Open Access Article
Open Access ArticleProtAgents: protein discovery via large language model multi-agent collaborations combining physics and machine learning†
Alireza
Ghafarollahi
a and
Markus J.
Buehler
 *ab
*ab
aLaboratory for Atomistic and Molecular Mechanics (LAMM), Massachusetts Institute of Technology, 77 Massachusetts Ave., Cambridge, MA 02139, USA. E-mail: mbuehler@MIT.EDU
bCenter for Computational Science and Engineering, Schwarzman College of Computing, Massachusetts Institute of Technology, 77 Massachusetts Ave., Cambridge, MA 02139, USA
First published on 17th May 2024
Abstract
Designing de novo proteins beyond those found in nature holds significant promise for advancements in both scientific and engineering applications. Current methodologies for protein design often rely on AI-based models, such as surrogate models that address end-to-end problems by linking protein structure to material properties or vice versa. However, these models frequently focus on specific material objectives or structural properties, limiting their flexibility when incorporating out-of-domain knowledge into the design process or comprehensive data analysis is required. In this study, we introduce ProtAgents, a platform for de novo protein design based on Large Language Models (LLMs), where multiple AI agents with distinct capabilities collaboratively address complex tasks within a dynamic environment. The versatility in agent development allows for expertise in diverse domains, including knowledge retrieval, protein structure analysis, physics-based simulations, and results analysis. The dynamic collaboration between agents, empowered by LLMs, provides a versatile approach to tackling protein design and analysis problems, as demonstrated through diverse examples in this study. The problems of interest encompass designing new proteins, analyzing protein structures and obtaining new first-principles data – natural vibrational frequencies – via physics simulations. The concerted effort of the system allows for powerful automated and synergistic design of de novo proteins with targeted mechanical properties. The flexibility in designing the agents, on one hand, and their capacity in autonomous collaboration through the dynamic LLM-based multi-agent environment on the other hand, unleashes great potentials of LLMs in addressing multi-objective materials problems and opens up new avenues for autonomous materials discovery and design.
1 Introduction
Proteins, the building blocks of life, serve as the fundamental elements of many biological materials emerging from natural evolution over the span of 300 million years. Protein-base biomaterials like silk, collagen and tissue assemblies such as skin exhibit diverse structural features and showcase unique combinations of material properties. The underlying sequences of amino acids (AAs) in a protein determines its unique there-dimensional structure, which, in turn, dictates its specific biological activity and associated outstanding properties. This inherent relationship has inspired scientists in the field of materials design and optimization to draw valuable insights from nature for creating novel protein-based materials. The diversity in protein design is immense, with over 20100 possible AA sequences for just a relatively small 100-residue protein. However, the natural evolutionary process has sampled only a fraction of this vast sequence space. This leaves a substantial portion uncharted, presenting a significant opportunity for the de novo design of proteins with potentially remarkable properties.1 Despite this potential, the extensive design space, coupled with the costs associated with experimental testing, poses formidable challenges in de novo protein design. Navigating this intricate landscape necessitates the development of a diverse set of effective tools enabling the targeted design of de novo proteins with specific structural features or properties.Over the past years, in the field of de novo protein design, data-driven and machine learning methods have emerged as powerful tools, offering valuable insights and accelerating the discovery of novel proteins with desired properties.2–15 These methods have opened great avenues for predicting structure, properties, and functions of proteins solely based on their underlying AA sequence. For instance, the development of deep learning (DL)-based AlphaFold 2 marked a significant breakthrough in the field of 3D folding protein prediction with a level of accuracy that in some cases rivaled expensive and time-consuming experimental techniques.16 Moreover, deep learning-based models have been developed to explore structure–property relationships in the analysis and design of proteins. These models encompass a broad spectrum of structural and mechanical properties, serving either as constraints or target values. For example, various DL-models developed predict the secondary structure of proteins from their primary sequences. Prediction of mechanical properties of spider silk protein sequences have been enabled by DL models.17–22 Moreover, DL-based models such as graph neural networks23 and transformer-based language models24 show enhanced accuracy in predicting the protein natural frequencies compared to physics-based all-atom molecular simulations. The development of such DL models significantly reduces the cost of screening the vast sequence space to target proteins with improved or optimized mechanical performance.
A frontier, however, that still exists is how we can create intelligent tools that can solve complex tasks and draw upon a diverse set of knowledge, tools and abilities. Another critical issue is that the combination of purely data-driven tools with physics-based modeling is important for accurate predictions. Moreover, such tools should ideally also be able to retrieve knowledge from, for instance, the literature or the internet. All these aspects must be combined in a nonlinear manner where multiple dependent steps in the iteration towards and answer are necessary to ultimately provide the solution to a task. As we will discuss in this study, such an integration of tools, methods, logic, reasoning and iterative solution can be implemented through the deployment of a multi-agent system driven by sophisticated Large Language Models (LLMs).
LLMs25,26 have represented a paradigm shift in modeling problems across a spectrum of scientific and engineering domains.8,27–41 Such models, built upon attention mechanism and transformer architectures,42 have emerged as powerful tools recently in the field of materials science and related areas, contributing to various aspects ranging from knowledge retrieval to modeling, design, and analysis. For example, models such as ChatGPT and the underlying GPT-4 architecture,43 part of the Generative Pretrained Transformer (GPT) class, demonstrate exceptional proficiency in mastering human language, coding,44 logic and reasoning.45 Recent studies highlight their ability to proficiently program numerical algorithms and troubleshoot code errors across several programming languages like Python, MATLAB, Julia, C, and C++.46 The GPT class of LLMs has also represented a new paradigm in simulating and predicting the materials behavior under different conditions,28 a field of materials science often reserved for conventional deep learning frameworks47 such as Convolutional Neural Networks,48,49 Generative Adversarial Networks,50–52 Recurrent Neural Networks22,54,55 (ref. 20, 53 and 54), and Graph Neural Networks.23,55–58 Moreover, due to their proficiency in processing and comprehending vast amount of different types of multimodal data, LLMs show promising capabilities in materials analysis and prediction application including key knowledge retrieval,35 general language tasks, hypothesis generation,29 and structure-to-property mapping.28,59
At the same time, LLMs are typically not best equipped to solve specific physics-based forward and inverse design tasks, and are often focused on leveraging their conversational capabilities. Here, LLMs have been instrumental in powering conversable AI agents, facilitating the transition from AI–human conversations to AI–AI or AI–tools interactions for increased autonomy.31,35,60–62 This capability represents a significant advancement, enabling intelligent mediation, fostering interdisciplinary collaboration, and driving innovation across disparate domains, including materials analysis, design, and manufacturing. The overall process could be deemed as adapting a problem-solving strategy dictated and directed by the AI system comprised of different agents. Thereby, the entire process can be AI automated with reduced or little human intervention. Depending on the complexity of the problem, using the idea of labor division, the agents have the capability to break the overall task into subtasks for which different agents or tools are used consecutively to iteratively solve the problem until all subtasks have accomplished and the solution has achieved. There is no intrinsic limitation in defining the type of tools, making the multi-agent model a versatile approach in addressing problems across scales and disciplines. The tools could range from a simple linear mathematical function to sophisticated deep neural network architectures. The use of llm-based multi-agent strategies for discovering new materials and automating scientific research has been examined in previous studies, with applications spanning mechanics,31 chemistry,63,64 and materials science.35 The comprehensive analysis by65 specifically addresses the implementation of multi-agent strategy in enhancing biomedical research and scientific discovery, underscoring their potential to transform the field.
In this paper, we propose a multi-agent strategy to the protein design problems by introducing ProtAgents, a multi-agent modeling framework to solve protein-related analysis and design problems by leveraging customized functions across domains and disciplines. The core underpinning concept of the multi-agent systems is the state-of-the-art LLMs combined with a series of other tools. The LLM backbone demonstrates exceptional abilities in analysis, rational thinking, and strategic planning, essential for complex problem-solving. Leveraged by these capabilities, the proposed model aims to reduce the need for human intervention and intelligence at different stages of protein design. The agent model consists a suite of AI and physics-based components such as:
• Physics simulators: obtain new physical data from simulations, specifically normal modes and vibrational properties by solving partial differential equations (PDEs).
• Generative AI model: conditional/unconditional de novo protein design, based on a denoising diffusion model.
• Fine-tuned transformer model: predict mechanical properties of proteins from their sequence.
• Retrieval agent: retrieve new data from a knowledge database of scientific literature.
The main contribution of our work is summarized as follows.
• We propose ProtAgents, a pioneering multi-agent modeling framework that combines state-of-the-art LLMs with diverse tools to tackle protein design and analysis problems.
• Our model harnesses the collective capabilities of agents with specialized expertise that interact autonomously and nonlinearly to solve the protein-related task.
• Equipped with various tools and functions, the model demonstrates an advanced ability to integrate new physical data from different disciplines, surpassing conventional deep learning models in versatility and problem-solving capacity in protein science.
• Our model significantly minimizes the need for human interference throughout different stages of the problem-solving process.
• ProtAgents operates on textual input, thereby enabling non-expert researchers to effectively address and analyze challenges within the realm of protein design.
The versatility of the approach in solving complex tasks is exhibited by providing a series of experiments in the context of proteins design, modeling, and data analysis.
The plan of this paper is as follows. In Section 2, we present an overview of the multi-agent framework developed to tackle multi-objective complex tasks. Subsequently, we delve into a series of experiments where each task is initially introduced, followed by a detailed examination of various aspects throughout the problem-solving process by the multi-agent teamwork. A comprehensive discussion regarding the multi-agent framework and future prospects is provided in Section 3.
2 Results and discussion
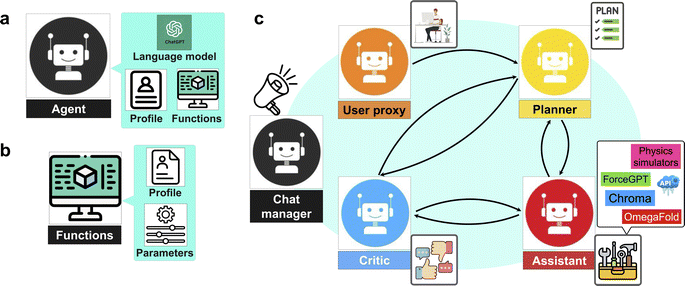
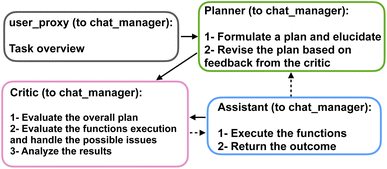
We present a series of computational experiments aimed at evaluating the effectiveness and potential of a multi-agent modeling framework for various challenges within the domain of protein modeling, design, and analysis. The multi-agent framework consists of a team of agents, each powered by a state-of-the-art general purpose large language model, GPT-4,43 accessed via the OpenAI API66 and characterized by a unique profile that details its role, and communication protocols, such as sharing information and engaging with humans via language as shown in Fig. 1a. Furthermore, agents are given access to a set of tools with various functionalities across domains. As shown in Fig. 1b each function is characterized by a descriptive profile and input parameters. The outline of the proposed multi-agent framework is shown in Fig. 1c, illustrating the collaborative efforts of a team of agents with the following entities.• “User”: human that poses the question.
