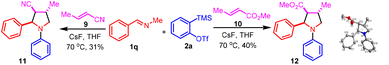A novel three-component coupling reaction of aryne, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) N derivatives, and acrylonitrile†
N derivatives, and acrylonitrile†
Yangang
Wu
,
Wenbo
Cai
,
Lulu
Yu
,
Junbiao
Chang
* and
Chuanjun
Song
 *
*
College of Chemistry, Zhengzhou University, Zhengzhou 450001, China. E-mail: changjunbiao@zzu.edu.cn; chjsong@zzu.edu.cn
First published on 13th November 2023
Abstract
A literature-unprecedented three-component aryne coupling reaction with pyrroline, imine, dihydroisoquinoline, imidazoline or oxazoline as a nucleophile, and acrylonitrile as a third component, acting as both a proton donor and a nucleophile, has been developed. With imidazoline or oxazoline as the substrate, the initially formed three-component reaction product could react further to provide tetrahydro-1,4-diazepine or tetrahydro-1,4-oxazepine, respectively.
The aza-Baylis–Hillman reaction1,2 is an atom economical and operationally simple carbon–carbon bond forming reaction between a Michael acceptor and an imine in the presence of a Lewis base, affording α-methylene-β-aminocarbonyl derivatives. It has found wide application in the synthesis of natural products3 and biologically active molecules.1b,c,4 Unfortunately, the reaction rates are sluggish, and the substrate scope is limited to activated aldimines (N-sulfonylimines, N-sulfinylimines, N-phosphinylimines) only. Thus, the development of an efficient strategy with a broad substrate scope is still desirable.
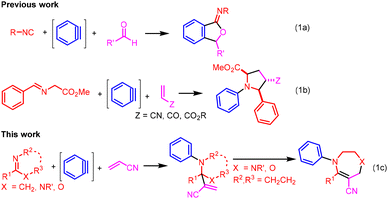
Over the last few decades, arynes5 have emerged as useful synthetic intermediates in organic synthesis.6 Because of their low-lying LUMO, arynes are readily attacked by neutral nucleophiles including isocyanides, imines, and amides to form zwitterionic species. In 2004, Yoshida et al. developed a novel three-component coupling reaction for the synthesis of iminoisobenzofurans by trapping with aldehydes the zwitterion generated from the nucleophilic attack of isocyanides on arynes (Scheme 1a).7 With a judicious choice of nucleophile and electrophile combinations, various benzoannulated heterocycles and multisubstituted arenes could be constructed via the three-component aryne coupling reactions.8–10 In 2015, Hwu and co-workers demonstrated a three-component reaction of benzyne, glycine Schiff base, and an electron-deficient alkene for the synthesis of pyrrolidines, which proceeded via a [3 + 2] cycloaddition pathway between the electron-deficient alkene and the azomethine ylide generated in situ from the aryne and glycine Schiff base (Scheme 1b).11 The presence of the electron-withdrawing ester group was key for the transformation of the initially formed zwitterionic species to the azomethine ylide for the [3 + 2] cycloaddition reaction. It is against this background we wonder that without such an electron-withdrawing group, will the zwitterion from the nucleophilic attack of an imine on an aryne react with an electron-deficient alkene by [4 + 2] cycloaddition to provide tetrahydroquinoline species. However, our studies indicate that the three-component coupling reactions of benzyne, acrylonitrile, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N derivatives result in the formation of aza-Baylis–Hillman type reaction products instead (Scheme 1c). In these transformations, acrylonitrile acts as both a proton donor and a nucleophile (vide infra). Herein, we report the details of these novel three-component aryne coupling reactions. With imidazolines or oxazolines as nucleophiles, the initially formed three-component coupling products could react further to yield 1,4-diazepines or 1,4-oxazepines, respectively. These structural motifs are found in nature,12 and many synthetic analogues have shown a broad spectrum of biological activities as anticonvulsants,13 dopamine D4 receptor ligands,14 immunosuppressants,15 glycosidase inhibitors,16 nitric oxide synthase inhibitors,17 and antifungal, antibacterial, and anticancer agents.18 Our work provides a new method toward the synthesis of these pharmaceutically important heterocyclic systems.
