Development of enzyme–inorganic hybrid nanoflower-modified electrodes and a smartphone-controlled electrochemical analyzer for point-of-care testing of salivary amylase in saliva†
Cong
Liu
a,
Xia
Gong
a,
Xiao
Yang
b,
Zipei
Yu
a,
Weihao
Li
b,
Guangyi
Liao
b,
Chuanquan
Lin
*b,
Lelun
Jiang
*a and
Changqing
Yi
 *a
*a
aGuangdong Provincial Engineering and Technology Center of Advanced and Portable Medical Devices, School of Biomedical Engineering, Sun Yat-Sen University, Guangzhou, 510275, PR China. E-mail: yichq@mail.sysu.edu.cn; jianglel@mail.sysu.edu.cn
bScience and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405, PR China. E-mail: chuanquanlin@gzucm.edu.cn
First published on 21st November 2023
Abstract
Quantitation of salivary alpha-amylase (sAA) plays a significant role in not only theoretical studies but also clinical practice. This study reports a quantitative point-of-care testing (POCT) system for sAA quantitation anywhere, anytime and by anyone, which consists of customized electrodes and a smartphone-controlled electrochemical analyzer. Organic–inorganic hybrid nanoflowers (NFs) encapsulating α-glucosidase (AG) and glucose dehydrogenase (GDH) have been synthesized and modified onto screen-printed electrodes (SPCEs) to fabricate the customized electrodes. The SPCEs integrated with the smartphone-controlled electrochemical analyzer exhibit good analytical performance for sAA with a low detection limit of 5.02 U mL−1 and a wide dynamic range of 100–2000 U mL−1 using chronoamperometry. The reported POCT system has been successfully demonstrated for quantitation of sAA in clinical saliva samples, and the quantitation results correlated well with those of the Bernfeld method which is extensively used in clinics. More importantly, this study reveals the great potential of sAA as an early warning indicator of abnormal glucose metabolism in obese individuals. Considering the non-invasive saliva sampling process as well as the easy-to-use and cost-effectiveness features of this quantitative POCT system, quantitation of salivary sAA at home by laypersons might become an appealing choice for obese individuals to monitor their glucose metabolism status anytime.
1. Introduction
Diabetes mellitus is a chronic systemic disease that disrupts glucose metabolism and leads to complications in multiple body systems, such as the cardiovascular, cerebrovascular, renal, and neurological systems.1–3 The number of individuals with diabetes is projected to reach 120 million by 2035, which poses challenges to healthcare, the economy, and social development.4 Accurate diagnosis and management of diabetes rely on measuring the blood glucose level (BGL). However, collecting blood samples from the finger for BGL testing is painful and carries the risk of transmitting blood-borne diseases, resulting in poor patient compliance. Consequently, there is growing interest in developing less invasive or non-invasive techniques for glucose monitoring.5–9Saliva has gained attention as a potential biological medium for monitoring physiological and pathological conditions due to its non-invasive sampling process and ease of storage.10–14 Saliva contains various components, including enzymes, electrolytes, proteins, nucleic acids, hormones, cytokines, and antibodies.15 Salivary amylase (sAA), in particular, has been identified as a biomarker for neurological, digestive, and metabolic disorders.16 Elevated sAA concentrations, for instance, can indicate the presence of pancreatic cancer, acute pancreatitis, bile duct obstruction, salivary gland infection, or gastroenteritis.17 Additionally, several studies have revealed a close relationship between altered sAA activity and pathophysiological changes in diabetic patients.18–20 Therefore, quantifying sAA activity not only contributes to theoretical research but also holds significance in clinical practice.
Immunoassays have been extensively developed for quantifying sAA activity, utilizing specific binding reactions between sAA and its antibodies.21,22 However, these immunoassays have limitations such as the use of expensive and unstable antibodies, as well as cross-reactions with other compounds in the sample matrix. An alternative approach is to indirectly quantitate sAA by measuring the consumption of starch or production of maltose during enzymatic reactions.23–26 For instance, the Bernfeld method, commonly used in clinical practice, quantifies sAA by measuring the absorbance of color products of maltose and 3,5-dinitrosalicylic acid at 540 nm. The principle behind the Bernfeld method is that maltose can reduce dinitrosalicylic acid to brownish-red 3-amino-5-nitrosalicylic acid whose absorbance is proportional to the concentration of maltose within a certain range. However, these methods are time-consuming, require specialized technicians and equipment, and are not suitable for resource-limited regions and home healthcare monitoring.
Enzyme-based electrochemical methods offer a promising solution for on-site quantitation of biomarkers in saliva, including sAA, ketones, and alcohols. These methods have advantages such as high sensitivity and specificity, low cost, simplicity of equipment, and ease of operation.8,9,17,25–29 For example, Zhang et al. developed a smartphone-based sensing system that measures enzymatically produced maltose in the presence of K3[Fe(CN)6] to quantify sAA activity. Maltose can reduce the electron mediator [Fe(CN)6]3− to [Fe(CN)6]4−, resulting in a potential change following the Nernst equation.25 In another study, Garcia and co-workers designed an amperometric method for direct detection of maltose produced by sAA hydrolysis of starch, utilizing Cu/CuO electrodes.26 These studies demonstrated the feasibility of using enzyme-based electrochemical methods for accurate quantitation of sAA activity in a cost-effective and portable manner, facilitating disease diagnosis in resource-limited regions and home healthcare monitoring.
Herein, we have developed a smartphone-based quantitative sensing system for point-of-care testing of sAA (Scheme 1). This system incorporates enzyme–inorganic hybrid nanoflower (NF)-modified electrodes as disposable sensing chips and a smartphone-controlled electrochemical analyzer as portable equipment. The system has been successfully demonstrated for on-site quantitation of sAA in clinical saliva samples and exhibits better accuracy and precision than the standard clinical method. Additionally, preliminary clinical studies have shown that sAA is a promising biomarker for early warning of type 2 diabetes mellitus (T2DM). The study also highlights the advantages of enzyme electrodes with enhanced stability, a cost-effective electrochemical analyzer, and the ease-of-use and accessibility of the system for performing biomarker quantitation anywhere, anytime, and by anyone. This has significant implications for the development of diverse point-of-care testing applications and the collection of massive amounts of data for AI-driven discoveries of new biomarkers.
