Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced efficiency of dye-sensitized solar cells via controlled thickness of the WO3 Langmuir–Blodgett blocking layer in the Debye length regime†
Neeraj
Kumar
ad,
Sipra
Choudhury
 bd,
Aman
Mahajan
bd,
Aman
Mahajan
 c and
Vibha
Saxena
c and
Vibha
Saxena
 *ad
*ad
aTechnical Physics Division, Bhabha Atomic Research Centre, Trombay, Mumbai 40085, India. E-mail: vibhas@barc.gov.in
bChemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai 40085, India
cDepartment of Physics, Guru Nanak Dev University, Amritsar 143005, India
dHomi Bhabha National Institute, Mumbai 40094, India
First published on 23rd August 2024
Abstract
Generally, a thin (>50 nm) transition metal oxide (TMO) film (such as TiO2) is employed as an interfacial layer (also called a blocking layer, BL) between FTO and mesoporous TiO2 in a dye-sensitized solar cell (DSC). The function of this layer is to provide full coverage of FTO so as to reduce back electron recombination without compromising transparency and charge transport properties. Recent research has demonstrated that WO3 has better charge transport and stability than TiO2 and therefore has the potential to be employed as a hole blocking layer. Furthermore, ultra-thin TMO films having thickness less than the Debye length (characteristic length of the space charge layer in the WO3, LD) are expected to provide better interfacial and optical properties. However, reducing the thickness in such thin films has been challenging owing to available preparation methods. In this work, the Langmuir–Blodgett (LB) technique has been adopted to prepare high quality crystalline BL of WO3 of controlled thickness in the range of LD (4–20 nm). The interfacial properties such as charge transport and blocking behaviour of WO3 LB films improved significantly when the thickness was reduced to ≤14 nm, which is comparable to LD. As a result, DSCs fabricated with thinner WO3 BL (thickness ≤14 nm) showed significant efficiency (η) improvement (22%) leading to η up to 9.79% vis à vis DSCs with WO3 BL having thickness 20 nm.
1. Introduction
In the last decade, dye sensitized solar cells (DSCs) have emerged as potential alternatives to Si solar cell technology on account of their various advantages, such as flexibility, low cost, simple fabrication techniques, lightweight and efficient working even under diffused light.1,2 The key challenges in developing highly efficient DSCs have been to simultaneously improve the light-harvesting of the active layer and to reduce the recombination losses at various interfaces. Conventionally, a thin transition metal oxide (TMO) film, such as TiO2 (>50 nm), is employed as an interfacial layer between the FTO substrate and mesoporous TiO2 (m-TiO2) layer (blocking layer, BL) in DSCs, which prevents undesired recombination of photon-generated electrons. This BL also helps in tuning the work function and providing better interfacial properties to enable efficient electron transport.3–5 In addition to TiO2, other metal oxide films such as SnO2, Fe2O3, ZnO and Nb2O5 have also been employed as BL in DSCs in order to enhance efficiency.6WO3 has been used extensively for a variety of applications, such as smart windows,7 photocatalysts and gas sensors, because of its excellent chemical stability, high work function (∼6.2 eV) and carrier mobility (5–12 cm2 V s−1), and tunability of electronic structures through the polymorph.8–11 However, WO3 films in solar cells have not been used much as interfacial layers due to low conductivity, amorphous structure, mediocre interfacial charge transfer and unfavorable conduction band (CB) position.12,13 Therefore, it is essential to achieve high crystallinity, uniformity, and transparency with suitable work function in WO3 films so that it can provide preferential percolation paths for the charge transport without compromising optical transmittance.14,15 Furthermore, it has been reported in the literature that reducing the thickness of a uniform TMO film to the Debye length (characteristic length of the space charge layer in the TMO, LD) will yield higher electrical conductivity without compromising optical transmittance.16 The LD is dependent on the dielectric constant (ε) and carrier density (ND) as follows:
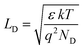 | (1) |
The LD of the WO3 is reported to be in the range of 2–20 nm and therefore, it is anticipated that WO3 films having thickness in this range (<20 nm) could lead to charge transport behavior different from what is observed for thicker films (>20 nm).17 Moreover, for TMO having thicknesses ≤LD, the space charge layer (SCL) can extend beyond the film thickness. This will eventually lead to different charge distributions near the film–substrate interface and hence, influence the interfacial properties such as charge transport and blocking behavior. Even though reducing the BL thickness is known to be effective for improving the interfacial properties without loss of optical transmittance, preparing such an ultra-thin (<20 nm) uniform film is challenging.
Amongst available techniques to deposit ultra-thin films, sputtering, pulsed layer deposition (PLD), atomic layer deposition (ALD) and molecular beam epitaxy (MBE) have been used widely18–22 for depositing WO3 films. The films, prepared by thermal evaporation, resulted in amorphous structure and rough morphology. Post annealing (>300 °C) employed to induce crystallization, however, led to shrinkage of the material, forming wide cracks owing to the increase in overall residual stresses in the film.23 Spark ablation was also used to prepare ultra-thin WO3 films, and enhanced NO2 gas sensing was reported.24 However, the films had many cracks in as-deposited as well as annealed films. Mattoni et al. reported crystalline ultra-thin WO3 (9 nm) on top of TiO2-terminated SrTiO3(001) substrates using the PLD technique.25 The film decorated with Pt resulted in increased sub-ppm hydrogen sensing owing to the crystalline structure and high surface area–volume ratio.26 Ultra-thin WO3 films were also produced using ALD from different precursors such as WF6, in situ-generated hexavalent tungsten oxyfluoride, (tBuN)2(NMe2)2W, and hexacarbonyl precursor W(CO)6.27 However, low growth per cycle (<0.2 Å), high impurity incorporation, and non-stoichiometric composition limited their use for device applications. Furthermore, a plasma-enhanced ALD process was reported, however, a polycrystalline film of monoclinic phase could be obtained only at elevated temperature ∼400 °C.28 Though ALD has the advantages of atomic level conformal coverage of a high quality and crystalline film on the substrate, high material cost and consumption as well as high wastage limits the industrial applicability of this technique. Therefore, present techniques for depositing ultra-thin WO3 films have the following disadvantages: (i) limited control over the quality of deposited films of different thicknesses, (ii) the necessity of specific substrates, corrosive precursor or co-reactants, (iii) the requirement of high vacuum and high deposition temperature and (iv) non-scalability. Therefore, the reported literature on BL materials in solar cells has mainly been focused on materials which could provide full coverage of the FTO substrate without loss of optical transmittance. It is to be emphasized here that the effect of BL thickness on the DSC performance has not been studied extensively for film thicknesses <20 nm due to the limitation of uniform film coverage by most of the reported preparation techniques6,18,19 and therefore, there is a lot of room for developing alternative materials for enhancing device efficiency through maximizing intrinsic BL properties.
