Open Access Article
Open Access ArticleBetanin dye extracted from ayrampo (Opuntia soehrensii) seeds to develop dye-sensitized solar cells†
Heather V. Flinta,
Harry Anderson Rivera Titob,
Richard D. Jamesa,
Fabio Cucinottaa,
Elizabeth Gibson a and
María Esther Quintana Caceda
a and
María Esther Quintana Caceda *b
*b
aEnergy Materials Laboratory, School of Natural and Environmental Science, Newcastle University, Newcastle-upon-Tyne, NE1 7RU, UK. E-mail: elizabeth.gibson@newcastle.ac.uk
bCenter for the Development of Advanced Materials and Nanotechnology, National University of Engineering, Av. Tupac Amaru 210, Lima 25, Peru. E-mail: mquintana@uni.edu
First published on 25th March 2024
Abstract
Pigments extracted from ayrampo seeds of the Peruvian-native prickly pear (Opuntia soehrensii) were used in dye-sensitized solar cells with promising efficiencies. The performance of the solar cells was then improved via the addition of citric acid to stabilise the photosensitive dye, and an efficiency of 1.41% was achieved with current output remaining stable after 7 days. Upon testing in low-light conditions, the solar conversion efficiency of devices increased to 4%. This paper not only highlights the potential of natural sensitizers in DSSCs but also shows that simple extraction and gentle handling methods can contribute to the device performance.
Introduction
The first working dye-sensitised solar cells (DSSCs) were produced by Graetzel and O'Regan in 1991.1 The principal components of a DSSC include the dye, electrolyte, semiconductor, and counter electrode. DSSCs utilize dyes to absorb photons of specific wavelengths and inject the resulting excited electrons into the conduction band of the semiconductor to cause charge to flow and generate a photocurrent. The redox electrolyte reduces the photo-oxidised dye and regenerates the ground state by transporting electrons from the counter electrode to the photoelectrode surface. Since the semiconductor components in the devices are nanocrystalline, charge separation within the material is dependent on diffusion rates to the front and back contacts in the device. DSSCs also perform relatively well in diffuse light conditions and high temperatures, with limitless possibilities for integration and application into everyday life due to their varied colours, shapes and transparency.2Over 3 million people in Peru do not have direct access to electricity, many of whom live in remote regions with no access to the mainline power grid. Installation of off-grid energy sources such as solar panels, or the replacement of widely used oil lamps with LEDs, could help reduce energy poverty and could assist in the improvement of the general health and amenities available to those living in rural areas in Peru. Yet there are ancient traditions that are practiced widely by artisans in Peru and Bolivia that can tie directly into the manufacture of dye-sensitized solar cells (DSSC). Weavers use ancient practices handed down through the generations to dye sheep and alpaca wool using simple methods and easy-to-find mordants. These mordants help to set the dye into the fiber and can traditionally include lemon juice, alum, ferrous sulfate, copper sulfate, or even urine. The solvent is usually water, and the dyeing process is often in large batches with minimal waste. The colour of the resulting wool products will be dependent on factors including the natural pH of the dye material, the temperature, and the type of mordants used. However, much knowledge surrounding natural dying processes is not recorded quantitively. Information is passed with a ‘common sense’ approach and there is very little exact data to be found in what is, culturally, an art form.