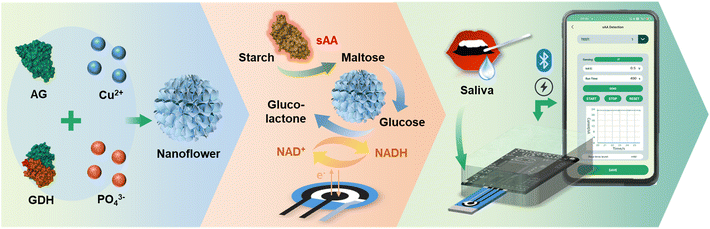 | ||
| Scheme 1 Schematic representation of NF synthesis, the sAA sensing mechanism, and the procedure for point-of-care testing of salivary sAA. | ||
2. Experimental
2.1. Ethics statement
The study protocol received ethical approval from the Research Ethics Committee of the First Affiliated Hospital of Guangzhou Chinese Medical University and adhered to the Ethical Review Criteria for Biomedical Research Involving Human Subjects (CAP) (AF/JD-02/04). The study was conducted in compliance with the ethical standards set forth in the 1964 Declaration of Helsinki and its subsequent revisions. Participants provided written informed consent before participating in the study.2.2. Reagents
α-Glucosidase, glucose dehydrogenase, soluble starch, chitosan, and Nafion (perfluorinated ion exchange resin, 5 wt% solution in a mixture of low-grade fatty alcohol and water) were purchased from Maclean's Reagent (Shanghai, China). sAA (derived from Bacillus subtilis), β-nicotinamide adenine dinucleotide hydrate, and β-nicotinamide adenine dinucleotide were purchased from Sigma-Aldrich (St Louis, MO, USA). Multi-walled carbon nanotubes (MWNTs) were purchased from Xianfeng Nano (Nanjing, China). Acetate buffer solutions (0.20 M) at different pH values were prepared by mixing acetic acid with sodium acetate. Phosphate buffer solutions (0.010 M) at different pH values were prepared by mixing sodium dihydrogen phosphate and disodium hydrogen phosphate. Chemicals such as CuSO4 and potassium ferricyanide were provided by Damao Chemical Reagent Factory. Deionized (DI) water was prepared using a Milli-Q plus system (Millipore Inc., Bedford, MA).2.3. Synthesis of NFs
Enzyme–inorganic hybrid NFs were prepared via a facile one-pot synthesis method.30 In brief, 20.0 μL of CuSO4 solution (0.20 M) was added to 1.0 mL of 10 mM PBS (pH 7.4) containing 0.30 mg of AG (50 U mg−1) and 0.10 mg of GDH (100 U mg−1). The resulting mixture was then incubated at 25 °C for 72 hours, followed by the removal of the supernatant through centrifugation at 8000 rpm for 5 minutes. The precipitate of NFs was washed three times using DI water, before being resuspended in 200 μL of DI water. Finally, the resulting suspension of NFs was stored at 4 °C for subsequent use.2.4. Fabrication of sensing chips
The maltose sensing chips were fabricated by modifying commercially available SPCEs. Prior to electrode modification, SPCEs were subjected to ultrasonic cleaning in solutions of anhydrous ethanol and ultrapure water for 1 minute each. Then, three functional layers were successively modified onto the surface of SPCEs. First, a layer of chitosan–MWNTs (CHIT–MWNTs) was modified onto the electrode surface by dropwise addition of 5.0 μL of the CHIT–MWNT mixture followed by air drying at room temperature. The CHIT–MWNT mixture was prepared by dispersing 1.0 mg of MWCNTs into 1.0 mL of CHIT solution (1.0 wt%) under ultrasonic oscillation. Then, a layer of dual enzyme-encapsulated NFs was modified onto the electrode surface by dropwise addition of 5.0 μL of NF suspension (1.0 mg mL−1) followed by desiccation overnight at 4 °C. Finally, 4.0 μL of Nafion solution (0.05 wt%) was added dropwise onto the electrode surface to prepare the outermost layer. The sensing chips were then ready for use and stored at 4 °C in a refrigerator. As a control, free enzyme/CHIT–MWNTs/SPCE was prepared using the same drop coating strategy. First, 5 μL of the dual enzyme mixed solution (0.30 mg mL−1 AD and 0.10 mg mL−1 GDH) was drop-coated onto CHIT–MWNTs/SPCE, and then the free enzyme was covered with the Nafion layer.2.5. Design of the smartphone-controlled electrochemical analyzer
A handheld electrochemical analyzer was developed to provide stable excitation voltage and collect current signals. The schematic diagram of the analyzer is shown in Fig. S1.† The PCB layout and component welding were designed using Altium Designer, resulting in a double panel. The analyzer consists of four main functional modules: a power management module, a microprogrammed control unit (MCU) (STM32F103VET6 chip), an electrochemical analog front end (AFE), and a Bluetooth module (HC-05). The power management module is connected to the smartphone via an OTG system to power the entire equipment. It converts the 5 V output from smartphones to the required 3.3 V for the microcontroller and other op-amps. The HC-05 Bluetooth module enables two-way communication between the analyzer and smartphones. The MCU module is responsible for analyzing and executing instructions from the smartphone. It provides constant potential for the AFE module, processes the digitized signals from the AFE module, and transmits them to smartphones. The programming for the MCU module was done using Keil uVision5 and STM32CubeMX. The electrochemical AFE module generates a stable excitation voltage and maintains a constant potential between the working electrode (WE) and the reference electrode (RE). It also filters, amplifies, and digitizes the analog current signals produced in the electrochemical redox reactions of NAD+/NADH. To control the electrochemical analyzer wirelessly and perform amperometric measurements, real-time plotting of i–t curves, and data analysis, a user-friendly Android application named “sAA detection” was developed. This application allows for easier control of the electrochemical analyzer and visualization of the results. The reliability of the quantitative sensing system was evaluated by measuring a set of saliva samples (N = 10) with unknown concentrations of sAA. The quantitation results were then compared with those obtained using the CHI630 electrochemical workstation (Chenhua Inc., Shanghai, China).2.6. Saliva sample testing
To collect saliva samples, we placed 1.0 cm × 1.0 cm test paper coated with 10% citric acid on the front 1/3 of each volunteer's tongue for 30 seconds to stimulate saliva secretion. The saliva was then collected using a cotton column and transferred to a saliva collection tube. The whole saliva sampling process can be found in Video S1.†For sAA quantitation, we filtered 100 μL of the saliva samples using medium-speed filter paper with a pore size of 30–50 μm. The filtered saliva was then added to a centrifuge tube containing 400 μL of acetate buffer (0.2 M, pH 6.0) with 0.5% amylose. After allowing the mixture to react for 15 minutes, we added 5 μL of 100.0 mM NAD+ to the buffer. The modified SPCEs were immersed in the mixture for 2 minutes before applying an excitation voltage of +0.4 V (vs. Ag/AgCl) to the sensing chip. The current value was recorded using the smartphone-controlled electrochemical analyzer and the CHI630 electrochemical workstation for 100 s, and the exact current value at 100 s was used for quantitative analysis. Video S2† shows the entire detection process. All measurements were performed in triplicate at room temperature (20–25 °C).
