The effect and mechanism of a microbial agent used for corrosion control in circulating cooling water†
Yu
Wang
 a,
Hongfeng
Liao
b,
Li
Gan
b,
Zhengxiu
Liu
c,
Ziqiang
Tang
c,
Xiaoran
Zhao
c,
Yubin
Zeng
*a and
Chunsong
Ye
*a
a,
Hongfeng
Liao
b,
Li
Gan
b,
Zhengxiu
Liu
c,
Ziqiang
Tang
c,
Xiaoran
Zhao
c,
Yubin
Zeng
*a and
Chunsong
Ye
*a
aDepartment of Energy Chemistry Engineering, School of Power and Mechanical Engineering, Wuhan University, Wuhan 430072, China. E-mail: zengyubin@whu.edu.cn; csye@whu.edu.cn
bJingneng Shiyan Thermal Power Co., Ltd., Shiyan 442000, China
cBeijing Jingneng Energy Technology Research Co., Ltd., Beijing 100022, China
First published on 16th November 2023
Abstract
Corrosion control is vital for the safe operation of a circulating cooling water system. The biological method is a novel treatment method performed by adding a microbial agent to achieve corrosion control. In this study, microbial agents, including Bacillus cereus, Pseudomonas, Bacillus subtilis, and Thiobacillus denitrificans, were selected to investigate the anti-corrosion effect and mechanism of the biological method. The results indicated that the anti-corrosion efficiency of the microbial agent on Q235B carbon steel ranged from 8.06–9.02%, while that for 316L stainless steel was 62.80%. The anti-corrosion mechanisms of functional bacteria were also revealed, which included (a) the formation of a biofilm to isolate oxygen and corrosive substances, (b) the inhibition of a cathodic reduction reaction and reduction of corrosion current, and (c) the inhibition of sulfide corrosion.
Water impactCorrosion control is vital for the safe operation of a circulating cooling water system. Biological treatment is a novel, efficient, and environmentally friendly method used for corrosion control achieved through adding a microbial agent. Our study provides a deeper understanding of the roles of functional bacteria, allowing for the advanced development and application of the microbial agent used for water treatment. |
1. Introduction
Carbon steel and stainless steel are commonly used metal materials for pipelines and heat exchangers in circulating cooling water systems used in power plants. Under the action of oxygen and aggressive ions in circulating cooling water, carbon steel, and stainless steel may undergo oxygen consumption corrosion, pitting corrosion, and other corrosion behaviors.1 Corrosion could cause condenser tube perforation, leakage of circulating cooling water to the thermal system, and other adverse effects.2 Therefore, processes that foster the anti-corrosion of metals are of great significance to the safe operation of circulating cooling water systems.Adding a chemical corrosion inhibitor is a common method for circulating cooling water treatment.3 Organic phosphonates, chromate, and molybdate are common corrosion inhibitors, which are usually used in conjunction with scale inhibitors. Chemical methods have good anti-corrosion and anti-scaling effects.4,5 However, the extensive use of chemical agents may cause problems, such as deterioration of water quality. Related studies have shown that the application of corrosion and scale inhibitors led to the total phosphorus content of circulating cooling water exceeding 3 mg L−1.6 This could increase the risk of the water quality exceeding effluent standards. Therefore, the development of an environmentally friendly method for corrosion control has become a research priority in circulating cooling water treatment.
The biological method is used for circulating cooling water treatment by means of adding a specific microbial agent and utilizing the synergistic effect of functional bacteria metabolism.7 Chen et al.8 used compound microbial preparation (CMP) with nitrobacteria, Bacillus subtilis (B. subtilis), photosynthetic bacteria, and Thiobacillus denitrificans (T. denitrificans, TDN), to treat circulating cooling water. The results showed that CMP had good corrosion and scale inhibition effect, and the corrosion inhibition rate on Q235 carbon steel reached 99.69%. Hu et al.9,10 evaluated the corrosion inhibition behavior of Bacillus cereus (B. cereus) on Q235 carbon steel in simulated cooling water, whose anti-corrosion efficiency was 73.88%. The results showed that the addition of B. cereus increased the charge transfer resistance of the metal and reduced the corrosion current density, allowing it to attach to the surface of Q235 carbon steel to form a tight biofilm.
Some research studied the anti-corrosion effect of microbial secretions. Li et al.11 studied soluble extracellular polymeric substances (s-EPS) secreted by B. cereus as a new biological agent with anti-corrosion and anti-scaling effects. The results showed that the corrosion rate of s-EPS (40 mg L−1) on 316L stainless steel in an artificial seawater medium reached 91.16% in 30 d. Jayaraman et al.12 studied the corrosion inhibition of aluminum 2024 using the formation of biofilms by B. subtilis WB600 and Bacillus licheniformis, respectively. The results indicated that the polyaspartate and γ-polyglutamate, which originated from biofilms, slowed down the pitting corrosion. The carboxylic acid group could be capable of chelating or coupling aluminum ions or aluminum oxides that were present at the metal–solution interface via hydrogen bonds, dipole–dipole, and coulombic interactions. Gunasekaran et al.13 found that the corrosion rate of carbon steel significantly decreased due to the formation of a surface biofilm in a phosphate-buffered basal salt solution containing Pseudomonas cichorii. The FTIR results proved that the biofilm protects the metal due to the ferrous ions and EPS that formed the Fe-EPS organo-metal complex layer. However, differences exist in the anti-corrosion effect of biofilm formed by microbial functional bacteria on different metals. The process of biofilm formation by functional bacteria and the interaction between biofilm and metal have not yet been clearly recognized. Therefore, it is important to clarify the anti-corrosion mechanism of functional bacteria for engineering the application of microbial agents.
In this paper, Q235B carbon steel and 316L stainless steel were selected to study the anti-corrosion effect of microbial agents and reveal the mechanism of functional bacteria. The corrosion rates of metal coupons with and without the microbial agent were compared by a rotary coupon test. A corrosion electrochemistry test monitored the attachment process of microorganisms onto the metal surface and calculated the anti-corrosion effect of the microbial agent. A biofilm observation test observed the formation process of the biofilm. Our study provides a deeper understanding of the anti-corrosion mechanism of functional bacteria, allowing for the advanced development and application of the microbial agent used for corrosion control in circulating cooling water.