• “Planner”: develops a plan to solve the task. Also suggests the functions to be executed.
• “Assistant”: who has access to all the customized functions, methods, and APIs and executes them to find or compute the relevant data necessary to solve the task.
• “Critic”: responsible for providing feedback about the plan developed by “planner” as well as analyzing the results and handling the possible mistakes and providing the output to the user.
The agents are organized into a team structure, overseen by a manager who coordinates overall communication among the agents. Table 1 lists the full profile for the agents recruited in our multi-agent framework. Moreover, a generic structure showing the dynamic collaboration between the team of agents proposed in the current study is depicted in Fig. 2. Further details can be found in the Materials and methods section 4.
| Agent# | Agent role | Agent profile |
|---|---|---|
| 1 | user_proxy | user_proxy. Plan execution needs to be approved by user_proxy |
| 2 | Planner | Planner. You develop a plan. Begin by explaining the plan. Revise the plan based on feedback from the critic and user_proxy, until user_proxy approval. The plan may involve calling custom function for retrieving knowledge, designing proteins, and computing and analyzing protein properties. You include the function names in the plan and the necessary parameters. If the plan involves retrieving knowledge, retain all the key points of the query asked by the user for the input message |
| 3 | Assistant | Assistant. You have access to all the custom functions. You focus on executing the functions suggested by the planner or the critic. You also have the ability to prepare the required input parameters for the functions |
| 4 | Critic | user_proxy. You double-check the plan, especially the functions and function parameters. Check whether the plan included all the necessary parameters for the suggested function. You provide feedback |
| 5 | Group chat manager | You repeat the following steps: dynamically selecting a speaker, collecting responses, and broadcasting the message to the group |
It is noteworthy that critical issues in the realm of protein design surpass the capabilities of mere Python code writing and execution. Instead, addressing these challenges necessitates the utilization of external tools specifically tailored for protein design and analysis, and the writing, adaptation, correction and execution of code depends nonlinearly on the progression of the solution strategy that is developed by the system.
The tools are incorporated into the model via the assistant agent who oversees executing the tools. To assess the performance of the multi-agent framework in handling complex interdisciplinary tasks, we have defined a rich library of functions each with special powers in solving the protein problems. Each function has a distinct profile that describes its role and requires one or more entities as inputs, each of which is also profiled to specify its identity and type such as string or integer. This helps the agents not only understand which function to choose but also how to provide the input parameters in the correct format. The functions provide the ability to, for instance, retrieve knowledge, perform protein folding, analyze the secondary structure, and predict some parameters through a pre-trained autoregressive language model. Additionally, a function can carry out simulations to compute the protein natural frequencies, thus allowing the model to integrate the new physics-based data. A full list of functions implemented in the current study is provided in Table S1 in the ESI.† It is worth mentioning that all the tools implemented in our multi-agent system are fixed, predefined functions, and the agents have not been given the ability to modify them.
Given the complexities residing in the protein design problems, the primary contribution of our multi-agent framework lies in assessing whether the team of agents can discern the requisite tools for a given query and evaluating the framework's capability to initiate the execution of these tools, along with providing the necessary inputs. The designated tasks are intentionally designed to be sufficiently complex, involving multiple subtasks where, for some cases, the execution of each depends on the successful completion of the preceding ones. This design showcases the model's capacity for the automated handling of intricate tasks, eliminating or substantially reducing the need for human intervention. Although the multi-agent framework allows for the human intervention at different stages, we skip that to further examine the team's capability in handling different possible situations, for instance in case of a failure.
2.1 Experiment I: knowledge retrieval, computations, and analysis
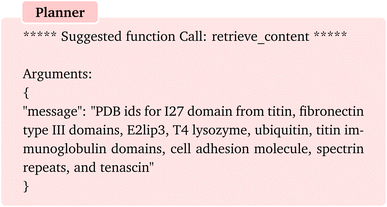
As the first example, we pose a task which involves a multi-step knowledge retrieval, analysis, and computations for a set of protein PDB identifiers. We start the first round of conversation by posing the following question:The planer then correctly suggests the function “retrieve_content” to be executed with the argument “examples of protein names whose mechanical properties have been studied through experiments”. Upon execution of the function, the assistant provides us with a list of protein names. Upon inspection, we find that the agent has successfully identified experimentally studied proteins, despite an abundance of information on proteins studied theoretically, for instance, through coarse-grain simulations. Since we are interested in the PDB ids, we continue the chat by a follow-up question “Can you provide me with the PDB ids for these proteins?” when “user_proxy” is being asked to provide feedback to chat manager. Again, the planner suggests “retrieve_content” function with the following message.
The “Assistant” agent then calls the function and gives the following output:
Upon careful examination of the results, we observe that, despite all the PDB ids exist in the source database, the PDB ids do not quite match with the protein names except for a few cases (1ubq, 1ten). Nevertheless, note that the error is caused by the poor performance of the “retrieve_content” function, which implements Llama index, and the team of agents cannot mitigate that as they have no access to the knowledge database. In fact, the entire retrieval augmented generation process is solely performed by Llama index and the agents merely contribute to this process by providing the query, calling the “retrieve_content” function, and returning the results. As such, we continue to test the agent-team capability in more challenging queries centered around computational tasks and physics-based simulations by assigning the following task in the next round of conversation.
The above is a complex multi-step analysis and computation task encompasses aspects such as secondary structures analysis, natural frequency calculations, and structure classification. Additionally, the task is subject to an initial condition that must be satisfied before proceeding through the next sequence of steps, adding an extra layer of complexity. In response, the planner comes up with a detailed plan which consists of all the actions that need to be taken to complete the task. Moreover, the plan mentions all the corresponding functions that need to be executed to accomplish the task. More importantly, the “planner” perfectly realizes to fetch the protein structures first before starting to analyze the secondary structure, although it was not explicitly mentioned in the task query.
The teamwork proceeds by a follow-up feedback provided by the “critic” agent about all the plan steps and functions which is concluded by the following statement.
Therefore, the positive feedback from the “critic” further supports the good performance of the planner in addressing all the critical steps required to accomplish the tasks.
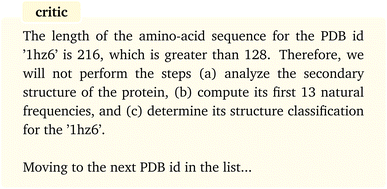
The “assistant” agent then follows the plan by calling and executing the corresponding functions, starting with AA length calculation, until all the steps have been undertaken. The results show that all the inputs to the functions are properly identified and provided and the functions are executed without any error. The conditional statement included in the tasks is also correctly satisfied for each protein, that is the computations are conducted only if the sequence length is less than 128 and are emitted otherwise. For instance, for the protein with PDB id “1hz6” the AA length is returned as 216 by the “assistant” which is then followed by the following message from the “critic”
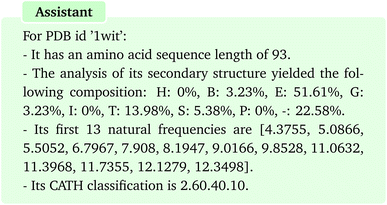
After completion of all the tasks, the assistant returns a summary of all the results for each protein as representatively shown below for PDB id “1wit”:
The results suggest that the framework effectively retains all outputs, demonstrating its strong memory even in the face of diverse and extended results. As the last round of conversation, we ask to save all the results which allows us to load them at later time for other purposes:
In response, the planner suggests to call the python function “save_to_csv_file”. The main task here is to generate the dictionary of results in JSON and with appropriate structure as instructed by the user. However, we see that upon generating the JSON data and inputting it into the function by the “assistant” agent, the following error occurs:
Without any human intervention, the agent team is able to resolve the issue by mutual correction. In particular, the “critic” identifies the cause of error by writing.
Guided by the feedback from the critic, the “assistant” then reconstructs the JSON file from the output results and is able to successfully execute the function and thus save the results in a csv file as shown in Table 2. The complete group chat records can be found in Table S2 of the ESI.†
| Protein ID# | Amino acid length | Secondary structure | First 13 frequencies | CATH classification |
|---|---|---|---|---|
| 1wit | 93 | [‘H’: 0.0, ‘B’: 3.23, ‘E’: 51.61, ‘G’: 3.23, ‘I’: 0.0, ‘T’: 13.98, ‘S’: 5.38, ‘P’: 0.0, ‘—‘: 22.58] | [4.3755, 5.0866, 5.5052, 6.7967, 7.908, 8.1947, 9.0166, 9.8528, 11.0632, 11.3968, 11.7355, 12.1279, 12.3498] | 2.60.40.10 |
| 1ubq | 76 | [‘H’: 15.79, ‘B’: 2.63, ‘E’: 31.58, ‘G’: 7.89, ‘I’: 0.0, ‘T’: 15.79, ‘S’: 5.26, ‘P’: 5.26, ‘—‘: 15.79] | [0.7722, 1.0376, 1.5225, 1.6534, 2.5441, 2.9513, 3.2873, 3.7214, 4.1792, 4.3437, 4.3908, 4.6551, 5.1631] | 3.10.20.90 |
| 1nct | 106 | [‘H’: 0.0, ‘B’: 4.08, ‘E’: 35.71, ‘G’: 0.0, ‘I’: 0.0, ‘T’: 2.04, ‘S’: 21.43, ‘P’: 0.0, ‘—‘: 36.73] | [3.6644, 4.425, 6.5351, 6.7432, 7.1409, 7.1986, 9.0207, 9.2223, 10.3163, 10.7313, 11.5299, 11.6373, 12.5606] | 2.60.40.10 |
| 1tit | 98 | [‘H’: 0.0, ‘B’: 1.12, ‘E’: 35.96, ‘G’: 0.0, ‘I’: 0.0, ‘T’: 6.74, ‘S’: 17.98, ‘P’: 0.0, ‘—‘: 38.2] | [5.5288, 5.9092, 8.2775, 8.6267, 9.3391, 9.8783, 10.1607, 11.451, 11.5896, 11.7052, 12.1498, 12.6082, 13.8622] | 2.60.40.10 |
| 1qjo | 80 | [‘H’: 0.0, ‘B’: 2.5, ‘E’: 40.0, ‘G’: 0.0, ‘I’: 0.0, ‘T’: 8.75, ‘S’: 13.75, ‘P’: 0.0, ‘—‘: 35.0] | [3.8578, 4.4398, 5.4886, 5.7815, 6.6332, 6.9269, 7.2329, 7.6453, 8.2545, 8.3076, 8.6118, 8.7135, 8.8546] | 2.40.50.100 |
| 2ptl | 78 | [‘H’: 15.38, ‘B’: 1.28, ‘E’: 30.77, ‘G’: 0.0, ‘I’: 0.0, ‘T’: 7.69, ‘S’: 19.23, ‘P’: 0.0, ‘—‘: 25.64] | [0.0386, 0.1161, 0.2502, 0.5921, 1.1515, 1.5257, 2.0924, 2.6793, 3.4292, 3.9289, 4.2172, 4.6878, 4.8022] | 3.10.20.10 |
The main outcomes of this experiment are as follows.
