Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A ring-fluorinated heptamethine cyanine dye: synthesis, photophysical properties, and vapochromic properties in response to ammonia†
Shouhei
Ajioka
a,
Yuto
Hagiyama
a,
Yuki
Uehashi
a,
Tomohiro
Agou
 b,
Yasuhiro
Kubota
a,
Toshiyasu
Inuzuka
c and
Kazumasa
Funabiki
b,
Yasuhiro
Kubota
a,
Toshiyasu
Inuzuka
c and
Kazumasa
Funabiki
 *a
*a
aDepartment of Chemistry and Biomolecular Science, Gifu University, 1-1 Yanagido, Gifu 501-1193, Japan. E-mail: funabiki.kazumasa.m6@f.gifu-u.ac.jp
bDepartment of Material Science, Graduate School of Science, University of Hyogo, 3-2-1 Koto, Kamigori-cho, Ako, Hyogo 678-1297, Japan
cDivision of Instrumental Analysis, Life Science Research Center, Gifu University, 1-1 Yanagido, Gifu 501-1193, Japan
First published on 13th November 2024
Abstract
Heptamethine cyanine dyes (HMCDs) have attracted considerable attention in biological and energy applications owing to their unique near-infrared (NIR) photophysical properties. Therefore, the development of molecules that change both visible and fluorescent colours in a stimulus-responsive manner by exploiting the NIR optical properties of HMCDs has been a subject of increasing interest. Most research results are based on a highly nucleophilic anion addition or reversible intramolecular addition reaction of a weakly nucleophilic neutral nucleophile with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the terminal indol-1-ium moiety. Examples of intermolecular addition of weakly neutral nucleophiles and the use of solid or polymer materials are not available. Here, we report the synthesis of a NIR-absorbing ring-perfluorinated HMCD. The HMCD's unique properties in various solvents and rapid and reversible vapochromic response to various amines, including NH3, based on the noteworthy structural modification induced by fluorine atoms on the aromatic ring are also presented. The ring-fluorinated HMCD adsorbed on neutral filter paper responds quickly to even low-nucleophilicity NH3 vapour. Repeatability tests on filter paper adsorbed with the ring-fluorinated HMCD and NH3 and HCl vapours show excellent reproducibility in 13 blue-green and yellow colour transitions. These results are the first examples of intermolecular addition of weakly neutral nucleophiles into HMCDs and stimulus responsiveness not in solutions.
N bond of the terminal indol-1-ium moiety. Examples of intermolecular addition of weakly neutral nucleophiles and the use of solid or polymer materials are not available. Here, we report the synthesis of a NIR-absorbing ring-perfluorinated HMCD. The HMCD's unique properties in various solvents and rapid and reversible vapochromic response to various amines, including NH3, based on the noteworthy structural modification induced by fluorine atoms on the aromatic ring are also presented. The ring-fluorinated HMCD adsorbed on neutral filter paper responds quickly to even low-nucleophilicity NH3 vapour. Repeatability tests on filter paper adsorbed with the ring-fluorinated HMCD and NH3 and HCl vapours show excellent reproducibility in 13 blue-green and yellow colour transitions. These results are the first examples of intermolecular addition of weakly neutral nucleophiles into HMCDs and stimulus responsiveness not in solutions.
Introduction
Polymethine cyanine dyes having azaheterocycles at both ends of the polymethine backbone offer advantages such as narrow absorption bands, high absorption coefficients, and readily tunable maximum absorption wavelengths (λabs) and maximum fluorescence wavelengths (λem) within the visible and near-infrared (NIR) regions.1 In particular, heptamethine cyanine dyes (HMCDs), which exhibit absorption and fluorescence emissions in the NIR region, are attracting considerable attention as one of the most promising molecules for photo-science and -technology using NIR light, such as imaging,2 therapy,3 and organic solar cells.4,5The development of molecules that change both the colours of the solutions or films and fluorescent colours in a stimulus-responsive manner by exploiting the NIR optical properties of HMCDs has been a subject of increasing interest. Changing both the colours of the solutions and fluorescent colours of the HMCD reported thus far is mostly based on (1) highly nucleophilic anion addition, such as cyanide anion (CN−), to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the terminal indol-1-ium moiety (Fig. 1(a))6 or (2) a reversible intramolecular addition reaction of weakly nucleophilic neutral nucleophiles, such as nitrogen, sulfur, and oxygen atoms, with the C
N bond of the terminal indol-1-ium moiety (Fig. 1(a))6 or (2) a reversible intramolecular addition reaction of weakly nucleophilic neutral nucleophiles, such as nitrogen, sulfur, and oxygen atoms, with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the indol-1-ium moiety or the C
N bond of the indol-1-ium moiety or the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond at the meso position, depending on the pH of the solution (Fig. 1(b)).7 However, intermolecular addition of weakly neutral nucleophiles is not reported. In addition, all HMCDs were in solution, and no examples of the use of solid or polymer materials are available.
C double bond at the meso position, depending on the pH of the solution (Fig. 1(b)).7 However, intermolecular addition of weakly neutral nucleophiles is not reported. In addition, all HMCDs were in solution, and no examples of the use of solid or polymer materials are available.
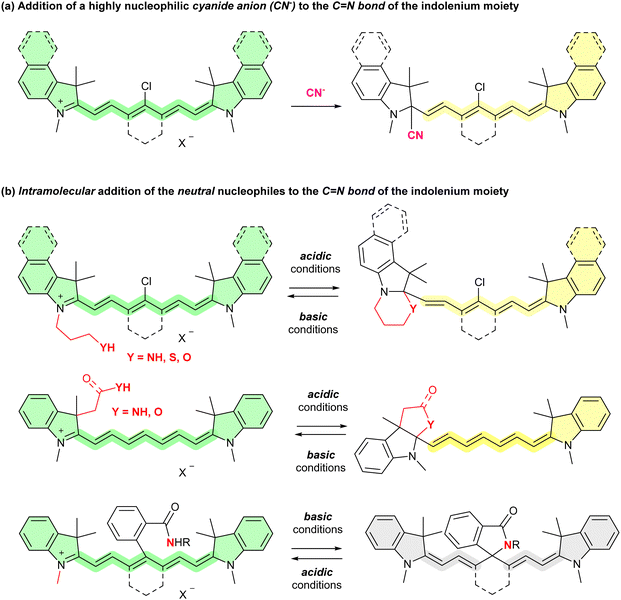 | ||
| Fig. 1 Previous methods for changing the colour or fluorescence of HMCDs in solutions by intermolecular addition of cyanide anion (a) or intramolecular addition of neutral nucleophiles (b). | ||
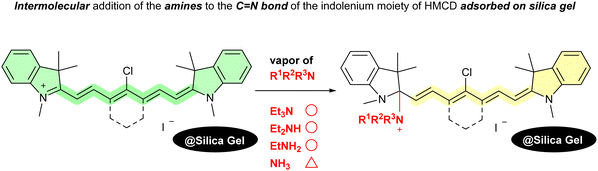
Ammonia (NH3) is currently attracting attention as a fuel that can replace oil, coal, and natural gas for power generation because it does not emit carbon dioxide when burned. Ammonia is expected to be an energy carrier for the transport and storage of hydrogen.8 Although many studies on the vapochromism of organic dyes or metal complexes towards NH3 have been reported,9 to the best of our knowledge, only one study has been conducted on the reversible vapochromic responsiveness of HMCDs adsorbed on silica gels to various amines, as shown in Fig. 2.10 The results revealed that the vapochromism due to the intermolecular addition of weakly nucleophilic neutral amines to the HMCD can be attributed to the adsorption of the dye on weakly acidic silica gel. However, the NH3 vapour of the HMCD adsorbed on the weakly acidic silica gel was insufficient because NH3 is not sufficiently nucleophilic.
The unique functions of organic dyes can be incorporated by introducing heteroatoms such as sulfur,11 phosphorous,12 and silicon13 into the electron-conjugated system or on the aromatic rings of the functional dyes. The functional dyes carrying partially ring-fluorinated aromatics or heteroaromatics have garnered attention as fluorescent dyes,14 organic field-effect transistors,15 organic photovoltaic cells,16 and amine-response dyes17 owing to their excellent properties, including their atomic size that is almost as small as that of a hydrogen atom, their highest electronegativity, their strong carbon–fluorine bond energy, and some interactions with other elements.18 However, only limited examples of stimulus-responsive NIR-absorbing molecules or materials carrying ring-fluorinated aromatics or heteroaromatics are available.19 Although only a few patents on the preparation of ring-fluorinated HMCDs exist, the structure and optical and other properties of such HMCDs have not yet been reported.20 Here, we report (1) the synthesis of a NIR-absorbing ring-perfluorinated HMCD and its (2) crystal structure, (3) unique properties in various solvents, and (4) rapid and reversible vapochromic response to various amines including NH3, based on the noteworthy structural modification induced by fluorine atoms on the aromatic ring. The results reveal the first examples of intermolecular addition of weakly neutral nucleophiles to HMCD and stimulus responsiveness not in solutions.
Results and discussion
Synthesis
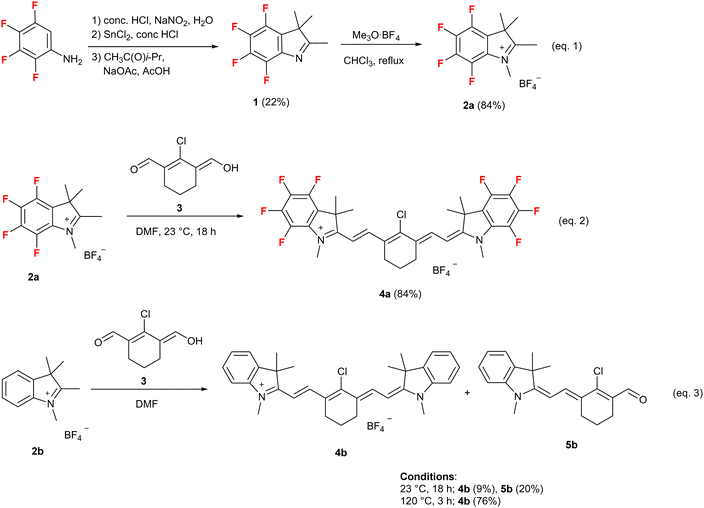
As shown in Scheme 1 (eqn (1), successive treatment of a commercially available 2,3,4,5-tetrafluoroaniline with sodium nitrite in water at −10 °C and tin chloride(II) hydrate at room temperature in the presence of concentrated hydrochloric acid yields 2,3,4,5-tetrafluorophenyl hydrazine hydrochloride. The obtained crude hydrazine hydrochloride reacts with 3-methyl-2-butanone in acetic acid at 120 °C in the presence of sodium acetate and results in the formation of 4,5,6,7-tetrafluoro-2,3,3-trimethyl-3H-indole (1) in 22% yield from 2,3,4,5-tetrafluoroaniline.The reaction between 1 and an excess amount of the most reactive haloalkane, iodomethane, does not proceed because the nucleophilicity of 1 is significantly reduced owing to the strong electron-withdrawing property of the fluorine atom. Treatment of 1 with 2 equiv. of the Meerwein reagent (trimethyloxonium tetrafluoroborate) in chloroform for 24 h under reflux results in the formation of 4,5,6,7-tetrafluoro-1,2,3,3-tetramethyl-3H-indol-1-ium tetrafluoroborate (2a) in 84% yield. The tetrafluoroindolium salt (2a) smoothly reacts with dialdehyde (3) in dimethylformamide (DMF) even at 23 °C, which is a very low temperature compared with a typical reaction temperature (120 °C), for 18 h and results in the formation of 2-((E)-2-((E)-2-chloro-3-(2-((E)-4,5,6,7-tetrafluoro-1,3,3-trimethylindolin-2-ylidene)ethylidene)cyclohex-1-en-1-yl)vinyl)-4,5,6,7-tetrafluoro-1,3,3-trimethyl-3H-indol-1-ium tetrafluoroborate (4a) in 84% yield (Scheme 1, eqn (2)).21 The reaction of a fluorine-free indolium salt (2b) with 3 in DMF under the same reaction conditions (at 23 °C for 18 h) results in the formation of 2-((E)-2-((E)-2-chloro-3-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)cyclohex-1-en-1-yl)vinyl)-1,3,3-trimethyl-3H-indol-1-ium tetrafluoroborate (4b) with only 9% yield, together with a 20% yield of (E)-2-chloro-3-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)cyclohex-1-ene-1-carbaldehyde (5b) (Scheme 1, eqn (3)). The high-temperature (120 °C) conditions allow the reaction of 2b with 3 in DMF to proceed smoothly for 3 h, giving a satisfactory yield (76%) of nonfluorinated HMCD (4b). These results may be attributed to an increase in the acidity of the hydrogen atom on the methyl group at the 2-position of 2a owing to the strong electron-withdrawing property of the four fluorine atoms on the aromatic ring. Consequently, the formation of the enamine from 2a and successive condensation with 3 occur smoothly, even at room temperature to result in the formation of the corresponding ring-fluorinated HMCD (4a) in good yield.
