Synthesis of imidazo[1,2-a]pyridinones via a visible light-photocatalyzed functionalization of alkynes/nitrile insertion/cyclization tandem sequence using micro-flow technology†
Minghui
Wei
a,
Jiankun
Chen
a,
Chengkou
Liu
a,
Zhao
Yang
b,
Hong
Qin
a,
Yujing
Hu
a,
Jindian
Duan
a,
Yuguang
Li
d,
Zheng
Fang
 *ac and
Kai
Guo
*ac and
Kai
Guo
 *ac
*ac
aCollege of Biotechnology and Pharmaceutical Engineering Nanjing Tech University, 30 Puzhu Rd S., Nanjing, 211816, China. E-mail: guok@njtech.edu.cn; fzcpu@163.com; Fax: +86 255813 9935; Tel: +86 25 5813 9926
bCollege of Engineering, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing, 210003, China. E-mail: yzcpu@163.com
cState Key Laboratory of Materials-Oriented Chemical Engineering, 30 Puzhu Rd S., Nanjing, 211816, China
dInstitute of Nanjing Advanced Biomaterials & Processing Equipment, China. E-mail: Liyuguang@njibp.com
First published on 17th November 2023
Abstract
We successfully developed an unprecedented route for imidazo[1,2-a]pyridinone synthesis through a visible light-photocatalysed functionalization of alkynes/nitrile insertion/cyclization tandem sequence in a microchannel reactor. This novel protocol features extremely mild conditions, broad substrate scope and high reaction efficiency. In addition, a reasonable reaction mechanism was proposed based on control experiments.
Introduction
Imidazo[1,2-a]pyridinone compounds, which are a class of very important nitrogen-containing condensed heterocyclic compounds, have become the research focus of organic chemists and pharmaceutical chemists on account of their specific physiological activities and structural similarity with indole, azaindole, etc.1 Many imidazo[1,2-a]pyridinone compounds have obvious inhibitory effects on many target enzymes,2–4 and have antiviral, antibacterial, and antimicrobial properties. Some imidazo[1,2-a]pyridinone compounds can be used as drugs to treat gastric ulcers, diabetes and psychosis,5–7 especially in the treatment of tumors.8,9 Therefore, rapid and efficient assembly of ring-containing structures has become an important synthesis method in modern organic synthesis as they are widespread in biologically active molecules and natural products.10,11 For decades, great endeavors have been devoted to the exploration of efficient and practical strategies employing radical cascade sequences for ring-forming transformations that deliver targeted molecular assemblies.12,13 Among various synthetic approaches, relay processes mediated by radical 1,n-hydrogen atom transfer (1,n-HAT, n = 5 or 6) displayed a diversity of synthetic tools to assemble cyclic structural scaffolds with remarkable features.14–23 In this context, as a versatile class, iminyl radicals have been involved in a wide range of synthetic applications and have gained increasing attention recently in synthetic chemistry because of their promising potential in 1,n-HAT-mediated relay processes for the construction of N-containing cyclic molecules (Scheme 1a).16–21,24–28 Recently, Liao and co-workers29 reported a new copper-catalyzed alkene-trifluoromethylation-triggered nitrile insertion/remote functionalization relay process (Scheme 1b).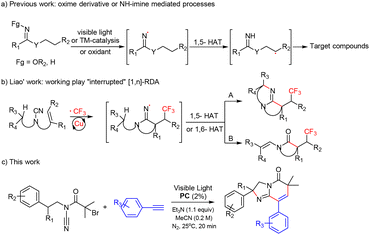 | ||
| Scheme 1 Approaches for the preparation of azaheterocycles via radical cyclization involving iminyl radicals. | ||
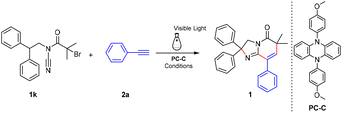
Despite the excellent achievement of these strategies, most of them suffer from some limitations, such as the use of metal catalysts, harsh reaction conditions, and poor efficiencies, thereby restricting their use. Accordingly, an environmentally friendly and novel process for imidazo[1,2-a]pyridinone construction is in urgent demand. Inspired by these studies and due to our continuous efforts devoted to photochemical reactions, we present an unprecedented route for imidazo[1,2-a]pyridinone synthesis through a visible light-photocatalysed functionalization of alkynes/nitrile insertion/cyclization tandem sequence. This novel protocol features extremely mild conditions, broad substrate scope and high reaction efficiency (Scheme 1c).
