Explicating conjugated polymer extraction used for the differentiation of single-walled carbon nanotubes†
Dominik
Just
*a,
Tomasz
Wasiak
a,
Andrzej
Dzienia
 a,
Karolina Z.
Milowska
a,
Karolina Z.
Milowska
 bc,
Anna
Mielańczyk
bc,
Anna
Mielańczyk
 a and
Dawid
Janas
a and
Dawid
Janas
 *a
*a
aDepartment of Chemistry, Silesian University of Technology, B. Krzywoustego 4, 44-100, Gliwice, Poland. E-mail: dominik.just@polsl.pl; dawid.janas@polsl.pl
bCIC nanoGUNE, Donostia-San Sebastián 20018, Spain
cIkerbasque, Basque Foundation for Science, Bilbao 48013, Spain
First published on 19th September 2024
Abstract
Single-walled carbon nanotubes (SWCNTs) are synthesized as mixtures of various SWCNT types, exhibiting drastically different properties, and thereby making the material of limited use. Fluorene-based polymers are successful agents for purifying such blends by means of conjugated polymer extraction (CPE), greatly increasing their application potential. However, a limited number of studies have devoted attention to understanding the effects of the polyfluorene backbone and side chain structure on the selectivity and separation efficiency of SWCNTs. Regarding the impact of the polymer backbone, it was noted that the ability to extract SWCNTs with conjugated polymers could be significantly enhanced by using fluorene-based copolymers that exhibit dramatically different interactions with SWCNTs depending on the types of monomers combined. However, the role of monomer side chains remains much less explored, and the knowledge generated so far is fragmentary. Herein, we present a new approach to tailor polymer selectivity by creating copolymers of polyfluorene bearing mixed-length alkyl chains. Their thorough and systematic analysis by experiments and modeling revealed considerable insight into the impact of the attached functional groups on the capacity of conjugated polymers for the purification of SWCNTs. Interestingly, the obtained results contradict the generally accepted conclusion that polyfluorene-based polymers and copolymers with longer chains always prefer SWCNTs of larger diameters. Besides that, we report that the capacity of such polymers for sorting SWCNTs may be substantially enhanced using specific low molecular weight compounds. The carried-out research provides considerable insight into the behavior of polymers and carbon-based materials at the nanoscale.
New conceptsDespite tremendous efforts from the scientific community, single-walled carbon nanotubes (SWCNTs) are still synthesized as heterogeneous mixtures containing many types of drastically different characteristics. Consequently, these blends require sorting to unleash this material's notably high implementation capacity. Several SWCNT differentiation techniques have been developed to unlock access to various SWCNTs. Regrettably, despite the merits of these methods, their mechanisms remain poorly understood, limiting their potential. In this work, we synthesized a wide range of previously unreported copolymers of polyfluorenes bearing mixed-length alkyl chains and used them to sort complex SWCNT mixtures. Meticulous analysis of the structure–activity relationships of these polymers by experimentation and modeling discovered crucial insight into the modus operandi of conjugated polymer extraction, eagerly employed for SWCNT purification. The results uncovered that the binding strength of the polymer macromolecules and other solutes present in the liquid medium to the SWCNTs determines the outcome of the sorting procedure. By capitalizing on this finding, the concentration of the harvested near monochiral (7,5) SWCNTs was increased three-fold while the exceptionally high purity of the material was maintained. With this in mind, the contribution offers valuable information about the behavior of macromolecular species, such as conjugated polymers and SWCNTs at the nanoscale. |
Introduction
Nanocarbon has shown remarkable characteristics, such as notable thermal1 and electrical conductivity,2 as well as tunable optical properties.3 Among them, single-walled carbon nanotubes (SWCNTs) are particularly valuable since their properties are highly dependent on the structure of the material, thus providing an opportunity for matching their performance with the targeted application. Each SWCNT type is classified by a chiral index (n,m), which describes the diameter and quantifies the specific order of carbon atoms in space (referred to as chirality). While progress has been made in synthesizing chirality-enriched SWCNT materials,4–8 achieving the purity required for high-end applications still necessitates post-synthetic purification strategies.9–19 Among the available purification methods, conjugated polymer extraction (CPE) is a useful one-step technique for enriching specific types of SWCNTs that hinges upon the affinity of conjugated polymers for chosen SWCNT chiralities, enabling their selective extraction from multi-component mixtures.19–27 However, despite its merits, CPE is not routinely exploited. The broader adoption of CPE is hindered by the lack of commercially available polymers with optimal molecular weights and the scarcity of productive combinations of polymers and conditions for selective SWCNT partitioning to obtain sufficiently large amounts of monochiral SWCNTs other than (6,5),20,28,29 (7,3),30 and (7,5),19,28 which are currently harvested using only poly[(9,9-dioctylfluorenyl-2,7-diyl)-alt-co-(6,6′-{2,2′-bipyridine})] (PFO-BPy), poly(9,9-dioctylfluorene-alt-benzothiadiazole) (PFO-BT), and poly(9,9-dioctylfluorene-2,7-diyl) (PFO), respectively. Thus, to solve this problem, more research is needed to elucidate the factors influencing the selectivity and yield of CPE.Interestingly, all the above mentioned SWCNT types are extracted using conjugated polymers containing an alkyl-grafted polyfluorene moiety in the backbone. So far, it is known that the attachment of shorter alkyl chains to the fluorene units lowers the SWCNT solubilization capacity of these polymers. Conversely, grafting 12- and 14-carbon chains alleviates this problem and provides considerable enrichment of SWCNT suspensions with larger species, e.g., (7,5), (7,6), or (8,6).31 However, further elongation of the side chains to 18 carbon atoms makes the samples more polydisperse as substantial amounts of various SWCNTs are solubilized in addition to the previously indicated chiralities. Hence, the length of the side chains is somewhat correlated with the diameter of the harvested SWCNTs and the concentration of the resulting dispersion. Other studies also performed using fluorene-based polymers supports these findings.31–33 Overall, it was noted that polyfluorenes with side chains shorter than 10 carbon atoms are ineffective in dispersing SWCNTs with large diameters.33 On the other hand, such polymers with longer alkyl chains promote higher optical density of SWCNT suspensions and facilitate the preferential isolation of large-diameter SWCNT species.