Unlike artificial sensitizers, which are often designed with specific charge transfer or absorption properties, dyes from vegetation have developed with the organism in mind. This means that the focus of research in DSSCs with natural dyes is not on the optimization of the dye molecules, but rather the selection of sources and extraction of dye components, followed by extensive optimisation of the systems surrounding the dye (including but not limited to the semiconductor morphology, electrolyte composition, pH and concentrations). Yet due to their versatility and photochemical properties, natural products in photovoltaic devices have been studied before, using beets, grapes, blackberry, hibiscus, and other natural products as the source material for the photosensitizers.3 Now, sensitizers from nature are used to demonstrate the principles of DSSC and renewable energy sources in classrooms and teaching labs around the world.4
Most natural dyes can be categorised into four groups: anthocyanins, betalains, carotenoids and chlorophylls.5 Most work has focussed on the more common anthocyanin and chlorophyll dyes. DSSCs containing anthocyanin reported in literature reach almost 2% efficiency using raw extracts, with good results found using citric acid as a solvent and/or stabilizer in some instances.6 Losses in the efficiency were attributed by Zhang et al. to either increased dye aggregation on the films, or electron recapture by the redox electrolyte, but these observations could be also attributed to most natural (or indeed synthetic) dye systems, not just the anthocyanins studied.7 Extensive analysis of chlorophyll derivatives used in TiO2 based-DSSC was carried out by Kay and Graetzel in 1993, where Chl-a was found to bind to the TiO2 surface only poorly due to the weak interaction of its ester and keto carbonyl groups with the hydrophilic oxide layer. The use of solvents with lower polarity such as hexane or diethyl ether improved dye absorption significantly, but a strong broadening and redshift of the film's absorption spectrum compared to the dye in solvent suggested that there was an aggregation of the dye on the surface.8 Further investigations concluded that only chlorophyll sensitizers bearing carboxylic acids directly conjugated to π-electrons of the chromophore could exhibit an effective electron injection into the TiO2 electrode. Wang et al. investigated the common chlorophyll variants from wakame seaweed extracts, and their findings agreed that reduced dye aggregation due to the favourable configuration of the peripheral vinyl carboxyl group was instrumental in reaching the high (η ∼3.4–4.6%) efficiencies achieved.9 However, most investigations into the compounds' properties have been in highly purified or synthetic samples and not with raw extracts.
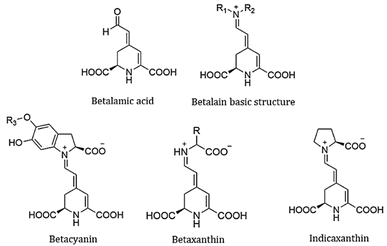
The focus of this work is a family of molecules known as Achkiy,10 which have a variety of colours depending on their structure. The core structure is an iminium derivative of betalamic acid and the dyes within this group are classified according to the substituents, R1 and R2 in Fig. 1 for example, betacyanins (usually purple) are the product of condensation between the backbone and cyclo-DOPA, whereas condensation with amines or other amino acids gives rise to the betaxanthins (often yellow in colour). Prickly pear contains betanin and indicaxanthin (proline-betaxanthin) and is orange, while the Bonel variety of red beet contains betanin and vulgaxanthin (glutamine-betaxanthin), which exhibits an intense red coloration with high absorption coefficients.11 The red-shift in absorption of betacyanins in comparison to the betaxanthins can be attributed to the extended aromatic structure in the cyclo-DOPA condensate. The strong absorption in the visible spectrum, makes them promising light harvesting pigments in solar cell devices. Additionally, the carboxyl functional groups bind the dyes to the semiconductor nanostructure and many betalain structures host multiple acid moieties. The molecules are also stable in the pH range 2–7, which makes them useful in mildly acidic and neutral conditions, and they are also more resistant to hydrolysis, making them an ideal dye system for green solvent use.
There are significantly fewer varieties of betalains compared to the anthocyanins, and a corresponding lack of research dedicated to their application in DSSC. Red beet was considered the only edible source of betalain dye for a long time, which limited the amount of attention the family received, yet in recent years betalains have been analysed and extracted from other sources such as Bougainvillea, amaranth, prickly pear, dragon fruit and beetroot.12,13 Betalain pigments in one fruit of the prickly pear genus (Sicilian prickly pear, Opuntia ficus-indica) have been characterized and indicaxanthin was found to be a major compound in the fruit, followed by betanin and isobetanin.14 The lack of betanin side products identified in some studies were attributed to gentle work-up conditions and led to higher purity betanin samples.11 Calogero et al. achieved a solar cell efficiency of 1.26% using an optimized TiO2 thin film as the photoanode substrate and a purple extract from a Sicilian prickly pear.15 Zhang et al. investigated uptake of dye from red beet onto TiO2 films, with limited success. The efficiency of their device was increased by purifying the dye extract via column chromatography to remove the yellow betaxanthins and the utilization of an acidic pre-treatment on the TiO2 to increase dye uptake, yet devices failed to surpass 1% efficiency.16 Sandquist and McHale proposed that separation of the red/purple betanin from the yellow indicaxanthin derived from red beetroot would improve dye uptake, since they had discovered that the yellow dye preferentially binds to TiO2. Their devices attained an efficiency of 2.7% through medium pressure chromatographic purification of the dyes and optimization of the photoanode to reduce recombination.17 Surprisingly, the team showed that aggregated betanin on the TiO2 surface enhanced the efficiency, which reached 3%.18
