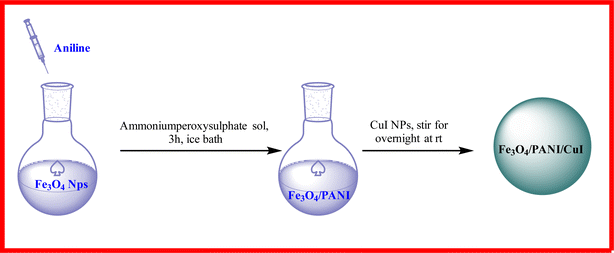
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fe3O4/PANI/CuI as a sustainable heterogeneous nanocatalyst for A3 coupling†
Nisha
a,
Sahil
Kohli
bg,
Snigdha
Singh
a,
Neera
Sharma
*c and
Ramesh
Chandra
 *adef
*adef
aDrug Discovery & Development Laboratory, Department of Chemistry, University of Delhi, Delhi-110007, India. E-mail: acbrdu@hotmail.com
bDepartment of Chemistry, School of Basic Sciences, Galgotias University, Greater Noida-203201, Uttar Pradesh, India
cDepartment of Chemistry, Hindu College, University of Delhi, Delhi-110019, India
dDr. B. R. Ambedkar Centre for Biomedical Research (ACBR), University of Delhi, Delhi-110007, India
eInstitute of Nanomedical Science (INMS), University of Delhi, Delhi-110007, India
fMaharaja Surajmal Brij University, Bharatpur-321201, Rajasthan, India
gManav Rachna International Institute of Research & Studies, Faridabad, Haryana-121004, India
First published on 22nd July 2024
Abstract
The prepared copper iodide nanoparticles were impregnated on the support of ferrite nanoparticles functionalized with polyaniline, resulting in a magnetically recoverable heterogeneous nanocomposite. The activity of the prepared nanocomposite was investigated in the synthesis of propargylamine derivatives via A3 coupling under mild conditions. Techniques such as FESEM, EDAX, XRD, XPS, TEM, BET and FTIR were used to characterize the effective and unique heterogeneous Fe3O4/PANI/CuI nanocomposite developed in this work. This method used in the current study has several advantages, including a short reaction time, neat conditions, good product yield, ideal green matrices values, reusability for up to seven cycles, and magnetic retrievability.
Introduction
Magnetic nanoparticles (MNPs) are of great importance due to their various applications in catalysis, magnetic resonance imaging (MRI), magnetic fluids, and biotechnology.1 MNPs can serve as magnetically recoverable catalysts for a variety of catalytic reactions because of their insoluble and paramagnetic character, which allows for easy separation from the reaction medium. Moreover, magnetic separation has evolved into one of the most significant and well-known catalytic methods in organic chemistry without the need for filtering, centrifugation, or other laborious workup procedures, simply by using an external magnet.2 However, bare MNPs have some limitations such as the tendency to easily agglomerate, colloidal instability, and dissolution in acids.3 The colloidal instability of MNPs leads to their agglomeration due to magnetic dipole–dipole interaction.4,5 This issue can be resolved by surface functionalization of MNPs using protective shells or coatings such as silica, carbon, or organic polymers.3 Furthermore, these coatings allow the covalent attachment of organic compounds on distinct nanoparticles, facilitating applications such as drug carriers, heterogeneous catalysis, and absorption media.3 The magnetic nature of nanoparticles permits facile recovery of nanocatalysts from reaction mixtures through magnets.5One of the polymeric shells synthesized via oxidative polymerization is polyaniline (PANI).6 The choice of polyaniline is because of its various properties such as facileness of synthesis, conductivity as a polymer, low cost, and a porous structure that can enhance the catalytic activity of nanoparticles. The Fe3O4/PANI hybrid shell can be considered a multifunctional support for metal nanocatalysts with significant catalytic performance.7
The benefits of a metal nanoparticle supported on nano-size heterogeneous material include good selectivity, minimal accumulation of metal nanoparticles, high dispersion in a liquid medium, and excellent reusability.8 Copper-based nanoparticles not only enhance the physicochemical characteristics of the nanoparticles but also reinforce the interface between the metal and the support.9 Cu-based nanocatalysts have abundant applications in nanotechnology due to their special properties and features such as catalysing organic transformations, electrocatalysis, and photocatalysis.10 Supported copper nanoparticles, such as CuO/NiO,9 CuO/Al2O3,11 ZnO/CuI/PPy,12 and Cu–MgO,13 have been used in many organic transformations. Copper-based nanocatalysts are found to be useful in various reactions including C–H activation of alkynes, oxygen arylation reaction, Suzuki reaction, Click reaction, Knoevenagel condensation-Michael addition cyclization reaction, Heck reaction and producing copper-acetylated species in situ to afford propargylamines.14
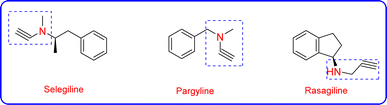
Propargylamines are crucial building blocks for organic synthesis because they can be utilized as synthetic precursors for synthesizing various medicinally essential compounds.8 Propargylamines are formed via a three-component reaction known as A3 coupling, which comprises a terminal alkyne, an aldehyde, and an amine.15 Moreover, a variety of propargylamines have been used to cure neuropsychiatric conditions like anxiety, Parkinson's disease, and depression.16 Various approved drugs, such as pargyline, selegiline, and rasagiline (Fig. 1), have a propargylamine scaffold.8,17 Late transition metals such as Au, Cu, and Ag are used to catalyse A3 reactions via one-pot synthesis.18
Over the past two decades, a catalytic variation of A3 coupling has attracted chemists' attention15 A3 coupling catalysed by various nanocatalysts such as Fe3O4@R. tinctorum/Ag,19 Cu/C,16 Fe3O4@SiO2@DNHCS-Tr@CuI,20 Au nanoparticles,21 Fe3O4–MoO3 (ref. 22) and CuO/GNS23 have been reported. However, these methods involve use of harmful reagents, prolonged reaction time, use of additives and costly reagents. Hence, there is a need for a sustainable heterogeneous nanocatalyst for the facile synthesis of propargylamines via A3 coupling.