N derivatives result in the formation of aza-Baylis–Hillman type reaction products instead (Scheme 1c). In these transformations, acrylonitrile acts as both a proton donor and a nucleophile (vide infra). Herein, we report the details of these novel three-component aryne coupling reactions. With imidazolines or oxazolines as nucleophiles, the initially formed three-component coupling products could react further to yield 1,4-diazepines or 1,4-oxazepines, respectively. These structural motifs are found in nature,12 and many synthetic analogues have shown a broad spectrum of biological activities as anticonvulsants,13 dopamine D4 receptor ligands,14 immunosuppressants,15 glycosidase inhibitors,16 nitric oxide synthase inhibitors,17 and antifungal, antibacterial, and anticancer agents.18 Our work provides a new method toward the synthesis of these pharmaceutically important heterocyclic systems.
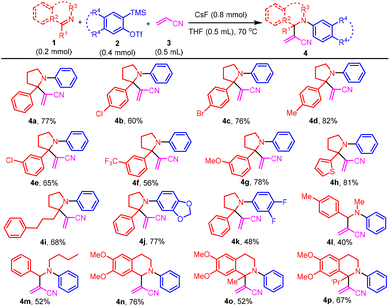
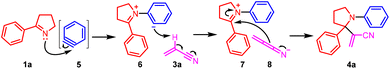
Our initial attempt of the three-component coupling reaction of 2-phenyl-1-pyrroline191a (0.2 mmol), 2-(trimethylsilyl)phenyl triflate 2a (0.4 mmol), and acrylonitrile 3 (0.5 mL) in the presence of CsF (0.8 mmol) in THF (0.5 mL) at 70 °C afforded compound 4a in 77% isolated yield (Scheme 2). The optimization of reaction conditions has been described in detail in the ESI.† The key to the success of the reaction was the use of a large excess of acrylonitrile. Otherwise, the product 4a′ resulting from benzyne-triggered hydrolysis of 1a was dominant (ESI).† With acrylonitrile-2-d3 as the substrate, the corresponding 4a-D was obtained in 69% isolated yield (Scheme 3). Based on this result, a plausible mechanism was proposed (Scheme 4). The nucleophilic attack on benzyne 5 (generated from 2a) by pyrroline 1a afforded zwitterion 6. Subsequent deprotonation of acrylonitrile 3 with 6 provided cation 7 and anion 8, which reacted to furnish 4a. Intrigued by this novel three-component coupling reaction, we examined the substrate scope. 2-Aryl-1-pyrrolines with either an electron-withdrawing group or electron-donating group substituted benzene ring could tolerate the reaction conditions, and resulted in the formation of the three-component coupling products 4b–4g in moderate to good isolated yields. Compound 4h with a thiophenyl moiety and 4i with an alkyl side-chain could be obtained. The aryne precursors were examined. While 4j from methylenedioxybenzyne was obtained in a comparable yield to 4a, compound 4k from difluorobenzyne was isolated in a much lower yield. Under the conditions, simple imines as well as dihydroisoquinolines performed as expected to provide compounds 4l–4p in moderate to good isolated yields. Finally, we tested the three-component reaction of crotonitrile 9 with imine 1q and benzyne (Scheme 5). The reaction gave a mixture of products, from which the [3 + 2] cycloaddition product 11 was isolated.11 However, it was contaminated with a minor inseparable impurity and a clean NMR spectrum could not be obtained. Fortunately, with methyl crotonate 10 as a component, pyrrolidine-3-carboxylate 12 was isolated in 40% yield as a single diastereoisomer, the relative stereochemistry of which was confirmed by X-ray single crystal diffraction.20
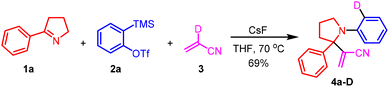 | ||
| Scheme 3 Three-component coupling reaction of pyrroline 1a, 2-(trimethylsilyl)phenyl triflate 2a, and acrylonitrile-2-d3. | ||
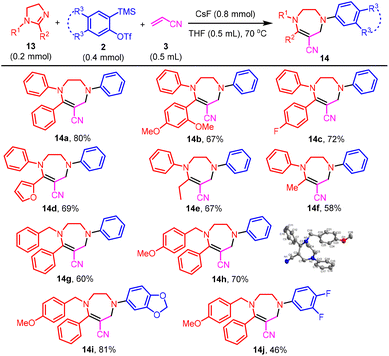
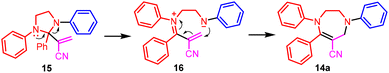