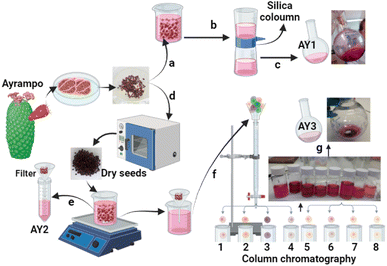
This work focuses on ayrampo fruit from a prickly pear species (Opuntia soehrensii) native to Peru. The flesh and seeds are known to be a good natural source of betalains, and they work as a powerful antioxidant against pyroxyl and nitric oxide radicals.19 Along with their intense red colouring, this has led to their use as natural pigment for textiles, in natural remedies and as a fashionable ingredient of gourmet food. Recently, the ayrampo dye has been applied in Organic Light-Emitting Diode (OLED) devices and surpassed the power output of the commonly used anthocyanin sources.10 The small selection of papers that report the application of betalains in DSSCs, used extracts from red beets. Here we show, through experiment and theory that the ayrampo seeds (AY) (Fig. 2) have potential as a native source of sensitizers that have favourable properties for solar cell devices. We also have compared the difference between the raw extract and a partially purified extract since conflicting results have been reported in the literature.20
We have tested the devices at full sun and 10% sun conditions to show the potential for efficient solar to power conversion under low light conditions. We have also tested the stability of the devices over a period of one week, which reveals the dual benefits of acid treatment in enhancing both the performance and stability of the solar cells.
Materials and methods
Extraction of pigments AY1, AY2 and AY3
Raw AY were purchased in the central market in Cusco, Peru. They were stored in dark, sealed bags in a refrigerator at 15 °C to avoid contamination and discoloration until used for further experimentation. Use of solvents such as methoxypropionitrile (MPN), dichloromethane (DCM), or acetonitrile (MeCN) to run columns or perform any kind of extraction not only gave instant color changes to the dye solution, but also would not be a sustainable alternative and would not be suitable for upscale in rural areas. Therefore, only solvents such as water or ethanol were used for the extraction and dyeing processes. To fully analyse the natural dye potential in devices, a compromise was reached to optimize both speed of extraction and the cleanliness of the resultant liquor. In the first method, the seeds were immersed in deionized (DI) water and a visible colour change of the seed cuticle from deep purple to pink/yellow was observed and a pigmented solution. To minimize the time taken to extract the dye and reduce losses, a short plug column of silica was used to clean the raw extract, remove large particles, and potentially reduce the betaxanthin concentration in the sample. This partially purified solution was labeled AY1 (Fig. 2c) and was taken on for further testing. To further separate and extract the pigments, seeds were first dried overnight at 60 °C. Afterwards 4 g of the dried seeds were added to 20 mL DI water and shaken. The final solution was filtered to produce a raw, highly pigmented solution and this was designated as AY2 (Fig. 2d). 15 mL of AY2 was separated into 8 distinct fractions via column chromatography with DI water. Solvent was then removed from each fraction using a rotary evaporator. Fraction 8 is labeled AY3 (Fig. 2g).Pigments obtained from the ayrampo solution in the forms of precipitate, supernatant or solution were characterized via UV-visible spectroscopy (Ocean Optics, USB 4000 equipped with a UV-VIS-NIR light source model DH-2000-BAL) and fluorescence spectroscopy (lifetime measurements were run using FLS1000 Photoluminescence Spectrometer, slit width 3 mm, and Fluoracle software used for fitting).
Solar cell fabrication and characterization
For the solar cells, the working electrode was assembled on TEC 15 (NSG) fluorine-doped tin oxide-coated glass. The glass surface was coated with a compact blocking layer that was deposited by immersing the substrates into 20 mM TiCl4 solution in ethanol and heating to 75 °C for 30 min, then washing with DI water and ethanol. TiO2 films were prepared by a doctor blading method in which a transparent TiO2 paste (DSL 18NR-T, Dyesol) was deposited by spreading with a glass rod over the glass which was masked with Scotch Magic Tape to control the thickness (ca. 6 μm) and film area (0.25 cm2). The films were dried by heating to 80 °C on a hotplate for 5 minutes. In some cases, TiO2 scattering layer (ca. 5 μm, Ti Nanoxide R/SP, Solaronix) was applied. The films were sintered (450 °C, 30 min, 30 min ramp time) in a Nabertherm furnace and upon cooling, another TiCl4 treatment was applied. The films were placed into dye solutions described above and left in the dark for 24 hours before testing (Fig. 3a). For comparison, a set of electrodes were immersed in a 0.3 mM solution of N719 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of t-butanol and MeCN.