The molecular characteristics of the polymer backbone also play a crucial role in the selectivity and yield of SWCNT suspensions. Short polymers and oligomers may exhibit selectivity but lack stability due to weak interactions with SWCNTs.21 Thus, while they can isolate particular SWCNTs in theory, in practice, after centrifugation, the amount of harvested SWCNTs is usually small because of the instability of the complexes formed between SWCNTs and short polymers. Conversely, excessively long polymers can face entanglement and solubility issues, negatively affecting extraction selectivity and yield.34 Therefore, molecular weight should be adjusted to tune CPE selectivity. We previously confirmed this effect by studying the effect of molecular weight on the isolation of (7,3) using PFO-BT, which showed that isolation with high yield and selectivity hinges upon the application of a polymer of optimum molecular weight.30
Another important aspect is the binding affinity between the SWCNT and the polymer. Studying the extraction of SWCNTs with various polymers (PFO, F8T2, F8BT, and P3HT) revealed that the binding strength follows the order of PFO < F8T2 ∼ F8BT < P3HT, inversely correlating with their selectivity.19,35–38 PFO exhibits weak binding to SWCNTs through π–π stacking. Concurrently, polymers with heteroatoms, such as polythiophenes (e.g., P3HT), bind to SWCNTs not only due to π–π stacking but also through lone electron pairs.39 However, these increased solubilization capabilities often come at the expense of selectivity. Consequently, achieving substantial amounts of monochiral SWCNT purification is challenging when these two factors are typically mutually exclusive. Recently, we proposed a solution to this problem by demonstrating high-yield separation of (6,5) SWCNTs using a mixture of selective polymer (serving the role of extractor) and heteroatom-bearing small organic solubilizers (enhancing the extraction yield),29 which facilitated polymer wrapping around the desired SWCNT species.
In this work, the effect of the structure of polyfluorene derivatives on the partitioning of SWCNTs using CPE was studied to gain insight into the elusive mechanism of this purification method. A mixed extraction strategy, involving more than one component at a time, was explored to reach this goal using polyfluorene derivatives and various additives. A detailed analysis of the generated SWCNT suspensions uncovered polymer-SWCNT-solvent interactions essential for SWCNT purification. Based on the newly obtained knowledge, the extraction system was tuned to harvest concentrated dispersions of near monochiral (7,5) SWCNTs. Besides the utility of the obtained materials, the identified polymer-SWCNT interactions provide essential know-how for making high-performance conductive composites.
Results and discussion
Impact of polyfluorene structure on sorting of SWCNTs by single-component CPE
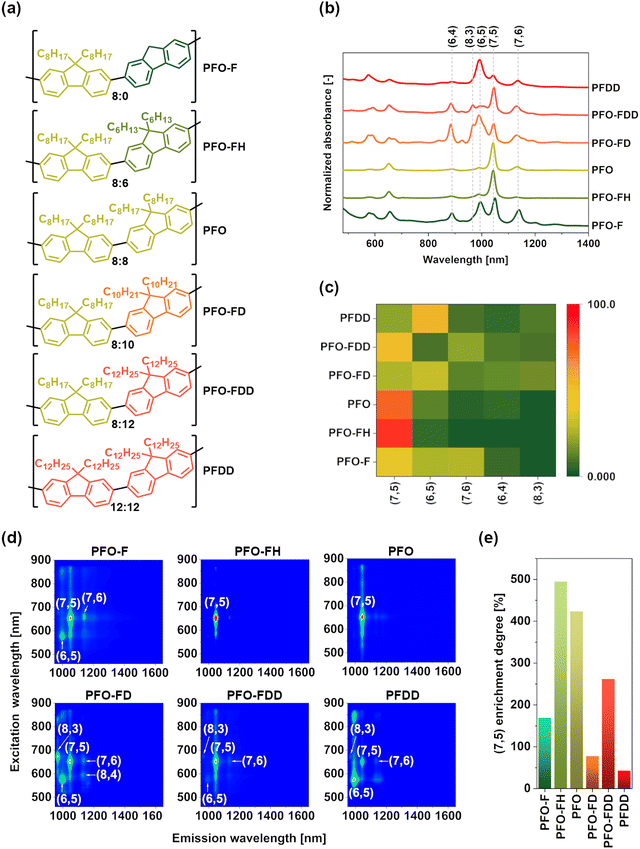
We synthesized hybrid polymers composed of two fluorene subunits with various alkyl chains and used them for sorting (6,5)-enriched CoMoCAT SWCNTs by CPE (Fig. 1a). The raw material contains a range of small SWCNTs, such as (6,5), (7,5), (7,6), (8,3), and (8,4).40 The goal was to investigate how such novel conjugated polymers can extract specific SWCNTs depending on the side chain structure. The obtained results showed that the alkyl chain length significantly influenced the composition of the resulting polymer-suspended SWCNTs (Fig. 1b). It appeared that not only PFO (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8), as reported in the literature, was capable of isolating nearly monochiral (7,5) SWCNT fractions, but also PFO-FH (8
8), as reported in the literature, was capable of isolating nearly monochiral (7,5) SWCNT fractions, but also PFO-FH (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), containing alkyl chains shorter by two methylene groups in one of the subunits. Moreover, PFO-FDD (8
6), containing alkyl chains shorter by two methylene groups in one of the subunits. Moreover, PFO-FDD (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) also extracted (7,5) SWCNTs preferentially, but the generated suspension was contaminated with other species, such as (6,4), (6,5), (7,6), and (8,3). Interestingly, the polymer demonstrated a distinct lack of affinity for (6,5) SWCNTs. Typically, when (6,5)-enriched raw SWCNTs are sorted, like in this work, and the polymer is non-selective, this chirality is abundant in the suspension,28,29 which was not the case. Another peculiar observation was made using PFDD (12
12) also extracted (7,5) SWCNTs preferentially, but the generated suspension was contaminated with other species, such as (6,4), (6,5), (7,6), and (8,3). Interestingly, the polymer demonstrated a distinct lack of affinity for (6,5) SWCNTs. Typically, when (6,5)-enriched raw SWCNTs are sorted, like in this work, and the polymer is non-selective, this chirality is abundant in the suspension,28,29 which was not the case. Another peculiar observation was made using PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12), which contains the most extended alkyl chains in its structure. In contrast to previous reports,31 PFDD, with its long side chains, exhibited a marked preference for smaller-diameter SWCNTs, namely, (6,5) (d = 0.76 nm), (7,5) (d = 0.83 nm), and (8,3) (0.78 nm), over the expected larger ones, e.g., (7,6) (d = 0.89 nm). Similar behavior was noted for PFO-FD (8
12), which contains the most extended alkyl chains in its structure. In contrast to previous reports,31 PFDD, with its long side chains, exhibited a marked preference for smaller-diameter SWCNTs, namely, (6,5) (d = 0.76 nm), (7,5) (d = 0.83 nm), and (8,3) (0.78 nm), over the expected larger ones, e.g., (7,6) (d = 0.89 nm). Similar behavior was noted for PFO-FD (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Lastly, PFO-F (8
10). Lastly, PFO-F (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), free from alkyl side chains in every second monomer, turned out to be the least selective polymer, as it demonstrated a preference for harvesting a broad spectrum of SWCNTs. Therefore, alkyl side chains in the polymer structure seemed crucial for stabilizing the polymer on the SWCNT surface. Without the two side chains, the polymer extracted various SWCNTs. Quantification of the composition of the resulting SWCNT suspensions based on the recorded and deconvoluted optical absorbance spectra confirmed that only two types of polymers exhibited a selective nature – PFO and PFO-FH (Fig. 1c; the deconvolution procedure is described in ESI† and visualized in Fig. S12). The application of PFO or, as noted for the first time, PFO-FH, produced optically pure SWCNT suspensions containing almost exclusively (7,5) chirality.