At the beginning, the reaction of 2-bromo-N-cyano-N-(2,2-diphenylethyl)-2-methylpropanamide (1k) and phenylacetylene (2a) was selected as the model reaction to determine the suitable reaction conditions through screening a series of reaction parameters. First, the model reaction was carried out under a N2 atmosphere for 12 hours in the presence of light of various wavelengths. The results are presented in Table 1. A 67% yield was obtained when the wavelength of light was 420 nm–430 nm (Table 1, entry 4).
| Entry | Wavelength of light | Yield of 1b (%) |
|---|---|---|
| a Reaction conditions: 1k (1.0 mmol, 1.0 equiv.), 2a (2.0 mmol, 2.0 equiv.), PC-C (2.0 mol%), K3PO4 (1.1 mmol, 1.1 equiv.), solvent: DCE (5 mL), N2, 25 °C, 12 h; model of lamps used: 10 W, 220 V, LED. b Isolated yield. | ||
| 1 | 360–370 nm | 55 |
| 2 | 380–385 nm | 48 |
| 3 | 390–398 nm | 53 |
| 4 | 420–430 nm | 67 |
| 5 | 435–445 nm | 35 |
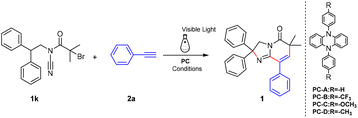
Then the reaction conditions were further optimized. The results are presented in Table 2. First, the model reaction was carried out under a N2 atmosphere for 12 hours in the presence of various organic photocatalysts, such as PC-A, PC-B, PC-C, and PC-D,30,31 and K3PO4 (1.1 equiv.), resulting in the formation of the desired product (1) in 46% to 69% yields (Table 2, entries 1–4). And the reaction could not occur in the absence of either a photocatalyst or visible light (Table 2, entries 5 and 6). Then investigation of bases showed that a weak base (Et3N) was slightly more suitable for this transformation (Table 2, entry 10). And the yield of the reaction is significantly reduced without a base (Table 2, entry 14). Subsequently, a series of solvents were screened including DCE, MeCN, THF, DMA, DMF, cyclohexane, and 1,4-dioxane. Gratifyingly, when the MeCN solvent is selected, the yield of the target product 1 is significantly higher (Table 2, entries 15–20). Then, the substrate concentration and the amount of catalyst were further optimized. Delightfully, an 82% yield was obtained when 0.2 M MeCN and 2% of PC-B were used. Finally, reaction times of 1 h–12 h were also screened (ESI†). When the reaction time was increased to 4 h, an 81% yield of the target product 1 was obtained. As the reaction time went on, the yield of 1 was able to slightly increase in the batch reaction, with the yield being just 82%.
| Entry | PC (equiv.) | Base (1.1 equiv.) | Solvent (0.25 M) | Time (h) | Yield of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1k (1.0 mmol, 1.0 equiv.), 2a (2.0 mmol, 2.0 equiv.), N2, 25 °C; blue light (10 W, 220 V, LED, wavelength 420 nm–430 nm). b Isolated yield. c No light. | |||||
| 1 | PC-A (2%) | K3PO4 | DCE | 12 | 58 |
| 2 | PC-B (2%) | K3PO4 | DCE | 12 | 69 |
| 3 | PC-C (2%) | K3PO4 | DCE | 12 | 67 |
| 4 | PC-D (2%) | K3PO4 | DCE | 12 | 46 |
| 5 | None | K3PO4 | DCE | 12 | None |
| 6c | PC-B (2%) | K3PO4 | DCE | 12 | None |
| 7 | PC-B (2%) | K2CO3 | DCE | 12 | 53 |
| 8 | PC-B (2%) | NaHCO3 | DCE | 12 | 49 |
| 9 | PC-B (2%) | LiOtBu | DCE | 12 | 42 |
| 10 | PC-B (2%) | Et3N | DCE | 12 | 71 |
| 11 | PC-B (2%) | DMAP | DCE | 12 | 48 |
| 12 | PC-B (2%) | DBU | DCE | 12 | 36 |
| 13 | PC-B (2%) | Pyridine | DCE | 12 | 44 |
| 14 | PC-B (2%) | None | DCE | 12 | 37 |
| 15 | PC-B (2%) | Et3N | MeCN | 12 | 82 |
| 16 | PC-B (2%) | Et3N | THF | 12 | 55 |
| 17 | PC-B (2%) | Et3N | DMA | 12 | 43 |
| 18 | PC-B (2%) | Et3N | DMF | 12 | 41 |
| 19 | PC-B (2%) | Et3N | Cyclohexane | 12 | 50 |
| 20 | PC-B (2%) | Et3N | 1,4-Dioxane | 12 | 78 |
Through the above experiments, although the metal residue problem has been solved, the reaction time is relatively long and there are more by-products in the batch reaction for the synthesis of piperidones. This may be caused by uneven illumination and the low mass transfer efficiency in batch reactions.