1 ratio of t-butanol and MeCN.
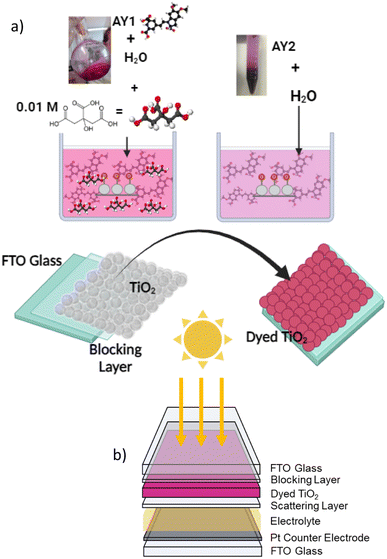 | ||
| Fig. 3 (a) Coloration process of solar cells, colored solution was prepared separately for each fraction. (b) Solar cell structure. | ||
Cells were constructed using the as-prepared films sandwiched with a Pt-coated FTO glass counter electrode (TEC 8), using 60 μm Surlyn as a spacer. An iodine redox electrolyte composed of 0.6 M TBAI, 0.03 M I2, 0.1 M guanidinium thiocyanate and 0.5 M t-butyl pyridine in MeCN was introduced into cells via vacuum and the cells were then sealed (Fig. 3b). To measure the photovoltaic parameters of devices, an Ivium CompactStat potentiostat was used in conjunction with a Xenon light source (Newport, 300 W) calibrated to 100 mW cm−2 with a Si photodiode. A black aperture was used to mask the device working area and the current was measured at applied bias between open circuit and short circuit at a rate of 5 mV s−1.
Results and discussion
Experimental details outlining the preparation of the seed material and extraction of pigments AY are described by Rivera et al.10 For this work, we set up others without using ketone summarised in Fig. 2. As indicated previously, we seek to carry out a process that can be repeated in rural areas. In this sense, two methods were used to extract the pigments from the seeds. In the first method, the aqueous solution containing the pigments was filtered through a short plug of silica to separate the purple/red portion (labeled AY1) from yellow bands. In the second method, the seeds were first dried overnight at 60 °C to enhance the extraction of the pigments and the raw extract was filtered through filter paper, rather than silica, to produce a highly pigmented solution, labeled AY2. Previously published analysis has shown the purple/red fraction to be rich in betacyanins. This fraction was chosen for further study since more visible light can be collected from sunlight using photosensitizers with an absorption onset at longer wavelength compared to more blue-absorbing pigments.21 The redox potential of the major betacyanins pigment was 0.38 V vs. Ag/AgCl (AY1).10,22,23 The excited state redox potential is approximately −1.76 V vs. Ag/AgCl (estimated by subtracting the energy required to excite an electron from the ground state to the excited state, ca. 2.14 eV). This is much higher than the conduction band edge of TiO2 (ca. −0.7 V vs. Ag/AgCl), making charge transfer highly favourable.23 The UV-visible spectrum of the raw solution AY2 is provided in Fig. 4, two distinct transitions can be observed in the spectrum (Initial), one at ca. 470 nm in the region expected for indicaxanthin and a second at ca. 532 nm in the region expected for betacyanin.17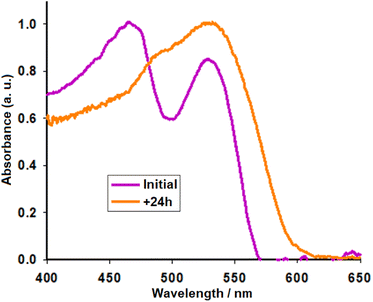 | ||
| Fig. 4 Normalized UV-visible absorption spectrum of the AY2 solution before and after 24 hours in daylight. | ||
Upon submersion of TiO2 films in the raw extract AY2, the absorption peak round 470 nm representing the presence of indicaxanthin recedes after only 24 hours and gives way to the betalain peaks (+24 h). Alternatively, Treat et al. observed dimerization and aggregation of betanin, characterised by blue- and red-shifted bands (470 and 607 nm) relative to the monomer (532 nm) spectrum.18 Excitation at 380 nm gave an emission band with a maximum at 470 nm (Fig. S1a,† solid and dotted black line). The excited state was shown to decay with a lifetime of ca. 7.9 ns (Fig S1b†). Therefore, the yellow component contains a different dye or a more rigid isomer, which is emissive. To characterise the red/purple dye it was necessary to separate the components by column chromatography. The excitation and emission spectra for fraction 8, labeled AY3, is shown in (Fig. S1a,† solid and dotted gray line).