Single-crystal structural analysis
Single crystals of 4a and 4b were prepared using the vapour diffusion method with dichloromethane (DCM) and hexane. Single-crystal X-ray analysis results of the obtained HMCDs (4a: CCDC No. 1987380, monoclinic, P21/c space group; 4b: CCDC No. 1987381, orthorhombic, Pca21 space group) are shown in Fig. 3 and 4.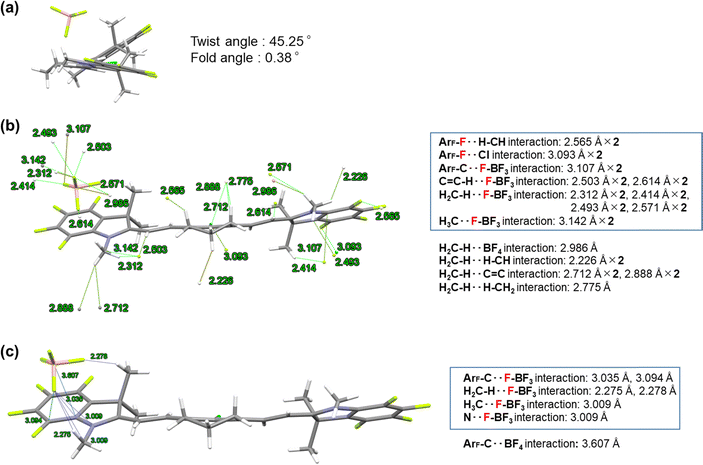 | ||
| Fig. 3 X-ray diffraction structures of HMCD 4a: twist and fold angles (a), intermolecular short contacts (b), and intramolecular short contacts (c). | ||
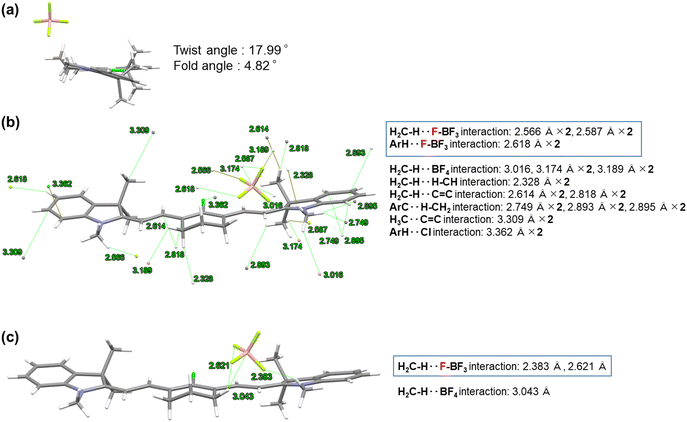 | ||
| Fig. 4 X-ray diffraction structures of the ring-fluorinated HMCD (4b): twist and fold angles (a), intermolecular short contacts (b), and intramolecular short contacts (c). | ||
The twist and fold angles of the two indolium planes of HMCD 4b21 or the two ring-fluorinated indolium planes of HMCD 4a were measured by using the Olex2 software22 and are shown in Fig. 3(a) and 4(a). The twist angle between the two ring-fluorinated indolium planes of HMCD 4a is 45.25°, which is considerably larger than that (17.99°) between the two non-fluorinated indolium planes of HMCD 4b. These results are consistent with those of the ring-fluorinated trimethine cyanine dye.23 The fold angle between the two ring-fluorinated indolium planes of HCMD 4a is 0.38°, which is smaller than that (4.82°) between the non-fluorinated indolium planes of HMCD 4b. These results are not consistent with those of the ring-fluorinated trimethine cyanine dye.23 As shown in Fig. 4(b) and (c), compared with the ring-fluorinated HMCD 4a, in the structure of HMCD 4b, less intramolecular and intermolecular interactions occur between the fluorine atom and other atoms. By contrast, ring-fluorinated HMCD 4a exhibits six types of intramolecular interactions between fluorine atoms and other atoms, such as hydrogen, chlorine, and carbon atoms (Fig. 3(b)), and four types of intermolecular interactions between fluorine atoms and hydrogen, carbon, and nitrogen atoms (Fig. 3(c)). The packing of the molecules observed in the single-crystal X-ray analysis of HMCDs 4a and 4b is shown in Fig. S1 and S2 (ESI†). Molecular orientations are indicated with four colors: red, blue, magenta, and green, in both HMCDs. As shown in Fig. S1(a) (ESI†), in the case of ring-perfluorinated HMCD 4a, both the blue and magenta molecules and the red and green molecules are arranged in the same direction. In addition, the blue and magenta molecular arrangements and the red and green molecular arrangements are opposite. As shown in Fig. S1(g), (h), (i), and (j) (ESI†), the distances between the indolium ring and the methine double bond are 4.988, 5.091, and 6.439 Å, and the distance between the two indolium rings is ∼5.5 Å. No π–π stacking is observed. As shown in Fig. S2 (ESI†), in the case of fluorine-free HMCD 4b, the blue and magenta molecules and the red and green molecules are arranged in a herringbone shape. The distances between the indolium ring and the methine double bond are 4.274 and 5.918 Å, as depicted in Fig. S2(b) and (f) (ESI†). No π–π stacking is observed.
Ultraviolet-visible (UV-vis)-NIR absorption and fluorescence spectra and behaviour in various solvents
The UV-vis-NIR absorption (solid line) and fluorescence (dotted line) spectra of HMCDs 4a and 4b in DCM (1 × 10−6 M) are shown in Fig. 5.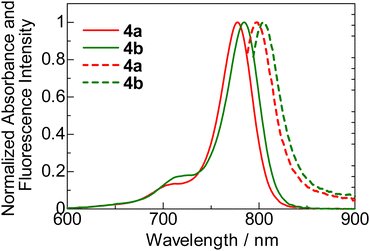 | ||
| Fig. 5 Normalised UV-vis-NIR absorption (solid line) and fluorescence (dotted line) spectra of HMCDs 4a and 4b in DCM (1 × 10−6 M). | ||
The λabs values of 4a and 4b in DCM are within the NIR region (778 and 785 nm, respectively). The λabs value of ring-fluorinated HMCD 4a is 7 nm blue-shifted compared with that of non-fluorinated HMCD 4b. The molar absorption coefficients (ε) of 4a and 4b are 318![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 331
000 and 331![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, respectively. Similar to λabs, λem of ring-fluorinated HMCD 4a is 5 nm blue-shifted compared with that of non-fluorinated HMCD 4b. The Stokes shifts (SS) of HMCDs 4a and 4b are small (322 and 301 cm−1, respectively). The fluorescence quantum yields (Φf) of both HMCDs are low, and the τf of ring-fluorinated HMCD 4a is slightly smaller than that of non-fluorinated HMCD 4b. The fluorescence lifetime (τs) of ring-fluorinated HMCD 4a is slightly shorter than that of non-fluorinated HMCD 4b. These results may be attributed to ring-fluorinated HMCD 4a having a higher non-radiative rate constant than non-fluorinated HMCD 4b (Table 1).
000, respectively. Similar to λabs, λem of ring-fluorinated HMCD 4a is 5 nm blue-shifted compared with that of non-fluorinated HMCD 4b. The Stokes shifts (SS) of HMCDs 4a and 4b are small (322 and 301 cm−1, respectively). The fluorescence quantum yields (Φf) of both HMCDs are low, and the τf of ring-fluorinated HMCD 4a is slightly smaller than that of non-fluorinated HMCD 4b. The fluorescence lifetime (τs) of ring-fluorinated HMCD 4a is slightly shorter than that of non-fluorinated HMCD 4b. These results may be attributed to ring-fluorinated HMCD 4a having a higher non-radiative rate constant than non-fluorinated HMCD 4b (Table 1).
| Dye | λ abs (nm) | ε (M−1 cm−1) | λ em (nm) | SS (cm−1) | Φ f | τ s (ns) | k f (109 s−1) | k nr (109 s−1) |
|---|---|---|---|---|---|---|---|---|
| a Measured in DCM (1 × 10−6 M). b Measured using an integrating sphere method. c Measured using a single-photon-counting method. d Radiative rate constant (kf = Φf/τs). e Non-radiative rate constant (knr = (1 − Φf)τs). | ||||||||
| 4a | 778 | 318![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
798 | 322 | 0.11 | 0.9 | 0.13 | 1.01 |
| 4b | 785 | 331![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
804 | 301 | 0.14 | 1.3 | 0.13 | 0.77 |
The UV-vis-NIR absorption and fluorescence spectra of non-fluorinated HMCD 4b in various solvents, such as methanol (MeOH), ethanol (EtOH), 2-propanol (i-PrOH), acetonitrile (MeCN), and acetone, were recorded after preparing the solution and leaving it at room temperature for 3 h. The spectra and images are shown in Fig. 6 and summarised in Table 2. Similar to previous results with other anions,22λabs (774–785 nm) and λem (795–804 nm) are in the NIR region, and negative solvatochromism is observed in the UV-vis-NIR and fluorescence spectra.
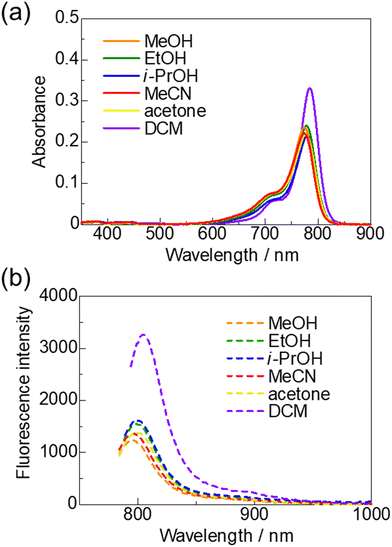 | ||
| Fig. 6 UV-vis-NIR absorption spectra (a) and fluorescence spectra (b) of non-fluorinated HMCD 4b in various solvents (1 × 10−6 M). | ||
| Dye | Solvent | λ abs (nm) | ε (M−1 cm−1) |
|---|---|---|---|
| a Measured in solvents (1 × 10−6 M). | |||
| 4a | MeOH | 400 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| EtOH | 402 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| i-PrOH | 399 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| MeCN | 404, 770 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 103 000, 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Acetone | 406, 773 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 15 000, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| DCM | 778 | 318![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| 4b | MeOH | 774 | 235![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| EtOH | 778 | 239![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| i-PrOH | 779 | 216![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| MeCN | 774 | 222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Acetone | 774 | 218![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| DCM | 785 | 331![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
By contrast, ring-fluorinated HMCD 4a exhibits unique properties in various solvents. The spectra and photographs in various solvents are shown in Fig. 7 and summarised in Table 2. The peaks at approximately 774–779 nm disappear in the case of MeOH and EtOH, and a new peak appears at approximately 400 nm (Fig. 7(a)). As displayed in Fig. 7(b), the peaks around 774–779 nm are small in the case of i-PrOH, MeCN, and acetone. Furthermore, as shown in Fig. 7(d), fluorescence spectra of ring-fluorinated HMCD 4a excited at λabs (770–778 nm) in DCM, MeCN, and acetone are observed at 790–798 nm. However, note that no fluorescence spectrum exists at λabs (399–406 nm) in the case of MeOH, EtOH, i-PrOH, MeCN, and acetone.