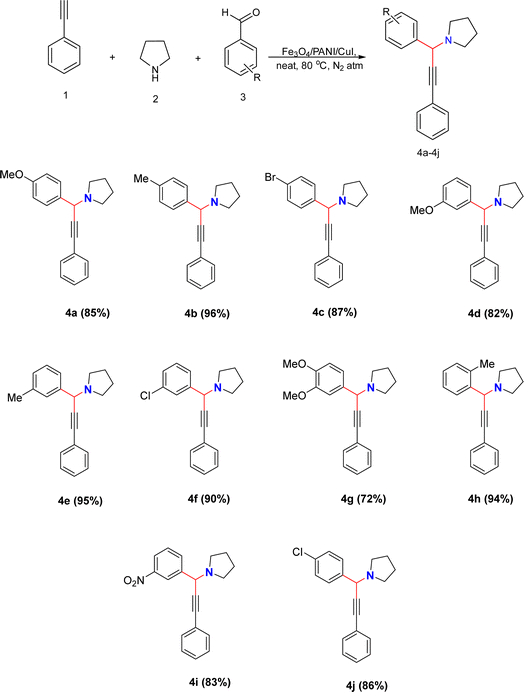
In the current work, we successfully develop a novel heterogeneous nanocatalyst, Fe3O4/PANI/CuI, for the synthesis of propargylamines via A3 coupling using pyrrolidine, phenylacetylene, and different benzaldehydes under neat conditions at 80 °C in a N2 atmosphere. The reaction was completed in 10 min with a high yield of the desired product. The fabricated nanocatalyst was easily recoverable and reusable with high catalytic efficiency for the synthesis of propargylamines.
Results and discussion
Synthesis and characterisation
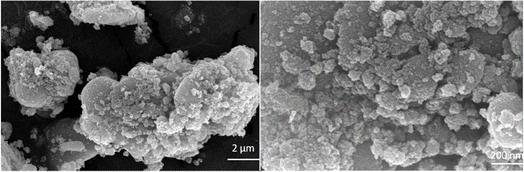
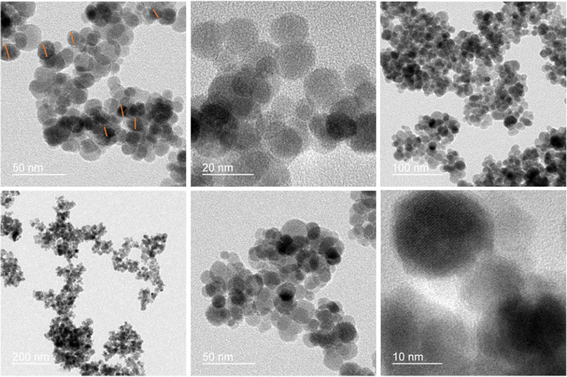
The field-emission scanning electron microscopy (FESEM) technique reveals the spherical morphology of the nanocomposite (Fig. 4). The transmission electron microscopy (TEM) analysis of the Fe3O4/PANI/CuI nanocomposite indicates that CuI is well embedded over the core–shell structure of Fe3O4 nanoparticles and the average size of nanoparticles is 42.6 nm, as shown in Fig. 5.
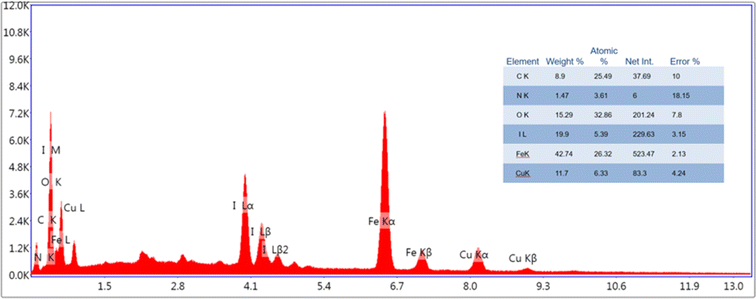
The energy-dispersive X-ray analysis of the Fe3O4/PANI/CuI nanocatalyst revealed the presence of iron (42.74 wt%), nitrogen (1.47 wt%), copper (11.7 wt%), oxygen (15.29 wt%), iodine (19.9 wt%), and carbon (8.9 wt%), as can be seen in Fig. 6.