In this context, the Langmuir–Blodgett (LB) technique is a very simple, environment-friendly, and versatile method for preparing ordered multilayers of a wide range of materials, such as carbon nanomaterials, conductive polymers, etc. with low consumption of materials. In addition, this technique allows control over the morphology, thickness and molecular architecture of films through suitable manipulation of the subphase pH, barrier speed, deposition speed, and post-transfer treatments along with the possibility of low temperature processing. In the present work, crystalline ultra-thin (4–20 nm) WO3 films were prepared from the thermal decomposition of the WO3-octadecyl amine (ODA) complex (WO3,ODA) of multilayers, using the LB technique. The interfacial properties of the prepared films were thoroughly characterized using UV-Vis spectroscopy, electrochemical techniques, ellipsometry, Kelvin-probe and field-emission scanning electron microscopy (FESEM). A significant improvement in blocking and charge transport behavior is observed for films thinner than ≤14 nm (within LD regime) as compared with thick 20 nm WO3 films (beyond the LD regime). The films, when employed in the fabrication of DSCs, show significant improvement in efficiency (η) up to 9.79% as compared to those with thicker (>LD) WO3 BL films (η ∼ 8.02%). The electrochemical impedance spectroscopy (EIS) and intensity-modulated photocurrent/photovoltage spectroscopy (IMVS/IMPS) data suggested improved charge transport and reduced recombination in DSCs with thinner (≤LD) WO3 BL as compared with those with thick BL (>LD).
2. Experimental section
Chloroform and other chemicals were of analytical grade or higher purity and were used without any further purification. Deionized (DI) water (Milli-Q, Millipore) having resistance 18 MΩ was used to prepare solutions. The preparation of LB multilayers of WO3,ODA was carried out using a KSV-Nima Langmuir trough equipped with two Teflon barriers with a total area of 850 cm2. The absence of surface-active contaminants was verified by compressing the subphase to achieve surface pressure ≤0.1 mN m−1. Then, 50 μL of octadecyl amine solution in chloroform (1 mg mL−1) was spread on the subphase containing 0.15 mM sodium tungstate solution (pH ∼ 7) in DI water, using a Hamilton micro-syringe. After 20 mins to evaporate solvent, the layer was simultaneously compressed by two compression barriers at the rate of 5 mN m−1 min−1. The FTO substrates were cleaned by successive sonication in warm Hellmnax soap, DI water, acetone, methanol, and isopropyl alcohol and then dried with nitrogen gas, whereas Si substrates were cleaned using a Piranha solution. All substrates were further cleaned by using atmospheric plasma (Harrick Plasma, model PDC-002) in ambient air for 10 min before carrying out multilayer deposition. Multilayers were transferred in successive downstrokes and upstrokes with a 10 min interval between the upstroke and the subsequent downstroke. The dipping rate was 5 mm min−1 in both directions. The transfer of the LB multilayers was Y type and the transfer ratio was 90–100% onto FTO and Si substrates. The films were subsequently thermally decomposed at 400 °C for 5 h. In order to compare the results, WO3 films were also prepared by spin casting, using a neutral surfactant-(polyethylene glycol) assisted tungsten precursor as reported earlier.29 The thickness of the prepared films on Si substrates was estimated using variable angle spectroscopic ellipsometry (Sentech SE400adv) at wavelength 632 nm. For device fabrication, a slightly modified procedure of preparing photoanode in our earlier work was adopted.30 The photoanode was prepared by doctor blading mesoporous TiO2 paste (Solaronix, Ti-nanoxide T/SP) and a scattering layer of TiO2 paste (Solaronix, Ti-nanoxide R/SP) on the plasma treated WO3 BL/FTO substrate. The films were sintered at 500 °C using a multi-step temperature program. This was followed by a post-treatment with 40 mM TiCl4 aqueous solution at 70 °C for ½ h and subsequently annealing at 500 °C for ½ h. The photoanodes were sensitized using a solution of 0.5 mM N719 dye (Solaronix) with 5 mM cholic acid in tert-butyl alcohol/acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The devices (area 0.12 cm2) were fabricated using a pre-drilled platinum coated counter electrode. The electrochemical as well as photovoltaic characterization was performed using a Potentiostat/Galvanostat (PGSTAT 30, Autolab Eco Chemie, Netherlands). The surface morphology was analyzed using a FESEM (Carl Zeiss Supra 55, SEM micrograph) as well as atomic force microscopy (AFM) (Anton Paar Tosca TM 400) in tapping mode. UV-Vis spectroscopy was carried out using a double beam UV-Vis spectrophotometer (Jasco, V 530). The Fourier transform infrared spectroscopy (FTIR) of LB films deposited on Si substrates was carried out, in reflectance mode, using a Bruker spectrometer (model: Vertex 80 V) at a resolution of 4 cm−1. X-ray photoelectron spectroscopy (XPS) data measurements were carried out using the RIBER MBE system, which was equipped with a Mg Kα source. The binding energy scale was calibrated to the Au 4f7/2 line of 83.95 eV. Raman spectra of LB films, deposited on Si substrates, were recorded on a Jobin-Yvon micro-Raman spectrometer (LabRAM HR800), in the 180° backscattering geometry, with a spectral resolution of 1 cm−1 using a HeNe Laser. The laser beam was focused onto the sample using a ×100 objective lens and the laser spot size was ∼5 μm. Kelvin probe scanning microscopy (model: KP Technology), having resolution of ∼0.003 eV, was employed to measure the work function of spin cast and LB WO3 films on FTO substrates. Electrochemical characterizations of all BLs were carried out using a one-compartment, three-electrode electrochemical cell: FTO or FTO/WO3 performed as the working electrode, while Ag/AgCl and platinum electrodes were used as reference and counter electrodes, respectively. The EIS measurements were done in a range from 100 mHz to 500 kHz by applying an AC voltage of 10 mV superimposed on a dc voltage. For dark EIS measurements, data were collected at dc bias of open-circuit voltage (VOC), VOC ± 0.02 V and VOC ± 0.04 V. The results were analyzed using an equivalent circuit model fitted with ZSimpWin 3.22 software. IMPS/IMVS measurements were carried out under a Green LED (wavelength 530 nm), which provided the DC illumination bias, superimposed with AC modulation ∼10% of the DC intensity. The measurements were performed between 1 kHz and 100 mHz, 20 data points per decade. The current–voltage (J–V) characteristics of the DSCs were recorded under 1 sun simulated AM 1.5G illumination using a solar simulator (G2V, Canada). The intensity of the source was calibrated using a reference silicon cell before recording the characteristics.