To visualise the electronic orbital distribution in the ground and excited states of the betalain component, time-dependent density functional theory was utilised, and the results are summarised in Fig. 5 and Table S1.† The structure was simplified to reduce computational costs by substitution of the sugar rings of the betanin with methoxy groups. The geometry of the betanidin-derived molecule was calculated in vacuum and the energy calculations were run in MeCN to represent the interaction of the dye with electrolyte, using the IEFPCM solvent model. The basis set for both optimization and energy was B3LYP 6-311g ++ (d,p), to attain a good balance between depth of the calculation and computational cost. The lowest-energy transition was predicted to be intense HOMO–LUMO π to π* transition with a small amount of charge transfer character towards the core and one of the carboxylic acid groups. The calculated HOMO–LUMO transition wavelength (532 nm) was consistent with the experimentally measured UV-visible absorption band in Fig. 4, the second electronic transition corresponding to 447 nm, was calculated to have a much weaker oscillator strength of 0.0645 compared to 1.1172 for the lowest energy transition. This transition contained two components: HOMO-1 to LUMO with a 98% contribution, and HOMO-3 to LUMO with 2% contribution. As seen in Fig. 5, the HOMO-1 shows more electron density around the indolium group. The electron density of the LUMO is located more on the betalamic acid part. While there are several possible binding modes to TiO2, Oprea et al.20 have calculated the proton affinities of the carboxylic acids of betalains and found that the most probable binding site would be on the betalamic acid. Therefore, not only are these pigments energetically suitable for photosensitization of TiO2, but also the push–pull electronic structure favours charge transfer to the electrode.
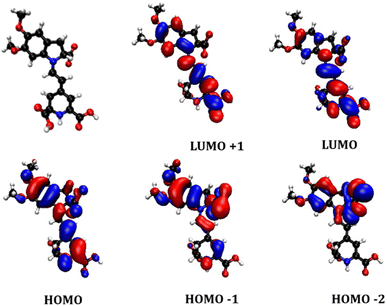 | ||
| Fig. 5 Optimized geometry (red balls = oxygen, blue = nitrogen, white = hydrogen, black = carbon) and calculated molecular orbitals for the proposed key betalain component of AY. | ||
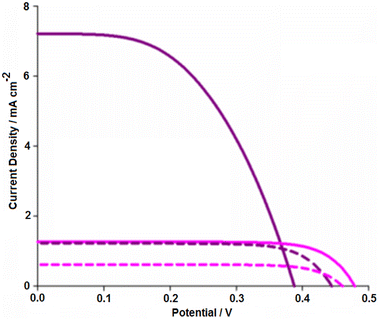
Samples of AY1 and AY2, as well as N719 as a reference dye, were subsequently used to produce DSSCs in order to assess the capability of AY extracts as sensitizers. A series of experiments were performed to elucidate the optimal device configuration, which is described in the solar cell fabrication and characterization section. In short, devices presented in this section comprise of an optimised 3-layer TiO2 photoanode with both a spin-coated blocking layer and a compact scattering layer (Fig. 3b). The electrolyte used for these devices was an iodine-based electrolyte (0.05 M I2, 1 M 1, 2, dimethyl-3-propylimidizolium iodide, 0.5 M LiI, 0.5 M 4-tert-butylpyridine in MeCN) and all devices were completed with a platinised counter electrode. Once an architecture was established, further experiments were carried out to deduce the effects of the natural dye sample preparation on the performance of the resulting DSSC. The parameters for these DSSCs and the following devices are listed in Table 1. In the first round of testing, the AY1 sample was compared with AY2 (Fig. S2†). The AY1 (filtered through silica) gave improved performance compared to AY2 (filtered through filter paper) and the performances of these devices are summarised in Table 1. The better performance of AY1 is likely to be due to improved dye loading arising from less competition between the betacyanin pigments and less photoactive compounds, such as indicaxanthin, in the crude material. This was consistent with the findings of Oprea et al.20 in their study, on the purification of the pigments extracted from red beetroots, the short circuit current rose from 0.38 mA to 2.95 mA, but the VOC decreased slightly from 358 mV to 357 mV and the FF decreased from 0.687 to 0.300, and the efficiency rose from 0.119 to 0.402%. While the VOC and FF values of the AY cells were reasonable, low current output limited the overall device performance. The next step was to improve this without hindering the sustainability of the device. Both the work of Prabavthy with anthocyanins6 and Calogero's prickly pear devices14 used acidic solvents or treatments to the TiO2 film in order to increase the photocurrent density (similar to the established processes of dying fibres with acidic or basic mordants). As shown in Fig. 3a, the addition of 0.01 M citric acid to the aqueous dye bath solution drastically increased the JSC (Fig. 6) and, therefore, the overall efficiency of the devices tested.