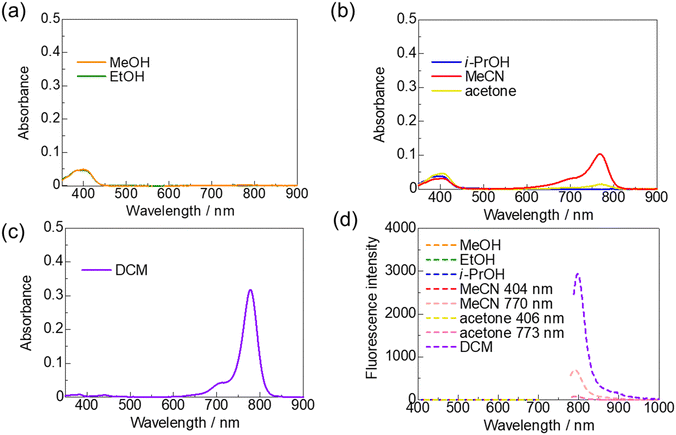 | ||
| Fig. 7 UV-vis-NIR absorption spectra in MeOH and EtOH (a), in i-PrOH, MeCN, and acetone (b), and in DCM (c). Fluorescence spectra (d) of ring-fluorinated HMCD 4a in various solvents (1 × 10−6 M). | ||
Electrochemical and thermal properties
Thermogravimetry-differential thermal analysis (TG-DTA) was performed to determine the decomposition temperatures (Tdt) of HMCDs 4a and 4b. The results of Tdt are shown in Fig. S3 (ESI†) and listed in Table 3. Ring-perfluorinated HMCD 4a shows a lower Tdt (238.0 °C) than non-fluorinated HMCD 4b (244.9 °C).| Dye | T dt (°C) | E ox (V vs. SCE) | HOMOc (eV) | λ onset (nm) | HOMO–LUMO gape (eV) | LUMOf (eV) |
|---|---|---|---|---|---|---|
| a Decomposition temperatures (Tdt) were determined by conducting TG-DTA. b Measured in MeCN containing the dyes (1 × 10−3 M) and Bu4NClO4 (0.1 M) and recalculated as Eox (V vs. SCE). c HOMO (eV) = − (Eox (V vs. SCE) + 4.4). d Measured in CH2Cl2 (1 × 10−6 M). e HOMO–LUMO gap (eV) = 1240/λonsetabs (nm). f LUMO (eV) = HOMO − (HOMO–LUMO gap). | ||||||
| 4a | 238.0 | 0.693 | −5.09 | 875 | 1.42 | −3.67 |
| 4b | 244.9 | 0.478 | −4.88 | 883 | 1.41 | −3.47 |
HMCDs 4a and 4b undergo oxidation at 0.693 and 0.478 V (versus the saturated calomel electrode (SCE)) in the cyclic voltammograms, respectively, as shown in Fig. S6 (ESI†). The EHOMO values for HMCDs 4a and 4b were calculated using the values of Eox in the cyclic voltammograms, and the ELUMO values for HMCDs 4a and 4b were obtained by performing calculations using the EHOMO values and the bandgap values, which can be obtained from the onset of the absorption spectra. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energies of aromatic ring-perfluorinated HMCD 4a were much lower than those of non-fluorinated HMCD 4b because of the high electronegativity of the fluorine atom.
Density functional theory (DFT) calculations
DFT calculations in the gas phase were performed at the B3LYP/6-31G(d,p) level with Gaussian 16 for HMCDs 4a and 4b performed to examine the electronic states of the HOMO and LUMO levels and bandgaps, and the results are illustrated in Fig. 8. Although a significant difference in the electron distribution between the HOMO and LUMO in HMCDs 4a and 4b is unlikely, the introduction of fluorine atoms into the aromatic ring of the dye lowers the levels of the HOMO and LUMO, especially the HOMO. Therefore, the bandgap of ring-fluorinated HMCD 4a is wider than that of non-fluorinated HMCD 4b. These results support that the λabs (778 nm) of ring-fluorinated HMCD 4a is blue-shifted compared with that of non-fluorinated HMCD 4b (785 nm).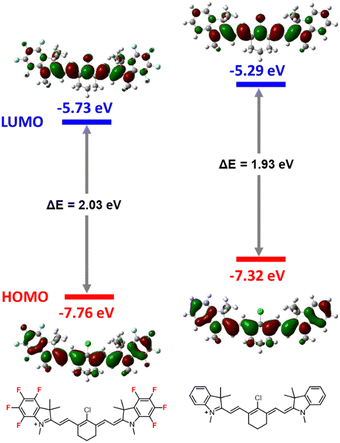 | ||
| Fig. 8 Energy diagram and molecular orbitals of the cationic parts of HMCDs 4a and 4b calculated using DFT at the B3LYP/6-31G(d,p). | ||
Photostability
As presented in Fig. 9(a)–(c), the photostabilities of ring-fluorinated HMCD 4a and non-fluorinated HMCD 4b in an incubator at 25 °C under white-light-emitting diode (LED) irradiation (8.5 W) are measured in CH2Cl2 solutions (1.0 × 10−6 M). The results for the condition when the HMCDs are left in the dark without LED irradiation in CH2Cl2 are depicted in Fig. 9(d). CH2Cl2 is used as the solvent because the lifetime of singlet oxygen is longer in CH2Cl2 than in other solvents, such as alcohols, benzene, and alkanes. The residual rates of HMCD 2a and 2b are calculated from the changes at λabs in the UV-vis-NIR absorption spectra.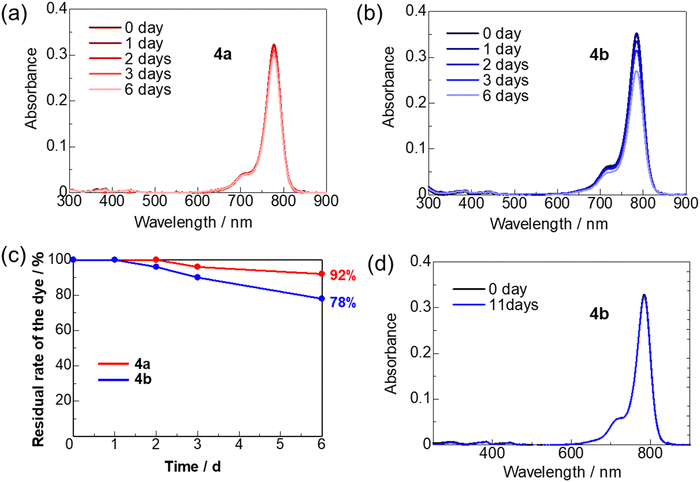 | ||
| Fig. 9 Photostabilities of HMCDs 4a and 4b under white LED irradiation (8.5 W) in an incubator at 25 °C in CH2Cl2 solutions (1 × 10−6 M). | ||
After 6 days of light irradiation, the residual rates of HMCDs 4a and 4b in CH2Cl2 are 92% and 78%, respectively (Fig. 9(c)). The photostability of ring-fluorinated HMCD 4a is higher than that of 2b. Non-fluorinated HMCD 4b in the CH2Cl2 solution kept in the dark does not degrade at all, and its residual rate is 99%. These results indicate that the introduction of a strong electron-withdrawing fluorine atom into the indolenine portion of HMCD stabilises the HOMO and the LUMO and reduces the electron density of the double bond, thus improving the photostability of the dye.23
Stimulus response to amines
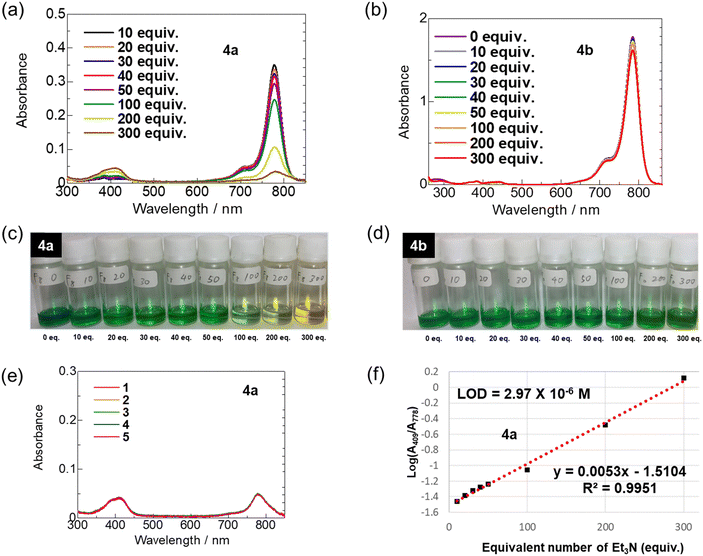
Based on the interesting absorption properties of ring-fluorinated HMCD 4a in various solvents, the stimulus responsiveness of ring-fluorinated HMCD 4a to other neutral nucleophiles such as triethylamine (TEA) in solutions was evaluated, as shown in Fig. 10.Various amounts (10, 20, 30, 40, 50, 100, 200, and 300 equiv.) of TEA were added to a highly concentrated DCM solution (5 × 10−6 M) of HMCDs 4a and 4b at room temperature, and UV-vis-NIR spectra were recorded after the solution was prepared and left at room temperature for 4 h, as presented in Fig. 10 and Table 4.
| Dye | Equiv. of TEA | λ abs (nm) |
|---|---|---|
| a Measured in CH2Cl2 (5 × 10−6 M). | ||
| 4a | 0 | 778 |
| 10 | 778, 416 | |
| 20 | 778, 413 | |
| 30 | 778, 412 | |
| 40 | 778, 412 | |
| 50 | 778, 412 | |
| 100 | 778, 409 | |
| 200 | 782, 409 | |
| 300 | 409 | |
| 4b | 0 | 785 |
| 10 | 784 | |
| 20 | 784 | |
| 30 | 784 | |
| 40 | 784 | |
| 50 | 784 | |
| 100 | 784 | |
| 200 | 784 | |
| 300 | 784 | |
The results of the UV-vis-NIR spectra and the photographs obtained under white LED irradiation are shown in Fig. 10. Although the values (784–785 nm) of λabs in the DCM solution of non-fluorinated HMCD 4b in the presence of various amounts of TEA are almost unchanged in the UV-vis-NIR spectra, the λabs of ring-fluorinated HMCD 4a shifts from 778–782 nm to 409–416 nm, and the colour of the DCM solution of the HMCD 4a changes from green to yellow (Fig. 10(c)). An excellent linear relationship between the absorption log (A409/A778) and the equiv. of TEA is observed, as shown in Fig. 10(f). Based on this linearity, the limit of detection (LOD) for 4a is determined as LOD = 3s/k, where s is the standard deviation (Fig. 10(e)) obtained from five measurements, and k is the slope of the line. The results indicate that the LOD of ring-fluorinated HMCD 4a is 2.97 × 10−6 M.
1H, 13C, and 19F nuclear magnetic resonance (NMR) spectra of ring-fluorinated HMCD 4a in deuterochloroform (CDCl3) without or with an excess amount of TEA were recorded to assess the structural changes in ring-fluorinated HMCD 4a in a DCM solution. The results of not only 1H, 19F, and 13C NMR spectroscopy of ring-fluorinated HMCD 4a without or with an excess amount of TEA but also high-resolution mass spectrometry (HRMS) are shown in Fig. 11. Two types of vinyl protons (Ha and Hb) in 1H NMR of 4a, four types of aromatic fluorine atoms in 19F NMR, and four types of alkyl carbons and an imine carbon atom (173 ppm) (magenta-filled circles) in 13C NMR are observed,17b because ring-fluorinated HMCD 4a has a resonance structure, that is, a particular electronic structure arising from a symmetric, positively charged, amino-terminated, 7-numbered polymethine chain.
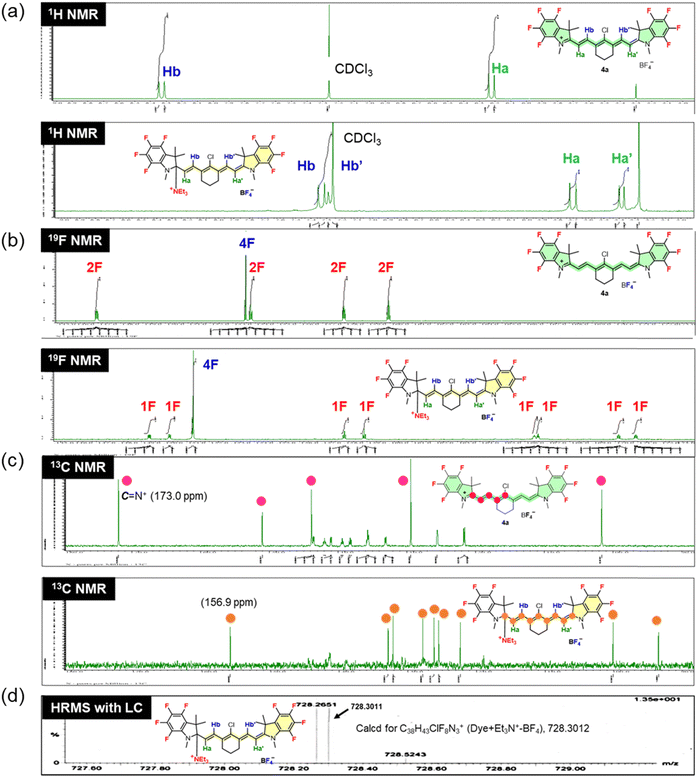 | ||
| Fig. 11 1H NMR spectra (a), 19F NMR spectra (b), and 13C NMR spectra (c) of ring-fluorinated HMCD 4a without or with an excess amount of TEA in CDCl3 and HRMS results (d). | ||
Because of the collapse of the symmetric resonance structure caused by the addition of TEA to aromatic ring-fluorinated HMCD 4a, four types of vinyl protons, Ha, Ha′, Hb, and Hb′, appear in the 1H NMR spectra, similar to our previously reported 1H NMR spectra of ring-fluorinated trimethine cyanine dye with n-hexylamine added to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond.17b Eight types of aromatic fluorine atoms in the 19F NMR spectra and nine types of alkyl carbons (orange-filled circles) in the 13C NMR spectra are observed. The peak at 173.0 ppm corresponding to the iminium carbon disappears and shifts to 156.9 ppm in the 13C NMR spectra. These results suggest that the addition of TEA to ring-fluorinated HMCD 4a proceeds smoothly with CDCl3 at room temperature to yield the corresponding TEA adduct with a molecular weight of 728.3011, as confirmed by performing HRMS with high-performance liquid chromatography (Fig. 11(d)).
