DOI:
10.1039/D4MA00617H
(Paper)
Mater. Adv., 2024,
5, 8238-8253
Multifunctional NaEu(WO4)2: defect-tuned red emission and acetone sensing at room temperature†
Received
15th June 2024
, Accepted 15th September 2024
First published on 18th September 2024
Abstract
Rare-earth double tungstate NaEu(WO4)2 was synthesized via a trisodium citrate (Na3cit)-assisted hydrothermal technique, followed by calcination, to promote crystallinity and detailed investigations on their crystal structures and luminescence properties. In this study, the structural evolution of our samples synthesized with different amounts of Na3cit was studied by employing X-ray diffraction, Rietveld refinement, Fourier transform infrared and Raman spectroscopy techniques. It was found that NaEu(WO4)2 belongs to the scheelite family with Na and Eu atoms occupying the same sites and  antisite defects deforming EuO8 dodecahedra. The modulation of W–O, Eu–O and angle splitting in the presence of
antisite defects deforming EuO8 dodecahedra. The modulation of W–O, Eu–O and angle splitting in the presence of  antisite defects was identified. From in-depth X-ray photoelectron spectroscopy, we validated the deformation of the EuO8 dodecahedron due to the presence of oxygen vacancies (VOs), which originated from
antisite defects was identified. From in-depth X-ray photoelectron spectroscopy, we validated the deformation of the EuO8 dodecahedron due to the presence of oxygen vacancies (VOs), which originated from  antisite defects. Herein, we show that the band gap of NaEu(WO4)2 is highly sensitive to defects; however, the 5D0–7F2 transition of Eu3+ at 615 nm with color coordinates (0.67, 0.33) is very robust, making NaEu(WO4)2 a suitable red phosphor material for near UV-type light-emitting devices (LEDs). We also identified that VOs present in the EuO8 dodecahedron act as active sites for acetone sensing (∼68% response to 100 ppm) with a response and recovery time of ∼3.3/10 s at room temperature, suggesting the potency of NaEu(WO4)2 as a multifunctional material with applications in LEDs and acetone sensors. In order to validate our experimental observations theoretically, we calculated the band structure and density of states of bare and
antisite defects. Herein, we show that the band gap of NaEu(WO4)2 is highly sensitive to defects; however, the 5D0–7F2 transition of Eu3+ at 615 nm with color coordinates (0.67, 0.33) is very robust, making NaEu(WO4)2 a suitable red phosphor material for near UV-type light-emitting devices (LEDs). We also identified that VOs present in the EuO8 dodecahedron act as active sites for acetone sensing (∼68% response to 100 ppm) with a response and recovery time of ∼3.3/10 s at room temperature, suggesting the potency of NaEu(WO4)2 as a multifunctional material with applications in LEDs and acetone sensors. In order to validate our experimental observations theoretically, we calculated the band structure and density of states of bare and  antisite defects containing NaEu(WO4)2 using ab initio density functional theory and identified the sensing mechanism. We believe that our studies will be helpful in introducing new multifunctional applications of NaEu(WO4)2, while theoretical calculations will provide new electronic insights that may be used to understand the features of other double rare-earth tungstate materials.
antisite defects containing NaEu(WO4)2 using ab initio density functional theory and identified the sensing mechanism. We believe that our studies will be helpful in introducing new multifunctional applications of NaEu(WO4)2, while theoretical calculations will provide new electronic insights that may be used to understand the features of other double rare-earth tungstate materials.
1. Introduction
Motivated by multifunctional materials in nature such as the human skin and bird feathers, the design and development of materials having multiple functionalities has become an upcoming field for various sustainable applications and has gained the attention of researchers in the recent past. Owing to their multiple features, scheelite-type alkali rare-earth double tungstates (AILn(WO4)2, A = alkali metal, Ln = lanthanide rare-earth metal) with C4h symmetry and the I41/a space group have emerged as promising materials in many technologically diverse areas such as catalysis, energy storage, scintillators, and laser hosts. Because of the strong covalence of the W–O bond, scheelites act as good hosts that promote high solubility for lanthanide (Ln) doping, where they exhibit narrow emission characteristics of high spectral purity due to the strong f–f or f–d transition of Ln3+ ions.1 It is worth mentioning that the absorption coefficient of Ln3+ ions is usually very low, resulting in a weak emission signal.2 However, tungstates, because of their strong absorption coefficient, facilitate energy transfer to Ln3+, and subsequently, the emission intensity gets enhanced.3 Because of this, Ln3+-doped tungstate scheelites show maximum performance in the field of solid-state lighting devices, including field emission displays, plasma display panels, liquid crystal displays, and light-emitting diodes (LEDs). These lighting systems have several advantages of biocompatibility, low power consumption, long lifetime, remarkably high brightness, etc., and hence have high market value. As different Ln3+ ions have different energy levels and accordingly produce different colors, the choice of Ln3+ is most important to tune the color output of the lighting devices as per requirements and applications.4 In general, the energy transfer between tungstate and Ln3+ ions significantly depends on the type of Ln3+ and host matrix, wherein efforts are mostly given to explore different tungstate hosts for improved energy transfer. In contrast to Ln3+-doped tungstates, self-contained tungstate–lanthanide hosts have been found to be highly efficient for this process, wherein scheelite-structured alkali metals, i.e., Ln-binary tungstate with the generic formula AILn(WO4)2, a representative of new scheelite materials with high density and low phonon threshold,5 have drawn worldwide attention. Much more improved emission has been detected from AILn(WO4)2, suggesting their suitability for several lighting systems including laser crystals and phosphors.6 It has been found that ‘A’ is commonly responsible for crystal field distortion around Ln3+ ions, relaxing the selection rules for f–f or f–d transitions, which enhance emission intensity.7 Among several Ln3+ ions such as Gd3+, Lu3+, Tm3+, and Dy3+, several works have been carried out with Eu3+-doped binary tungstate as the designing red phosphor due to its high absorption coefficient in UV/near UV region, which circumvents low color rendering index in comparison with other Eu3+-doped metal oxides/sulphides (e.g., ZnO, SnO2, and ZnS) and scheelites (e.g., SrWO4).8–11 Nevertheless, there is still a need to improve the Eu3+-red phosphor, wherein researchers are actively involved. In this regard, it may be stated that few fundamental studies are likely to explore the structure and optical emissions from NaEu(WO4)2, indicating its potency as a red phosphor material. As an example, J. Huang et al. have investigated the effect of alkali metal ions on the local structure of AEu(WO4)2 (A = Li, Na, K), where they identified broader red emission at 614 nm due to an effective charge transfer excitation in KEu(WO4)2 in comparison with that of LiEu(WO4)2 and NaEu(WO4)2.12 Neeraj et al. observed a significant improvement in the light output in NaY0.5Eu0.5(WO4)(MoO4) with reference to the commercial red phosphor Y2O3S:Eu3+.13 Commonly, the AILn(WO4)2 class of materials is synthesised by several techniques such as solid state reaction, hydrothermal, solvothermal, and microwave synthesis.14 Several researchers, including our group, have found that defects depending on the synthesis protocols play an important role in tuning the distortion around Ln3+ ions and accordingly, the selection rules get relaxed. Therefore, it may be stated that emission is significantly dependent on the synthesis methods, where some studies are going on actively.15 As an example, the use of water and ethylene glycol during the solvothermal synthesis of NaEu(WO4)2 significantly alters the Eu–O bond lengths, which in consequence could cause a blue shift of the charge transfer band,16 while Munirathnappa et al. tuned the defects by electron beam radiation and observed a significant improvement of the luminescence property of NaEu(WO4)2.17 A careful literature review reveals that few efforts were undertaken to generate various micro/nanostructures of NaEu(WO4)2 using different surfactants such as ethylenediaminetetraacetic acid (EDTA), cetyltrimethyl ammonium bromide (CTAB), trisodium citrate (Na3cit), polyvinylpyrrolidone (PVP), and ethylene glycol (EG) and the morphological influences on the emission characteristics were studied. However, there is no report to illustrate the effect of surfactant on the defects, particularly on the distortion of the crystal field around Eu3+ ions and related emissions.
Based on our previous investigations18 and motivated by the fact that defects can be tuned by varying the surfactant during the hydrothermal process, an attempt has been made for the first time to examine the influence of Na3cit on the defects and related deformation around the Eu3+ ions within NaEu(WO4)2. In this study, we have identified that Na3cit plays a significant role to tune the  antisite defects, which are subsequently counterbalanced by the oxygen vacancy (VO). We have also emphasized the VO-induced modification of the bond length and mixing of Eu 4f orbitals, which synergistically impact the emission properties and chromaticity coordinates. Most importantly, by varying the synthesis condition, we achieved the chromaticity coordinate (0.667, 0.333) from one of our synthesized samples, which perfectly matches with that of the National Television Standard Committee (NTSC)-prescribed chromaticity coordinate for red phosphor.
antisite defects, which are subsequently counterbalanced by the oxygen vacancy (VO). We have also emphasized the VO-induced modification of the bond length and mixing of Eu 4f orbitals, which synergistically impact the emission properties and chromaticity coordinates. Most importantly, by varying the synthesis condition, we achieved the chromaticity coordinate (0.667, 0.333) from one of our synthesized samples, which perfectly matches with that of the National Television Standard Committee (NTSC)-prescribed chromaticity coordinate for red phosphor.
In the recent past, there has been an increasing interest in the detection of life-threatening toxic gases. Although techniques like as gas/liquid chromatography exist, because of their high cost, their wide application is limited. Herein, chemo-resistive-type gas sensors based on metal oxides have been viewed as one of the most economical, effective, and fast techniques for sensing applications. However, major issues still exist in their high sensitivity and high temperature (∼250–400 °C) operation.19 Hence, it is worthwhile to develop a room temperature sensor with low power consumption, safety, and long lifetime. Though sensors operating at higher temperature are largely reported, room temperature sensors are very rare. However, efforts are continuously underway to find out suitable materials for room-temperature sensors. Our previous investigations reveal that VO often leads to the gas sensing property;15,18 hence, we checked the gas sensing property of NaEu(WO4)2 and, most importantly, acetone sensing has been achieved at room temperature. Apart from the experimental investigations on the optical and gas sensing properties, ab initio density functional theory has been adopted to acquired knowledge of the band structure and partial density of states (PDOS) of NaEu(WO4)2 in order to understand  antisite defect-induced changes in the optical and acetone sensing properties of NaEu(WO4)2. To the best of our knowledge, no such study exists for NaEu(WO4)2; hence, we believe that these experimental and theoretical findings will be helpful in realizing the multifunctional applications of NaEu(WO4)2.
antisite defect-induced changes in the optical and acetone sensing properties of NaEu(WO4)2. To the best of our knowledge, no such study exists for NaEu(WO4)2; hence, we believe that these experimental and theoretical findings will be helpful in realizing the multifunctional applications of NaEu(WO4)2.
2. Experimental section
2.1. Synthesis of NaEu(WO4)2
Sodium europium double tungstate (NaEu(WO4)2) was synthesized by a facile hydrothermal method. Initially, 1.00 mmol (0.338 g) of europium nitrate hydrate [Eu(NO3)3·H2O, Alfa Aesar] and tri-sodium citrate dihydrate [Na3cit, Merck] were dissolved in 50.00 mL DI solution and stirred for 20 minutes. Another 20.00 mL aqueous solution containing 2.00 mmol (0.660 g) sodium tungstate dihydrate [Na2WO4·2H2O, Sigma Aldrich] was added dropwise to the previously prepared solution and stirred (30 minutes) for homogeneous mixing. Then, the mixture solution was transferred to a Teflon-lined stainless-steel autoclave of 100.00 mL capacity for hydrothermal reaction, which was carried out at 180 °C for 24 h. After the reaction, the temperature was cooled down and the powder sample was collected by means of centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, followed by washing with DI water and ethanol twice and drying at 70 °C for 24 h. The obtained powder was calcined at 800 °C under nitrogen (N2) atmosphere for 5 h and the final product was obtained. In order to examine the effect of Na3cit on the final product, we varied the Na3cit (ca. 0.75, 0.50 and 0.25 mmol) amount during the hydrothermal reaction, keeping all other parameters same. Accordingly, they were named as NEWO0.75, NEWO0.50 and NEWO0.25, respectively.
000 rpm, followed by washing with DI water and ethanol twice and drying at 70 °C for 24 h. The obtained powder was calcined at 800 °C under nitrogen (N2) atmosphere for 5 h and the final product was obtained. In order to examine the effect of Na3cit on the final product, we varied the Na3cit (ca. 0.75, 0.50 and 0.25 mmol) amount during the hydrothermal reaction, keeping all other parameters same. Accordingly, they were named as NEWO0.75, NEWO0.50 and NEWO0.25, respectively.
2.2. Characterization techniques
Structural analysis and phase confirmation were using the X-ray diffraction (XRD) patterns collected on a Rigaku (Japan) Ultima III powder diffractometer equipped with a Cu Kα radiation source (λ = 1.5406 Å). The Rietveld refinement was done with the help of FullProf Suite software to determine the structural and microstructural parameters. The background was fitted with linear interpolation, while the Pseudo-Voigt profile with asymmetry was used for the peak shape. The morphology was examined by field emission scanning electron microscopy (FESEM, S-4800, Hitachi Japan). Vibrational analysis was done using Fourier transform infrared spectroscopy (Shimadzu, IRPrestige-21) and Raman spectroscopy (alpha 300 Witec, 530 nm laser, 3 mW power and 2 m spot size). Elemental analysis was performed using X-ray photoelectron spectroscopy (PHI 5000 Versa Prob II, FEI Inc., Al Kα radiation, 1486 eV). The optical properties were investigated by a UV-vis (V-630, JASCO) and a photoluminescence spectrophotometer (RF-5301, Shimadzu). The measurement of luminescence lifetime was carried out with the help of the time-correlated single photon counting set up by Horiba Jobin-Yvon. The luminescence decay time was collected through a Hamamatsu MCP photomultiplier (R3809) and analysed using IBH DAS6 software.
2.3. Measurement of the gas-sensing properties of NaEu(WO4)2
Taguchi-type sensor modules were fabricated with a cylindrical alumina substrate wherein Pt wires and conducting gold paste were used for electroplating the alumina substrates. By mixing the as-synthesised materials with isopropyl alcohol, a slurry paste of desired consistency was prepared and the paste was drop-coated on the substrate and dried for 6 h to get rid of residue solvents. Ni–Cr wire, used as heating coils, was inserted inside the hollow of the alumina substrate. Then, the sensor modules, prepared by wielding the substrates on a six-pin socket, were used to evaluate the gas sensing properties in the presence of different volatile organic compounds (VOCs), namely, ammonia, acetone, ethanol, formalin, methanol, and isopropyl alcohol. The response was evaluated using the formulae 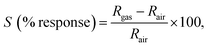 where Rgas and Rair denote the resistance of the sensor in the absence and presence of the target gas, respectively. The response and recovery time of the sensors were determined from the time taken by the sensor to reach the 90% resistance change with respect to the equilibrium state resistance after exposing and removing the target gas.
where Rgas and Rair denote the resistance of the sensor in the absence and presence of the target gas, respectively. The response and recovery time of the sensors were determined from the time taken by the sensor to reach the 90% resistance change with respect to the equilibrium state resistance after exposing and removing the target gas.
2.4.
Ab initio calculation using density functional theory
The first principles density functional theory (DFT) calculation was carried out in the plane wave pseudo-potential basis, as implemented in the Vienna ab initio simulation package the (VASP) using generalized gradient approximation (GGA) exchange–correlation potential. The cutoff energy of the plane wave basis was set to 500 eV, which was sufficient to achieve convergence, while 5 × 5 × 2 Monkhorst–Pack mesh was used for Brillouin zone sampling. The applied Hellmann–Feynman forces on each atom and EDIFF parameter were fixed to 0.015 eV Å−1 and 10−6 eV for these calculations.
Furthermore, the adsorption energy of acetone on the slab surface was calculated to overcome the antisite defect-induced gas sensing operation. Herein, we considered a vacuum region of 15 Å to avoid interlayer interactions along the z-axis, while the 5 × 5 × 1 Monkhorst–Pack mesh and 500 eV cut off energy were employed to get converged free energy for the surface calculation. To understand the adsorption mechanism, isolated acetone molecules were modelled above the cleaved (0 0 4) surface (most stable surface with minimum free energy), keeping acetone at the centre of the unit cell using three configurations (i.e., vertical, parallel to the x-axis and parallel to the y-axis). The adsorption was determined from eqn (1)20
| | | Eads = Eslab+gas − (Eslab + Egas) | (1) |
where,
Eslab+gas,
Eslab and
Egas represent the total energy of the slab including gas, total energy of the slab and energy of the isolated gas,
i.e., acetone. Within this definition, a negative binding energy is attributed to an exothermic process.