Studies have shown that microfluidic devices are very useful chemical synthesis platforms32 and have a wide range of applications in green chemistry.33 Compared with traditional chemical batch reactors, microfluidic reactors have finer reaction channels, which can increase the illumination area, improve mass and heat transfer, increase the reaction rate and effectively avoid the possibility of polymerization side reactions.34 Hence, we envisioned that phenethylamine-derived bromides might directly undergo cyclization tandem sequence reactions with alkynes via diaryldihydrophenazine catalysis to assemble imidazo[1,2-a]pyridinones under visible light conditions in a microchannel reactor (see Scheme 1). The overall process can be carried out under mild and metal-free conditions.
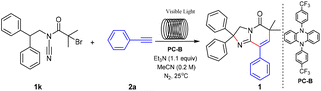
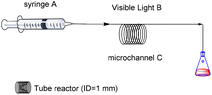
Initially, the reaction of 2-bromo-N-cyano-N-(2,2-diphenylethyl)-2-methylpropanamide (1k) and phenylacetylene (2a) was selected as the model reaction to identify appropriate reaction conditions through screening a series of reaction parameters in a microchannel reactor under simulated sunlight conditions. And the results are summarized in Table 3. As shown in Table 3, the microchannel reactor consisted of syringe pumps (A), visible light (B), and a microchannel (C). The volume of the syringe is 10 mL. The reaction residence time can be modulated by adjusting the flow rate of the syringe. First, the amount of catalyst was investigated (Table 3, entries 1–4). Obviously, a series of experiments revealed that a 93% yield was obtained when 1 mol% of PC-B was used (Table 3, entry 2). Then the length of the quartz tube was tested (Table 3, entries 5–7). However, a 93% yield was obtained when the length of the quartz tube is 2 meters (Table 3, entry 2). In order to further optimize the reaction conditions, the residence time in the microchannel reactor was screened (Table 3, entries 8–11). The optimal residence time is 20 minutes (Table 3, entry 10).