N bond.17b Eight types of aromatic fluorine atoms in the 19F NMR spectra and nine types of alkyl carbons (orange-filled circles) in the 13C NMR spectra are observed. The peak at 173.0 ppm corresponding to the iminium carbon disappears and shifts to 156.9 ppm in the 13C NMR spectra. These results suggest that the addition of TEA to ring-fluorinated HMCD 4a proceeds smoothly with CDCl3 at room temperature to yield the corresponding TEA adduct with a molecular weight of 728.3011, as confirmed by performing HRMS with high-performance liquid chromatography (Fig. 11(d)).
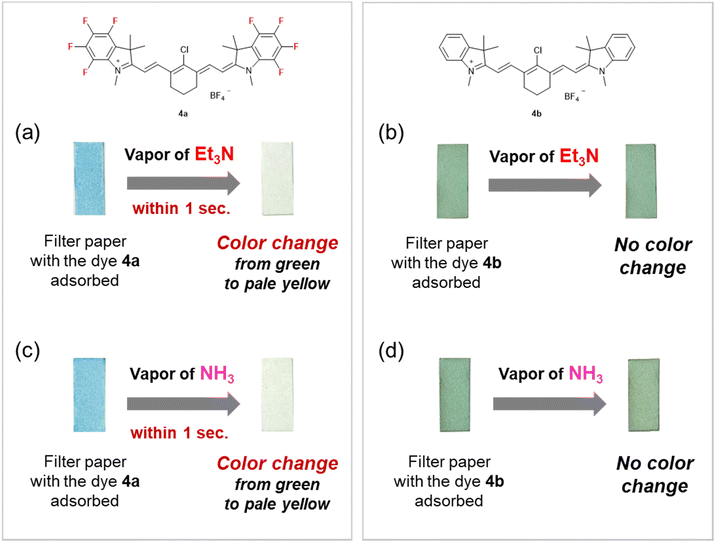
Finally, the reversible vapochromic responses of the filter papers adsorbing ring-fluorinated HMCD 4a and non-fluorinated HMCD 4b to amine vapour were investigated. The filter papers adsorbing ring-fluorinated HMCD 4a and non-fluorinated HMCD 4b were prepared by soaking white filter papers in a DCM solution (5 × 10−4 M) of HMCDs 4a and 4b overnight and drying at room temperature overnight. The green colour of the filter paper adsorbing non-fluorinated HMCD 4b did not change in the presence of TEA vapour (Fig. 12(b)). However, notably, when the blueish-green filter paper adsorbing ring-fluorinated HMCD 4a was exposed to TEA or low-nucleophilicity ammonia vapour, the green filter paper responded within 1 s and showed a rapid and visible colour change from blueish-green to pale yellow (Fig. 12(a)). Non-fluorinated HMCD 4b adsorbed on weakly acidic silica gel changed only slightly in response to NH3 vapor,10 whereas ring-fluorinated HMCD 4a adsorbed on the neutral filter paper reacted quickly, even with low-nucleophilicity NH3 vapour.
Exposure of ring-fluorinated HMCD 4a adsorbed on the filter paper to the vapour of other primary amines, such as ethylamine, n-propylamine, and n-butylamine, and secondary amines, such as diethylamine, pyrrolidine, and piperidine, instantly causes the same vapochromic-responsive behaviour, along with a colour change from blue to green to pale yellow (Fig. 13(a) and (b)). No response is observed when ring-fluorinated HMCD 4a adsorbed on the filter paper is exposed to an aromatic amine, such as aniline (Fig. 13(d)).
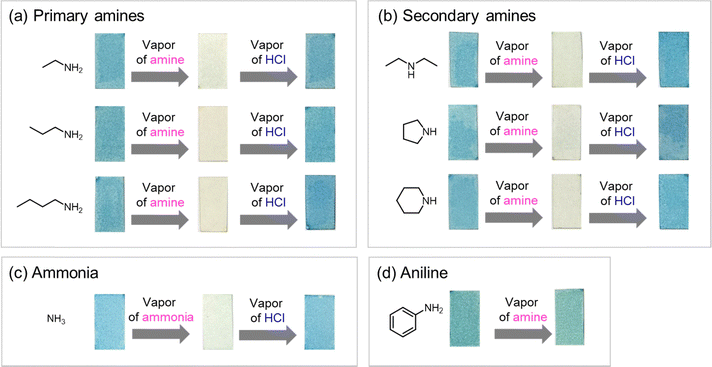 | ||
| Fig. 13 Vapochromic discolouration and colouration test of ring-fluorinated HMCD 4a adsorbed on the filter paper with various primary amines (a), secondary amines (b), and aniline (c). | ||
However, the colour of the filter paper adsorbing ring-fluorinated HMCD 4a does not revert from pale yellow to blueish-green when left in air. However, ring-fluorinated HMCD 4a adsorbed on the filter paper is reversibly converted to the original HMCD when exposed to HCl vapour, as shown in Fig. 13(a)–(c). Fig. 14 presents the results of the repeatability tests with NH3 and HCl vapours on ring-fluorinated HMCD 4a adsorbed on the filter paper. The filter paper adsorbing ring-fluorinated HMCD 4a exhibits excellent repeatability in terms of colour switching, which occurs 13 times.
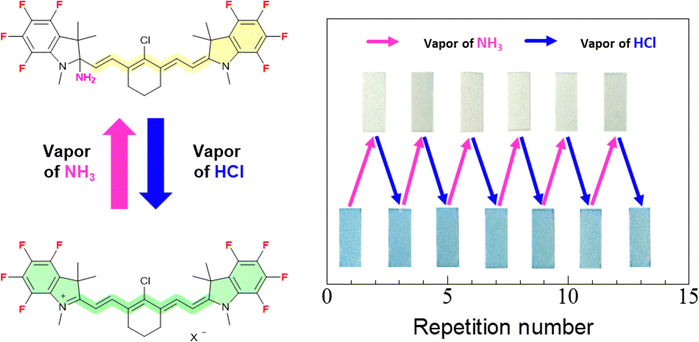 | ||
| Fig. 14 Vapochromic discolouration and colouration repeatability test of ring-fluorinated HMCD 4a adsorbed on the filter paper with NH3 and HCl vapours. | ||
The unique vapochromic-responsive phenomenon, along with the colour change towards various primary and secondary amines including ammonia, can be ascribed to the nucleophile addition into the iminium double bond of the aromatic ring-fluorinated HMCD (4a) on the filter paper, similar to that in the DCM or CDCl3 solution.
Conclusions
The introduction of fluorine atoms into the aromatic ring of HMCDs reduced the HOMO and LUMO levels of the HMCDs. Consequently, various primary alcohols, despite being weakly neutral nucleophiles, were added to ring-fluorinated HMCD 4a in an alcohol solvent, and the colour of the solution changed from green to yellow. This phenomenon was also observed when TEA was added to a DCM solution of ring-fluorinated HMCD 4a. The addition of TEA to the iminium double bond of HMCD 4a was confirmed by performing 1H, 13C, and 19F NMR and HRMS on the solution. Based on the unique properties of ring-fluorinated HMCD 4a, for the first time, we developed an HMCD adsorption filter paper, a near-infrared absorbent exhibiting reversible vapochromic response to weakly nucleophilic ammonia. These results are the first examples of intermolecular addition of weakly neutral nucleophiles into HMCDs and stimulus responsiveness not in solutions.Experimental
Measurements
1H NMR spectra were recorded at 392 or 400 MHz in CDCl3, hexadeuteroacetone ((CD3)2CO), and hexadeuterodimethyl sulfoxide ((CD3)2SO) solutions, with tetramethylsilane (Me4Si) as an internal standard, using a JEOL ECS-400 or ECX-400P FT-NMR spectrometer. 13C NMR spectra were obtained at 99 or 101 MHz in CDCl3, (CD3)2CO, and (CD3)2SO solutions, with Me4Si as an internal standard, using the JEOL ECS-400 or ECX-400P FT-NMR spectrometer. 19F NMR spectra were recorded at 369 or 376 MHz in CDCl3, (CD3)2CO, and (CD3)2SO solutions, with CFCl3 as an external standard, using the JEOL ECS-400 or ECX-400P FT-NMR spectrometer. The data were reported as s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, m = multiplet, br s = broad singlet, coupling constant(s), and integration. The melting points were determined using Yanagimoto MP-S3 micro-melting-point apparatus and were uncorrected. Electrospray ionisation mass spectroscopy in MeOH was performed using a JEOL JMS-T100LP instrument (Accu TOF LC-plus). UV-vis absorption spectra were recorded on Hitachi U-4100 and PerkinElmer Lamda950 instruments. Fluorescence spectra were recorded using a Jasco FP-8600 spectrofluorometer. The absolute fluorescence quantum yields were obtained using a HAMAMATSU Quantaurus-QY C11347-01 instrument. The fluorescence lifetimes were measured using a HAMAMATSU Quantaurus-Tau compact fluorescence lifetime spectrometer (C11367-01). Cyclic voltammetry was performed by employing an automatic polarisation system (HSV-110). TG-DTA was performed on an SII EXSTAR 6000 TG/DTA 6300 system under nitrogen after applying heat treatment at 100 °C under vacuum for 12 h, and the measured values were uncorrected. The X-ray crystal structure was evaluated using a Rigaku AFC 10 (CCD: Saturn 724 +) + VariMax Mo Optic system. 4,5,6,7-Tetrafluoro-2,3,3-trimethyl-3H-indole (1),21 1,2,3,3-tetramethyl-3H-indol-1-ium tetrafluoroborate (2b),24 and (E)-2-chloro-3-(hydroxymethylene)cyclohex-1-enecarbaldehyde (3)25 were prepared according to previously reported methods.Synthesis
Yield 84%; m.p. 151–155 °C; Rf 0.65 (hexane/dichloromethane = 2/1); IR (KBr) 1523 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1; HRMS (ESI) found: m/z 246.0892; calcd for C12H12NF4: M-BF4, 246.0906; 1H NMR (acetone-d6) δ 1.70 (s, 6H, CH3 × 2), 2.82 (s, 3H, CH3), 4.13 (s, 3H, NCH3); 13C NMR (acetone-d6) δ 14.2 (s), 20.7 (s), 38.7 (s), 57.2 (s), 125.6 (d, J = 18.7 Hz), 127.2 (s), 138.7 (dm, J = 256.9 Hz), 142.1 (dm, J = 255.4 Hz), 143.8 (dd, J = 250.2, 12.5 Hz), 200.6 (s); 19F NMR (acetone-d6) δ −155.7 (dd, J = 19.2, 3.0 Hz, 1F), −155.5 (td, J = 19.2, 3.0 Hz, 1F), −152.3 (tm, J = 18.1 Hz, 1F), −151.9 (s, 4F), −147.1 (tm, J = 18.1 Hz, 1F).