0), free from alkyl side chains in every second monomer, turned out to be the least selective polymer, as it demonstrated a preference for harvesting a broad spectrum of SWCNTs. Therefore, alkyl side chains in the polymer structure seemed crucial for stabilizing the polymer on the SWCNT surface. Without the two side chains, the polymer extracted various SWCNTs. Quantification of the composition of the resulting SWCNT suspensions based on the recorded and deconvoluted optical absorbance spectra confirmed that only two types of polymers exhibited a selective nature – PFO and PFO-FH (Fig. 1c; the deconvolution procedure is described in ESI† and visualized in Fig. S12). The application of PFO or, as noted for the first time, PFO-FH, produced optically pure SWCNT suspensions containing almost exclusively (7,5) chirality.
Furthermore, the corresponding photoluminescence (PL) excitation–emission maps (Fig. 1d) not only corroborated the optical absorption spectra discussed above, but also proved that the harvested SWCNTs were essentially free of defects (E11* and E112* peaks were absent),41 confirming their high quality. Besides that, the analysis of the degree of enrichment of the obtained suspensions with (7,5) SWCNTs (Fig. 1e) validated high utility of PFO and PFO-FH for extraction of (7,5) SWCNTs. The proportion of (7,5) SWCNTs in the raw material, being ca. 14–15%,30 was considerably improved. In particular, the abundance of this SWCNT type after purification with PFO-FH increased by 495%, meaning that the concentration of this species grew nearly six-fold because of its application, approaching 90% purity.
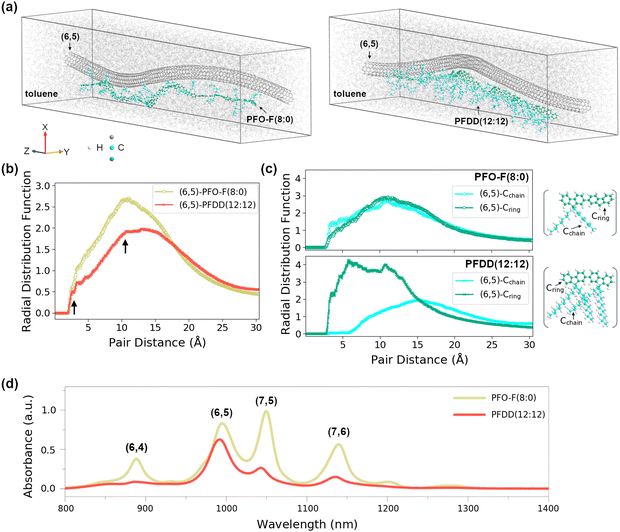
To elucidate the impact of the side chain length on the type of SWCNT preferred by the polymer, we used molecular dynamics (MD) modeling (see ESI,† section 2.4 for details) and correlated them with the optical absorption spectra of the respective SWCNT dispersions. For this purpose, PFO-F (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and PFDD (12
0) and PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12), which differed the most from the structural point of view and produced polydisperse SWCNT suspensions with a considerably dissimilar affinity to (6,5) and (7,5) SWCNTs, were examined. The modeling results presented in Fig. 2 indicated that the interactions between the (6,5) SWCNT and the polymer strongly depended on the length of the side chain. As presented in the left panel of Fig. 2a, PFO-F (8
12), which differed the most from the structural point of view and produced polydisperse SWCNT suspensions with a considerably dissimilar affinity to (6,5) and (7,5) SWCNTs, were examined. The modeling results presented in Fig. 2 indicated that the interactions between the (6,5) SWCNT and the polymer strongly depended on the length of the side chain. As presented in the left panel of Fig. 2a, PFO-F (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) preferentially oriented its backbone next to the (6,5) SWCNT and tried to wrap its side chains around the SWCNTs. On the other hand, after the same time of MD simulations (Fig. 2a, right panel), the PFDD (12
0) preferentially oriented its backbone next to the (6,5) SWCNT and tried to wrap its side chains around the SWCNTs. On the other hand, after the same time of MD simulations (Fig. 2a, right panel), the PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) kept its side chains away from the SWCNT surface while most of its backbone seems to be positioned closer to the SWCNT than in case of PFO-F (8
12) kept its side chains away from the SWCNT surface while most of its backbone seems to be positioned closer to the SWCNT than in case of PFO-F (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0). Because of the PFDD (12
0). Because of the PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12)'s preference to deposit the main polymer chain on the SWCNT surface rather than the alkyl side chains (these moieties are non-selective due to their inability to form chirality-specific π–π interactions with the SWCNTs), it provided a more homogeneous SWCNT suspension mainly made of (6,5) SWCNTs (Fig. 2d).
12)'s preference to deposit the main polymer chain on the SWCNT surface rather than the alkyl side chains (these moieties are non-selective due to their inability to form chirality-specific π–π interactions with the SWCNTs), it provided a more homogeneous SWCNT suspension mainly made of (6,5) SWCNTs (Fig. 2d).
The differences in interactions between (6,5) SWCNT and these two polyfluorene derivatives with different side chains were also reflected in the radial distribution function (RDF) analysis, which describes the probability of finding polymer or its distinct part at a certain distance from SWCNT. Comparison between PFO-F(8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0)-(6,5) SWCNT and PFDD(12
0)-(6,5) SWCNT and PFDD(12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12)-(6,5) SWCNT RDFs shows that the probability of finding PFO-F (8
12)-(6,5) SWCNT RDFs shows that the probability of finding PFO-F (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) polymer next to the (6,5) SWCNT was higher than the probability of finding PFDD (12
0) polymer next to the (6,5) SWCNT was higher than the probability of finding PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) polymer next to the same SWCNT type (cf. the magnitudes of both RDF peaks displayed in Fig. 2b). This result stayed in accordance with the characterization of material by optical absorption spectroscopy (Fig. 2d) showing that PFO-F provided a more concentrated SWCNT suspension (higher absorption values). Hence, we concluded that positioning of the alkyl chains toward the SWCNTs improved the dispersion capacity of PFO-F at the expense of selectivity.
12) polymer next to the same SWCNT type (cf. the magnitudes of both RDF peaks displayed in Fig. 2b). This result stayed in accordance with the characterization of material by optical absorption spectroscopy (Fig. 2d) showing that PFO-F provided a more concentrated SWCNT suspension (higher absorption values). Hence, we concluded that positioning of the alkyl chains toward the SWCNTs improved the dispersion capacity of PFO-F at the expense of selectivity.