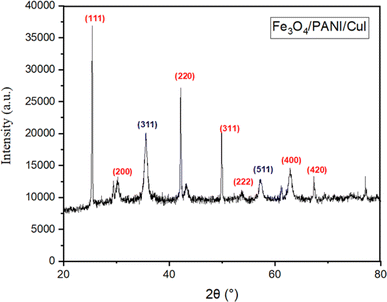
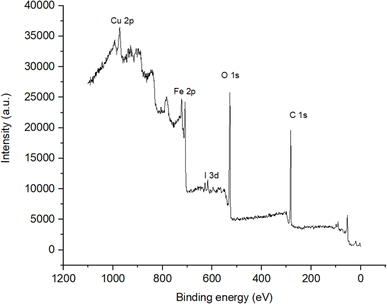
Fig. 7 illustrates the X-ray photoelectron spectra (XPS) of the Fe3O4/PANI/CuI nanocomposite. The spectra revealed the presence of Cu 2p1/2 and Cu 2p3/2 with binding energies at 952.88 and 932.44 eV, respectively, and the presence of I 3d3/2 and 3d5/2 with binding energies at 631 and 619.23 eV, respectively. The values for copper and iodine resemble the reported binding energy values of CuI, which confirm the +1 oxidation state of copper in the nanocomposite.27 The peak at 284.78 eV corresponds to the binding energy value of C 1s. The values of binding energies at 724.53 and 710.63 eV resemble the reported values of Fe 2p3/2 and Fe 2p1/2, respectively, while the peak at 530.17 eV corresponds to O 1s, confirming the presence of Fe3O4 in the nanocomposite. The broadness of the iron peaks indicates the presence of both oxidation states (Fe2+ and Fe3+) in Fe3O4.28
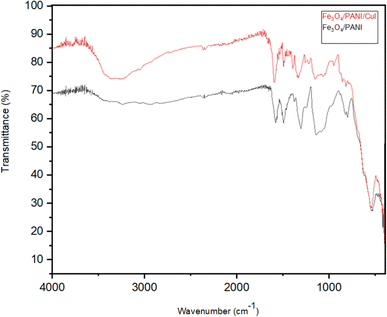
Fig. 8 shows the FTIR spectrum of Fe3O4/PANI/CuI; it depicts a peak at 3311 cm−1, which is attributed to the presence of the surface OH group in the nanocomposite.29 The peaks at 1598 and 1494 cm−1 are attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of a quinoid and benzenoid ring, respectively.30 A peak that appeared at 1374 cm−1 is similarly typical of polyaniline and is considered to be a consequence of C–N stretching vibrations near a quinonoid ring.31 The peak at 1161 cm−1 is due to the C–N stretching vibration.30 The peak at 553 cm−1 is the characteristic peak of ferrite nanoparticles.30
C stretching vibrations of a quinoid and benzenoid ring, respectively.30 A peak that appeared at 1374 cm−1 is similarly typical of polyaniline and is considered to be a consequence of C–N stretching vibrations near a quinonoid ring.31 The peak at 1161 cm−1 is due to the C–N stretching vibration.30 The peak at 553 cm−1 is the characteristic peak of ferrite nanoparticles.30
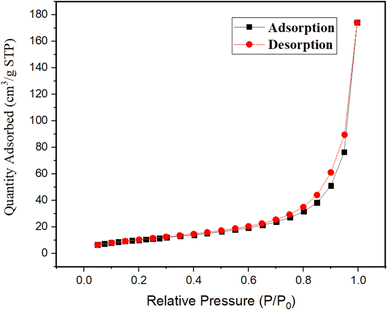
N2-Adsorption desorption isotherm was collected using the Brunauer–Emmett–Teller (BET) technique, which is portrayed as a H3 hysteresis loop of isotherm and shows a surface area of 38.471 m2 g−1, pore radius of 2.16 nm, and pore volume of 0.076 cm3 g−1 (Fig. 9).