To validate the results, we also applied the standard clinical method, the Bernfeld method, to the same human saliva samples to determine sAA activity. The saliva sample was diluted 2000 times with pure water and then transferred to a centrifugation tube containing 1.0 mL of 1.0% soluble starch solution. The tube was placed in a 37 °C water bath for 3 minutes. After that, 1.0 mL of a color developing agent solution containing 3,5-dinitrosalicylic acid was added, and the mixture was heated in boiling water for 15 min, followed by cooling in ice water for 3 min. Finally, 9 mL of pure water was added and thoroughly mixed. The absorbance of the mixture at 540 nm was measured using an ultraviolet spectrophotometer (UVmin-1240, Shimadzu). The maltose produced by the reaction of sAA with starch in the saliva samples was obtained by converting the standard curve of absorbance and maltose concentration established previously. The activity level of sAA in the samples was obtained by converting the standard of releasing 1.0 mg of maltose with 1.0 active unit (U) of sAA at 37 °C and pH 6.9 after digesting starch for 3 minutes.
2.7. Statistical methods
The measurement consistency between the smartphone-controlled electrochemical analyzer and the CHI630 electrochemical workstation was analyzed using the paired Student's t-test. Bland–Altman analysis was conducted to evaluate the quantitation consistency between the reported method and the Bernfeld standard method. Additionally, a paired Student's t-test was conducted to determine if there were significant differences in sAA activity levels between individuals with normal and abnormal glucose metabolism.3. Results and discussion
3.1. Rationale
The rationale behind this study was based on the utilization of a smartphone-controlled electrochemical analyzer and the electrochemical redox reactions of NAD+/NADH conducted on the NF-modified electrodes (Scheme 1). The quantitation of sAA is achieved indirectly through the measurement of enzymatic production of maltose. For this purpose, a bi-enzyme system consisting of AG and GDH is designed for maltose sensing. In this system, AG hydrolyzes maltose specifically to glucose. Subsequently, GDH oxidizes glucose to glucolactone in the presence of coenzyme NAD+, resulting in the reduction of NAD+ to NADH.31–33 The generated NADH can be electrochemically oxidized to regenerate NAD+, leading to an immediate and sensitive amperometric signal. Based on these sequential enzymatic reactions, sAA activity can be correlated with the electrochemical behavior of NADH, where a higher sAA activity corresponds to a stronger electrolytic current of NAD+/NADH.Multienzyme cascade catalysis is a major category of chemical transformations and plays a crucial role in biological signal transduction and metabolic pathways. The spatial organization of enzymes in confined structures provides a universal method for designing biocatalytic cascades. The guiding principle is to direct the product of one enzyme towards serving as the substrate of the second enzyme, thereby facilitating inter-enzyme communication and creating localized high substrate concentrations. This principle enhances the efficiency of biocatalytic cascades compared to that of biocatalytic environments controlled by nonlocal diffusion.46
To address the issue of improving the stability of AG and GDH on electrodes in the designed sensing scheme, enzyme–inorganic hybrid nanoflowers (NFs) are synthesized. The advantages of these 3D NFs are that they offer (1) efficient and reproducible enzyme immobilization due to their facile and mild synthesis, (2) a large surface area with more active sites for immobilizing more enzyme molecules, allowing for immediate and sensitive determination, and (3) strong interaction between the protein and metal ions in the interstices of the petals, which significantly reduces enzyme leakage and enhances enzyme stability. The potential of NFs in improving enzyme stability has been demonstrated in a previous study where uricase loaded onto NFs showed only a 20% decrease in activity after 7 days of storage at 55 °C.34 It is worth noting that the formation of NFs is not limited by enzyme type and they can be combined with various enzyme molecules in different reactions.
3.2. Synthesis and characterization of enzyme–inorganic hybrid NFs
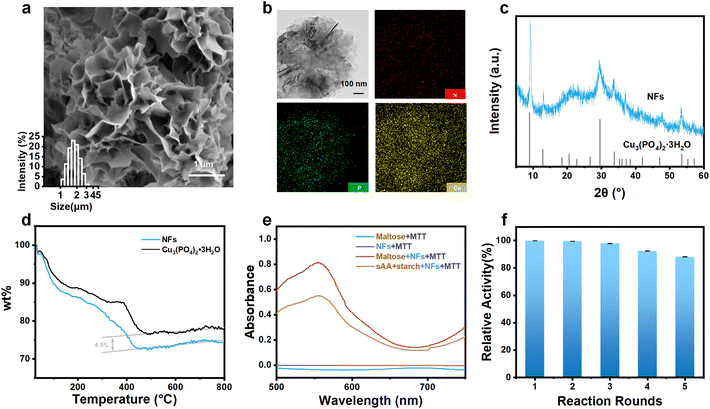
It has been well-documented that enzyme–inorganic hybrid 3D NFs can substantially enhance the stability of the immobilized enzymes for various sensing applications.34–36 In this study, AG and GDH played a crucial role in the NF synthesis by providing nucleation sites for the formation of copper phosphate crystals (Cu3(PO4)2·3H2O) which are the key component of the as-prepared NFs. The amide group within the main chain of AG (Fig. S2a–c†) and GDH (Fig. S2d–f†) coordinated with Cu2+ ions, facilitating the gradual growth of separate petal-like structures. The as-prepared NFs exhibited a flower-like multistage nanostructure composed of staggered nanosheets, as shown in Fig. 1a, under SEM analysis. The average size of these nanoflowers was found to be 1.8 ± 0.2 μm (inset of Fig. 1a). TEM images revealed a well-defined petal-like hierarchical structure of the NFs (Fig. 1b).The X-ray diffraction (XRD) pattern confirmed that the main component of the NFs is the Cu3(PO4)2·3H2O (JPSCD 00-022-0548) crystal (Fig. 1c). Energy dispersive spectroscopy (EDS) analysis provided evidence of successful loading of enzymes onto the NFs, showing a uniform distribution of the nitrogen (N) element (Fig. 1b), which is present in the enzymes only. Additionally, EDS analysis detected the presence of carbon (C), copper (Cu), phosphorus (P), and oxygen (O) in the NFs (Fig. S3†).