N) cm−1; HRMS (ESI) found: m/z 246.0892; calcd for C12H12NF4: M-BF4, 246.0906; 1H NMR (acetone-d6) δ 1.70 (s, 6H, CH3 × 2), 2.82 (s, 3H, CH3), 4.13 (s, 3H, NCH3); 13C NMR (acetone-d6) δ 14.2 (s), 20.7 (s), 38.7 (s), 57.2 (s), 125.6 (d, J = 18.7 Hz), 127.2 (s), 138.7 (dm, J = 256.9 Hz), 142.1 (dm, J = 255.4 Hz), 143.8 (dd, J = 250.2, 12.5 Hz), 200.6 (s); 19F NMR (acetone-d6) δ −155.7 (dd, J = 19.2, 3.0 Hz, 1F), −155.5 (td, J = 19.2, 3.0 Hz, 1F), −152.3 (tm, J = 18.1 Hz, 1F), −151.9 (s, 4F), −147.1 (tm, J = 18.1 Hz, 1F).
Yield 84%; Tdt 238.0 °C; Rf 0.43 (dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); IR (KBr) 1558 (C
1); IR (KBr) 1558 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1; HRMS (ESI) found: m/z 627.1813; calcd for C32H28ClF8N2: M-BF4, 627.1808; 1H NMR (CDCl3) δ 1.82 (s, 12H, CH3 × 4), 1.89–1.92 (m, 2H, –CH2CH2CH2–), 2.67 (t, J = 6.00 Hz, 4H, –CH2CH2CH2–), 3.79 (s, 6H, NCH3 × 2), 6.16 (d, J = 14.2 Hz, 2H, vinyl H), 8.30 (d, J = 14.2 Hz, 2H, vinyl H); 13C NMR (CDCl3) δ 20.6 (s), 26.4 (s, 2C), 34.6 (d, J = 9.3 Hz), 50.8 (s), 102.6 (s), 122.6 (d, J = 15.9 Hz), 126.6 (s), 130.4 (s), 135.4 (dd, J = 249.4, 13.2 Hz), 138.0 (dt, J = 252.3, 14.9 Hz), 141.7 (dt, J = 252.3, 14.9 Hz), 143.3 (dd, J = 249.4, 13.2 Hz), 144.9 (s), 152.0 (s), 173.0 (s); 19F NMR (CDCl3) δ −159.5 (t, J = 19.9 Hz, 2F), −157.5 (dd, J = 19.9, 14.2 Hz, 2F), −153.4 (tm, J = 19.9 Hz, 2F), −153.2 (s, 4F), −146.5 (dd, J = 19.9, 14.2 Hz, 2F).
N) cm−1; HRMS (ESI) found: m/z 627.1813; calcd for C32H28ClF8N2: M-BF4, 627.1808; 1H NMR (CDCl3) δ 1.82 (s, 12H, CH3 × 4), 1.89–1.92 (m, 2H, –CH2CH2CH2–), 2.67 (t, J = 6.00 Hz, 4H, –CH2CH2CH2–), 3.79 (s, 6H, NCH3 × 2), 6.16 (d, J = 14.2 Hz, 2H, vinyl H), 8.30 (d, J = 14.2 Hz, 2H, vinyl H); 13C NMR (CDCl3) δ 20.6 (s), 26.4 (s, 2C), 34.6 (d, J = 9.3 Hz), 50.8 (s), 102.6 (s), 122.6 (d, J = 15.9 Hz), 126.6 (s), 130.4 (s), 135.4 (dd, J = 249.4, 13.2 Hz), 138.0 (dt, J = 252.3, 14.9 Hz), 141.7 (dt, J = 252.3, 14.9 Hz), 143.3 (dd, J = 249.4, 13.2 Hz), 144.9 (s), 152.0 (s), 173.0 (s); 19F NMR (CDCl3) δ −159.5 (t, J = 19.9 Hz, 2F), −157.5 (dd, J = 19.9, 14.2 Hz, 2F), −153.4 (tm, J = 19.9 Hz, 2F), −153.2 (s, 4F), −146.5 (dd, J = 19.9, 14.2 Hz, 2F).
Yield 76%; Tdt 244.9 °C; Rf 0.43 (dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); IR (KBr) 1551 (C
1); IR (KBr) 1551 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1; HRMS (ESI) found: m/z 483.2547; calcd for C32H36ClN2: M-BF4, 483.2562; 1H NMR (acetone-d6) δ 1.77 (s, 12H, CH3 × 4), 1.91–1.97 (m, 2H, –CH2CH2CH2–), 2.77 (t, J = 6.20 Hz, 4H, –CH2CH2CH2–), 3.79 (s, 6H, NCH3 × 2), 6.41 (d, J = 14.2 Hz, 2H, vinyl H), 7.32 (td, J = 7.3, 1.4 Hz, 2H, aryl H), 7.41–7.49 (m, 4H, aryl H), 7.62 (d, J = 7.3 Hz, 2H, aryl H), 8.44 (d, J = 14.20 Hz, 2H, vinyl H); 13C NMR (acetone-d6) δ 21.6 (s), 26.9 (s), 28.0 (s), 32.0 (s), 50.1 (s), 102.5 (s), 112.0 (s), 123.2 (s), 126.2 (s), 127.4 (s), 129.6 (s), 142.2 (s), 144.1 (s), 144.7 (s), 150.0 (s), 174.3 (s); 19F NMR (acetone-d6) δ −151.7 (s, 4F).
N) cm−1; HRMS (ESI) found: m/z 483.2547; calcd for C32H36ClN2: M-BF4, 483.2562; 1H NMR (acetone-d6) δ 1.77 (s, 12H, CH3 × 4), 1.91–1.97 (m, 2H, –CH2CH2CH2–), 2.77 (t, J = 6.20 Hz, 4H, –CH2CH2CH2–), 3.79 (s, 6H, NCH3 × 2), 6.41 (d, J = 14.2 Hz, 2H, vinyl H), 7.32 (td, J = 7.3, 1.4 Hz, 2H, aryl H), 7.41–7.49 (m, 4H, aryl H), 7.62 (d, J = 7.3 Hz, 2H, aryl H), 8.44 (d, J = 14.20 Hz, 2H, vinyl H); 13C NMR (acetone-d6) δ 21.6 (s), 26.9 (s), 28.0 (s), 32.0 (s), 50.1 (s), 102.5 (s), 112.0 (s), 123.2 (s), 126.2 (s), 127.4 (s), 129.6 (s), 142.2 (s), 144.1 (s), 144.7 (s), 150.0 (s), 174.3 (s); 19F NMR (acetone-d6) δ −151.7 (s, 4F).
(E)-2-Chloro-3-(2-((E)-1,3,3-trimethylindolin-2-ylidene)ethylidene)cyclohex-1-ene-1-carbaldehyde (5b):27Rf 0.21 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane = 1
dichloromethane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); IR (KBr) 1643 (C
2); IR (KBr) 1643 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (chloroform-d) δ 1.66 (s, 6H, CH3 ×2), 1.74–1.81 (m, 2H, -CH2CH2CH2-), 2.48 (t, J = 5.84 Hz, 2H, -CH2CH2CH2-), 2.58 (tm, J = 5.84 Hz, 2H, -CH2CH2CH2-), 3.23 (s, 3H, -NCH3), 5.46 (d, J = 12.8 Hz, 1H, vinyl H), 6.72 (d, J = 7.79 Hz, 1H, aryl H), 6.94 (tm, J = 7.79 Hz, 1H, aryl H), 7.19–7.24 (m, 2H, aryl H) 7.82 (d, J = 12.8 Hz, 1H, vinyl H), 10.3 (s, 1H, CHO); 13C NMR (chloroform-d) δ 21.0 (s), 24.7 (s), 26.8 (s), 28.4 (s), 29.5 (s), 46.5 (s), 93.0 (s), 106.8 (s), 121.0 (s), 121.8 (s), 123.4 (s), 128.0 (s), 128.7 (s), 131.2 (s), 139.3 (s), 144.5 (s), 148.8 (s), 163.0 (s), 191.1 (s).
O) cm−1; 1H NMR (chloroform-d) δ 1.66 (s, 6H, CH3 ×2), 1.74–1.81 (m, 2H, -CH2CH2CH2-), 2.48 (t, J = 5.84 Hz, 2H, -CH2CH2CH2-), 2.58 (tm, J = 5.84 Hz, 2H, -CH2CH2CH2-), 3.23 (s, 3H, -NCH3), 5.46 (d, J = 12.8 Hz, 1H, vinyl H), 6.72 (d, J = 7.79 Hz, 1H, aryl H), 6.94 (tm, J = 7.79 Hz, 1H, aryl H), 7.19–7.24 (m, 2H, aryl H) 7.82 (d, J = 12.8 Hz, 1H, vinyl H), 10.3 (s, 1H, CHO); 13C NMR (chloroform-d) δ 21.0 (s), 24.7 (s), 26.8 (s), 28.4 (s), 29.5 (s), 46.5 (s), 93.0 (s), 106.8 (s), 121.0 (s), 121.8 (s), 123.4 (s), 128.0 (s), 128.7 (s), 131.2 (s), 139.3 (s), 144.5 (s), 148.8 (s), 163.0 (s), 191.1 (s).
Preparation of the dye-adsorbed filter paper
The filter paper was dipped in a DCM solution (5 × 10−4 M) of the 4a and 4b dyes and allowed to stand in a refrigerator overnight to allow each dye to be adsorbed on the filter paper. After being removed and air-dried, the filter paper samples were exposed to the vapours of various amines.Author contributions
SA performed the experiments on the synthesis and photophysical properties, analysed the data, and wrote part of the manuscript. TA and YU examined the single-crystal X-ray structure and analysed and summarised the data. YH performed the experiments on optical properties. YK and TI contributed useful discussions and comments on the synthesis, photophysical properties, and single-crystal X-ray structural analyses. KF conceived and designed the study and revised the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by JSPS KAKENHI (JP20K05647 and 23H02003 to KF). We would also like to thank Editage (https://www.editage.com) for pre-submission English language editing.References
- For selected reviews, see.
(a) Y. Zhang and Y. Jia, NIR-II Cyanine@albumin Fluorophore for Deep Tissue Imaging and Imaging-Guided Surgery, SmartMat, 2024, 5, e1245 CrossRef CAS;
(b) X. Ma, Y. Huang, A. Li, X. Zeng, S. H. Liu, J. Yin and G.-F. Yang, The Aggregates of Near-Infrared Cyanine Dyes in Phototherapy, ChemMedChem, 2023, 18, e202300204 CrossRef CAS PubMed;
(c) N. G. Medeiros, C. A. Braga, V. S. Câmara, R. C. Duarte and F. S. Rodembusch, Asian J. Org. Chem., 2022, 11, e202200095 CrossRef CAS;
(d) K. Ilina and M. Henary, Cyanine Dyes Containing Quinoline Moieties: History, Synthesis, Optical Properties, and Applications, Chem. – Eur. J., 2021, 27, 4230–4248 CrossRef CAS PubMed;
(e) R. M. Exner, F. Cortezon-Tamarit and S. I. Pascu, Explorations into the Effect of Meso-Substituents in Tricarbocyanine Dyes: A Path to Diverse Biomolecular Probes and Materials, Angew. Chem., Int. Ed., 2021, 60, 6230–6241 CrossRef CAS PubMed;
(f) L. Feng, W. Chen, X. Ma, S. H. Liu and J. Yin, Near-Infrared Heptamethine Cyanines (Cy7): from Structure, Property to Application, Org. Biomol. Chem., 2020, 18, 9385–9397 RSC;
(g) A. Mishra, R. K. Behera, P. K. Behera, B. K. Mishra and G. B. Behera, Cyanines During the 1990s:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A Review, Chem. Rev., 2000, 100, 1973–2012 CrossRef CAS PubMed.
A Review, Chem. Rev., 2000, 100, 1973–2012 CrossRef CAS PubMed. - For selected recent reviews, see. (a) S. M. Usama and K. Burgess, Hows and Whys of Tumor-Seeking Dyes, Acc. Chem. Res., 2021, 54, 2121–2131 CrossRef CAS PubMed; (b) S. Wang, B. Li and F. Zhang, Molecular Fluorophores for Deep-Tissue Bioimaging, ACS Cent. Sci., 2020, 6, 1302–1316 CrossRef CAS PubMed; (c) B. Li, M. Zhao and F. Zhang, Rational Design of Near-Infrared-II Organic Molecular Dyes for Bioimaging and Biosensing, ACS Mater. Lett., 2020, 2, 905–917 CrossRef CAS; (d) A. P. Gorka, R. R. Nani and M. J. Schnermann, Cyanine Polyene Reactivity: Scope and Biomedical Applications, Org. Biomol. Chem., 2015, 13, 7584–7598 RSC.
