. Zhang and C. Wang, Nat. Commun., 2018, 9, 2437 Search PubMed.
- G.-T. Park, H.-H. Ryu, N.-Y. Park, C. S. Yoon and Y.-K. Sun, J. Power Sources, 2019, 442, 227242 Search PubMed.
- Z. Guo, X. Shi, L. Cao, J. Zhang, X. Zhang, J. Yao, Y.-J. Cheng and Y. Xia, ACS Appl. Mater. Interfaces, 2024, 16, 731 Search PubMed.
- K. Chen, P. Barai, O. Kahvecioglu, L. Wu, K. Z. Pupek, M. Ge, L. Ma, S. N. Ehrlich, H. Zhong, Y. Zhu, V. Srinivasan, J. Bai and F. Wang, Nat. Commun., 2024, 15, 430 Search PubMed.
- P. Kurzhals, F. Riewald, M. Bianchini, H. Sommer, H. A. Gasteiger and J. Janek, J. Electrochem. Soc., 2021, 168, 110518 Search PubMed.
- L. Yu, H. Zhao, J. Sun, Q. Han, J. Zhu and J. Lu, Adv. Funct. Mater., 2022, 32, 2204931 Search PubMed.
- U.-H. Kim, J.-H. Kim, J.-Y. Hwang, H.-H. Ryu, C. S. Yoon and Y.-K. Sun, Mater. Today, 2019, 23, 26 Search PubMed.
- F. Fan, R. Zheng, T. Zeng, H. Xu, X. Wen, X. Wang, G. Tian, S. Wang, C. Zeng, W. Xiang and C. Shu, Chem. Eng. J., 2023, 477, 147181 Search PubMed.
- N.-Y. Park, G. Cho, S.-B. Kim and Y.-K. Sun, Adv. Energy Mater., 2023, 13, 2204291 Search PubMed.
- U.-H. Kim, G.-T. Park, P. Conlin, N. Ashburn, K. Cho, Y.-S. Yu, D. A. Shapiro, F. Maglia, S.-J. Kim, P. Lamp, C. S. Yoon and Y.-K. Sun, Energy Environ. Sci., 2021, 14, 1573 Search PubMed.
- X. Mu, X. Hui, M. Wang, K. Wang, Y. Li, Y. Zhang, M. Sui and P. Yan, ACS Appl. Energy Mater., 2023, 6, 5018 Search PubMed.
- J. Oh, S.-Y. Lee, H. Kim, J. Ryu, B. Gil, J. Lee and M. Kim, Adv. Sci., 2022, 9, 2203639 Search PubMed.
- Q. Lin, W. Guan, J. Zhou, J. Meng, W. Huang, T. Chen, Q. Gao, X. Wei, Y. Zeng, J. Li and Z. Zhang, Nano Energy, 2020, 76, 105021 Search PubMed.
- Y. Jiang, P. Yan, M. Yu, J. Li, H. Jiao, B. Zhou and M. Sui, Nano Energy, 2020, 78, 105364 Search PubMed.
- H. Chung, Y. Li, M. Zhang, A. Grenier, C. Mejia, D. Cheng, B. Sayahpour, C. Song, M. H. Shen, R. Huang, E. A. Wu, K. W. Chapman, S. J. Kim and Y. S. Meng, Chem. Mater., 2022, 34, 7302 Search PubMed.
- C. S. Yoon, S. J. Kim, U.-H. Kim, K.-J. Park, H.-H. Ryu, H.-S. Kim and Y.-K. Sun, Adv. Funct. Mater., 2018, 28, 1802090 Search PubMed.
- M. Legros, G. Dehm, E. Arzt and T. J. Balk, Science, 2008, 319, 1646 Search PubMed.
- L. de Biasi, B. Schwarz, T. Brezesinski, P. Hartmann, J. Janek and H. Ehrenberg, Adv. Mater., 2019, 31, 1900985 Search PubMed.
- L. Liang, W. Zhang, F. Zhao, D. K. Denis, F. U. Zaman, L. Hou and C. Yuan, Adv. Mater. Interfaces, 2020, 7, 1901749 Search PubMed.
- C. Yang, R. Shao, Q. Wang, T. Zhou, J. Lu, N. Jiang, P. Gao, W. Liu, Y. Yu and H. Zhou, Energy Storage Mater., 2021, 35, 62 Search PubMed.
- S. Y. Park, W. J. Baek, S. Y. Lee, J. A. Seo, Y.-S. Kang, M. Koh and S. H. Kim, Nano Energy, 2018, 49, 1 Search PubMed.
- S. E. Renfrew and B. D. McCloskey, J. Am. Chem. Soc., 2017, 139, 17853 Search PubMed.
- A. T. S. Freiberg, J. Sicklinger, S. Solchenbach and H. A. Gasteiger, Electrochim. Acta, 2020, 346, 136271 Search PubMed.
- W. M. Dose, I. Temprano, J. P. Allen, E. Björklund, C. A. O’Keefe, W. Li, B. L. Mehdi, R. S. Weatherup, M. F. L. De Volder and C. P. Grey, ACS Appl. Mater. Interfaces, 2022, 14, 13206 Search PubMed.
- J. Hu, L. Li, Y. Bi, J. Tao, J. Lochala, D. Liu, B. Wu, X. Cao, S. Chae, C. Wang and J. Xiao, Energy Storage Mater., 2022, 47, 195 Search PubMed.
- L. Ni, H. Chen, J. Gao, Y. Mei, H. Wang, W. Deng, G. Zou, H. Hou and X. Ji, ACS Nano, 2023, 17, 12759 Search PubMed.
- J. Kim, H. Ma, H. Cha, H. Lee, J. Sung, M. Seo, P. Oh, M. Park and J. Cho, Energy Environ. Sci., 2018, 11, 1449 Search PubMed.
- H.-H. Ryu, H.-W. Lim, G.-C. Kang, N.-Y. Park and Y.-K. Sun, ACS Energy Lett., 2023, 8, 1354 Search PubMed.