Since enzyme–inorganic hybrid NFs are synthesized to immobilize enzymes for the sensing purpose, the amount of enzymes loaded on the NFs is a crucial parameter in determining the analytical performance of the NF-modified electrodes. Therefore, thermogravimetric analysis (TGA) was performed, and results revealed that the combustion of the enzymes loaded on the NFs commences at approximately 100 °C and concludes at 450 °C. By comparing the mass fraction of the NFs and Cu3(PO4)2·3H2O before and after combustion, the amount of enzymes loaded on the NFs was determined to be ∼4.5% (w/w).
The most important parameter in determining the analytical performance of the NF-modified electrodes is the activity of enzymes loaded on the NFs. NADH, the end product of enzymatic cascade reactions induced by AG and GDH, can reduce thiazolium blue (MTT) to form formazan, which exhibits maximum absorbance at a wavelength of ∼570 nm when dissolved in dimethyl sulfoxide (DMSO).37 Therefore, the activity of AG and GDH loaded on the NFs could be examined by recording the absorbance at 570 nm in the presence of maltose and MTT. As shown in Fig. 1e, when the NFs were mixed with maltose and MTT, a distinct absorbance near 570 nm was observed, confirming the occurrence of enzymatic cascade reactions and validating the retained activity of AG and GDH loaded on the NFs. In addition, on mixing the NFs with sAA, starch and MTT, the same phenomenon was observed, suggesting that the product of the catalytic reaction between sAA and starch could also trigger enzymatic cascade reactions. This result directly verifies the sensing rationale of the study.
The activity of enzymes loaded on the NFs can be influenced by various factors during their synthesis, such as temperature, pH, and reaction time.38 To maintain the activity of the loaded enzymes to the greatest extent possible, the synthesis of the enzyme-loaded NFs was optimized. The results showed that the enzyme-loaded NFs synthesized at pH 7.4 (Fig. S4a†) and 25 °C (Fig. S4b†) for 72 hours (Fig. S4c†) exhibited the highest absorbance at 570 nm. These conditions were considered optimal and were used in subsequent experiments.
Furthermore, the NFs provide protection for the loaded enzymes, allowing for their activity to be maintained during repeated reactions. It was observed that even after five cycles of reaction with maltose, there was only a ∼12% decrease in enzyme activity (Fig. 1f), indicating good stability of AG and GDH loaded on the NFs.
3.3. Preparation and characterization of the NF-modified screen-printed electrode (SPCE)
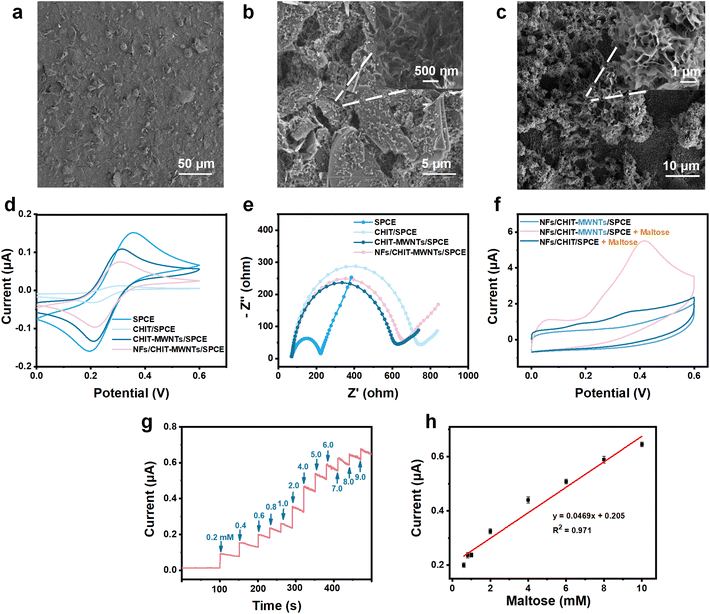
In this study, SPCEs were conditioned as sAA sensors by modifying with three different layers. The first layer of modification consisted of chitosan (CHIT) and MWNTs. In this design, CHIT served a dual function as a biocompatible dock for enzymes and as an anti-fouling reagent for minimization of electrode biocontamination. While MWNTs acted as electron transfer accelerators, counteracting the adverse effects of poor conductivity of CHIT. Compared to the rough surface of the bare SPCE (Fig. 2a), the surface of CHIT–MWNT-modified SPCEs presented typical MWNTs (Fig. 2b), indicating successful modification of the first layer. As shown in Fig. 2d and e, when SPCEs were modified with CHIT only, the redox peak currents of Fe(CN)63−/Fe(CN)64− significantly decreased, and the resistance of the electrode substantially increased. This is caused by the poor conductivity of CHIT, which hinders electron transfer. However, the addition of MWNTs significantly counteracted the adverse effects of increased resistance caused by CHIT. Fig. 2d and e clearly show that the CHIT–MWNT-modified SPCEs exhibit significantly stronger redox peak currents of Fe(CN)63−/Fe(CN)64− and substantially lower resistance than the CHIT-modified SPCEs. These results also provided evidence for the successful modification of the first layer.The second layer of modification involved dual enzyme-loaded NFs to achieve specific recognition of maltose, the product of enzymatic reaction between sAA and starch. Fig. 2c clearly shows a distinct 3D flower-like structure, indicating successful modification of the second layer. This construction of an enzyme cascade system based on the NFs, with its high specific surface area and unique layered structure for co-loading AG and GDH, improves enzyme stability while maximizing their catalytic activity. After the modification of the second layer, the NFs/CHIT–MWNT-modified SPCEs exhibited slightly decreased redox peak currents of Fe(CN)63−/Fe(CN)64− as well as slightly increased resistance, compared with the CHIT–MWNT-modified SPCEs (Fig. 2d and e). This is reasonable because the conductivity of enzymes is also not good. It is worth noting that compared to the bare SPCEs, obviously narrowed ΔEp of Fe(CN)63−/Fe(CN)64− was observed for the CHIT–MWNT-modified SPCEs and the NFs/CHIT–MWNT-modified SPCEs. This result suggested a reduction in electrode surface polarization, an improvement in the surface status of the electrode and an increased likelihood of the redox reaction of Fe(CN)63−/Fe(CN)64−. Finally, to further prevent possible leakage of enzymes and ensure the stability of sAA detection, a thin Nafion layer was coated onto SPCEs as the third layer of modification.