1 v/v). The devices (area 0.12 cm2) were fabricated using a pre-drilled platinum coated counter electrode. The electrochemical as well as photovoltaic characterization was performed using a Potentiostat/Galvanostat (PGSTAT 30, Autolab Eco Chemie, Netherlands). The surface morphology was analyzed using a FESEM (Carl Zeiss Supra 55, SEM micrograph) as well as atomic force microscopy (AFM) (Anton Paar Tosca TM 400) in tapping mode. UV-Vis spectroscopy was carried out using a double beam UV-Vis spectrophotometer (Jasco, V 530). The Fourier transform infrared spectroscopy (FTIR) of LB films deposited on Si substrates was carried out, in reflectance mode, using a Bruker spectrometer (model: Vertex 80 V) at a resolution of 4 cm−1. X-ray photoelectron spectroscopy (XPS) data measurements were carried out using the RIBER MBE system, which was equipped with a Mg Kα source. The binding energy scale was calibrated to the Au 4f7/2 line of 83.95 eV. Raman spectra of LB films, deposited on Si substrates, were recorded on a Jobin-Yvon micro-Raman spectrometer (LabRAM HR800), in the 180° backscattering geometry, with a spectral resolution of 1 cm−1 using a HeNe Laser. The laser beam was focused onto the sample using a ×100 objective lens and the laser spot size was ∼5 μm. Kelvin probe scanning microscopy (model: KP Technology), having resolution of ∼0.003 eV, was employed to measure the work function of spin cast and LB WO3 films on FTO substrates. Electrochemical characterizations of all BLs were carried out using a one-compartment, three-electrode electrochemical cell: FTO or FTO/WO3 performed as the working electrode, while Ag/AgCl and platinum electrodes were used as reference and counter electrodes, respectively. The EIS measurements were done in a range from 100 mHz to 500 kHz by applying an AC voltage of 10 mV superimposed on a dc voltage. For dark EIS measurements, data were collected at dc bias of open-circuit voltage (VOC), VOC ± 0.02 V and VOC ± 0.04 V. The results were analyzed using an equivalent circuit model fitted with ZSimpWin 3.22 software. IMPS/IMVS measurements were carried out under a Green LED (wavelength 530 nm), which provided the DC illumination bias, superimposed with AC modulation ∼10% of the DC intensity. The measurements were performed between 1 kHz and 100 mHz, 20 data points per decade. The current–voltage (J–V) characteristics of the DSCs were recorded under 1 sun simulated AM 1.5G illumination using a solar simulator (G2V, Canada). The intensity of the source was calibrated using a reference silicon cell before recording the characteristics.
3. Results and discussion
3.1 Surface properties of the WO3,ODA monolayer and structure of WO3,ODA Langmuir–Blodgett multilayers
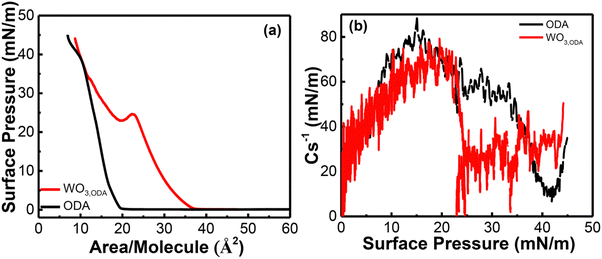
Since the lateral organization of the ODA molecules on the subphase may influence the WO3,ODA monolayer formation and subsequent multilayer deposition, the surface properties of monolayer organization and packing were investigated by recording surface pressure–mean molecular area (π–A) isotherms and are presented in Fig. 1(a). At large area, i.e. at the start of barrier compression, (zero surface pressure) a gas phase at the air–water interface is observed. The presence of WO42− ions in the subphase results in an increased expansion of the ODA monolayer as evident by an increase in the lift-off area (36.8 Å2) of the ODA monolayer spread on tungstate salt subphase. Distinct transition phases can be observed in the presence of WO42− ions in the subphase, which indicates an interaction between WO42− ions and ODA. This interaction occurs because the ODA molecules undergo protonation in the presence of water resulting in positive head groups (R-NH3+). This positive charge interacts with anionic WO42−, resulting in an electrostatic interaction between the two groups. In the range 10–20 mN m−1, it is more clearly discerned in the presence of WO42− ions than in pure water isotherms where transition phases occur suddenly because of fast condensation of the ODA molecules. Moreover, an additional slope change takes place around 25 mN m−1, where a kink is observed. When ODA is compressed beyond this, a steep slope of the π–A curve is observed, indicating a phase transition. This is different for the isotherm of the ODA monolayer, for which the lift off area ∼21 Å2 per molecule is estimated, consistent with the reported values found in the literature.31 Transition phases are not observed in this isotherm, suggesting that a direct transition from a gas to a condensed phase takes place. In order to further elucidate phase transitions and to characterize the interactions of WO42− ions with the ODA monolayer, as well as to examine changes in the interfacial packing properties, two-dimensional compressibility of the Langmuir monolayers was investigated. The compressibility coefficient was calculated as per the following equation: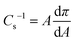 | (2) |
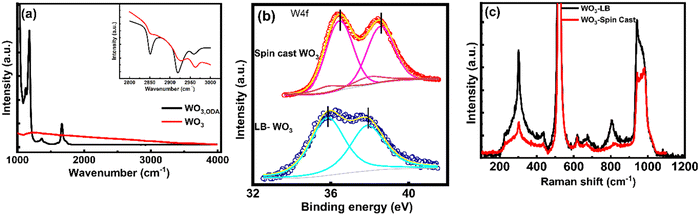 | ||
| Fig. 2 (a) FTIR of the WO3 LB film after thermal decomposition, (b) XPS and (c) Raman spectra of the spin cast and WO3 LB film. | ||