• This experiment exemplifies how multi-agent strategy enables us to go beyond the knowledge of pre-trained LLMs by retrieving new knowledge from external sources.
• This experiment shows an instructive collaboration between the AI–AI agents in problem-solving as well as error handling.
• Multi-agent systems enable us to provide feedback at various stages and pose follow-up questions throughout the process.
• The experiment highlights the failure of the model in extracting correct knowledge from external sources.As discussed above, the last point directly stems from the failure of the function responsible for knowledge retrieval. To circumvent this, two main strategies can be adopted: (a) we can guide the agents to provide feedback on the reliability of the generated content, although this may be limited by the constraints of the pre-trained language models' knowledge; (b) we can implement more advanced Retrieval-Augmented Generation (RAG) models. Since knowledge retrieval is not the main focus of this paper, we will leave this aspect for future work.
2.2 Experiment II: de novo protein design using chroma
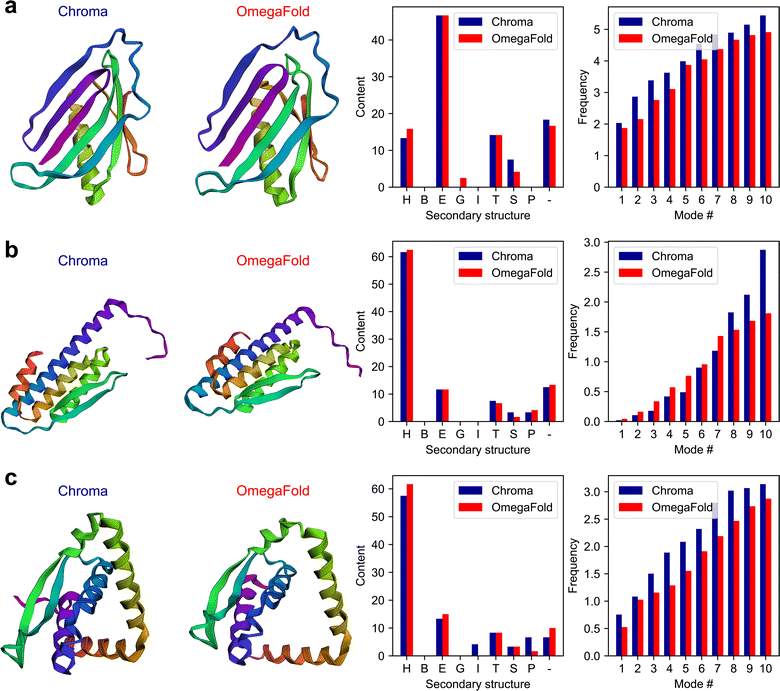
An important characteristic of the multi-agent model is its capability in handling very complex tasks in the context of protein design and analysis. This partly stems from the possibility of incorporating customized functions in the model for various purposes from knowledge retrieval to performing physics-based simulations. In this example, we explore the model's performance in using the state-of-the art models in de novo protein design, Chroma,15 and protein folding, OmegaFold.4 Chroma offers a unified approach for protein design that can directly sample novel protein structures and sequences with the possibility to direct the generative design towards the desired functions and properties by appropriate conditioning. OmegaFold is a state-of-the-art folding method without the need of using multiple sequence alignments (MSA) to predict the three-dimensional folded protein structure given its AA sequence.In this experiment, we formulate a complex multi-step task with the objective of comparing the two models based on various structural and physical features derived from the folded structures obtained through Chroma and OmegaFold2. We pose the following task through the “user_proxy” agent:
The “planner” then suggests the following plan.
At first glance, the plan seems to cover all the details necessary to accomplish the tasks included in the problem statement. However, the “critic” agent who is responsible for giving feedback about the plan spots a minuscule error in the saving part of the plan as follows:
The correction made by the “critic”concerning the sequence length underscores its notable proficiency in comprehending how diverse functions and parameters influence various aspects within the realm of protein design.
The “user_proxy” agent is then asked to confirm the plan. The “assistant” then takes the stage and starts following the plan by calling and executing the functions until all the steps have been undertaken. An overview of the work performed by the “assistant” is depicted in Fig. 3. At the end of the computations, the results are formatted into a JSON dictionary to fed into the “save_to_csv_file” function. However, an error related to the JSON dictionary format occurs when executing the function as follows:
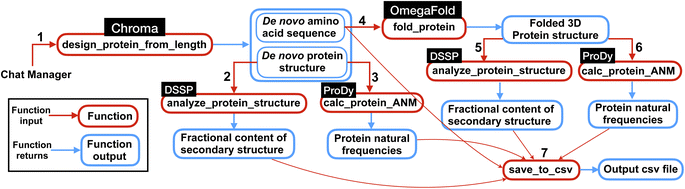 | ||
| Fig. 3 Overview of the multi-agent work to solve the complex task posed in Experiment II, Section 2.2. First the multi-agent uses Chroma to generate de novo protein sequences and then computes natural frequencies and secondary structures content for the generated structures. Next, from de novo AA sequences, the model finds the 3D folded structures using OmegaFold and finally computes the frequencies and secondary structure content for the protein structures. Finally, the results are saved in a csv file as shown in Table 3. The numbers represent the sequence in which the functions are executed within the workflow. | ||
The “critic” then steps in by making the following comment and suggesting a plan to fix the error as follows:
The critic makes the necessary corrections and suggest the corrected JSON dictionary for the “assistant” to execute the “save_to_csv_file” function. This time, the function is successfully executed and the results are saved into a csv file as shown in Table 3. At last, the “critic” gives an evolution about the whole process:
| Protein number# | Amino acid sequence | Secondary structure (pre-fold) | Frequencies (pre-fold) | Secondary structure (post-fold) | Frequencies (post-fold) |
|---|---|---|---|---|---|
| 1 | MIIINIKTENGLSIT | ‘H’: 13.33, ‘B’: 0.0, ‘E’: 46.66, ‘G’: 0.0, ‘I’: 0.0, ‘T’: 14.16, ‘S’: 7.5, ‘P’: 0.0, ‘—‘: 18.33 | [2.0337, 2.8678, 3.3843, 3.6263, 3.9904, 4.5381, 4.8373, 4.8956, 5.1492, 5.4416] | ‘H’: 15.83, ‘B’: 0.0, ‘E’: 46.66, ‘G’: 2.5, ‘I’: 0.0, ‘T’: 14.16, ‘S’: 4.16, ‘P’: 0.0, ‘—‘: 16.66 | [1.8739, 2.1563, 2.7611, 3.1086, 3.8712, 4.0481, 4.3759, 4.6717, 4.8183, 4.9126] |
| YNSDEKKLELKYTP | |||||
| VKSPEDFKFPEDAK | |||||
| ATISEVEYKGKKVI | |||||
| KIDAKLYVSPDLSK | |||||
| AKLTIEVNADISQE | |||||
| EADKIIDEFIKLLES | |||||
| LGNIKLKVTKDGN | |||||
| KYTIEVE | |||||
| 2 | GSPLPRPPLSPEEQ | ‘H’: 61.66, ‘B’: 0.0, ‘E’: 11.66, ‘G’: 0.0, ‘I’: 0.0, ‘T’: 7.5, ‘S’: 3.33, ‘P’: 3.33, ‘—‘: 12.5 | [0.0207, 0.1058, 0.1782, 0.4189, 0.49, 0.9015, 1.1832, 1.8257, 2.1212, 2.8726] | ‘H’: 62.5, ‘B’: 0.0, ‘E’: 11.66, ‘G’: 0.0, ‘I’: 0.0, ‘T’: 6.66, ‘S’: 1.66, ‘P’: 4.16, ‘—‘: 13.33 | [0.0444, 0.1641, 0.3379, 0.5724, 0.765, 0.9568, 1.4306, 1.5344, 1.6834, 1.8099] |
| EALRKKAQEKYNE | |||||
| FVSKIKELLRRAAD | |||||
| RVRRGEPVELIEKT | |||||
| IKIGDYEYKIVATSP | |||||
| EEAKELENLIKEMI | |||||
| DLGFKPSKEFSDKL | |||||
| VEAARLIREGRVD | |||||
| EALRLLDEM | |||||
| 3 | APLDPDDLSAQLR | ‘H’: 57.50, ‘B’: 0.0, ‘E’: 13.33, ‘G’: 0.0, ‘I’: 4.16, ‘T’: 8.33, ‘S’: 3.33, ‘P’: 6.66, ‘—‘: 6.66 | [0.7546, 1.0836, 1.5026, 1.8874, 2.0844, 2.3192, 2.7975, 3.0199, 3.0669, 3.1382] | ‘H’: 61.66, ‘B’: 0.0, ‘E’: 15.0, ‘G’: 0.0, ‘I’: 0.0, ‘T’: 8.33, ‘S’: 3.33, ‘P’: 1.66, ‘—‘: 10.0 | [0.5256, 1.0278, 1.1566, 1.2877, 1.5521, 1.9111, 2.1887, 2.4664, 2.734, 2.8731] |
| AAIDELVRLGYEEE | |||||
| VSKPEFIEALRLYA | |||||
| LDLGLKEVVLRRVT | |||||
| PAPASQPGVYTVE | |||||
| DVTVDLEALRKQE | |||||
| LSPEEQARLEKIRA | |||||
| KYDEMLADPEFQA | |||||
| LLDEVLARARAA |
The main findings of this experiment are summarized as follows:
• This experiment showcases a good example of multi-agent power in developing workflows to autonomously solve complex tasks in the context of de novo protein design and analysis.
• This experiment demonstrates how new physics can be retrieved from physics simulators and integrated into the model.
• The experiment shows the great capability of the “critic” agent in providing valuable feedback to other working agents at different stages of the problem-solving endeavor, further assisting the team of agents in handling possible errors without the need for human involvement.