The modified SPCEs with NFs/CHIT–MWNTs displayed a clear electrochemical response to maltose. When the sensor was immersed in an acetate buffer solution containing 5.0 mM maltose and 2.0 mM NAD+, a distinct anodic peak near 0.4 V was observed (Fig. 2f). This indicated successful maltose response, confirming the functionality of the NFs/CHIT–MWNT-modified SPCEs as maltose sensing chips. In contrast, in the absence of maltose, no significant electrochemical redox peak was observed. Therefore, the potential for maltose detection in chronoamperometry mode was determined to be fixed at 0.4 V.
Then, the parameters for maltose detection were optimized for sAA quantitation. The NFs/CHIT–MWNT-modified SPCEs were fabricated using 1.0% (w/w) CHIT, 1.0% (w/w) MWNTs and an AG-to-GSH ratio of 1.5, which showed the highest electrochemical response to maltose (Fig. S5a–c†). The pH of the buffer solution was also optimized, with values ranging from 6.0 to 7.0 yielding the best results (Fig. S5d†). The real-time amperometric response of the NFs/CHIT–MWNT-modified SPCEs to the gradual addition of maltose is shown in Fig. 2g, with the amperometric current increasing in the concentration range of 0.2 to 10 mM. The limit of detection (LOD) was determined to be 0.20 mM (Fig. 2h).
3.4. Analytical performance of the NFs/CHIT–MWNT-modified SPCEs for sAA detection
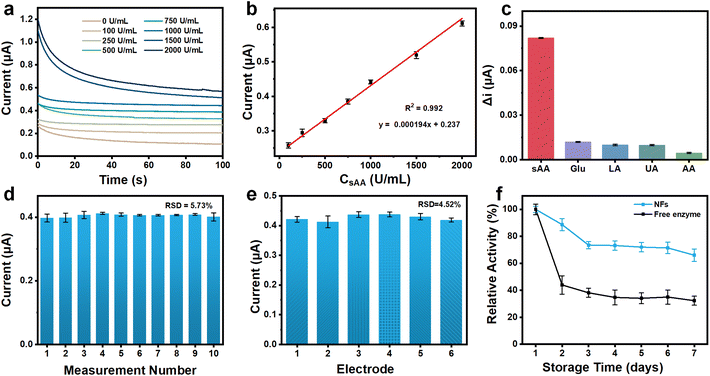
To enable sAA sensing, the NFs/CHIT–MWNT-modified SPCEs were further conditioned, considering that a specific catalytic reaction between sAA and starch could generate maltose. The parameters for sAA detection, including temperature, the amount of starch, and the reaction time between sAA and starch, were optimized. Fig. S6a† demonstrates that the electrochemical response increased with increasing temperature in the range of 5–37 °C. Although the highest response was observed at 37 °C, the electrochemical current obtained at 25 °C was approximately 87% of that obtained at 37 °C. This result indicated that even at 25 °C, the assay could still provide satisfactory sensitivity. Therefore, for convenient and on-site detection, room temperature (25 °C) was chosen as the optimum temperature for sAA detection. Regarding starch concentration, Fig. S6b† shows that higher concentrations resulted in stronger electrochemical responses. However, it should be noted that high concentrations of starch may result in incomplete dissolution and adherence on the electrode surface due to its low solubility in aqueous solutions. To balance these factors, 0.5% was selected as the optimal concentration for starch. Furthermore, Fig. S6c† illustrates that the electrochemical response underwent dramatic changes within the first 15 min and then stabilized throughout the monitoring period. As a result, 15 min was determined as the optimal reaction time between sAA and starch.Under the optimized conditions, the NFs/CHIT–MWNT-modified SPCEs were used to detect a standard solution containing 0.5% starch and various concentrations of sAA (0, 100, 250, 500, 750, 1000, 1500, and 2000 U mL−1). The chronoamperometric current increased with increasing sAA concentration (Fig. 3a), showing good linearity in the concentration range of 100–2000 U mL−1 (Fig. 3b). The LOD was determined to be 5.02 U mL−1. This sAA sensing assay demonstrated comparable detection sensitivity to and a wider dynamic range than previously reported assays (Table S1†).
Due to the high specificity of the cascade-connected enzymatic reactions between sAA and starch, AG and maltose, as well as GDH and glucose, this sAA sensing assay is anticipated to have high specificity. Fig. 3c confirms that potential coexisting substances in saliva, such as glucose (Glu), uric acid (UA), ascorbic acid (AA), and lactic acid (LA), cause only minimal changes in the amperometric signal. This excellent specificity and anti-interference capacity enable direct detection of clinical saliva samples with minimal pretreatment.
The repeatability, reproducibility, and long-term stability of the NFs/CHIT–MWNT-modified SPCEs were assessed by measuring their amperometric response to a solution containing 800 U mL−1 sAA and 0.50% starch. To test repeatability, the electrode was thoroughly rinsed with ultra-pure water and dried after each measurement. Fig. 3d shows that repeated measurements produced nearly identical results with only slight deviations. The relative standard deviation (RSD) value of the 10 measurements was calculated to be 5.73%, indicating good repeatability. To evaluate reproducibility, six different electrodes fabricated at different times were used to detect the same sample. Results showed good reproducibility, with an RSD of 4.52% (Fig. 3e).