Fe3O4/PANI/CuI as heterogeneous nanocatalysts for the synthesis of propargylamine derivatives
We synthesized propargylamine derivatives using Fe3O4/PANI/CuI to investigate its catalytic properties in organic transformations (Scheme 1). For optimization, a model reaction was performed involving phenylacetylene (1), pyrrolidine (2), and 4-methyl benzaldehyde (3) using the nanocatalyst in various solvents or under neat conditions at 80 °C in a nitrogen atmosphere for the preparation of the desired product 4b, as shown in Table 1. We examined how different catalyst loading amounts, solvent concentrations, and temperatures affected the reaction kinetics, as presented in Table 1. Initially, the model reaction was performed in toluene (Table 1, entry 1), resulting in a 47% yield of the product. Subsequently, the reaction was carried out in polar aprotic solvents such as THF, acetonitrile, DMSO, and DMF. The desired product did not form in both acetonitrile and THF (Table 1, entries 2 and 3). However, the product was obtained with a 40% yield in DMF (Table 1, entry 4). The reaction was then monitored in environmentally friendly solvents such as ethanol, water, and ethylene glycol (EG), yielding no product in water (Table 1, entry 5), trace amounts of product in ethanol, and 30% product yield in EG (Table 1, entry 7). The product was isolated in good yield in neat conditions (Table 1, entry 8). By altering the catalyst loading, the % yield was found to remain unchanged on lowering or increasing the catalyst amount respectively (Table 1, entries 9 and 10). Further, we studied the influence of temperature on development of reaction. On raising the temperature, there was no change in product yield (Table 1 entry 11), while on decreasing the temperature, there was a reduction in the product yield (Table 1, entry 12).| S. no. | Nanocatalyst (mg) | Solvent | Temp. (°C) | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: catalyst (5–20 mg), 1 (1.0 mmol), 2 (1 mmol), 3 (1 mmol), solvent (2–3 mL), N2 atm, 80 °C, 10 min. | |||||
| 1 | Fe3O4/PANI/CuI (10) | Toluene | 80 | 10 | 47 |
| 2 | Fe3O4/PANI/CuI (10) | CH3CN | 80 | 10 | — |
| 3 | Fe3O4/PANI/CuI (10) | THF | 80 | 10 | — |
| 4 | Fe3O4/PANI/CuI (10) | DMF | 80 | 10 | 40 |
| 5 | Fe3O4/PANI/CuI (10) | Water | 80 | 10 | — |
| 6 | Fe3O4/PANI/CuI (10) | Ethanol | 80 | 10 | Trace |
| 7 | Fe3O4/PANI/CuI (10) | EG | 80 | 10 | 30 |
| 8 | Fe3O4/PANI/CuI (10) | Neat | 80 | 10 | 96 |
| 9 | Fe3O4/PANI/CuI (5) | Neat | 80 | 10 | 53 |
| 10 | Fe3O4/PANI/CuI (20) | Neat | 80 | 10 | 96 |
| 11 | Fe3O4/PANI/CuI (10) | Neat | 110 | 10 | 96 |
| 12 | Fe3O4/PANI/CuI (10) | Neat | 50 | 10 | 22 |
| 13 | CuI (10) | Neat | 80 | 10 | 41 |
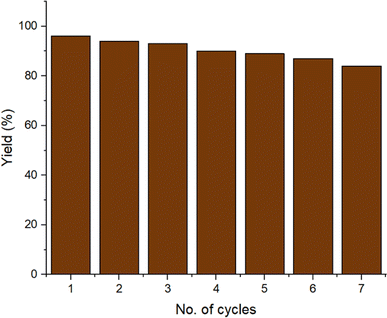
Under optimized conditions, we examined the recyclability of the catalyst to produce the product 4b. Once the reaction was completed, the catalyst was recovered from the reaction using a magnet and then washed many times with water and ethanol before being dried in the oven. The recovered catalyst was then used for seven cycles (Fig. 10). The stability of the recycled catalyst after seven cycles was confirmed by XRD, SEM, FTIR, EDAX and TEM, which confirmed that there was no change in the activity and morphology of the catalyst (ESI Fig. S1–S5†). An ICP study of the filtrate was done after catalyst recovery and showed the leached metal concentrations of copper and iron ion to be 2.08 and 0.12 ppm, respectively, which are lower than the authentic values of the respective ions according to WHO terms.32
The existing methodology demonstrates sustainability and eco-friendliness, as evidenced by the green metrics values, as shown in Table 2 (refer to calculations in the ESI†), which closely approach the ideal values.
| Catalyst | Reaction mass efficiency | E-Factor | Process mass intensity | Carbon efficiency |
|---|---|---|---|---|
| Fe3O4/PANI/CuI | 90% | 0.10 | 1.10 | 96% |
Table 3 provides a summary of the literature review, listing the previously established methods for producing propargyl derivatives, including the reaction conditions and corresponding yields.