In contrast to the RDF between PFO-F (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and (6,5) SWCNT, the RDF between PFDD (12
0) and (6,5) SWCNT, the RDF between PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) and (6,5) SWCNT possessed two clearly separated peaks (see arrows in Fig. 2b), indicating that two distinct parts of PFDD (12
12) and (6,5) SWCNT possessed two clearly separated peaks (see arrows in Fig. 2b), indicating that two distinct parts of PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) polymer were positioned at different distances from the SWCNT. More detailed RDF analysis showed (Fig. 2c) that, indeed, the minimal distance between the (6,5) SWCNT and the longer side chains of PFDD (12
12) polymer were positioned at different distances from the SWCNT. More detailed RDF analysis showed (Fig. 2c) that, indeed, the minimal distance between the (6,5) SWCNT and the longer side chains of PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) was much larger than between the SWCNT of the same chirality and the side chains of PFO-F (8
12) was much larger than between the SWCNT of the same chirality and the side chains of PFO-F (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) but the PFDD (12
0) but the PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) backbone appeared to be closer to the SWCNT than the PFO-F (8
12) backbone appeared to be closer to the SWCNT than the PFO-F (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) backbone. That would suggest a stronger interaction between PFDD (12
0) backbone. That would suggest a stronger interaction between PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) and the (6,5) SWCNT compared with the PFO-F (8
12) and the (6,5) SWCNT compared with the PFO-F (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) – (6,5) case, explaining its much-improved chiral selectivity.
0) – (6,5) case, explaining its much-improved chiral selectivity.
To understand better the character of interactions between both polymers and (6,5) SWCNT, we performed additional density functional-based tight-binding (DFTB) calculations of 2 units of (6,5) SWCNT interacting with four monomers of PFO-F (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) or PFDD (12
0) or PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) polymers surrounded by toluene molecules (see ESI,† section 2.4 for details). Comparison of the interaction energy (Eint) of both systems, calculated as the difference between the total energy of the interacting systems and the total energies of their non-interacting components, also validated the above claims. Eint(6,5) + PFDD(12
12) polymers surrounded by toluene molecules (see ESI,† section 2.4 for details). Comparison of the interaction energy (Eint) of both systems, calculated as the difference between the total energy of the interacting systems and the total energies of their non-interacting components, also validated the above claims. Eint(6,5) + PFDD(12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) + toluene was more negative (−17.785 eV) than Eint(6,5) + PFO-F(8
12) + toluene was more negative (−17.785 eV) than Eint(6,5) + PFO-F(8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) + toluene (−14.114 eV), confirming that the interactions in the first system were stronger. Moreover, detailed electronic structure analysis revealed that PFO-F(8
0) + toluene (−14.114 eV), confirming that the interactions in the first system were stronger. Moreover, detailed electronic structure analysis revealed that PFO-F(8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) induced greater modifications in the electrostatic potential around the (6,5) SWCNT (Fig. S3, ESI†), introduced more polymer states close to the Fermi level, and lowered the Fermi energy of the system more significantly compared to the PFDD (12
0) induced greater modifications in the electrostatic potential around the (6,5) SWCNT (Fig. S3, ESI†), introduced more polymer states close to the Fermi level, and lowered the Fermi energy of the system more significantly compared to the PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) polymer.
12) polymer.
Lastly, we also calculated the diffusion coefficients of (6,5), (7,5) and (7,6) SWCNTs interacting with ten units of FDD(12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) polymers in toluene (Fig. S1, ESI†). The coefficients were found to be D(6,5) = 2.31 × 10−7 ± 1.16 × 10−8 cm2 s−1 (R2 = 0.985), D(7,5) = 9.77 × 10−7 ± 7.04 × 10−8 cm2 s−1 (R2 = 0.985) and D(7,6) = 1.29 × 10−5 ± 1.84 × 10−6 cm2 s−1 (R2 = 0.983), confirming the different selectivity of PFDD (12
12) polymers in toluene (Fig. S1, ESI†). The coefficients were found to be D(6,5) = 2.31 × 10−7 ± 1.16 × 10−8 cm2 s−1 (R2 = 0.985), D(7,5) = 9.77 × 10−7 ± 7.04 × 10−8 cm2 s−1 (R2 = 0.985) and D(7,6) = 1.29 × 10−5 ± 1.84 × 10−6 cm2 s−1 (R2 = 0.983), confirming the different selectivity of PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) polymer. The diffusion coefficient of (7,6) SWCNT was two orders of magnitude larger than of (6,5) and (7,5) SWCNTs, revealing that SWCNTs of larger diameters, such as (7,6) SWCNT, had higher molecular mobility in the presence of PFDD (12
12) polymer. The diffusion coefficient of (7,6) SWCNT was two orders of magnitude larger than of (6,5) and (7,5) SWCNTs, revealing that SWCNTs of larger diameters, such as (7,6) SWCNT, had higher molecular mobility in the presence of PFDD (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) polymer. This finding corroborated our experimental observations, which showed a preference of PFDD for smaller SWCNTs. Since the outcome of CPE was heavily affected by the diffusivity of the SWCNTs in the solvent during centrifugation, this factor likely had a major impact on the SWCNT separation result. The more mobile (7,6) and (7,5) SWCNTs could be more effectively precipitated, thus enriching the suspension with (6,5) SWCNTs.
12) polymer. This finding corroborated our experimental observations, which showed a preference of PFDD for smaller SWCNTs. Since the outcome of CPE was heavily affected by the diffusivity of the SWCNTs in the solvent during centrifugation, this factor likely had a major impact on the SWCNT separation result. The more mobile (7,6) and (7,5) SWCNTs could be more effectively precipitated, thus enriching the suspension with (6,5) SWCNTs.
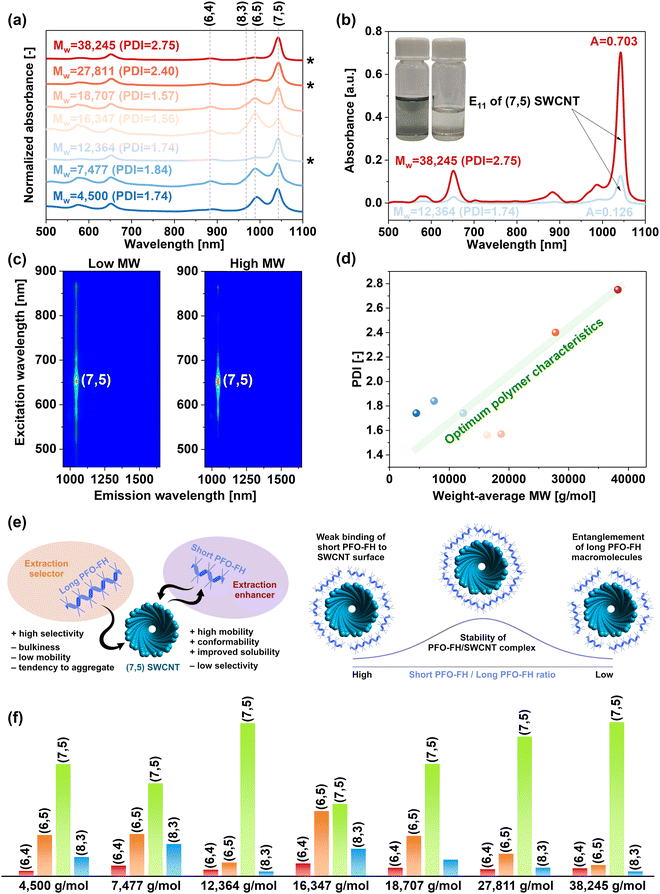
To explore whether molecular weight of the conjugated polymers is crucial for establishing strong interactions with the SWCNTs, which are at the heart of selective extraction thereof, we evaluated PFO-FH polymers with various characteristics (Fig. 3a). The results showed that PFO-FH samples with Mw values of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 364 g mol−1, 27
364 g mol−1, 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 811 g mol−1, and 38
811 g mol−1, and 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 245 g mol−1 all gave (7,5) suspensions of very high purity. At the same time, polymers with other Mw values underperformed and produced fractions contaminated with (6,5) SWCNTs. The increase in the molecular weight of PFO-FH favorably affected the separation yield. By changing the Mw from 12
245 g mol−1 all gave (7,5) suspensions of very high purity. At the same time, polymers with other Mw values underperformed and produced fractions contaminated with (6,5) SWCNTs. The increase in the molecular weight of PFO-FH favorably affected the separation yield. By changing the Mw from 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 364 g mol−1 to 38
364 g mol−1 to 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 245 g mol−1, the concentration of the collected near monochiral (7,5) SWCNTs increased by a factor of ∼6 (Fig. 3b). The conducted characterization by PL confirmed that the yield enhancement did not occur at the expense of purity (Fig. 3c), as both fractions showed only signals from (7,5) SWCNTs.