| Sensitizer | VOC/V | JSC/mA cm−2 | FF | η/% |
|---|---|---|---|---|
| AY1 | 0.48 | 1.27 | 0.75 | 0.45 |
| AY2 | 0.49 | 0.88 | 0.68 | 0.29 |
| AY1 + citric acid | 0.39 | 7.21 | 0.50 | 1.41 |
| N719 | 0.56 | 15.4 | 0.55 | 4.75 |
Therefore, citric acid was found to be promising in terms of JSC, with the added benefits of being cheap, widely available and non-toxic. The pH of the dyebath dropped upon addition of the acid to a value of around 2–3, compatible with the stability of the betalains. Consistent with Zhang et al., the films became darker when citric acid was added, suggesting increased dye-loading which should lead to higher JSC.16 The efficiency of the device using AY1 and citric acid reached 1.41%, which is comparable to the state of the art for betalains, and surpasses the efficiency of many natural chlorophyll and anthocyanin dyes found in literature.3 Low VOC and high series resistance, leading to low FF, limited the efficiency in this device. However, the increase in JSC, with decrease in FF and VOC was consistent with Zhang et al.,16 for HCl-treated TiO2 devices sensitized by betanin from extracted from beets (where JSC increased from 1.35 to 2.42 mA cm−2, VOC decreased from 0.45 to 0.44 V, FF decreased from 0.65 to 0.63 and efficiency increased from 0.40 to 0.67%). The drop in FF was also consistent with Treat et al., who reported VOC between 0.350–0.375 V, FF between 0.47–0.45, JSC 14–15 mA cm−2 for betanin from red beets.18 The JSC was also lower than the optimized betanin-sensitized devices reported by Sandquist et al. (up to 13.91 mA cm−2), and the VOC and FF values were slightly lower (up to 0.44 V and 0.57 reported).17 The authors attributed high charge recombination as the cause of these losses. We also note that the presence of water is known to adjust the energy of the conduction band edge of TiO2, leading to increased dark current and lower VOC compared to devices made under dry conditions.24
Decreasing the light intensity to 10% of its normal value is more representative of the amount of light that reaches the floor of rainforests, which are unsurprisingly lacking in power infrastructure. It was then found that upon testing the device under low light conditions, these efficiencies reach up to 4% for the citric acid treated device (Table 2).
| DSSC | Voc/V | Jsc/mA cm−2 | FF | η/% |
|---|---|---|---|---|
| AY1 | 0.47 | 0.61 | 0.72 | 2.1 |
| AY1 + citric acid | 0.45 | 1.23 | 0.74 | 4 |
An investigation of the device stability over a period of one week was undertaken (Fig. S3†). Each sample was measured under ‘1 sun’ intensity in intervals of 0, 1 and 7 days. The devices that dried out or were otherwise compromised during the time period were excluded from subsequent measurements. The N719 device exhibited an expected decrease in both VOC and JSC after 1 day, followed by a plateau in performance (Fig. S3c†). Both the AY1 and AY2 devices suffered from a steady decline in performance over the week (Fig. S3b and d†). However, the acid-stabilized device retained its high performance after a week, even after continued exposure to sunlight (Fig. S3a†). It is thought that the acid group could act as a stabilizer by decreasing breakdown of the dye via hydrolysis mechanisms. Further investigation onto the stability of these devices is required to identify the processes, whether physical or chemical, behind the loss of pigmentation.