| S. no. | Nanocatalyst | Reaction conditions | Time | % Yield | Ref. |
|---|---|---|---|---|---|
| 1 | Fe3O4@SiO2–Se-T/CuI | Neat, 80 °C | 2 h | 95 | 33 |
| 2 | ZSM-5/APTMS/(E)-4-((pyridine-2-ylimino)methyl)benzaldehyde/Cu-NPs | K2CO3, H2O, 60 °C | 2 h | 94 | 34 |
| 3 | UIO-66-NH2G1@PdNPs | Toluene, N2 gas, 110 °C | 3 h | 93 | 35 |
| 4 | [Fe3O4@bisimidazolium-Pd]2Cl− | PEG-400, 100 °C | 2 h | 98 | 36 |
| 5 | Fe3O4@starch-Acr@Cu(II) | H2O, reflux | 35 min | 99 | 37 |
| 6 | g-C3N4-TCT-2AEDSEA-Ag-Cu-Ni | Toluene, 80 °C | 8 h | 91 | 38 |
| 7 | Fe3O4@SiO2-di-(pyridin-2-yl)amine-Cu | H2O, reflux | 2 h | 99 | 39 |
| 8 | Co2+-Cu@SA(0)-600 | Toluene, 110 °C | 1 h | 89 | 40 |
| 9 | MMT-K10/Fe3O4/CuO | Toluene, 80 °C | 8 h | 91 | 41 |
| 10 | o-Cu2O-PVP | Neat, 100 °C | 5 min | 80 | 42 |
| 11 | Fe3O4/PANI/CuI | Neat, 80 °C | 10 min | 96 | Our work |
The plausible mechanism for the synthesis of propargylamine via A3 coupling catalysed by the Fe3O4/PANI/CuI nanocomposite is shown in Fig. 11. The copper-based nanocatalyst activates the phenylacetylene ring and proceeds through an attack on the carbon of the iminium ion, which is formed from the aldehyde and amine and results in the formation of the desired product as well as catalyst regeneration.33,39
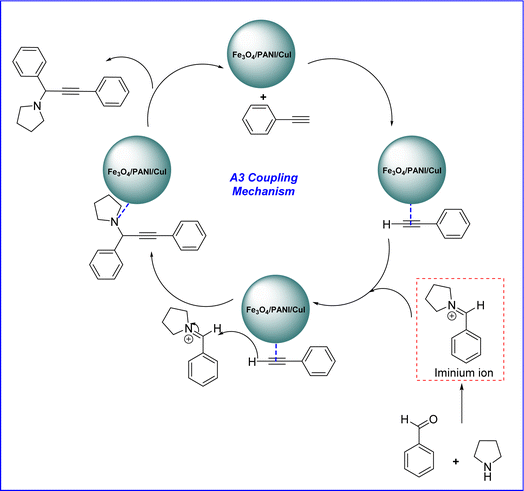 | ||
| Fig. 11 Mechanism for Fe3O4/PANI/CuI catalysed synthesis of propargylamine derivative via A3 coupling. | ||
General procedure for the synthesis of propargyl derivatives
In general, a mixture of phenylacetylene (1 mmol), pyrrolidine (1 mmol), aromatic aldehyde (1 mmol), and catalyst (10 mg) was added to a 50 mL round-bottom flask and stirred continuously at 80 °C. TLC was used to monitor the progress of the reaction. After the completion of the reaction, the reaction mixture was cooled and diluted with ethyl acetate, and the catalyst was separated with the aid of a magnet. The crude product was extracted with ethyl acetate and purified by column chromatography using basic alumina as a stationary phase and ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane as an eluent. The obtained pure product was confirmed by 1H and 13C NMR spectroscopy.
hexane as an eluent. The obtained pure product was confirmed by 1H and 13C NMR spectroscopy.
Conclusion
In summary, we have developed a sustainable heterogeneous copper-based magnetic nanocatalyst for the one-pot synthesis of propargylamine derivatives under solvent-free conditions with a short reaction time. The designed nanocatalyst is easily magnetically recoverable and can be recycled for up to seven runs without any drastic reduction in product yield. This protocol provides a shorter reaction time to obtain products with high yield and good catalytic activity under mild reaction conditions as compared to previously reported methods.Data availability
The data that support the findings of this study are available from the corresponding author following reasonable request.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
RC and SS acknowledges the Institution of Eminence at the University of Delhi and the Institute of Nanomedical Science (INMS) for their assistance. Nisha is obliged to USIC, University of Delhi for instrumental facilities and CSIR for awarding her a Junior Research Fellowship (09/045(1792)/2020-EMR-I). RC and SS are thankful to Indo-Russia DSTRFBR: INT/RUS/RFBR/389 and SS is thankful to SERB-TARE: TAR/2022/000618 for their financial assistance.References
- A. H. Lu, E. E. Salabas and F. Schuth, Magnetic nanoparticles: synthesis, protection, functionalization, and application, Angew. Chem., Int. Ed., 2007, 46(8), 1222–1244 CrossRef CAS PubMed.
- M. Abedi, M. Hosseini, A. Arabmarkadeh and M. Kazemi, Magnetic nanocatalysts in A3-coupling reactions, Synth. Commun., 2021, 51(6), 835–855 CAS.
- T. E. Saraswati, A. Ogino and M. Nagatsu, Plasma-activated immobilization of biomolecules onto graphite-encapsulated magnetic nanoparticles, Carbon, 2012, 50(3), 1253–1261 CrossRef CAS.