245 g mol−1, the concentration of the collected near monochiral (7,5) SWCNTs increased by a factor of ∼6 (Fig. 3b). The conducted characterization by PL confirmed that the yield enhancement did not occur at the expense of purity (Fig. 3c), as both fractions showed only signals from (7,5) SWCNTs.
Plotting the Mw of the employed polymers against their PDIs indicated that these parameters must be proportional to ensure a high quality of the generated SWCNT suspensions (Fig. 3d). We recently showed that extraction of (6,5) SWCNTs with a different polymer, PFO-BPy, can be enhanced by introducing non-selective PFO-T oligomers, improving their stability in the liquid medium.29 Herein, we see that PFO-FH may exhibit a self-enhancing effect, eliminating the need to add a second polymer to produce a pristine (7,5) SWCNT dispersion of high concentration. For such a phenomenon to occur, there must be a balance between the long- and short-polymer fractions. The former fraction serves the role of a selector, enabling selective SWCNT solubilization. At the same time, the latter enhances the stability of the preferentially dispersed SWCNT types in the liquid medium, reducing their tendency to precipitate during centrifugation (Fig. 3e). In such a case, relatively long polymer chains with insufficient PDI suspend SWCNTs non-selectively. They contain a surplus of small molecular weight fractions, which impede the deposition of the long polymers on the SWCNT side wall, making the suspended material more polydisperse since it is primarily solubilized with short, non-selective polymers. On the other hand, short polymers with excessive PDI also exhibit non-selective extraction capacity because of the overabundance of the long polymer chains, which are prone to entanglement.42–44 This effect is described in more detail in the ESI† (Section 4.1, Fig. S17). To sum up, only when a suitable ratio of short to long polymers is established the SWCNT suspensions produced are composed of nearly exclusively of the targeted (7,5) SWCNTs in appreciable concentrations (Fig. 3f). The fact that low polymer polydispersity is not inevitably beneficial for making composites with SWCNTs has much broader implications extending the scope of this work.
Enhancing the yield of (7,5) SWCNT extraction using two-component CPE
To gain insight into the complex behavior of polymers at the nanoscale we created two-component CPE systems not only based on raw SWCNTs, a single polymer, and solvent medium. We mixed PFO with other components to analyze its extraction capabilities while, hopefully, preserving selectivity for (7,5) SWCNTs. Firstly, we studied PFO-FH as a potential (7,5) extraction promoter since it exhibited superb affinity to this type of SWCNTs, as reported in the previous chapter. We combined PFO with the low MW batch of this polymer at various weight ratios (Fig. 4a). In each experiment, we obtained (7,5) SWCNT suspensions of excellent purity (Fig. S19, ESI†), The most suitable ratio of PFO to PFO-FH was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 mg (Fig. 4a). For such conditions, the absorbance value changed from 0.68 to 1.16, so the concentration of (7,5) SWCNTs was increased nearly two-fold without compromising the superb quality of the suspensions.
6 mg (Fig. 4a). For such conditions, the absorbance value changed from 0.68 to 1.16, so the concentration of (7,5) SWCNTs was increased nearly two-fold without compromising the superb quality of the suspensions.
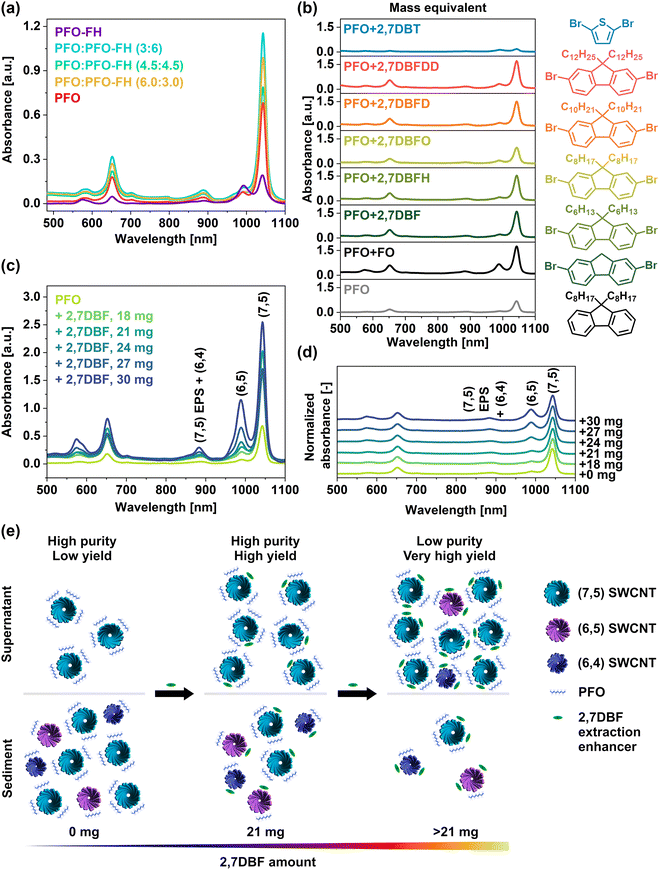 | ||
| Fig. 4 (a) As-recorded optical absorbance spectra of SWCNTs suspended with PFO, PFO-FH, and mixtures of these polymers in the specified ratios given in milligrams. Molecular parameters for the specified polymers may be found in Table S2 (ESI†) (PFO – P12, PFO-FH – P3), (b) optical absorbance spectra of SWCNT suspensions prepared using the specified polymers and extraction enhancers visualized on the right, (c) as-recorded and (d) normalized and offset optical absorbance spectra of SWCNT suspensions created using PFO and 2,7DBF at various weight ratios, (e) illustration of the underlying interactions responsible for separation outcome. | ||
To gain deeper insight into the extraction process of (7,5) SWCNTs using PFO, we investigated the possibility of boosting the separation system with small molecular weight compounds to identify possible SWCNT differentiation promoters. Previously, we noted that 2,5-dibromothiophene (2,5DBT) was the most potent enhancer of yield and selectivity for extraction of (6,5) SWCNT with PFO-BPy6,6′ among the tested chemicals.29 We, therefore, used this enhancer to facilitate the isolation of (7,5) SWCNT species with PFO. Surprisingly, the system's selectivity was lost, and the concentration of the collected SWCNTs deteriorated (Fig. 4b). The application of structural isomers with bromine atoms in different, i.e., 2,4DBT and 3,4DBT, was also unsuccessful (Fig. S21, ESI†). Hence, sulfur-bearing compounds were not suitable for enhancing the (7,5) isolation capacity of PFO.