2. Materials and methods
2.1 Materials pretreatment
In this study, a dry powder of the microbial agent was purchased from Yuanneng Chemical Co., Ltd. (Tieling, China). The microbial agent was composed of B. cereus, Pseudomonas, B. subtilis, and T. denitrificans, and the proportion was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Moreover, carbohydrates, used as an assistant agent, were used to meet the needs for microbial growth, which included saccharose, arabinose and glucose. The microbial agent of dry powder was pretreated to activate the microorganism, which was mixed with carbohydrates and normal saline. The mixed liquid was heated in a water bath at 36 °C for 24 h. The liquid microbial agent was prepared and used for future experiments. The effective microbial content was varied in the range of 109–1010 CFU L−1. Other chemicals were of analytical grade throughout the experiments. The circulating cooling water of a power plant was used as the test water, and the water quality depended on temperature and other factors. The water quality index is shown in Table S1.†
1. Moreover, carbohydrates, used as an assistant agent, were used to meet the needs for microbial growth, which included saccharose, arabinose and glucose. The microbial agent of dry powder was pretreated to activate the microorganism, which was mixed with carbohydrates and normal saline. The mixed liquid was heated in a water bath at 36 °C for 24 h. The liquid microbial agent was prepared and used for future experiments. The effective microbial content was varied in the range of 109–1010 CFU L−1. Other chemicals were of analytical grade throughout the experiments. The circulating cooling water of a power plant was used as the test water, and the water quality depended on temperature and other factors. The water quality index is shown in Table S1.†
2.2 Rotary coupon test
The aim of the rotary coupon test is to study the anti-corrosion effect of the microbial agent on Q235B carbon steel and 316L stainless steel. The Q235B and 316L coupons should be pretreated according to the Chinese Standard GB/T 18175-2014.14 The coupons were installed in the beaker of the rotary hanging-piece corrosion device. The circulating cooling water was used as a test solution. In one group, a liquid microbial agent was added with a concentration of 50 mL L−1 and carbohydrate daily with a concentration of 75 mg L−1. The other group did not add the microbial agent. The solution was kept at a constant temperature of 36 °C for 30 d, and the rotation speed was set to 100 r min−1. Three parallel samples were tested in each group. The corrosion rate (va) and anti-corrosion efficiency (ηc) of the microbial agent could be calculated according to eqn (1) and (2).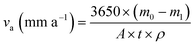 | (1) |
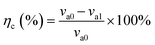 | (2) |
3650 is the conversion factor; m0 and m1 are the weights of the coupon before and after corrosion (g); A is the surface area of the coupon (cm2); t is the corrosion time (d); ρ is the density of the material coupon (g cm−3); va1 and va0 are the average corrosion rate with and without the microbial agent (mm a−1), respectively.
The coupon with attachment after corrosion was dried under natural conditions. The surface attachment on the coupon was detected by energy dispersive spectroscopy (Aztec Energy EDS, Oxford Instruments) for the elemental component and analyzed by XPert Pro X-ray diffraction (XRD, Malvern Panalytical) and K-Alpha X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific) for the material component. The coupon was further pickled according to the Chinese Standard GB/T 18175-2014.15 The pickling of the coupon was conducted to remove the corrosion product and surface attachment. The coupons after corrosion and after pickling were observed by MIRA 3 field emission scanning electron microscopy (SEM, Tescan) for microscopic morphology.
2.3 Corrosion electrochemistry test
The conventional three-electrode apparatus measured the open circuit potential (OCP), electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization. The working electrode material used Q235B carbon steel and 316L stainless steel. The working electrode had a working area of 0.5 cm2 and a material density of 7.85 g cm−3 (Q235B carbon steel) and 8 g cm−3 (316L stainless steel). Before the test, the working electrode was mechanically polished and washed with deionized water. The reference electrode used a saturated calomel electrode (SCE) and was placed in an L-shaped salt bridge with the saturated KCl solution. The auxiliary electrode was a 10 mm × 10 mm platinum sheet electrode.The working electrode was installed in the beaker of the rotary hanging-piece corrosion device, and the working surface was immersed in solution. The circulating cooling water was used as a test solution. In one group, a liquid microbial agent was added with a concentration of 50 mL L−1 and carbohydrate daily with a concentration of 75 mg L−1. The other group did not add an agent. The solution was kept at a constant temperature of 36 °C for 30 d, and the rotation speed was set to 100 r min−1. The working electrode was removed at regular intervals for the electrochemistry test. The test solution was taken out from the beaker as a test electrolyte. All electrochemical tests were performed at the electrochemical workstation.
The OCP was the electrode potential measured when the metal was not carrying current. The EIS test was performed at a stable OCP and a sinusoidal signal of 10 mV was applied to the electrode. The frequency range was 0.1–105 Hz. The OCP and EIS tests were non-destructive, which allowed for the continuous monitoring of the influence of microorganisms on the electrode and the analysis of the corrosion behavior of the electrode. The potentiodynamic polarization method was scanned at the potential range of −0.5 V to +0.5 V (vs. OCP) with a scan rate of 1 mV s−1. Potentiodynamic polarization was a destructive test and only measured at the end of the test. The anti-corrosion efficiency (ηc) could be calculated according to corrosion current density (icorr), and the equation was as follows:
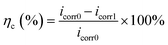 | (3) |
2.4 Biofilm observation test
A biofilm observation test was performed with the intent of observing the biofilm formation process. The 316L stainless steel coupon was selected and pretreated in the same way as the rotary coupon test. The 316L coupon was installed in the beaker of the rotary hanging-piece corrosion device. The circulating cooling water was used as a test solution. A liquid microbial agent with a concentration of 50 mL L−1 was added to the solution, and carbohydrate with a concentration of 75 mg L−1 was added daily. The solution was kept at 36 °C for 30 d, and the speed was set to 100 r min−1.The coupon-attached biofilm was removed from the beaker every 5 d, and soaked in a phosphate buffer solution containing 3% glutaraldehyde for 4 h to immobilize the biofilm. Then the coupon was dehydrated successively with an ethanol gradient: 25%, 50%, 75%, 90%, and 100% for 10 min.15,16 The coupon-attached biofilm was observed by SEM and optical surface profilometer (OSP). The organic component of the biofilm was analyzed by Fourier transform infrared spectroscopy (FTIR), where a diamond crystal was used as the reflection accessory.
3. Results and discussion
3.1 Coupon corrosion characteristic
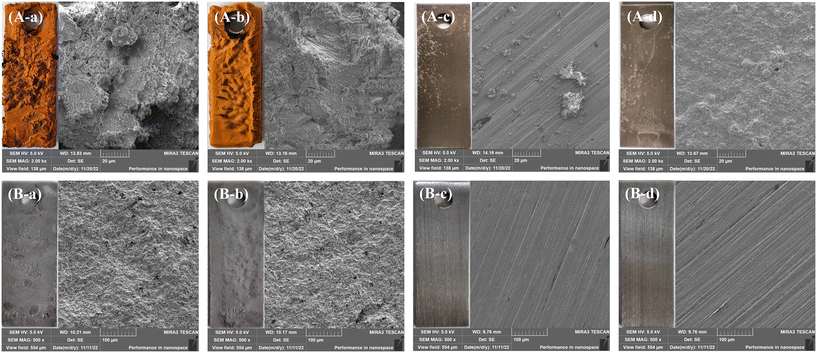
Table S2† shows the corrosion rate of Q235B and 316L coupons after 30 d. The average corrosion rate of Q235B without microbial agent was 0.7261 ± 0.0167 mm a−1, which was slightly higher than that with microbial agent (0.6606 ± 0.0098 mm a−1). The anti-corrosion efficiency of the microbial agent on Q235B carbon steel was 9.02%. However, the corrosion rate of Q235B carbon steel with and without microbial agent exceeded the limit value of the Chinese Standard GB/T 50050-2017.17 Q235B carbon steel corroded under the water quality condition of circulating cooling water. The average corrosion rates of 316L with and without microbial agent were 0.0005 ± 0.0002 and 0.0006 ± 0.0001 mm a−1, respectively. The corrosion rate of 316L stainless steel with and without the microbial agent was not different and was much lower than the limit value of 0.005 mm a−1. The 316L stainless steel did not incur the obvious corrosion behavior under the circulating cooling water.Fig. 1 shows the appearance image and SEM image of Q235B and 316L coupons after corrosion and pickling. There was no significant difference between the surface of the Q235B coupon with and without microbial agent after corrosion. The Q235B coupon generated a corrosion product layer that covered the whole surface of the coupon. The scattered attachment appeared on the 316L coupon surface without the microbial agent after corrosion. This could be a particle impurity originating from the circulating cooling water, which remained on the metal surface. A layer of film appeared on the 316L coupon surface with microbial agent after corrosion because the functional bacteria could attach to the metal surface and form a biofilm layer. The surface of the Q235B coupon after pickling with and without microbial agent was uneven and had no metallic luster, but the surface had no obvious pit. The corrosion process of Q235B carbon steel with and without microbial agent in circulating cooling water was uniform corrosion, rather than pitting corrosion. The surface of the 316L coupon after pickling with and without microbial agent was flat and had metallic luster. The 316L stainless steel with and without microbial agent in circulating cooling water had no obvious corrosion behavior.