- S. P. Yeap, J. Lim, B. S. Ooi and A. L. Ahmad, Agglomeration, colloidal stability, and magnetic separation of magnetic nanoparticles: collective influences on environmental engineering applications, J. Nanopart. Res., 2007, 19(11), 1–15 Search PubMed.
- (a) R. A. Bohara, N. D. Thorat and S. H. Pawar, Role of functionalization: strategies to explore potential nano-bio applications of magnetic nanoparticles, RSC Adv., 2016, 6(50), 43989–44012 RSC; (b) S. Singh, T. Goel, A. Singh, H. Chugh, N. Chakraborty, I. Roy, M. Tiwari and R. Chandra, Synthesis and characterization of Fe3O4@SiO2@PDA@Ag core–shell nanoparticles and biological application on human lung cancer cell line and antibacterial strains, Artif. Cells, Nanomed., Biotechnol., 2024, 52(1), 46–58 CrossRef CAS PubMed; (c) M. Kazemi and M. Mohammadi, Magnetically recoverable catalysts: catalysis in synthesis of polyhydroquinolines, Appl. Organomet. Chem., 2020, 34(3), e5400 CrossRef CAS.
- (a) J. P. L. Morais, D. V. Bernardino, B. d. S. Batista, W. O. Pereira, F. M. B. Amaral, M. C. M. P. Branca and F. P. Gasparin, Conductive polymer blend based on polyaniline and galactomannan: optical and electrical properties, Synth. Met., 2023, 295, 117346 CrossRef CAS; (b) S. Kohli, G. Rathee, S. Hooda and R. Chandra, Al2O3/CuI/PANI nanocomposite catalyzed green synthesis of biologically active 2-substituted benzimidazole derivatives, Dalton Trans., 2021, 50(22), 7750–7758 RSC.
- (a) J. Chenjing, H. Jie, C. Fangyuan, W. Xiaoxia and G. Rong, Fe3O4@PANI hybrid shell as a multifunctional support for Au nanocatalysts with a remarkably improved catalytic performance, Langmuir, 2017, 33, 4520–4527 CrossRef PubMed; (b) Nisha, S. Kohli, N. Sharma and R. Chandra, Development of ZnO/PANI/Ag nanocomposite for synthesis of bioactive xanthene-1, 8 (2H)-dione derivatives, Appl. Organomet. Chem., 2023, 37, e7049 CrossRef.
- (a) E. Zarenezhad, R. Taghavi, P. Kamrani, M. Farjam and S. Rostamnia, Gold nanoparticle decorated dithiocarbamate modified natural boehmite as a catalyst for the synthesis of biologically essential propargylamines, RSC Adv., 2022, 12(49), 31680–31687 RSC; (b) P. Munnik, P. E. De Jongh and K. P. De Jong, Recent developments in the synthesis of supported catalysts, Chem. Rev., 2015, 115(14), 6687–6718 CrossRef CAS PubMed; (c) M. Mohammadi, M. Khodamorady, B. Tahmasbi, K. Bahrami and A. Ghorbani-Choghamarani, Boehmite nanoparticles as versatile support for organic–inorganic hybrid materials: synthesis, functionalization, and applications in eco-friendly catalysis, J. Ind. Eng. Chem., 2021, 97, 1–78 CrossRef CAS; (d) X. Zheng, P. Li, S. Dou, W. Sun, H. Pan, D. Wang and Y. Li, Non-carbon-supported single-atom site catalysts for electrocatalysis, Energy Environ. Sci., 2021, 14(5), 2809–2858 RSC.
- M. Rawat and D. S. Rawat, CuO@NiO nanocomposite catalyzed synthesis of biologically active indenoisoquinoline derivatives, ACS Sustain. Chem. Eng., 2020, 36, 13701–13712 CrossRef.
- N. K. Ojha, G. V. Zyryanov, A. Majee, V. N. Charushin, O. N. Chupakhin and S. Santra, Copper nanoparticles as inexpensive and efficient catalyst: A valuable contribution in organic synthesis, Coord. Chem. Rev., 2017, 353, 1–57 CrossRef CAS.
- S. Khan, S. S. Shah, N. K. Janjua, A. B. Yurtcan, M. T. Nazir, K. M. Katubi and N. S. Alsaiari, Alumina supported copper oxide nanoparticles (CuO/Al2O3) as high-performance electrocatalysts for hydrazine oxidation reaction, Chemosphere, 2023, 137659 CrossRef CAS PubMed.
- S. Kohli, Nisha, G. Rathee, S. Hooda and R. Chandra, Exploring the untapped catalytic application of a ZnO/CuI/PPy nanocomposite for the green synthesis of biologically active 2,4,5-trisubstituted imidazole scaffolds, Nanoscale Adv., 2023, 5(8), 2352–2360 RSC.
- K. N. Patil, P. Manikanta, R. R. Nikam, P. M. Srinivasappa, A. H. Jadhav, H. P. Aytam, K. S. R. Rao and B. M. Nagaraja, Effect of precipitating agents on activity of co-precipitated Cu–MgO catalysts towards selective furfural hydrogenation and cyclohexanol dehydrogenation reactions, Results Eng., 2023, 17, 100851 CrossRef CAS.