Thiophene-based polymers (PFO-T, PFO-3DDT) display non-selective interactions with SWCNTs.28,29,35 Their high but indifferent affinity for SWCNTs is likely caused by the free electron pairs on the sulfur atom, which may boost π–π stacking29 of such molecules on the SWCNTs. In such a case, the thiophene-derived structures are deposited on all SWCNT species available in the dispersion, regardless of chirality, so they cannot purify SWCNT mixtures. PFO binds to SWCNTs relatively weakly,29,36,37 so it can be easily displaced from the surface with such compounds, which exhibit a potent affinity to SWCNT. This relation may explain the loss of purity of the suspensions generated by PFO in the presence of thiophene derivatives.
Because of these reasons, we then studied the influence of other compounds on the separation of SWCNTs. For this experiment, we selected building blocks (free of sulfur atoms) used in this study for the synthesis of SWCNT-selective polyfluorene derivatives, such as dioctylfluorene (FO), as well as several dibromofluorene derivatives, including 2,7-dibromofluorene (2,7DBF), 2,7-dibromo-9,9-dihexylfluorene (2,7DBFH), 2,7-dibromo-9,9-dioctylfluorene (2,7DBFO), 2,7-dibromo-9,9-decylfluorene (2,7DBFD), and 2,7-dibromo-9,9-dodecylfluorene (2,7DBFDD). We introduced 18 mg of each additive into the PFO/SWCNT/toluene system and observed their impact on the polymer behavior (Fig. 4b). Interestingly, all the evaluated additives showed the potential to increase the yield of (7,5) SWCNTs. This result can be attributed to the inherent high affinity of the fluorene structure for this specific chirality, analogously as poly(9,9-dioctylfluorene-2,7-diyl) (PFO) harvests exclusively (7,5) SWCNTs. Among the tested monomers, 2,7DBF showed the best effect. Notably, the tested fluorene-based enhancers substantially increased the harvested SWCNTs’ concentration, and the SWCNT dispersions they produced were nearly monochiral (except when FO was used). Interestingly, 2,7DBFH and 2,7DBFD improved the SWCNT extraction to a similar degree, even though the copolymers prepared from them with PFO (PFO-FH and PFO-FD) exhibited a completely different SWCNT differentiation capacity (Fig. 1b).
The most beneficial effect of 2,7DBF was observed when its content was increased to 21 mg (Fig. 4c and d). The absorbance at the S11 optical transition of (7,5) SWCNT chirality increased threefold from 0.68 to 2.00 with respect to the sole application of PFO. Importantly, a high concentration of (7,5) SWCNTs was achieved without decreasing the purity of the material. This result was superior to the previously discussed improvements caused by incorporating PFO-FH into the PFO/SWCNT/toluene system, for which the absorbance values almost doubled from 0.68 to 1.16. We hypothesize that such an entity (2,7DBF) can fit more easily into the interstitial spaces between SWCNT and PFO (rather than the macromolecular PFO-FH) without disrupting the relatively weak interaction between the two elements.
It should be noted that the excessive addition of 2,7DBF to the dispersion was unfavorable. Even though the concentration of SWCNT suspension increased, the material was contaminated with (6,5) SWCNTs. Based on the results obtained, it is reasonable to assume that these additives improved the solubilization of the undesired SWCNTs, as depicted in Fig. 4e. Initially, the suspension contained almost solely (7,5) SWCNTs, but their concentration was relatively low as most SWCNTs were precipitated. Adding 2,7DBF molecules improved the concentration of dispersed (7,5) SWCNT species by coating their surface, thereby minimizing their precipitation. However, due to the molecular size of these enhancers, they were not selective, so they also deposited on other SWCNT types. Therefore, at high 2,7DBF content (>21 mg), other SWCNT chiralities were solubilized similarly. Thus, while the overall yield of extraction was the highest under such conditions, the purity was not satisfactory.
To elucidate the interactions enabling enhancement of the capacity of the CPE system for the purification of SWCNTs due to the introduction of additives, energy levels of two such systems, (6,5) SWCNT/PFO-BPy6,6′ (enhanced with 2,5DBT)29 and PFO (enhanced with 2,7DBT, according to this study), were calculated in the framework of spin-polarized density functional theory (DFT) (Fig. 5a).45,46 We previously reported that 2,5DBT exhibits favorable electronic interactions with PFO-BPy6,6′ (formation of type-I heterojunction), positively affecting its folding around (6,5) SWCNTs. Still, the results of this research reveal that charge transfer alone cannot explain the enhancement of the extraction degree of harvested SWCNTs. On the one hand, 2,7DBF, PFO, and (7,5) SWCNTs, which form an analogous system to 2,5DBT, PFO-BPy6,6′, and (6,5) SWCNTs, from the energy level point of view, also gave an improvement in the extraction yield. On the other hand, however, we also tested 2,5DBT as a potential enhancer of extraction of (7,5) SWCNTs with PFO (Fig. 4b), but, to our surprise, both the purity and yield of the generated suspension were low. If the appropriate energy level of such an additive was sufficient to cause the enhancement, we should have observed it because 2,5DBT has similar HOMO and LUMO levels to 2,7DBF.