3.2 Optical, morphology and blocking properties of ultra-thin WO3 films
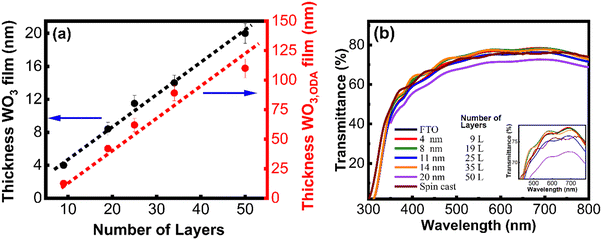
After establishing the fact that thermal decomposition of the WO3,ODA multilayered films yields a stoichiometric and crystalline WO3 structure, we prepared WO3 films of various thicknesses by controlling the deposition cycle of WO3,ODA monolayers on the substrate. Different numbers of monolayers, 9, 19, 25, 35 and 50 layers, were deposited and hereafter will be referred to as 9 L, 19 L, 25 L, 35 L and 50 L, respectively. The optical, morphology and interfacial properties of the prepared WO3 film were studied before employing them as BLs in DSC fabrication and the results are presented as follows:Since the optical transmittance is strongly affected by dielectric constants, we estimated thickness and optical constants (refractive index, n and extinction coefficient, k) using ellipsometric measurements and fitted data using the model as reported earlier.30 The estimated n and k of the as-deposited films were found to be in 1.56 and 0.04, respectively. After thermal decomposition, the refractive index (∼2.8) increases and the absorption coefficient decreases (∼0.006), suggesting conversion of the WO3,ODA LB multilayers to WO3. The high refractive index of all WO3 films suggests compact and uniform deposition of the film. The thicknesses of the WO3 multilayers, transferred on Si substrates, were estimated using estimated n and k values and are presented in Fig. 3(a) as a function of number of layers. The thickness of the as-WO3 film was found to shrink approximately 1/6th after thermal decomposition. The estimated data reveal that a monolayer of WO3,ODA is ∼2.4 nm thick and after thermal decomposition, it reduces to ∼0.4 nm. It should be noted that as-deposited films were featureless and transparent up to 19 L, and afterwards a semi-transparent film having a brownish tinge was observed. After thermal decomposition, all WO3 LB films of thickness ≤14 nm show enhanced optical transmittance as presented in Fig. 3(b). This might be due to better matching of the refractive index at the FTO/WO3/air interface than the FTO/air interface (refractive index for FTO ∼ 2.4), which reduces internal reflection and therefore results in enhanced transmittance. Slightly reduced transmittance observed for the 20 nm thick film might be due to increased thickness. Typical fringes observed in the spectra of all WO3 LB films suggest smooth surface morphology of the prepared films; in contrast, such fringes were absent in the spin cast WO3 film.
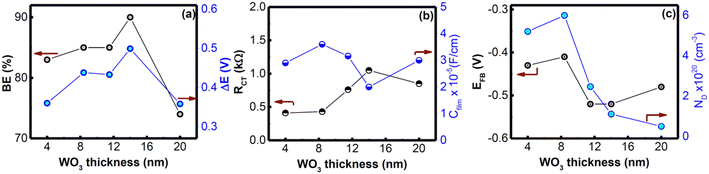
The smooth morphologies of the prepared LB films as compared with that of the spin cast film were also confirmed from recorded FESEM images, as depicted in Fig. 4. The FESEM images of WO3 films are slightly blurred as compared with FTO (inset of Fig. 4(a)) owing to the ultra-thin nature. As compared to the FESEM image of the spin cast WO3 film, LB deposited WO3 films were found to be more uniform, pin-hole free and homogeneous. It should be noted that the thickness variation of the WO3 film from 4 nm to 20 nm did not result in different surface morphologies. The uniformity in the LB deposited film is also supported by the AFM image, which shows a compact film comprising small crystallites after thermal decomposition of WO3,ODA multilayers (Fig. S1, ESI†). It should be noted that though uniform morphologies for WO3 crystalline films are observed in the SEM and AFM images, lateral uniformity in cm2 area has been assessed in the best manner using cyclic voltammetry.30,36 Therefore, the thickness dependence of the interfacial properties, such as blocking and charge transport behaviour, were studied by analyzing their cyclic voltammogram (CV), EIS and Mott–Schottky (MS) plots as described in earlier reports30 (Fig. S2, ESI†). The effectiveness of the blocking effect (BE) of the WO3 film was estimated by taking into account the percentage suppression of the anodic current in CV as compared to that for the FTO substrate. As shown in Fig. S2(a) (ESI†), all WO3 LB films showed (i) anodic/cathodic peaks, and (ii) significant suppression of the anodic current as compared to bare FTO and (iii) enhanced cathodic peak, which is due to the electron accumulation below the flat band potential and corresponding reduction of Fe(CN)63− at the FTO/WO3 surface. It is to be noted that WO3,ODA multilayers do not show such features owing to the presence of insulating ODA in the complex. When the film thickness is increased from 4 to 11 nm, good blocking behavior >80% is observed, despite the fact that the thickness of the film is very low (Fig. 5(a)). For 14 nm thick film, the blocking effect is slightly increased and then slightly decreases for 20 nm thick films. The peak-to-peak separation (ΔE) of the anodic and cathodic peaks in the CV follows the trend of blocking effect. This is expected as enhanced thickness of the WO3 layer poses more and more resistance to charge transfer reaction and therefore the oxidation peak shifts towards positive potential and therefore, results in increased ΔE.