| Entry | PC-B (equiv.) | Tube diameter (mm) | Tube length (m) | Residence time (minutes) | Yield of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1k (1.0 mmol,1.0 equiv.), 2a (1.0 mmol, 1.0 equiv.), Et3N (1.1 mmol, 1.1 equiv.), 5 mL MeCN (0.2 M) solution, N2, 25 °C; blue light (10 W, 220 V, LED, wavelength 420 nm–430 nm). b Isolated yield. | |||||
| 1 | 2% | 1 | 2 | 30 | 85 |
| 2 | 1% | 1 | 2 | 30 | 93 |
| 3 | 0.5% | 1 | 2 | 30 | 53 |
| 4 | 0.1% | 1 | 2 | 30 | 34 |
| 5 | 1% | 1 | 3 | 30 | 84 |
| 6 | 1% | 1 | 1 | 30 | 81 |
| 7 | 1% | 1 | 0.5 | 30 | 68 |
| 8 | 1% | 1 | 2 | 5 | 37 |
| 9 | 1% | 1 | 2 | 10 | 45 |
| 10 | 1% | 1 | 2 | 20 | 91 |
| 11 | 1% | 1 | 2 | 40 | 92 |
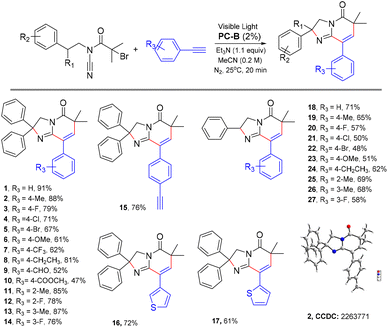
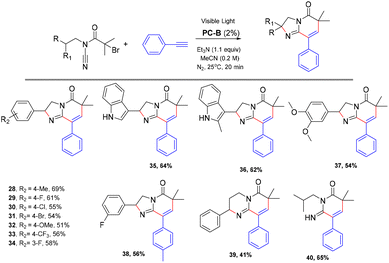
After optimising the conditions, we probed the scope of alkynes that could be used in this photocatalytic cyclization tandem sequence reaction. As shown in Scheme 2, varying the electronic properties of the benzene ring substituent did not significantly influence the reaction efficiency. Phenylethynyl with electron-rich substituents (e.g., -Me) and electron-deficient substituents (e.g., –F, –Cl, –Br, –OCH3 and –CF3) performed well, affording the desired products in generally high yields (Scheme 2, 2–15, 18–27). The structure of 2 was confirmed using single crystal X-ray diffraction analysis (more details are shown in the ESI†).
Subsequently, we probed the scope of phenethylamine-derived bromides that could be used in this photocatalytic cyclization tandem sequence reaction. As shown in Scheme 3, varying the electronic properties of the benzene ring substituent did not significantly influence the reaction efficiency. Except for the methoxy group, phenethylamine-derived bromides with electron-rich substituents (e.g., –Me) and electron-deficient substituents (e.g., –F, –Cl, –Br and –CF3) performed well, affording the desired products in generally high yields (Scheme 3, 28–33). In addition, we found that amphetamine-derived bromides can also cyclize with phenylacetylene to form the corresponding products (Scheme 3, 39).
To explore the practical value of this method, a scale-up continuous flow reactor was set up (Scheme 4). The reaction proceeded effectively in this continuous flow reactor system, and products 1, 2 and 3 (more details are shown in the ESI†) were obtained in 89%, 81% and 72% yields, respectively.
 | ||
| Scheme 4 Reaction conditions: 1k (10 mmol), 2a (1 equiv.), MeCN (20 mL), Et3N (1.1 equiv.), and PC-B (1% equiv.) at room temperature for 20 minutes. Isolated yield. | ||
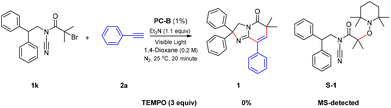
Subsequently, to explore the mechanism of this reaction, a few control experiments were carried out (Scheme 5). When there is no light or photocatalyst PC-B, the yield of 1 is 0% (Table 2, entries 5 and 6). Therefore, the reaction can occur normally only when the organic photocatalyst PC-B and visible light exist simultaneously. Then, when the radical scavenger TEMPO (3 equiv.) was added to the reaction under the optimized conditions, the transformation was strongly inhibited, indicating that the reaction process goes through a radical pathway (Scheme 5).
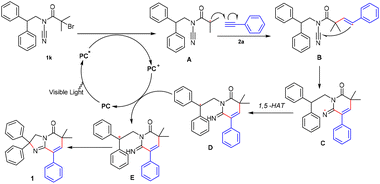
On the basis of these studies, control experiments, and previous reports,30,35–39 a plausible mechanism was proposed (Scheme 6). First, the organic photocatalyst PC-B was exposed to visible light to form an excited state PC-B*. At this time, PC-B* has a strong enough reducing power to induce 2-bromo-1-(1H-indol-1-yl)-2-methylpropan-1-one to form free radical A. Then free radical A and phenylethynyl combined to form free radical B. Then free radical B attacked the 2-position of indole to form free radical C. Finally, free radical C formed the target product 1 under the action of the organic photocatalyst PC-B+ and Et3N.