Next, we tested the three-component coupling reaction of imidazoline, benzyne, and acrylonitrile. The reaction of 1,2-diphenylimidazoline 13a with benzyne and acrylonitrile 3 generated tetrahydro-1,4-diazepine 14a, rather than the corresponding formal aza-Baylis–Hillman reaction product as shown in Scheme 2 (Scheme 6). We postulated that 14a was yielded from the initially formed formal aza-Baylis–Hillman reaction product 15via a ring-opening/closing process (Scheme 7). The reaction of representative 2-aryl-1-phenylimidazolines and 2-alkyl-1-phenylimidazolines proceeded smoothly to provide tetrahydro-1,4-diazepines 14b–14f in good isolated yields. Next, 1-benzyl-2-phenylimidazoline was evaluated. Of the two possible tetrahydro-1,4-diazepines, only 14g was obtained, and no trace of the alternative product was evident on the TLC plate. Similarly, with 1-(4-methoxybenzyl)-2-phenylimidazoline as the substrate, the sole product isolated was 14h, the structure of which was unambiguously confirmed by X-ray single crystal diffraction.21 With 1-(4-methoxybenzyl)-2-phenylimidazoline as a nucleophile, aryne precursors were examined. It was observed that electron-donating groups favored product formation (14ivs.14j).
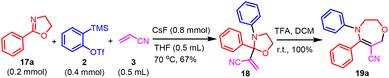
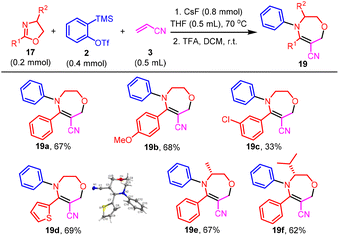
Finally, we explored the three-component coupling reaction of oxazoline, benzyne, and acrylonitrile. The reaction of 2-phenyloxazoline 17a with benzyne and acrylonitrile provided oxazolidine 18 as a stable compound, which could be isolated and fully characterized (Scheme 8). On standing, 18 gradually transformed into tetrahydro-1,4-oxazepine 19a. This process could complete in 10 min by treatment of the former with TFA at ambient temperature. Therefore, we applied the two-step procedure for the rest of our study of the three-component reaction to obtain tetrahydro-1,4-oxazepine directly. As shown in Scheme 9, while the reaction of 2-(4-methoxyphenyl)oxazoline and 2-(thiophen-2-yl)oxazoline proceeded smoothly to provide 19b and 19d in good isolated yields, the reaction of the substrate bearing an electron-deficient chlorophenyl moiety was less satisfactory, giving 19c in 33% isolated yield. The structure of 19d was unambiguously confirmed by X-ray single crystal diffraction.22 Chiral oxazolines could tolerate the reaction conditions to afford 19e and 19f in good isolated yields without racemization of the stereogenic center as determined with chiral HPLC.
Conclusions
In summary, we have developed a novel three-component coupling reaction of benzyne, acrylonitrile, and various nucleophiles including pyrroline, imine, dihydroisoquinoline, imidazoline, and oxazoline. Interestingly, the imidazolidine and oxazolidine intermediates could react further to provide tetrahydro-1,4-diazepine and tetrahydro-1,4-oxazepine, respectively. Biological studies of the compounds synthesized are currently underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the NSFC (82130103, 82373724) for financial support.References
- For reviews, see: (a) V. Declerck, J. Martinez and F. Lamaty, Aza-Baylis−Hillman Reaction, Chem. Rev., 2009, 109, 1–48 CrossRef CAS PubMed; (b) Y. Wei and M. Shi, Recent Advances in Organocatalytic Asymmetric Morita-Baylis-Hillman/aza-Morita-Baylis-Hillman Reactions, Chem. Rev., 2013, 113, 6659–6690 CrossRef CAS PubMed; (c) Y. L. Shi and M. Shi, Aza–Baylis–Hillman Reactions and Their Synthetic Applications, Eur. J. Org. Chem., 2007, 2905–2916 CrossRef CAS; (d) G. Masson, C. Housseman and J. Zhu, The Enantioselective Morita–Baylis–Hillman Reaction and Its aza Counterpart, Angew. Chem., Int. Ed., 2007, 46, 4614–4628 CrossRef CAS PubMed.