The long-term stability of the enzymes was assessed by comparing the NFs/CHIT–MWNT-modified SPCEs with the control group, where the enzymes were directly coated onto the CHIT–MWNT layer without the NFs. Both types of electrodes were stored at room temperature for seven consecutive days. Fig. 3f shows that the enzyme activity decreased by approximately 25% and 60% in the first three days for the NFs/CHIT–MWNT-modified SPCEs and the control group, respectively. However, the enzyme activity on the NFs/CHIT–MWNT-modified SPCEs remained relatively stable for the next four days, maintaining about 70% of the original activity. On the other hand, the control group only retained about 40% of its original activity. These results clearly demonstrate that the enzymes loaded onto the NFs exhibit improved stability, and the NFs/CHIT–MWNT-modified SPCEs show good long-term stability.
3.5. Validation of the smartphone-controlled electrochemical analyzer
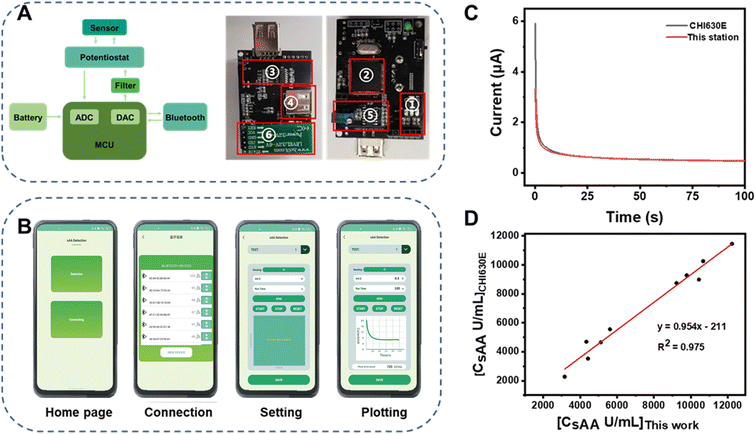
The use of smartphones for portable instrumentation has become popular due to their powerful data-processing capability, user-friendly interface, and convenient connectivity.38–43 In this study, a portable electrochemical analyzer controlled using a smartphone was developed for on-site quantitation. The system diagram and photos of the double-sided electrochemical detection circuit are illustrated in Fig. 4a. The key components included an ARM STM32 microcontroller, a potentiostat circuit, a current and voltage converter, an energy conversion module, and a Bluetooth module. The system was compact, measuring 55 mm × 45 mm × 2 mm and weighing approximately 30 g. It could be easily manufactured at a cost of around $10.The portable electrochemical analyzer was controlled using a smartphone via a self-developed application (App) (Fig. 4b). For typical detection, the Home Page pops up at first when clicking on the icon of the App (Fig. 4bI). After pressing the button of “Connection”, a list of hardware with Bluetooth around the smartphone displays on the screen (Fig. 4bII). The portable analyzer should be chosen from the list to establish wireless connection with the smartphone. Then, measurement parameters such as the potential and the time of electrolysis performed in the chronoamperometry mode can be set at this stage and sent to the analyzer via Bluetooth (Fig. 4bIII). Subsequently, the analyzer applies a constant excitation voltage to SPCEs to generate a chrono-amperometric signal, which is amplified, converted, and transmitted to the smartphone also via Bluetooth. Finally, the App processes the received data and plots real-time chrono-amperometric curves (Fig. 4bIV). A demo video of the operation of this portable instrument is supplemented in the ESI (Video S2†). The user-friendly interface of the APP enables this portable electrochemical analyzer to be operated by laypersons. It is worth noting that the App can be installed and run on Android smartphones of various brands including OPPO, Huawei, Xiaomi, etc. This portable analyzer offers advantages such as low power consumption, affordability, and convenience, enabling on-site quantitation anywhere, anytime and by anyone.
To validate the reliability of the smartphone-controlled electrochemical analyzer, a comparison was made with a CHI630 electrochemical workstation. As shown in Fig. 4c, the chronoamperometric curve generated by the smartphone-controlled electrochemical analyzer was almost the same as that generated by CHI630. Furthermore, the same set of samples (N = 10) with unknown sAA concentrations ranging from 120–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 619 U mL−1 was determined using both instruments. The results obtained from these two instruments (Fig. 4d) showed a strong correlation (R2 = 0.975) with a slope of 0.95, indicating good consistency between them. The high accuracy demonstrated by the portable electrochemical analyzer in quantitating sAA was comparable to that of CHI630.
619 U mL−1 was determined using both instruments. The results obtained from these two instruments (Fig. 4d) showed a strong correlation (R2 = 0.975) with a slope of 0.95, indicating good consistency between them. The high accuracy demonstrated by the portable electrochemical analyzer in quantitating sAA was comparable to that of CHI630.
3.6. Determination of sAA in clinical saliva samples
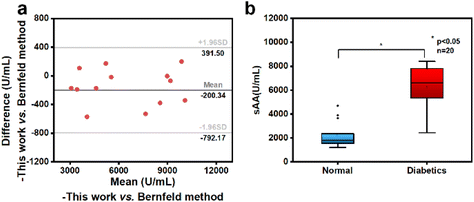
In order to assess the practicality and applicability of the sensing system, saliva samples from 13 volunteers were quantitatively analyzed using the smartphone-controlled electrochemical analyzer integrated with the NFs/CHIT–MWNT-modified SPCEs. The same samples were also analyzed using the Bernfeld method. The quantitation results from both methods were compared and are tabulated in Table S2.† Bland–Altman analysis was performed and the results are presented in Fig. 5a. All the data points were within the 95% confidence level consistency limit, indicating good quantitation consistency between the reported sensing system and the Bernfeld method. This result confirms that the sensing system can be effectively used for sAA quantitation with clinically acceptable accuracy.It has been well-documented that sAA activity in saliva of healthy people is typically in the range of 200–800 U mL−1.24–26 However, in the saliva samples of the 13 volunteers admitted to the hospital due to obesity and metabolic issues, sAA activity ranged from 3000 to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1, significantly higher than the normal level. And evidence is constantly accumulating that sAA activity is directly involved in the development of obesity and T2DM.44,45 Enlightened by these findings and with the help of clinicians, we further evaluated the feasibility of salivary sAA activity as a potential warning indicator of T2DM.
