Open Access Article
Open Access ArticleImprovement of the rate capability of all-solid-state cells with Fe-based polysulfide positive electrode materials by modifying the microstructure†
Tomonari Takeuchi *a,
Noboru Taguchi
*a,
Noboru Taguchi a,
Mitsunori Kitta
a,
Mitsunori Kitta a,
Toyonari Yajib,
Misae Otoyama
a,
Toyonari Yajib,
Misae Otoyama a,
Kentaro Kuratani
a,
Kentaro Kuratani a and
Hikari Sakaebe
a and
Hikari Sakaebe ac
ac
aNational Institute of Advanced Industrial Science and Technology (AIST), Midorigaoka 1-8-31, Ikeda, Osaka 563-8577, Japan. E-mail: takeuchi.tomonari@aist.go.jp
bSynchrotron Radiation Center, Ritsumeikan University, Kusatsu, Shiga 525-8577, Japan
cKyushu University, 6-1 Kasuga koen, Kasuga-shi, Fukuoka 816-8580, Japan
First published on 28th February 2024
Abstract
We successfully prepared an Fe- and Li-containing polysulfide positive electrode material (Li8FeS5–Li2FeS2 composite) that shows a high specific capacity (>500 mA h g−1) with improved rate capability in all-solid-state cells. High-resolution TEM analysis indicated the coexistence of small crystallites of high-conductivity Li2FeS2 and FeS, as well as low-crystallinity Li2S, in the composite, and this microstructure is responsible for the improved battery performance.
Introduction
Recently, there has been an increasing demand for high-energy storage systems, particularly those applicable in electric vehicles. Currently, next-generation batteries are required with a much higher energy density than that of the conventional lithium-ion batteries consisting of oxide-based cathode materials (practically ca. 200 W h kg−1).1,2 To date, several candidates such as lithium–oxygen batteries (theoretically ca. 3500 W h kg−1),3,4 lithium–sulphur (Li–S) batteries (ca. 2600 W h kg−1),5 and Zn–oxygen batteries (ca. 1100 W h kg−1)6 have been included in next-generation batteries. Among them, the Li–S battery is a promising system that generates high energy density in a closed system (without introducing gaseous components).Lithium sulphide (Li2S) is a potential cathode active material in Li–S cells with a high theoretical capacity (ca. 1170 mA h g−1) and has the advantage that a variety of anode materials such as graphite and silicon can be used in practical battery systems.7–13 However, Li2S shows high electrical resistivity, which gives rise to poor material usage in the cells. In order to enhance the conductivity of Li2S, several attempts such as forming composites with carbon (Li2S–C)10–12 or metals (Li2S–Fe, Li2S–Cu, and Li2S–V)7–9 have been made. Along with the latter material design, we developed the Fe-containing polysulfide material LixFeSy, which showed a relatively high specific capacity of ca. 730 mA h g−1 for the Li8FeS5 cell with a non-aqueous liquid electrolyte.9 However, these LixFeSy cells showed capacity degradation with cycling, as observed often for Li–S cells with a liquid electrolyte, partly because of the side reactions between the sulphide electrode material and the liquid electrolyte and partly because of the dissolution of polysulfides formed during electrochemical charge/discharge reactions into the liquid electrolyte. Replacing the liquid electrolyte with the solid electrolyte is a promising approach to solve these problems, and there have been many reports on all-solid-state cells with metal polysulfide cathode materials such as Li2TiS3, Li3NbS4, and Li3CuS2, showing superior electrochemical performances.14–16 Much recently, V-containing polysulfide materials (LixVSy) have been developed, and their all-solid-state cells showed superior rate capability with a higher specific capacity (>600 mA h g−1), which originated from its higher electrical conductivity (>10−2 S cm−1).17
In this study, we prepared an Fe-based polysulfide electrode material (LixFeSy) in an attempt to show superior rate capability with high specific capacity in all-solid-state cells. Fe-based electrode materials are advantageous from the standpoints of resource abundance and cost, which would be beneficial for application to batteries, particularly in electric vehicles. However, LixFeSy showed a relatively low conductivity of ca. 1.0 × 10−5 S cm−1 for Li8FeS5, implying a necessity for improvement in performance, particularly in rate capability. The lower conductivity of Li8FeS5 so far (denoted as Li8FeS5-H sample) is partly due to the rather homogeneous component originating from the preparation process of both heating and milling. We prepared “inhomogeneous” Li8FeS5, that is, coexisting with some high-conductivity components such as FeS and Li2FeS2 by “incomplete” milling of Li2S and FeS (denoted as Li8FeS5-MM sample) to improve its conductivity; particularly, Li2FeS2 has been reported to show a relatively high electronic conductivity and a high Li+-diffusion coefficient,18 the coexistence of which would be advantageous for improving the rate capability of Li8FeS5.