- B. Namkoong, N.-Y. Park, G.-T. Park, J.-Y. Shin, T. Beierling, C. S. Yoon and Y.-K. Sun, Adv. Energy Mater., 2022, 12, 2200615 Search PubMed.
- C. F. Lopez, J. A. Jeevarajan and P. P. Mukherjee, J. Electrochem. Soc., 2015, 162, A1905 Search PubMed.
- P. Lyu, X. Liu, J. Qu, J. Zhao, Y. Huo, Z. Qu and Z. Rao, Energy Storage Mater., 2020, 31, 195 Search PubMed.
- S. Mallick and D. Gayen, J. Energy Storage, 2023, 62, 106894 Search PubMed.
- P. T. Coman, E. C. Darcy, C. T. Veje and R. E. White, Appl. Energy, 2017, 203, 189 Search PubMed.
- Y. Li, X. Liu, L. Wang, X. Feng, D. Ren, Y. Wu, G. Xu, L. Lu, J. Hou, W. Zhang, Y. Wang, W. Xu, Y. Ren, Z. Wang, J. Huang, X. Meng, X. Han, H. Wang, X. He, Z. Chen, K. Amine and M. Ouyang, Nano Energy, 2021, 85, 105878 Search PubMed.
- P. V. Chombo and Y. Laoonual, J. Power Sources, 2020, 478, 228649 Search PubMed.
- F. Kong, C. Liang, L. Wang, Y. Zheng, S. Perananthan, R. C. Longo, J. P. Ferraris, M. Kim and K. Cho, Adv. Energy Mater., 2019, 9, 1802586 Search PubMed.
- S. Sharifi-Asl, J. Lu, K. Amine and R. Shahbazian-Yassar, Adv. Energy Mater., 2019, 9, 1900551 Search PubMed.
- S. Yoon, H. G. Park, S. Koo, J. Hwang, Y. Lee, K. Park and D. Kim, J. Alloys Compd., 2023, 939, 168531 Search PubMed.
- P. Hou, F. Li, Y. Sun, H. Li, X. Xu and T. Zhai, ACS Appl. Mater. Interfaces, 2018, 10, 24508 Search PubMed.
- D. Song, P. Hou, X. Wang, X. Shi and L. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 12864 Search PubMed.
- P. Y. Hou, L. Q. Zhang and X. P. Gao, J. Mater. Chem. A, 2014, 2, 17130 Search PubMed.
- J.-Y. Liao and A. Manthiram, J. Power Sources, 2015, 282, 429 Search PubMed.
- J.-L. Shi, R. Qi, X.-D. Zhang, P.-F. Wang, W.-G. Fu, Y.-X. Yin, J. Xu, L.-J. Wan and Y.-G. Guo, ACS Appl. Mater. Interfaces, 2017, 9, 42829 Search PubMed.
- S.-J. Yoon, S.-T. Myung, H.-J. Noh, J. Lu, K. Amine and Y.-K. Sun, ChemSusChem, 2014, 7, 3295 Search PubMed.
- U.-H. Kim, S.-B. Lee, N.-Y. Park, S. J. Kim, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2022, 7, 3880 Search PubMed.
- B. Wang, F. Cai, C. Chu, B. Fu, K. Świerczek, L. Li and H. Zhao, ACS Appl. Mater. Interfaces, 2024, 16, 12599 Search PubMed.
- A. Schilling, J. Schmitt, F. Dietrich and K. Dröder, Energy Technol., 2016, 4, 1502 Search PubMed.
- H.-Y. Tran, A. Lindner, W. Menesklou and W. Braunwarth, Energy Technol., 2023, 11, 2201092 Search PubMed.
- J. C. Stallard, L. Wheatcroft, S. G. Booth, R. Boston, S. A. Corr, M. F. L. De Volder, B. J. Inkson and N. A. Fleck, Joule, 2022, 6, 984 Search PubMed.
- M. Müller, L. Schneider, N. Bohn, J. R. Binder and W. Bauer, ACS Appl. Energy Mater., 2021, 4, 1993 Search PubMed.
- R. Xu, H. Sun, L. S. de Vasconcelos and K. Zhao, J. Electrochem. Soc., 2017, 164, A3333 Search PubMed.
- H.-R. Kim, S.-G. Woo, J.-H. Kim, W. Cho and Y.-J. Kim, J. Electroanal. Chem., 2016, 782, 168 Search PubMed.
- J. Moon, J. Y. Jung, T. D. Hoang, D. Y. Rhee, H. B. Lee, M.-S. Park and J.-S. Yu, J. Power Sources, 2021, 486, 229359 Search PubMed.
- S. Maeng, Y. Chung, S. Min and Y. Shin, J. Power Sources, 2020, 448, 227395 Search PubMed.
- Z. Huang, J. Yan, Z. Liu, W. Wang, Y. Tang, Z. Zhang, T. Yang, X. Wang, X. Li, Q. Kong, S. Lan, H. Zhu, Y. Ren and Q. Liu, Adv. Funct. Mater., 2024, 34, 2400956 Search PubMed.
- T. Karthik and S. Asthana, Mater. Lett., 2017, 190, 273 Search PubMed.
- J. Poetschke, V. Richter and R. Holke, Int. J. Refract. Met. Hard Mater., 2012, 31, 218 Search PubMed.
- I. A. Ovid’ko and A. G. Sheinerman, Acta Mater., 2004, 52, 1201 Search PubMed.
- I. A. Ovid'ko, Philos. Trans. R. Soc., A, 2015, 373, 20140129 Search PubMed.
- G.-T. Park, S.-M. Han, J.-H. Ryu, M.-C. Kim, D.-H. Kim, M.-S. Kim and Y.-K. Sun, ACS Energy Lett., 2023, 8, 3784 Search PubMed.
- S.-B. Lee, N.-Y. Park, G.-T. Park, U.-H. Kim, S.-J. Sohn, M.-S. Kang, R. M. Ribas, R. S. Monteiro and Y.-K. Sun, ACS Energy Lett., 2024, 9, 740 Search PubMed.