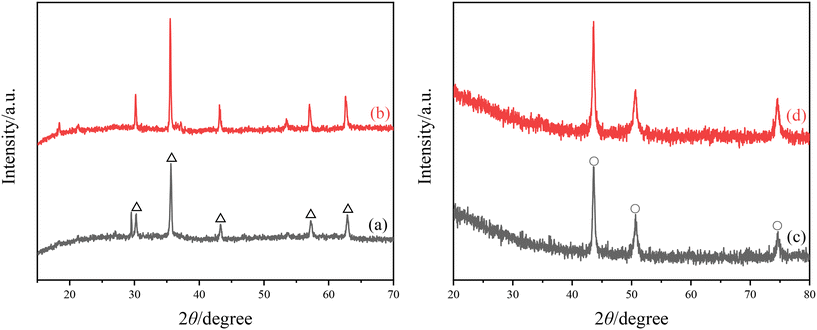
The XRD images of surface attachment on the Q235B and 316L coupons are shown in Fig. 2. The diffraction peaks of Fe3O4 magnetite crystal (PDF Card No. 99-0073) appeared in attachment of Q235B coupon with and without microbial agent, corresponding to 30.43°(220), 35.43°(311), 43.05°(400), 56.94°(511), and 62.52°(440), respectively. The results indicate that Q235B carbon steel underwent oxygen consumption corrosion and generated the corrosion products of Fe3O4. The diffraction peaks of Cr0.19Fe0.7Ni0.11 austenite crystal (PDF Card No. 33-0397) appeared in the attachment of 316L coupon with and without microbial agent, corresponding to 43.58°(111), 50.79°(200), and 74.68°(220), respectively. In fact, a Cr0.19Fe0.7Ni0.11 austenite crystal was the base of 316L stainless steel rather than an attachment.
The elemental component for the surface attachment of Q235B and 316L coupons by EDS analysis is shown in Table 1. Four elements of C, O, S, and Fe were present in the attachment of the Q235B coupon after corrosion. The C, O, and Fe were derived from the Fe3O4 corrosion product, which was confirmed by the XRD results. The element, S, was detected because Q235B carbon steel may have generated a small amount of FeS corrosion product. Sulfate-reducing bacteria (SRB) is a common corrosive microorganism in circulating cooling water. It could consume cathodic hydrogen through an enzyme called hydrogenase to generate H2S. The accumulation of corrosive H2S on metal leads to metal corrosion, which generates the FeS corrosion product.18,19 The growth environment of TDN of functional bacteria is similar to SRB. TDN could convert the corrosive H2S into non-corrosive SO42−, thereby reducing the corrosion of sulfide to metals.20 EDS results show that the content of S for Q235B attachment decreased after adding the microbial agent. This proves that the proportion of FeS in the corrosion product decreased and functional bacteria had the effect of inhibiting sulfide corrosion.
| Element | Without microbial agent | With microbial agent | ||
|---|---|---|---|---|
| Quality percentage/% | Atomic percentage/% | Quality percentage/% | Atomic percentage/% | |
| (a) Q235B coupon | ||||
| C | 3.65 | 9.83 | 3.90 | 10.10 |
| O | 23.65 | 47.87 | 26.12 | 50.83 |
| S | 0.62 | 0.50 | 0.18 | 0.14 |
| Fe | 72.08 | 41.80 | 69.81 | 38.93 |
| (b) 316L coupon | ||||
| C | 4.21 | 16.39 | 33.54 | 61.57 |
| N | — | — | 0.57 | 0.84 |
| O | 0.94 | 2.74 | 11.02 | 15.16 |
| P | — | — | 1.22 | 0.84 |
| Si | 0.43 | 0.72 | 0.22 | 0.16 |
| S | 0.58 | 0.85 | 0.97 | 0.66 |
| Cr | 17.05 | 15.34 | 9.63 | 4.08 |
| Mn | 1.04 | 0.88 | 0.33 | 0.12 |
| Fe | 66.2 | 55.46 | 37.16 | 14.61 |
| Ni | 9.55 | 7.61 | 5.33 | 1.97 |
Eight elements, C, O, Si, S, Cr, Mn, Fe, and Ni, were present in the attachment of 316L coupon without microbial agent after corrosion, which was mainly derived from the 316L stainless steel base. After adding the microbial agent, the extra elements, N and P, were detected in the attachment. Furthermore, the content of C and O significantly increased. This may be due to the formation of biofilm on the surface of 316L stainless steel by functional bacteria. The main components are microbial flora and an extracellular polymeric substance (EPS) secreted by functional bacteria (B. cereus, Pseudomonas, and B. subtilis). An EPS is mainly composed of polysaccharides, proteins, and small amounts of phospholipids, humic acids, and nucleic acids.21 Therefore, the content proportion of C, N, O, and P is higher in the attachment of 316L coupon with microbial agent.
The material proportion of Fe for the surface attachment of Q235B coupon by XPS analysis is shown in Fig. 3. The characteristic peaks of Fe3O4 and FeS appeared at 710.8 eV and 713.6 eV, respectively.22–24 Therefore, the main components of the corrosion product for Q235B carbon steel are Fe3O4 and FeS. In addition, the proportion of FeS in the corrosion product with the microbial agent was 7.27%, which was lower than that without the microbial agent (16.64%). The functional bacteria could inhibit the production of FeS, which has an anti-corrosion effect on Q235B carbon steel. This is consistent with the conclusion of the EDS analysis.
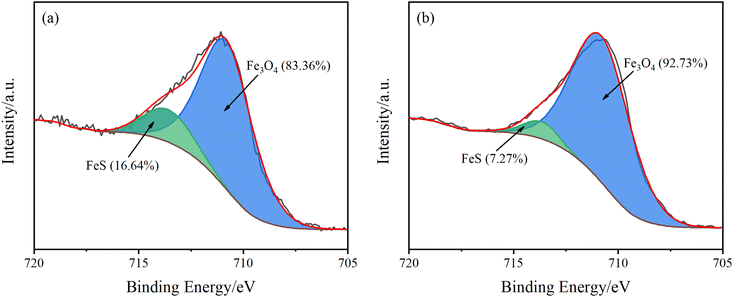 | ||
| Fig. 3 High resolution Fe 2p3/2 spectra for the attachment of Q235B coupon under different conditions. (a) Without microbial agent; (b) with microbial agent. | ||
Furthermore, the proportion of Fe3O4 in corrosion products was much higher than that of FeS. Therefore, oxygen consumption corrosion is the main type of corrosion for Q235B carbon steel in circulating cooling water, rather than sulfide corrosion. Although the functional bacteria have the capacity to inhibit the corrosion of sulfide, the anti-corrosion efficiency on Q235B carbon steel is low. The functional bacteria could not inhibit the occurrence of oxygen consumption corrosion.