000 U mL−1, significantly higher than the normal level. And evidence is constantly accumulating that sAA activity is directly involved in the development of obesity and T2DM.44,45 Enlightened by these findings and with the help of clinicians, we further evaluated the feasibility of salivary sAA activity as a potential warning indicator of T2DM.
Twenty obese volunteers were recruited from the First Affiliated Hospital of Guangzhou University of Chinese Medicine for this study. Saliva and blood samples were collected from these volunteers to measure sAA activity using the reported sensing system and glycosylated hemoglobin (HbA1c) levels using fluorescence immunochromatography, respectively. The volunteers’ Body Mass Index (BMI) was also calculated. The data, including sAA activity, HbA1c levels, and BMI, were recorded and are presented in Table S3.† Based on their HbA1c levels, the 20 volunteers were divided into two sub-groups: one group with abnormal glucose metabolism (HbA1c > 6.5%) comprising 12 volunteers and another group with normal glucose metabolism comprising 8 volunteers. An independent sample t-test was then conducted to determine if there was a significant difference in sAA activity between these two groups. As shown in Fig. 5b, the mean sAA activity in the saliva samples of the volunteers with abnormal glucose metabolism was significantly higher than that in the group with normal glucose metabolism. This difference was statistically significant at the 0.05 level (p < 0.05). It is worth noting that even among volunteers with HbA1c levels below 6.5%, classified as having normal glucose metabolism, their salivary sAA activity was significantly higher than that of healthy individuals (200–800 U mL−1). These findings strongly suggest that salivary sAA activity holds promise as an early warning indicator of abnormal glucose metabolism in obese individuals. Considering the non-invasive nature of saliva sampling and the ease-of-use and cost-effectiveness of the sensing system used in this study, quantifying salivary sAA activity at home by laypersons could be an attractive option for obese individuals to monitor their glucose metabolism status anywhere and anytime.
4. Conclusions
In summary, on-site quantitation of sAA has been successfully demonstrated using a new quantitative POCT system which consists of the NFs/CHIT–MWNTs/SPCEs and a smartphone-controlled electrochemical analyzer. Three different layers of modification were made onto SPCEs to prepare the NFs/CHIT–MWNTs/SPCEs: a CHIT–MWNT layer in which CHIT served a dual function as a biocompatible enzyme dock and as a semi-permeable layer to minimize biocontamination on the electrode surface, while MWNTs acted as electron transfer accelerators; double enzyme (AG and GDH)-loaded NFs for specific recognition of maltose with enhanced stability; and a Nafion layer for electrode surface protection. sAA can specifically catalyze starch to produce maltose, which triggers cascade enzymatic reactions of AG–maltose and GDH–glucose in the presence of coenzyme NAD+ on the NFs/CHIT–MWNTs/SPCEs. To enable quantitation anywhere and anytime, a smartphone-controlled electrochemical analyzer with a weight of ∼30 g, which can implement chronoamperometric measurements, is manufactured at a cost of ∼10.0 USD. Quantitation of sAA in clinical saliva samples using the reported POCT system can be achieved in 20 min with clinically acceptable accuracy. A preclinical study on 20 obese volunteers reveals the great potential of sAA as an early warning indicator of abnormal glucose metabolism in obese individuals. This study offers a highly efficient and simple platform for developing diverse POCT applications and a scalable solution for home-health monitoring.Author contributions
Cong Liu: investigation, data curation, writing – original draft. Xia Gong: investigation and software. Xiao Yang: investigation and formal analysis. Zipei Yu: formal analysis and writing – review and editing. Weihao Li and Guangyi Liao: formal analysis. Chuanquan Lin and Lelun Jiang: methodology, supervision and validation. Changqing Yi: project administration, supervision, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Science and Technology Project of Guangzhou (No. 2023B03J1258, 2023B03J1276) and the Guangdong Provincial Key Laboratory of Sensing Technology and Biomedical Instruments (2020B1212060077).References
- R. M. Mizelle, Lancet, 2021, 397, 1256–1257 CrossRef.
- T. Costacou, J. Guo, R. G. Miller and T. J. Orchard, Lancet, 2019, 393, 985 CrossRef PubMed.
- P. Bjornstad, K. L. Drews, S. Caprio, R. Gubitosi-Klug, D. M. Nathan, B. Tesfaldet, J. Tryggestad, N. H. White and P. Zeitler, N. Engl. J. Med., 2021, 385, 416–426 CrossRef CAS.
- Diabetes, https://www.who.int/zh/news-room/fact-sheets/detail/diabetes, (accessed August 2023).
- J. Yang, J. Yang, X. Gong, Y. Zheng, S. Yi, Y. Cheng, Y. Li, B. Liu, X. Xie, C. Yi and L. Jiang, Adv. Healthcare Mater., 2022, 11, 2102547 CrossRef CAS.
- W. Villena Gonzales, A. T. Mobashsher and A. Abbosh, Sensors, 2019, 19, 800 CrossRef PubMed.
- I. Lee and D. Probst, Biosens. Bioelectron., 2021, 181, 113054 CrossRef CAS.
- J. Yang, X. Gong, S. Chen, Y. Zheng, L. Peng, B. Liu, Z. Chen, X. Xie, C. Yi and L. Jiang, ACS Sens., 2023, 8, 1241–1251 CrossRef CAS PubMed.
- Y. Cheng, X. Gong, J. Yang, G. Zheng, Y. Zheng, Y. Li, Y. Xu, G. Nie, X. Xie, M. Chen, C. Yi and L. Jiang, Biosens. Bioelectron., 2022, 203, 114026 CrossRef CAS.
- R. Goldoni, M. Farronato, S. T. Connelly, G. M. Tartaglia and W.-H. Yeo, Biosens. Bioelectron., 2021, 171, 112723 CrossRef CAS.
- I. T. Gug, M. Tertis, O. Hosu and C. Cristea, Trends Anal. Chem., 2019, 113, 301–316 CrossRef CAS.
- B. Ciui, M. Tertis, C. N. Feurdean, C. N. Feurdean, A. Ilea, R. Sandulescu, J. Wang and C. Cristea, Sens. Actuators, B, 2019, 281, 399–407 CrossRef CAS.