- S.-H. Lee, B.-S. Jin and H.-S. Kim, Sci. Rep., 2019, 9, 17541 Search PubMed.
- Z. Li, X. Huang, J. Liang, J. Qin, R. Wang, J. Cheng and D. Wang, J. Energy Chem., 2023, 77, 461 Search PubMed.
- X. Chen, X. Jia, Y. Qu, D. Li, D. Chen and Y. Chen, New J. Chem., 2018, 42, 5868 Search PubMed.
- L. Chang, Y. Lu, D. Lei, H. Liu, L. He, Y. Ni and Y. Li, J. Power Sources, 2021, 494, 229777 Search PubMed.
- W. Hua, Z. Wu, M. Chen, M. Knapp, X. Guo, S. Indris, J. R. Binder, N. N. Bramnik, B. Zhong, H. Guo, S. Chou, Y.-M. Kang and H. Ehrenberg, J. Mater. Chem. A, 2017, 5, 25391 Search PubMed.
- J. Zheng, P. Yan, L. Estevez, C. Wang and J.-G. Zhang, Nano Energy, 2018, 49, 538 Search PubMed.
- S. Yang, B. Yan, L. Lu and K. Zeng, RSC Adv., 2016, 6, 94000 Search PubMed.
- C. Schwab, A. Höweling, A. Windmüller, J. Gonzalez-Julian, S. Möller, J. R. Binder, S. Uhlenbruck, O. Guillon and M. Martin, Phys. Chem. Chem. Phys., 2019, 21, 26066 Search PubMed.
- Y. Liu, Y. Zhu and Y. Cui, Nat. Energy, 2019, 4, 540 Search PubMed.
- C. Mao, R. E. Ruther, J. Li, Z. Du and I. Belharouak, Electrochem. Commun., 2018, 97, 37 Search PubMed.
- M. Weiss, R. Ruess, J. Kasnatscheew, Y. Levartovsky, N. R. Levy, P. Minnmann, L. Stolz, T. Waldmann, M. Wohlfahrt-Mehrens, D. Aurbach, M. Winter, Y. Ein-Eli and J. Janek, Adv. Energy Mater., 2021, 11, 2101126 Search PubMed.
- Z. Yang, H. Charalambous, Y. Lin, S. E. Trask, L. Yu, J. Wen, A. Jansen, Y. Tsai, K. M. Wiaderek, Y. Ren and I. Bloom, J. Power Sources, 2022, 521, 230961 Search PubMed.
- L. Zou, W. Zhao, Z. Liu, H. Jia, J. Zheng, G. Wang, Y. Yang, J.-G. Zhang and C. Wang, ACS Energy Lett., 2018, 3, 2433 Search PubMed.
- J. Hu, B. Wu, X. Cao, Y. Bi, S. Chae, C. Niu, B. Xiao, J. Tao, J. Zhang and J. Xiao, J. Power Sources, 2020, 454, 227966 Search PubMed.
- S. Ma, X. Zhang, S. Wu, E. Fan, J. Lin, R. Chen, F. Wu and L. Li, Energy Storage Mater., 2023, 55, 556 Search PubMed.
- J.-M. Lim, T. Hwang, D. Kim, M.-S. Park, K. Cho and M. Cho, Sci. Rep., 2017, 7, 39669 Search PubMed.
- Y. Zhang, Z. Yang and C. Tian, J. Mater. Chem. A, 2019, 7, 23628 Search PubMed.
- Y. Yang, R. Xu, K. Zhang, S.-J. Lee, L. Mu, P. Liu, C. K. Waters, S. Spence, Z. Xu, C. Wei, D. J. Kautz, Q. Yuan, Y. Dong, Y.-S. Yu, X. Xiao, H.-K. Lee, P. Pianetta, P. Cloetens, J.-S. Lee, K. Zhao, F. Lin and Y. Liu, Adv. Energy Mater., 2019, 9, 1900674 Search PubMed.
- T. Liu, L. Yu, J. Lu, T. Zhou, X. Huang, Z. Cai, A. Dai, J. Gim, Y. Ren, X. Xiao, M. V. Holt, Y. S. Chu, I. Arslan, J. Wen and K. Amine, Nat. Commun., 2021, 12, 6024 Search PubMed.
- Y. Xia, A. Chen, K. Wang, Z. Xiao, Q. Mao, X. Lu, G. Wang, C. Lu, J. Zhang, H. Huang, Y. Gan, X. He, X. Xia and W. Zhang, Mater. Today Energy, 2023, 34, 101292 Search PubMed.
- X. Cheng, Y. Li, G. Zhao, W. Qiang and B. Huang, Ceram. Int., 2022, 48, 27849 Search PubMed.
- L. A. Sitnikova, A. A. Savina, A. V. Morozov, A. A. Golubnichiy, E. A. Dolzhikova, I. A. Moiseev, S. Y. Luchkin and A. M. Abakumov, J. Power Sources, 2024, 602, 234302 Search PubMed.
- Y. Mo, L. Guo, H. Jin, B. Du, B. Cao, Y. Chen, D. Li and Y. Chen, J. Power Sources, 2020, 468, 228405 Search PubMed.
- R. Yang, Z. Wang, L. Dai and L. Chen, Mater. Chem. Phys., 2005, 93, 149 Search PubMed.
- Z. Li, H. Bao, X. Miao and X. Chen, J. Colloid Interface Sci., 2011, 357, 286 Search PubMed.
- N.-Y. Park, H.-H. Ryu, L.-Y. Kuo, P. Kaghazchi, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2021, 6, 4195 Search PubMed.
- Y.-K. Sun, S.-T. Myung, M.-H. Kim, J. Prakash and K. Amine, J. Am. Chem. Soc., 2005, 127, 13411 Search PubMed.
- Y.-K. Sun, S.-T. Myung, H.-S. Shin, Y. C. Bae and C. S. Yoon, J. Phys. Chem. B, 2006, 110, 6810 Search PubMed.