3.2 Corrosion electrochemistry
Fig. 4 shows the change of OCP for the Q235B electrode and 316L electrode with and without microbial agent. The results show that the Q235B electrode had a positive shift of OCP with and without microbial agent. Q235B carbon steel could be corroded under the water quality condition of circulating cooling water and the functional bacteria has difficulty forming biofilm on the metal surface. The influence of the corrosion process for OCP is larger than the attachment process of microorganisms. The change of OCP for the Q235B electrode is mainly caused by the formation of the corrosion product.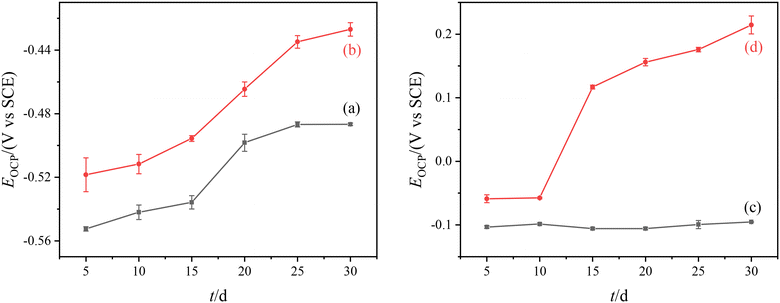 | ||
| Fig. 4 Change of OCP for different electrodes. (a) Q235B without microbial agent; (b) Q235B with microbial agent; (c) 316L without microbial agent; (d) 316L with microbial agent. | ||
There was a significant difference between the change of OCP for the 316L electrode with and without microbial agent. The OCP of the 316L electrode without microbial agent remained unchanged, while that with microbial agent was positively shifted. This positive shift of OCP indicates that the microorganisms have attached to the metal surface, and the shift value is positively related to the number of microorganisms attached to the electrode surface.25 The change of OCP for the 316L electrode is mainly caused by the attachment process of functional bacteria rather than the corrosion process. The OCP remained unchanged for 0–10 d, and the functional bacteria slowly attached to the electrode. After 10 d, the positive shift in OCP was obvious as the amount of functional bacteria increased and biofilm gradually formed on the electrode surface. For the 316L stainless steel electrode, the functional bacteria could attach to the metal surface and form biofilm.
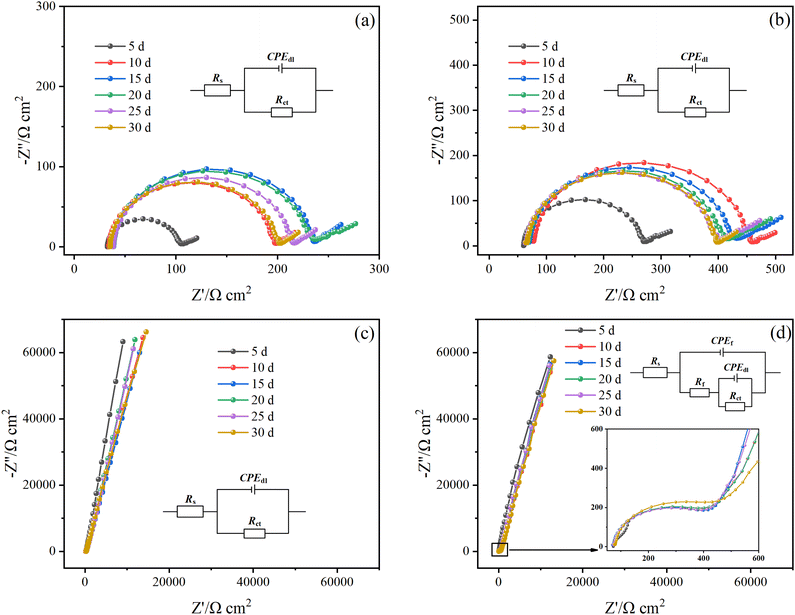
Fig. 5 shows the Nyquist diagram of the Q235B electrode and the 316L electrode with and without microbial agent at different times. There was no significant difference between the Nyquist diagram of the Q235B electrode with and without microbial agent. The extra semicircle occurred in the high-frequency region of the 316L electrode after adding a microbial agent, which was caused by functional bacteria forming a biofilm on the electrode surface. The corresponding EIS data were fitted using the equivalent circuit, as shown in Fig. 5. The one-time constant was used to represent the process of oxide film formation on the electrode of Q235B without microbial agent, Q235B with microbial agent, and 316L without microbial agent. The two-time constant was used to represent the process of double film on the electrode of 316L with microbial agent. The outer layer was biofilm and the inner layer was oxide film. CPE was usually used instead of the capacitor element when the data deviated from ideal capacitance behavior due to the non-uniformity of the scale layer. In the equivalent circuit, Rs represents the solution resistance, Rct represents the charge transfer resistance, CPEdl represents the constant phase element of the metal/medium double layer, Rf represents the biofilm resistance, and CPEf represents the constant phase element of the biofilm layer.
According to the equivalent circuit, the impedance data were fitted in Zview software and the relevant parameters are shown in Table 2. The Rs value of the solution changed slightly and was in the range of 32.79–79.16 Ω cm2. The Rct value of the Q235B electrode with and without microbial agent shows a trend of increasing first before slightly decreasing because the oxide film of the corrosion product gradually forms on the Q235B electrode during the initial 0–15 d. As a consequence, the corrosion is inhibited. However, this oxide film is unstable, and its protective effect of metal gradually weakens with time.