3. Results and discussion
3.1. Phase, crystal structure and micro-structural analyses of NaEu(WO4)2 by XRD and FESEM
The XRD patterns of all the samples (as shown in Fig. S1, ESI†) consist of strong, sharp diffraction peaks matched closely with tetragonal NaEu(WO4)2 (JCPDS no. 04-002-3849, space group I41/a).12 The absence of any deleterious secondary peak in the diffraction patterns is imperative to point out the formation of the pure phase of the samples. Meanwhile, a crystallite size of ∼33–43 nm was obtained from the well-known Scherer's relation for all the samples, while the strain (ε), calculated using the relation 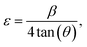 where β and θ denote the full width at half maxima (FWHM) and Bragg's angle respectively, was calculated to be ∼3.40 × 10−3, 3.14 × 10−3, and 2.59 × 10−3 for NEWO0.75, NEWO0.50, and NEWO0.25, respectively. It is noteworthy that Na3cit does not have a significant effect on the particle size during the hydrothermal reaction; rather, it has significant impact on ‘ε’, which may be related to the structural distortion.
where β and θ denote the full width at half maxima (FWHM) and Bragg's angle respectively, was calculated to be ∼3.40 × 10−3, 3.14 × 10−3, and 2.59 × 10−3 for NEWO0.75, NEWO0.50, and NEWO0.25, respectively. It is noteworthy that Na3cit does not have a significant effect on the particle size during the hydrothermal reaction; rather, it has significant impact on ‘ε’, which may be related to the structural distortion.
Rietveld refinement (shown in Fig. 1(a)–(c)), adopted to study long-range structural order–disorder, provides lattice parameters, unit cell volumes, and lengths of the links between (W–O)/(Na/Eu–O) of all the samples, as summarized in Table 1. Note that these parameters are very close to the data published in the literature12,16 as well as goodness-of-fit (e.g., χ2), which indicates the reliability of the refinement process. The unit cell of NaEu(WO4)2, modelled using the refined parameters for the visualization in the electronic and structural analysis (VESTA) program, consists of the [WO4] tetrahedron with Td symmetry group and the (Na/Eu)O8 dodecahedron, where Na+ and Eu3+ are randomly distributed over the same site positions with S4 symmetry (without an inversion symmetry) (as shown in Fig. 1(d)) and corresponds to two different Eu–O bond lengths (shorter and longer), denoted by [Eu–O]s and [Eu–O]l, respectively, indicating the distortion of the (Na/Eu)O8 dodecahedron.21 This distortion (e.g., ΔNa/Eu–O), estimated from 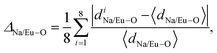 where 〈dNa/Eu–O〉 denotes average Na/Eu–O bond length, (schematically shown in Fig. 1(e) for all the samples), decreases monotonically from NEWO0.75 to NEWO0.25.21 The peculiarity of the hydrothermal method, where varying the Na3cit concentration commonly influences the organization of the [(Na/Eu)O8] dodecahedron, which causes various structural defects in the form of VO, distortion of the bonds, stresses, and strain on the crystallite lattices. Presently ΔNa/Eu–O, ascribed to ‘ε’, is believed to be dependent on VO, which can be explained as follows: we have noticed an enhancement in the (Na/Eu)–O bond length and reverse trend for W–O, which, according to Li et al., can be attributed to the decreasing dipole–dipole interactions due to charge entrapment at the VO site, which gets formed due to the
where 〈dNa/Eu–O〉 denotes average Na/Eu–O bond length, (schematically shown in Fig. 1(e) for all the samples), decreases monotonically from NEWO0.75 to NEWO0.25.21 The peculiarity of the hydrothermal method, where varying the Na3cit concentration commonly influences the organization of the [(Na/Eu)O8] dodecahedron, which causes various structural defects in the form of VO, distortion of the bonds, stresses, and strain on the crystallite lattices. Presently ΔNa/Eu–O, ascribed to ‘ε’, is believed to be dependent on VO, which can be explained as follows: we have noticed an enhancement in the (Na/Eu)–O bond length and reverse trend for W–O, which, according to Li et al., can be attributed to the decreasing dipole–dipole interactions due to charge entrapment at the VO site, which gets formed due to the  antisite defect at the 4a site, as given by
antisite defect at the 4a site, as given by 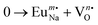 .22,23 Hence, the gradual decrease of ΔNa/Eu–O from NEWO0.75 to NEWO0.25 may be ascribed to the reduced
.22,23 Hence, the gradual decrease of ΔNa/Eu–O from NEWO0.75 to NEWO0.25 may be ascribed to the reduced  antisite defect. In our case, the hydrothermal reaction of Na+, Eu3+, and WO42− ions resulted in the formation of NaEu(WO4)2 precipitates, which can be shown in eqn (2)–(6) below.24–26
antisite defect. In our case, the hydrothermal reaction of Na+, Eu3+, and WO42− ions resulted in the formation of NaEu(WO4)2 precipitates, which can be shown in eqn (2)–(6) below.24–26
| | | Na2WO4(aq) → 2Na+(aq) + (WO4)2−(aq) | (2) |
| | | Eu(NO3)3(aq) → Eu3+(aq) + 3NO−(aq) | (3) |
| | | Na3cit(aq) → 3Na+(aq) + cit3−(aq) | (4) |
| | | Eu3+(aq) + cit3−(aq) → [Eu3+–cit3−] complex(aq) + NaNO3(aq) | (5) |
| | | 2[Eu3+–cit3−] complex(aq) + 2Na+(aq) + 2(WO4)2−(aq) → 2 NaEu(WO4)2(S) + 2cit3−(aq) | (6) |
The [Eu
3+–cit
3−] complex plays a pivotal role in the synthesis of NaEu(WO
4)
2, specifically on

. Low Na
3cit concentration inhibits the formation of the [Eu
3+–cit
3−] complex, which as a result reduces the

antisite defect. According to Shannon's data, the ionic radius of Na
+ (1.18 Å) is larger than that of Eu
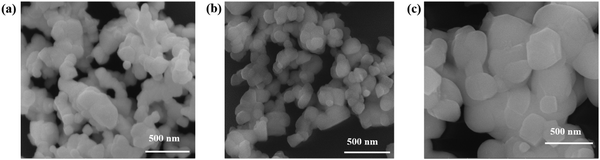
3+ (0.947 Å); therefore, the equivalent radius of A-site
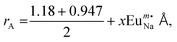 27
27 where ‘
x’ represents the fraction of the antisite defect, suggesting that a decrease of the

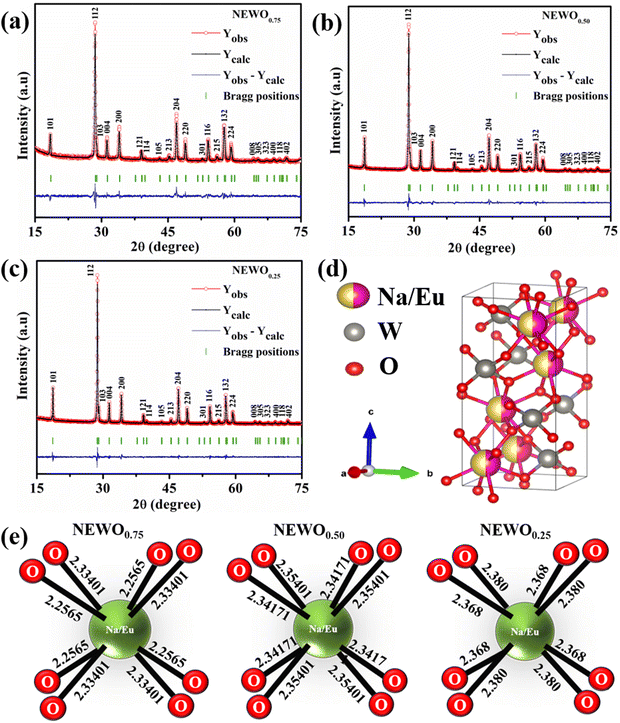
antisite defect will increase the (Na/Eu)–O bond length, in good agreement with our experimental findings. The FESEM images of the samples shown in
Fig. 2(a)–(c) illustrates the agglomerated nature of the particle, which may be attributed to the calcination effect at 800 °C.
 |
| | Fig. 1 The observed and refined XRD pattern of NaEu(WO4)2 samples: (a) NEWO0.75, (b) NEWO0.50, (c) NEWO0.25, (d) unit cell of the NaEu(WO4)2 tetragonal structure and (e) the schematic representation of the variation in the bond lengths of the Na/Eu–O dodecahedron in NaEu(WO4)2. | |
Table 1 Refined parameters from the Rietveld refinement method using FullProf suite software
| Parameters |
NEWO0.75 |
NEWO0.50 |
NEWO0.25 |
| Lattice parameters |
a = b (Å) |
5.2586 (2) |
5.24393 (8) |
5.25153 (6) |
|
c (Å) |
11.4182 (6) |
11.3766 (3) |
11.3979 (2) |
| Unit cell volume |
V (Å−3) |
315.74 (2) |
312.843 (14) |
314.3378 (8) |
| Bond length (in Å) |
Na/Eu–O |
2.2565 (8) |
2.34171 (5) |
2.368 (9) |
| 2.33401 (9) |
2.35401 (4) |
2.380 (9) |
| W–O |
2.05794 (7) |
1.94063 (3) |
1.914 (8) |
| Bond distance distortion index (ΔNa/Eu–O) |
16.88 |
2.62 |
2.53 |
|
R
p (%) |
5.10 |
4.63 |
4.74 |
|
R
wp (%) |
6.60 |
6.11 |
6.17 |
|
R
exp (%) |
5.75 |
4.90 |
5.59 |
|
χ
2
|
1.32 |
1.56 |
1.22 |
 |
| | Fig. 2 FESEM image of the NaEu(WO4)2 samples: (a) NEWO0.75, (b) NEWO0.50 and (c) NEWO0.25. | |
3.2. Investigations of the defects in NaEu(WO4)2 by FTIR, Raman and X-ray photoelectron spectroscopy techniques
In order to judge the structural distortion of the dodecahedron due to the  antisite defect, the FTIR and Raman spectra were taken as these spectroscopic techniques invaluably illustrate the short-range order–disorder phenomenon.28 The FTIR spectra (Fig. S2, ESI†), measured in the range of 400–1000 cm−1, consists of four absorption bands at 412–418, 438–440, 714–725 and 775–852 cm−1 indexed to internal O–W–O symmetric stretching, O–W–O symmetric bending, O–W–O anti-symmetric stretching (1Eu), and O–W–O anti-symmetric stretching (1Au) vibrations, respectively,28,29 while the band at 515–555 cm−1 is attributed to the Eu–O stretching vibration.30,31 Deconvolution reveals two distinct peaks (shown in the inset of Fig. 3(a)–(c)) measured at 524.5 and 548.1 cm−1 for NEWO0.75, 524.3 and 547.2 cm−1 for NEWO0.50, and 521.9 and 542.3 cm−1 for NEWO0.25. Herein, we believe that these two peaks originate from the deformed EuO8 dodecahedra containing the
antisite defect, the FTIR and Raman spectra were taken as these spectroscopic techniques invaluably illustrate the short-range order–disorder phenomenon.28 The FTIR spectra (Fig. S2, ESI†), measured in the range of 400–1000 cm−1, consists of four absorption bands at 412–418, 438–440, 714–725 and 775–852 cm−1 indexed to internal O–W–O symmetric stretching, O–W–O symmetric bending, O–W–O anti-symmetric stretching (1Eu), and O–W–O anti-symmetric stretching (1Au) vibrations, respectively,28,29 while the band at 515–555 cm−1 is attributed to the Eu–O stretching vibration.30,31 Deconvolution reveals two distinct peaks (shown in the inset of Fig. 3(a)–(c)) measured at 524.5 and 548.1 cm−1 for NEWO0.75, 524.3 and 547.2 cm−1 for NEWO0.50, and 521.9 and 542.3 cm−1 for NEWO0.25. Herein, we believe that these two peaks originate from the deformed EuO8 dodecahedra containing the  antisite defect, i.e., [EuO8]deformed and EuO8 dodecahedra without the antisite defect, i.e., [EuO8]ordered.32 [EuO8]deformed was estimated from the FTIR spectra using the relation
antisite defect, i.e., [EuO8]deformed and EuO8 dodecahedra without the antisite defect, i.e., [EuO8]ordered.32 [EuO8]deformed was estimated from the FTIR spectra using the relation 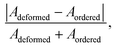 where Adeformed and Aordered denote the area under the curves corresponding to [EuO8]deformed and [EuO8]ordered, showing a decreasing trend (∼0.80, 0.51 and 0.19 for NEWO0.75, NEWO0.50 and NEWO0.25, respectively), which is in good agreement with the Rietveld results of the decreasing
where Adeformed and Aordered denote the area under the curves corresponding to [EuO8]deformed and [EuO8]ordered, showing a decreasing trend (∼0.80, 0.51 and 0.19 for NEWO0.75, NEWO0.50 and NEWO0.25, respectively), which is in good agreement with the Rietveld results of the decreasing  antisite defect.
antisite defect.
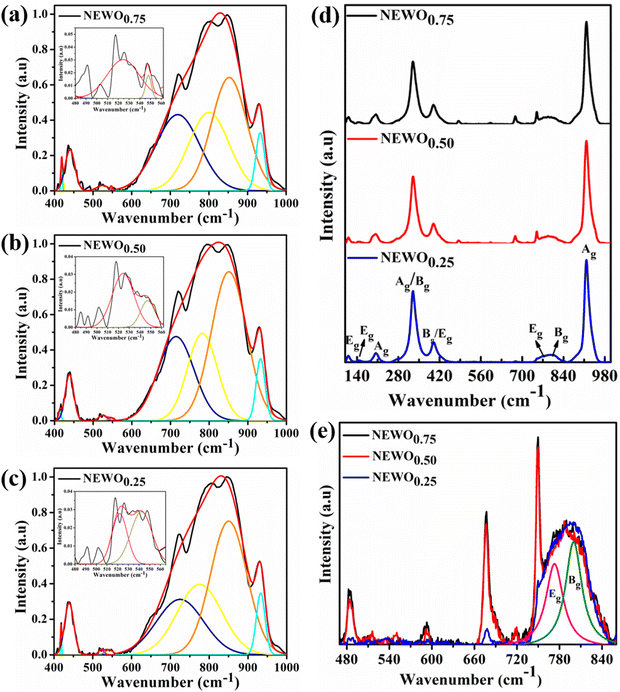 |
| | Fig. 3 Deconvoluted peaks of the FTIR spectrum: (a) NEWO0.75, (b) NEWO0.50, and (c) NEWO0.25. (d) Raman spectra of NEWO0.75, and NEWO0.50, NEWO0.25. (e) Magnified Raman spectra (480–840 cm−1). | |
According to group theory calculation, only thirteen Raman vibrational modes of tetragonal scheelite materials at the Γ-point are active and represented by eqn (7)28
where non-degenerate A
g, B
g modes and doubly degenerate E
g mode originate from various internal vibrations of [WO
4]
2− with the
S4 point symmetry or external vibrations of the (Na/Eu)O
8 dodecahedron with
D2d point symmetry. Herein, Raman spectrum of all sample (
Fig. 3(d)), measured in between 100–1000 cm
−1, comprises of eight peaks, measured at 111, 147, 203, 329, 399, 763, 800 and 917 cm
−1, while NEWO
0.50 and NEWO
0.75 show three additional peaks. Very briefly, the peak at 111 cm
−1 is assigned to the E
g stretching vibration of the (Na/Eu)O
8 dodecahedron or the translational mode of Na
+ and Eu
3+,
33 while the peaks at 147 and 203 cm
−1 are indexed to the E
g and A
g free rotation of [WO
4]
2−, respectively.
34 The peaks at 329 and 399 cm
−1 are ascribed to the A
g anti-symmetric and B
g symmetric bending of O–W–O,
35 while the peaks at 763 and 800 cm
−1 are ascertained to the B
g and E
g anti-symmetric stretching of the O–W–O vibrations, respectively.
36 The peak at 918 cm
−1 is attributed to the A
g symmetric O–W–O stretching.
37 In contrast to NEWO
0.25, three additional peaks of comparatively low intensity (zoomed-in image shown in
Fig. 3(e)) have been identified at 484, 677 and 750 cm
−1 for NEWO
0.50 and NEWO
0.75, and they can be readily ascribed to [(Na/Eu)O
8]
deformed of NEWO
0.50 and NEWO
0.75, thus validating previous studies.