- For selected recent examples, see: (a) B. Yang, M. Shen, X. Ji, Z. Xu, H. Sun, B. Jiang and G. Li, Chiral N-Phosphonyl Imines for an Aza-Morita-Baylis-Hillman Reaction via Group-Assisted Purification (GAP) Chemistry, J. Org. Chem., 2016, 81, 2488–2493 CrossRef CAS; (b) S. Grélaud, J. Lusseau, S. Massip and Y. Landais, Acyl Radical Addition onto aza-Baylis-Hillman Adducts: A Stereocontrolled Access to 2,3,5-Trisubstituted Pyrrolidines, Adv. Synth. Catal., 2017, 359, 2434–2441 CrossRef; (c) X. Lu and U. Schneider, Aza-Morita-Baylis-Hillman Reactions Catalyzed by a Cyclopropenylidene, Chem. Commun., 2016, 52, 12980–12983 RSC; (d) T. Milcent, J. Hao, K. Kawada, V. A. Soloshonok, S. Ongeri and B. Crousse, Highly Stereoselective Aza-Baylis-Hillman Reactions of CF3-Sulfinylimines: Straightforward Access to α-Methylene β-CF3 β-Amino Acids, Eur. J. Org. Chem., 2014, 3072–3075 CrossRef CAS; (e) K. Hyodo, S. Nakamura and N. Shibata, Enantioselective Aza-Morita-Baylis-Hillman Reactions of Acrylonitrile Catalyzed by Palladium(II) Pincer Complexes Having C2-symmetric Chiral Bis(imidazoline) Ligands, Angew. Chem., Int. Ed., 2012, 51, 10337–10341 CrossRef CAS PubMed.
- (a) I. P. Andrews and O. Kwon, Enantioselective Total Synthesis of (+)-ibophyllidine via an Asymmetric Phosphine-catalyzed [3 + 2] Annulation, Chem. Sci., 2012, 3, 2510–2514 RSC; (b) S. Ishikawa, F. Noguchi and A. Kamimura, Asymmetric Synthesis of 2-alkyl-substituted 2,5-dihydropyrroles from Optically Active Aza-Baylis-Hillman Adducts. Formal Synthesis of (-)-Trachelanthamidine, J. Org. Chem., 2010, 75, 3578–3586 CrossRef CAS PubMed; (c) G. Sirasani, T. Paul, W. Dougherty Jr., S. Kassel and R. B. Andrade, Concise Total Syntheses of (+/-)-Strychnine and (+/-)-Akuammicine, J. Org. Chem., 2010, 75, 3529–3532 CrossRef CAS PubMed; (d) G. Sirasani and R. B. Andrade, Sequential One-Pot Cyclizations: Concise Access to the ABCE Tetracyclic Framework of Strychnos Alkaloids, Org. Lett., 2009, 11, 2085–2088 CrossRef CAS PubMed; (e) G. Sirasani and R. B. Andrade, Total Synthesis of (−)-Leuconicine A and B, Org. Lett., 2011, 13, 4736–4737 CrossRef CAS; (f) L. S. Hutchings-Goetz, C. Yang, J. W. B. Fyfe and T. N. Snaddon, Enantioselective Syntheses of Strychnos and Chelidonium Alkaloids through Regio- and Stereocontrolled Cooperative Catalysis, Angew. Chem., Int. Ed., 2020, 59, 17556–17564 CrossRef CAS PubMed.
- D. Basavaiah, A. J. Rao and T. Satyanarayana, Recent Advances in the Baylis−Hillman Reaction and Applications, Chem. Rev., 2003, 103, 811–892 CrossRef CAS PubMed.
- (a) J. Shi, L. Li and Y. Li, o-Silylaryl Triflates: A Journey of Kobayashi Aryne Precursors, Chem. Rev., 2021, 121, 3892–4044 CrossRef CAS PubMed; (b) T. Kitamura, Synthetic Methods for the Generation and Preparative Application of Benzyne, Aust. J. Chem., 2010, 63, 987–1001 CrossRef CAS.