- Y.-K. Sun, D.-H. Kim, C. S. Yoon, S.-T. Myung, J. Prakash and K. Amine, Adv. Funct. Mater., 2010, 20, 485 Search PubMed.
- L. de Biasi, A. O. Kondrakov, H. Geßwein, T. Brezesinski, P. Hartmann and J. Janek, J. Phys. Chem. C, 2017, 121, 26163 Search PubMed.
- W. Li, H. Y. Asl, Q. Xie and A. Manthiram, J. Am. Chem. Soc., 2019, 141, 5097 Search PubMed.
- B.-B. Lim, S.-J. Yoon, K.-J. Park, C. S. Yoon, S.-J. Kim, J. J. Lee and Y.-K. Sun, Adv. Funct. Mater., 2015, 25, 4673 Search PubMed.
- J. H. Lee, C. S. Yoon, J.-Y. Hwang, S.-J. Kim, F. Maglia, P. Lamp, S.-T. Myung and Y.-K. Sun, Energy Environ. Sci., 2016, 9, 2152 Search PubMed.
- H. Wu, X. Zhou, C. Yang, D. Xu, Y.-H. Zhu, T. Zhou, S. Xin and Y. You, ACS Appl. Mater. Interfaces, 2023, 15, 18828 Search PubMed.
- H.-H. Ryu, H.-W. Lim, S. G. Lee and Y.-K. Sun, Nat. Energy, 2024, 9, 47 Search PubMed.
- N.-Y. Park, S.-B. Kim, M.-C. Kim, S.-M. Han, D.-H. Kim, M.-S. Kim and Y.-K. Sun, Adv. Energy Mater., 2023, 13, 2301530 Search PubMed.
- C. Geng, D. Rathore, D. Heino, N. Zhang, I. Hamam, N. Zaker, G. A. Botton, R. Omessi, N. Phattharasupakun, T. Bond, C. Yang and J. R. Dahn, Adv. Energy Mater., 2021, 12, 2103067 Search PubMed.
- F. Lv, Y. Zhang, M. Wu and Y. Gu, Appl. Phys. Lett., 2021, 119, 023903 Search PubMed.
- S. Lee, H. W. Park, J. P. Hong, S.-C. Nam, D. H. Jeon and J.-H. Song, Solid State Ionics, 2022, 386, 116042 Search PubMed.
- X. Yang, Y. Tang, J. Zheng, G. Shang, J. Wu, Y. Lai, J. Li and Z. Zhang, Electrochim. Acta, 2019, 320, 134587 Search PubMed.
- C.-K. Yang, L.-Y. Qi, Z. Zuo, R.-N. Wang, M. Ye, J. Lu and H.-H. Zhou, J. Power Sources, 2016, 331, 487 Search PubMed.
- B. Du, Y. Mo, H. Jin, X. Li, Y. Qu, D. Li, B. Cao, X. Jia, Y. Lu and Y. Chen, ACS Appl. Energy Mater., 2020, 3, 6657 Search PubMed.
- D. Han, J. Zhang, M. Yang, K. Xie, J. Peng, O. Dolotko, C. Huang, Y. Wu, L. Shao, W. Hua and W. Tang, Energy Mater., 2024, 4, 400001 Search PubMed.
- B. Ying, J. R. Fitzpatrick, Z. Teng, T. Chen, T. W. B. Lo, V. Siozios, C. A. Murray, H. E. A. Brand, S. Day, C. C. Tang, R. S. Weatherup, M. Merz, P. Nagel, S. Schuppler, M. Winter and K. Kleiner, Chem. Mater., 2023, 35, 1514 Search PubMed.
- J. Guo and W. Li, ACS Appl. Energy Mater., 2022, 5, 397 Search PubMed.
- Z. Zhang, D. Chen and C. Chang, RSC Adv., 2017, 7, 51721 Search PubMed.
- D. Rathore, C. Geng, N. Zaker, I. Hamam, Y. Liu, P. Xiao, G. A. Botton, J. Dahn and C. Yang, J. Electrochem. Soc., 2021, 168, 120514 Search PubMed.
- B. Huang, L. Cheng, X. Li, Z. Zhao, J. Yang, Y. Li, Y. Pang and G. Cao, Small, 2022, 18, 2107697 Search PubMed.
- S. Lee, C. Li and A. Manthiram, Adv. Energy Mater., 2024, 14, 2400662 Search PubMed.
- D. Wang, C. Xin, M. Zhang, J. Bai, J. Zheng, R. Kou, J. Y. Peter Ko, A. Huq, G. Zhong, C.-J. Sun, Y. Yang, Z. Chen, Y. Xiao, K. Amine, F. Pan and F. Wang, Chem. Mater., 2019, 31, 2731 Search PubMed.
- M. J. Zhang, G. Teng, Y. K. Chen-Wiegart, Y. Duan, J. Y. P. Ko, J. Zheng, J. Thieme, E. Dooryhee, Z. Chen, J. Bai, K. Amine, F. Pan and F. Wang, J. Am. Chem. Soc., 2018, 140, 12484 Search PubMed.
- H.-H. Ryu, G.-T. Park, C. S. Yoon and Y.-K. Sun, J. Mater. Chem. A, 2019, 7, 18580 Search PubMed.
- M. Bianchini, M. Roca-Ayats, P. Hartmann, T. Brezesinski and J. Janek, Angew. Chem., Int. Ed., 2019, 58, 10434 Search PubMed.
- F. Xin, H. Zhou, Y. Zong, M. Zuba, Y. Chen, N. A. Chernova, J. Bai, B. Pei, A. Goel, J. Rana, F. Wang, K. An, L. F. J. Piper, G. Zhou and M. S. Whittingham, ACS Energy Lett., 2021, 6, 1377 Search PubMed.
- C. Liang, F. Kong, R. C. Longo, C. Zhang, Y. Nie, Y. Zheng and K. Cho, J. Mater. Chem. A, 2017, 5, 25303 Search PubMed.