38
To accumulate more information about the chemical bonding characteristics of the constituent elements in the presence of [(Na/Eu)O8]deformed, we adopted XPS to examine the valence state of Na, Eu, W and O. Herein, all the measured binding energy data have been corrected with reference to C 1s with a binding energy at 284.6 eV, which appears as an environmental carbon. The XPS survey spectra (Fig. S3, ESI†), recorded in between 0 and 1100 eV, represents a core binding energy of Na, Eu, W and O of NEWO0.75, NEWO0.50, and NEWO0.25, while the high-resolution spectra of Eu and O, W and Na were also recorded. As illustrated in Fig. 4(a), (c) and (e), the high-resolution Eu spectrum consists of two peaks at 135.9 and 141.8 eV corresponding to the spin–orbit splitting of 4d5/2 and 4d3/2 orbitals, respectively, in accordance with its trivalent oxidation state.39 As these spectra are asymmetric, hence, a careful deconvolution demonstrates two peaks (denoted by Ea and Eb) for 4d5/2 orbitals with a binding energy of 135.0, 136.8 eV for NEWO0.75, 135.2, 137.0 eV for NEWO0.5 and 136.0, 137.5 eV for NEWO0.25. Similarly, the binding energies of 4d3/2 orbitals, after deconvolution (e.g., peak Ec and Ed), were obtained at 140.4, 142.2 eV and 141.0, 142.5 and 141.5, 143.1 eV for the three respective samples. Herein, 4d5/2 with lower binding energy (e.g., 135.0, 135.2 and 136.0 eV for NEWO0.75, NEWO0.5 and NEWO0.25 respectively) is assigned to [EuO8]ordered, while a higher binding energy (e.g., 136.8, 137.0, and 137.5 eV for NEWO0.75, NEWO0.5 and NEWO0.25, respectively) corresponds to [EuO8]deformed. Two different binding energies denote the presence of Eu3+ with two different polarizing fields, which can be normally expressed by 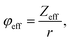 where Zeff and ‘r’ denote nuclear charge and ionic radius in the respective ligand field, respectively, while φeff is the effective ionic potential and denotes the polarizability of the Eu3+ ion.40 In brief, due to the less difference between the electronegativity of O (3.44) and Eu (1.2), the Eu–O bond is covalent, where electrons are shared by Eu and O. In case of the [EuO8]ordered dodecahedron, the Eu3+ ions are surrounded by eight O atoms, whereas this number is less in the case of [EuO8]deformed due to the presence of
where Zeff and ‘r’ denote nuclear charge and ionic radius in the respective ligand field, respectively, while φeff is the effective ionic potential and denotes the polarizability of the Eu3+ ion.40 In brief, due to the less difference between the electronegativity of O (3.44) and Eu (1.2), the Eu–O bond is covalent, where electrons are shared by Eu and O. In case of the [EuO8]ordered dodecahedron, the Eu3+ ions are surrounded by eight O atoms, whereas this number is less in the case of [EuO8]deformed due to the presence of 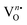 , suggesting different ‘r’ for these two different dodecahedrons; rather, it will be less in [EuO8]deformed. Therefore, the higher binding energy of Eu 4d orbitals in [EuO8]deformed is ascribed to the higher φeff in the deformed dodecahedron. The [EuO8]deformed:[EuO8]ordered ratio, an indication of the polarizability around Eu3+ ions, was estimated from the area under the 4d5/2 orbital, and this ratio was found to be 1.0, 0.7 and 0.6 for NEWO0.75, NEWO0.50 and NEWO0.25, respectively, indicating a monotonic decrease of [EuO8]deformed. The covalence of the dodecahedron host, which primarily depends on ‘ϕeff’, is believed to be decrease from NEWO0.75 to NEWO0.25 as the decrease in the
, suggesting different ‘r’ for these two different dodecahedrons; rather, it will be less in [EuO8]deformed. Therefore, the higher binding energy of Eu 4d orbitals in [EuO8]deformed is ascribed to the higher φeff in the deformed dodecahedron. The [EuO8]deformed:[EuO8]ordered ratio, an indication of the polarizability around Eu3+ ions, was estimated from the area under the 4d5/2 orbital, and this ratio was found to be 1.0, 0.7 and 0.6 for NEWO0.75, NEWO0.50 and NEWO0.25, respectively, indicating a monotonic decrease of [EuO8]deformed. The covalence of the dodecahedron host, which primarily depends on ‘ϕeff’, is believed to be decrease from NEWO0.75 to NEWO0.25 as the decrease in the  antisite defect makes the dodecahedron more ionic due to the larger electronegativity difference between Na (0.93) and O (3.44). In addition, the increasing binding energy of both 4d5/2 and 4d3/2 orbitals from NEWO0.75 to NEWO0.25 is correlated to the enhancement of the Eu–O bond lengths in good agreement with Rietveld analysis.
antisite defect makes the dodecahedron more ionic due to the larger electronegativity difference between Na (0.93) and O (3.44). In addition, the increasing binding energy of both 4d5/2 and 4d3/2 orbitals from NEWO0.75 to NEWO0.25 is correlated to the enhancement of the Eu–O bond lengths in good agreement with Rietveld analysis.
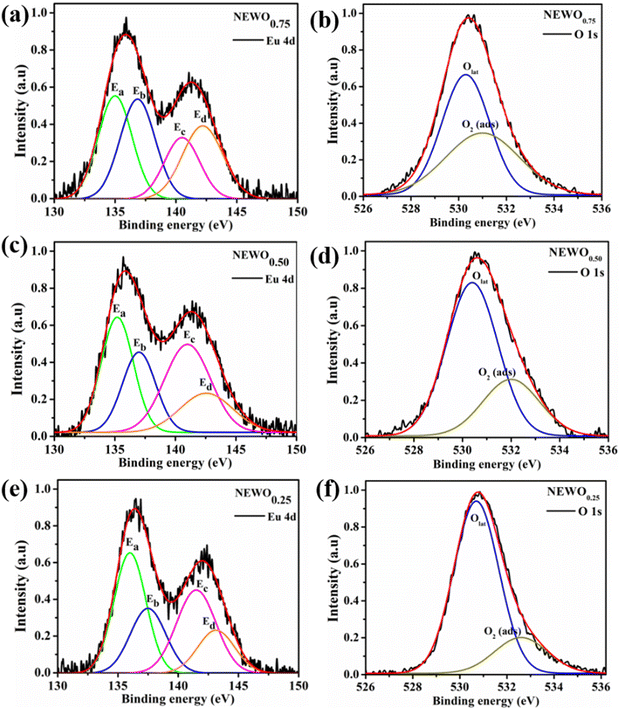 |
| | Fig. 4 High-resolution XPS spectra of the Eu 4d state and O 1s state of (a) and (b) NEWO0.75. (c) and (d) NEWO0.50 and (e) and (f) NEWO0.25. | |
For oxygen, the high-resolution O 1s spectra of the three samples are shown in Fig. 4(b), (d) and (f), which is measured at 530.0 eV of the typical asymmetric characteristics, indicating the presence of 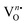 in the samples.41 After deconvolution, two different bonding states of oxygen with binding energies of 530.3, 531.0 eV for NEWO0.75, 530.4, 532.0 eV for NEWO0.50 and 530.7, 532.6 eV for NEWO0.25 were observed. Low binding energy corresponds to the lattice oxygen (Olat) species of [EuO8]ordered, while the higher binding energies are attributed to the adsorbed oxygen (O2(ads)) species at the
in the samples.41 After deconvolution, two different bonding states of oxygen with binding energies of 530.3, 531.0 eV for NEWO0.75, 530.4, 532.0 eV for NEWO0.50 and 530.7, 532.6 eV for NEWO0.25 were observed. Low binding energy corresponds to the lattice oxygen (Olat) species of [EuO8]ordered, while the higher binding energies are attributed to the adsorbed oxygen (O2(ads)) species at the 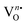 site within [EuO8]deformed. An increase in the binding energy of Olat is ascribed to the reduced deformation of the [EuO8]deformed dodecahedron. However, the O2(ads)/Olat molar ratio was calculated to be ∼0.44, 0.29 and 0.20 for NEWO0.75, NEWO0.50 and NEWO0.25, respectively; hence, the result indicates a monotonic decrease in
site within [EuO8]deformed. An increase in the binding energy of Olat is ascribed to the reduced deformation of the [EuO8]deformed dodecahedron. However, the O2(ads)/Olat molar ratio was calculated to be ∼0.44, 0.29 and 0.20 for NEWO0.75, NEWO0.50 and NEWO0.25, respectively; hence, the result indicates a monotonic decrease in 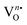 from NEWO0.75 to NEWO0.25. As XPS is highly sensitive to the surface, therefore, we believe that these
from NEWO0.75 to NEWO0.25. As XPS is highly sensitive to the surface, therefore, we believe that these 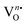 mostly resides on the sample surface. In contrast, the spectra of Na and W have been found in the Gaussian shape and were deconvoluted (as shown in Fig. S4, ESI†). From the above FTIR and XPS experiments, we have confirmed that the antisite defect mainly arose due to the Eu elements, which play a key role in the defects.
mostly resides on the sample surface. In contrast, the spectra of Na and W have been found in the Gaussian shape and were deconvoluted (as shown in Fig. S4, ESI†). From the above FTIR and XPS experiments, we have confirmed that the antisite defect mainly arose due to the Eu elements, which play a key role in the defects.
3.3. Investigation of the optical properties of NaEu(WO4)2
In order to gain insight into the mechanism on the effect of the  antisite defect on the optical properties, the UV-vis absorption spectra (shown in Fig. S5, ESI†) were recorded and analysed to calculate the band gap (Eg) using Tauc plot.42 Herein, the direct Eg of NEWO (discussed later) was estimated to be ∼2.24, 2.27 and 2.58 eV for NEWO0.75, NEWO0.50, and NEWO0.25, respectively (shown in Fig. 5(a)–(c)). Such a trend of increasing Eg may be assigned to a decrease in the covalence of the host lattice due to [EuO8]deformed and can be explained as follows: mostly, Eg depends on the polarizability of cations and deformation of anions. Greater the cationic polarization and anionic deformation, stronger the covalent links between the anion and cation, thus narrowing the Eg. Our XPS studies reveal that the covalence of the host matrix decreases from NEWO0.75 to NEWO0.25; thus, the enhancement of Eg can be certainly ascribed to the reduced covalence of the host matrix.
antisite defect on the optical properties, the UV-vis absorption spectra (shown in Fig. S5, ESI†) were recorded and analysed to calculate the band gap (Eg) using Tauc plot.42 Herein, the direct Eg of NEWO (discussed later) was estimated to be ∼2.24, 2.27 and 2.58 eV for NEWO0.75, NEWO0.50, and NEWO0.25, respectively (shown in Fig. 5(a)–(c)). Such a trend of increasing Eg may be assigned to a decrease in the covalence of the host lattice due to [EuO8]deformed and can be explained as follows: mostly, Eg depends on the polarizability of cations and deformation of anions. Greater the cationic polarization and anionic deformation, stronger the covalent links between the anion and cation, thus narrowing the Eg. Our XPS studies reveal that the covalence of the host matrix decreases from NEWO0.75 to NEWO0.25; thus, the enhancement of Eg can be certainly ascribed to the reduced covalence of the host matrix.
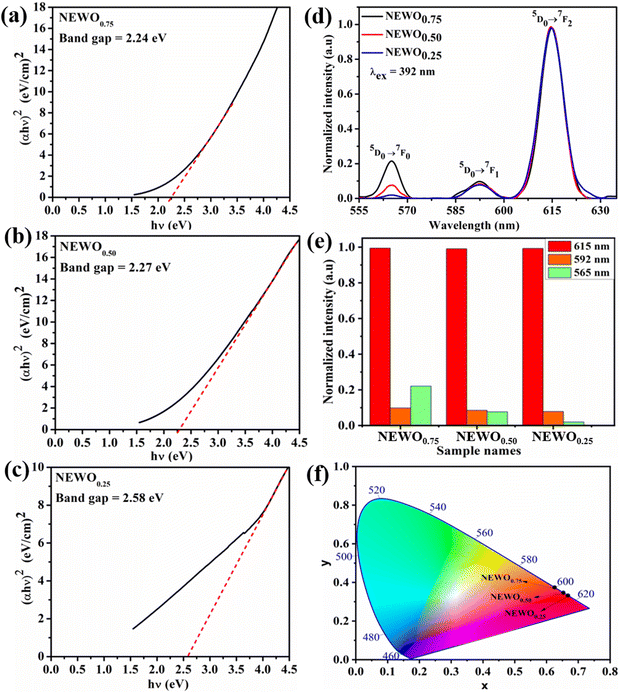 |
| | Fig. 5 Tauc plot: (a) NEWO0.75, (b) NEWO0.50, and (c) NEWO0.25. (d) PL spectra of NEWO0.75, NEWO0.50, and NEWO0.25. (e) Bar diagram of the normalized PL intensity of samples and (f) CIE diagram of NEWO0.75, NEWO0.50, and NEWO0.25 samples. | |
Prior to examining the emission, we recorded the photoluminescence excitation (PLE) spectra of the as-synthesized samples (normalized spectra are shown in Fig. S6(a), ESI†). It is clearly visible that the PLE spectra of all the as-prepared materials consist of three peaks, measured at 380, 392 and 463 nm, which are assigned to 7F0–5L7, 7F0–5L6 and 7F0–5D2 intra-configurational 4f6 transitions of Eu3+. One peak, recorded at 360 nm for NEWO0.50 and NEWO0.25, is ascribed to the 7F0–5D4 transition of Eu3+, while another peak of relatively low intensity at 413 nm, observed only for NEWO0.25, demonstrates the 7F0–5D3 transition of Eu3+. Careful literature survey reveals that our measured PLE spectra are not very sharp in comparison with other observations and may be ascribed to the distorted dodecahedron.43 In addition, a sharp peak was also identified at 306 nm, which may be assigned to the charge transfer band (CTB) from the surrounding oxygen anions to the WO4 tetrahedron. In this context, it may be stated that this is an interesting result for this particular phosphor material because of the fact that they have intense excitation peaks at 380, 392 and 463 nm in the ultra-violet (UV), near-UV, and UV-blue region, respectively, can act as efficient pumping sources for the red emission from Eu3+ ions.
Normalized photoluminescence signals under 392 nm excitation wavelength are presented in (Fig. 5(d)). Herein, the spectrum of each sample shows a typical emission band of Eu3+ in the range of 555–635 nm, comprising one prominent peak at 615 nm and two relatively less intense peaks at 565 and 592 nm. The peak at 615 nm corresponds to the 5D0 → 7F2 electric dipole transition of Eu3+, which is hypersensitive to the local structures of Eu3+, particularly in the non-centrosymmetric crystal site, according to Judd–Ofelt's theory. In contrast, the emission peak at 592 nm, assigned to the 5D0 → 7F1 magnetic dipole transition of Eu3+, does not depend on the surrounding environment.44 It is noteworthy that the ground state electronic configuration of Eu3+ has 7F0 non-degenerate and non-overlapping 2S+1LJ multiplets; thus, Eu3+ is mostly used as an optical probe to investigate the crystal field surrounding them, where the intensity ratio between 5D0 → 7F2 and 5D0 → 7F1 is used to estimate the asymmetry of the local environment.45 A very careful estimation reveals that the area under the 615 nm emission curve increases from NEWO0.75 to NEWO0.25, indicating the reduction of [EuO8]deformed, in good agreement with the previous XRD and FTIR results. Hence, it may be concluded that the lowering of the Na3cit concentration reduces the  antisite defect as well as
antisite defect as well as 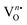 , which, as a result, increase the symmetry of the crystal structure. The emission peak at 565 nm, attributed to the 5D0 → 7F0 transition of Eu3+, has been observed in few other NaEu(WO4)2-like host matrices including the LaAlO3:Eu3+ nanophosphor, LaPO4:Eu3+ nanoparticles, SrB2O4:Eu3+ phosphor, and potassium–aluminoborotellurite.46–48 In principle, he 5D0 → 7F0 transition of Eu3+ is prohibited according to the selection rule of magnetic dipole transition (J = 0 → J′ = 0). However, the presence of this transition indicates the violation of the Judd–Ofelt's selection rule, which may be ascribed to the asymmetry of the [EuO8]deformed dodecahedron.49 Herein, we believe that the asymmetry of the crystal lattice leads to wavefunction mixing between the 7F0 and 7F2 states (discussed later). In this context, the second-order parameter (B20) of the crystal field expansion plays a pivotal role in the mixing of the wavefunctions and is related to the intensity of 5D0 → 7F0 and 5D0 → 7F2 transitions by the following eqn (8).50
, which, as a result, increase the symmetry of the crystal structure. The emission peak at 565 nm, attributed to the 5D0 → 7F0 transition of Eu3+, has been observed in few other NaEu(WO4)2-like host matrices including the LaAlO3:Eu3+ nanophosphor, LaPO4:Eu3+ nanoparticles, SrB2O4:Eu3+ phosphor, and potassium–aluminoborotellurite.46–48 In principle, he 5D0 → 7F0 transition of Eu3+ is prohibited according to the selection rule of magnetic dipole transition (J = 0 → J′ = 0). However, the presence of this transition indicates the violation of the Judd–Ofelt's selection rule, which may be ascribed to the asymmetry of the [EuO8]deformed dodecahedron.49 Herein, we believe that the asymmetry of the crystal lattice leads to wavefunction mixing between the 7F0 and 7F2 states (discussed later). In this context, the second-order parameter (B20) of the crystal field expansion plays a pivotal role in the mixing of the wavefunctions and is related to the intensity of 5D0 → 7F0 and 5D0 → 7F2 transitions by the following eqn (8).50
| | 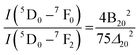 | (8) |
where
Δ20 (=1439 cm
−1) denotes the energy gap between the
7F
0 and
7F
2 levels. In this calculation,
B20 was found to be 623, 1393 and 2492 cm
−1 for NEWO
0.25, NEWO
0.50 and NEWO
0.75, respectively. These values of the
B20 parameter are within the range of few other materials such as YPO4:Eu
3+ (1365 cm
−1), Ca(PO
3)
2:Eu
3+ (1600 cm
−1), and K–Al–B–Te:Eu
3+ (829 cm
−1), which suggests the reliability of our estimated
B20 parameters.