- (a) A. V. Dubrovskiy, N. A. Markina and R. C. Larock, Use of Benzynes for The Synthesis of Heterocycles, Org. Biomol. Chem., 2013, 11, 191–218 RSC; (b) M. Sarmah, A. Sharma and P. Gogoi, Exploration of Kobayashi's Aryne Precursor: A Potent Reactive Platform for The Synthesis of Polycyclic Aromatic Hydrocarbons, Org. Biomol. Chem., 2021, 19, 722–737 RSC; (c) H. Takikawa, A. Nishii, T. Sakai and K. Suzuki, Aryne-based Strategy in the Total Synthesis of Naturally Occurring Polycyclic Compounds, Chem. Soc. Rev., 2018, 47, 8030–8056 RSC; (d) T. Roy and A. T. Biju, Recent Advances in Molecular Rearrangements Involving Aryne Intermediates, Chem. Commun., 2018, 54, 2580–2594 RSC; (e) A. E. Goetz, T. K. Shah and N. K. Garg, Pyridynes and Indolynes as Building Blocks for Functionalized Heterocycles and Natural Products, Chem. Commun., 2015, 51, 34–45 RSC; (f) P. M. Tadross and B. M. Stoltz, A Comprehensive History of Arynes in Natural Product Total Synthesis, Chem. Rev., 2012, 112, 3550–3577 CrossRef CAS; (g) C. M. Gampe and E. M. Carreira, Arynes and Cyclohexyne in Natural Product Synthesis, Angew. Chem., Int. Ed., 2012, 51, 3766–3778 CrossRef CAS; (h) S. S. Bhojgude and A. T. Biju, Arynes in Transition-Metal-Free Multicomponent Coupling Reactions, Angew. Chem., Int. Ed., 2012, 51, 1520–1522 CrossRef CAS PubMed; (i) H. Yoshida and K. Takaki, Aryne Insertion Reactions into Carbon-Carbon σ-Bonds, Synlett, 2012, 1725–1732 CrossRef CAS.
- H. Yoshida, H. Fukushima, J. Ohshita and A. Kunai, Arynes in a Three-component Coupling Reaction: Straightforward Synthesis of Benzoannulated Iminofurans, Angew. Chem., Int. Ed., 2004, 43, 3935–4028 CrossRef CAS PubMed.
- (a) A. Bhunia, D. Porwal, R. G. Gonnade and A. T. Biju, Multicomponent Reactions Involving Arynes, Quinolines, and Aldehydes, Org. Lett., 2013, 15, 4620–4623 CrossRef CAS PubMed; (b) K. M. Allan, C. D. Gilmore and B. M. Stoltz, Benzannulated Bicycles by Three-Component Aryne Reactions, Angew. Chem., Int. Ed., 2011, 50, 4488–4491 CrossRef CAS; (c) H. Yoshida, T. Morishita and J. Ohshita, Direct Access to Anthranilic Acid Derivatives via CO2 Incorporation Reaction Using Arynes, Org. Lett., 2008, 10, 3845–3847 CrossRef CAS; (d) H. Yoshida, T. Morishita, H. Fukushima, J. Ohshita and A. Kunai, Three-Component Coupling of Arynes, Aminosilanes, and Aldehydes, Org. Lett., 2007, 9, 3367–3370 CrossRef CAS PubMed; (e) H. Yoshida, H. Fukushima, T. Morishita, J. Ohshita and A. Kunai, Three-component Coupling using Arynes and Isocyanides: Straightforward Access to Benzo-annulated Nitrogen or Oxygen Heterocycles, Tetrahedron, 2007, 63, 4793–4805 CrossRef CAS; (f) H. Yoshida, H. Fukushima, J. Ohshita and A. Kunai, CO2 Incorporation Reaction Using Arynes: Straightforward Access to Benzoxazinone, J. Am. Chem. Soc., 2006, 128, 11040–11041 CrossRef CAS PubMed; (g) H. Yoshida, H. Fukushima, J. Ohshita and A. Kunai, Straightforward Access to 2-Iminoisoindolines via Three-component Coupling of Arynes, Isocyanides and Imines, Tetrahedron Lett., 2004, 45, 8659–8662 CrossRef CAS.