- For a recent review, see. (a) P. J. Choi, T. I-H. Park, E. Cooper, M. Dragunow, W. A. Denny and J. Jose, Heptamethine Cyanine Dye Mediated Drug Delivery: Hype or Hope, Bioconjugate Chem., 2020, 31, 1724–1739 CrossRef CAS PubMed; (b) M. M. Leitao, D. de Melo-Diogo, C. G. Alves, R. Lima-Sousa and I. J. Correia, Prototypic Heptamethine Cyanine Incorporating Nanomaterials for Cancer Phototheragnostic, Adv. Healthcare Mater., 2020, 9, 1901665 CrossRef CAS PubMed; (c) Y. Zhang, A. C. Yen and Y. Zhao, Polymeric Nanocarriers Incorporating Near-Infrared Absorbing Agents for Potent Photothermal Therapy of Cancer, Poly. J., 2016, 48, 589–603 CrossRef CAS . For recent examples, see. ; (d) A. St. Lorenz, E. R. Buabeng, O. Taratula and M. Henary, Near-Infrared Heptamethine Cyanine Dyes for Nanoparticle-Based Photoacoustic Imaging and Photothermal Therapy, J. Med. Chem., 2021, 64, 8798–8805 CrossRef CAS PubMed; (e) H. Qian, Q. Cheng, Y. Tian, H. Dang, C. Teng and L. Yan, An Anti-Aggregation NIR-II Heptamethine-Cyanine Dye with a Stereo-Specific Cyanine for Imaging-Guided Photothermal Therapy, J. Mater. Chem. B, 2021, 9, 2688–2696 RSC; (f) F. Wu, Y. Lu, X. Mu, Z. Chen, S. Liu, X. Zhou, S. Liu and Z. Li, Intriguing H-Aggregates of Heptamethine Cyanine for Imaging-Guided Photothermal Cancer Therapy, ACS Appl. Mater. Interfaces, 2020, 12, 32388–32396 CrossRef CAS; (g) X. Zhao, Z. Fan, Y. Qiao, Y. Chen, S. Wang, X. Yue, T. Shen, W. Liu, J. Yang, H. Gao, X. Zhan, L. Shang, Y. Yin, W. Zhao, D. Ding, R. Xi and M. Meng, AIEgens Conjugation Improves the Photothermal Efficacy and Near-Infrared Imaging of Heptamethine Cyanine IR-780, ACS Appl. Mater. Interfaces, 2020, 12, 16114–16124 CrossRef CAS; (h) S. Siriwibool, N. Kaekratoke, K. Chansaenpak, K. Siwawannapong, P. Panajapo, K. Sagarik, P. Noisa, R.-Y. Lai and A. Kamkaew, Near-Infrared Fluorescent pH Responsive Probe for Targeted Photodynamic Cancer Therapy, Sci. Rep., 2020, 10, 1283 CrossRef CAS PubMed; (i) X. Mu, Y. Lu, F. Wu, Y. Wei, H. Ma, Y. Zhao, J. Sun, S. Liu, X. Zhou and Z. Li, Supramolecular Nanodiscs Self-Assembled from Non-Ionic Heptamethine Cyanine for Imaging-Guided Cancer Photothermal Therapy, Adv. Mater., 2020, 32, 1906711 CrossRef CAS PubMed.
- For examples of organic photovoltaics (OPV), see. (a) C. J. Traverse, M. Young, J. Suddard-Bangsund, T. Patrick, M. Bates, P. Chen, B. Wingate, S. Y. Lunt, A. Anctil and R. R. Lunt, Anions for Near-Infrared Selective Organic Salt Photovoltaics, Sci. Rep., 2017, 7, 16399 CrossRef PubMed; (b) J. Suddard-Bangsund, C. J. Traverse, M. Young, T. J. Patrick, Y. Zhao and R. R. Lunt, Organic Salts as a Route to Energy Level Control in Low Bandgap, High Open-Circuit Voltage Organic and Transparent Solar Cells that Approach the Excitonic Voltage Limit, Adv. Energy Mater., 2016, 6, 1501659 CrossRef; (c) Y. Zhao, G. A. Meek, B. G. Levine and R. R. Lunt, Near-Infrared Harvesting Transparent Luminescent Solar Concentrators, Adv. Opt. Mater., 2014, 2, 606–611 CrossRef CAS; (d) H. Zhang, S. Jenatsch, J. De Jonghe, F. Nüesch, R. Steim, A. C. Véron and R. Hany, Transparent Organic Photodetector using a Near-Infrared Absorbing Cyanine Dye, Sci. Rep., 2015, 5, 9439 CrossRef CAS; (e) A. C. Véron, H. Zhang, A. Linden, F. Nüesch, J. Heier, R. Hany and T. Geiger, NIR-Absorbing Heptamethine Dyes with Tailor-Made Counterions for Application in Light to Energy Conversion, Org. Lett., 2014, 16, 1044–1047 CrossRef PubMed; (f) H. Zhang, G. Wicht, C. Gretener, M. Nagel, F. Nüesch, Y. Romanyuk, J.-N. Tisserant and R. Hany, Semitransparent Organic Photovoltaics Using a Near-Infrared Absorbing Cyanine Dye, Sol. Energy Mater. Sol. Cells, 2013, 118, 157–164 CrossRef CAS; (g) P.-A. Bouit, D. Rauh, S. Neugebauer, J. L. Delgado, E. D. Piazza, S. Rigaut, O. Maury, C. Andraud, V. Dyakonov and N. A. Martin, Cyanine-Cyanine” Salt Exhibiting Photovoltaic Properties, Org. Lett., 2009, 11, 4806–4809 CrossRef CAS PubMed.
- For examples of dye-sensitized solar cells (DSSC), see. (a) W. Naim, V. Novelli, I. Nikolinakos, N. Barbero, I. Dzeba, F. Grifoni, Y. Ren, T. Alnasser, A. Velardo, R. Borrelli, S. Haacke, S. M. Zakeeruddin, M. Graetzel, C. Barolo and F. Sauvage, Transparent and Colorless Dye-Sensitized Solar Cells Exceeding 75% Average Visible Transmittance, JACS Au, 2021, 1, 409–426 CrossRef CAS PubMed; (b) T. Geiger, I. Schoger, D. Rentsch, A. C. Veron, F. Ostwald, T. Meyer and F. Nuesch, Unsymmetrical Heptamethine Dyes for NIR Dye-Sensitized Solar Cells, Int. J. Photoenergy, 2014, 258984 Search PubMed; (c) K. Funabiki, H. Mase, A. Hibino, N. Tanaka, N. Mizuhata, Y. Sakuragi, A. Nakashima, T. Yoshida, Y. Kubota and M. Matsui, Synthesis of a Novel Heptamethine-Cyanine Dye for Use in Near-Infrared Active Dye-Sensitized Solar Cells with Porous Zinc Oxide Prepared at Low Temperature, Energy Environ. Sci., 2011, 4, 2186–2192 RSC; (d) T. Ono, T. Yamaguchi and H. Arakawa, Study on Dye-Sensitized Solar Cell using Novel Infrared Dye, Sol. Energy Mater. Sol. Cells, 2009, 93, 831–835 CrossRef CAS; (e) A. Otsuka, K. Funabiki, N. Sugiyama, H. Mase, T. Yoshida, H. Minoura and M. Matsui, Chem. Lett., 2008, 37, 176–177 CrossRef CAS; (f) M. Matsui, Y. Hashimoto, K. Funabiki, J.-Y. Jin, T. Yoshida and H. Minoura, Synth. Met., 2005, 148, 147–153 CrossRef CAS.
- For a cyanide anion, see. (a) L. Yijiang, Q. Dali, P. Hua, L. Mengnan, C. Hongbiao and L. Huaming, A Highly Selective Fluorescent Probe for Colorimetric Recognition of Cyanide Anion Based on Heptamethine Cyanine-Triphenylamine Conjugate, J. Photochem. Photobiol., A, 2018, 364, 151–158 CrossRef; (b) D. Qiu, Y. Liu, M. Li, H. Chen and H. Li, Near-Infrared Chemodosimetric Probes Based on Heptamethine Cyanine Dyes for the “Naked-Eye” Detection of Cyanide in Aqueous Media, J. Lumin., 2017, 185, 286–291 CrossRef CAS; (c) Y. Wang, S.-Y. Gwon, Y.-A. Son and S.-H. Kim, Spectroscopic Characterization of Heptamethine Cyanine Dyes for the Interaction with the CN− and F−, Mol. Cryst. Liq. Cryst., 2012, 566, 61–66 CrossRef CAS.
- (a) A. Martin and P. Rivera-Fuentes, A general strategy to develop fluorogenic polymethine dyes for bioimaging, Nat. Chem., 2024, 16, 28–35 CrossRef CAS PubMed; (b) A. Sakama, H. Seo, J. Hara, Y. Shindo, Y. Ikeda, K. Oka, D. Citterio and Y. Hiruta, Rational design of pH-responsive near-infrared spirocyclic cyanines: the effects of substituents and the external environment, Chem. Commun., 2024, 60, 5984–5987 RSC; (c) A. Mukherjee, P. C. Saha, R. S. Das, T. Bera and S. Guha, Acidic pH-Activatable Visible to Near-Infrared Switchable Ratiometric Fluorescent Probe for Live-Cell Lysosome Targeted Imaging, ACS Sens., 2021, 6, 2141–2146 CrossRef CAS PubMed; (d) H. Mu, K. Miki, H. Harada, K. Tanaka, K. Nogita and K. Ohe, pH-Activatable Cyanine Dyes for Selective Tumor Imaging Using Near-Infrared Fluorescence and Photoacoustic Modalities, ACS Sens., 2021, 6, 123–129 CrossRef CAS PubMed; (e) M. Oe, K. Miki and K. Ohe, An Enzyme-Triggered Turn-On Fluorescent Probe Based on Carboxylate-Induced Detachment of a Fluorescence Quencher, Org. Biomol. Chem., 2020, 18, 8620–8624 RSC; (f) M. Oe, K. Miki, H. Mu, H. Harada, A. Morinibu and K. Ohe, pH-Responsive Cy5 Dyes Having Nucleophilic Substituents for Molecular Imaging, Tetrahedron Lett., 2018, 59, 3317–3321 CrossRef CAS; (g) H. Mu, K. Miki, Y. Takahashi, N. Teshima, M. Oe, K. Kojima and K. Ohe, pH Responsiveness of Near-Infrared Fluorescent Cyanine Dyes Encapsulated in Self-Assemblies Composed of Various Amphiphiles, Chem. Lett., 2018, 47, 1147–1150 CrossRef CAS; (h) K. Miki, K. Kojima, K. Oride, H. Harada, A. Morinibu and K. Ohe, pH-Responsive Near-Infrared Fluorescent Cyanine Dyes for Molecular Imaging Based on pH Sensing, Chem. Commun., 2017, 53, 7792–7795 RSC ; Other type of pH-responsive intramolecular addition at meso-position, see: ; (i) L. He, W. Lin, Q. Xu, M. Ren, H. Wei and J.-Y. Wang, A Simple and Effective “Capping” Approach to Readily Tune the Fluorescence of Near-Infrared Cyanines, Chem. Sci., 2015, 6, 4530–4536 RSC.
- For reviews, see: F. Schüth, R. Palkovits, R. Schlögl and D. S. Su, Energy Environ. Sci., 2012, 5, 6278–6289 RSC; A. Klerke, C. H. Christensen, J. K. Nørskov and T. Vegge, J. Mater. Chem., 2008, 18, 2304–2310 RSC ; For a recent example, see: Y. Ofuchi, K. Mitarai, S. Doi, K. Saegusa, M. Hayashi, H. Sampei, T. Higo, J. G. Seo and Y. Sekine, Chem. Sci. 10.1039/D4SC04790G.