- (a) S. Cao, B. Zou, J. Yang, J. Wang and H. Feng, Hollow CuO–CeO2 Nanospheres for an effectively catalytic annulation/A3-coupling reaction sequence, ACS Appl. Nano Mater., 2022, 5(8), 11689–11698 CrossRef CAS; (b) M. J. Ndolomingo, N. Bingwa and R. Meijboom, Review of supported metal nanoparticles: synthesis methodologies, advantages and application as catalysts, J. Mater. Sci., 2020, 55(15), 6195–6241 CrossRef CAS; (c) F. Ghobakhloo, D. Azarifar, M. Mohammadi, H. Keypour and H. Zeynali, Copper (II) Schiff-base complex modified UiO-66-NH2 (Zr) metal–organic framework catalysts for Knoevenagel condensation–Michael addition–cyclization reactions, Inorg. Chem., 2022, 61(12), 4825–4841 CrossRef CAS PubMed.
- B. V. Rokade, J. Barker and P. J. Guiry, Development of and recent advances in asymmetric A3 coupling, Chem. Soc. Rev., 2019, 48(18), 4766–4790 RSC.
- P. V. Rathod, J. M. C. Puguan and H. Kim, Solvent-free synthesis of propargyl amines via A3 coupling reaction and organic pollutant degradation in aqueous condition using Cu/C catalyst, Appl. Organomet. Chem., 2020, 34(12), e5986 CrossRef CAS.
- O. Weinreb, T. Amit, O. Bar-Am and M. B. Youdim, Rasagiline: a novel anti-Parkinsonian monoamine oxidase-B inhibitor with neuroprotective activity, Prog. Neurobiol., 2010, 92(3), 330–344 CrossRef CAS PubMed.
- J. Farhi, I. N. Lykakis and G. E. Kostakis, Metal catalysed A3 coupling methodologies: classification and visualization, Catalysts, 2022, 12, 660 CrossRef CAS.
- H. Veisi, L. Mohammadi, S. Hemmati, T. Tamoradi and P. Mohammadi, In situ immobilized silver nanoparticles on Rubia tinctorum extract-coated ultrasmall iron oxide nanoparticles: an efficient nanocatalyst with magnetic recyclability for synthesis of propargylamines by A3 coupling reaction, ACS Omega, 2019, 4(9), 13991–14003 CrossRef CAS.
- S. Peiman, R. Baharfar and R. Hosseinzadeh, CuI NPs immobilized on a ternary hybrid system of magnetic nanosilica, PAMAM dendrimer and trypsin, as an efficient catalyst for A3-coupling reaction, Res. Chem. Intermed., 2022, 48(4), 1365–1382 CrossRef CAS.
- M. Kidwai, V. Bansal, A. Kumar and S. Mozumdar, The first Au-nanoparticles catalyzed green synthesis of propargylamines via a three-component coupling reaction of aldehyde, alkyne and amine, Green Chem., 2007, 9(7), 742–745 RSC.
- M. B. Gawande, P. S. Branco, I. D. Nogueira, C. A. A. Ghumman, N. Bundaleski, A. Santos, O. M. Teodoro and R. Luque, Catalytic applications of a versatile magnetically separable Fe–Mo (nanocat-Fe–Mo) nanocatalyst, Green Chem., 2013, 15(3), 682–689 RSC.
- M. Gopiraman, D. Deng, S. Ganesh Babu, T. Hayashi, R. Karvembu and I. S. Kim, Sustainable and versatile CuO/GNS nanocatalyst for highly efficient base free coupling reactions, ACS Sustain. Chem. Eng., 2015, 3(10), 2478–2488 CrossRef CAS.
- A. G. Karunanayake, C. M. Navarathna, S. R. Gunatilake, M. Crowley, R. Anderson, D. Mohan and T. Mlsna, Fe3O4 nanoparticles dispersed on Douglas fir biochar for phosphate sorption, ACS Appl. Nano Mater., 2019, 2(6), 3467–3479 CrossRef CAS.
- V. A. J. Silva, P. L. Andrade, M. P. C. Silva, L. D. L. S. Valladares and J. A. Aguiar, Synthesis and characterization of Fe3O4 nanoparticles coated with fucan polysaccharides, J. Magn. Magn. Mater., 2013, 343, 138–143 CrossRef CAS.
- M. Rawat and D. S. Rawat, CuI@Al2O3 catalyzed synthesis of 2-aminonicotinonitrile derivatives under solvent free condition, Tetrahedron Lett., 2019, 60(16), 1153–1157 CrossRef CAS.
- X. Wang, Y. Shen, A. Xie, L. Qiu, S. Li and Y. Wang, Novel structure CuI/PANI nanocomposites with bifunctions: superhydrophobicity and photocatalytic activity, J. Mater. Chem., 2011, 21(26), 9641–9646 RSC.