Experimental
The Li8FeS5-MM sample was prepared by mechanical milling technique; a blended powder of Li2S and FeS in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio was mechanically milled (MM) for 5–40 h using a planetary ball mill apparatus (Fritsch Pulverisette 7) under a rotating speed of 400 rpm to yield Li8FeS5-MM (5–40 h) samples. The previously reported Li8FeS5-H with a rather homogeneous component was also prepared by heating (600 °C) and subsequent milling for 40 h, for comparison.13 Since Li2S and the resulting Li8FeS5 are very sensitive to atmospheric moisture, all the procedures except for the heating and mechanical milling were carried out in an argon-filled glove box; the heating and mechanical milling were carried out under atmospheric conditions using an argon-filled container and a pot wherein Li2S and Li8FeS5 were enclosed. We also prepared the Li8FeS5–Li2FeS2 composite by mechanically milling the blended powder of the above-mentioned Li8FeS5-MM (40 h) and Li2FeS2 in a 6
1 molar ratio was mechanically milled (MM) for 5–40 h using a planetary ball mill apparatus (Fritsch Pulverisette 7) under a rotating speed of 400 rpm to yield Li8FeS5-MM (5–40 h) samples. The previously reported Li8FeS5-H with a rather homogeneous component was also prepared by heating (600 °C) and subsequent milling for 40 h, for comparison.13 Since Li2S and the resulting Li8FeS5 are very sensitive to atmospheric moisture, all the procedures except for the heating and mechanical milling were carried out in an argon-filled glove box; the heating and mechanical milling were carried out under atmospheric conditions using an argon-filled container and a pot wherein Li2S and Li8FeS5 were enclosed. We also prepared the Li8FeS5–Li2FeS2 composite by mechanically milling the blended powder of the above-mentioned Li8FeS5-MM (40 h) and Li2FeS2 in a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 weight ratio for 1 h (Li2FeS2 was prepared by heating Li2S and FeS in a 1
4 weight ratio for 1 h (Li2FeS2 was prepared by heating Li2S and FeS in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio at 1000 °C).
1 molar ratio at 1000 °C).
The phase purity of the sample was checked using X-ray diffraction (XRD) measurements (RINT TTR-III, Rigaku, Japan) using a monochromatic Cu Kα radiation within the 2θ range of 10–80°. Before the measurements, each sample was covered with a Kapton film in an argon-filled glove box, and the measurements were carried out within 1 h to minimize the reaction with atmospheric moisture. Structural refinement by X-ray Rietveld analysis was carried out using the RIETAN-2000 program.19 The microstructure of the sample was examined using a high-resolution TEM (Talos F200X, ThermoFisher Scientific) operating at 300 kV in scanning mode with a probe current of 300 pA. The valence state and local structure of S atoms for the sample powders were examined by S K-edge X-ray absorption fine structure (XAFS) measurements, which were carried out at the soft X-ray double crystal monochromator beamline, BL-10, of the Synchrotron Radiation Center, Ritsumeikan University.20 The total electron yield (TEY) method was used, and the incident X-ray beam was monochromatized with a Ge (111) crystal (2d = 6.532 Å) pair. The photon energy was calibrated with the strong resonance of K2SO4 (S 1s → t2) appearing at 2481.7 eV.21 All samples were sealed in an argon-filled transfer vessel.20
The electrical conductivity of each sample was measured using an electrochemical test device (Celltest 1470E, Solartron Analytical) with applied voltages of 50, 100, and 150 mV, after the sample powder was cold-pressed into a pellet with a diameter of 10 mm and thickness of 0.7 mm. The all-solid-state cells (10 mm in diameter) were assembled using the above-mentioned Li8FeS5-MM or Li8FeS5–Li2FeS2 by uniaxial pressing in the same manner as described previously.17 Argyrodite-type sulfide solid electrolyte (SE) powder (80 mg) was pelletized and the positive electrode powder (5 mg), which was prepared by blending Li8FeS5-MM (or Li8FeS5–Li2FeS2), SE, and acetylene black (AB) in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio, was loaded on the above-mentioned SE pellet, which were then pressed together under 360 MPa for 5 min into a laminated pellet. After an indium foil (9 mm diameter and 0.3 mm thick) and a lithium foil (8 mm diameter and 0.2 mm thick) were attached on the opposite side as a negative electrode, it was pressed under 90 MPa for 2 min. The unit cell element was then fastened using stainless steel rods and sealed into a solid-state cell. The electrochemical measurements were carried out at 25 °C initially with charging, after standing for 1 h on open circuit, using a TOSCAT-3100 instrument (Toyo System, Japan) at a current density of 0.13 mA cm−2 (50 mA g−1, corresponding to ca. 0.1 C) when charging, and those of 0.13, 0.25, 0.64, and 1.3 mA cm−2 when discharging, between 3.0 and 1.0 V. Cyclic voltammetry (CV) of the cell was also conducted in the voltage range of 1.0–3.0 V (vs. Li–In) using a potentiostat/galvanostat (Model 1400, Solartron Analytical) at scan rates of 0.1 and 0.5 mV s−1.