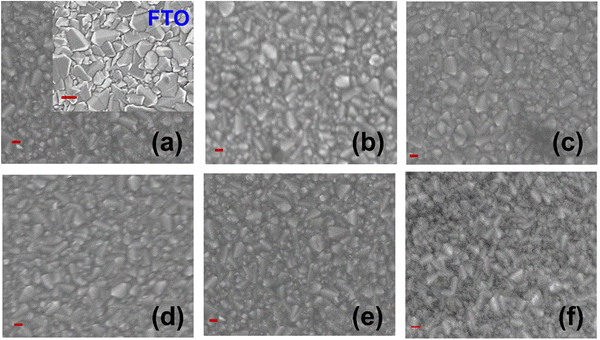 | ||
| Fig. 4 Top-view FE-SEM images of the prepared WO3 LB films of thicknesses (a) 4 nm, (b) 8 nm, (c) 11 nm, (d) 14 nm, (e) 20 nm, and (f) the spin cast film, scale bar ∼200 nm. | ||
In order to investigate further the thickness effect on blocking behaviour, the EIS spectra of various films were recorded (Fig. S2(b), ESI†). The measured data were fitted using Randle's equivalent circuit model (R([RCTW]Q)), where R is the overall contribution of the intrinsic resistance of the WO3 film, ionic resistance of the electrolyte, and contact resistance comprising connections to the WO3 electrode, and RCT, Q and W are the charge transfer resistance, constant phase element and Warburg impedance corresponding to WO3 film, respectively. The capacitance (Cfilm) of the WO3 film was estimated using estimated Q and RCT values as described earlier36 and is presented in Fig. 5(b). As shown in the figure, the RCT value increased while the Cfilm decreased with increasing the thickness from 4 nm to 14 nm. These results are indicative of the formation of dense and compact layers. When the film thickness is increased to 20 nm, a slight decrease in charge transfer resistance is observed. The results are consistent with the cyclic voltammetric results where a similar drop in the blocking effect and ΔE has been observed. In order to probe further the thickness dependent interfacial properties of FTO/WO3, we estimated the capacitance (CSC) of the space charge region, which plays a significant role in the charge distribution at the semiconductor–electrolyte interface. The potential drop in the space charge region, and therefore, the extent of band bending, referred to as ϕSC (as depicted schematically in ESI,† Fig. S3) is given by the following equation:37
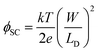 | (3) |
3.3 Influence of WO3 BL thickness on photovoltaic properties
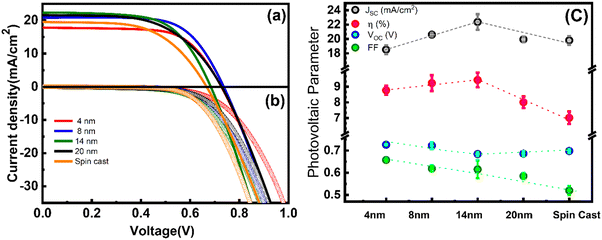
The effect of the variation of the thickness of the blocking WO3 layer on DSCs performance was studied by recording current density (J)–voltage (V) curves under AM 1.5 illumination and in the dark (Fig. 6a and b). All devices showed high efficiency (8% or greater) as compared with the DSC fabricated with spin cast WO3 BL, which shows low efficiency (∼7%) due to low fill factor (FF) and VOC. The DSCs prepared using spin cast films show good short-circuit current density (JSC), which is comparable with that of WO3 LB. This might be due to the fact that the work function (Φ) values for spin cast and LB films are close to the ionization potential of FTO (ΦFTO∼5.1 eV) (Fig. S4, ESI†). Owing to the small difference, ohmic-like contact (Fig. S3a, ESI†) forms in both cases, resulting in comparable JSC. Furthermore, there is a clear distinction in efficiency for DSCs fabricated with WO3 BL thickness ≤14 nm and those with thickness ∼20 nm. The JSC, FF and VOC decrease with increase in the WO3 blocking layer thickness to 20 nm, which is well above the LD and is attributable to the bulk-effect.Similar observations were reported for perovskite solar cells fabricated with ultra-thin films of NiO and SnO2 as interfacial layers.16,43 Seo et al. found that when the NiO film thicknesses decreased from 10–15 to 2.5–7.5 nm, the short-circuit current (JSC ∼16.5 to ∼18.5 mA cm−2) increased due to the improved conductivity of the film as the thickness is sufficient to be overlapped by LD. In the case of the SnO2 film, when the thickness is increased just above the LD value, improvement in all photovoltaic parameters was observed due to enhanced charge extraction.43 In both cases, the device efficiency decreased when the thicknesses of the buffer layer increased much higher than the Debye length. Since the thickness of the prepared WO3 BL (4–14 nm) is less than the reported Debye length of WO3, the improvement in the JSC of the devices is due to nano effects. Beyond this thickness, the bulk property dominates and therefore, the JSC decreases owing to the decrease in conductivity. Slightly reduced JSC observed in DSCs fabricated with 4 nm WO3 BL might be due to the limitation of FTO surface roughness, therefore resulting in slightly reduced efficiency. Nevertheless, improved VOC and FF obtained for these devices are attributed to the fact that the thickness of BL is of the order of LD. In this case, the WO3 film is fully depleted, having minimal potential drop in the BL, as discussed in the earlier section for MS analysis. Improved charge transport and reduced recombination for DSCs employing thinner BLs (≤LD) as compared to that having thicker BL (>LD) therefore gives rise to improved JSC and FF and thereby enhanced efficiency. In all, high efficiency (∼9.79%) observed for DSCs employing 14 nm thick WO3 BL is the result of enhanced carrier density, conductivity, and the uniform and homogeneous surface, as well as excellent blocking behaviour as observed earlier. It is to be emphasized here that even a 4 nm thick WO3 blocking layer is sufficient to serve the purpose of the blocking layer without compromising VOC and FF.
To further investigate the influence of BL thickness on the device performance, we focus on dark current (Fig. 6(b)), which is generally dominated by the recombination through nanocrystalline TiO2 particles or the exposure of the FTO substrate to the electrolyte. The onset potential for 4 nm thick WO3 film is observed at voltage >0.56 V and shifts to lower potential 0.48 V for a WO3 film thickness of 20 nm, in accordance with the JV data where a slight decrease in FF is observed for DSCs employing WO3 BL. This result is consistent with earlier reports where thinner films have provided better charge extraction due to the space charge region effect.43 In all, DSCs fabricated with BL of thickness ≤14 nm reveal better JSC, FF, VOC and efficiency as well as reduced dark current as compared to DSCs employed with BL of thickness ∼20 nm.
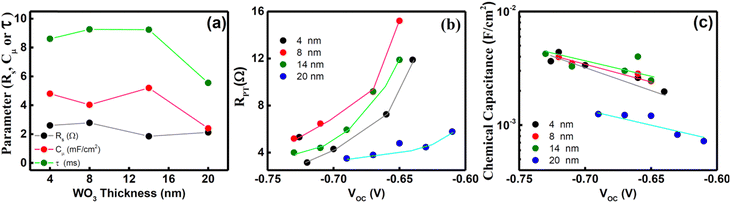
To substantiate the above findings, we investigated the devices further using impedance spectroscopy, under illumination as well as dark conditions (Fig. S5, ESI†). Various parameters, such as chemical capacitance (Cμ), recombination time, series resistance (Rs) and charge transfer resistance (RPT) were estimated by fitting the Nyquist plots using the electrical circuit as described in Fig. S5(f) (ESI†)3,4 and are presented in Fig. 7. The electrical circuit elements contributing to the photoanode impedance are dependent on the illumination as well as potential conditions as discussed below:
(i) EIS under 1 sun:
Under 1 sun illumination and at a potential equal to the open circuit potential, the chemical capacitance Cμ, represents the density of photogenerated electrons arising due to charge accumulation at the photoanode/electrolyte interface. The estimated Rs for DSCs comprises the bulk resistance of the photoanode, and contact resistance of all interfaces and the counter electrode as well as the connection resistance. Since all DSCs have the same layers except that the thickness of WO3 BL is varied, any variation in Rs value may be attributable to the FTO/WO3 and WO3/m-TiO2 interface. As shown in the figure, a slightly higher Rs is observed for DSCs having BL of thickness ≤9 nm than that for BL of thickness 14 nm, and this is attributable to the presence of fully depleted regions in thinner WO3 BL as evident from MS results discussed earlier in Section 3.2. Furthermore, the recombination time and Cμ does not change much for DSCs fabricated with BL thickness ≤14 nm. However, a sudden drop is observed for DSCs based on 20 nm WO3 BL. The high values of chemical capacitance and recombination suggest better charge transport and reduced recombination, leading to higher JSC, VOC and FF for DSCs employing WO3 BL of thickness ≤14 nm. This is consistent with our earlier observation on the CV and EIS results of WO3, where a high carrier density and charge transfer resistance is associated with thinner WO3 films (thickness ≤14 nm) and corroborates JV data.