Conclusions
To conclude this work has focussed on only one of the vast arrays of photoactive sensitizers available in the natural world. Findings from this section not only highlight the potential of natural sensitizers in DSSCs but helped us understand that simple extraction and gentle handling methods can contribute to improved device performance. It has also highlighted the capability of the devices in low light conditions, with up to 4% efficiency reached for the citric acid stabilized AY1 sample, with only small losses in the device performance over an extended period. In this sense, the Ayrampo dye shows a wide gap to develop dye-sensitized solar cells whose efficiency potential lies in the decrease of its pH using organic acid. It is important to add that this natural dye is not 100% used in Peru in the industry, leading to its non-utilization, while this solar cell alternative will help to incorporate Ayrampo to a sustainable economic chain.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We thank the EPSRC Global Challenges Research Fund (GCRF) grant BH173540, for providing financial support for the exchange of students and also for carrying out this research; EAG and HVF thanks ERC starting grant pTYPE 715354; EPSRC ISCF North East Centre for Energy Materials EP/R021503. Likewise, H. A. R. T. acknowledges the Thin Films Laboratory of the National University of Engineering for all the facilities to carry out the experiments. Also, M. E. Q. C acknowledges the 204-2020-FONDECYT project from PROCIENCIA.References
- B. O'regan and M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films, Nature, 1991, 6346, 737–740 CrossRef.
- A. Hagfeld, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Dye-Sensitized Solar Cells, Chem. Rev., 2010, 11, 6595–6663 CrossRef.
- G. Calogero, A. Bartolotta, G. Di Marco, A. Di Carlo and F. Bonaccorso, Vegetable-based dye-sensitized solar cells, Chem. Soc. Rev., 2015, 44, 3244–3294 RSC.
- G. P. Smestad and M. Gratzel, Demonstrating Electron Transfer and Nanotechnology: A Natural Dye-Sensitized Nanocrystalline Energy Converter, J. Chem. Educ., 1998, 6, 752 CrossRef.
- N. T. R. N. Kumara, A. Lim, Ch. M. Lim, M. I. Petra and P. Ekanayake, Recent progress and utilization of natural pigments in dye sensitized solar cells: A review, Renewable Sustainable Energy Rev., 2017, 78, 301–317 CrossRef CAS.
- N. Prabavathy, S. Shalini, R. Balasundaraprabhu, D. Velauthapillai, S. Prasanna, P. Walke and N. Muthukumarasamy, Effect of solvents in the extraction and stability of anthocyanin from the petals of Caesalpinia pulcherrima for natural dye sensitized solar cell applications, J. Mater. Sci.: Mater. Electron., 2017, 28, 9882–9892 CrossRef CAS.
- N. J. Cherepy, G. P. Smestad, M. Grätzel and J. Z. Zhang, Ultrafast Electron Injection: Implications for a Photoelectrochemical Cell Utilizing an Anthocyanin Dye-Sensitized TiO2 Nanocrystalline Electrode, J. Phys. Chem. B, 1997, 45, 9342–9351 CrossRef.
- A. Kay and M. Graetzel, Artificial photosynthesis. 1. Photosensitization of titania solar cells with chlorophyll derivatives and related natural porphyrins, J. Phys. Chem., 1993, 23, 6272–6277 CrossRef.
- X. F. Wang, C. H. Zhan, T. Maoka, Y. Wada and Y. Koyama, Fabrication of dye-sensitized solar cells using chlorophylls c1 and c2 and their oxidized forms and from Undaria pinnatifida (Wakame), Chem. Phys. Lett., 2007, 447, 79–85 CrossRef CAS.
- H. A. Rivera Tito, G. Hernández-Sosa, C. Romero-Nieto, E. Regulska, N. Jürgensen, J. Zimmermann, K. Salazar-Salinas and M. E. Quintana Caceda, Extraction of 2′-O-apiosyl-6′-O-crotonic acid-betanin from the ayrampo seed (Opuntia soehrensii) cuticle and its use as an emitting layer in an organic light-emitting diode, RSC Adv., 2020, 10, 36695–36703 RSC.