1 weight ratio, was loaded on the above-mentioned SE pellet, which were then pressed together under 360 MPa for 5 min into a laminated pellet. After an indium foil (9 mm diameter and 0.3 mm thick) and a lithium foil (8 mm diameter and 0.2 mm thick) were attached on the opposite side as a negative electrode, it was pressed under 90 MPa for 2 min. The unit cell element was then fastened using stainless steel rods and sealed into a solid-state cell. The electrochemical measurements were carried out at 25 °C initially with charging, after standing for 1 h on open circuit, using a TOSCAT-3100 instrument (Toyo System, Japan) at a current density of 0.13 mA cm−2 (50 mA g−1, corresponding to ca. 0.1 C) when charging, and those of 0.13, 0.25, 0.64, and 1.3 mA cm−2 when discharging, between 3.0 and 1.0 V. Cyclic voltammetry (CV) of the cell was also conducted in the voltage range of 1.0–3.0 V (vs. Li–In) using a potentiostat/galvanostat (Model 1400, Solartron Analytical) at scan rates of 0.1 and 0.5 mV s−1.
Results and discussion
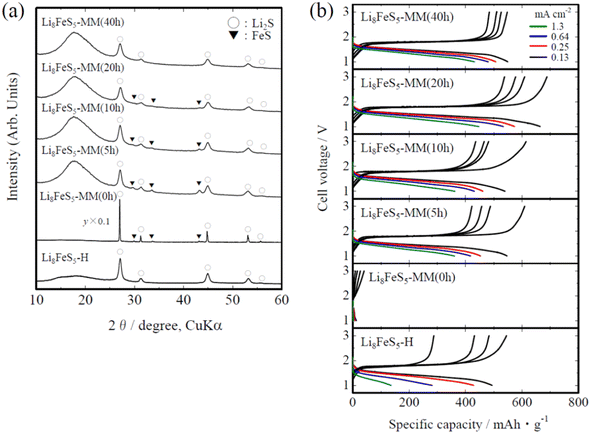
The obtained Li8FeS5-MM powders were greyish-black in appearance, and their XRD patterns are shown in Fig. 1(a). With increasing milling time, the initial crystalline Li2S and FeS changed to low-crystallinity Li2S and FeS, and finally, XRD peaks originating from FeS were not detected; the XRD pattern of Li8FeS5-MM (40 h) was very similar to that of the previous Li8FeS5-H sample. The FeS content, estimated by X-ray Rietveld analysis and listed in Table 1, decreased with milling time, accompanied by a slight decrease in the lattice parameter of Li2S. This indicates the decomposition of FeS and the partial substitution of smaller Fe2+ ions (0.66 Å) for Li+ ions (0.74 Å)22 in Li2S via the milling process. Therefore, the milling process decreases the crystallite size of initial Li2S and FeS, promoting the mutual chemical reaction (in particular, the incorporation of Fe2+ ions in Li2S) and resulting in finally forming low-crystallinity rather homogeneous Fe-substituted Li2S. The measured electrical conductivity of the Li8FeS5-MM samples was in the range of ca. 5 × 10−5–8 × 10−5 S cm−1, as listed in Table 1, which was higher than that of the Li8FeS5-H sample (ca. 1.0 × 10−5 S cm−1), probably due to the coexistence of small amounts of FeS (ca. 101 S cm−1)23 and the incorporation of Fe2+ ions into Li2S.| FeS content/mol% | a/Å | σ/S cm−1 | |
|---|---|---|---|
| Li8FeS5-MM (0 h) | 4(1) | 5.71112 (9) | <10−8 |
| Li8FeS5-MM (5 h) | 8(1) | 5.710 (7) | 5.0 × 10−5 |
| Li8FeS5-MM (10 h) | 4(1) | 5.709 (7) | 5.8 × 10−5 |
| Li8FeS5-MM (20 h) | 3(1) | 5.704 (8) | 8.4 × 10−5 |
| Li8FeS5-MM (40 h) | 0(1) | 5.704 (6) | 7.4 × 10−5 |
| Li8FeS5–Li2FeS2 | 6(1) | 5.711 (3) | 4.4 × 10−4 |
| Li8FeS5–H13 | 0(1) | 5.7048 (10) | 1.0 × 10−5 |