The plots of the generated results in this experiment including the 3D folded structures are shown in Fig. 4. The full conversations can be found in Table S3 in the ESI.†
2.3 Experiment III: de novo protein design conditioned on the protein CATH class
CATH is a hierarchical classification system for protein structures that consists of four main levels. The highest level in this hierarchy is the “Class” which primarily characterizes the secondary structure content of the protein. For example, C1, C2, and C3 correspond to proteins predominantly composed of α-helix, mainly β-sheet, and a combination of α and β secondary structures. Consequently, designing proteins based on the CATH class number, i.e. C1, C2, C3, can be understood as creating proteins with a specific fractional content of the secondary structure. Previous studies have demonstrated the importance of the protein secondary structures content, specially α-helix/β-sheet ratio, on the mechanical properties of the protein materials.67,68 For instance, α-helix-rich proteins tend to yield stretchy materials,69 while β-sheet-rich ones produce rigid materials.70–72 Chroma has the potential to conditionally generate proteins with specified folds according to CATH class annotations at three levels.15In this example, we task the multi-agent team with generating proteins based on their fractional content of the secondary structure and subsequently performing computational and structural analysis tasks. Specifically, in addition to secondary structure analysis and natural frequency calculations, as covered in previous examples, we instruct the team to compute the maximum unfolding force (maximum force in the unfolding force–separation curve) and unfolding energy (the area under the unfolding force–separation curve) for each generated protein. To accomplish the latter, we have equipped the multi-agent team with a custom function that utilizes a trained autoregressive transformer generative AI model, ForceGPT. In addition to maximum unfolding force and energy, the trained generative model is able to predict the entire unfolding force–separation curve based solely on the protein amino acid sequence. Furthermore, the model has the capability to perform inverse design tasks by generating protein AA sequences that yield desired unfolding behavior. Detailed information about the training of the model can be found in Materials and methods section4. The task given is:
Note that, as before, we do not specify any particular function or offer hints for selecting the appropriate function to accomplish the tasks. Instead, we empower the agents to formulate a plan, wherein they decide which functions to select and determine the input parameters. The planner outlines the following plan for the given task:
It can be seen that the planner demonstrates good performance in breaking the task into sub-tasks to be accomplished step by step. Moreover, it has identified and suggested the correct functions and corresponding input parameters for each sub-task. The plan is further supported by the “critic” who provides positive feedback as follows:
The multi-agent team then proceeds to execute the different steps outlined in the plan by calling and executing the functions. Specifically, the function ‘design_protein_from_CATH’ is executed with the appropriate ‘CATH_ANNOTATION’ for a specific protein structure design, as outlined in the plan. Following the generation of all proteins, the executions are followed by structural analysis and force and energy computations. It's noteworthy that the model exhibits good performance in restoring and memorizing the sequences of the generated proteins, which are essential for the force and energy calculations. Finally, the team successfully completes the task by computing the first 10 frequencies for each protein. An overview of the computations performed by the team of agents for this experiment is shown in Fig. 5.
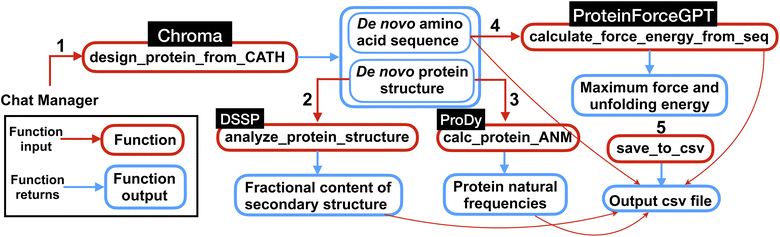 | ||
| Fig. 5 Overview of the multi-agent work to solve the complex task posed in Experiment III, Section 2.3. First the multi-agent uses Chroma to generate de novo protein sequences and structures conditioned on the input CATH class. Then using the generated protein structures, the natural frequencies and secondary structures content are computed. Next, the force (maximum force along the unfolding force-extension curve) and energy (the area under the force-extension curve) are computed from de novo AA sequences using ProteinForceGPT. Finally, the results are saved in a csv file as shown in Table 4. The numbers represent the sequence in which the functions are executed within the workflow. | ||
Given the complexity of the problem involving numerous computational tasks, a decent number of results have been generated in the first round of the conversation. In the next round, to evaluate the team's ability to memorize and restore the results, we present the following task:
In this task, we not only request the team to save the data but also require them to adhere to a customized format when storing the results. The model is proficient in creating a JSON dictionary that satisfies the specified format and saving the results to a CSV file, as illustrated in Table 4.
| Protein name# | AA sequence | Secondary structure | Unfolding energy | Max force | First 10 frequencies |
|---|---|---|---|---|---|
| mainly_alpha_protein_1 | SMKKIEDYIREKLKA | ‘H’: 89.0, ‘B’: 0.0, ‘E’: 0.0, ‘G’: 0.0, ‘I’: 0.0, ‘T’: 4.0, ‘S’: 1.0, ‘P’: 0.0, ‘—‘: 6.0 | 0.381 | 0.444 | [0.2329, 0.4901, 0.9331, 1.3741, 1.7347, 2.1598, 2.3686, 2.6359, 2.8555, 3.0364] |
| LGLSDEEIEERVKQL | |||||
| MEGIKNPKKFEKEL | |||||
| QKRNDRESLLIFKEA | |||||
| YALYEASKDKEKGK | |||||
| KLINKVQSERDKWE | |||||
| TEQAEAARAAAAA | |||||
| mainly_alpha_protein_2 | MSKKEIEELKKKLDE | ‘H’: 89.0, ‘B’: 0.0, ‘E’: 0.0, ‘G’: 0.0, ‘I’: 0.0, ‘T’: 5.0, ‘S’: 0.0, ‘P’: 0.0, ‘—‘: 6.0 | 0.376 | 0.536 | [1.6126, 2.0783, 2.3073, 2.4565, 3.399, 3.475, 4.1377, 4.7104, 4.8864, 5.2187] |
| IVETLKEYARQGDD | |||||
| ACKKAADLIEEVKKA | |||||
| LEEGNPEKYSQLKKK | |||||
| LTDAINKAIEEYRKR | |||||
| FEAEGKPEEAQKVID | |||||
| KLKKILDEITN | |||||
| mainly_beta_protein_1 | TTVTVTPPVADADG | ‘H’: 0.0, ‘B’: 0.0, ‘E’: 64.0, ‘G’: 0.0, ‘I’: 0.0, ‘T’: 10.0, ‘S’: 6.0, ‘P’: 0.0, ‘—‘: 20.0 | 0.462 | 0.533 | [1.2806, 1.5057, 1.9846, 2.1025, 2.4723, 2.702, 2.9931, 3.1498, 3.4432, 4.1685] |
| NEHSTVTAYGNKVT | |||||
| ITITCPSNCTVTETV | |||||
| DGVAKTLGTVSGNQ | |||||
| TITETRTIAPDEVVT | |||||
| RTYTCTPNASATSSK | |||||
| TQTVTIKGSQPAP | |||||
| mainly_beta_protein_2 | SLKAKNLEEMIKEAE | ‘H’: 58.00, ‘B’: 0.0, ‘E’: 8.0, ‘G’: 6.0, ‘I’: 0.0, ‘T’: 8.0, ‘S’: 4.0, ‘P’: 3.0, ‘—‘: 13.0 | 0.371 | 0.548 | [2.8864, 4.3752, 4.5928, 4.8295, 5.0854, 5.5618, 5.8646, 6.007, 6.3847, 7.1246] |
| KLGYSRDEVEKIINE | |||||
| IRDKFKKLGVKISEK | |||||
| TLAYIAYLRLLGVKID | |||||
| WDKIKKVKKATPAD | |||||
| FRVSEEDLKKPEIQKI | |||||
| LEKIKKEIN | |||||
| alpha_beta_protein_1 | APTVKTFEDTINGQK | ‘H’: 15.0, ‘B’: 0.0, ‘E’: 59.0, ‘G’: 3.0, ‘I’: 0.0, ‘T’: 12.0, ‘S’: 1.0, ‘P’: 0.0, ‘—‘: 10.0 | 0.424 | 0.535 | [2.4383, 2.5651, 3.3175, 3.8231, 3.9673, 4.2655, 4.6393, 5.1509, 5.6023, 5.9555] |
| VTVTVTASPGGKITI | |||||
| KTSPGYGDEVAKAFI | |||||
| EELKKQNVLESYKVE | |||||
| SAPGKETTISDVKVK | |||||
| SGATVTFYVINNGKK | |||||
| GKEYSVTVDA | |||||
| alpha_beta_protein_2 | MELKVTEKKGKGDY | ‘H’: 35.0, ‘B’: 0.0, ‘E’: 29.00, ‘G’: 0.0, ‘I’: 0.0, ‘T’: 3.0, ‘S’: 12.0, ‘P’: 3.0, ‘—‘: 18.0 | 0.376 | 0.543 | [2.8756, 3.8895, 4.0594, 4.2831, 4.5542, 5.171, 5.3661, 5.4312, 6.1964, 6.3066] |
| KVKVIELNTPDKRYII | |||||
| IESDASRESLIKAAEA | |||||
| LLQGKEVEPTPVNEK | |||||
| NVVLFEDEDVKTSIE | |||||
| RSKKLFKSDNPEENI | |||||
| KKALEYLLK |
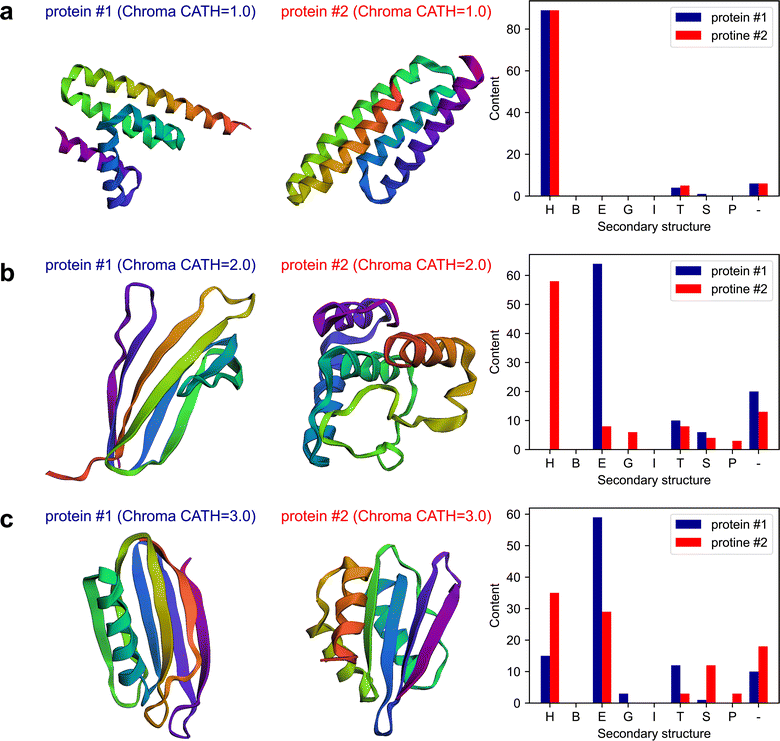
The plots of the obtained results are shown in Fig. 6. The results indicate that Chroma has done a poor performance in creating β-rich protein named mainly_beta_protein_2 which its structure is dominant in α-helix. As an attempt to test the capability of the multi-agent model in analyzing the results, in the last round of the conversation, we ask the model to assess Chroma's performance in generating the proteins conditioned on the secondary structure by posing the following question:
The “critic” agent conducts a thorough evaluation of Chroma's performance in generating proteins with targeted secondary structure content. Through a detailed analysis of each CATH structure, it reveals the inherent strengths and weaknesses in Chroma's capabilities. Specifically, addressing the limitations of Chroma's performance, the critic's evaluation provides the following observations for the mainly beta proteins:
The main findings of this experiment are as follows.