51 In this context, it may be stated that a smaller magnitude of the
B20 parameter indicates a low degree of crystal field perturbation (denoted by the asymmetric
5D
0 →
7F
0 transition at 565 nm, shown in the bar diagram in
Fig. 5(e)); thus, the decreasing trend of
B20 from NEWO
0.75 to NEWO
0.25 can be certainly attributed to the lowering of [EuO
8]
deformed in our synthesized samples and corroborates highly with the Rietveld, FTIR and XPS studies. The quantum yield (QY), of NEWO
0.25, calculated from the photoluminescence decay profile (shown in Fig. S6(b), ESI
†) using the relation
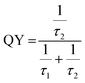
(where,
τ1 and
τ2 denote non-radiative and radiative recombination times), is ∼50%, which is slightly less than the QY of other scheelite materials.
52,53
Furthermore, the colour feature, indicated by the color coordinate (x, y) in the Commission International de I’Eclariage (CIE) chromaticity diagram (shown in Fig. 5(f)), has been examined for our synthesized samples. As presented in Table 2, the color coordinates of all the samples are observed within the red region, which may be attributed to the strong emission at 615 nm. Most importantly, NEWO0.25 exhibits improved colour coordinate from the commercially available red Y2O2S:Eu3+ phosphor (0.63, 0.35),54 which perfectly matches with the standard color coordinate (0.67, 0.33) according to NTSC. Colour purity (CP) of ∼86.7, 93.6 and 97.1% for NEWO0.75, NEWO0.50 and NEWO0.25 was calculated using the following eqn (9)38
| | 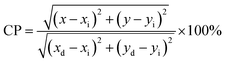 | (9) |
where (
x,
y) is the CIE coordinate of the NaEu(WO
4)
2 samples, (
xi,
yi) is the illuminate point of the 1931 CIE Standard Source C with the colour coordinates of (0.3101, 0.3162), and (
xd,
yd) is the colour coordinate of the dominant wavelength. Herein, the correlated colour temperatures (CCTs) of ∼1747, 2365 and 2957 K for NEWO
0.75, NEWO
0.50 and NEWO
0.25, respectively, were calculated using the McCamy relation
eqn (10)55| | | CCT = 449n3 + 3525n2 + 6823.3n + 5520.3 | (10) |
where
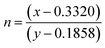
; (
x,
y) represent the chromaticity coordinate. These results suggest that NaEu(WO
4)
2 may be a potential candidate for red emission with excellent color purity and color coordinates close to the NTSC norms.
Table 2 List of the CIE co-ordinates of this work, commercial red phosphors and few synthesised red phosphors
| Materials name |
CIE coordinate |
Ref. |
|
x
|
y
|
| NEWO0.25 |
0.667 |
0.333 |
This work |
| NEWO0.50 |
0.653 |
0.347 |
This work |
| NEWO0.75 |
0.625 |
0.374 |
This work |
| NTSC value |
0.67 |
0.33 |
56
|
| Commercial red phosphors |
Y2O2S:Eu3+ |
0.63 |
0.35 |
54
|
| Y2O3:Eu3+ |
0.49 |
0.32 |
57
|
| Ba2Gd0.1Eu0.9(BO3)2Cl |
0.581 |
0.355 |
57
|
| Ba2Bi0.4Eu0.6V3O11 |
0.65 |
0.35 |
58
|
| KGd0.7Eu0.3TiO4 |
0.65 |
0.34 |
59
|
| Sr3Y0.94 (PO4)3:0.06 Eu3+ |
0.64 |
0.33 |
56
|
| Sr1.7Zn0.3CeO4:Eu3+ |
0.60 |
0.34 |
60
|
| Ca2Ga2GeO7:0.07Eu3+,0.07Na+ |
0.64 |
0.359 |
61
|
| CdWO4:0.5%Eu3+ |
0.6465 |
0.347 |
62
|
| Ca3(PO4)2:0.04Eu3+ |
0.5776 |
0.2840 |
63
|
| YBO3:Eu3+ |
0.655 |
0.345 |
64
|
| NaCaBO3:0.11Eu3+ |
0.608 |
0.387 |
65
|
| Sr2SiO4:Eu3+ |
0.6106 |
0.3517 |
66
|
4. Investigation of the gas sensing properties of NaEu(WO4)2
The gas sensing property of any metal oxide mostly depends on the gas–solid interaction with target gas molecules, wherein the adsorption/desorption of the gas molecule leads to a significant change in the resistance of the material. Previous studies by several researchers including our group reveal that VO traps electrons (e′), acts as a donor level within Eg and plays a pivotal role in both optical and gas sensing.15,18,67–72 For metal oxide sensors, VO facilitates receptor and transducer functions, which in turn tune the interaction of the target gas molecule on the sensor surface and transportation of the generated electronic signal within the sensor. It is an accepted mechanism that VO enhances the chemisorption of atmospheric O2, as described by O2(gas) + e− ↔ O2−(ads)73 and facilitates the receptor activity by oxidizing more of the target gas molecules. Herein, a careful literature survey shows that O2−(ads) can facilitate the following redox reactions between NEWO samples and acetone (CH3COCH3), ethanol (C2H5OH), ammonia (NH3), methanol (CH3OH), and formaldehyde (HCHO), as presented by eqn (11)–(15).| | | CH3COCH3(gas) + 8O2−(ads) ↔ 3H2O(gas) + 3CO2(gas) + 8e− | (11) |
| | | CH3CH2OH(gas) + O2−(ads) ↔ 2H2O(gas) + C2H2O + 2e− | (12) |
| | | 4NH3(gas) + 3O2−(ads) ↔ 6H2O(gas) + 2N2(gas) + 3e− | (13) |
| | | CH3OH(gas) + O2−(ads) ↔ 2H2O(gas) + CO(gas) + 2e− | (14) |
| | | HCHO(gas) + O2−(ads) ↔ H2O(gas) + CO2(gas) + 2e− | (15) |
These electrons released at the defect state at the conduction band minima act as donors, thus improving the response significantly. As XPS analyses reveals the presence of O2(ads) formed within the [EuO8]deformed dodecahedron, hence, the gas sensing property can be expected from NEWO samples. In order to validate the gas sensing ability of our synthesized NEWO samples as a proof-of-concept, we carried out a series of resistive-type sensing measurements using NEWO0.75, NEWO0.50 and NEWO0.25 as sensor probes against NH3, CH3COCH3, C2H5OH, CH3OH, HCHO and IPA (C3H8O) at room temperature. As shown in Fig. 6(a), NEWO0.75 exhibits highly selective sensitivity against CH3COCH3 in comparison with other target gases such as etc. as its resistance changes, while no significant variation was observed for other gases (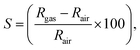 which was 5%, 3%, 8%, 4% and 6% for C2H5OH, NH3, HCHO, CH3OH and C3H8O, respectively). Room temperature operational condition is ascribed to the low bond-dissociation energy of CH3COCH3 (393 kJ mol−1), in comparison with C2H5OH (458 kJ mol−1), NH3 (435 kJ mol−1), and CH3OH (439 kJ mol−1), while the absence of sensing activity of NEWO0.50 and NEWO0.25 can be assigned to the low [EuO8]deformed content and can be explained as follows. The electron-depleted layer (LD), formed due to O2−(ads), also plays a pivotal role in transducing the electronic signal in sensing response. Normally, LD is prominent for the VO-enriched sample, leading to superior sensing response.71 Our XPS studies reveal the highest
which was 5%, 3%, 8%, 4% and 6% for C2H5OH, NH3, HCHO, CH3OH and C3H8O, respectively). Room temperature operational condition is ascribed to the low bond-dissociation energy of CH3COCH3 (393 kJ mol−1), in comparison with C2H5OH (458 kJ mol−1), NH3 (435 kJ mol−1), and CH3OH (439 kJ mol−1), while the absence of sensing activity of NEWO0.50 and NEWO0.25 can be assigned to the low [EuO8]deformed content and can be explained as follows. The electron-depleted layer (LD), formed due to O2−(ads), also plays a pivotal role in transducing the electronic signal in sensing response. Normally, LD is prominent for the VO-enriched sample, leading to superior sensing response.71 Our XPS studies reveal the highest 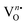 content of NEWO0.75, indicating the most efficient signal transduction in comparison with NEWO0.25 and NEWO0.50. Therefore, the high
content of NEWO0.75, indicating the most efficient signal transduction in comparison with NEWO0.25 and NEWO0.50. Therefore, the high 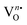 content of NEWO0.75 is believed to play a significant role in sensing CH3COCH3, one of the highly flammable VOCs that cause difficulties in breathing, nausea, vomiting and symptoms of neurological poisoning and also as a biomarker of diabetes found in exhaled human breath.74,75
content of NEWO0.75 is believed to play a significant role in sensing CH3COCH3, one of the highly flammable VOCs that cause difficulties in breathing, nausea, vomiting and symptoms of neurological poisoning and also as a biomarker of diabetes found in exhaled human breath.74,75
 |
| | Fig. 6 (a) Selectivity of theNEWO0.75 sensor toward acetone at RT. (b) Dynamic response curve of NEWO0.75 when exposed to 1–100 ppm acetone at RT. (c) Acetone response value of NEWO0.75 as a function of acetone concentration at RT (inset: isotherm fitting of the acetone response value of NEWO0.75 with linear regression). (d) Response and recovery time of the NEWO0.75 sensor for 100 ppm acetone at RT. (e) Response data at different %RH values to 100 ppm acetone at RT. (f) Long-term stability data of the NEWO0.75 sensor to 100 ppm acetone for 120 days. | |
Dynamic response, tested by exposing the sensor to different concentrations of CH3COCH3 ranging from 1 to 100 ppm (Fig. 6(b)), illustrates that the response increases gradually with increasing CH3COCH3 concentration, while Fig. 6(c) depicts the response of NEWO0.75 as a function of CH3COCH3 concentration. Herein, it may be concluded from Fig. 6(c) that the NEWO0.75 sensor has the capability to detect CH3COCH3 down to 1 ppm with changes in the resistance of ∼12% at room temperature, while the response increases to ∼68% at 100 ppm CH3COCH3 saturating above 100 ppm. To understand the adsorption/desorption mechanism, we fitted the response data with the Freundlich adsorption isotherm, as given by eqn (16) and (17).
| | i.e. log(Sg − 1) = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) log(Cg) log(Cg) | (17) |
where,
Sg and
Cg are the sensitivity and concentration of the target gas, while “
a” and “
b” denote the constants that depend on the electrical charge of the surface species and stoichiometry of the surface reaction. As is known from previous reports,
b < 0.5 indicates physisorption process, while
b > 1 denotes chemisorption mechanism.
76 Currently, the linear characteristic between log(
Sg − 1) and log(
Cg), as shown in the inset of
Fig. 6(c), provides
b = 0.38, indicating the physisorption of CH
3COCH
3 on the surface of NEWO
0.75. Response and recovery time of ∼3.3 s/10 s in the presence of 100 ppm CH
3COCH
3 indicate that the sensor is suitable for ultrafast application, as shown in
Fig. 6(d). The repeatability, studied by exposing the sensor to seven consecutive cycles of exposure to 10 ppm acetone at room temperature (shown in Fig. S7, ESI
†), gives almost similar characteristics (≤4% variation) for each measurement, yielding the excellent repeatability of NEWO
0.75 as a sensor material. The performance of the sensor was also checked under different humidity conditions, wherein our sensor shows highly robust performance under humidity (
Fig. 6(e)). The long-term stability of the senor (presented in
Fig. 6(f)), tested over 120 days, shows the negligible deterioration of the response. In this context, we compared the CH
3COCH
3 response characteristics in the previous literature and have presented a study in
Table 3. It can be stated here that NaEu(WO
4)
2 exhibits the best response characteristics (∼68% to 100 ppm acetone) with very short response–recovery time, and most importantly, it works at room temperature, indicating the reliability of the NaEu(WO
4)
2-based sensor for different non-invasive applications.
Table 3 The sensing performance of various metal oxides toward acetone gas
| Material name (type) |
Operating temperature (°C) |
Response/recovery time (s) |
Acetone response value (in ppm) |
Ref. |
| NEWO0.75 |
Room temperature |
3.3/10 |
68 (100 ppm) |
This work |
| 0.5% Fe–ZnO |
365 |
3/12 |
105.7 (100 ppm) |
77
|
| Pt–Fe2O3 |
139 |
3/22 |
25.7 (100 ppm) |
78
|
| ZnFe2O4 |
220 |
23/9 |
64.9 (50 ppm) |
79
|
| SnO2 |
370 |
9.7/5.8 |
50.2 (200 ppm) |
80
|
| WO3 |
320 |
5/5 |
32 (100 ppm) |
81
|
| TiO2 |
400 |
0.75/0.5 |
21.6 (200 ppm) |
82
|
| Co3O4 |
240 |
2/5 |
4.88 (500 ppm) |
83
|
| PrFeO3 |
180 |
5/5 |
32.5 (50 ppm) |
84
|
| In2O3 |
300 |
0.7/14 |
29.8 (50 ppm) |
85
|
| La0.7Sr0.3FeO3 |
275 |
20/270 |
0.7 (500 ppm) |
86
|
Presently, the high selectivity of NaEu(WO4)2 towards CH3COCH3 sensing can be understood as the lowering of bond dissociation energy (BDE) in comparison with other VOCs. According to the previous reports,87–90 the BDE of acetone is 393 kJ mol−1, whereas that of other gases is as follows: ethanol, 458 kJ mol−1; ammonia, 435 kJ mol−1; methanol, 439 kJ mol−1; formaldehyde, 364 kJ mol−1; and IPA, 440 kJ mol−1. During the adsorption of the gas molecules, acetone molecules will break more easily, allowing them to participate in the reaction process. However, the gas molecules having higher BDE would be less likely to react with the sensor material at low operating temperature (RT). It is interesting to note that even though the BDE of formaldehyde is in the range comparable to that of acetone, the sensor showed lower response to formaldehyde. This can be attributed to the electron donating effect during the reduction process of the target gases. As given in eqn (11)–(15), it is evident that during the reduction process of acetone, a greater number of electrons are released that contribute to the highest sensitivity and makes the sensor selective to acetone than any other target gas. Additionally, acetone molecules are known to have a large dipole moment (μ = 2.91 D).91,92 Therefore, there will be a strong interaction between acetone and the sensing layer of NEWO0.75, resulting in high response.