Conclusions
In summary, we successfully developed an unprecedented route for imidazo[1,2-a]pyridinone synthesis through a visible light-photocatalysed functionalization of alkynes/nitrile insertion/cyclization tandem sequence in a microchannel reactor. This novel protocol features extremely mild conditions, broad substrate scope and high reaction efficiency. In addition, the amplification experiment shows that this method has certain practicality. We believe that this study could provide researchers with inspiration for the synthesis of novel ring-containing structures.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was supported by the National Natural Science Foundation of China (Grant No. 22078150 and 22278221), the Guangdong Provincial Key R&D Programme (Grant No. 2021B0909060003), the Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture (Grant No. XTC2202), and the Jiangsu Province Industrial Prospects and Key Core Technologies-Competitive Projects (Grant No. BE2021083).References
- P. Vanelle, C. Castera and M. J. H. D. Crozet, An Efficient Synthetic Route to New Imidazo[1,2-a]pyridines by Cross-Coupling Reactions in Aqueous Medium, Heterocycles, 2005, 65, 2979 CrossRef.
- M. A. Ismail, R. Brun, T. Wenzler, F. A. Tanious, W. D. Wilson and D. W. Boykin, Novel dicationic imidazo 1,2-a pyridines and 5,6,7,8-tetrahydro-imidazo 1,2-a pyridines as antiprotozoal agents, Eur. J. Med. Chem., 2004, 47, 3658–3664 CrossRef CAS.
- A. M. Palmer, B. Grobbel, C. Jecke, C. Brehm, P. J. Zimmermann, W. Buhr, M. P. Feth, W. A. Simon and W. Kromer, Synthesis and evaluation of 7H-8,9-Dihydropyrano 2,3-c imidazo 1,2-a pyridines as potassium-competitive acid blockers, Eur. J. Med. Chem., 2007, 50, 6240–6264 CrossRef CAS PubMed.
- A. R. Katritzky, Y. J. Xu and H. B. Tu, Regiospecific synthesis of 3-substituted imidazo 1,2-a pyridines, imidazo 1,2-a pyrimidines, and imidazo 1,2-c pyrimidine, J. Org. Chem., 2003, 68, 4935–4937 CrossRef CAS PubMed.
- A. Scribner, R. Dennis, S. L. Lee, G. Ouvry, D. Perrey, M. Fisher, M. Wyvratt, P. Leavitt, P. Liberator, A. Gurnett, C. Brown, J. Mathew, D. Thompson, D. Schmatz and T. Biftu, Synthesis and biological activity of imidazopyridine anticoccidial agents: Part II, Eur. J. Med. Chem., 2008, 43, 1123–1151 CrossRef CAS PubMed.
- G.-B. Liang, X. Qian, D. Feng, M. Fisher, C. M. Brown, A. Gurnett, P. S. Leavitt, P. A. Liberator, A. S. Misura, T. Tamas, D. M. Schmatz, M. Wyvratt and T. Biftu, Synthesis and SAR studies of potent imidazopyridine anticoccidial agents, Bioorg. Med. Chem. Lett., 2007, 17, 3558–3561 CrossRef CAS PubMed.
- S. L. Colletti, J. L. Frie, E. C. Dixon, S. B. Singh, B. K. Choi, G. Scapin, C. E. Fitzgerald, S. Kumar, E. A. Nichols, S. J. O'Keefe, E. A. O'Neill, G. Porter, K. Samuel, D. M. Schmatz, C. D. Schwartz, W. L. Shoop, C. M. Thompson, J. E. Thompson, R. X. Wang, A. Woods, D. M. Zaller and J. B. Doherty, Hybrid-designed inhibitors of p38 MAP kinase utilizing N-arylpyridazinones, Eur. J. Med. Chem., 2003, 46, 349–352 CrossRef CAS.
- T. Ikemoto, T. Kawamoto, K. Tomimatsu and M. J. Tetrahedron, A Practical Synthesis of the Chronic Renal Disease Agent, 4,5-Dihydro-3H-1,4,8b-triazaacenaphthylen-3-one Derivatives, Using Regioselective Chlorination of Ethyl 5-methylimidazo[1,2-a]pyridine-3-carboxylate with N-Chlorosuccinimide, Tetrahedron, 2010, 56, 7915–7921 CrossRef.