- For recent examples, see. (a) J. Wang, Z. Zhou, Y. Luo, T. Xu, L. Xu and X. Zhang, Machine Learning-Assisted Janus Colorimetric Face Mask for Breath Ammonia Analysis, Anal. Chem., 2024, 96, 381–387 CrossRef PubMed; (b) T. Zhou, J. Chen, T. Wang, H. Yan, Y. Xu, Y. Li and W. Sun, One-Dimensional Chain Viologen-Based Lanthanide Multistimulus-Responsive Materials with Photochromism, Photoluminescence, Photomagnetism, and Ammonia/Amine Vapor Sensing, ACS Appl. Mater. Interfaces, 2022, 14, 57037–57046 CrossRef CAS PubMed; (c) S. Yu, A. Tian, Q. Lu, X. Xu, S. Ma, X. Wang and Z. Wang, Polyoxometalate–Viologen Thermochromic Hybrids for Organic Amine Detectors and Memristors with Temperature-Regulating Resistance Switching Characteristics, Inorg. Chem., 2023, 62, 1549–1560 CrossRef CAS PubMed; (d) I. Ahmad, A. A. Malik and A. A. Dar, Multi-Stimuli-Responsive Organo-Sulfonated Anil and Its Organic Complex, Cryst. Growth Des., 2022, 22, 6483–6492 CrossRef CAS; (e) K. M. Fürpaß, L. M. Peschel, J. A. Schachner, S. M. Borisov, H. Krenn, F. Belaj and N. C. Mösch-Zanetti, Vapochromism and Magnetochemical Switching of a Nickel(II) Paddlewheel Complex by Reversible NH3 Uptake and Release, Angew. Chem., Int. Ed., 2021, 60, 13401–13404 CrossRef PubMed; (f) X.-F. Wang, R.-L. Lin, W.-Q. Sun, J.-X. Liu, L.-X. Xu, C. Redshaw and X. Feng, Cucurbit[7]Uril-Based Self-Assembled Supramolecular Complex with Reversible Multistimuli-Responsive Chromic Behavior and Controllable Fluorescence, Adv. Opt. Mater., 2024, 12, 2400839 CrossRef CAS; (g) X.-F. Wang, C.-Y. Xu, R.-L. Lin, W.-Q. Sun, M.-F. Ye, L.-X. Xu and J.-X. Liu, Acid–base regulated inclusion complexes of β-cyclodextrin with 1-[2-(4-fluorophenyl)-2-oxoethyl]-4,4-bipyridinium dichloride displaying multistimuli-responsive chromic behaviors and photomodulable fluorescence, J. Mater. Chem. C, 2024, 12, 2764–2771 RSC; (h) S. Masuda, S. Kusumoto, M. Okamura, S. Hikichi, R. Tokunaga, S. Hayami, Y. Kim and Y. Koide, Thermosalient effect of a naphthalene diimide and tetrachlorocobaltate hybrid and changes of color and magnetic properties by ammonia vapor, Dalton Trans., 2023, 52, 10531–10536 RSC; (i) A. Rico, P. Le Poul, J. Rodríguez-López, S. Achelle and S. Gauthier, Exploring structural and optical properties of a new series of soft salts based on cyclometalated platinum complexes, Dalton Trans., 2024, 53, 11417–11425 RSC.
- M. Shibayama, Y. Uehashi, S. Ajioka, Y. Kubota, T. Inuzuka and K. Funabiki, Vapochromism of Indolenine-based Heptamethine Cyanine Dye Adsorbed on Silica Gel, New J. Chem., 2023, 47, 5262–5269 RSC.
- For recent examples, see. (a) S. Takahashi, M. Murai, Y. Hattori, S. Seki, T. Yanai and S. Yamaguchi, Sulfur-Bridged Cationic Diazulenomethenes: Formation of Charge-Segregated Assembly with High Charge-Carrier Mobility, J. Am. Chem. Soc., 2024, 146, 22642–22649 CrossRef CAS PubMed; (b) W. Wu, K. Yan, Z. He, L. Zhang, Y. Dong, B. Wu, H. Liu, S. Wang and F. Zhang, J. Am. Chem. Soc., 2024, 146, 11570–11576 CAS; (c) M. Taki, K. Kajiwara, E. Yamaguchi, Y. Sato and S. Yamaguchi, Fused Thiophene-S,S-dioxide-Based Super-Photostable Fluorescent Marker for Lipid Droplets, ACS Mater. Lett., 2021, 3, 42–49 CrossRef CAS; (d) S. Tian, H. Ma, X. Wang, A. Lv, H. Shi, Y. Geng, J. Li, F. Liang, Z.-M. Su, Z. An and W. Huang, Utilizing d–pπ Bonds for Ultralong Organic Phosphorescence, Angew. Chem., Int. Ed., 2019, 58, 6645–6649 CrossRef CAS PubMed; (e) J. Yang, X. Zhen, B. Wang, X. Gao, Z. Ren, J. Wang, Y. Xie, J. Li, Q. Peng, K. Pu and Z. Li, The Influence of the Molecular Packing on the Room Temperature Phosphorescence of Purely Organic Luminogens, Nat. Commun., 2018, 9, 840 CrossRef PubMed.
- For recent examples, see.
(a) K. Andoh, M. Murai, P.-A. Bouit, M. Hissler and S. Yamaguchi, Dithieno[3,2-b;
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2’,3’-f]phosphepinium-Based Near-Infrared Fluorophores: px–π* Conjugation Inherent to Seven-Membered Phosphacycles, Angew. Chem., Int. Ed., 2024, 136, e202410204 CrossRef;
(b) N. Inai, S. Yamaguchi and T. Yanai, Theoretical Insight into the Effect of Phosphorus Oxygenation on Nonradiative Decays: Comparative Analysis of P-Bridged Stilbene Analogs, ACS Phys. Chem. Au, 2023, 3, 540–552 CrossRef CAS PubMed;
(c) N. König, Y. Godínez-Loyola, H. Weiske, S. Naumov, P. Lönnecke, R. Tonner-Zech and C. A. Strassert, Evamarie Hey-Hawkins, Access to Strong Thieno[3,2-b]phosphole-Based Solid-State Emitters via Manganese(III)-Mediated Oxidative Annulation, Chem. Mater., 2023, 35, 8218–8228 CrossRef;
(d) H. Ogasawara, Y. Tanaka, M. Taki and S. Yamaguchi, Late-Stage Functionalisation of Alkyne-Modified Phospha-Xanthene Dyes: Lysosomal Imaging Using an Off-On-Off Type of pH Probe, Chem. Sci., 2021, 12, 7902–7907 RSC;
(e) M. A. Gonzalez, A. S. Walker, K. J. Cao, J. R. Lazzari-Dean, N. S. Settineri, E. J. Kong, R. H. Kramer and E. W. Miller, Voltage Imaging with a NIR-Absorbing Phosphine Oxide Rhodamine Voltage Reporter, J. Am. Chem. Soc., 2021, 143, 2304–2314 CrossRef CAS PubMed;
(f) M. Grzybowski, M. Taki, K. Kajiwara and S. Yamaguchi, Effects of Amino Group Substitution on the Photophysical Properties and Stability of Near-Infrared Fluorescent P-Rhodamines, Chem. – Eur. J., 2020, 26, 7912–7917 CrossRef CAS PubMed.
2’,3’-f]phosphepinium-Based Near-Infrared Fluorophores: px–π* Conjugation Inherent to Seven-Membered Phosphacycles, Angew. Chem., Int. Ed., 2024, 136, e202410204 CrossRef;
(b) N. Inai, S. Yamaguchi and T. Yanai, Theoretical Insight into the Effect of Phosphorus Oxygenation on Nonradiative Decays: Comparative Analysis of P-Bridged Stilbene Analogs, ACS Phys. Chem. Au, 2023, 3, 540–552 CrossRef CAS PubMed;
(c) N. König, Y. Godínez-Loyola, H. Weiske, S. Naumov, P. Lönnecke, R. Tonner-Zech and C. A. Strassert, Evamarie Hey-Hawkins, Access to Strong Thieno[3,2-b]phosphole-Based Solid-State Emitters via Manganese(III)-Mediated Oxidative Annulation, Chem. Mater., 2023, 35, 8218–8228 CrossRef;
(d) H. Ogasawara, Y. Tanaka, M. Taki and S. Yamaguchi, Late-Stage Functionalisation of Alkyne-Modified Phospha-Xanthene Dyes: Lysosomal Imaging Using an Off-On-Off Type of pH Probe, Chem. Sci., 2021, 12, 7902–7907 RSC;
(e) M. A. Gonzalez, A. S. Walker, K. J. Cao, J. R. Lazzari-Dean, N. S. Settineri, E. J. Kong, R. H. Kramer and E. W. Miller, Voltage Imaging with a NIR-Absorbing Phosphine Oxide Rhodamine Voltage Reporter, J. Am. Chem. Soc., 2021, 143, 2304–2314 CrossRef CAS PubMed;
(f) M. Grzybowski, M. Taki, K. Kajiwara and S. Yamaguchi, Effects of Amino Group Substitution on the Photophysical Properties and Stability of Near-Infrared Fluorescent P-Rhodamines, Chem. – Eur. J., 2020, 26, 7912–7917 CrossRef CAS PubMed. - For recent examples, see. (a) M. Murai, M. Abe, S. Ogi and S. Yamaguchi, Diazulenylmethyl Cations with a Silicon Bridge: A π-Extended Cationic Motif to Form J-Aggregates with Near-Infrared Absorption and Emission, J. Am. Chem. Soc., 2022, 144, 20385–20393 CrossRef CAS PubMed; (b) J. B. Grimm, L. Xie, J. C. Casler, R. Patel, A. N. Tkachuk, N. Falco, H. Choi, J. Lippincott-Schwartz, T. A. Brown, B. S. Glick, Z. Liu and L. D. Lavis, A General Method to Improve Fluorophores Using Deuterated Auxochromes, JACS Au, 2021, 1, 690–696 CrossRef CAS PubMed; (c) T. Tsuda, S.-M. Choi and R. Shintani, Palladium-Catalyzed Synthesis of Dibenzosilepin Derivatives via 1,n-Palladium Migration Coupled with anti-Carbopalladation of Alkyne, J. Am. Chem. Soc., 2021, 143, 1641–1650 CrossRef CAS PubMed; (d) N. B. Alexey, Modular Synthetic Approach to Silicon-Rhodamine Homologues and Analogues via Bis-aryllanthanum Reagents, Org. Lett., 2021, 23, 2604–2609 CrossRef PubMed; (e) A. S. Moses, O. R. Taratula, H. Lee, F. Luo, T. Grenz, T. Korzun, A. St. Lorenz, F. Y. Sabei, S. Bracha, A. W. G. Alani, O. D. Slayden and O. Taratula, Nanoparticle-Based Platform for Activatable Fluorescence Imaging and Photothermal Ablation of Endometriosis, Small, 2020, 16, 1906936 CrossRef CAS PubMed; (f) M. Pengshung, P. Neal, T. L. Atallah, J. Kwon, J. R. Caram, S. A. Lopez and E. M. Sletten, Silicon Incorporation in Polymethine Dyes, Chem. Commun., 2020, 56, 6110–6113 RSC.
- (a) J. B. Grimm, A. N. Tkachuk, R. Patel, S. T. Hennigan, A. Gutu, P. Dong, V. Gandin, A. M. Osowski, K. L. Holland, Z. J. Liu, T. A. Brown and L. D. Lavis, Optimized Red-Absorbing Dyes for Imaging and Sensing, J. Am. Chem. Soc., 2023, 145, 23000–23013 CrossRef CAS PubMed; (b) S. O. Oloo, G. Zhang, P. Bobadova-Parvanova, S. Al Horani, M. Al Horani, F. R. Fronczek, K. M. Smith, M. da Graça and H. Vicente, Synthesis and Regioselective Functionalization of Tetrafluorobenzo-[α]-Fused BOPYPY Dyes, Inorg. Chem., 2024, 63, 9164–9174 CrossRef CAS PubMed; (c) Y. Hayashi, N. Suzuki, T. Maeda, H. Fujiwara and S. Yagi, Photophysical Properties of 4-(5-Methylthiophen-2-yl)pyridiniumcyclic Enolate Betaine Dyes Tuned by Control of Twisted Intramolecular Transfer, New J. Chem., 2021, 42, 9770–9779 RSC; (d) T. D. Moseev, M. V. Varaksin, D. A. Gorlov, V. N. Charushin and O. N. Chupakhin, Transition-Metal-Free C-H/C-Li Coupling of Nonaromatic 2H-Imidazole 1-Oxides with Pentafluorophenyl Lithium in the Design of Novel Fluorophores with Intramolecular Charge Transfer Effect, J. Org. Chem., 2020, 85, 11124–11133 CrossRef CAS PubMed; (e) K. Santra, I. Geraskin, M. Nilsen-Hamilton, G. A. Kraus and J. W. Petrich, Characterization of the Photophysical Behavior of DFHBI Derivatives: Fluorogenic Molecules that Illuminate the Spinach RNA Aptamer, J. Phys. Chem. B, 2019, 123, 2536–2545 CrossRef CAS PubMed; (f) R. III Chavez, L. Diodati, D. L. Wheeler, J. Shaw, A. L. Tomlinson and M. Jeffries-El, Evaluating the Impact of Fluorination on the Electro-optical Properties of Cross-Conjugated Benzobisoxazoles, J. Phys. Chem. A, 2019, 123, 1343–1352 CrossRef PubMed.