- A. N. Ramdzan, M. Almeida, M. J. McCullough and S. D. Kolev, Anal. Chim. Acta, 2016, 919, 47–54 CrossRef CAS PubMed.
- C. Moonla and K. Sakdaphetsiri, Biosens. Bioelectron., 2023, 220, 114891 CrossRef CAS.
- M. Castagnola, P. M. Picciotti, I. Messana, C. Fanali, A. Fiorita, T. Cabras, L. Calò, E. Pisano, G. C. Passali, F. Iavarone, G. Paludetti and E. Scarano, Acta Otorhinolaryngol. Ital., 2011, 31, 347–357 CAS.
- U. M. Nater and N. Rohleder, Psychoneuroendocrinology, 2009, 34, 486–496 CrossRef CAS PubMed.
- E. Azzopardi, C. Lloyd, S. R. Teixeira, R. S. Conlan and I. S. Whitaker, Surgery, 2016, 160, 26–37 CrossRef PubMed.
- K. Nakajima, World J. Diabetes, 2016, 7, 112 CrossRef.
- R. E. G. Tiongco, E. S. Arceo, N. S. Rivera, C. C. D. Flake and A. R. Policarpio, Diabetes Metab. Syndr., 2019, 13, 2601–2605 CrossRef.
- P. D. G. Catherine and P. A. S. Breslin, Curr. Diabetes Rep., 2016, 16, 1–7 CrossRef.
- T. V. da Silva Santos, R. R. Teixeira, D. L. Franco, J. M. Madurro, A. G. Brito-Madurro and F. S. Espindola, Mater. Sci. Eng., C, 2012, 32, 530–535 CrossRef.
- B. D. Ventura, N. Sakač, R. Funari and R. Velotta, Talanta, 2017, 174, 52–58 CrossRef.
- I. Tsyrulneva, P. Alagappan and B. Liedberg, ACS Sens., 2019, 4, 865–873 CrossRef CAS.
- L. Zhang, W. Yang, Y. Yang, H. Liu and Z. Gu, Analyst, 2015, 140, 7399–7406 RSC.
- P. T. Garcia, L. N. Guimarães, A. A. Dias, C. J. Ulhoa and W. K. T. Coltro, Sens. Actuators, B, 2018, 258, 342–348 CrossRef CAS.
- P. T. Garcia, A. A. Dias, J. A. C. Souza and W. K. T. Coltro, Anal. Chim. Acta, 2018, 1041, 50–57 CrossRef CAS PubMed.
- S. Kurbanoglu, C. Erkmen and B. Uslu, Trends Anal. Chem., 2020, 124, 115809 CrossRef CAS.
- R. K. Mishra, J. R. Sempionatto, Z. Li, C. Brown, N. M. Galdino, R. Shah, S. Liu, L. J. Hubble, K. Bagot, S. Tapert and J. Wang, Talanta, 2020, 211, 120757 CrossRef CAS PubMed.
- T. Guang, W. Huang, N. Xu, Z. Xu, L. Jiang, M. Li, X. Wei, Y. Liu, X. Shen, X. Li and C. Yi, Sens. Actuators, B, 2019, 294, 132–140 CrossRef.
- J. Ge, J. Lei and R. N. Zare, Nat. Nanotechnol., 2012, 7, 428–432 CrossRef CAS.
- A. Liu, Q. Lang, B. Liang and J. Shi, Biosens. Bioelectron., 2017, 87, 25–30 CrossRef CAS PubMed.
- H. Teymourian, A. Barfidokht and J. Wang, Chem. Soc. Rev., 2020, 21, 7671–7709 RSC.
- H. Lee, Y. J. Hong, S. Baik, T. Hyeon and D.-H. Kim, Adv. Healthcare Mater., 2018, 7, 1701150 CrossRef.
- X. Xing, B. Yao, Q. Wu, R. Zhang, L. Yao, J. Xu, G. Gao and W. Chen, Biosens. Bioelectron., 2022, 198, 113804 CrossRef CAS.
- M. Zhang, Y. Zhang, C. Yang, C. Ma and J. Tang, Talanta, 2021, 224, 121840 CrossRef CAS PubMed.
- R. Jin, D. Kong, X. Zhao, H. Li, X. Yan, F. Liu, P. Sun, D. Du, Y. Lin and G. Lu, Biosens. Bioelectron., 2019, 141, 111473 CrossRef CAS.
- M. Ito, C. Codony-Servat, J. Codony-Servat, D. Lligé, I. Chaib, X. Sun, J. Miao, R. Sun, X. Cai, A. Verlicchi, M. Okada, M. A. Molina-Vila, N. Karachaliou, P. Cao and R. Rosell, Cell Commun. Signaling, 2019, 17, 139 CrossRef.
- Y. Li, X. Fei, L. Liang, J. Tian, L. Xu, X. Wang and Y. Wang, J. Mol. Catal., 2016, 133, 92–97 CrossRef CAS.
- D. Zhang and Q. Liu, Biosens. Bioelectron., 2016, 75, 273–284 CrossRef CAS.
- J. Liu, Z. Geng, Z. Fan, Ji. Liu and H. Chen, Biosens. Bioelectron., 2019, 132, 17–37 CrossRef CAS.
- S. Xia, J. Pan, D. Dai, Z. Dai, M. Yang and C. Yi, Chin. Chem. Lett., 2023, 34, 107799 CrossRef CAS.
- N. Xu, M. Xiao, Z. Yu, B. Jin, M. Yang and C. Yi, Food Chem., 2024, 431, 137107 CrossRef CAS PubMed.
- Y. Liu, M. Xiao, N. Xu, M. Yang and C. Yi, Sens. Actuators, B, 2022, 367, 132083 CrossRef CAS.
- D. G. C. Peyrot and P. A. Breslin, Curr. Diabetes Rep., 2016, 10, 102 CrossRef.
- S. E. Abd-Elraheem and H. H. Mansour, Diabetes Metab. Syndr., 2017, 11, S637–S641 CrossRef.
- W. H. Chen, M. Vázquez-González and A. Zoabi, et al. , Nat. Catal., 2018, 1, 689–695 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr04388f |
| This journal is © The Royal Society of Chemistry 2024 |