- F. Xin, A. Goel, X. Chen, H. Zhou, J. Bai, S. Liu, F. Wang, G. Zhou and M. S. Whittingham, Chem. Mater., 2022, 34, 7858 Search PubMed.
- L. Mu, R. Zhang, W. H. Kan, Y. Zhang, L. Li, C. Kuai, B. Zydlewski, M. M. Rahman, C.-J. Sun, S. Sainio, M. Avdeev, D. Nordlund, H. L. Xin and F. Lin, Chem. Mater., 2019, 31, 9769 Search PubMed.
- Y. Zhang, J. Liu, W. Xu, Y. Lu, H. Ma, F. Cheng and J. Chen, J. Power Sources, 2022, 535, 231445 Search PubMed.
- Y. Y. Wang, M. Y. Gao, S. Liu, G. R. Li and X. P. Gao, ACS Appl. Mater. Interfaces, 2021, 13, 7343 Search PubMed.
- H.-H. Ryu, H. H. Sun, S.-T. Myung, C. S. Yoon and Y.-K. Sun, Energy Environ. Sci., 2021, 14, 844 Search PubMed.
- J. L. Matthew Li, Science, 2020, 367, 979 Search PubMed.
- H.-H. Ryu, G.-C. Kang, R. Ismoyojati, G.-T. Park, F. Maglia and Y.-K. Sun, Mater. Today, 2022, 56, 8–15 Search PubMed.
- F. Tian, Y. Zhang, Z. Liu, R. de Souza Monteiro, R. M. Ribas, P. Gao, Y. Zhu, H. Yu, L. Ben and X. Huang, Solid State Ionics, 2021, 359, 115520 Search PubMed.
- F. Du, Q. Zhou, H. Cao, H. Dai, D. Hu, P. Sun, J. Adkins and J. Zheng, J. Power Sources, 2020, 478, 228737 Search PubMed.
- K. Zou, M. Jiang, Z. Zhao, S. Xie, T. Ning, L. Tan, H. Li, Y. Zhou, W. Wang, X. Wu and L. Li, Chem. Eng. J., 2023, 476, 146793 Search PubMed.
- P. Yan, J. Zheng, J. Liu, B. Wang, X. Cheng, Y. Zhang, X. Sun, C. Wang and J.-G. Zhang, Nat. Energy, 2018, 3, 600 Search PubMed.
- G.-T. Park, S.-B. Kim, B. Namkoong, J.-H. Ryu, J.-I. Yoon, N.-Y. Park, M.-C. Kim, S.-M. Han, F. Maglia and Y.-K. Sun, Angew. Chem., Int. Ed., 2023, 62, e202314480 Search PubMed.
- J. G. Chen, B. D. De Vries, J. T. Lewandowski and R. B. Hall, Catal. Lett., 1994, 23, 25 Search PubMed.
- T. Nohma, H. Kurokawa, M. Uehara, M. Takahashi, K. Nishio and T. Saito, J. Power Sources, 1995, 54, 522 Search PubMed.
- R. Jung, R. Morasch, P. Karayaylali, K. Phillips, F. Maglia, C. Stinner, Y. Shao-Horn and H. A. Gasteiger, J. Electrochem. Soc., 2018, 165, A132 Search PubMed.
- H. S. Liu, Z. R. Zhang, Z. L. Gong and Y. Yang, Electrochem. Solid-State Lett., 2004, 7, A190 Search PubMed.
- Y. Bi, Q. Li, R. Yi and J. Xiao, J. Electrochem. Soc., 2022, 169, 020521 Search PubMed.
- W. M. Seong, K.-H. Cho, J.-W. Park, H. Park, D. Eum, M. H. Lee, I.-S. S. Kim, J. Lim and K. Kang, Angew. Chem., Int. Ed., 2020, 59, 18662 Search PubMed.
- Z. Fan, F. Du, Q. Zhou, D. Hu, P. Sun, C. Mei, Q. Hao, T. Xu and J. Zheng, J. Power Sources, 2022, 523, 231044 Search PubMed.
- N. V. Faenza, L. Bruce, Z. W. Lebens-Higgins, I. Plitz, N. Pereira, L. F. J. Piper and G. G. Amatucci, J. Electrochem. Soc., 2017, 164, A3727 Search PubMed.
- J. Kim, Y. Hong, K. S. Ryu, M. G. Kim and J. Cho, Electrochem. Solid-State Lett., 2006, 9, A19 Search PubMed.
- X. Xiong, Z. Wang, P. Yue, H. Guo, F. Wu, J. Wang and X. Li, J. Power Sources, 2013, 222, 318 Search PubMed.
- J.-H. Park, B. Choi, Y.-S. Kang, S. Y. Park, D. J. Yun, I. Park, J. H. Shim, J.-H. Park, H. N. Han and K. Park, Energy Technol., 2018, 6, 1361 Search PubMed.
- K. Park and B. Choi, J. Alloys Compd., 2018, 766, 470 Search PubMed.
- I. A. Shkrob, J. A. Gilbert, P. J. Phillips, R. Klie, R. T. Haasch, J. Bareño and D. P. Abraham, J. Electrochem. Soc., 2017, 164, A1489 Search PubMed.
- L. Hartmann, D. Pritzl, H. Beyer and H. A. Gasteiger, J. Electrochem. Soc., 2021, 168, 070507 Search PubMed.
- W. Lee, S. Lee, E. Lee, M. Choi, R. Thangavel, Y. Lee and W.-S. Yoon, Energy Storage Mater., 2022, 44, 441 Search PubMed.
- J. Pan, Y. Sun, P. Wan, Z. Wang and X. Liu, Electrochem. Commun., 2005, 7, 857 Search PubMed.
- Y. Gao, R. Jiang, Z. Dai, Z. Du, Y. Jin, G. Li and S. Hou, ACS Appl. Energy Mater., 2022, 5, 15069 Search PubMed.
- D. Weber, Đ. Tripković, K. Kretschmer, M. Bianchini and T. Brezesinski, Eur. J. Inorg. Chem., 2020, 3117 Search PubMed.