- (a) X. Huang, W. Zhao, D.-L. Chen, Y. Zhan, T. Zeng, H. Jin and B. Peng, Benzyne-mediated Trichloromethylation of Chiral Oxazolines, Chem. Commun., 2019, 55, 2070–2073 RSC; (b) S.-J. Li, Y. Wang, J.-K. Xu, D. Xie, S.-K. Tian and Z.-X. Yu, Formal Insertion of Imines (or Nitrogen Heteroarenes) and Arynes into the C–Cl Bond of Carbon Tetrachloride, Org. Lett., 2018, 20, 4545–4548 CrossRef CAS; (c) J. Tan, B. Liu and S. Su, Aryne Triggered Dearomatization Reaction of Isoquinolines and Quinolines with Chloroform, Org. Chem. Front., 2018, 5, 3093–3097 RSC; (d) J.-K. Xu, S.-J. Li, H.-Y. Wang, W.-C. Xu and S.-K. Tian, Three-component Carboarylation of Unactivated Imines with Arynes and Carbon Nucleophiles, Chem. Commun., 2017, 53, 1708–1711 RSC; (e) H. Yoshida, Y. Asatsu, Y. Mimura, Y. Ito, J. Ohshita and K. Takaki, Three-Component Coupling of Arynes and Organic Bromides, Angew. Chem., Int. Ed., 2011, 50, 9676–9679 CrossRef CAS PubMed; (f) M. Jeganmohan, S. Bhuvaneswari and C.-H. Cheng, Synthesis of N-Arylated 1,2-Dihydroheteroaromatics Through the Three-Component Reaction of Arynes with N-Heteroaromatics and Terminal Alkynes or Ketones, Chem. – Asian J., 2010, 5, 153–159 CrossRef CAS; (g) F. Sha and X. Huang, A Multicomponent Reaction of Arynes, Isocyanides, and Terminal Alkynes: Highly Chemo- and Regioselective Synthesis of Polysubstituted Pyridines and Isoquinolines, Angew. Chem., Int. Ed., 2009, 48, 3458–3461 CrossRef CAS PubMed; (h) M. Jeganmohan and C.-H. Cheng, Reaction of arynes, N-heteroaromatics and nitriles, Chem. Commun., 2006, 2454–2456 RSC.
- (a) A. Guin, R. N. Gaykar, S. Deswal and A. T. Biju, Three-Component, Diastereoselective [6 + 3] Annulation of Tropone, Imino Esters, and Arynes, Org. Lett., 2021, 23, 7456–7461 CrossRef CAS; (b) H. Hazarika and P. Gogoi, Direct Synthesis of Ortho-methylthio Allyl and Vinyl Ethers via Three Component Reaction of Aryne, Activated Alkene and DMSO, Org. Biomol. Chem., 2020, 18, 2727–2738 RSC; (c) K. Liu, L.-L. Liu, C.-Z. Gu, B. Dai and L. He, Aryne-Induced Dearomatized Phosphonylation of Electron-deficient Azaarenes, RSC Adv., 2016, 6, 33606–33610 RSC; (d) S.-E. Suh and D. M. Chenoweth, Aryne Compatible Solvents are not Always Innocent, Org. Lett., 2016, 18, 4080–4083 CrossRef CAS PubMed; (e) H. Yoshida, Y. Ito and J. Ohshita, Three-component Coupling Using Arynes and DMF: Straightforward Access to Coumarins via Ortho-quinone Methides, Chem. Commun., 2011, 47, 8512–8514 RSC; (f) E. Yoshioka, S. Kohtani and H. Miyabe, A Multicomponent Coupling Reaction Induced by Insertion of Arynes into the C-O Bond of Formamide, Angew. Chem., Int. Ed., 2011, 50, 6638–6642 CrossRef CAS PubMed.
- S. P. Swain, Y.-C. Shih, S.-C. Tsay, J. Jacob, C.-C. Lin, K. C. Hwang, J.-C. Horng and J. R. Hwu, Aryne-Induced Novel Tandem 1,2-Addition/(3+2) Cycloaddition to Generate Imidazolidines and Pyrrolidines, Angew. Chem., Int. Ed., 2015, 54, 9926–9930 CrossRef CAS PubMed.
- (a) D. J. St Jean and C. Fotsch, Mitigating Heterocycle Metabolism in Drug Discovery, J. Med. Chem., 2012, 55, 6002–6020 CrossRef CAS PubMed; (b) F. Cabezas, A. RamÍrez, F. Viladomat, C. Codina and J. Bastida, Alkaloids from Eucharis amazonica (Amaryllidaceae), Chem. Pharm. Bull., 2003, 51, 315–317 CrossRef CAS PubMed; (c) B. I. Khodorov, Batrachotoxin as A Tool to Study Voltage-sensitive Sodium Channels of Excitable Membranes, Prog. Biophys. Mol. Biol., 1985, 45, 57–148 CrossRef CAS PubMed.