• This experiment showcases another successful application of multi-agent collaboration in designing proteins that can possess targeted secondary structure.
• This experiment underscores the power of the multi-agent system in retrieving new physics from pre-trained sophisticated transformer-based models.
• This illustration not only highlights the multi-agent model's proficiency in performing computational tasks but also underscores its intelligence in handling intricate data analyses—an aspect traditionally reserved for human.
• The experiment highlights a potential problem with multi-agent systems: the generation of undesired content, in this case, proteins that do not possess the intended secondary structures.
In the next experiment, we will propose a strategy to effectively circumvent the issue pertaining undesired data generation.
The full conversations for this experiment can be found in Table S4 of the ESI.†
2.4 Experiment IV: secondary structure effect on mechanical properties
Our previous experiments have shown that multi-agent modeling can simplify complex tasks in protein science into manageable sub-tasks. This involves the careful selection of suitable functions, the precise construction of input parameters, the execution of the functions, and returning the results to the user. In this study, we expand the capabilities of the multi-agent system beyond mere planning and function execution by introducing a task aimed at advancing scientific discovery. The possibility of developing an AI scientist that could make substantial contributions to protein science presents an exciting opportunity for further investigation.65 Future research should concentrate on improving the multi-agent's capacity to autonomously generate hypotheses and design simulations to test and refine these hypotheses. It should be noted that this experiment does not seek to make real scientific discoveries but rather demonstrates the potential of the multi-agent system in this domain.Before proceeding to the results, we should mention that we have slightly modified the profile of the planner to mitigate the approximations involved in generating CATH-conditioned proteins as observed in Experiment III. In more detail, we have added the following instruction to the planner's profile to ensure the generated proteins possess the targeted secondary structures:
If the plan involves using “design_protein_from_CATH” function, if the secondary structure does not meet expectation, you should re-design.
We pose the following task via user_proxy agent.
The planner then proposes the following plan and asks the Critic to approve the plan.
The above plan includes key features, indicating that the system has grasped two main aspects of the solution to the problem: (a) without explicit instructions, the planner decides to design three proteins with varying secondary structures using the ‘design_protein_from_CATH’ function; (b) unlike in previous experiments, the planner now takes an additional step to ensure that the secondary structures of the proteins meet the expectations.
Upon receiving approval from the Critic, the implementation of the plan commences, with functions being executed sequentially. Initially, two proteins with CATH_ANNOTATION values of 1 and 2 are generated, and their secondary structures are analyzed, revealing a high percentage of alpha-helix and beta-sheet, respectively. However, an unexpected error related to memory occurred during the execution of the third protein, which has a CATH_ANNOTATION of 3 (mixed protein):
In response, the Planner suggests retrying the function. However, the memory issue persists, and the Planner returns the following:
Since the problem persists, the Planner smartly revises the initial plan and proposes an alternative that omits the generation of the mixed protein, effectively bypassing the memory issue.
The plan implementation proceeds by calling “calculate_force_energy_from_seq” function and using the sequences of the previously designed proteins as the input. The function returns the force and energy values and the results are then saved into a csv file using the function “save_to_csv_file” which is executed successfully. Lastly, the “Planner” concludes the process by summarizing the results, reporting the main findings, and reminding the problem in designing the protein with mixed structure due to technical issues.
The main conclusions of this experiment are summarized as follows:
• The experiment highlights the capability of multi-agent modeling to create workflows that develop and execute research in protein science, potentially offering new scientific revelations.
• By issuing suitable directions, the model can be prompted to deliver more trustworthy outputs. This strategy is effective not only in decreasing the uncertainty in generative designs, as demonstrated here, but also in sidestepping errors due to hallucinations, a frequent issue in large language models.73,74
• LLM-based agents excel in responding to unexpected circumstances and devising alternative solutions, thus avoiding unforeseen errors.
3 Conclusions
Large Language Models (LLMs) have made remarkable strides, revealing their immense potential to potentially replicate human-like intelligence across diverse domains and modalities, demonstrating proficiency in comprehending extensive collective knowledge and proving adept at effectively applying this information. However, to reach intelligent problem-solving systems, these types of models are not yet sufficient and require integration with other methods. In this study we explored the capability of AI agents to solve protein design problems in an autonomous manner without the need for human intervention. The agents have been powered by a general purpose LLM model, GPT-4, which allows them to communicate via conversation. It should be noted that the general capabilities of the AI agents powered by the LLM plays an important role at different stages of the problem solving. In our case, GPT-4-powered agents showed excellent proficiency specially in problem understanding, strategy development, and criticizing the outcomes. Such an AI system is not limited to mere linguistic interactions between agents; they have the capacity to incorporate a variety of special-purpose modeling and simulation tools, human input, tools for knowledge retrieval, and even deep learning-based surrogate models to solve particular tasks. Furthermore, additional tools can be integrated into the multi-agent system with popular external APIs and up-to-date knowledge about special topics can be retrieved by searching and browsing the web through specialized API interfaces. By harnessing the collective abilities of agents, including reasoning, tool usage, criticism, mutual correction, adaptation to new observations, and communication this framework has proven highly effective in navigating intricate challenges including protein design.To achieve this goal we constructed a group of agents, each assigned a unique profile through initial prompts, to dynamically interact in a group chat via conversations and make decisions and take actions based on their observations. The agents profile outlines their attributes, roles, and functionalities within the system and describe communication protocols to exchange information with other agents in the system. Our team of agents include a user_proxy to pose the query, a planner to formulate a plan, an function-backed assistant to execute the functions, and a critic to evaluate the outcome and criticizing the performance. We also use a chat manager to lead the group chat by dynamically choosing the working agent based on the current outcome and the agents' roles. Through a series of experiments, we unleashed the power of agents in not only conducting the roles they were assigned to, but to autonomously collaborate by discussion powered by the all-purpose LLM. For example, the agent playing the role of a planner successfully identified all the tasks in the query and suggested a details plan including the necessary functions to accomplish them. Furthermore, the agent assigned the critic role, is able to give constructive feedback about the plan or provide suggestions in case of failure, to correct errors that may emerge. Our experiments have showcased the great potential of the multi-agent modeling framework in tackling complex tasks as well as integrating AI-agents into physics-based modeling.
It is worth mentioning there are similarities between llm-based multi-agent systems as employed here to non-llm-based autonomous multi-agent systems.75–78 These agents operate with significant autonomy, drawing on previous experiences and data-driven insights to navigate complex, high-dimensional decision spaces. Whether orchestrating materials discovery workflows or engaging in multi-agent interactions, these systems exemplify how intelligent agents can reduce the need for direct human oversight and enhance efficiency in diverse fields such as materials science and virtual simulations. Furthermore, an intriguing aspect of multi-agent modeling is its compatibility with the concept of federated learning,79,80 forming a robust framework for managing distributed data and learning tasks. This integration enables the use of distinct agents embedded in different systems, which may be located in physically distinct locales and possess varying levels of data access. This setup not only enhances data privacy and security but also improves the system's adaptability and responsiveness to changing environmental conditions.
Multi-agent modeling is a powerful technique that offers enhanced problem-solving capacity as shown here in various computational experiments in the realm of protein design, physics modeling, and analysis. Given a complex query comprising multi-objective tasks, using the idea of division of labor, the model excels at developing a strategy to break the task into sub-tasks and then, recruiting a set of agents to effectively engage in problem solving tasks in an autonomous fashion. Tool-backed agents have the capacity to execute tools via function execution. We equipped an agent with a rich library of tools that span a broad spectrum of functionalities including de novo protein design, protein folding, and protein secondary structure analysis among others. The fact that there is no intrinsic limitation in customizing the functions, allows us to integrate knowledge across different disciplines into our model and analysis, for instance by integrating knowledge retrieval systems or retrieving physical data via simulations. For instance, here we utilized coarse grained simulations to obtain natural frequencies of proteins but the model offers a high flexibility in defining functions that focus on other particular area simulation (e.g. an expert in performing Density Functional Theory, Molecular Dynamics, or even physics-inspired neural network solvers37,81,82). Multi-agent framework can also accelerate the discovery of de novo proteins with targeted mechanical properties by embracing the power of robust end-to-end deep models solving forward and inverse protein design problems17,24,59,83–86
Developing these models that connect some structural protein features, such as secondary structure, to a material property, such as toughness or strength have gained a lot of attention recently. Here, we used a pre-trained autoregressive transformer model to predict the maximum force and energy of protein unfolding, but other end-to-end models could also be utilized. In the context of inverse protein design problems, a team of two agents, one expert in the forward tasks and the other in the inverse task, can be collaborated to assist the cycle check wherein the de novo proteins certainly meet the specified property criteria. Along the same line, one could benefit from the multi-agent collaboration in evaluating the accuracy of generative models in conditional designing of proteins or compare the created 3D structures with the state-of-the-art folding tools.16,87,88 For example, through an automated process of protein generation and structure analysis, our ProtAgents framework revealed the shortcomings of Chroma in designing β-sheet-rich proteins. In another example, the folded 3D structures of Chroma were compared with those obtained by OmegaFold2. All these examples, demonstrate the capacity of multi-agent framework in a wide range of applications in the context of protein design and analysis. Lastly, the model enables integrating various information across scales, whether new protein sequences or physics simulations output in form of rich data structures, for inclusion in easily readable file formats (like JSON) to be used by other agents or to be stored for future analysis.