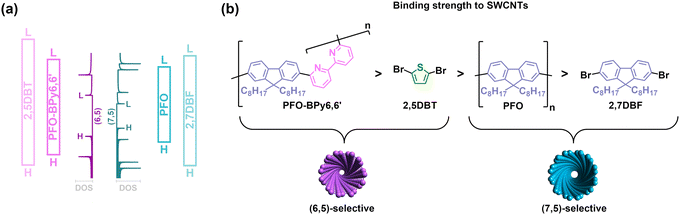 | ||
| Fig. 5 (a) Energy levels of (6,5) SWCNT, (7,5) SWCNT, PFO-BPy6,6′, PFO, 2,5DBT, and 2,7DBF. The density of states (DOS) for (6,5) and (7,5) SWCNTs (shown up to E55 transitions) were taken from ref. 48 and aligned with the Fermi level calculated in this study at the DFT/B3LYP/DZP level of theory. HOMO (H) and LUMO (L) levels of the specified compounds were also calculated in this study (cf. ESI†). (b) The deduced binding strength of the employed compounds and polymers to SWCNTs. | ||
Therefore, another essential aspect of this process needs to be considered, which is the binding strength of these additives to SWCNTs (Fig. 5b). The heteroatom containing 2,5DBT can bind to SWCNTs more firmly, easily displacing the PFO molecules weakly connected with the SWCNT surface. Hence, its application makes the quality of the PFO/SWCNT suspension deteriorate. Conversely, 2,7DBF displays even weaker binding capacity to SWCNTs than short PFO molecules, which were barely able to suspend SWCNTs.47 Consequently, its presence in the liquid medium does not interfere significantly with the relationship between PFO and (7,5) SWCNTs, enabling selective extraction. On that account, we concluded that these extraction additives are only effective if their effective binding strength to SWCNTs is lower than that of the polymer employed for harvesting the preferred SWCNT chirality. Consequently, they may supplement the action of the selective polymer by enhancing the stability of the CP-SWCNT complexes, possibly through the adjustment of the polymer conformation on the surface of SWCNTs. This, in turn, makes the conjugated polymer/SWCNT hybrids less prone to precipitation during post-extraction centrifugation, thereby increasing the concentration of the near monochiral SWCNT suspensions.
Experimental
All the information regarding the materials and methods used in this work is provided in the ESI.†Conclusions
In conclusion, we investigated the effects of the structure of self-synthesized polyfluorenes on their ability to differentiate complex mixtures of SWCNTs. An array of polymers with varying alkyl chain lengths was synthesized. It was noted that the type of attached functional groups had a considerable impact on the capacity of conjugated polymers for the purification of SWCNT. Contrary to published reports, polymers with long alkyl chains did not necessarily prefer large-diameter SWCNT species. The performed MD modeling indicated that, depending on the length of alkyl side chains, the polymer molecules tend to orient differently with respect to SWCNTs, which considerably affects the activity of these polymers in the context of SWCNT separation. Besides, it was shown that the macromolecular characteristics of conjugated polymers play a significant role in the purification of SWCNTs using CPE. Even though, due to synthetic limitations, it was not possible to obtain similar molecular weights for all examined copolymers of polyfluorene bearing mixed-length alkyl chains.Furthermore, we prepared two-component extraction systems to gain greater insight into the mechanism of CPE and the behavior of polymers at the nanoscale. Firstly, it was discovered that the polymers may exhibit a self-enhancing effect, boosting their capacity to extract particular SWCNTs, if the correct balance between short and long polymer fractions is established. Secondly, the performance of the extraction systems was improved dramatically by using small molecular enhancers. According to the results, such chemical compounds deposited on the uncovered parts of SWCNTs amplify their solubility in the liquid medium. Consequently, the extraction yield increased substantially. Further investigation revealed that the binding strength of polymers and enhancers to specific SWCNTs was a crucial parameter to consider. These small enhancers served their purpose only if their binding strength to SWCNTs was lower than that of the selective conjugated polymer. If this was not the case or the enhancers were introduced in excessive amounts, the possibility of the deposition of polymer molecules on SWCNTs to enable their targeted extraction was markedly decreased. Nonetheless, the application of the optimum extraction conditions determined in this study gave rise to a more than a three-fold increase in the concentration of extracted near monochiral (7,5) SWCNTs. Therefore, besides the significant insight into the interactions between polymers, SWCNTs, and solvent molecules presented in this study, the contribution offers a straightforward approach to making these highly desired materials more accessible. This achievement should facilitate the implementation of sorted SWCNTs in technologies that demand such materials with precisely defined characteristics. Besides that, the thorough interpretation of the polymer behavior paves the way for manufacturing of high-performance conductive composites filled with SWCNTs, which have a broad spectrum of potential applications.
Data availability
All data supporting this article have been included in the main text and ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank the National Science Centre, Poland (under the SONATA program, Grant agreement UMO-2020/39/D/ST5/00285) for supporting the research and the Rector of the Silesian University of Technology for facilitating this study through a Pro-Quality grant (04/020/RGJ24/1091). K. Z. M. would also like to acknowledge Marie Skłodowska-Curie Cofund Program of the European Commission (Grant no. H2020-MSCA-COFUND-2020-101034228-WOLFRAM2).References
- S. Berber, Y.-K. Kwon and D. Tománek, Phys. Rev. Lett., 2000, 84, 4613–4616 CrossRef CAS PubMed.
- G. J. Brady, Y. Joo, S. Singha Roy, P. Gopalan and M. S. Arnold, Appl. Phys. Lett., 2014, 104, 083107 CrossRef.
- H. Kataura, Y. Kumazawa, Y. Maniwa, I. Umezu, S. Suzuki, Y. Ohtsuka and Y. Achiba, Optical Properties of Single-Wall Carbon Nanotubes, Synth. Met., 1999, 103, 2555–2558 CrossRef CAS.
- S. D. Dutta, D. K. Patel and K.-T. Lim, Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems, Elsevier, 2020, pp. 505–535 Search PubMed.
- K. Awasthi, A. Srivastava and O. N. Srivastava, J. Nanosci. Nanotechnol., 2005, 5, 1616–1636 CrossRef CAS PubMed.
- Y. Yan, J. Miao, Z. Yang, F. X. Xiao, H. Bin Yang, B. Liu and Y. Yang, Chem. Soc. Rev., 2015, 44, 3295–3346 RSC.
- J. Liu, C. Wang, X. Tu, B. Liu, L. Chen, M. Zheng and C. Zhou, Nat. Commun., 2012, 3, 1199 CrossRef PubMed.
- S. M. Bachilo, L. Balzano, J. E. Herrera, F. Pompeo, D. E. Resasco and R. B. Weisman, J. Am. Chem. Soc., 2003, 125, 11186–11187 CrossRef CAS PubMed.
- X. Tu, S. Manohar, A. Jagota and M. Zheng, Nature, 2009, 460, 250–253 CrossRef CAS PubMed.
- M. Zheng and E. D. Semke, J. Am. Chem. Soc., 2007, 129, 6084–6085 CrossRef CAS PubMed.
- S. Ohmori, T. Saito, B. Shukla, M. Yumura and S. Iijima, ACS Nano, 2010, 4, 3606–3610 CrossRef CAS PubMed.
- X. Sun, S. Zaric, D. Daranciang, K. Welsher, Y. Lu, X. Li and H. Dai, J. Am. Chem. Soc., 2008, 130, 6551–6555 CrossRef CAS PubMed.
- D. A. Heller, R. M. Mayrhofer, S. Baik, Y. V. Grinkova, M. L. Usrey and M. S. Strano, J. Am. Chem. Soc., 2004, 126, 14567–14573 CrossRef CAS PubMed.