| Time/(d) | R s/(Ω cm2) | CPEdl/(F cm−2) | n dl | R ct/(Ω cm2) |
|---|---|---|---|---|
| (a) Q235B without microbial agent | ||||
| 5 | 32.79 | 4.22 × 10−6 | 0.99 | 70.95 |
| 10 | 34.50 | 1.67 × 10−6 | 0.99 | 161.98 |
| 15 | 39.02 | 1.58 × 10−6 | 0.99 | 195.98 |
| 20 | 38.26 | 1.72 × 10−6 | 0.98 | 194.55 |
| 25 | 38.68 | 1.56 × 10−6 | 0.99 | 175.46 |
| 30 | 35.52 | 1.62 × 10−6 | 0.99 | 164.09 |
| (b) Q235B with microbial agent | ||||
| 5 | 60.87 | 1.37 × 10−6 | 0.99 | 207.93 |
| 10 | 76.83 | 8.85 × 10−7 | 0.97 | 379.28 |
| 15 | 69.42 | 9.67 × 10−7 | 0.97 | 357.58 |
| 20 | 67.29 | 1.01 × 10−6 | 0.97 | 343.03 |
| 25 | 64.53 | 1.02 × 10−6 | 0.97 | 333.40 |
| 30 | 66.01 | 9.62 × 10−7 | 0.98 | 331.15 |
| (c) 316L without microbial agent | ||||
| 5 | 52.23 | 2.57 × 10−5 | 0.88 | 9.62 × 105 |
| 10 | 54.55 | 2.25 × 10−5 | 0.86 | 4.50 × 106 |
| 15 | 58.22 | 2.49 × 10−5 | 0.85 | 3.53 × 106 |
| 20 | 55.18 | 2.43 × 10−5 | 0.85 | 2.27 × 106 |
| 25 | 79.16 | 2.43 × 10−5 | 0.85 | 2.10 × 106 |
| 30 | 73.43 | 2.31 × 10−5 | 0.86 | 2.89 × 106 |
| Time/(d) | R s/(Ω cm2) | CPEf/(F cm−2) | n f | R f/(Ω cm2) | CPEdl/(F cm−2) | n dl | R ct/(Ω cm2) |
|---|---|---|---|---|---|---|---|
| (d) 316L with microbial agent | |||||||
| 5 | 71.60 | 8.92 × 10−6 | 0.93 | 194.14 | 2.60 × 10−5 | 0.90 | 1.63 × 106 |
| 10 | 74.02 | 1.51 × 10−6 | 0.92 | 468.40 | 2.45 × 10−5 | 0.86 | 7.51 × 106 |
| 15 | 69.84 | 1.52 × 10−6 | 0.92 | 464.74 | 2.44 × 10−5 | 0.88 | 7.76 × 106 |
| 20 | 74.44 | 1.51 × 10−6 | 0.92 | 470.47 | 2.52 × 10−5 | 0.88 | 7.18 × 106 |
| 25 | 72.12 | 1.55 × 10−6 | 0.92 | 455.63 | 2.46 × 10−5 | 0.85 | 7.24 × 106 |
| 30 | 76.57 | 1.41 × 10−6 | 0.93 | 526.48 | 2.46 × 10−5 | 0.85 | 7.44 × 106 |
The Rct value of the 316L electrode without microbial agent increased before slightly decreasing, which was consistent with the changing trend of the Q235B electrode. For 316L, the electrode with microbial agent, the CPEf value decreased from 8.92 × 10−6 to 1.41 × 10−6 F cm−2, and the Rf value increased from 194.14 to 526.48 Ω cm2 after 30 d. The increase in the Rf value indicates that the integrity of the biofilm gradually increased. Furthermore, some studies have shown that the CPEf value is inversely proportional to the film thickness.15,18,26 Therefore, the functional bacteria could attach to the 316L electrode surface and form the biofilm, which is consistent with the result of the OCP change. The Rct value of the 316L electrode with microbial agent reached a maximum of 7.76 × 106 Ω cm2 at 15 d and thereafter remained unchanged. Therefore, the biofilm can maintain a long and stable protective effect on 316L stainless steel. In addition, the Rct value of the 316L electrode with microbial agent is higher than that without microbial agent, indicating that the formation of biofilm inhibits the charge transfer and diffusion process of oxygen molecules. The functional bacteria have an anti-corrosion effect on 316L stainless steel.
The biofilm formation of functional bacteria on the metal surface is one of the important factors for anti-corrosion. The biofilm could isolate the metal from oxygen molecules and inhibit the cathodic reduction reaction; thereby, the biofilm acts as a physical barrier layer that prevents the corrosive substances in the circulating cooling water from coming in contact with the metal and inhibits the metal from being damaged by corrosive substances.27–29 Moreover, the type of metals and microorganisms influence the biofilm formation process. The results show that biofilm could only be formed on 316L stainless steel but experienced difficulty forming on Q235B carbon steel. When Q235B carbon steel comes in contact with the circulating cooling water, oxygen consumption corrosion will rapidly occur and generate the corrosion products of Fe3O4. The process of biofilm formation is relatively slow. The surface of Q235B carbon steel is covered by corrosion products before the attachment of functional bacteria. Therefore, the functional bacteria could not inhibit oxygen consumption corrosion by biofilm formation, and the anti-corrosion efficiency on Q235B carbon steel is low.
The new Q235B and 316L electrode and the electrode after 30 d of test were used for potentiodynamic polarization. Fig. S1† shows the potentiodynamic polarization curve of different electrodes. The corrosion current density (icorr), corrosion potential (Ecorr), anode Tafel slope (βa), and cathode Tafel slope (βc) could be calculated by fitting the Tafel lines. The fitting parameters are shown in Table 3. The icorr value of Q235B electrode with and without microbial agent after 30 d were 5.70 × 10−5 and 6.20 × 10−5 A cm−2, respectively. The anti-corrosion efficiency of the microbial agent on Q235B carbon steel was 8.06%, close to the rotary coupon test. The microbial agent had an anti-corrosion effect on Q235B carbon steel. However, the icorr value of the electrode after 30 d was higher than that of a new electrode. Q235B carbon steel was corroded in the circulating cooling water with and without microbial agent.
| Type | i corr/(A cm−2) | E corr/(V vs. SCE) | β a/(mV dec−1) | −βc/(mV dec−1) | |
|---|---|---|---|---|---|
| Q235B electrode | New electrode at 0 d | 7.21 × 10−6 | −0.63 | 182.31 | 578.95 |
| Without microbial agent after 30 d | 6.20 × 10−5 | −0.50 | 376.29 | 329.18 | |
| With microbial agent after 30 d | 5.70 × 10−5 | −0.42 | 405.98 | 318.29 | |
| 316L electrode | New electrode at 0 d | 1.70 × 10−7 | −0.09 | 848.04 | 134.81 |
| Without microbial agent after 30 d | 1.89 × 10−7 | −0.08 | 1115.20 | 135.66 | |
| With microbial agent after 30 d | 7.03 × 10−8 | 0.19 | 509.62 | 87.48 | |
The icorr values of the 316L electrode with and without microbial agent after 30 d were 7.03 × 10−8 and 1.89 × 10−7 A cm−2, respectively. In addition, the icorr value of the 316L electrode after adding a microbial agent decreased compared to that with the new electrode. The microbial agent had an anti-corrosion effect on 316L stainless steel, and the anti-corrosion efficiency was 62.80%. This is because the formation of biofilm inhibits the diffusion process of oxygen molecules, resulting in a decrease in the limiting diffusion current density (iL) of oxygen. Since the corrosion is controlled by the diffusion process of oxygen, the icorr is equal to iL. The results show that the icorr value of the electrode with microbial agent is lower, which proves the above conclusion.
3.3 Biofilm formation process
Fig. 6(a–g) show the SEM images of the 316L coupon surface at different times under 1000× magnification. The results show that the attachment on the coupon surface increased with time. The functional bacteria could attach to the coupon surface and gradually secrete EPS to form a biofilm. The sporadic and dispersed attachment appeared on the coupon surface at 5 d, which was the attachment process of functional bacteria. Then, the functional bacteria continued to reproduce and gradually gathered to form colonies and produce a large amount of EPS. The complete biofilm layer gradually covered the coupon surface after 20 d. Fig. 6(h) shows the SEM images of biofilm under 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification. The “mushroom-like” biological structure was observed, and the microorganisms cross-linked to form clusters, consistent with the biofilm morphology observed in relevant studies.15,30,31 SEM images show that the biofilm was composed of multi-layer microbial flora, and the secreted EPS closely linked each microbial flora.
000× magnification. The “mushroom-like” biological structure was observed, and the microorganisms cross-linked to form clusters, consistent with the biofilm morphology observed in relevant studies.15,30,31 SEM images show that the biofilm was composed of multi-layer microbial flora, and the secreted EPS closely linked each microbial flora.