- F. C. Stintzing, A. Schieber and R. Carle, Identification of Betalains from Yellow Beet (Beta vulgaris L.) and Cactus Pear [Opuntia ficus-indica (L.) Mill.] by High-Performance Liquid Chromatography−Electrospray Ionization Mass Spectrometry, J. Agric. Food Chem., 2002, 50, 2302–2307 CrossRef CAS.
- H. Zhou, L. Wu, Y. Gao and T. Ma, Dye-sensitized solar cells using 20 natural dyes as sensitizers, J. Photochem. Photobiol., A, 2011, 219, 188–194 CrossRef CAS.
- N. S. Ramli, P. Ismail and A. Rahmat, Influence of Conventional and Ultrasonic-Assisted Extraction on Phenolic Contents, Betacyanin Contents, and Antioxidant Capacity of Red Dragon Fruit (Hylocereus polyrhizus), Sci. World J., 2014, 964731 Search PubMed.
- J. A. Fernández-López and L. Almela, Application of high-performance liquid chromatography to the characterization of the betalain pigments in prickly pear fruits, J. Chromatogr. A, 2001, 913, 415–420 CrossRef PubMed.
- G. Calogero, G. Di Marco, S. Cazzanti, S. Caramori, R. Argazzi, A. Di Carlo and C. A. Bignozzi, Efficient Dye-Sensitized Solar Cells Using Red Turnip and Purple Wild Sicilian Prickly Pear Fruits, Int. J. Mol. Sci., 2010, 11, 254–267 CrossRef CAS PubMed.
- D. Zhang, S. M. Lanier, J. A. Downing, J. L. Avent, J. Lum and J. L. McHale, Betalain pigments for dye-sensitized solar cells, J. Photochem. Photobiol., A, 2008, 195, 72–80 CrossRef CAS.
- C. Sandquist and J. L. McHale, Improved efficiency of betanin-based dye-sensitized solar cells, J. Photochem. Photobiol., A, 2011, 221, 90–97 CrossRef CAS.
- N. A. Treat, F. J. Knorr and J. L. McHale, Templated Assembly of Betanin Chromophore on TiO2: Aggregation-Enhanced Light-Harvesting and Efficient Electron Injection in a Natural Dye-Sensitized Solar Cell, J. Phys. Chem. C, 2016, 120, 9122–9131 CrossRef CAS.
- D. Butera, L. Tesoriere, F. Di Gaudio, A. Bongiorno, M. Allegra, A. M. Pintaudi, R. Kohen and M. A. Livrea, Antioxidant Activities of Sicilian Prickly Pear (Opuntia ficus indica) Fruit Extracts and Reducing Properties of Its Betalains: Betanin and Indicaxanthin, J. Agric. Food Chem., 2002, 50, 6895–6901 CrossRef CAS PubMed.
- C. I. Oprea, A. Dumbravă, I. Enache, A. Georgescu and M. A. Gîrţu, A combined experimental and theoretical study of natural betalain pigments used in dye-sensitized solar cells, J. Photochem. Photobiol., A, 2012, 240, 5–13 CrossRef CAS.
- M. J. García-Salinas and M. J. Ariza, Optimizing a simple natural dye production method for dye-sensitized solar cells: examples for betalain (bougainvillea and beetroot extracts) and anthocyanin dyes, Appl. Sci., 2019, 12, 2515 CrossRef.
- S. Wybraniec, P. Stalica, A. Spórna, B. Nemzer, Z. Pietrzkowski and T. Michalowski, Antioxidant Activity of Betanidin: Electrochemical Study in Aqueous Media, J. Agric. Food Chem., 2011, 59, 12163–12170 CrossRef CAS PubMed.
- M. Grätzel, Dye-sensitized solar cells, J. Photochem. Photobiol., C, 2003, 2, 145–153 CrossRef.
- K. Zhu, J. Song-Rim and A. J. Frank, Effects of water intrusion on the charge-carrier dynamics, performance, and stability of dye-sensitized solar cells, Energy Environ. Sci., 2012, 11, 9492–9495 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08010b |
| This journal is © The Royal Society of Chemistry 2024 |