- W. Wang, L. Wu, Z. Li, K. Huang, J. Jiang, Z. Chen, X. Qi, H. Dou and X. Zhang, ACS Appl. Energy Mater., 2020, 3, 12423 Search PubMed.
- T. Sattar, S.-J. Sim, B.-S. Jin and H.-S. Kim, Sci. Rep., 2021, 11, 18590 Search PubMed.
- J. Park, Y. Kim, Y. Kim, J. Park, D. G. Lee, Y. Lee, J. Hwang, K.-Y. Park and D. Lee, Chem. Eng. J., 2023, 467, 143335 Search PubMed.
- J. Li, J. Xiang, G. Yi, Y. Tang, H. Shao, X. Liu, B. Shan and R. Chen, Coatings, 2022, 12, 84 Search PubMed.
- Y.-H. Du, H. Sheng, X.-H. Meng, X.-D. Zhang, Y.-G. Zou, J.-Y. Liang, M. Fan, F. Wang, J. Tang, F.-F. Cao, J.-L. Shi, X.-F. Cao and Y.-G. Guo, Nano Energy, 2022, 94, 106901 Search PubMed.
- Y. Xie, J. Li, M. Li, J. Cai, X. Huang, H. Nguyen, L. Yu, D. Huo, Z. Yang, N. Karami, B. A. S. Sulaiman, N. A. Chernova, S. Upreti, B. Prevel, Y. Liu, F. Wang and Z. Chen, Adv. Funct. Mater., 2024, 34, 2311551 Search PubMed.
- Y. Xie, M. Li, J. Li, X. Huang, J. Cai, Z. Yang, H. Nguyen, B. A. Shaik Sulaiman, N. Karami, N. A. Chernova, S. Upreti, B. Prevel, F. Wang and Z. Chen, Batteries, 2023, 9, 485 Search PubMed.
- South Korea's Battery Industry Speeding up the Process of Transitioning to the “Cobalt Zero” Era, https://english.etnews.com/20200828200001, (accessed July 2024).
- Kona EV Fires Underline Safety of SK Innovation's Batteries, https://www.businesskorea.co.kr/news/articleView.html?idxno=53563#google_vignette, (accessed July 2024).
- SK On to jointly invest in cathode material production facility with Ford and EcoPro BM, https://skinnonews.com/global/archives/10835, (accessed July 2024).
- ECOPROBM, SK ON, FORD INVESTING IN QUÉBEC; BUILDING CATHODE PLANT TO SOLIDIFY EV SUPPLY CHAIN IN NORTH AMERICA, https://media.ford.com/content/fordmedia/fna/us/en/news/2023/08/17/ecoprobm--sk-on--ford-investing-in-quebec--building-cathode-plan.html, (accessed July 2024).
- U.-H. Kim, E.-J. Lee, C. S. Yoon, S.-T. Myung and Y.-K. Sun, Adv. Energy Mater., 2016, 6, 1601417 Search PubMed.
- N.-Y. Park, M.-C. Kim, S.-M. Han, G.-T. Park, D.-H. Kim, M.-S. Kim and Y.-K. Sun, Angew. Chem., Int. Ed., 2024, 63, e202319707 Search PubMed.
- K.-J. Park, J.-Y. Hwang, H.-H. Ryu, F. Maglia, S.-J. Kim, P. Lamp, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2019, 4, 1394 Search PubMed.
- C. Wang, J. T. Kim, C. Wang and X. Sun, Adv. Mater., 2023, 35, e2209074 Search PubMed.
- Z. Zhang, W. Jia, Y. Feng, R. Ai, J. Yu, X. Bie, X. Zhai, T. Jiang, S. Yao and F. Du, Energy Environ. Sci., 2023, 16, 4453 Search PubMed.
- A. Banerjee, X. Wang, C. Fang, E. A. Wu and Y. S. Meng, Chem. Rev., 2020, 120, 6878 Search PubMed.
- T.-Y. Yu, H.-U. Lee, J. W. Lee, S.-M. Park, I.-S. Lee, H.-G. Jung and Y.-K. Sun, J. Mater. Chem. A, 2023, 11, 24629 Search PubMed.
- S. Schweidler, L. de Biasi, G. Garcia, A. Mazilkin, P. Hartmann, T. Brezesinski and J. Janek, ACS Appl. Energy Mater., 2019, 2, 7375 Search PubMed.
- Y. Zhu, X. He and Y. Mo, ACS Appl. Mater. Interfaces, 2015, 7, 23685 Search PubMed.
- Y. Nomura, K. Yamamoto, T. Hirayama, E. Igaki and K. Saitoh, ACS Energy Lett., 2020, 5, 2098 Search PubMed.
- J. Kim, W. Lee, J. Seok, E. Lee, W. Choi, H. Park, S. Yun, M. Kim, J. Lim and W.-S. Yoon, J. Energy Chem., 2022, 66, 226 Search PubMed.
- R. Koerver, W. Zhang, L. de Biasi, S. Schweidler, A. O. Kondrakov, S. Kolling, T. Brezesinski, P. Hartmann, W. G. Zeier and J. Janek, Energy Environ. Sci., 2018, 11, 2142 Search PubMed.
- S. H. Jung, U. H. Kim, J. H. Kim, S. Jun, C. S. Yoon, Y. S. Jung and Y. K. Sun, Adv. Energy Mater., 2019, 10, 1903360 Search PubMed.
- U.-H. Kim, T.-Y. Yu, J. W. Lee, H. U. Lee, I. Belharouak, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2023, 8, 809 Search PubMed.
- S. Kim, H. Cha, R. Kostecki and G. Chen, ACS Energy Lett., 2023, 8, 521 Search PubMed.
- X. Liu, B. Zheng, J. Zhao, W. Zhao, Z. Liang, Y. Su, C. Xie, K. Zhou, Y. Xiang, J. Zhu, H. Wang, G. Zhong, Z. Gong, J. Huang and Y. Yang, Adv. Energy Mater., 2021, 11, 2003583 Search PubMed.