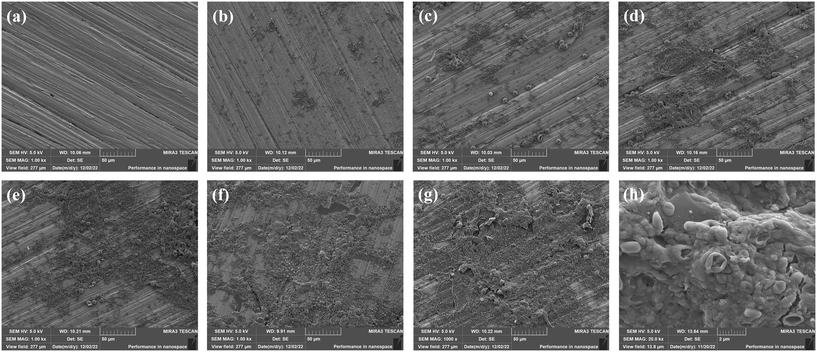 | ||
| Fig. 6 SEM images of the coupon surface at different times. (a) 0 d; (b) 5 d; (c) 10 d; (d) 15 d; (e) 20 d; (f) 25 d; (g) 30 d; (h) 30 d-high magnification. | ||
The 3D topography images were obtained by using OSP to observe the biofilm formed on the coupon surface at different times, as shown in Fig. 7. The coupon surface at 20 d was smooth and had no obvious fluctuation. Some convex regions appeared on the coupon surface with time, which reflected the continuous attachment of functional bacteria. The convex regions gradually increased, and the surface height of the coupon gradually increased. The attached functional bacteria could form a biofilm layer and gradually cover the coupon surface. To quantitatively describe the thickness of biofilm, the height distribution in the observation region was statistically analyzed. Table S3† shows the surface roughness parameters of the coupon at different times, including the surface arithmetical average height (Sa) and the surface root-mean-square height (Sq). The results show that the Sa and Sq values increased in 0–20 d, the surface roughness of the coupon increased, and the thickness of the biofilm increased. The values of Sa and Sq remained unchanged after 20 d. At 20 d, the functional bacteria formed a dense and complete biofilm layer structure on the surface of the 316L coupon, which is consistent with the SEM results. It could be inferred from the roughness parameters that the thickness of the biofilm layer is about 1 μm. The dense biofilm layer formed by functional bacteria could effectively prevent the corrosive substances in the circulating cooling water from making contact with the metal, thus achieving an anti-corrosion effect.
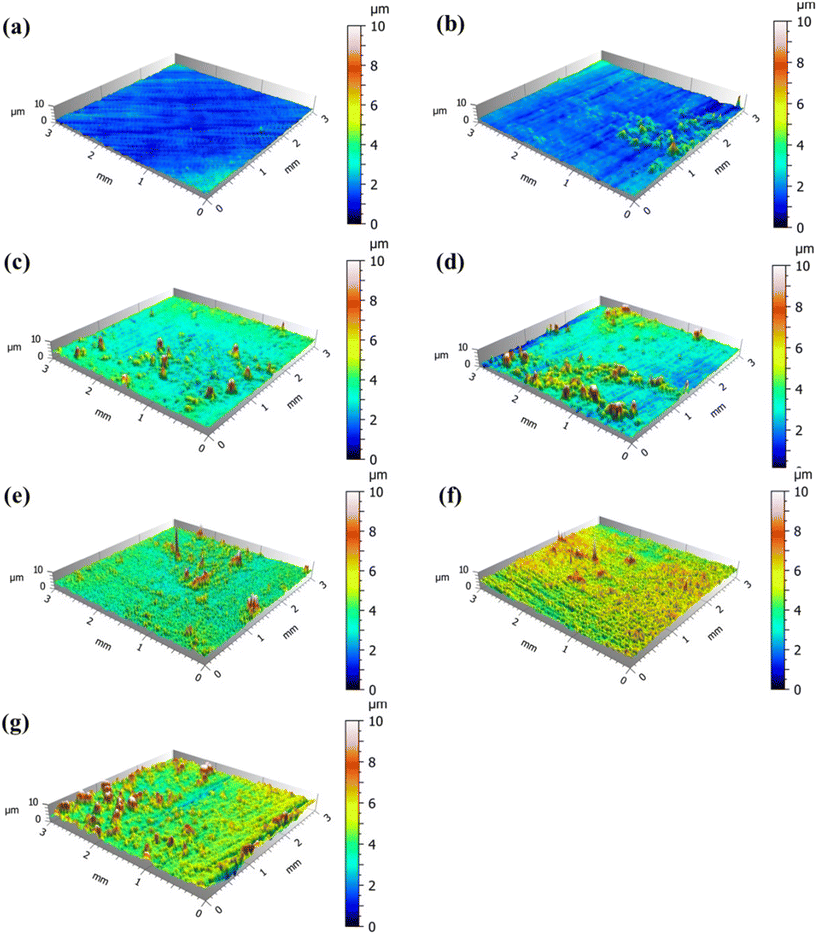 | ||
| Fig. 7 3D topography images of the coupon surface at different times. (a) 0 d; (b) 5 d; (c) 10 d; (d) 15 d; (e) 20 d; (f) 25 d; (g) 30 d. | ||
Fig. S2† shows the FTIR spectrum of biofilm on the coupon surface after 30 d. The results show that the characteristic absorption peaks of the N–H amino group and O–H hydroxyl group appeared at 3286 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond appeared at 1644 cm−1, NH2 bond appeared at 1539 cm−1, the amine appeared at 1026 cm−1, and carboxylic acid group appeared at 1411 cm−1. These were typical characteristic peaks of a protein secondary structure.32–34 The vibration band of C–O appeared at 1261 and 1082 cm−1, which was typical of the characteristic peak of a polysaccharide.35 In addition, the characteristic absorption peaks caused by the C–H bond stretching vibration appeared at 2958 and 798 cm−1. The results indicate that the components of biofilm were protein and polysaccharide, which was EPS secreted by functional bacteria.
O bond appeared at 1644 cm−1, NH2 bond appeared at 1539 cm−1, the amine appeared at 1026 cm−1, and carboxylic acid group appeared at 1411 cm−1. These were typical characteristic peaks of a protein secondary structure.32–34 The vibration band of C–O appeared at 1261 and 1082 cm−1, which was typical of the characteristic peak of a polysaccharide.35 In addition, the characteristic absorption peaks caused by the C–H bond stretching vibration appeared at 2958 and 798 cm−1. The results indicate that the components of biofilm were protein and polysaccharide, which was EPS secreted by functional bacteria.
3.4 Anti-corrosion mechanism analysis
The above results show that adding a microbial agent to circulating cooling water could control corrosion. As shown in Fig. 8, the functional bacteria could establish an anti-corrosion effect in the following three ways.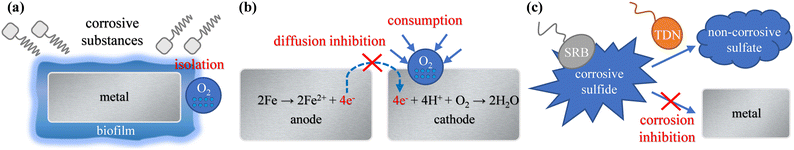 | ||
| Fig. 8 Schematic diagram of the anti-corrosion mechanism for functional bacteria. (a) Biofilm formation; (b) corrosion current reduction; (c) sulfide corrosion inhibition. | ||
The positive shift in the OCP value and the increase of the Rf value prove that biofilm formed on the 316L electrode after adding a microbial agent. The Rct value of the 316L electrode with the microbial agent is higher than that without microbial agent. This indicates that the formation of biofilm isolates the oxygen molecules and inhibits the consumption corrosion of metal. The SEM images and 3D topography images illustrate that the functional bacteria could attach to the 316L coupon surface and secrete EPS to form biofilm. A dense and complete biofilm layer covers the 316L coupon surface after 20 d. The thickness of biofilm is about 1 μm and the main components of the biofilm are protein and polysaccharide.