- For recent examples, see. (a) Y. Chang, Y.-S. Wu, S.-H. Tung, W.-C. Chen, C.-C. Chueh and C.-L. Liu, ACS Appl. Mater. Interfaces, 2023, 15, 15745–15757 CrossRef CAS PubMed; (b) K. Iguchi, T. Mikie, M. Saito, K. Komeyama, T. Seo, Y. Ie and I. Osaka, N-type Semiconducting Polymers Based on Dicyano Naphthobisthiadiazole: High Electron Mobility with Unfavorable Backbone Twist, Chem. Mater., 2021, 33, 2218–2228 CrossRef CAS; (c) K. Ortstein, S. Hutsch, A. Hinderhofer, J. Vahland, M. Schwarze, S. Schellhammer, M. Hodas, T. Geiger, H. Kleemann, H. F. Bettinger, F. Schreiber, F. Ortmann and K. Leo, Energy Level Engineering in Organic Thin Films by Tailored Halogenation, Adv. Funct. Mater., 2020, 30, 2002987 CrossRef CAS; (d) H. Wei, Y. Liu, Z. Liu, J. Guo, P.-A. Chen, X. Qiu, G. Dai, Y. Li, J. Yuan, L. Liao and Y. Hu, Effect of Backbone Fluorine and Chlorine Substitution on Charge-Transport Properties of Naphthalenediimide-Based Polymer, Semiconductors, Adv. Electron. Mater., 2020, 6, 1901241 CrossRef CAS; (e) T. Hodsden, K. J. Thorley, J. Panidi, A. Basu, A. V. Marsh, H. Dai, A. J. P. White, C. Wang, W. Mitchell, F. Glöcklhofer, T. D. Anthopoulos and M. Heeney, Core Fluorination Enhances Solubility and Ambient Stability of an IDT-Based n-Type Semiconductor in Transistor Devices, Adv. Funct. Mater., 2020, 30, 2000325 CrossRef CAS; (f) A. Y. Sosorev, V. A. Trukhanov, D. R. Maslennikov, O. V. Borshchev, R. A. Polyakov, M. S. Skorotetcky, N. M. Surin, M. S. Kazantsev, D. I. Dominskiy, V. A. Tafeenko, S. A. Ponomarenko and D. Y. Paraschuk, Fluorinated Thiophene-Phenylene Co-Oligomers for Optoelectronic Devices, ACS Appl. Mater. Interfaces, 2020, 12, 9507–9519 CrossRef CAS PubMed; (g) K. Yamamoto, S. Kato, H. Zajaczkowska, T. Marszalek, P. W. M. Blom and Y. Ie, Effects of Fluorine Substitution in Quinoidal Oligothiophenes for Use as Organic Semiconductors, J. Mater. Chem. C, 2020, 8, 3580–3588 RSC.
- For recent examples, see. (a) Z. Jia, S. Qin, L. Meng, Q. Ma, I. Angunawela, J. Zhang, X. Li, Y. He, W. Lai, N. Li, H. Ade, C. J. Brabec and Y. Li, High performance tandem organic solar cells via a strongly infrared-absorbing narrow bandgap acceptor, Nat. Commun., 2021, 12, 178 CrossRef CAS PubMed; (b) H. Ji, J. Li, M. Du, J. Yang, A. Tang, G. Li, Q. Guo and E. Zhou, Fluorination of the Quinoxaline-Based p-Type Polymer and n-Type Small Molecule for High VOC Organic Solar Cells, J. Phys. Chem. C, 2021, 125, 10876–10882 CrossRef CAS; (c) G.-U. Kim, C. Sun, J. S. Park, H. G. Lee, D. Lee, J.-W. Lee, H. J. Kim, S. Cho, Y.-H. Kim, S.-K. Kwon and B. J. Kim, Importance of Terminal Group Pairing of Polymer Donor and Small-Molecule Acceptor in Optimizing Blend Morphology and Voltage Loss of High-Performance Solar Cells, Adv. Funct. Mater., 2021, 31, 2100870 CrossRef CAS; (d) Y. Firdaus, V. M. Le Corre, S. Karuthedath, W. Liu, A. Markina, W. Huang, S. Chattopadhyay, M. M. Nahid, M. I. Nugraha, Y. Lin, A. Seitkhan, A. Basu, W. Zhang, I. McCulloch, H. Ade, J. Labram, F. Laquai, D. Andrienko, L. J. A. Koster and T. D. Anthopoulos, Long-Range Exciton Diffusion in Molecular Non-Fullerene Acceptors, Nat. Commun., 2020, 11, 5220 CrossRef CAS PubMed; (e) J. Yao, B. Qiu, Z.-G. Zhang, L. Xue, R. Wang, C. Zhang, S. Chen, Q. Zhou, C. Sun, C. Yang, M. Xiao, L. Meng and Y. Li, Cathode Engineering with Perylene-Diimide Interlayer Enabling over 17% Efficiency Single-Junction Organic Solar Cells, Nat. Commun., 2020, 11, 2726 CrossRef CAS PubMed; (f) A. D. Fenta, S.-F. Liao, S.-W. Li, C.-F. Lu and C.-T. Chen, Increasing the Fluorine Substituent of Thieno[3,4-c]pyrrole-4,6-dione Terthiophene Copolymers Progressively Narrows the Nanofibrils and Enhances the Efficiency of Fullerene-Based Polymer Photovoltaics, Macromolecules, 2020, 53, 7073–7083 CrossRef CAS; (g) Q. Nie, A. Tang, P. Cong, L. Chen, Q. Zhang, H. Ji, G. Li, Q. Guo and E. Zhou, Wide Band Gap Photovoltaic Polymer Based on Pyrrolo[3,4-f]benzotriazole-5,7-dione (TzBI) with Ultrahigh VOC Beyond 1.25 V, J. Phys. Chem. C, 2020, 124, 19492–19498 CrossRef CAS; (h) P. Lei, B. Zhang, Y. Chen, Y. Geng, Q. Zeng, A. Tang and E. Zhou, Gradual Fluorination on the Phenyl Side Chains for Benzodithiophene-Based Linear Polymers to Improve the Photovoltaic Performance, ACS Appl. Mater. Interfaces, 2020, 12, 38451–38459 CrossRef CAS PubMed; (i) S. Wen, Y. Li, N. Zheng, I. O. Raji, C. Yang and X. Bao, High-efficiency organic solar cells enabled by halogenation of polymers based on 2D conjugated benzobis(thiazole), J. Mater. Chem. A, 2020, 8, 13671–13678 RSC; (j) H. Jiang, X. Li, H. Wang, G. Huang, W. Chen, R. Zhang and R. Yang, Appropriate Molecular Interaction Enabling Perfect Balance Between Induced Crystallinity and Phase Separation for Efficient Photovoltaic Blends, ACS Appl. Mater. Interfaces, 2020, 12, 26286–26292 CrossRef CAS PubMed; (k) Y. Wang, H. Zhong, Y. Hong, T. Shan, K. Ding, L. Zhu, F. Liu, H. Wei, C. Yu and H. Zhong, Tailoring the Molecular Geometry of Polyfluoride Perylene Diimide Acceptors towards Efficient Organic Solar Cells, J. Mater. Chem. C, 2020, 8, 8224–8233 RSC; (l) T. Liu, Y. Zhang, Y. Shao, R. Ma, Z. Luo, Y. Xiao, T. Yang, X. Lu, Z. Yuan, H. Yan, Y. Chen and Y. Li, Asymmetric Acceptors with Fluorine and Chlorine Substitution for Organic Solar Cells toward 16.83% Efficiency, Adv. Funct. Mater., 2020, 30, 2000456 CrossRef CAS.
- For recent examples, see. (a) R. Kani, Y. Miwa, Y. Kubota, T. Inuzuka, S. Kutsumizu and K. Funabiki, Rapid and Dual Optical CO2-Responsive Polydimethylsiloxane Elastomer with Fluorinated Cyanine Dye, Chem. – Asian J., 2023, 18, e20230170 CrossRef PubMed; (b) R. Kani, Y. Kubota, T. Inuzuka and K. Funabiki, Aromatic Fluorine Atom-Induced Highly Amine-Sensitive Trimethine Cyanine Dye Showing Colorimetric and Ratiometric Fluorescence Change, RSC Adv., 2022, 12, 25587–25592 RSC.
- P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, 2013 Search PubMed; K. Uneyama, Organofluorine Chemistry, Blackwell Pubs. Oxford, 2006 Search PubMed; T. Hiyama, Organofluorine Compounds: Chemistry and Applications, Springer, Berlin, 2007 Search PubMed.
- (a) H. Ma, Y. Lu, Z. Huang, S. Long, J. Cao, Z. Zhang, X. Zhou, C. Shi, W. Sun, J. Du, J. Fan and X. Peng, ER-Targeting Cyanine Dye as an NIR Photoinducer to Efficiently Trigger Photoimmunogenic Cancer Cell Death, J. Am. Chem. Soc., 2022, 144, 3477–3486 CrossRef CAS PubMed; (b) F. Niedermair, S. M. Borisov, G. Zenkl, O. T. Hofmann, H. Weber, R. Saf and I. Klimant, Tunable Phosphorescent NIR Oxygen Indicators Based on Mixed Benzo- and Naphthoporphyrin Complexes, Inorg. Chem., 2010, 49, 9333–9342 CrossRef CAS PubMed.
- (a) M. Henary and W. D. Wilson, Carbocyanines for G-Quadruplex DNA Stabilization and Telomerase Inhibition, WO2013012886 A1 2013-01-24 Search PubMed; (b) M. E. Cooper, N. J. Gardner and P. G. Laughton, Water Soluble Fluoro-Substituted Cyanine Dyes as Reactive Fluorescence Labelling Reagents, WO2006111726 A1 2006-04-22 Search PubMed.
- (a) M. Eskandari, J. C. Roldao, J. Cerezo, B. Milian-Medina and J. Gierschner, Counterion-Mediated Crossing of the Cyanine Limit in Crystals and Fluid Solution: Bond Length Alternation and Spectral Broadening Unveiled by Quantum Chemistry, J. Am. Chem. Soc., 2020, 142, 2835–2843 CrossRef CAS PubMed; (b) P. A. Bouit, C. Aronica, L. Toupet, B. Le Guennic, C. Andraud and O. Maury, Continuous Symmetry Breaking Induced by Ion Pairing Effect in Heptamethine Cyanine Dyes: Beyond the Cyanine Limit, J. Am. Chem. Soc., 2010, 132, 4328–4335 CrossRef CAS PubMed; (c) W. Rettig and M. Dekhtyar, Merocyanines: Polyene-Polymethine Transition in Donor-Acceptor-Substituted Stilbenes and Polyenes, Chem. Phys., 2003, 293, 75–90 CrossRef CAS; (d) L. M. Tolbert and X. Zhao, Beyond the Cyanine Limit: Peierls Distortion and Symmetry Collapse in a Polymethine Dye, J. Am. Chem. Soc., 1997, 119, 3253–3258 CrossRef CAS.
- https://www.olexsys.org/olex2/ .
- K. Funabiki, Y. Saito, T. Kikuchi, K. Yagi, Y. Kubota, T. Inuzuka, Y. Miwa, M. Yoshida, O. Sakurada and S. Kutsumizu, Aromatic Fluorine-Induced One-pot Synthesis of Ring-Perfluorinated Trimethine Cyanine Dye and Its Remarkable Fluorescence Properties, J. Org. Chem., 2019, 84, 4372–4380 CrossRef CAS PubMed.
- T. Zimmermann and O. Brede, 3H-Indolium Salts Efficiently Prepared from N-Substituted Anilines and α-Branched Ketones by An One-Pot Synthesis, J. Heterocycl. Chem., 2004, 41, 103–108 CrossRef CAS.
- J.-W. Yan, J.-Y. Zhu, K.-X. Zhou, J.-S. Wang, H.-Y. Tan, Z.-Y. Xu, S.-B. Chen, Y.-T. Lu, M.-C. Cui and L. Zhang, Neutral Merocyanine Dyes: for in vivo NIR Fluorescence Imaging of Amyloid-β Plaques, Chem. Commun., 2017, 53, 9910–9913 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1987380 and 1987381. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ma00962b |
| This journal is © The Royal Society of Chemistry 2024 |