Fig. 1(b) shows the charge and discharge curves of the InLi/Li8FeS5-MM sample cells at different current densities. Data for the InLi/Li8FeS5-H sample cell are also shown for comparison. All the sample cells showed discharge plateaus at ca. 1.5 V, which corresponded to that at ca. 2.1 V in the previously reported Li/Li8FeS5-H sample cells with a non-aqueous liquid electrolyte.13 The Li8FeS5-MM sample cells showed a higher discharge capacity, particularly at a higher current density, than that of the Li8FeS5-H sample cell. The specific discharge capacity at a lower current density (0.13 mA cm−2) for the Li8FeS5-MM (20 h) sample cell (ca. 660 mA h g−1) was comparable to that of the previously reported Li8FeS5-H sample cell (ca. 730 mA h g−1) with a non-aqueous liquid electrolyte.13 In addition, the discharge capacity at higher current densities (ca. 450 mA h g−1 at 1.3 mA cm−2 for Li8FeS5-MM (20 h)) was comparable or superior to the previously reported values for all-solid-state Li–S batteries.14–17
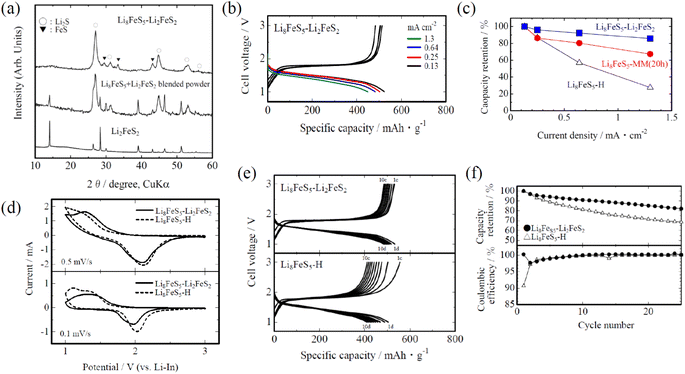
Thus, the coexistence of the conductive component (FeS) in Li8FeS5 was effective for improving the rate capability of the all-solid-state cell. We then intended to introduce another conductive component, Li2FeS2, to obtain the Li8FeS5–Li2FeS2 composite; Li2FeS2 additives would be advantageous for improving both the electronic conductivity and Li+ diffusion in the composite.17 Fig. 2(a) shows the XRD pattern of the obtained Li8FeS5–Li2FeS2 composite. As in the above-mentioned Li8FeS5-MM samples, the Li8FeS5–Li2FeS2 composite consisted of low-crystallinity Li2S and small amounts of FeS. No XRD peaks originating from Li2FeS2 were detected, probably due to its decomposition and conversion to FeS as well as its incorporation into Li2S (resulting in Fe-substituted Li2S) via the milling process. The FeS content and the lattice parameter of Li2S, estimated by the X-ray Rietveld analysis, are also listed in Table 1; judging from the data, the Li8FeS5–Li2FeS2 composite seems very similar to the Li8FeS5-MM (5–10 h) samples. Fig. 2(b) shows the charge and discharge curves of the Li8FeS5–Li2FeS2 sample cells at different current densities. Even at a higher current density, the cell showed high discharge capacity compared with the Li8FeS5-MM sample cells (Fig. 1(b)). Such improved rate capability is evident from the capacity retention, as shown in Fig. 2(c). CV measurements also showed consistent results; as shown in Fig. 2(d), the Li8FeS5-H sample cell showed a reductive peak at ca. 1.4 V under 0.1 mV s−1 but not obvious at a higher scan rate (0.5 mV s−1), whereas the Li8FeS5–Li2FeS2 sample cell showed an evident peak even under a higher scan rate. The measured electrical conductivity of the Li8FeS5–Li2FeS2 composite was ca. 4.4 × 10−4 S cm−1, higher than that of the Li8FeS5-MM samples (ca. 5–8 × 10−5 S cm−1). Such higher conductivity is responsible for the improved rate capability in the Li8FeS5–Li2FeS2 sample cell. In addition, the Li8FeS5–Li2FeS2 sample cell showed improved cycle performance, as shown in Fig. 2(e) and (f), partly due to its higher conductivity; higher conductivity could improve the utilization of the active materials as well as suppress the localized charging inhomogeneity. Because the XRD results showed similar estimated FeS contents and lattice parameters of Li2S for the Li8FeS5–Li2FeS2 and Li8FeS5-MM (5–10 h) samples (Table 1), there would be some characteristic microstructures that cause the difference in the XAFS measurements and TEM observations for the Li8FeS5–Li2FeS2 sample.