Designing de novo proteins that meet special objectives in term of mechanical or structural properties present unique challenges calling for new strategies. The prevailing strategies often rely on developing data-driven end-to-end deep learning models to find the complex mapping from protein constitutive structure to property or vice versa. However, these models often focus on specific properties, limiting their functionality in multi-objective design purposes where several criteria needs to be met. To overcome these challenges and propel the field forward, future research endeavors could revolve around the development of an integrated system of agents designed to automate the entire lifecycle of training deep neural networks for protein design. Each agent within this system could be assigned specific responsibilities, such as data generation through simulations, data curation for ensuring quality and relevance, and the execution of the code required for model training. Additionally, a critic agent could monitor and critique the training process, making decisions like early stopping or tuning hyperparameters to enhance the model's accuracy. This collaborative and automated approach would not only streamline the design process but also contribute to achieving higher or desired levels of accuracy in the generated models. Furthermore, this agent-based strategy can extend to on-the-fly active learning, where agents dynamically adapt the model based on real-time feedback, improving its performance iteratively.
Multi-agent modeling has the potential to transform the landscape of de novo protein design, enhancing efficiency, adaptability, and the capacity to meet diverse and complex design goals, thereby establishing a new paradigm in materials design workflows. To fully realize the transformative potential of multi-agent modeling in de novo protein design, it is important to address the gap in evaluation methodologies that currently exist in the field. A crucial element in the development of AI models involves evaluating their performance, typically done using well-established benchmarks. However, current benchmarks for assessing AI models do not yet incorporate multi-agent strategies and often rely on simplistic single-shot or multi-shot responses. Thus, developing a comprehensive benchmark specifically for evaluating multi-agent strategies in protein tasks presents an intriguing avenue for future research. The development of such benchmarks would greatly enhance the ability to evaluate the success and applicability of LLM-based multi-agent systems in the field of protein science.
4 Materials and methods
4.1 Agent design
As shown in Fig. 1a, we design AI agents using all-purpose LLM GPT-4 and dynamic multi-agent collaboration is implemented in Autogen framework,89 an open-source ecosystem for agent-based AI modeling.In our multi-agent system, the human user_proxy agent is constructed using UserProxyAgent class from Autogen, and Assistant, Planner, Critic agents are created via AssistantAgent class from Autogen; and the group chat manager is created using GroupChatManager class. Each agent is assigned a role through a profile description listed in Table 1, included as system_message at their creation.
4.2 Function and tool design
All the tools implemented in this work are defined as python functions. Each function is characterized by a name, a description, and input properties with a description as tabulated in Table S1 of the ESI.† The list of functions are incorporated into the multi-agent system, included as the function_map parameter in the Assistant agent at its creation.4.3 Autoregressive transformer model to predict protein unfolding force-extension from sequences
We use a special-purpose GPT-style model denoted as ProteinForceGPT, similar as in,85 here trained to predict force-extension curves from sequences along with other mechanical properties, and vice versa (https://huggingface.co/lamm-mit/ProteinForceGPT). The protein language model is based on the NeoGPT-X architecture and uses rotary positional embeddings (RoPE).90 The model has 16 attention heads, 36 hidden layers and a hidden size of 1024, an intermediate size of 4096 and uses GeLU activation functions.Pre-training was conducted based on a dataset of ∼800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 amino acid sequences, using next-token predictions using a “Sequence” task (https://huggingface.co/datasets/lamm-mit/GPTProteinPretrained):
000 amino acid sequences, using next-token predictions using a “Sequence” task (https://huggingface.co/datasets/lamm-mit/GPTProteinPretrained):
The ProteinForceGPT model was then fine-tuned bidirectionally, to predict mechanical properties of proteins from their sequence, as well as sequence candidates that meet a required force-extension behavior and various other properties. Fine-tuning is conducted using a dataset derived from molecular dynamics (MD) simulations.91 Sample tasks for the model include:
Sample results from validation of the model are shown in Fig. S2.† We only use forward predictions for use in the agent model reported here.
4.4 Software versions and hardware
We develop our multi-agent models using local workstations with NVIDIA GPUs. We use Python 3.10 and pyautogen-0.2.2.89 Additional implementation details are included in the code.4.5 Visualization
We use Py3DMol92 for visualization of the protein structures.4.6 Secondary structure analysis
We use the dictionary of protein secondary structure (DSSP)93 module via BioPython94 to analyze the secondary structure content of the proteins from its geometry.4.7 Natural vibrational frequency calculations
We perform Anisotropic Network Model (ANM)95,96 calculations as implemented in ProDy97 for normal mode analysis. The problem is solved by considering the protein as a network of interactions, defined within a cutoff distance for which spring-like potentials are assumed to define molecular interactions.4.8 Retrieval augmented generation
We use Llama Index98 as a tool to implement RAG where the full text of papers cited as ref. 67 and 68 are used as external sources from which information can be retrieved by the system in real-time.Data and code availability
All data and codes are available on GitHub at https://github.com/lamm-mit/ProtAgents. Alternatively, they will be provided by the corresponding author based on reasonable request.Author contributions
MJB and AG conceived the study and developed the multi-agent models. AG performed the tests for various problems, analyzed the results and prepared the first draft of the paper. MJB supported the analysis, revised and finalized the paper with AG.Conflicts of interest
The author declares no conflict of interest.Acknowledgements
We acknowledge support from USDA (2021-69012-35978), DOE-SERDP (WP22-S1-3475), ARO (79058LSCSB, W911NF-22-2-0213 and W911NF2120130) as well as the MIT-IBM Watson AI Lab, MIT's Generative AI Initiative, and Google. Additional support from NIH (U01EB014976 and R01AR077793) ONR (N00014-19-1-2375 and N00014-20-1-2189) is acknowledged. AG gratefully acknowledges the financial support from the Swiss National Science Foundation (#P500PT_214448).Notes and references
- P. S. Huang, S. E. Boyken and D. Baker, Nature, 2016, 537(7620), 320–327 CrossRef CAS PubMed.
- P. Notin, M. Dias, J. Frazer, J. M. Hurtado, A. N. Gomez, D. Marks and Y. Gal, Tranception: Protein Fitness Prediction with Autoregressive Transformers and Inference-time Retrieval, 2022, https://proceedings.mlr.press/v162/notin22a.html.
- J. Ingraham, V. K. Garg, R. Barzilay and T. Jaakkola, Adv. Neural Inf. Process. Syst., 2019, 32, 1–12 Search PubMed.
- K. E. Wu, K. K. Yang, R. vanden Berg, J. Y. Zou, A. X. Lu and A. P. Amini, arXiv, 2022, preprint, arXiv:2209.15611v2, DOI:10.48550/arXiv.2209.15611.
- N. Anand and T. Achim, arXiv, 2022, preprint, arXiv:2205.15019, DOI:10.48550/arXiv.2205.15019.
- R. R. Eguchi, C. A. Choe and P. S. Huang, PLoS Comput. Biol., 2022, 18, e1010271 CrossRef CAS PubMed.
- A. Rives, J. Meier, T. Sercu, S. Goyal, Z. Lin, J. Liu, D. Guo, M. Ott, C. L. Zitnick, J. Ma and R. Fergus, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2016239118 CrossRef CAS PubMed.
- A. Madani, B. McCann, N. Naik, N. S. Keskar, N. Anand, R. R. Eguchi, P.-S. Huang and R. Socher, arXiv, 2020, preprint, arXiv:2004.03497, DOI:10.48550/arXiv.2004.03497.
- N. Anand, R. Eguchi, I. I. Mathews, C. P. Perez, A. Derry, R. B. Altman and P. S. Huang, Nat. Commun., 2022, 13(1), 1–11 Search PubMed.
- J. G. Greener, L. Moffat and D. T. Jones, Sci. Rep., 2018, 8(1), 1–12 CAS.
- A. J. Riesselman, J. B. Ingraham and D. S. Marks, Nat. Methods, 2018, 15(10), 816–822 CrossRef CAS PubMed.
- E. C. Alley, G. Khimulya, S. Biswas, M. AlQuraishi and G. M. Church, Nat. Methods, 2019, 16(12), 1315–1322 CrossRef CAS PubMed.
- J. L. Watson, D. Juergens, N. R. Bennett, B. L. Trippe, J. Yim, H. E. Eisenach, W. Ahern, A. J. Borst, R. J. Ragotte, L. F. Milles, B. I. Wicky, N. Hanikel, S. J. Pellock, A. Courbet, W. Sheffler, J. Wang, P. Venkatesh, I. Sappington, S. V. Torres, A. Lauko, V. D. Bortoli, E. Mathieu, S. Ovchinnikov, R. Barzilay, T. S. Jaakkola, F. DiMaio, M. Baek and D. Baker, Nature, 2023, 620(7976), 1089–1100 CrossRef CAS PubMed.
- I. Anishchenko, S. J. Pellock, T. M. Chidyausiku, T. A. Ramelot, S. Ovchinnikov, J. Hao, K. Bafna, C. Norn, A. Kang, A. K. Bera, F. DiMaio, L. Carter, C. M. Chow, G. T. Montelione and D. Baker, Nature, 2021, 600(7889), 547–552 CrossRef CAS PubMed.
- J. B. Ingraham, M. Baranov, Z. Costello, K. W. Barber, W. Wang, A. Ismail, V. Frappier, D. M. Lord, C. Ng-Thow-Hing, E. R. V. Vlack, S. Tie, V. Xue, S. C. Cowles, A. Leung, J. V. Rodrigues, C. L. Morales-Perez, A. M. Ayoub, R. Green, K. Puentes, F. Oplinger, N. V. Panwar, F. Obermeyer, A. R. Root, A. L. Beam, F. J. Poelwijk and G. Grigoryan, Nature, 2023, 623, 1070–1078 CrossRef CAS PubMed.
- J. Jumper, R. Evans, A. Pritzel, T. Green, M. Figurnov, O. Ronneberger, K. Tunyasuvunakool, R. Bates, A. Žídek, A. Potapenko, A. Bridgland, C. Meyer, S. A. Kohl, A. J. Ballard, A. Cowie, B. Romera-Paredes, S. Nikolov, R. Jain, J. Adler, T. Back, S. Petersen, D. Reiman, E. Clancy, M. Zielinski, M. Steinegger, M. Pacholska, T. Berghammer, S. Bodenstein, D. Silver, O. Vinyals, A. W. Senior, K. Kavukcuoglu, P. Kohli and D. Hassabis, Nature, 2021, 596(7873), 583–589 CrossRef CAS PubMed.
- C. H. Yu, W. Chen, Y. H. Chiang, K. Guo, Z. M. Moldes, D. L. Kaplan and M. J. Buehler, ACS Biomater. Sci. Eng., 2022, 8, 1156–1165 CrossRef CAS PubMed.