- (a) G. Sharma, J. Y. Park and M. S. Park, Synthesis and Anticonvulsant Evaluation of 6-amino-1,4-oxazepane-3,5-dione Derivatives, Arch. Pharmacal Res., 2008, 31, 838–842 CrossRef CAS PubMed; (b) G. Sharma, J. Y. Park and M. S. Park, Design and Synthesis of 6-amino-1,4-oxazepane-3,5-dione Derivatives as Novel Broad Spectrum Anticonvulsants, Bioorg. Med. Chem. Lett., 2008, 18, 3188–3191 CrossRef CAS PubMed.
- K. Audouze, E. Ø. Nielsen and D. Peters, New Series of Morpholine and 1,4-Oxazepane Derivatives as Dopamine D4 Receptor Ligands: Synthesis and 3D-QSAR Model, J. Med. Chem., 2004, 47, 3089–3104 CrossRef CAS PubMed.
- F. E. Koehn, O. J. McConnell, R. E. Longley, S. H. Sennett and J. K. Reed, Analogs of the Marine Immunosuppressant Microcolin A: Preparation and Biological Activity, J. Med. Chem., 1994, 37, 3181–3186 CrossRef CAS PubMed.
- P. A. Burland and H. M. I. Osborn, Synthesis and Glycosidase Inhibitory Profiles of Functionalised Morpholines and Oxazepanes, Bioorg. Med. Chem., 2011, 19, 5679–5692 CrossRef CAS.
- K. Shankaran, K. L. Donnelly, S. K. Shah, C. G. Caldwell, P. Chen, W. K. Hagmann, M. MacCoss, J. L. Humes, S. G. Pacholok, T. M. Kelly, S. K. Grant and K. K. Wong, Synthesis of Analogs of (1,4)-3- and 5-imino oxazepane, Thiazepane, and Diazepane as Inhibitors of Nitric Oxide Synthases, Bioorg. Med. Chem. Lett., 2004, 14, 5907–5911 CrossRef CAS PubMed.
- (a) X.-H. Liu, Y.-M. Jia, B.-A. Song, Z.-X. Pang and S. Yang, Design and Synthesis of Novel 2-Methyl-4,5-substitutedbenzo[f]-3,3a,4,5-tetrahydro-pyrazolo[1,5-d][1,4]oxazepin-8(7H)-one Derivatives as Telomerase Inhibitors, Bioorg. Med. Chem. Lett., 2013, 23, 720–723 CrossRef CAS PubMed; (b) S. Chandrasekhar, M. Seenaiah, A. Kumar, C. R. Reddy, S. K. Mamidyala, C. G. Kumar and S. Balasubramanian, Intramolecular Copper(I)-catalyzed 1,3-dipolar Cycloaddition of Azido-alkynes: Synthesis of Triazolo-benzoxazepine Derivatives and Their Biological Evaluation, Tetrahedron Lett., 2011, 52, 806–808 CrossRef CAS; (c) S. Kaneko, M. Arai, T. Uchida, T. Harasaki, T. Fukuoka and T. Konosu, Synthesis and Evaluation of N-substituted 1,4-oxazepanyl Sordaricins as Selective Fungal EF-2 inhibitors, Bioorg. Med. Chem. Lett., 2002, 12, 1705–1708 CrossRef CAS.
- H. Huang, Q. Yang, Q. Zhang, J. Wu, Y. Liu, C. Song and J. Chang, A Hofmann Rearrangement–Ring Expansion Cascade for the Synthesis of 1-Pyrrolines: Application to the Synthesis of 2,3-Dihydro-1H-pyrrolo[2,1-a]isoquinolinium Salts, Adv. Synth. Catal., 2016, 358, 1130–1135 CrossRef CAS.
- Crystallographic data for structural analysis have been deposited with the Cambridge Crystallographic Data Center, CCDC 2189779.†.
- Crystallographic data for structural analysis have been deposited with the Cambridge Crystallographic Data Center, CCDC 2190017.†.
- Crystallographic data for structural analysis have been deposited with the Cambridge Crystallographic Data Center, CCDC 2190240.†.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and NMR spectra. CCDC 2189779; 2190017; 2190240. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo01454a |
| This journal is © the Partner Organisations 2024 |