(ii) Dark EIS:
Under dark conditions, the photoanode is insulating as no photoexcited electrons are injected into the conduction band of mesoporous TiO2 and the value of recombination resistance is higher than that in 1 sun. This is due to the fact that the I3− is formed in proximity to the counter electrode and then it has to diffuse through the electrolyte before reaching the photoanode. The chemical capacitance (Cm-TiO2) in this case arises mainly due to localized electron trapping states and therefore, accounts for the electron recombination at the m-TiO2/electrolyte interface. Furthermore, the Cm-TiO2 of the FTO/WO3/m-TiO2 photoanode in the dark shows an exponential rise with decrease in applied voltage. This is a behavior typical of the photoanode and is related to the total density of electrons in the dark, n, by the expression:44
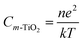 | (4) |
The parameters deduced from dark EIS measurements, such as charge transfer resistance (RPT) and Cm-TiO2 of the m-TiO2/electrolyte interface corroborate the EIS results under 1 sun. A clear distinction between DSCs employing BL having thickness ≤14 nm and 20 nm is observed in the RPT–VOC and Cm-TiO2–VOC curves, as shown in Fig. 7(b) and (c). The RPT value increases exponentially with increase in the VOC, and is consistent with earlier reports.44 Higher RPT values obtained for DSCs having WO3 BL of thickness ≤14 nm, suggests reduced charge recombination at the m-TiO2/electrolyte interface. A high value of Cm-TiO2 observed in DSCs having BL of thickness ≤14 nm indicates reduced charge carrier density and therefore fewer defects as compared with that of DSCs with BL of thickness 20 nm.
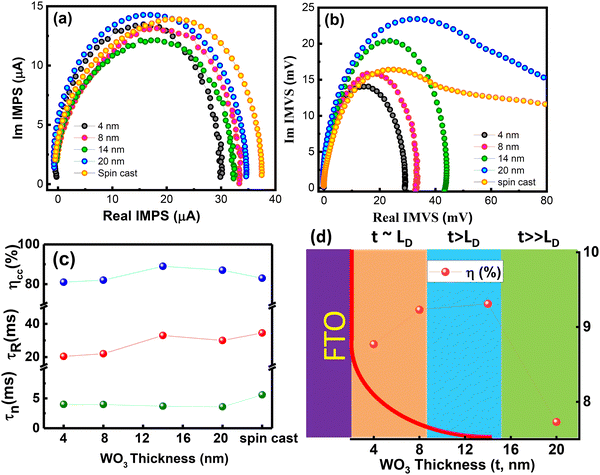
The improved interfacial properties of the DSCs fabricated with BL of thickness ≤14 nm are further confirmed from IMVS and IMPS measurements (Fig. 8). IMPS and IMVS curves do not show much variation in shape when the thickness of the BL in DSC is varied from 4 nm to 14 nm. However, the DSCs fabricated with 20 nm and spin cast WO3 film revealed development of an additional arc at lower frequency suggesting changes in the kinetics at the FTO/WO3 interface. The appearance of an arc may be related to recombination due to the bulk-nature of the film.45 The transport and recombination time for the device were calculated from the expression Tn = 1/2πfn and TR =1/2πfR, respectively, where fn (or fR) is the characteristic frequency minimum of the imaginary component from the IMPS (or IMVS) data. The figure reveals that (i) the Tn and TR remain almost constant with thickness for DSCs fabricated with WO3 BL ≤ 8 nm. (ii) A slight improvement in TR and Tn is observed for DSC fabricated with BL ≥ 14 nm thick film and (iii) high Tn and low TR values for DSCs employing spin cast films. These data are consistent with JV and EIS results of DSCs, which reveal higher JSC and FF for DSCs based on WO3 LB as compared with that of the spin cast film. Another important parameter of the DSCs, viz, electron collection efficiency can be estimated from the Tn and TR values as follows:
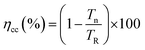 | (5) |
4. Conclusion
In summary, we have successfully prepared ultra-thin compact BL (4–20 nm) in the range of Debye length by the LB method and studied the influence of BL thickness on DSC performance. A clear distinction in charge transport and blocking properties was observed between thin films (≤14 nm) and thick films (>20 nm), which were related to LD. Better interfacial properties as well as charge transport and blocking properties of DSC devices based on thin WO3 blocking layers (≤14 nm) result in higher efficiency up to ∼9.79% in DSCs devices as compared to DSCs having WO3 BL of 20 nm thickness (8.02%) as well as those prepared with spin cast films (7%). This work demonstrates that employing WO3 BL of the Debye length regime results in the enhancement of the efficiency of the solar cells without compromising optical transmittance and blocking properties.Author contributions
N. Kumar: data curation, formal analysis. S. Choudhury: resources, validation. A. Mahajan: resources, validation. Vibha Saxena: conceptualization, data curation, formal analysis, project administration, resources, supervision, validation, visualization, writing – original draft writing – review & editing. All authors have read and agreed to the published version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank Dr S. M. Yusuf, Dr L. M. Pant and Dr A. Singh for the support of this work.References
- E. Pulli, E. Rozzi and F. Bella, Transparent photovoltaic technologies: current trends towards upscaling, Energy Convers. Manage., 2020, 219, 112982 CrossRef.
- J.-M. Ji, H. Zhou, Y. K. Eom, C. H. Kim and H. K. Kim, 14.2% Efficiency Dye-Sensitized Solar Cells by Co-sensitizing Novel Thieno[3,2-b]indole-Based Organic Dyes with a Promising Porphyrin Sensitizer, Adv. Energy Mater., 2020, 10(15), 2000124 CrossRef CAS.
- P. Ledezma, J. Jermakka, J. Keller and S. Freguia, Impact of film thickness of ultra-thin dip-coated compact TiO2 layers on the performance of mesoscopic perovskite solar cells, Front. Microbiol., 2017, 8, 750 CrossRef PubMed.