Moreover, related studies have shown that not all EPS produced by microorganisms results in a protective effect on metal. Stadler et al.36 found that EPS produced by Desulfovibrio alaskensis had a corrosion inhibition effect on pure iron and carbon steel under anaerobic conditions; however, EPS produced by Desulfovibrio vulgaris and Desulfovibrio indonesiensis did not protect metals. Therefore, it is of great significance to select functional bacteria that could produce EPS that do protect metal. In our study, the B. cereus, Pseudomonas, and B. subtilis were used as functional bacteria, proving that they could form biofilm and produce an anti-corrosion effect on 316L stainless steel.
Suma et al.37 reported that the protective action of Pseudomonas putida RSS on a mild steel surface was enhanced by biofilm formation. The results indicate that no trace of corrosion was present even after 12 months of immersion, with a negligible corrosion rate of 3.01 × 10−2 mmpy. However, this property of biofilm producing/biopassivating did not appear in our study. The Q235B carbon steel is corroded and covered by corrosion products before the attachment of functional bacteria. The biofilm secreted by functional bacteria is difficult to form on Q235B carbon steel.
4. Conclusion
This study aimed to evaluate the anti-corrosion effect of a microbial agent and reveal the mechanism of functional bacteria. The results showed that the anti-corrosion efficiency of a microbial agent on Q235B carbon steel was 8.06–9.02% and 62.80% on 316L stainless steel. The anti-corrosion mechanism of functional bacteria included biofilm formation, corrosion current reduction, and sulfide corrosion inhibition.The results show that functional bacteria could form biofilm on the metal surface, thereby inhibiting corrosion. However, biofilm could only be formed on 316L stainless steel, and it had difficulty forming on Q235B carbon steel. The positive shift of the OCP value and the increase in the Rf value proved the formation of biofilm on the 316L electrode after adding a microbial agent. The SEM images and 3D topography images show that a dense and complete biofilm layer covered the 316L coupon surface after 20 d. The thickness of biofilm was about 1 μm and the main components of biofilm were protein and polysaccharide. The change in the Rct value of the 316L electrode after adding a microbial agent indicated that the formation of biofilm isolated the oxygen molecule and corrosive substances, which inhibited the metal corrosion.
The attached functional bacteria and biofilm on metal surfaces could consume oxygen molecules through respiration and inhibit the diffusion process of oxygen. The icorr value of the 316L electrode with microbial agent was lower, which proved that functional bacteria could inhibit the cathodic reduction reaction and reduce the corrosion current.
The TDN of functional bacteria could convert the corrosive sulfide produced by SRB into a non-corrosive sulfate, thereby inhibiting the corrosion of sulfide to metal. The analyses of EDS and XPS showed that the proportion of FeS corrosion products produced by Q235B carbon steel decreased after adding a microbial agent. Although the functional bacteria had the effect of sulfide corrosion inhibition, the anti-corrosion efficiency on Q235B carbon steel was low. The functional bacteria could not inhibit oxygen consumption corrosion because the biofilm experienced difficulty forming on Q235B carbon steel.
Author contributions
Yu Wang: methodology, writing – original draft, writing – review & editing; Hongfeng Liao: methodology, resources; Li Gan: validation, resources; Zhengxiu Liu: data curation, resources; Ziqiang Tang: visualization, supervision; Xiaoran Zhao: writing – review & editing; Yubin Zeng: supervision, writing – review & editing; Chunsong Ye: supervision, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Beijing Jingneng Energy Technology Research Co., Ltd.References
- J. D. Choundraj, R. G. Kelly, R. Monikandan, P. M. Singh and J. Kacher, Influence of native oxide film on corrosion behavior of additively manufactured stainless steel 316l, Corros. Sci., 2023, 217, 111098 CrossRef CAS.
- N. Xu, N. Ding, F. Zaïri, L. Liu, W. Guo, X. Wu, H. Ma, H. Xu and C. Lawrence Wu, Leakage failure of a stainless steel spiral plate condenser, Eng. Failure Anal., 2022, 132, 105921 CrossRef CAS.
- D. B. Alexander and A. A. Moccari, Evaluation of corrosion-inhibitors for component cooling water-systems, Corrosion, 1993, 49(11), 921–928 CrossRef CAS.
- J. L. Fang, Y. Li, X. R. Ye, Z. W. Wang and Q. Liu, Passive films and corrosion protection due to phosphonic acid inhibitors, Corrosion, 1993, 49(4), 266–271 CrossRef CAS.
- C. Pan, Y. Song, W. Jin, Z. Qin, S. Song, W. Hu and D. Xia, Enhancing the stability of passive film on 304 ss by chemical modification in alkaline phosphate-molybdate solutions, Trans. Tianjin Univ., 2020, 26(2), 135–141 CrossRef CAS.
- Z. Zhou, W. Qiao, Y. Lin, X. Shen, D. Hu, J. Zhang, L. Jiang and L. Wang, Phosphonate removal from discharged circulating cooling water using iron–carbon micro-electrolysis, Water Sci. Technol., 2014, 70(3), 524–532 CrossRef CAS.
- Y. Wang, T. Wang and C. Ye, Advance on mechanism of water quality conditioning for recirculating cooling water by microorganisms, Ind. Water Treat., 2022, 42(2), 60–66 Search PubMed.
- C. Chen, Y. Wang, S. Liu, R. Feng, X. Gu and C. Qiao, Research on the application of compound microorganism preparation in reusing urban reclaimed water in circulating cooling water system, Water Sci. Technol., 2019, 80(9), 1763–1773 CrossRef PubMed.
- Y. Hu, C. Chen, S. Liu, Y. Zhou, W. Jia and Y. Cao, Biofilm-induced corrosion inhibition of q235 carbon steel by anaerobic bacillus cereus inoculum in simulated cooling water, Environ. Sci. Pollut. Res., 2022, 30, 20833–20848 CrossRef.
- Y. Hu, C. Chen and S. Liu, Evaluation of microbial agents as corrosion and scale inhibitor for industrial cooling water applications, Water Sci. Technol., 2022, 85(6), 1904–1919 CrossRef CAS PubMed.
- S. Li, Q. Qu, L. Li, K. Xia, Y. Li and T. Zhu, Bacillus cereus s-eps as a dual bio-functional corrosion and scale inhibitor in artificial seawater, Water Res., 2019, 166, 115094 CrossRef CAS.
- D. Ornek, A. Jayaraman, B. C. Syrett, C. H. Hsu, F. B. Mansfeld and T. K. Wood, Pitting corrosion inhibition of aluminum 2024 by bacillus biofilms secreting polyaspartate or gamma-polyglutamate, Appl. Microbiol. Biotechnol., 2002, 58(5), 651–657 CrossRef CAS.