- A. Elnaggar, M. Heinzinger, C. Dallago, G. Rehawi, Y. Wang, L. Jones, T. Gibbs, T. Feher, C. Angerer, M. Steinegger, D. Bhowmik and B. Rost, IEEE Trans. Pattern Anal. Mach. Intell., 2022, 44, 7112–7127 Search PubMed.
- C. Mirabello and G. Pollastri, Bioinformatics, 2013, 29, 2056–2058 CrossRef CAS PubMed.
- G. Pollastri, D. Przybylski, B. Rost and P. Baldi, Proteins: Struct., Funct., Bioinf., 2002, 47, 228–235 CrossRef CAS PubMed.
- B. Zhang, J. Li and Q. Lü, BMC Bioinf., 2018, 19, 1–13 CrossRef PubMed.
- G. Pollastri and A. McLysaght, Bioinformatics, 2005, 21, 1719–1720 CrossRef CAS PubMed.
- K. Guo and M. J. Buehler, Digital Discovery, 2022, 1, 277–285 RSC.
- Y. Hu and M. J. Buehler, ACS Nano, 2022, 16, 20656–20670 CrossRef CAS.
- J. Wei, Y. Tay, R. Bommasani, C. Raffel, B. Zoph, S. Borgeaud, D. Yogatama, M. Bosma, D. Zhou, D. Metzler, E. H. Chi, T. Hashimoto, O. Vinyals, P. Liang, J. Dean and W. Fedus, arXiv, 2022, preprint, arXiv:2206.07682, DOI:10.48550/arXiv.2206.07682.
- Y. Chang, X. U. Wang, Y. Wu, H. Chen, W. Ye, Y. Zhang, Y. I. Chang, P. S. Yu, X. Yi, Y. Chang, Q. Yang, H. Kong, X. Wang, J. Wang, L. Yang, K. Zhu, C. Wang, Y. Wang and X. Xie, J. ACM, 2023, 37, 42 Search PubMed.
- K. M. Jablonka, Q. Ai, A. Al-Feghali, S. Badhwar, J. D. Bocarsly, A. M. Bran, S. Bringuier, L. C. Brinson, K. Choudhary, D. Circi, S. Cox, W. A. deJong, M. L. Evans, N. Gastellu, J. Genzling, M. V. Gil, A. K. Gupta, Z. Hong, A. Imran, S. Kruschwitz, A. Labarre, J. Lála, T. Liu, S. Ma, S. Majumdar, G. W. Merz, N. Moitessier, E. Moubarak, B. Mouriño, B. Pelkie, M. Pieler, M. C. Ramos, B. Ranković, S. G. Rodriques, J. N. Sanders, P. Schwaller, M. Schwarting, J. Shi, B. Smit, B. E. Smith, J. V. Herck, C. Völker, L. Ward, S. Warren, B. Weiser, S. Zhang, X. Zhang, G. A. Zia, A. Scourtas, K. J. Schmidt, I. Foster, A. D. White and B. Blaiszik, Digital Discovery, 2023, 2, 1233–1250 RSC.
- M. J. Buehler, J. Mech. Phys. Solids, 2023, 181, 105454 CrossRef.
- M. J. Buehler, Appl. Mech. Rev., 2023, 1–82 CAS.
- J. Boyko, J. Cohen, N. Fox, M. H. Veiga, J. I.-H. Li, J. Liu, B. Modenesi, A. H. Rauch, K. N. Reid, S. Tribedi, A. Visheratina and X. Xie, arXiv, 2023, preprint, arXiv:2311.04929, DOI:10.48550/arXiv.2311.04929.
- B. Ni and M. J. Buehler, Extreme Mech. Lett., 2024, 102131 CrossRef.
- N. R. Brodnik, S. Carton, C. Muir, S. Ghosh, D. Downey, M. P. Echlin, T. M. Pollock and S. Daly, J. Appl. Mech., 2023, 90, 101008 CrossRef.
- R. Tinn, H. Cheng, Y. Gu, T. Naumann, J. Gao, H. P. Correspondence, N. Usuyama, X. Liu and H. Poon, Patterns, 2023, 4, 100729 CrossRef CAS PubMed.
- Y. Hu and M. J. Buehler, APL Mach. Learn., 2023, 1, 10901 CrossRef.
- M. J. Buehler, ACS Eng. Au, 2023, 1–11 CAS.
- R. K. Luu and M. J. Buehler, J. Appl. Mech., 2023, 90, 090801 CrossRef.
- G. C. Peng, M. Alber, A. B. Tepole, W. R. Cannon, S. De, S. Dura-Bernal, K. Garikipati, G. Karniadakis, W. W. Lytton, P. Perdikaris, L. Petzold and E. Kuhl, Arch. Comput. Methods Eng., 2021, 28, 1017–1037 CrossRef PubMed.
- T. B. Brown, B. Mann, N. Ryder, M. Subbiah, J. Kaplan, P. Dhariwal, A. Neelakantan, P. Shyam, G. Sastry, A. Askell, S. Agarwal, A. Herbert-Voss, G. Krueger, T. Henighan, R. Child, A. Ramesh, D. M. Ziegler, J. Wu, C. Winter, C. Hesse, M. Chen, E. Sigler, M. Litwin, S. Gray, B. Chess, J. Clark, C. Berner, S. Mccandlish, A. Radford, I. Sutskever and D. Amodei, Adv. Neural Inf. Process. Syst., 2020, 33, 1877–1901 Search PubMed.
- M. Bates, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 9977–9982 CrossRef CAS.
- R. Thoppilan, D. D. Freitas, J. Hall, N. Shazeer, A. Kulshreshtha, H.-T. Cheng, A. Jin, T. Bos, L. Baker, Y. Du, Y. Li, H. Lee, H. S. Zheng, A. Ghafouri, M. Menegali, Y. Huang, M. Krikun, D. Lepikhin, J. Qin, D. Chen, Y. Xu, Z. Chen, A. Roberts, M. Bosma, V. Zhao, Y. Zhou, C.-C. Chang, I. Krivokon, W. Rusch, M. Pickett, P. Srinivasan, L. Man, K. Meier-Hellstern, M. R. Morris, T. Doshi, R. D. Santos, T. Duke, J. Soraker, B. Zevenbergen, V. Prabhakaran, M. Diaz, B. Hutchinson, K. Olson, A. Molina, E. Hoffman-John, J. Lee, L. Aroyo, R. Rajakumar, A. Butryna, M. Lamm, V. Kuzmina, J. Fenton, A. Cohen, R. Bernstein, R. Kurzweil, B. Aguera-Arcas, C. Cui, M. Croak, E. Chi and Q. Le, arXiv, 2022, preprint, arXiv:2201.08239, DOI:10.48550/arXiv.2201.08239.
- A. Chowdhery, S. Narang, J. Devlin, M. Bosma, G. Mishra, A. Roberts, P. Barham, H. W. Chung, C. Sutton, S. Gehrmann, P. Schuh, K. Shi, S. Tsvyashchenko, J. Maynez, A. Rao, P. Barnes, Y. Tay, N. Shazeer, V. Prabhakaran, E. Reif, N. Du, B. Hutchinson, R. Pope, J. Bradbury, J. Austin, M. Isard, G. Gur-Ari, P. Yin, T. Duke, A. Levskaya, S. Ghemawat, S. Dev, H. Michalewski, X. Garcia, V. Misra, K. Robinson, L. Fedus, D. Zhou, D. Ippolito, D. Luan, H. Lim, B. Zoph, A. Spiridonov, R. Sepassi, D. Dohan, S. Agrawal, M. Omernick, A. M. Dai, T. S. Pillai, M. Pellat, A. Lewkowycz, E. Moreira, R. Child, O. Polozov, K. Lee, Z. Zhou, X. Wang, B. Saeta, M. Diaz, O. Firat, M. Catasta, J. Wei, K. Meier-Hellstern, D. Eck, J. Dean, S. Petrov and N. Fiedel, J. Mach. Learn. Res., 2023, 24, 1–113 Search PubMed.
- A. Vaswani, N. Shazeer, N. Parmar, J. Uszkoreit, L. Jones, A. N. Gomez, Ł. Kaiser and I. Polosukhin, Adv. Neural Inf. Process. Syst., 2017, 30, 1–11 Search PubMed.
- OpenAI, J. Achiam, S. Adler, S. Agarwal, L. Ahmad, I. Akkaya, F. L. Aleman, D. Almeida, J. Altenschmidt, S. Altman, S. Anadkat, R. Avila, I. Babuschkin, S. Balaji, V. Balcom, P. Baltescu, H. Bao, M. Bavarian, J. Belgum, I. Bello, J. Berdine, G. Bernadett-Shapiro, C. Berner, L. Bogdonoff, O. Boiko, M. Boyd, A.-L. Brakman, G. Brockman, T. Brooks, M. Brundage, K. Button, T. Cai, R. Campbell, A. Cann, B. Carey, C. Carlson, R. Carmichael, B. Chan, C. Chang, F. Chantzis, D. Chen, S. Chen, R. Chen, J. Chen, M. Chen, B. Chess, C. Cho, C. Chu, H. W. Chung, D. Cummings, J. Currier, Y. Dai, C. Decareaux, T. Degry, N. Deutsch, D. Deville, A. Dhar, D. Dohan, S. Dowling, S. Dunning, A. Ecoffet, A. Eleti, T. Eloundou, D. Farhi, L. Fedus, N. Felix, S. P. Fishman, J. Forte, I. Fulford, L. Gao, E. Georges, C. Gibson, V. Goel, T. Gogineni, G. Goh, R. Gontijo-Lopes, J. Gordon, M. Grafstein, S. Gray, R. Greene, J. Gross, S. S. Gu, Y. Guo, C. Hallacy, J. Han, J. Harris, Y. He, M. Heaton, J. Heidecke and C. Hesse, arXiv, 2023, preprint, arXiv:2303.08774, DOI:10.48550/arXiv.2303.08774.
- M. Chen, J. Tworek, H. Jun, Q. Yuan, H. P. deOliveiraPinto, J. Kaplan, H. Edwards, Y. Burda, N. Joseph, G. Brockman, A. Ray, R. Puri, G. Krueger, M. Petrov, H. Khlaaf, G. Sastry, P. Mishkin, B. Chan, S. Gray, N. Ryder, M. Pavlov, A. Power, L. Kaiser, M. Bavarian, C. Winter, P. Tillet, F. P. Such, D. Cummings, M. Plappert, F. Chantzis, E. Barnes, A. Herbert-Voss, W. H. Guss, A. Nichol, A. Paino, N. Tezak, J. Tang, I. Babuschkin, S. Balaji, S. Jain, W. Saunders, C. Hesse, A. N. Carr, J. Leike, J. Achiam, V. Misra, E. Morikawa, A. Radford, M. Knight, M. Brundage, M. Murati, K. Mayer, P. Welinder, B. McGrew, D. Amodei, S. McCandlish, I. Sutskever and W. Zaremba, arXiv, 2021, preprint, arXiv:2107.03374, DOI:10.48550/arXiv.2107.03374.