- I. P. Liu, W.-H. Lin, C.-M. Tseng-Shan and Y.-L. Lee, Importance of Compact Blocking Layers to the Performance of Dye-Sensitized Solar Cells under Ambient Light Conditions, ACS Appl. Mater. Interfaces, 2018, 10(45), 38900–38905 CrossRef CAS PubMed.
- W. Zhang, Y. Wu, H. W. Bahng, Y. Cao, C. Yi, Y. Saygili, J. Luo, Y. Liu, L. Kavan and J.-E. Moser, et al., Comprehensive control of voltage loss enables 11.7% efficient solid-state dye-sensitized solar cells, Energy Environ. Sci., 2018, 11(7), 1779–1787, 10.1039/C8EE00661J.
- M.-E. Yeoh and K.-Y. Chan, Efficiency Enhancement in Dye-Sensitized Solar Cells with ZnO and TiO2 Blocking Layers, J. Electron. Mater., 2019, 48(7), 4342–4350 CrossRef CAS.
- M. A. Arvizu, H.-Y. Qu, U. Cindemir, Z. Qiu, E. A. Rojas-González, D. Primetzhofer, C. G. Granqvist, L. Österlund and G. A. Niklasson, Electrochromic WO3 thin films attain unprecedented durability by potentiostatic pretreatment, J. Mater. Chem. A, 2019, 7(6), 2908–2918, 10.1039/C8TA09621J.
- L. You, B. Liu, T. Liu, B. Fan, Y. Cai, L. Guo and Y. Sun, Organic Solar Cells Based on WO2.72 Nanowire Anode Buffer Layer with Enhanced Power Conversion Efficiency and Ambient Stability, ACS Appl. Mater. Interfaces, 2017, 9(14), 12629–12636 CrossRef CAS PubMed.
- R. Shakoury, A. Arman, S. Rezaee, A. G. Korpi, S. Kulesza, C. Luna, M. Bramowicz and M. Mardani, Optical properties and morphology analysis of hexagonal WO3 thin films obtained by electron beam evaporation, J. Mater. Sci.: Mater. Electron., 2021, 32(1), 798–805 CrossRef CAS.
- J. M. Berak and M. J. Sienko, Effect of oxygen-deficiency on electrical transport properties of tungsten trioxide crystals, J. Solid State Chem., 1970, 2(1), 109–133 CrossRef CAS.
- M. Miyakawa, K. Ueda and H. Hosono, Carrier generation in highly oriented WO3 films by proton or helium implantation, J. Appl. Phys., 2002, 92(4), 2017–2022 CrossRef CAS.
- S. S. Patil, R. M. Mane, K. V. Khot, S. S. Mali, C. Kook Hong and P. N. Bhosale, Surfactant assisted approach to development of efficient WO3 photoanode for natural dye sensitized solar cell, Sol. Energy, 2021, 220, 371–383 CrossRef CAS.
- J. Zhang, C. Shi, J. Chen, Y. Wang and M. Li, Preparation of ultra-thin and high-quality WO3 compact layers and comparision of WO3 and TiO2 compact layer thickness in planar perovskite solar cells, J. Solid State Chem., 2016, 238, 223–228 CrossRef CAS.
- D. Liu, Q. Wang, C. J. Traverse, C. Yang, M. Young, P. S. Kuttipillai, S. Y. Lunt, T. W. Hamann and R. R. Lunt, Impact of Ultrathin C60 on Perovskite Photovoltaic Devices, ACS Nano, 2018, 12(1), 876–883 CrossRef CAS PubMed.
- M. T. Masood, C. Weinberger, J. Sarfraz, E. Rosqvist, S. Sandén, O. J. Sandberg, P. Vivo, G. Hashmi, P. D. Lund and R. Österbacka, et al., Impact of Film Thickness of Ultrathin Dip-Coated Compact TiO2 Layers on the Performance of Mesoscopic Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2017, 9(21), 17906–17913 CrossRef CAS PubMed.
- S. Seo, I. J. Park, M. Kim, S. Lee, C. Bae, H. S. Jung, N.-G. Park, J. Y. Kim and H. Shin, An ultra-thin, un-doped NiO hole transporting layer of highly efficient (16.4%) organic–inorganic hybrid perovskite solar cells, Nanoscale, 2016, 8(22), 11403–11412, 10.1039/C6NR01601D.
- J. Kukkola, M. Mohl, A.-R. Leino, J. Mäklin, N. Halonen, A. Shchukarev, Z. Konya, H. Jantunen and K. Kordas, Room temperature hydrogen sensors based on metal decorated WO3 nanowires, Sens. Actuators, B, 2013, 186, 90–95 CrossRef CAS.
- H. Cheng, Y. Liu, B. Cai, C. Hägglund, T. Kubart, G. Boschloo and H. Tian, Atomic Layer Deposition of SnO2 as an Electron Transport Material for Solid-State P-type Dye-Sensitized Solar Cells, ACS Appl. Energy Mater., 2022, 5(10), 12022–12028 CrossRef CAS.
- Y. Li, L. Ma, Y. Yoo, G. Wang, X. Zhang and M. J. Ko, Atomic layer deposition: A versatile method to enhance TiO2 nanoparticles interconnection of dye-sensitized solar cell at low temperature, J. Ind. Eng. Chem., 2019, 73, 351–356 CrossRef CAS.
- S. Seo, S. Jeong, H. Park, H. Shin and N.-G. Park, Atomic layer deposition for efficient and stable perovskite solar cells, Chem. Commun., 2019, 55(17), 2403–2416, 10.1039/C8CC09578G.
- Y. Kuang, V. Zardetto, R. van Gils, S. Karwal, D. Koushik, M. A. Verheijen, L. E. Black, C. Weijtens, S. Veenstra and R. Andriessen, et al., Low-Temperature Plasma-Assisted Atomic-Layer-Deposited SnO2 as an Electron Transport Layer in Planar Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2018, 10(36), 30367–30378 CrossRef CAS PubMed.
- S. Balasubramanyam, A. Sharma, V. Vandalon, H. C. M. Knoops, W. M. M. Kessels and A. A. Bol, Plasma-enhanced atomic layer deposition of tungsten oxide thin films using (tBuN)2(Me2N)2W and O2 plasma, J. Vac. Sci. Technol., A, 2018, 36(1), 01B103 CrossRef.
- A. Jelinska, K. Bienkowski, M. Jadwiszczak, M. Pisarek, M. Strawski, D. Kurzydlowski, R. Solarska and J. Augustynski, Enhanced Photocatalytic Water Splitting on Very Thin WO3 Films Activated by High-Temperature Annealing, ACS Catal., 2018, 8(11), 10573–10580 CrossRef CAS.