- M. Hayakawa, H. Kaizawa, K. Kawaguchi, N. Ishikawa, T. Koizumi, T. Ohishi, M. Yamano, M. Okada, M. Ohta, S. J. B. Tsukamoto and M. Chemistry, Synthesis and biological evaluation of imidazo[1,2-a]pyridine derivatives as novel PI3 kinase p110alpha inhibitors, Bioorg. Med. Chem., 2007, 15, 403 CrossRef CAS.
- M. Aldeghi, S. Malhotra, D. L. Selwood and A. W. E. Chan, Two-and Three-dimensional Rings in Drugs, Chem. Biol. Drug Des., 2014, 83, 450–461 CrossRef CAS.
- R. D. Taylor, M. MacCoss and A. D. G. Lawson, Rings in Drugs, Eur. J. Med. Chem., 2014, 57, 5845–5859 CrossRef CAS.
- A. Studer and D. P. Curran, Catalysis of Radical Reactions: A Radical Chemistry Perspective, Angew. Chem., Int. Ed., 2016, 55, 58–102 CrossRef CAS.
- H. M. Huang, M. H. Garduno-Castro, C. Morrill and D. J. Procter, Catalytic cascade reactions by radical relay, Chem. Soc. Rev., 2019, 48, 4626–4638 RSC.
- J. Robertson, J. Pillai and R. K. Lush, Radical translocation reactions in synthesis, Chem. Soc. Rev., 2001, 30, 94–103 RSC.
- Z. Cekovic, Reactions of delta-carbon radicals generated by 1,5-hydrogen transfer to alkoxyl radicals, Tetrahedron, 2003, 59, 8073–8090 CrossRef CAS.
- S. Chiba and H. Chen, sp(3) C-H oxidation by remote H-radical shift with oxygen- and nitrogen-radicals: a recent update, Org. Biomol. Chem., 2014, 12, 4051–4060 RSC.
- X. Q. Hu, J. R. Chen and W. J. Xiao, Controllable Remote C-H Bond Functionalization by Visible-Light Photocatalysis, Angew. Chem., Int. Ed., 2017, 56, 1960–1962 CrossRef CAS.
- J. Davies, S. P. Morcillo, J. J. Douglas and D. Leonori, Hydroxylamine Derivatives as Nitrogen-Radical Precursors in Visible-Light Photochemistry, Chem. – Eur. J., 2018, 24, 12154–12163 CrossRef CAS.
- J. C. K. Chu and T. Rovis, Complementary Strategies for Directed C(sp(3))-H Functionalization: A Comparison of Transition-Metal-Catalyzed Activation, Hydrogen Atom Transfer, and Carbene/Nitrene Transfer, Angew. Chem., Int. Ed., 2018, 57, 62–101 CrossRef CAS.
- X. X. Wu and C. Zhu, Recent advances in alkoxy radical-promoted C-C and C-H bond functionalization starting from free alcohols, Chem. Commun., 2019, 55, 9747–9756 RSC.
- H. Chen and S. Y. Yu, Remote C-C bond formation via visible light photoredox-catalyzed intramolecular hydrogen atom transfer, Org. Biomol. Chem., 2020, 18, 4519–4532 RSC.
- K. Löffler and C. Freytag, Über eine neue Bildungsweise von N–alkylierten Pyrrolidinen, Ber. Dtsch. Chem. Ges., 2006, 42, 3427–3431 CrossRef.
- A. W. Hofmann, Ueber die Einwirkung des Broms in alkalischer Lösung auf die Amine, Ber. Dtsch. Chem. Ges., 2006, 16, 558–560 CrossRef.
- S. Z. Zard, Recent progress in the generation and use of nitrogen-centred radicals, Chem. Soc. Rev., 2008, 37, 1603–1618 RSC.
- T. Xiong and Q. Zhang, New amination strategies based on nitrogen-centered radical chemistry, Chem. Soc. Rev., 2016, 45, 3069–3087 RSC.
- J. R. Chen, X. Q. Hu, L. Q. Lu and W. J. Xiao, Visible light photoredox-controlled reactions of N-radicals and radical ions, Chem. Soc. Rev., 2016, 45, 2044–2056 RSC.