- S. Chongdar, G. Gunasekaran and P. Kumar, Corrosion inhibition of mild steel by aerobic biofilm, Electrochim. Acta, 2005, 50(24), 4655–4665 CrossRef CAS.
- Chinese Standard GB/T 18175-2014 Determination of corrosion inhibition performance of water treatment agents-Rotation specimen method, Beijing, China, 2014 Search PubMed.
- H. Mehta, G. Kaur, G. R. Chaudhary and N. Prabhakar, Assessment of bio-corrosion inhibition ability of hafnium based cationic metallosurfactant on iron surface, Corros. Sci., 2021, 179, 109101 CrossRef CAS.
- J. Wu, D. Zhang, P. Wang, Y. Cheng, S. Sun, Y. Sun and S. Chen, The influence of desulfovibrio sp. And pseudoalteromonas sp. On the corrosion of q235 carbon steel in natural seawater, Corros. Sci., 2016, 112, 552–562 CrossRef CAS.
- Chinese Standard GB/T 50050-2017 Code for design of industrial recirculating cooling water treatment, Beijing, China, 2017 Search PubMed.
- J. Li, W. Zhang, J. Li, D. Jiao and L. Cui, Comparative study of microbiologically influenced corrosion of stainless steels in reclaimed water in power plant, Proceedings of the Chinese Society of Electrical Engineering, 2010, 30(32), 63–70 Search PubMed.
- M. Lv and M. Du, A review: microbiologically influenced corrosion and the effect of cathodic polarization on typical bacteria, Rev. Environ. Sci. Bio/Technol., 2018, 17(3), 431–446 CrossRef CAS.
- Y. Tian, X. Pei, X. Zhu, A. A. G. Jose, J. Li, C. Zhao and L. Liang, Microbial inhibition of metal corrosion: a review, Microbiology, 2020, 47(12), 4260–4268 Search PubMed.
- G. Sheng, H. Yu and X. Li, Extracellular polymeric substances (eps) of microbial aggregates in biological wastewater treatment systems: a review, Biotechnol. Adv., 2010, 28(6), 882–894 CrossRef CAS PubMed.
- S. J. Yuan and S. O. Pehkonen, Microbiologically influenced corrosion of 304 stainless steel by aerobic pseudomonas ncimb 2021 bacteria: afm and xps study, Colloids Surf., B, 2007, 59(1), 87–99 CrossRef CAS.
- Y. Qi, J. Li, R. Liang, S. Ji, J. Li and M. Liu, Chemical additives affect sulfate reducing bacteria biofilm properties adsorbed on stainless steel 316l surface in circulating cooling water system, Front. Environ. Sci. Eng., 2017, 11(2), 14 CrossRef.
- S. Yuan, B. Liang, Y. Zhao and S. O. Pehkonen, Surface chemistry and corrosion behaviour of 304 stainless steel in simulated seawater containing inorganic sulphide and sulphate-reducing bacteria, Corros. Sci., 2013, 74, 353–366 CrossRef CAS.
- W. Wang, X. Li, J. Wang, H. Xu and J. Wu, Influence of biofilms growth on corrosion potential of metals immersed in seawater, Mater. Corros., 2004, 55(1), 30–35 CrossRef CAS.
- E. Kuş, K. Nealson and F. Mansfeld, The bacterial battery and the effect of different exposure conditions on biofilm properties, Electrochim. Acta, 2008, 54(1), 47–52 CrossRef.
- H. Liu, D. Xiangqian, J. Duan, X. Zhai and B. Hou, Effect of microorganism on initial corrosion behavior of 5083 aluminum alloy in natural seawater, Fushi Kexue Yu Fanghu Jishu, 2016, 28(1), 51–57 CAS.
- B. Little and R. Ray, A perspective on corrosion inhibition by biofilms, Corrosion, 2002, 58(5), 424–428 CrossRef CAS.
- B. E. Torres Bautista, A. J. Wikieł, I. Datsenko, M. Vera, W. Sand, A. Seyeux, S. Zanna, I. Frateur and P. Marcus, Influence of extracellular polymeric substances (eps) from pseudomonas ncimb 2021 on the corrosion behaviour of 70cu-30ni alloy in seawater, J. Electroanal. Chem., 2015, 737, 184–197 CrossRef CAS.
- Q. Qu, Y. He, L. Wang, H. Xu, L. Li, Y. Chen and Z. Ding, Corrosion behavior of cold rolled steel in artificial seawater in the presence of bacillus subtilis c2, Corros. Sci., 2015, 91, 321–329 CrossRef CAS.
- Y. Chu, P. Xu, Y. Ou, P. Bai and Z. Wei, Corrosion behavior and interaction of mixed bacteria on carbon steel in reclaimed water, Sci. Total Environ., 2020, 718, 136679 CrossRef CAS.
- D. Wei, M. Li, X. Wang, F. Han, L. Li, J. Guo, L. Ai, L. Fang, L. Liu, B. Du and Q. Wei, Extracellular polymeric substances for zn (ii) binding during its sorption process onto aerobic granular sludge, J. Hazard. Mater., 2016, 301, 407–415 CrossRef CAS PubMed.
- A. Rajasekar and Y. Ting, Role of inorganic and organic medium in the corrosion behavior of bacillus megaterium and pseudomonas sp. In stainless steel ss 304, Ind. Eng. Chem. Res., 2011, 50(22), 12534–12541 CrossRef CAS.
- J. Wang, Q. Li, M. Li, T. Chen, Y. Zhou and Z. Yue, Competitive adsorption of heavy metal by extracellular polymeric substances (eps) extracted from sulfate reducing bacteria, Bioresour. Technol., 2014, 163, 374–376 CrossRef CAS PubMed.
- Z. Wang, Z. Wu, X. Yin and L. Tian, Membrane fouling in a submerged membrane bioreactor (mbr) under sub-critical flux operation: membrane foulant and gel layer characterization, J. Membr. Sci., 2008, 325(1), 238–244 CrossRef CAS.
- R. Stadler, W. Fuerbeth, K. Harneit, M. Grooters, M. Woellbrink and W. Sand, First evaluation of the applicability of microbial extracellular polymeric substances for corrosion protection of metal substrates, Electrochim. Acta, 2008, 54(1), 91–99 CrossRef CAS.
- M. S. Suma, R. Basheer, B. R. Sreelekshmy, V. Vipinlal, M. A. Sha, P. Jineesh, A. Krishnan, S. R. Archana, V. S. Saji and S. M. A. Shibli, Pseudomonas putida rss biopassivation of mild steel for long term corrosion inhibition, Int. Biodeterior. Biodegrad., 2019, 137, 59–67 CrossRef CAS.
- M. Dubiel, C. H. Hsu, C. C. Chien, F. Mansfeld and D. K. Newman, Microbial iron respiration can protect steel from corrosion, Appl. Environ. Microbiol., 2002, 68(3), 1440–1445 CrossRef CAS PubMed.
- H. Yuan, A. Gong, X. Li, L. Qiu, X. Lang and Y. Li, Study on growth characteristics of Thiobacillus Denitrificans and its defence effect on the microbiological corrosion of carbon steel caused by SRB, Chemistry & Bioengineering, 2009, 26, 50–54 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ew00629h |
| This journal is © The Royal Society of Chemistry 2024 |