- H. Zhu, Z. Wang, L. Chen, Y. Hu, H. Jiang and C. Li, Adv. Mater., 2023, 35, 2209357 Search PubMed.
- H. Kim, Y. Kong, W. M. Seong and A. Manthiram, ACS Appl. Mater. Interfaces, 2023, 15, 26585 Search PubMed.
- K. Yang, Y. Sun, Q. Su, Y. Lu, K. Liu, Z. Li, H. Liu and L. Zhang, Chem. Eng. J., 2023, 471, 144405 Search PubMed.
- C. Zhan, T. Wu, J. Lu and K. Amine, Energy Environ. Sci., 2018, 11, 243 Search PubMed.
- W. Li, U.-H. Kim, A. Dolocan, Y.-K. Sun and A. Manthiram, ACS Nano, 2017, 11, 5853 Search PubMed.
- W. Li, Y. Jie, Y. Chen, M. Yang, Y. Chen, X. Li, Y. Guo, X. Meng, R. Cao and S. Jiao, Nano Res., 2023, 16, 8417 Search PubMed.
- M. Evertz, F. Horsthemke, J. Kasnatscheew, M. Börner, M. Winter and S. Nowak, J. Power Sources, 2016, 329, 364 Search PubMed.
- W. Xue, M. Huang, Y. Li, Y. G. Zhu, R. Gao, X. Xiao, W. Zhang, S. Li, G. Xu, Y. Yu, P. Li, J. Lopez, D. Yu, Y. Dong, W. Fan, Z. Shi, R. Xiong, C.-J. Sun, I. Hwang, W.-K. Lee, Y. Shao-Horn, J. A. Johnson and J. Li, Nat. Energy, 2021, 6, 495 Search PubMed.
- J. Langdon and A. Manthiram, Adv. Mater., 2022, 34, 2205188 Search PubMed.
- J.-Y. Hwang, S.-J. Park, C. S. Yoon and Y.-K. Sun, Energy Environ. Sci., 2019, 12, 2174 Search PubMed.
- H. Kim, S.-H. Lee, J.-M. Kim, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2023, 8, 2970 Search PubMed.
- J. Zheng, M. H. Engelhard, D. Mei, S. Jiao, B. J. Polzin, J.-G. Zhang and W. Xu, Nat. Energy, 2017, 2, 17012 Search PubMed.
- S. Chen, J. Zheng, L. Yu, X. Ren, M. H. Engelhard, C. Niu, H. Lee, W. Xu, J. Xiao, J. Liu and J.-G. Zhang, Joule, 2018, 2, 1548 Search PubMed.
- X. Ren, L. Zou, X. Cao, M. H. Engelhard, W. Liu, S. D. Burton, H. Lee, C. Niu, B. E. Matthews, Z. Zhu, C. Wang, B. W. Arey, J. Xiao, J. Liu, J.-G. Zhang and W. Xu, Joule, 2019, 3, 1662 Search PubMed.
- R. Weber, M. Genovese, A. J. Louli, S. Hames, C. Martin, I. G. Hill and J. R. Dahn, Nat. Energy, 2019, 4, 683 Search PubMed.
- C. Niu, H. Lee, S. Chen, Q. Li, J. Du, W. Xu, J.-G. Zhang, M. S. Whittingham, J. Xiao and J. Liu, Nat. Energy, 2019, 4, 551 Search PubMed.
- J. Liu, Z. Bao, Y. Cui, E. J. Dufek, J. B. Goodenough, P. Khalifah, Q. Li, B. Y. Liaw, P. Liu, A. Manthiram, Y. S. Meng, V. R. Subramanian, M. F. Toney, V. V. Viswanathan, M. S. Whittingham, J. Xiao, W. Xu, J. Yang, X.-Q. Yang and J.-G. Zhang, Nat. Energy, 2019, 4, 180 Search PubMed.
- C. Fang, B. Lu, G. Pawar, M. Zhang, D. Cheng, S. Chen, M. Ceja, J.-M. Doux, H. Musrock, M. Cai, B. Liaw and Y. S. Meng, Nat. Energy, 2021, 6, 987 Search PubMed.
- D. T. Boyle, W. Huang, H. Wang, Y. Li, H. Chen, Z. Yu, W. Zhang, Z. Bao and Y. Cui, Nat. Energy, 2021, 6, 487 Search PubMed.
- H. Kwon, H. Kim, J. Hwang, W. Oh, Y. Roh, D. Shin and H.-T. Kim, Nat. Energy, 2024, 9, 57 Search PubMed.
- S. C. Kim, J. Wang, R. Xu, P. Zhang, Y. Chen, Z. Huang, Y. Yang, Z. Yu, S. T. Oyakhire, W. Zhang, L. C. Greenburg, M. S. Kim, D. T. Boyle, P. Sayavong, Y. Ye, J. Qin, Z. Bao and Y. Cui, Nat. Energy, 2023, 8, 814 Search PubMed.
- Z. Huang, J.-C. Lai, S.-L. Liao, Z. Yu, Y. Chen, W. Yu, H. Gong, X. Gao, Y. Yang, J. Qin, Y. Cui and Z. Bao, Nat. Energy, 2023, 8, 577 Search PubMed.
- J.-Y. Hwang, S.-T. Myung, J. U. Choi, C. S. Yoon, H. Yashiro and Y.-K. Sun, J. Mater. Chem. A, 2017, 5, 23671 Search PubMed.
- X. Liang and Y.-K. Sun, Adv. Funct. Mater., 2022, 32, 2206154 Search PubMed.
- T.-Y. Yu, H.-H. Ryu, G. Han and Y.-K. Sun, Adv. Energy Mater., 2020, 10, 2001609 Search PubMed.
- M. Sathiya, Q. Jacquet, M.-L. Doublet, O. M. Karakulina, J. Hadermann and J.-M. Tarascon, Adv. Energy Mater., 2018, 8, 1702599 Search PubMed.