5. Computational analysis of ordered and disordered NaEu(WO4)2
To perceive a deep insight on the effect of the  antisite defect on optical and gas sensing properties, we carried out a series of ab initio calculations of electronic structure using density functional theory. Prior to examining the
antisite defect on optical and gas sensing properties, we carried out a series of ab initio calculations of electronic structure using density functional theory. Prior to examining the  antisite effects, we calculated the electronic structure along high symmetry points within the Brillouin zone, total density of states (TDOS), and angular momentum projected partial density of states (PDOS) of perfectly ordered NEWO (NEWOorder). In this calculation, we optimized the unit cell structure and obtained lattice parameters a = b ∼ 5.215 Å and c ∼ 11.511 Å, Eu–O bond lengths ∼2.461 and 2.460 Å, W–O ∼1.783 Å and ΔEu–O = 0.04, which match closely with the experimentally measured parameters, indicating the good choice of the exchange potential for our calculations. In these calculations, the top of the valence band maxima (VBM) was set at zero. The electronic band structure (shown in Fig. 7(a)) of NEWOorder exhibits Eg ∼ 3.29 eV with both valence band maxima (VBM) and conduction band minima (CBM) at the Γ-point. TDOS and PDOS calculations (shown in Fig. 7(b)–(f)) demonstrate the predominant contribution of O 2px, 2py and Eu 4f orbitals in following order 4fz3 > 4fxz2 > 4fzx3 > 4fy3x2 > 4fyz2, 4fx3, 4fxyz to the upper part of the valence band, while the lower part mostly consists of W 4dxz, 4dz2, 4dxy and O 2pz. The lower part of the conduction band mostly comprises the hybridization of W 4dz2, 4dx2 O 2pz orbitals, whereas the upper part mostly consists of Eu 4f–O 2px, 2py orbitals. As the electronegativity of Eu (1.2) is lower than that of W (2.36) on the empirical Pauling electronegativity scale, hence, Eu 4f orbitals should lie above the W 4d orbitals, which corroborates well with our calculations. Among different orbitals, the VBM and CBM of NEWO mostly originate from the hybridization of Eu 4fxyz–O 2px, 2py and W 4dz2–O 2pz, respectively. Therefore, it may be stated that the VB to CB electronic transition is associated with the charge transfer from the EuO8 dodecahedron to the WO4 tetrahedron. The band structure, TDOS and PDOS calculations of the disordered samples (shown in Fig. S8–S10, ESI†) give Eg ∼ 1.77, 1.47 and 0.77 eV for NEWO0.25, NEWO0.50 and NEWO0.75, respectively; thus, the decreasing trend of Eg agrees well with the experimental results. Though the VBM remains unchanged at Γ-point for all the samples, however, the CBM is shifted to the M-point for NEWO0.50 and NEWO0.75, indicating a change in the Eg from direct to indirect. PDOS and TDOS illustrate that all the orbitals get delocalized in the disordered samples with respect to NEWOorder, indicating enhanced bonding hybridization among the Eu 4f, O 2p and W 4d orbitals. Therefore, the observed phenomenon of enhanced second-order parameter (B20) is certainly ascribed to the mixing of the orbitals 4fz3, 4fxz2, 4fy3x2, 4fyz2, 4fx3, 4fxyz while, most importantly, the 4fzx2 orbital does not contribute to this variation. However, significant modifications have been noted for the conduction band; more specifically, the delocalization and red shifting observed for W 4dz2, 4dx2 and O 2pz orbitals are attributed to the increase in the W–O bond length; thus, the reduction of Eg is assigned to the red shifting of the W 4dz2, 4dx2 and O 2pz states. The high density of states of Eu 4f in the conduction band, found in the deformed samples and assigned to the higher
antisite effects, we calculated the electronic structure along high symmetry points within the Brillouin zone, total density of states (TDOS), and angular momentum projected partial density of states (PDOS) of perfectly ordered NEWO (NEWOorder). In this calculation, we optimized the unit cell structure and obtained lattice parameters a = b ∼ 5.215 Å and c ∼ 11.511 Å, Eu–O bond lengths ∼2.461 and 2.460 Å, W–O ∼1.783 Å and ΔEu–O = 0.04, which match closely with the experimentally measured parameters, indicating the good choice of the exchange potential for our calculations. In these calculations, the top of the valence band maxima (VBM) was set at zero. The electronic band structure (shown in Fig. 7(a)) of NEWOorder exhibits Eg ∼ 3.29 eV with both valence band maxima (VBM) and conduction band minima (CBM) at the Γ-point. TDOS and PDOS calculations (shown in Fig. 7(b)–(f)) demonstrate the predominant contribution of O 2px, 2py and Eu 4f orbitals in following order 4fz3 > 4fxz2 > 4fzx3 > 4fy3x2 > 4fyz2, 4fx3, 4fxyz to the upper part of the valence band, while the lower part mostly consists of W 4dxz, 4dz2, 4dxy and O 2pz. The lower part of the conduction band mostly comprises the hybridization of W 4dz2, 4dx2 O 2pz orbitals, whereas the upper part mostly consists of Eu 4f–O 2px, 2py orbitals. As the electronegativity of Eu (1.2) is lower than that of W (2.36) on the empirical Pauling electronegativity scale, hence, Eu 4f orbitals should lie above the W 4d orbitals, which corroborates well with our calculations. Among different orbitals, the VBM and CBM of NEWO mostly originate from the hybridization of Eu 4fxyz–O 2px, 2py and W 4dz2–O 2pz, respectively. Therefore, it may be stated that the VB to CB electronic transition is associated with the charge transfer from the EuO8 dodecahedron to the WO4 tetrahedron. The band structure, TDOS and PDOS calculations of the disordered samples (shown in Fig. S8–S10, ESI†) give Eg ∼ 1.77, 1.47 and 0.77 eV for NEWO0.25, NEWO0.50 and NEWO0.75, respectively; thus, the decreasing trend of Eg agrees well with the experimental results. Though the VBM remains unchanged at Γ-point for all the samples, however, the CBM is shifted to the M-point for NEWO0.50 and NEWO0.75, indicating a change in the Eg from direct to indirect. PDOS and TDOS illustrate that all the orbitals get delocalized in the disordered samples with respect to NEWOorder, indicating enhanced bonding hybridization among the Eu 4f, O 2p and W 4d orbitals. Therefore, the observed phenomenon of enhanced second-order parameter (B20) is certainly ascribed to the mixing of the orbitals 4fz3, 4fxz2, 4fy3x2, 4fyz2, 4fx3, 4fxyz while, most importantly, the 4fzx2 orbital does not contribute to this variation. However, significant modifications have been noted for the conduction band; more specifically, the delocalization and red shifting observed for W 4dz2, 4dx2 and O 2pz orbitals are attributed to the increase in the W–O bond length; thus, the reduction of Eg is assigned to the red shifting of the W 4dz2, 4dx2 and O 2pz states. The high density of states of Eu 4f in the conduction band, found in the deformed samples and assigned to the higher  antisite defect, agree well with the enhanced emission corresponding to the f–f transitions of Eu3+ ions.
antisite defect, agree well with the enhanced emission corresponding to the f–f transitions of Eu3+ ions.
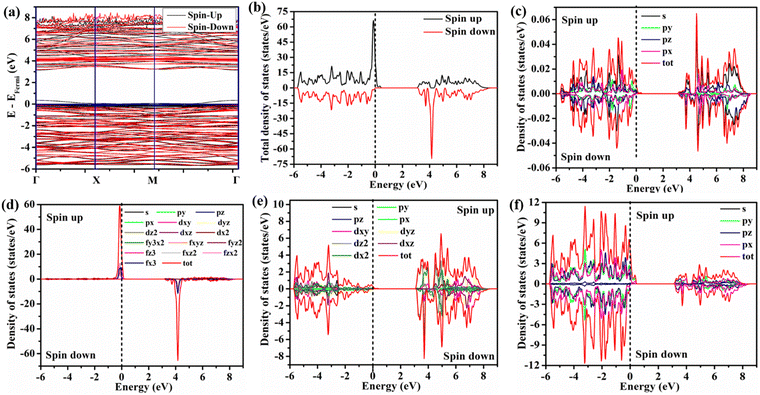 |
| | Fig. 7 DFT of NEWOorder: (a) band structure, (b) TDOS, (c) DOS of Na, (d) DOS of Eu, (e) DOS of W, and (f) DOS of O. | |
Furthermore, in order to understand the effect of  antisite effects on the sensing performances, we examined CH3COCH3 adsorption on NEWOorder and NEWO0.75, while the calculation was performed using three different orientations of the CH3COCH3 molecule (shown in Fig. 8(a)–(c)) on the (0 0 4) plane as the model plane for adsorption. As depicted in Table 4, CH3COCH3 adsorption has been found to be stable in vertical configuration and most importantly, we notice the most negative CH3COCH3 adsorption energy for NEWO0.75 in comparison with NEWOorder, indicating the greater stability of CH3COCH3 on the (0 0 4) plane of NEWO0.75. We observed an optimized distance between the adsorbed CH3COCH3 and sample surface of ∼2.9–3.2 Å, which is larger than the sum of the corresponding atomic covalent radii; hence, it may be stated that no direct chemical is formed between CH3COCH3 and the sample. It is noted (shown in Fig. 8(d)–(f)) that CH3COCH3 adsorption gives rise to additional O 2px, 2py and 2pz orbitals as impurity levels in between the valence and conduction bands. In addition, we also observed that the PDOS calculations of Eu 4f and W 4d orbitals get red-shifted, indicating a significant change in the conductivity of NEWO in the presence of CH3COCH3 (shown in Fig. S11 and S12, ESI†). However, this modification is found to be more significant in NEWO0.75, suggesting the higher sensing performance of NEWO0.75 with respect to NEWOorder. Therefore, it may be concluded that the
antisite effects on the sensing performances, we examined CH3COCH3 adsorption on NEWOorder and NEWO0.75, while the calculation was performed using three different orientations of the CH3COCH3 molecule (shown in Fig. 8(a)–(c)) on the (0 0 4) plane as the model plane for adsorption. As depicted in Table 4, CH3COCH3 adsorption has been found to be stable in vertical configuration and most importantly, we notice the most negative CH3COCH3 adsorption energy for NEWO0.75 in comparison with NEWOorder, indicating the greater stability of CH3COCH3 on the (0 0 4) plane of NEWO0.75. We observed an optimized distance between the adsorbed CH3COCH3 and sample surface of ∼2.9–3.2 Å, which is larger than the sum of the corresponding atomic covalent radii; hence, it may be stated that no direct chemical is formed between CH3COCH3 and the sample. It is noted (shown in Fig. 8(d)–(f)) that CH3COCH3 adsorption gives rise to additional O 2px, 2py and 2pz orbitals as impurity levels in between the valence and conduction bands. In addition, we also observed that the PDOS calculations of Eu 4f and W 4d orbitals get red-shifted, indicating a significant change in the conductivity of NEWO in the presence of CH3COCH3 (shown in Fig. S11 and S12, ESI†). However, this modification is found to be more significant in NEWO0.75, suggesting the higher sensing performance of NEWO0.75 with respect to NEWOorder. Therefore, it may be concluded that the  antisite defect related
antisite defect related  plays the most important role in CH3COCH3 sensing by NaEu(WO4)2 at room temperature, while conductivity changes mostly involve Eu 4f orbitals.
plays the most important role in CH3COCH3 sensing by NaEu(WO4)2 at room temperature, while conductivity changes mostly involve Eu 4f orbitals.
 |
| | Fig. 8 Three possible layouts of the acetone molecule and the (0 0 4) plane surface of the NEWO0.75 material: (a) vertical position, (b) parallel to x-axis and (c) parallel to y-axis position. PDOS plot of the (0 0 4) slab surface + acetone molecule: (d) O 2px orbitals, (e) O 2py orbitals, and (f) O 2pz orbitals. | |
Table 4 Adsorption energy of acetone gas on the (0 0 4) plane surface of targeted nanomaterials
| Target materials |
Adsorption energy |
| Vertical position |
Parallel X position |
Parallel Y position |
| NEWOorder |
−1.20 |
+0.45 |
+0.42 |
| NEWO0.75 |
−1.30 |
+0.19 |
+0.18 |
6. Conclusion
In summary, the present study reports the synthesis and characterization of the  antisite defect containing NaEu(WO4)2 synthesized at various concentrations of Na3cit. Herein, we have identified that the
antisite defect containing NaEu(WO4)2 synthesized at various concentrations of Na3cit. Herein, we have identified that the  antisite defects that can be controlled by Na3cit concentration during hydrothermal reaction have a significant effect on the structural deformation of the EuO8 dodecahedron, covalence of the tungstate host and polarization around the Eu3+ ions. Due to the variation of the polarized field around Eu3+ ions, a significant mixing of the Eu 4f orbitals, followed by the relaxation of the f–f transition rule, was observed and because of this relaxation, NaEu(WO4)2 exhibits strong luminescence at 615 nm with colour coordinate (0.667, 0.333) and color purity ∼97.1% under near-UV excitation (392 nm), indicating its potency as a red phosphor material in LEDs. We identified the presence of
antisite defects that can be controlled by Na3cit concentration during hydrothermal reaction have a significant effect on the structural deformation of the EuO8 dodecahedron, covalence of the tungstate host and polarization around the Eu3+ ions. Due to the variation of the polarized field around Eu3+ ions, a significant mixing of the Eu 4f orbitals, followed by the relaxation of the f–f transition rule, was observed and because of this relaxation, NaEu(WO4)2 exhibits strong luminescence at 615 nm with colour coordinate (0.667, 0.333) and color purity ∼97.1% under near-UV excitation (392 nm), indicating its potency as a red phosphor material in LEDs. We identified the presence of  s and associated
s and associated  antisite defects, and they act as the active sites for O2 adsorption, which in consequence facilitates the CH3COCH3 sensing property. Most importantly, we have found the sensing property at room temperature, which is very rare but has tremendous technological importance due to the low power consumption. Our ab initio calculations show a deep understanding of the electronic structure of NaEu(WO4)2, wherein the VBM and CBM originate from Eu–O and W–O hybridizations, respectively, indicating a charge transfer optical band. Finally, it may be concluded that the prepared materials could be multifunctional materials that can work as both red phosphor and room-temperature acetone gas sensor through the tuning of the antisite defect with the control of the Na3cit concentration.
antisite defects, and they act as the active sites for O2 adsorption, which in consequence facilitates the CH3COCH3 sensing property. Most importantly, we have found the sensing property at room temperature, which is very rare but has tremendous technological importance due to the low power consumption. Our ab initio calculations show a deep understanding of the electronic structure of NaEu(WO4)2, wherein the VBM and CBM originate from Eu–O and W–O hybridizations, respectively, indicating a charge transfer optical band. Finally, it may be concluded that the prepared materials could be multifunctional materials that can work as both red phosphor and room-temperature acetone gas sensor through the tuning of the antisite defect with the control of the Na3cit concentration.
Data availability
The data supporting the findings of this work are available within the article and its ESI.† Raw data that support the findings of this work are available from the corresponding authors (C. K. G.) upon reasonable request.
Conflicts of interest
There is no conflicts to declare.
Acknowledgements
One of the authors (K. R. S.) thanks UGC, Govt. of India (NTA Ref. no.: 191620049221) for financial support during execution of this work. The authors would also like to extend their appreciation to the Researchers Supporting Project number (RSPD2024R956), King Saud University, Riyadh, Saudi Arabia.
References
- M. Yin, Y. Liu, L. Mei, M. S. Molokeev, Z. Huang and M. Fang, RSC Adv., 2015, 5, 73077–73082 RSC.
- M. Laguna, N. O. Nuñez, A. I. Becerro and M. Ocaña, CrystEngComm, 2017, 19, 1590–1600 RSC.
- K. G. Sharma and N. R. Singh, New J. Chem., 2013, 37, 2784–2791 RSC.
- H. Zhu, C. Liang and W. Huang, Phys. B, 2020, 582, 411999 CrossRef CAS.
- X. Yu, Y. Jiang, X. Li, Z. Song, X. Zhang, H. Liu, B. Zhao, T. Ye, L. Duan and J. Fan, CrystEngComm, 2022, 24, 805–817 RSC.
- Y. Ding, J. Liu, M. Zeng, X. Wang, J. Shi, W. Wang, Y. Miao and X. Yu, Dalton Trans., 2018, 47, 8697–8705 RSC.
- V. Mahalingam and J. Thirumalai, RSC Adv., 2016, 6, 80390–80397 RSC.
- R. Singh, A. King and B. B. Nayak, Optik, 2021, 247, 167870 CrossRef CAS.
- Y. Yang, C. Chen and Q. Wang, J. Alloys Compd., 2024, 175042 CrossRef CAS.
- V. Chauhan, P. Dixit, P. K. Pandey, S. Chaturvedi and P. C. Pandey, Methods Appl. Fluoresc., 2023, 12, 015002 CrossRef PubMed.
- L. Kong, H. Sun, Y. Nie, Y. Yan, R. Wang, Q. Ding, S. Zhang, H. Yu and G. Luan, Molecules, 2023, 28, 2681 CrossRef CAS PubMed.
- J. Huang, J. Xu, H. Luo, X. Yu and Y. Li, Inorg. Chem., 2011, 50, 11487–11492 CrossRef CAS PubMed.
- S. Neeraj, N. Kijima and A. K. Cheetham, Chem. Phys. Lett., 2004, 387, 2–6 CrossRef CAS.
- N. Haldar, T. Mondal, A. Dutta, D. Sarkar, U. K. Ghorai and C. K. Ghosh, Appl. Phys. A, 2023, 129, 708 CrossRef CAS.
- N. Haldar, T. Mondal, T. Das, D. Sarkar, M. Pal and C. K. Ghosh, CrystEngComm, 2023, 25, 3514–3527 RSC.
- A. K. Munirathnappa, A. K, A. K. Sinha and N. G. Sundaram, Cryst. Growth Des., 2018, 18, 253–263 CrossRef CAS.
- A. K. Munirathnappa, V. C. Petwal, J. Dwivedi and N. G. Sundaram, New J. Chem., 2018, 42, 2726–2732 RSC.
- N. Haldar, T. Mondal, T. Das, D. Sarkar, M. Pal, A. H. Seikh and C. K. Ghosh, Mater. Adv., 2024, 5, 4480–4490 RSC.
- S. Brahma, Y. W. Yeh, J. L. Huang and C. P. Liu, Appl. Surf. Sci., 2021, 564, 150351 CrossRef CAS.
- C. Li, H. Zhou, S. Yang, L. Wei, Z. Han, Y. Zhang and H. Pan, ACS Appl. Nano Mater., 2019, 2, 6144–6151 CrossRef CAS.
- F. A. Rabuffetti, S. P. Culver, L. Suescun and R. L. Brutchey, Inorg. Chem., 2014, 53, 1056–1061 CrossRef CAS PubMed.
- L. Li, Y. Su and G. Li, Appl. Phys. Lett., 2007, 90, 054105 CrossRef.
- G. M. Kuz'Micheva, I. A. Kaurova, V. B. Rybakov, P. A. Eistrikh-Geller, E. V. Zharikov, D. A. Lis and K. A. Subbotin, CrystEngComm, 2016, 18, 2921–2928 RSC.
- Z. Xu, C. Li, G. Li, R. Chai, C. Peng, D. Yang and J. Lin, J. Phys. Chem. C, 2010, 114, 2573–2582 CrossRef CAS.