- M. D. Karkas, Photochemical Generation of Nitrogen-Centered Amidyl, Hydrazonyl, and Imidyl Radicals: Methodology Developments and Catalytic Applications, ACS Catal., 2017, 7, 4999–5022 CrossRef.
- W. Q. Yin and X. L. Wang, Recent advances in iminyl radical-mediated catalytic cyclizations and ring-opening reactions, New J. Chem., 2019, 43, 3254–3264 RSC.
- J. M. Xi, Y. H. Sun, W. C. Li, Y. H. Wu, Z. L. Wei and W. W. Liao, Radical Alkene-Trifluoromethylation-Triggered Nitrile Insertion/Remote Functionalization Relay Processes: Diverse Synthesis of Trifluoromethylated Azaheterocycles Enabled by Copper Catalysis, Org. Lett., 2022, 24, 1110–1115 CrossRef CAS PubMed.
- J. C. Theriot, C. H. Lim, H. Yang, M. D. Ryan, C. B. Musgrave and G. M. Miyake, Organocatalyzed atom transfer radical polymerization driven by visible light, Sci, 2016, 352, 1082–1086 CrossRef CAS.
- K. O. Puffer, D. A. Corbin and G. M. Miyake, Impact of Alkyl Core Substitution Kinetics in Diaryl Dihydrophenazine Photoredox Catalysts on Properties and Performance in O-ATRP, ACS Catal., 2023, 13, 14042–14051 CrossRef CAS.
- K. S. Elvira, X. C. i. Solvas, R. C. R. Wootton and A. J. deMello, The past, present and potential for microfluidic reactor technology in chemical synthesis, Nat. Chem., 2013, 5, 905–915 CrossRef CAS.
- S. Mozharov, A. Nordon, D. Littlejohn, C. Wiles, P. Watts, P. Dallin and J. M. Girkin, Improved Method for Kinetic Studies in Microreactors Using Flow Manipulation and Noninvasive Raman Spectrometry, J. Am. Chem. Soc., 2011, 133, 3601–3608 CrossRef CAS.
- C. Wiles and P. Watts, Translation of microwave methodology to continuous flow for the efficient synthesis of diaryl ethers via a base-mediated SNAr reaction, Beilstein J. Org. Chem., 2011, 7, 1360–1371 CrossRef CAS.
- K. J. Bian, Y. Li, K. F. Zhang, Y. He, T. R. Wu, C. Y. Wang and X. S. Wang, Iron-catalyzed remote functionalization of inert C(sp(3))-H bonds of alkenesvia1,n-hydrogen-atom-transfer by C-centered radical relay, Chem. Sci., 2020, 11, 10437–10443 RSC.
- G. H. Lonca, D. Y. Ong, T. M. H. Tran, C. Tejo, S. Chiba and F. Gagosz, Anti-Markovnikov Hydrofunctionalization of Alkenes: Use of a Benzyl Group as a Traceless Redox-Active Hydrogen Donor, Angew. Chem., Int. Ed., 2017, 56, 11440–11444 CrossRef CAS PubMed.
- P. Yu, J. S. Lin, L. Li, S. C. Zheng, Y. P. Xiong, L. J. Zhao, B. Tan and X. Y. Liu, Enantioselective C-H Bond Functionalization Triggered by Radical Trifluoromethylation of Unactivated Alkene, Angew. Chem., Int. Ed., 2014, 53, 11890–11894 CrossRef CAS.
- Z. Li, Y.-H. Wu, J.-M. Xi, Z.-L. Wei and W.-W. Liao, Copper-Catalyzed Difluoroalkylation of Alkene/Nitrile Insertion/Cyclization Tandem Sequences: Construction of Difluorinated Bicyclic Amidines, Org. Lett., 2021, 23, 9591–9596 CrossRef CAS PubMed.
- M. Wei, C. Liu, C.-S. Wang, Y. Li, P. Qiu, Q. Dong, Z. Yang, Z. Fang and K. Guo, Synthesis of pyrido[1,2-a]indol-6(7H)-ones via a visible light-photocatalyzed formal (4 + 2) cycloaddition of indole-derived bromides and alkenes or alkynes, Green Chem., 2023, 25, 2453–2457 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2263771. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo01508d |
| This journal is © the Partner Organisations 2024 |