- S. Kohli, G. Rathee, S. Hooda and R. Chandra, An efficient approach for the green synthesis of biologically active 2,3-dihydroquinazolin-4 (1H)-ones using a magnetic EDTA coated copper based nanocomposite, RSC Adv., 2023, 13(3), 1923–1932 RSC.
- S. Rajendran, R. Pachaiappan, T. K. Hoang, S. Karthikeyan, L. Gnanasekaran, S. Vadivel, M. Soto-Moscoso and M. A. Gracia-Pinilla, CuO-ZnO-PANI a lethal pnp combination in degradation of 4-chlorophenol under visible light, J. Hazard. Mater., 2021, 416, 125989 CrossRef CAS PubMed.
- J. Alam, U. Riaz and S. Ahmad, Effect of ferrofluid concentration on electrical and magnetic properties of the Fe3O4/PANI nanocomposites, J. Magn. Magn. Mater., 2007, 314, 93–99 CrossRef CAS.
- J. Marek, J. Vilcakova, N. E. Kazantseva, J. Prokes, M. Trchova and J. Stejskal, Conducting and magnetic hybrid polyaniline/nickel composites, Synth. Met., 2022, 291, 117165 CrossRef.
- W. Yang, B. Vogler, Y. Lei and T. Wu, Metallic ion leaching from heterogeneous catalysts: an overlooked effect in the study of catalytic ozonation processes, Environ. Sci.: Water Res. Technol., 2017, 3(6), 1143–1151 RSC.
- Y. Rangraz, F. Nemati and A. Elhampour, Design, synthesis, and characterization of a novel magnetically recoverable copper nanocatalyst containing organoselenium ligand and its application in the A3 coupling reaction, Ind. Eng. Chem. Res., 2019, 58(37), 17308–17318 CrossRef CAS.
- M. Leila, M. Hosseinifard, M. R. Vaezi and S. Rostamnia, Stabilization of copper nanoparticles onto the double Schiff-base-functionalized ZSM-5 for A3 coupling reaction catalysis aimed under mild conditions, RSC Adv., 2023, 13, 4843–4858 RSC.
- C. Mansoureh, H. Alinezhad and S. Ghasemi, Post-synthetic modification of UIO-66-NH2 as a highly efficient and recyclable nanocatalyst in the three-component coupling (A3) reaction for the synthesis of propargylamine derivatives, J. Organomet. Chem., 2023, 1002, 122903 CrossRef.
- H. R. Althomali, M. K. Abbood, F. M. Altalbawy, E. A. M. Saleh, S. S. Abdullaev, A. J. Ibrahim, S. A. Ansari and R. M. R. Parra, A novel nanomagnetic palladium (II) complex of bisimidazolium-based N-heterocyclic carbene an efficient heterogeneous catalyst for A3 coupling reactions, J. Mol. Struct., 2023, 135911 CrossRef.
- T. Mahmood, F. Mazhari and M. Mavvaji, Copper (II)-immobilized on starch-coated nanomagnetite as an efficient and magnetically recoverable catalyst for the synthesis of propargyl amines through one-pot A3 coupling reaction, Org. Prep. Proced. Int., 2023, 55, 251–264 CrossRef.
- M. Zarei, I. Mohammadzadeh, K. Saidi and H. Sheibani, Synthesis of Ag–Cu–Ni nanoparticles stabilized on functionalized g–C3N4 and investigation of its catalytic activity in the A3-coupling reaction, ACS Omega, 2023, 8, 18685–18694 CrossRef CAS PubMed.
- S. Ahmad, A. M. Ahmad, O. S. Raed, Z. M. Mustafa, K. W. Ali and A. J. Mohammed, Magnetic nanoparticles modified with di (pyridin-2-yl) amine ligand supported copper complex: a novel and efficient magnetically reusable catalyst for A3 coupling and CS cross-coupling reactions, Polycyclic Aromat. Compd., 2023, 43, 4407–4425 CrossRef.
- M. Kaur, S. Sharma, A. Choudhary and S. Paul, Tuning the catalytic performance of a Cu supported silica modified γ-Al2O3 nanocatalyst via cobalt-doping for A3-coupling, React. Chem. Eng., 2023, 8, 2141–2155 RSC.
- H. B. Fatemeh, H. Khabazzadeh, M. Fayazi and M. Rezaeipour, Synthesis of CuO and Cu2O nanoparticles stabilized on the magnetic Fe3O4-Montmorillonite-K10 and comparison of their catalytic activity in A3 coupling reaction, J. Iran. Chem. Soc., 2023, 20, 1439–1456 CrossRef.
- H. Bao, A. Y. Li, V. Kairouz and A. Moores, Ultra-fast Cu-based A3-coupling catalysts: faceted Cu2O microcrystals as efficient catalyst-delivery systems in batch and flow conditions, Can. J. Chem., 2022, 100(3), 217–223 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4na00448e |
| This journal is © The Royal Society of Chemistry 2024 |