- Y. Chen, S. W. Park, B. K. Moon, B. C. Choi, J. H. Jeong and C. Guo, CrystEngComm, 2013, 15, 8255–8261 RSC.
- S. Suwanboon, W. Somraksa, P. Amornpitoksuk and C. Randorn, J. Alloys Compd., 2020, 832, 154963 CrossRef CAS.
- J. Guo, D. Zhou, Y. Li, T. Shao, Z. M. Qi, B. B. Jin and H. Wang, Dalton Trans., 2014, 43, 11888–11896 RSC.
- R. F. Gonçalves, L. S. Cavalcante, I. C. Nogueira, E. Longo, M. J. Godinho, J. C. Sczancoski, V. R. Mastelaro, I. M. Pinatti, I. L. V. Rosa and A. P. A. Marques, CrystEngComm, 2015, 17, 1654–1666 RSC.
- V. V. Popov, Y. V. Zubavichus, A. P. Menushenkov, A. A. Yastrebtsev, B. R. Gaynanov, S. G. Rudakov, A. A. Ivanov, F. E. Dubyago, R. D. Svetogorov, E. V. Khramov and N. A. Tsarenko, Crystals, 2022, 12, 892 CrossRef CAS.
- Z. Mo, Z. Deng, R. Guo, Q. Fu, C. Feng, P. Liu and Y. Sun, Mater. Sci. Eng., B, 2012, 177, 121–126 CrossRef CAS.
- H. Yang, D. Zhang, L. Shi and J. Fang, Acta Mater., 2008, 56, 955–967 CrossRef CAS.
- R. Gutkowski, C. Khare, F. Conzuelo, Y. U. Kayran, A. Ludwig and W. Schuhmann, Energy Environ. Sci., 2017, 10, 1213–1221 RSC.
- J. V. B. Moura, C. Luz-Lima, G. S. Pinheiro and P. T. C. Freire, Spectrochim. Acta, Part A, 2019, 208, 229–235 CrossRef CAS PubMed.
- L. S. Cavalcante, J. C. Sczancoski, J. W. M. Espinosa, J. A. Varela, P. S. Pizani and E. Longo, J. Alloys Compd., 2009, 474, 195–200 CrossRef CAS.
- N. Dirany, E. McRae and M. Arab, CrystEngComm, 2017, 19, 5008–5021 RSC.
- S. S. Bhat, D. Swain, C. Narayana, M. Feygenson, J. C. Neuefeind and N. G. Sundaram, Cryst. Growth Des., 2014, 14, 835–843 CrossRef CAS.
- A. K. Munirathnappa, D. Dwibedi, J. Hester, P. Barpanda, D. Swain, C. Narayana and N. G. Sundaram, J. Phys. Chem. C, 2018, 123, 1041–1049 CrossRef.
- B. P. Singh, J. Singh and R. A. Singh, RSC Adv., 2014, 4, 32605–32621 Search PubMed.
- H. Sun, B. Zhang, Q. Zhao, Y. Liu, R. Wu, S. Zhang, Y. Li and A. Chang, J. Am. Ceram. Soc., 2022, 105, 3715–3724 CrossRef CAS.
-
P. W. Atkins, T. L. Overton, J. P. Rourke, M. T. Weller and F. A. Armstrong, Inorg. Chem, Oxford University Press, New York, 5th edn, 2010, p. 16 Search PubMed.
- S. K. Gupta, M. Sahu, P. S. Ghosh, D. Tyagi, M. K. Saxena and R. M. Kadam, Dalton Trans., 2015, 44, 18957–18969 RSC.
- X. Wang, Z. Zhao, Q. Wu, C. Wang, Q. Wang, L. Yanyan and Y. Wang, J. Mater. Chem. C, 2016, 4, 8795–8801 RSC.
- P. Jena, S. K. Gupta, N. K. Verma, A. K. Singh and R. M. Kadam, New J. Chem., 2017, 41, 8947–8958 RSC.
- B. Xu, X. Cao, G. Wang, Y. Li, Y. Wang and J. Su, Dalton Trans., 2014, 43, 11493–11501 RSC.
- B. P. Singh, P. V. Ramakrishna, S. Singh, V. K. Sonu, S. Singh, P. Singh, A. Bahadur, R. A. Singh and S. B. Rai, RSC Adv., 2015, 5, 55977–55985 RSC.
- X. Dong, Z. Fu, Y. Yu, S. Li and Z. Dai, Mater. Lett., 2012, 74, 140–142 CrossRef CAS.
- G. Rui, Q. Dong and L. Wei, Trans. Nonferrous Met. Soc. China, 2010, 20, 432–436 CrossRef.
- L. S. Zhao, J. Liu, Z. C. Wu and S. P. Kuang, Spectrochim. Acta, Part A, 2012, 87, 228–231 CrossRef CAS PubMed.
- Z. Wang, J. Zhong, H. Jiang, J. Wang and H. Liang, Cryst. Growth Des., 2014, 14, 3767–3773 CrossRef CAS.
- A. K. Parchur and R. S. Ningthoujam, RSC Adv., 2012, 2, 10859–10868 RSC.
- G. Nishimura and T. Kushida, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 9075 CrossRef CAS PubMed.
- S. K. Gupta, P. S. Ghosh, M. Sahu, K. Bhattacharyya, R. Tewari and V. Natarajan, RSC Adv., 2015, 5, 58832–58842 RSC.
- V. Mahalingam and J. Thirumalai, J. Mater. Sci.: Mater. Electron., 2016, 27, 8884–8890 CrossRef CAS.
- L. Jinglei, Y. U. Yan, L. Lingjie, S. Cheng, L. I. Guanyu and L. I. Nianbing, J. Rare Earths, 2012, 30, 330–334 CrossRef.
- M. Rai, G. Kaur, S. K. Singh and S. B. Rai, Dalton Trans., 2015, 44, 6184–6192 RSC.
- B. Yang, Z. Yang, Y. Liu, F. Lu, P. Li, Y. Yang and X. Li, Ceram. Int., 2012, 38, 4895–4900 CrossRef CAS.
- H. Jing, C. Guo, N. Zhang, Z. Ren and J. Bai, ECS J. Solid State Sci. Technol., 2012, 2, R1 Search PubMed.
- J. Zhao, C. Guo, T. Li, X. Su, N. Zhang and J. Chen, Dyes Pigm., 2016, 132, 159–166 CrossRef CAS.
- N. Zhang, C. Guo, J. Zheng, X. Su and J. Zhao, J. Mater. Chem. C, 2014, 2, 3988–3994 RSC.
- H. Li, R. Zhao, Y. Jia, W. Sun, J. Fu, L. Jiang, S. Zhang, R. Pang and C. Li, ACS Appl. Mater. Interfaces, 2014, 6, 3163–3169 CrossRef CAS PubMed.
- Y. Li, N. Li, P. Zhang, Z. Wei, Z. Wang, L. Zhao and W. Chen, Spectrochim. Acta, Part A, 2021, 248, 119247 CrossRef CAS PubMed.
- M. You, J. Xu, Z. Zhang and Y. Zhou, Ceram. Int., 2014, 40, 16189–16194 CrossRef CAS.
- X. Zhang, L. Zhou and M. Gong, Opt. Mater., 2013, 35, 993–997 CrossRef.
- R. S. Yadav, R. K. Dutta, M. Kumar and A. C. Pandey, J. Lumin., 2009, 129, 1078–1082 CrossRef CAS.
- X. Zhang, Y. Chen, S. Zeng, L. Zhou, J. Shi and M. Gong, Ceram. Int., 2014, 40, 14537–14541 CrossRef CAS.
- H. Liu, Y. Hao, H. Wang, J. Zhao, P. Huang and B. Xu, J. Lumin., 2011, 131, 2422–2426 CrossRef CAS.
- H. Ullah, Z. H. Yamani, A. Qurashi, J. Iqbal and K. Safeen, J. Mater. Sci.: Mater. Electron., 2020, 31, 17474–17481 CrossRef CAS.
- P. P. Ortega, B. Hangai, H. Moreno, L. S. R. Rocha, M. A. Ramírez, M. A. Ponce, E. Longo and A. Z. Simões, J. Alloys Compd., 2021, 888, 161517 CrossRef CAS.
- B. Sanches de Lima, P. R. Martinez-Alanis, F. Guell, W. A. dos Santos Silva, M. I. Bernardi, N. L. Marana, E. Longo, J. R. Sambrano and V. R. Mastelaro, ACS Appl. Electron. Mater., 2021, 3, 1447–1457 CrossRef CAS.
- Q. Zhang, G. Xie, M. Duan, Y. Liu, Y. Cai, M. Xu, K. Zhao, H. Tai, Y. Jiang and Y. Su, ACS Appl. Nano Mater., 2023, 6, 17445–17456 CrossRef CAS.
- R. T. Parayil, B. Bhagat, S. K. Gupta, K. Mukherjee and M. Mohapatra, Phys. Chem. Chem. Phys., 2024, 26, 7424–7434 RSC.
- R. T. Parayil, S. K. Gupta, R. Rohilla, J. Prakash, K. Sudarshan and M. Mohapatra, ACS Appl. Electron. Mater., 2023, 5, 5151–5163 CrossRef CAS.
- T. Das, S. Mojumder, S. Chakraborty, D. Saha and M. Pal, Appl. Surf. Sci., 2022, 602, 154340 CrossRef CAS.
- J. Wu, Z. Zheng, H. Chi, J. Jiang, L. Zhu and Z. Ye, ACS Appl. Mater. Interfaces, 2024, 16, 9126–9136 CrossRef CAS PubMed.
- K. Yuan, C. Y. Wang, L. Y. Zhu, Q. Cao, J. H. Yang, X. X. Li, W. Huang, Y. Y. Wang, H. L. Lu and D. W. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 14095–14104 CrossRef CAS PubMed.
- T. Das, S. Mojumder, D. Saha and M. Pal, Sens. Actuators, B, 2024, 406, 135358 CrossRef CAS.
- Y. Chen, H. Li, D. Huang, X. Wang, Y. Wang, W. Wang, M. Yi, Q. Cheng, Y. Song and G. Han, Mater. Sci. Semicond. Process., 2022, 148, 106807 CrossRef CAS.
- S. Zhang, M. Yang, K. Liang, A. Turak, B. Zhang, D. Meng, C. Wang, F. Qu, W. Cheng and M. Yang, Sens. Actuators, B, 2019, 290, 59–67 CrossRef CAS.
- Z. Wen, H. Ren, D. Li, X. Lu, S. W. Joo and J. Huang, Sens. Actuators, B, 2023, 379, 133287 CrossRef CAS.
- D. Chen, J. Xu, Z. Xie and G. Shen, ACS Appl. Mater. Interfaces, 2011, 3, 2112–2117 CrossRef CAS PubMed.
- J. Lu, C. Xu, L. Cheng, N. Jia, J. Huang and C. Li, Mater. Sci. Semicond. Process., 2019, 101, 214–222 CrossRef CAS.
- W. Ge, S. Jiao, Z. Chang, X. He and Y. Li, ACS Appl. Mater. Interfaces, 2020, 12, 13200–13207 CrossRef CAS PubMed.
- T. Zhou, T. Zhang, J. Deng, R. Zhang, Z. Lou and L. Wang, Sens. Actuators, B, 2017, 242, 369–377 CrossRef CAS.
- L. Ma, S. Y. Ma, X. F. Shen, T. T. Wang, X. H. Jiang, Q. Chen, Z. Qiang, H. M. Yang and H. Chen, Sens. Actuators, B, 2018, 255, 2546–2554 CrossRef CAS.
- X. Sun, H. Ji, X. Li, S. Cai and C. Zheng, Mater. Lett., 2014, 120, 287–291 CrossRef CAS.
- P. A. Murade, V. S. Sangawar, G. N. Chaudhari, V. D. Kapse and A. U. Bajpeyee, Curr. Appl. Phys., 2011, 11, 451–456 CrossRef.
- H. Avireddy, H. Kannan, P. Shankar, G. K. Mani, A. J. Kulandaisamy and J. B. B. Rayappan, Mater. Chem. Phys., 2018, 212, 394–402 CrossRef CAS.
- H. Y. Lee, J. H. Bang, S. M. Majhi, A. Mirzaei, K. Y. Shin, D. J. Yu, W. Oum, S. Kang, M. L. Lee, S. S. Kim and H. W. Kim, Sens. Actuators, B, 2022, 359, 131550 CrossRef CAS.
- S. Zhang, P. Song, J. Zhang, H. Yan, J. Li, Z. Yang and Q. Wang, Sens. Actuators, B, 2017, 242, 983–993 CrossRef CAS.
- G. Feng, Y. Che, S. Wang, S. Wang, J. Hu, J. Xiao, C. Song and L. Jiang, Sens. Actuators, B, 2022, 367, 132087 CrossRef CAS.
- C. N. Wang, M. X. Peng, L. J. Yue, X. Y. Yang and Y. H. Zhang, Sens. Actuators, A, 2022, 342, 113650 CrossRef CAS.
- S. Neogi and R. Ghosh, Sens. Actuators, B, 2024, 415, 135980 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a
*a
 antisite defects deforming EuO8 dodecahedra. The modulation of W–O, Eu–O and angle splitting in the presence of
antisite defects deforming EuO8 dodecahedra. The modulation of W–O, Eu–O and angle splitting in the presence of  antisite defects was identified. From in-depth X-ray photoelectron spectroscopy, we validated the deformation of the EuO8 dodecahedron due to the presence of oxygen vacancies (VOs), which originated from
antisite defects was identified. From in-depth X-ray photoelectron spectroscopy, we validated the deformation of the EuO8 dodecahedron due to the presence of oxygen vacancies (VOs), which originated from  antisite defects. Herein, we show that the band gap of NaEu(WO4)2 is highly sensitive to defects; however, the 5D0–7F2 transition of Eu3+ at 615 nm with color coordinates (0.67, 0.33) is very robust, making NaEu(WO4)2 a suitable red phosphor material for near UV-type light-emitting devices (LEDs). We also identified that VOs present in the EuO8 dodecahedron act as active sites for acetone sensing (∼68% response to 100 ppm) with a response and recovery time of ∼3.3/10 s at room temperature, suggesting the potency of NaEu(WO4)2 as a multifunctional material with applications in LEDs and acetone sensors. In order to validate our experimental observations theoretically, we calculated the band structure and density of states of bare and
antisite defects. Herein, we show that the band gap of NaEu(WO4)2 is highly sensitive to defects; however, the 5D0–7F2 transition of Eu3+ at 615 nm with color coordinates (0.67, 0.33) is very robust, making NaEu(WO4)2 a suitable red phosphor material for near UV-type light-emitting devices (LEDs). We also identified that VOs present in the EuO8 dodecahedron act as active sites for acetone sensing (∼68% response to 100 ppm) with a response and recovery time of ∼3.3/10 s at room temperature, suggesting the potency of NaEu(WO4)2 as a multifunctional material with applications in LEDs and acetone sensors. In order to validate our experimental observations theoretically, we calculated the band structure and density of states of bare and  antisite defects containing NaEu(WO4)2 using ab initio density functional theory and identified the sensing mechanism. We believe that our studies will be helpful in introducing new multifunctional applications of NaEu(WO4)2, while theoretical calculations will provide new electronic insights that may be used to understand the features of other double rare-earth tungstate materials.
antisite defects containing NaEu(WO4)2 using ab initio density functional theory and identified the sensing mechanism. We believe that our studies will be helpful in introducing new multifunctional applications of NaEu(WO4)2, while theoretical calculations will provide new electronic insights that may be used to understand the features of other double rare-earth tungstate materials. antisite defects, which are subsequently counterbalanced by the oxygen vacancy (VO). We have also emphasized the VO-induced modification of the bond length and mixing of Eu 4f orbitals, which synergistically impact the emission properties and chromaticity coordinates. Most importantly, by varying the synthesis condition, we achieved the chromaticity coordinate (0.667, 0.333) from one of our synthesized samples, which perfectly matches with that of the National Television Standard Committee (NTSC)-prescribed chromaticity coordinate for red phosphor.
antisite defects, which are subsequently counterbalanced by the oxygen vacancy (VO). We have also emphasized the VO-induced modification of the bond length and mixing of Eu 4f orbitals, which synergistically impact the emission properties and chromaticity coordinates. Most importantly, by varying the synthesis condition, we achieved the chromaticity coordinate (0.667, 0.333) from one of our synthesized samples, which perfectly matches with that of the National Television Standard Committee (NTSC)-prescribed chromaticity coordinate for red phosphor. antisite defect-induced changes in the optical and acetone sensing properties of NaEu(WO4)2. To the best of our knowledge, no such study exists for NaEu(WO4)2; hence, we believe that these experimental and theoretical findings will be helpful in realizing the multifunctional applications of NaEu(WO4)2.
antisite defect-induced changes in the optical and acetone sensing properties of NaEu(WO4)2. To the best of our knowledge, no such study exists for NaEu(WO4)2; hence, we believe that these experimental and theoretical findings will be helpful in realizing the multifunctional applications of NaEu(WO4)2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, followed by washing with DI water and ethanol twice and drying at 70 °C for 24 h. The obtained powder was calcined at 800 °C under nitrogen (N2) atmosphere for 5 h and the final product was obtained. In order to examine the effect of Na3cit on the final product, we varied the Na3cit (ca. 0.75, 0.50 and 0.25 mmol) amount during the hydrothermal reaction, keeping all other parameters same. Accordingly, they were named as NEWO0.75, NEWO0.50 and NEWO0.25, respectively.