- H.-R. Yao, W.-J. Lv, Y.-X. Yin, H. Ye, X.-W. Wu, Y. Wang, Y. Gong, Q. Li, X. Yu, L. Gu, Z. Huang and Y.-G. Guo, ACS Appl. Mater. Interfaces, 2019, 11, 22067 Search PubMed.
- C. Xu, P. J. Reeves, Q. Jacquet and C. P. Grey, Adv. Energy Mater., 2021, 11, 2003404 Search PubMed.
- J.-Y. Hwang, S.-T. Myung, C. S. Yoon, S.-S. Kim, D. Aurbach and Y.-K. Sun, Adv. Funct. Mater., 2016, 26, 8083 Search PubMed.
- J.-Y. Hwang, S.-M. Oh, S.-T. Myung, K. Y. Chung, I. Belharouak and Y.-K. Sun, Nat. Commun., 2015, 6, 6865 Search PubMed.
- L. Lin, L. Zhang, S. Wang, F. Kang and B. Li, J. Mater. Chem. A, 2023, 11, 7867 Search PubMed.
- J. Xie, A. D. Sendek, E. D. Cubuk, X. Zhang, Z. Lu, Y. Gong, T. Wu, F. Shi, W. Liu, E. J. Reed and Y. Cui, ACS Nano, 2017, 11, 7019 Search PubMed.
- C. Delmas and I. Saadoune, Solid State Ionics, 1992, 53, 370 Search PubMed.
- X. Liang, J.-Y. Hwang and Y.-K. Sun, Adv. Energy Mater., 2023, 13, 2301975 Search PubMed.
- S.-T. Myung, K. Izumi, S. Komaba, Y.-K. Sun, H. Yashiro and N. Kumagai, Chem. Mater., 2005, 17, 3695 Search PubMed.
- S. Jang, S.-H. Kang, K. Amine, Y. Bae and Y.-K. Sun, Electrochim. Acta, 2005, 50, 4168 Search PubMed.
- Y. Sun, J. Han, S. Myung, S. Lee and K. Amine, Electrochem. Commun., 2006, 8, 821 Search PubMed.
- H.-H. Sun, J.-Y. Hwang, C. S. Yoon, A. Heller and C. B. Mullins, ACS Nano, 2018, 12, 12912 Search PubMed.
- Y. K. Sun, M. J. Lee, C. S. Yoon, J. Hassoun, K. Amine and B. Scrosati, Adv. Mater., 2012, 24, 1192 Search PubMed.
- X. Liang, T.-Y. Yu, H.-H. Ryu and Y.-K. Sun, Energy Storage Mater., 2022, 47, 515 Search PubMed.
- Y. Wu, X. Liu, L. Wang, X. Feng, D. Ren, Y. Li, X. Rui, Y. Wang, X. Han, G.-L. Xu, H. Wang, L. Lu, X. He, K. Amine and M. Ouyang, Energy Storage Mater., 2021, 37, 77 Search PubMed.
- K. Edström, T. Gustafsson and J. O. Thomas, Electrochim. Acta, 2004, 50, 397 Search PubMed.
- V. Etacheri, O. Haik, Y. Goffer, G. A. Roberts, I. C. Stefan, R. Fasching and D. Aurbach, Langmuir, 2012, 28, 965 Search PubMed.
- Z. Zhu, Y. Tang, Z. Lv, J. Wei, Y. Zhang, R. Wang, W. Zhang, H. Xia, M. Ge and X. Chen, Angew. Chem., Int. Ed., 2018, 57, 3656 Search PubMed.
- J. Chen, X. Fan, Q. Li, H. Yang, M. R. Khoshi, Y. Xu, S. Hwang, L. Chen, X. Ji, C. Yang, H. He, C. Wang, E. Garfunkel, D. Su, O. Borodin and C. Wang, Nat. Energy, 2020, 5, 386 Search PubMed.
- S.-J. Park, J.-Y. Hwang, C. S. Yoon, H.-G. Jung and Y.-K. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 17985 Search PubMed.
- Y. Lee, T. K. Lee, S. Kim, J. Lee, Y. Ahn, K. Kim, H. Ma, G. Park, S.-M. Lee, S. K. Kwak and N.-S. Choi, Nano Energy, 2020, 67, 104309 Search PubMed.
- X. Fan, L. Chen, O. Borodin, X. Ji, J. Chen, S. Hou, T. Deng, J. Zheng, C. Yang, S.-C. Liou, K. Amine, K. Xu and C. Wang, Nat. Nanotechnol., 2018, 13, 715 Search PubMed.
- S. Tan, Z. Shadike, J. Li, X. Wang, Y. Yang, R. Lin, A. Cresce, J. Hu, A. Hunt, I. Waluyo, L. Ma, F. Monaco, P. Cloetens, J. Xiao, Y. Liu, X.-Q. Yang, K. Xu and E. Hu, Nat. Energy, 2022, 7, 484 Search PubMed.
- T. Deng, X. Fan, L. Cao, J. Chen, S. Hou, X. Ji, L. Chen, S. Li, X. Zhou, E. Hu, D. Su, X.-Q. Yang and C. Wang, Joule, 2019, 3, 2550 Search PubMed.
- B. S. Parimalam and B. L. Lucht, J. Electrochem. Soc., 2018, 165, A251 Search PubMed.
- J. Cha, J.-G. Han, J. Hwang, J. Cho and N.-S. Choi, J. Power Sources, 2017, 357, 97 Search PubMed.
- J. Xiao, F. Shi, T. Glossmann, C. Burnett and Z. Liu, Nat. Energy, 2023, 8, 329 Search PubMed.
- Y. S. Meng, V. Srinivasan and K. Xu, Science, 2022, 378, 1065 Search PubMed.
Footnotes |
| † These authors contributed equally to this work. |
| ‡ Present address: Department of Chemical Engineering and Applied Chemistry, Chungnam National University, Daejeon, 34134, South Korea. |
| This journal is © The Royal Society of Chemistry 2024 |