- N. A. Isaac, M. Valenti, A. Schmidt-Ott and G. Biskos, Characterization of Tungsten Oxide Thin Films Produced by Spark Ablation for NO2 Gas Sensing, ACS Appl. Mater. Interfaces, 2016, 8(6), 3933–3939 CrossRef CAS PubMed.
- G. Mattoni, A. Filippetti, N. Manca, P. Zubko and A. D. Caviglia, Charge doping and large lattice expansion in oxygen-deficient heteroepitaxial WO3, Phys. Rev. Mater., 2018, 2(5), 053402 CrossRef CAS.
- G. Mattoni, B. de Jong, N. Manca, M. Tomellini and A. D. Caviglia, Single-Crystal Pt-Decorated WO3 Ultrathin Films: A Platform for Sub-ppm Hydrogen Sensing at Room Temperature, ACS Appl. Nano Mater., 2018, 1(7), 3446–3452 CrossRef CAS PubMed.
- M. A. Mamun, K. Zhang, H. Baumgart and A. A. Elmustafa, Nanomechanical and Morphological Characterization of Tungsten Trioxide (WO3) Thin Films Grown by Atomic Layer Deposition, ECS J. Solid State Sci. Technol., 2015, 4(9), P398 CrossRef CAS.
- S. Balasubramanyam, A. Sharma, V. Vandalon, H. C. M. Knoops, W. M. M. Kessels and A. A. Bol, Plasma-enhanced atomic layer deposition of tungsten oxide thin films using (tBuN)2(Me2N)2W and O2 plasma, J. Vac. Sci. Technol., A, 2017, 36(1), 01B103 CrossRef.
- O. Prakash, V. Saxena, R. K. Bedi, A. K. Debnath and A. Mahajan, Solution processable transition metal oxide ultra-thin films as alternative electron transport and hole blocking layers in dye sensitized solar cells, J. Photochem. Photobiol., A, 2021, 418, 113385 CrossRef CAS.
- V. Saxena, N. Bhagat, S. Choudhury, A. Mahajan and A. Singh, Phase Variation of Ultrathin WO3 Electron-Transport Layer Prepared by Scalable Langmuir–Blodgett Technique to Boost Efficiency of Dye Sensitized Solar Cells, Solar RRL, 2022, 6(8), 2200222 CrossRef CAS.
- Z. Avazbaeva, W. Sung, J. Lee, M. D. Phan, K. Shin, D. Vaknin and D. Kim, Origin of the Instability of Octadecylamine Langmuir Monolayer at Low pH, Langmuir, 2015, 31(51), 13753–13758 CrossRef CAS PubMed.
- J. T. Davies, E. K. Rideal and S. A. Rice, Interfacial Phenomena, Phys. Today, 1962, 15(4), 78 CrossRef.
- J. Li, Y. Liu, Z. Zhu, G. Zhang, T. Zou, Z. Zou, S. Zhang, D. Zeng and C. Xie, A full-sunlight-driven photocatalyst with super long-persistent energy storage ability, Sci. Rep., 2013, 3(1), 2409 CrossRef PubMed.
- O. Prakash, V. Saxena, S. Choudhury, Tanvi, A. Singh, A. K. Debnath, A. Mahajan, K. P. Muthe and D. K. Aswal, Low temperature processable ultra-thin WO3 Langmuir-Blodgett film as excellent hole blocking layer for enhanced performance in dye sensitized solar cell, Electrochim. Acta, 2019, 318, 405–412 CrossRef CAS.
- C. V. Ramana, S. Utsunomiya, R. C. Ewing, C. M. Julien and U. Becker, Structural Stability and Phase Transitions in WO3 Thin Films, J. Phys. Chem. B, 2006, 110(21), 10430–10435 CrossRef CAS PubMed.
- Tanvi, V. Saxena, A. Singh, O. Prakash, A. Mahajan, A. K. Debnath, K. P. Muthe and S. C. Gadkari, Improved performance of dye sensitized solar cell via fine tuning of ultra-thin compact TiO2 layer, Sol. Energy Mater., Sol. Cells, 2017, 170, 127–136 CrossRef CAS.
- R. Memming, Semiconductor Electrochemistry, Wiley, 2015 Search PubMed.
- Y. Matsumoto, Y. Miura and S. Takata, Thickness-Dependent Flat Band Potential of Anatase TiO2(001) Epitaxial Films on Nb:SrTiO3(001) Investigated by UHV-Electrochemistry Approach, J. Phys. Chem. C, 2016, 120(3), 1472–1477 CrossRef CAS.
- P. Xu, T. J. Milstein and T. E. Mallouk, Flat-Band Potentials of Molecularly Thin Metal Oxide Nanosheets, ACS Appl. Mater. Interfaces, 2016, 8(18), 11539–11547 CrossRef CAS PubMed.
- A. Sacco, Electrochemical impedance spectroscopy: fundamentals and application in dye-sensitized solar cells, Renewable Sustainable Energy Rev., 2017, 79, 814–829 CrossRef CAS.
- L. Kavan, N. Tétreault, T. Moehl and M. Grätzel, Electrochemical Characterization of TiO2 Blocking Layers for Dye-Sensitized Solar Cells, J. Phys. Chem. C, 2014, 118(30), 16408–16418 CrossRef CAS.
- J. Premkumar, Development of Super-Hydrophilicity on Nitrogen-Doped TiO2 Thin Film Surface by Photoelectrochemical Method under Visible Light, Chem. Mater., 2004, 16(21), 3980–3981 CrossRef CAS.
- S. S.-O. Youn, J. Kim, J. Na, W. Jo and G. Y. Kim, Understanding the Space-Charge Layer in SnO2 for Enhanced Electron Extraction in Hybrid Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2022, 14(42), 48229–48239 CrossRef CAS PubMed.
- F. Fabregat-Santiago, J. Bisquert, G. Garcia-Belmonte, G. Boschloo and A. Hagfeldt, Influence of electrolyte in transport and recombination in dye-sensitized solar cells studied by impedance spectroscopy, Sol. Energy Mater. Sol. Cells, 2005, 87(1), 117–131 CrossRef CAS.
- A. Pockett, M. Spence, S. K. Thomas, D. Raptis, T. Watson and M. J. Carnie, Beyond the First Quadrant: Origin of the High Frequency Intensity-Modulated Photocurrent/Photovoltage Spectroscopy Response of Perovskite Solar Cells, Solar RRL, 2021, 5(5), 2100159 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ma00063c |
| This journal is © The Royal Society of Chemistry 2024 |