000 rpm, followed by washing with DI water and ethanol twice and drying at 70 °C for 24 h. The obtained powder was calcined at 800 °C under nitrogen (N2) atmosphere for 5 h and the final product was obtained. In order to examine the effect of Na3cit on the final product, we varied the Na3cit (ca. 0.75, 0.50 and 0.25 mmol) amount during the hydrothermal reaction, keeping all other parameters same. Accordingly, they were named as NEWO0.75, NEWO0.50 and NEWO0.25, respectively.
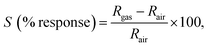 where Rgas and Rair denote the resistance of the sensor in the absence and presence of the target gas, respectively. The response and recovery time of the sensors were determined from the time taken by the sensor to reach the 90% resistance change with respect to the equilibrium state resistance after exposing and removing the target gas.
where Rgas and Rair denote the resistance of the sensor in the absence and presence of the target gas, respectively. The response and recovery time of the sensors were determined from the time taken by the sensor to reach the 90% resistance change with respect to the equilibrium state resistance after exposing and removing the target gas.
 where β and θ denote the full width at half maxima (FWHM) and Bragg's angle respectively, was calculated to be ∼3.40 × 10−3, 3.14 × 10−3, and 2.59 × 10−3 for NEWO0.75, NEWO0.50, and NEWO0.25, respectively. It is noteworthy that Na3cit does not have a significant effect on the particle size during the hydrothermal reaction; rather, it has significant impact on ‘ε’, which may be related to the structural distortion.
where β and θ denote the full width at half maxima (FWHM) and Bragg's angle respectively, was calculated to be ∼3.40 × 10−3, 3.14 × 10−3, and 2.59 × 10−3 for NEWO0.75, NEWO0.50, and NEWO0.25, respectively. It is noteworthy that Na3cit does not have a significant effect on the particle size during the hydrothermal reaction; rather, it has significant impact on ‘ε’, which may be related to the structural distortion.
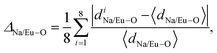 where 〈dNa/Eu–O〉 denotes average Na/Eu–O bond length, (schematically shown in Fig. 1(e) for all the samples), decreases monotonically from NEWO0.75 to NEWO0.25.21 The peculiarity of the hydrothermal method, where varying the Na3cit concentration commonly influences the organization of the [(Na/Eu)O8] dodecahedron, which causes various structural defects in the form of VO, distortion of the bonds, stresses, and strain on the crystallite lattices. Presently ΔNa/Eu–O, ascribed to ‘ε’, is believed to be dependent on VO, which can be explained as follows: we have noticed an enhancement in the (Na/Eu)–O bond length and reverse trend for W–O, which, according to Li et al., can be attributed to the decreasing dipole–dipole interactions due to charge entrapment at the VO site, which gets formed due to the
where 〈dNa/Eu–O〉 denotes average Na/Eu–O bond length, (schematically shown in Fig. 1(e) for all the samples), decreases monotonically from NEWO0.75 to NEWO0.25.21 The peculiarity of the hydrothermal method, where varying the Na3cit concentration commonly influences the organization of the [(Na/Eu)O8] dodecahedron, which causes various structural defects in the form of VO, distortion of the bonds, stresses, and strain on the crystallite lattices. Presently ΔNa/Eu–O, ascribed to ‘ε’, is believed to be dependent on VO, which can be explained as follows: we have noticed an enhancement in the (Na/Eu)–O bond length and reverse trend for W–O, which, according to Li et al., can be attributed to the decreasing dipole–dipole interactions due to charge entrapment at the VO site, which gets formed due to the  antisite defect at the 4a site, as given by
antisite defect at the 4a site, as given by 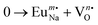 .22,23 Hence, the gradual decrease of ΔNa/Eu–O from NEWO0.75 to NEWO0.25 may be ascribed to the reduced
.22,23 Hence, the gradual decrease of ΔNa/Eu–O from NEWO0.75 to NEWO0.25 may be ascribed to the reduced  antisite defect. In our case, the hydrothermal reaction of Na+, Eu3+, and WO42− ions resulted in the formation of NaEu(WO4)2 precipitates, which can be shown in eqn (2)–(6) below.24–26
antisite defect. In our case, the hydrothermal reaction of Na+, Eu3+, and WO42− ions resulted in the formation of NaEu(WO4)2 precipitates, which can be shown in eqn (2)–(6) below.24–26 . Low Na3cit concentration inhibits the formation of the [Eu3+–cit3−] complex, which as a result reduces the
. Low Na3cit concentration inhibits the formation of the [Eu3+–cit3−] complex, which as a result reduces the  antisite defect. According to Shannon's data, the ionic radius of Na+ (1.18 Å) is larger than that of Eu3+ (0.947 Å); therefore, the equivalent radius of A-site
antisite defect. According to Shannon's data, the ionic radius of Na+ (1.18 Å) is larger than that of Eu3+ (0.947 Å); therefore, the equivalent radius of A-site 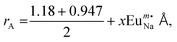 27 where ‘x’ represents the fraction of the antisite defect, suggesting that a decrease of the
27 where ‘x’ represents the fraction of the antisite defect, suggesting that a decrease of the  antisite defect will increase the (Na/Eu)–O bond length, in good agreement with our experimental findings. The FESEM images of the samples shown in Fig. 2(a)–(c) illustrates the agglomerated nature of the particle, which may be attributed to the calcination effect at 800 °C.
antisite defect will increase the (Na/Eu)–O bond length, in good agreement with our experimental findings. The FESEM images of the samples shown in Fig. 2(a)–(c) illustrates the agglomerated nature of the particle, which may be attributed to the calcination effect at 800 °C.
 antisite defect, the FTIR and Raman spectra were taken as these spectroscopic techniques invaluably illustrate the short-range order–disorder phenomenon.28 The FTIR spectra (Fig. S2, ESI†), measured in the range of 400–1000 cm−1, consists of four absorption bands at 412–418, 438–440, 714–725 and 775–852 cm−1 indexed to internal O–W–O symmetric stretching, O–W–O symmetric bending, O–W–O anti-symmetric stretching (1Eu), and O–W–O anti-symmetric stretching (1Au) vibrations, respectively,28,29 while the band at 515–555 cm−1 is attributed to the Eu–O stretching vibration.30,31 Deconvolution reveals two distinct peaks (shown in the inset of Fig. 3(a)–(c)) measured at 524.5 and 548.1 cm−1 for NEWO0.75, 524.3 and 547.2 cm−1 for NEWO0.50, and 521.9 and 542.3 cm−1 for NEWO0.25. Herein, we believe that these two peaks originate from the deformed EuO8 dodecahedra containing the
antisite defect, the FTIR and Raman spectra were taken as these spectroscopic techniques invaluably illustrate the short-range order–disorder phenomenon.28 The FTIR spectra (Fig. S2, ESI†), measured in the range of 400–1000 cm−1, consists of four absorption bands at 412–418, 438–440, 714–725 and 775–852 cm−1 indexed to internal O–W–O symmetric stretching, O–W–O symmetric bending, O–W–O anti-symmetric stretching (1Eu), and O–W–O anti-symmetric stretching (1Au) vibrations, respectively,28,29 while the band at 515–555 cm−1 is attributed to the Eu–O stretching vibration.30,31 Deconvolution reveals two distinct peaks (shown in the inset of Fig. 3(a)–(c)) measured at 524.5 and 548.1 cm−1 for NEWO0.75, 524.3 and 547.2 cm−1 for NEWO0.50, and 521.9 and 542.3 cm−1 for NEWO0.25. Herein, we believe that these two peaks originate from the deformed EuO8 dodecahedra containing the  antisite defect, i.e., [EuO8]deformed and EuO8 dodecahedra without the antisite defect, i.e., [EuO8]ordered.32 [EuO8]deformed was estimated from the FTIR spectra using the relation
antisite defect, i.e., [EuO8]deformed and EuO8 dodecahedra without the antisite defect, i.e., [EuO8]ordered.32 [EuO8]deformed was estimated from the FTIR spectra using the relation  where Adeformed and Aordered denote the area under the curves corresponding to [EuO8]deformed and [EuO8]ordered, showing a decreasing trend (∼0.80, 0.51 and 0.19 for NEWO0.75, NEWO0.50 and NEWO0.25, respectively), which is in good agreement with the Rietveld results of the decreasing
where Adeformed and Aordered denote the area under the curves corresponding to [EuO8]deformed and [EuO8]ordered, showing a decreasing trend (∼0.80, 0.51 and 0.19 for NEWO0.75, NEWO0.50 and NEWO0.25, respectively), which is in good agreement with the Rietveld results of the decreasing  antisite defect.
antisite defect.

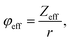 where Zeff and ‘r’ denote nuclear charge and ionic radius in the respective ligand field, respectively, while φeff is the effective ionic potential and denotes the polarizability of the Eu3+ ion.40 In brief, due to the less difference between the electronegativity of O (3.44) and Eu (1.2), the Eu–O bond is covalent, where electrons are shared by Eu and O. In case of the [EuO8]ordered dodecahedron, the Eu3+ ions are surrounded by eight O atoms, whereas this number is less in the case of [EuO8]deformed due to the presence of
where Zeff and ‘r’ denote nuclear charge and ionic radius in the respective ligand field, respectively, while φeff is the effective ionic potential and denotes the polarizability of the Eu3+ ion.40 In brief, due to the less difference between the electronegativity of O (3.44) and Eu (1.2), the Eu–O bond is covalent, where electrons are shared by Eu and O. In case of the [EuO8]ordered dodecahedron, the Eu3+ ions are surrounded by eight O atoms, whereas this number is less in the case of [EuO8]deformed due to the presence of  , suggesting different ‘r’ for these two different dodecahedrons; rather, it will be less in [EuO8]deformed. Therefore, the higher binding energy of Eu 4d orbitals in [EuO8]deformed is ascribed to the higher φeff in the deformed dodecahedron. The [EuO8]deformed:[EuO8]ordered ratio, an indication of the polarizability around Eu3+ ions, was estimated from the area under the 4d5/2 orbital, and this ratio was found to be 1.0, 0.7 and 0.6 for NEWO0.75, NEWO0.50 and NEWO0.25, respectively, indicating a monotonic decrease of [EuO8]deformed. The covalence of the dodecahedron host, which primarily depends on ‘ϕeff’, is believed to be decrease from NEWO0.75 to NEWO0.25 as the decrease in the
, suggesting different ‘r’ for these two different dodecahedrons; rather, it will be less in [EuO8]deformed. Therefore, the higher binding energy of Eu 4d orbitals in [EuO8]deformed is ascribed to the higher φeff in the deformed dodecahedron. The [EuO8]deformed:[EuO8]ordered ratio, an indication of the polarizability around Eu3+ ions, was estimated from the area under the 4d5/2 orbital, and this ratio was found to be 1.0, 0.7 and 0.6 for NEWO0.75, NEWO0.50 and NEWO0.25, respectively, indicating a monotonic decrease of [EuO8]deformed. The covalence of the dodecahedron host, which primarily depends on ‘ϕeff’, is believed to be decrease from NEWO0.75 to NEWO0.25 as the decrease in the  antisite defect makes the dodecahedron more ionic due to the larger electronegativity difference between Na (0.93) and O (3.44). In addition, the increasing binding energy of both 4d5/2 and 4d3/2 orbitals from NEWO0.75 to NEWO0.25 is correlated to the enhancement of the Eu–O bond lengths in good agreement with Rietveld analysis.
antisite defect makes the dodecahedron more ionic due to the larger electronegativity difference between Na (0.93) and O (3.44). In addition, the increasing binding energy of both 4d5/2 and 4d3/2 orbitals from NEWO0.75 to NEWO0.25 is correlated to the enhancement of the Eu–O bond lengths in good agreement with Rietveld analysis.
 in the samples.41 After deconvolution, two different bonding states of oxygen with binding energies of 530.3, 531.0 eV for NEWO0.75, 530.4, 532.0 eV for NEWO0.50 and 530.7, 532.6 eV for NEWO0.25 were observed. Low binding energy corresponds to the lattice oxygen (Olat) species of [EuO8]ordered, while the higher binding energies are attributed to the adsorbed oxygen (O2(ads)) species at the
in the samples.41 After deconvolution, two different bonding states of oxygen with binding energies of 530.3, 531.0 eV for NEWO0.75, 530.4, 532.0 eV for NEWO0.50 and 530.7, 532.6 eV for NEWO0.25 were observed. Low binding energy corresponds to the lattice oxygen (Olat) species of [EuO8]ordered, while the higher binding energies are attributed to the adsorbed oxygen (O2(ads)) species at the  site within [EuO8]deformed. An increase in the binding energy of Olat is ascribed to the reduced deformation of the [EuO8]deformed dodecahedron. However, the O2(ads)/Olat molar ratio was calculated to be ∼0.44, 0.29 and 0.20 for NEWO0.75, NEWO0.50 and NEWO0.25, respectively; hence, the result indicates a monotonic decrease in
site within [EuO8]deformed. An increase in the binding energy of Olat is ascribed to the reduced deformation of the [EuO8]deformed dodecahedron. However, the O2(ads)/Olat molar ratio was calculated to be ∼0.44, 0.29 and 0.20 for NEWO0.75, NEWO0.50 and NEWO0.25, respectively; hence, the result indicates a monotonic decrease in  from NEWO0.75 to NEWO0.25. As XPS is highly sensitive to the surface, therefore, we believe that these
from NEWO0.75 to NEWO0.25. As XPS is highly sensitive to the surface, therefore, we believe that these 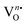 mostly resides on the sample surface. In contrast, the spectra of Na and W have been found in the Gaussian shape and were deconvoluted (as shown in Fig. S4, ESI†). From the above FTIR and XPS experiments, we have confirmed that the antisite defect mainly arose due to the Eu elements, which play a key role in the defects.
mostly resides on the sample surface. In contrast, the spectra of Na and W have been found in the Gaussian shape and were deconvoluted (as shown in Fig. S4, ESI†). From the above FTIR and XPS experiments, we have confirmed that the antisite defect mainly arose due to the Eu elements, which play a key role in the defects. antisite defect on the optical properties, the UV-vis absorption spectra (shown in Fig. S5, ESI†) were recorded and analysed to calculate the band gap (Eg) using Tauc plot.42 Herein, the direct Eg of NEWO (discussed later) was estimated to be ∼2.24, 2.27 and 2.58 eV for NEWO0.75, NEWO0.50, and NEWO0.25, respectively (shown in Fig. 5(a)–(c)). Such a trend of increasing Eg may be assigned to a decrease in the covalence of the host lattice due to [EuO8]deformed and can be explained as follows: mostly, Eg depends on the polarizability of cations and deformation of anions. Greater the cationic polarization and anionic deformation, stronger the covalent links between the anion and cation, thus narrowing the Eg. Our XPS studies reveal that the covalence of the host matrix decreases from NEWO0.75 to NEWO0.25; thus, the enhancement of Eg can be certainly ascribed to the reduced covalence of the host matrix.
antisite defect on the optical properties, the UV-vis absorption spectra (shown in Fig. S5, ESI†) were recorded and analysed to calculate the band gap (Eg) using Tauc plot.42 Herein, the direct Eg of NEWO (discussed later) was estimated to be ∼2.24, 2.27 and 2.58 eV for NEWO0.75, NEWO0.50, and NEWO0.25, respectively (shown in Fig. 5(a)–(c)). Such a trend of increasing Eg may be assigned to a decrease in the covalence of the host lattice due to [EuO8]deformed and can be explained as follows: mostly, Eg depends on the polarizability of cations and deformation of anions. Greater the cationic polarization and anionic deformation, stronger the covalent links between the anion and cation, thus narrowing the Eg. Our XPS studies reveal that the covalence of the host matrix decreases from NEWO0.75 to NEWO0.25; thus, the enhancement of Eg can be certainly ascribed to the reduced covalence of the host matrix.
 antisite defect as well as
antisite defect as well as 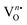 , which, as a result, increase the symmetry of the crystal structure. The emission peak at 565 nm, attributed to the 5D0 → 7F0 transition of Eu3+, has been observed in few other NaEu(WO4)2-like host matrices including the LaAlO3:Eu3+ nanophosphor, LaPO4:Eu3+ nanoparticles, SrB2O4:Eu3+ phosphor, and potassium–aluminoborotellurite.46–48 In principle, he 5D0 → 7F0 transition of Eu3+ is prohibited according to the selection rule of magnetic dipole transition (J = 0 → J′ = 0). However, the presence of this transition indicates the violation of the Judd–Ofelt's selection rule, which may be ascribed to the asymmetry of the [EuO8]deformed dodecahedron.49 Herein, we believe that the asymmetry of the crystal lattice leads to wavefunction mixing between the 7F0 and 7F2 states (discussed later). In this context, the second-order parameter (B20) of the crystal field expansion plays a pivotal role in the mixing of the wavefunctions and is related to the intensity of 5D0 → 7F0 and 5D0 → 7F2 transitions by the following eqn (8).50
, which, as a result, increase the symmetry of the crystal structure. The emission peak at 565 nm, attributed to the 5D0 → 7F0 transition of Eu3+, has been observed in few other NaEu(WO4)2-like host matrices including the LaAlO3:Eu3+ nanophosphor, LaPO4:Eu3+ nanoparticles, SrB2O4:Eu3+ phosphor, and potassium–aluminoborotellurite.46–48 In principle, he 5D0 → 7F0 transition of Eu3+ is prohibited according to the selection rule of magnetic dipole transition (J = 0 → J′ = 0). However, the presence of this transition indicates the violation of the Judd–Ofelt's selection rule, which may be ascribed to the asymmetry of the [EuO8]deformed dodecahedron.49 Herein, we believe that the asymmetry of the crystal lattice leads to wavefunction mixing between the 7F0 and 7F2 states (discussed later). In this context, the second-order parameter (B20) of the crystal field expansion plays a pivotal role in the mixing of the wavefunctions and is related to the intensity of 5D0 → 7F0 and 5D0 → 7F2 transitions by the following eqn (8).50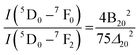
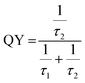 (where, τ1 and τ2 denote non-radiative and radiative recombination times), is ∼50%, which is slightly less than the QY of other scheelite materials.52,53
(where, τ1 and τ2 denote non-radiative and radiative recombination times), is ∼50%, which is slightly less than the QY of other scheelite materials.52,53
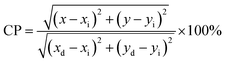
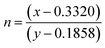 ; (x, y) represent the chromaticity coordinate. These results suggest that NaEu(WO4)2 may be a potential candidate for red emission with excellent color purity and color coordinates close to the NTSC norms.