- S. Bubeck, V. Chandrasekaran, R. Eldan, J. Gehrke, E. Horvitz, E. Kamar, P. Lee, Y. T. Lee, Y. Li, S. Lundberg, H. Nori, H. Palangi, M. T. Ribeiro and Y. Zhang, arXiv, 2023, preprint, arXiv:2303.12712, DOI:10.48550/arXiv.2303.12712.
- J. Austin, A. Odena, M. Nye, M. Bosma, H. Michalewski, D. Dohan, E. Jiang, C. Cai, M. Terry, Q. Le, et al., arXiv, 2021, preprint arXiv:2108.07732, DOI:10.48550/arXiv.2108.07732.
- K. Guo, Z. Yang, C. H. Yu and M. J. Buehler, Mater. Horiz., 2021, 8, 1153–1172 RSC.
- Z. Li, F. Liu, W. Yang, S. Peng and J. Zhou, IEEE Trans. Neural Netw. Learn. Syst., 2022, 33, 6999–7019 Search PubMed.
- K. O'Shea and R. Nash, Int. J. Res. Appl. Sci. Eng. Technol., 2015, 10, 943–947 Search PubMed.
- E. L. Buehler and M. J. Buehler, Biomed. Eng. Adv., 2022, 4, 100038 CrossRef.
- Z. Yang, C. H. Yu and M. J. Buehler, Sci. Adv., 2021, 7, 1–10 Search PubMed.
- D. Repecka, V. Jauniskis, L. Karpus, E. Rembeza, I. Rokaitis, J. Zrimec, S. Poviloniene, A. Laurynenas, S. Viknander, W. Abuajwa, O. Savolainen, R. Meskys, M. K. Engqvist and A. Zelezniak, Nat. Mach. Intell., 2021, 3, 324–333 CrossRef.
- R. Cao, C. Freitas, L. Chan, M. Sun, H. Jiang and Z. Chen, Molecules, 2017, 22, 1732 CrossRef PubMed.
- Y.-C. Hsu, C.-H. Yu and M. J. Buehler, Matter, 2020, 3, 197–211 CrossRef.
- W. Lu, Z. Yang and M. J. Buehler, J. Appl. Phys., 2022, 132, 74703 CrossRef CAS.
- A. Strokach, D. Becerra, C. Corbi-Verge, A. Perez-Riba and P. M. Kim, Cell Syst., 2020, 11, 402–411.e4 CrossRef CAS PubMed.
- R. You, S. Yao, H. Mamitsuka and S. Zhu, Bioinformatics, 2021, 37, i262–i271 CrossRef CAS PubMed.
- Z. Yang and M. J. Buehler, npj Comput. Mater., 2022, 8(1), 1–13 CrossRef.
- W. Lu, D. L. Kaplan and M. J. Buehler, Adv. Funct. Mater., 2023, 2311324 Search PubMed.
- H. Zhang, W. Du, J. Shan, Q. Zhou, Y. Du, J. B. Tenenbaum, T. Shu and C. Gan, arXiv, 2023, preprint, arXiv:2307.02485, DOI:10.48550/arXiv.2307.02485.
- L. Wang, C. Ma, X. Feng, Z. Zhang, H. Yang, J. Zhang, Z. Chen, J. Tang, X. Chen, Y. Lin, W. X. Zhao, Z. Wei and J.-R. Wen, Front. Comput. Sci., 2024, 18, 186345 CrossRef.
- Z. Xi, W. Chen, X. Guo, W. He, Y. Ding, B. Hong, M. Zhang, J. Wang, S. Jin, E. Zhou, R. Zheng, X. Fan, X. Wang, L. Xiong, Y. Zhou, W. Wang, C. Jiang, Y. Zou, X. Liu, Z. Yin, S. Dou, R. Weng, W. Cheng, Q. Zhang, W. Qin, Y. Zheng, X. Qiu, X. Huang and T. Gui, arXiv, 2023, preprint, arXiv:2309.07864, DOI:10.48550/arXiv.2309.07864.
- D. A. Boiko, R. MacKnight, B. Kline and G. Gomes, Nature, 2023, 624, 570–578 CrossRef CAS PubMed.
- A. M. Bran, S. Cox, O. Schilter, C. Baldassari, A. D. White and P. Schwaller, Nat. Mach. Intell., 2024, 6, 525–535 CrossRef PubMed.
- S. Gao, A. Fang, Y. Huang, V. Giunchiglia, A. Noori, J. R. Schwarz, Y. Ektefaie, J. Kondic and M. Zitnik, Empowering Biomedical Discovery with AI Agents, 2024 Search PubMed.
- OpenAI API, https://openai.com/blog/openai-api.
- M. Sikora, J. I. Sułkowska and M. Cieplak, PLoS Comput. Biol., 2009, 5, e1000547 CrossRef PubMed.
- J. I. Sułkowska and M. Cieplak, J. Phys.: Condens. Matter, 2007, 19, 283201 CrossRef.
- T. Ackbarow, X. Chen, S. Keten and M. J. Buehler, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16410–16415 CrossRef CAS PubMed.
- T. P. Knowles and M. J. Buehler, Nat. Nanotechnol., 2011, 6(8), 469–479 CrossRef CAS PubMed.
- Z. Xu and M. J. Buehler, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2010, 81, 061910 CrossRef PubMed.
- Z. Qin and M. J. Buehler, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2010, 82, 061906 CrossRef PubMed.
- T. Guo, X. Chen, Y. Wang, R. Chang, S. Pei, N. V. Chawla, O. Wiest and X. Zhang, Large Language Model based Multi-Agents: A Survey of Progress and Challenges, arXiv, 2024, preprint, arXiv:2402.01680, DOI:10.48550/arXiv.2402.01680.
- L. Huang, W. Yu, W. Ma, W. Zhong, Z. Feng, H. Wang, Q. Chen, W. Peng, X. Feng, B. Qin and T. Liu, A Survey on Hallucination in Large Language Models: Principles, Taxonomy, Challenges, and Open Questions, arXiv, 2023, preprint, arXiv:2311.05232, DOI:10.48550/arXiv.2311.05232.
- A. Dorri, S. S. Kanhere and R. Jurdak, IEEE Access, 2018, 6, 28573–28593 Search PubMed.
- C. P. Gomes, J. Bai, Y. Xue, J. Björck, B. Rappazzo, S. Ament, R. Bernstein, S. Kong, S. K. Suram and R. B. van Dover etal, MRS Commun., 2019, 9, 600–608 CrossRef CAS.
- J. H. Montoya, K. T. Winther, R. A. Flores, T. Bligaard, J. S. Hummelshøj and M. Aykol, Chem. Sci., 2020, 11, 8517–8532 RSC.
- A. G. Kusne and A. McDannald, Matter, 2023, 6, 1880–1893 CrossRef.
- Q. Yang, Y. Liu, T. Chen and Y. Tong, ACM Trans. Intell. Syst. Technol., 2019, 10, 1–19 Search PubMed.
- C. Zhang, Y. Xie, H. Bai, B. Yu, W. Li and Y. Gao, Knowl.-Based Syst., 2021, 216, 106775 CrossRef.
- I. E. Lagaris, A. Likas and D. I. Fotiadis, IEEE Trans. Neural Netw., 1998, 9, 987–1000 CrossRef CAS PubMed.
- M. Raissi, P. Perdikaris and G. E. Karniadakis, J. Comput. Phys., 2019, 378, 686–707 CrossRef.
- F. Y. Liu, B. Ni and M. J. Buehler, Extreme Mech. Lett., 2022, 55, 101803 CrossRef.
- C. H. Yu and M. J. Buehler, APL Bioeng., 2020, 4, 16108 CrossRef CAS PubMed.
- M. J. Buehler, J. Appl. Phys., 2023, 134, 084902 CrossRef CAS.
- B. Ni, D. L. Kaplan and M. J. Buehler, Chem, 2023, 9, 1828–1849 CAS.
- M. AlQuraishi, Curr. Opin. Chem. Biol., 2021, 65, 1–8 CrossRef CAS PubMed.
- W. Gao, S. P. Mahajan, J. Sulam and J. J. Gray, Patterns, 2020, 1, 100142 CrossRef CAS PubMed.
- Q. Wu, G. Bansal, J. Zhang, Y. Wu, B. Li, E. Zhu, L. Jiang, X. Zhang, S. Zhang, J. Liu, A. Awadallah, R. W. White, D. Burger and C. Wang, arXiv, 2023, preprint, arXiv:2308.08155, DOI:10.48550/arXiv.2308.08155.
- J. Su, M. Ahmed, Y. Lu, S. Pan, W. Bo and Y. Liu, Neurocomputing, 2024, 568, 127063 CrossRef.
- B. Ni, D. L. Kaplan and M. J. Buehler, Sci. Adv., 2023, 1–15 CrossRef.
- N. Rego and D. Koes, Bioinformatics, 2015, 31, 1322–1324 CrossRef PubMed.
- W. Kabsch and C. Sander, Biopolymers, 1983, 22, 2577–2637 CrossRef CAS PubMed.
- P. J. Cock, T. Antao, J. T. Chang, B. A. Chapman, C. J. Cox, A. Dalke, I. Friedberg, T. Hamelryck, F. Kauff, B. Wilczynski and M. J. D. Hoon, Bioinformatics, 2009, 25, 1422 CrossRef CAS PubMed.
- A. R. Atilgan, S. R. Durell, R. L. Jernigan, M. C. Demirel, O. Keskin and I. Bahar, Biophys. J., 2001, 505–515 CrossRef CAS PubMed.
- P. Doruker, A. R. Atilgan and I. Bahar, Proteins: Struct., Funct., Bioinf., 2000, 512–524 CrossRef CAS.
- A. Bakan, L. M. Meireles and I. Bahar, Bioinformatics, 2011, 27, 1575–1577 CrossRef CAS PubMed.
- LlamaIndex (formerly GPT Index), a data framework for LLM applications, https://github.com/run-llama/llama_index.
Footnote |
| † Electronic supplementary information (ESI) available: The full records of different conversation experiments along with additional materials are provided as supplementary materials. See DOI: https://doi.org/10.1039/d4dd00013g |
| This journal is © The Royal Society of Chemistry 2024 |