- S. Niyogi, C. G. Densmore and S. K. Doorn, J. Am. Chem. Soc., 2009, 131, 1144–1153 CrossRef CAS PubMed.
- R. B. Weisman and S. M. Bachilo, Nano Lett., 2003, 3, 1235–1238 CrossRef CAS.
- M. Zheng and B. A. Diner, J. Am. Chem. Soc., 2004, 126, 15490–15494 CrossRef CAS PubMed.
- M. S. Arnold, A. A. Green, J. F. Hulvat, S. I. Stupp and M. C. Hersam, Nat. Nanotechnol., 2006, 1, 60–65 CrossRef CAS PubMed.
- X. Peng, N. Komatsu, T. Kimura and A. Osuka, J. Am. Chem. Soc., 2007, 129, 15947–15953 CrossRef CAS PubMed.
- A. Nish, J. Y. Hwang, J. Doig and R. J. Nicholas, Nat. Nanotechnol., 2007, 2, 640–646 CrossRef CAS PubMed.
- H. Ozawa, N. Ide, T. Fujigaya, Y. Niidome and N. Nakashima, Chem. Lett., 2011, 40, 239–241 CrossRef CAS.
- H. Ozawa, T. Fujigaya, Y. Niidome, N. Hotta, M. Fujiki and N. Nakashima, J. Am. Chem. Soc., 2011, 133, 2651–2657 CrossRef CAS PubMed.
- H. Ozawa, N. Ide, T. Fujigaya, Y. Niidome and N. Nakashima, Chem. - Eur. J., 2011, 17, 13438–13444 CrossRef CAS PubMed.
- H. W. Lee, Y. Yoon, S. Park, J. H. Oh, S. Hong, L. S. Liyanage, H. Wang, S. Morishita, N. Patil, Y. J. Park, J. J. Park, A. Spakowitz, G. Galli, F. Gygi, P. H. S. Wong, J. B. H. Tok, J. M. Kim and Z. Bao, Nat. Commun., 2011, 2, 541 CrossRef PubMed.
- J. Gao, M. A. Loi, E. J. F. De Carvalho and M. C. Dos Santos, ACS Nano, 2011, 5, 3993–3999 CrossRef CAS PubMed.
- M. Tange, T. Okazaki and S. Iijima, ACS Appl. Mater. Interfaces, 2012, 4, 6458–6462 CrossRef CAS PubMed.
- M. Tange, T. Okazaki and S. Iijima, J. Am. Chem. Soc., 2011, 133, 11908–11911 CrossRef CAS PubMed.
- M. Tange, T. Okazaki and S. Iijima, Nanoscale, 2014, 6, 248–254 RSC.
- A. Dzienia, D. Just and D. Janas, Nanoscale, 2023, 15, 9510–9524 RSC.
- D. Just, A. Dzienia, K. Z. Milowska, A. Mielańczyk and D. Janas, Mater. Horiz., 2024, 11, 758–767 RSC.
- A. Dzienia, D. Just, P. Taborowska, A. Mielanczyk, K. Z. Milowska, S. Yorozuya, S. Naka, T. Shiraki and D. Janas, Small, 2023, 19, 2304211 CrossRef CAS PubMed.
- J. Ouyang, H. Shin, P. Finnie, J. Ding, C. Guo, Z. Li, Y. Chen, L. Wei, A. J. Wu, S. Moisa, F. Lapointe and P. R. L. Malenfant, ACS Appl. Polym. Mater., 2022, 4, 6239–6254 CrossRef.
- Z. Ouyang, Z. Takáts, T. A. Blake, B. Gologan, A. J. Guymon, J. M. Wiseman, J. C. Oliver, V. J. Davisson and R. G. Cooks, Science, 2003, 301, 1351–1354 CrossRef CAS PubMed.
- J. Ding, Z. Li, J. Lefebvre, F. Cheng, G. Dubey, S. Zou, P. Finnie, A. Hrdina, L. Scoles, G. P. Lopinski, C. T. Kingston, B. Simard and P. R. L. Malenfant, Nanoscale, 2014, 6, 2328–2339 RSC.
- W. Gomulya, G. D. Costanzo, E. J. F. De Carvalho, S. Z. Bisri, V. Derenskyi, M. Fritsch, N. Fröhlich, S. Allard, P. Gordiichuk, A. Herrmann, S. J. Marrink, M. C. Dos Santos, U. Scherf and M. A. Loi, Adv. Mater., 2013, 25, 2948–2956 CrossRef CAS PubMed.
- T. Schuettfort, H. J. Snaith, A. Nish and R. J. Nicholas, Nanotechnology, 2010, 21, 025201 CrossRef CAS PubMed.
- S. D. Stranks, S. N. Habisreutinger, B. Dirks and R. J. Nicholas, Adv. Mater., 2013, 25, 4365–4371 CrossRef CAS PubMed.
- S. D. Stranks, A. M. R. Baker, J. A. Alexander-Webber, B. Dirks and R. J. Nicholas, Small, 2013, 9, 2245–2249 CrossRef CAS PubMed.
- S. N. Habisreutinger, T. Leijtens, G. E. Eperon, S. D. Stranks, R. J. Nicholas and H. J. Snaith, J. Phys. Chem. Lett., 2014, 5, 4207–4212 CrossRef CAS PubMed.
- G. A. Rance, D. H. Marsh, R. J. Nicholas and A. N. Khlobystov, Chem. Phys. Lett., 2010, 493, 19–23 CrossRef CAS.
- A. Dzienia, D. Just, T. Wasiak, K. Z. Milowska, A. Mielańczyk, N. Labedzki, S. Kruss and D. Janas, Adv. Sci., 2024, 11, 2402176 CrossRef CAS PubMed.
- J. Zaumseil, Adv. Opt. Mater., 2022, 10, 2101576 CrossRef CAS.
- N. Berton, F. Lemasson, F. Hennrich, M. M. Kappes and M. Mayor, Chem. Commun., 2012, 48, 2516–2518 RSC.
- F. Jakubka, S. P. Schießl, S. Martin, J. M. Englert, F. Hauke, A. Hirsch and J. Zaumseil, ACS Macro Lett., 2012, 1, 815–819 CrossRef CAS PubMed.
- P. Imin, F. Cheng and A. Adronov, Polym. Chem., 2011, 2, 1404–1408 RSC.
- P. Hohenberg and W. Kohn, Phys. Rev., 1964, 136, B864–B871 CrossRef.
- W. Kohn and L. J. Sham, Phys. Rev., 1965, 140, A1133–A1138 CrossRef.
- T. Wasiak, D. Just, A. Dzienia, D. Łukowiec, S. Wacławek, A. Mielańczyk, S. Kodan, A. Bansal, R. Chandra and D. Janas, Sci. Rep., 2024, 14, 2336 CrossRef CAS PubMed.
- Shigeo Maruyama, 1D DOS (van Hove singularity), https://www.photon.t.u-tokyo.ac.jp/~maruyama/kataura/1D_DOS.html.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nh00427b |
| This journal is © The Royal Society of Chemistry 2024 |