; (x, y) represent the chromaticity coordinate. These results suggest that NaEu(WO4)2 may be a potential candidate for red emission with excellent color purity and color coordinates close to the NTSC norms.
 which was 5%, 3%, 8%, 4% and 6% for C2H5OH, NH3, HCHO, CH3OH and C3H8O, respectively). Room temperature operational condition is ascribed to the low bond-dissociation energy of CH3COCH3 (393 kJ mol−1), in comparison with C2H5OH (458 kJ mol−1), NH3 (435 kJ mol−1), and CH3OH (439 kJ mol−1), while the absence of sensing activity of NEWO0.50 and NEWO0.25 can be assigned to the low [EuO8]deformed content and can be explained as follows. The electron-depleted layer (LD), formed due to O2−(ads), also plays a pivotal role in transducing the electronic signal in sensing response. Normally, LD is prominent for the VO-enriched sample, leading to superior sensing response.71 Our XPS studies reveal the highest
which was 5%, 3%, 8%, 4% and 6% for C2H5OH, NH3, HCHO, CH3OH and C3H8O, respectively). Room temperature operational condition is ascribed to the low bond-dissociation energy of CH3COCH3 (393 kJ mol−1), in comparison with C2H5OH (458 kJ mol−1), NH3 (435 kJ mol−1), and CH3OH (439 kJ mol−1), while the absence of sensing activity of NEWO0.50 and NEWO0.25 can be assigned to the low [EuO8]deformed content and can be explained as follows. The electron-depleted layer (LD), formed due to O2−(ads), also plays a pivotal role in transducing the electronic signal in sensing response. Normally, LD is prominent for the VO-enriched sample, leading to superior sensing response.71 Our XPS studies reveal the highest  content of NEWO0.75, indicating the most efficient signal transduction in comparison with NEWO0.25 and NEWO0.50. Therefore, the high
content of NEWO0.75, indicating the most efficient signal transduction in comparison with NEWO0.25 and NEWO0.50. Therefore, the high  content of NEWO0.75 is believed to play a significant role in sensing CH3COCH3, one of the highly flammable VOCs that cause difficulties in breathing, nausea, vomiting and symptoms of neurological poisoning and also as a biomarker of diabetes found in exhaled human breath.74,75
content of NEWO0.75 is believed to play a significant role in sensing CH3COCH3, one of the highly flammable VOCs that cause difficulties in breathing, nausea, vomiting and symptoms of neurological poisoning and also as a biomarker of diabetes found in exhaled human breath.74,75
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) log(Cg)
log(Cg) antisite defect on optical and gas sensing properties, we carried out a series of ab initio calculations of electronic structure using density functional theory. Prior to examining the
antisite defect on optical and gas sensing properties, we carried out a series of ab initio calculations of electronic structure using density functional theory. Prior to examining the  antisite effects, we calculated the electronic structure along high symmetry points within the Brillouin zone, total density of states (TDOS), and angular momentum projected partial density of states (PDOS) of perfectly ordered NEWO (NEWOorder). In this calculation, we optimized the unit cell structure and obtained lattice parameters a = b ∼ 5.215 Å and c ∼ 11.511 Å, Eu–O bond lengths ∼2.461 and 2.460 Å, W–O ∼1.783 Å and ΔEu–O = 0.04, which match closely with the experimentally measured parameters, indicating the good choice of the exchange potential for our calculations. In these calculations, the top of the valence band maxima (VBM) was set at zero. The electronic band structure (shown in Fig. 7(a)) of NEWOorder exhibits Eg ∼ 3.29 eV with both valence band maxima (VBM) and conduction band minima (CBM) at the Γ-point. TDOS and PDOS calculations (shown in Fig. 7(b)–(f)) demonstrate the predominant contribution of O 2px, 2py and Eu 4f orbitals in following order 4fz3 > 4fxz2 > 4fzx3 > 4fy3x2 > 4fyz2, 4fx3, 4fxyz to the upper part of the valence band, while the lower part mostly consists of W 4dxz, 4dz2, 4dxy and O 2pz. The lower part of the conduction band mostly comprises the hybridization of W 4dz2, 4dx2 O 2pz orbitals, whereas the upper part mostly consists of Eu 4f–O 2px, 2py orbitals. As the electronegativity of Eu (1.2) is lower than that of W (2.36) on the empirical Pauling electronegativity scale, hence, Eu 4f orbitals should lie above the W 4d orbitals, which corroborates well with our calculations. Among different orbitals, the VBM and CBM of NEWO mostly originate from the hybridization of Eu 4fxyz–O 2px, 2py and W 4dz2–O 2pz, respectively. Therefore, it may be stated that the VB to CB electronic transition is associated with the charge transfer from the EuO8 dodecahedron to the WO4 tetrahedron. The band structure, TDOS and PDOS calculations of the disordered samples (shown in Fig. S8–S10, ESI†) give Eg ∼ 1.77, 1.47 and 0.77 eV for NEWO0.25, NEWO0.50 and NEWO0.75, respectively; thus, the decreasing trend of Eg agrees well with the experimental results. Though the VBM remains unchanged at Γ-point for all the samples, however, the CBM is shifted to the M-point for NEWO0.50 and NEWO0.75, indicating a change in the Eg from direct to indirect. PDOS and TDOS illustrate that all the orbitals get delocalized in the disordered samples with respect to NEWOorder, indicating enhanced bonding hybridization among the Eu 4f, O 2p and W 4d orbitals. Therefore, the observed phenomenon of enhanced second-order parameter (B20) is certainly ascribed to the mixing of the orbitals 4fz3, 4fxz2, 4fy3x2, 4fyz2, 4fx3, 4fxyz while, most importantly, the 4fzx2 orbital does not contribute to this variation. However, significant modifications have been noted for the conduction band; more specifically, the delocalization and red shifting observed for W 4dz2, 4dx2 and O 2pz orbitals are attributed to the increase in the W–O bond length; thus, the reduction of Eg is assigned to the red shifting of the W 4dz2, 4dx2 and O 2pz states. The high density of states of Eu 4f in the conduction band, found in the deformed samples and assigned to the higher
antisite effects, we calculated the electronic structure along high symmetry points within the Brillouin zone, total density of states (TDOS), and angular momentum projected partial density of states (PDOS) of perfectly ordered NEWO (NEWOorder). In this calculation, we optimized the unit cell structure and obtained lattice parameters a = b ∼ 5.215 Å and c ∼ 11.511 Å, Eu–O bond lengths ∼2.461 and 2.460 Å, W–O ∼1.783 Å and ΔEu–O = 0.04, which match closely with the experimentally measured parameters, indicating the good choice of the exchange potential for our calculations. In these calculations, the top of the valence band maxima (VBM) was set at zero. The electronic band structure (shown in Fig. 7(a)) of NEWOorder exhibits Eg ∼ 3.29 eV with both valence band maxima (VBM) and conduction band minima (CBM) at the Γ-point. TDOS and PDOS calculations (shown in Fig. 7(b)–(f)) demonstrate the predominant contribution of O 2px, 2py and Eu 4f orbitals in following order 4fz3 > 4fxz2 > 4fzx3 > 4fy3x2 > 4fyz2, 4fx3, 4fxyz to the upper part of the valence band, while the lower part mostly consists of W 4dxz, 4dz2, 4dxy and O 2pz. The lower part of the conduction band mostly comprises the hybridization of W 4dz2, 4dx2 O 2pz orbitals, whereas the upper part mostly consists of Eu 4f–O 2px, 2py orbitals. As the electronegativity of Eu (1.2) is lower than that of W (2.36) on the empirical Pauling electronegativity scale, hence, Eu 4f orbitals should lie above the W 4d orbitals, which corroborates well with our calculations. Among different orbitals, the VBM and CBM of NEWO mostly originate from the hybridization of Eu 4fxyz–O 2px, 2py and W 4dz2–O 2pz, respectively. Therefore, it may be stated that the VB to CB electronic transition is associated with the charge transfer from the EuO8 dodecahedron to the WO4 tetrahedron. The band structure, TDOS and PDOS calculations of the disordered samples (shown in Fig. S8–S10, ESI†) give Eg ∼ 1.77, 1.47 and 0.77 eV for NEWO0.25, NEWO0.50 and NEWO0.75, respectively; thus, the decreasing trend of Eg agrees well with the experimental results. Though the VBM remains unchanged at Γ-point for all the samples, however, the CBM is shifted to the M-point for NEWO0.50 and NEWO0.75, indicating a change in the Eg from direct to indirect. PDOS and TDOS illustrate that all the orbitals get delocalized in the disordered samples with respect to NEWOorder, indicating enhanced bonding hybridization among the Eu 4f, O 2p and W 4d orbitals. Therefore, the observed phenomenon of enhanced second-order parameter (B20) is certainly ascribed to the mixing of the orbitals 4fz3, 4fxz2, 4fy3x2, 4fyz2, 4fx3, 4fxyz while, most importantly, the 4fzx2 orbital does not contribute to this variation. However, significant modifications have been noted for the conduction band; more specifically, the delocalization and red shifting observed for W 4dz2, 4dx2 and O 2pz orbitals are attributed to the increase in the W–O bond length; thus, the reduction of Eg is assigned to the red shifting of the W 4dz2, 4dx2 and O 2pz states. The high density of states of Eu 4f in the conduction band, found in the deformed samples and assigned to the higher  antisite defect, agree well with the enhanced emission corresponding to the f–f transitions of Eu3+ ions.
antisite defect, agree well with the enhanced emission corresponding to the f–f transitions of Eu3+ ions.

 antisite effects on the sensing performances, we examined CH3COCH3 adsorption on NEWOorder and NEWO0.75, while the calculation was performed using three different orientations of the CH3COCH3 molecule (shown in Fig. 8(a)–(c)) on the (0 0 4) plane as the model plane for adsorption. As depicted in Table 4, CH3COCH3 adsorption has been found to be stable in vertical configuration and most importantly, we notice the most negative CH3COCH3 adsorption energy for NEWO0.75 in comparison with NEWOorder, indicating the greater stability of CH3COCH3 on the (0 0 4) plane of NEWO0.75. We observed an optimized distance between the adsorbed CH3COCH3 and sample surface of ∼2.9–3.2 Å, which is larger than the sum of the corresponding atomic covalent radii; hence, it may be stated that no direct chemical is formed between CH3COCH3 and the sample. It is noted (shown in Fig. 8(d)–(f)) that CH3COCH3 adsorption gives rise to additional O 2px, 2py and 2pz orbitals as impurity levels in between the valence and conduction bands. In addition, we also observed that the PDOS calculations of Eu 4f and W 4d orbitals get red-shifted, indicating a significant change in the conductivity of NEWO in the presence of CH3COCH3 (shown in Fig. S11 and S12, ESI†). However, this modification is found to be more significant in NEWO0.75, suggesting the higher sensing performance of NEWO0.75 with respect to NEWOorder. Therefore, it may be concluded that the
antisite effects on the sensing performances, we examined CH3COCH3 adsorption on NEWOorder and NEWO0.75, while the calculation was performed using three different orientations of the CH3COCH3 molecule (shown in Fig. 8(a)–(c)) on the (0 0 4) plane as the model plane for adsorption. As depicted in Table 4, CH3COCH3 adsorption has been found to be stable in vertical configuration and most importantly, we notice the most negative CH3COCH3 adsorption energy for NEWO0.75 in comparison with NEWOorder, indicating the greater stability of CH3COCH3 on the (0 0 4) plane of NEWO0.75. We observed an optimized distance between the adsorbed CH3COCH3 and sample surface of ∼2.9–3.2 Å, which is larger than the sum of the corresponding atomic covalent radii; hence, it may be stated that no direct chemical is formed between CH3COCH3 and the sample. It is noted (shown in Fig. 8(d)–(f)) that CH3COCH3 adsorption gives rise to additional O 2px, 2py and 2pz orbitals as impurity levels in between the valence and conduction bands. In addition, we also observed that the PDOS calculations of Eu 4f and W 4d orbitals get red-shifted, indicating a significant change in the conductivity of NEWO in the presence of CH3COCH3 (shown in Fig. S11 and S12, ESI†). However, this modification is found to be more significant in NEWO0.75, suggesting the higher sensing performance of NEWO0.75 with respect to NEWOorder. Therefore, it may be concluded that the  antisite defect related
antisite defect related  plays the most important role in CH3COCH3 sensing by NaEu(WO4)2 at room temperature, while conductivity changes mostly involve Eu 4f orbitals.
plays the most important role in CH3COCH3 sensing by NaEu(WO4)2 at room temperature, while conductivity changes mostly involve Eu 4f orbitals. antisite defect containing NaEu(WO4)2 synthesized at various concentrations of Na3cit. Herein, we have identified that the
antisite defect containing NaEu(WO4)2 synthesized at various concentrations of Na3cit. Herein, we have identified that the  antisite defects that can be controlled by Na3cit concentration during hydrothermal reaction have a significant effect on the structural deformation of the EuO8 dodecahedron, covalence of the tungstate host and polarization around the Eu3+ ions. Due to the variation of the polarized field around Eu3+ ions, a significant mixing of the Eu 4f orbitals, followed by the relaxation of the f–f transition rule, was observed and because of this relaxation, NaEu(WO4)2 exhibits strong luminescence at 615 nm with colour coordinate (0.667, 0.333) and color purity ∼97.1% under near-UV excitation (392 nm), indicating its potency as a red phosphor material in LEDs. We identified the presence of
antisite defects that can be controlled by Na3cit concentration during hydrothermal reaction have a significant effect on the structural deformation of the EuO8 dodecahedron, covalence of the tungstate host and polarization around the Eu3+ ions. Due to the variation of the polarized field around Eu3+ ions, a significant mixing of the Eu 4f orbitals, followed by the relaxation of the f–f transition rule, was observed and because of this relaxation, NaEu(WO4)2 exhibits strong luminescence at 615 nm with colour coordinate (0.667, 0.333) and color purity ∼97.1% under near-UV excitation (392 nm), indicating its potency as a red phosphor material in LEDs. We identified the presence of  s and associated
s and associated  antisite defects, and they act as the active sites for O2 adsorption, which in consequence facilitates the CH3COCH3 sensing property. Most importantly, we have found the sensing property at room temperature, which is very rare but has tremendous technological importance due to the low power consumption. Our ab initio calculations show a deep understanding of the electronic structure of NaEu(WO4)2, wherein the VBM and CBM originate from Eu–O and W–O hybridizations, respectively, indicating a charge transfer optical band. Finally, it may be concluded that the prepared materials could be multifunctional materials that can work as both red phosphor and room-temperature acetone gas sensor through the tuning of the antisite defect with the control of the Na3cit concentration.
antisite defects, and they act as the active sites for O2 adsorption, which in consequence facilitates the CH3COCH3 sensing property. Most importantly, we have found the sensing property at room temperature, which is very rare but has tremendous technological importance due to the low power consumption. Our ab initio calculations show a deep understanding of the electronic structure of NaEu(WO4)2, wherein the VBM and CBM originate from Eu–O and W–O hybridizations, respectively, indicating a charge transfer optical band. Finally, it may be concluded that the prepared materials could be multifunctional materials that can work as both red phosphor and room-temperature acetone gas sensor through the tuning of the antisite defect with the control of the Na3cit concentration.