Fig. S1† shows the S K-edge X-ray absorption near-edge structure (XANES) spectra of the Li8FeS5–Li2FeS2 and Li8FeS5-H samples. Both samples showed similar spectra with three characteristic absorption peaks at 2469 eV (assigned to the bound state resonance due to an electronic transition between the S 1s and p-hybridized Fe 3d bands), 2472–2473 eV (originating from the 1s → 3p electronic transition in sulphur atoms), and 2476 eV (originating from the electronic transition of the 1s electron in S2− to the unoccupied orbital with S 3p and Li 2s characters).13,24 These spectral similarities indicate that the valence state and local structure around the S atoms are nearly consistent among these samples; that is, the Fe, Li, and S atoms surrounding the S atoms coordinate in a similar configuration on average while including slight fluctuations individually.
In contrast to the XAFS results, TEM observations showed a characteristic microstructure in the Li8FeS5–Li2FeS2 sample. As shown in Fig. 3(a), the high-resolution TEM images showed the presence of some lattice fringes with domain sizes of ca. 10–20 nm distributed randomly in the amorphous (non-crystalline) background in the Li8FeS5–Li2FeS2 sample. In order to confirm the structure of each lattice fringe, the fast Fourier transform (FFT) pattern (pseudo diffraction pattern) of the TEM image was obtained (Fig. 3(b)), where several Debye–Scherrer rings and a halo pattern were observed. By careful comparison of the lattice spacings with previously reported crystallographic data,25 they were assigned to the Li2S and FeS components (for example, ca. 0.29 nm for Li2S(002) and ca. 0.27 nm for FeS(011)). A notable point is that the extra component, not assigned to Li2S and FeS, remained, and it was assigned to Li2FeS2 (for example, ca. 0.63 nm for Li2FeS2(001)). Although no peaks ascribed to Li2FeS2 were detected in the XRD pattern (Fig. 2(a)), there remained very small (or close to amorphous) Li2FeS2 crystallites in the Li8FeS5–Li2FeS2 sample. By inverse Fourier transformation of the FFT pattern, the locations of these three components (Li2S, FeS, and Li2FeS2) were approximately identified in the TEM image and denoted in Fig. 3(a). Such a microstructure would be responsible for the higher conductivity and improved rate capability of the all-solid-state cell. Particularly, the difference in rate capability between the Li8FeS5-MM and Li8FeS5–Li2FeS2 sample cells (Fig. 2(c)) would originate from the coexistence of small crystallites of Li2FeS2 having higher Li+ diffusion. In addition, the difference between the Li8FeS5-MM (40 h) and Li8FeS5-H sample cells (Fig. 1(b)) (both samples showed no FeS content estimated in XRD) might be due to the coexistence of small crystallites of FeS in the Li8FeS5-MM (40 h) sample; the higher conductivity of the Li8FeS5-MM (40 h) sample indicates the coexistence of small crystallites of FeS (XRD is usually detectable for a crystallite of size more than 10 nm). Thus, designing a microstructure, in which small crystallites of high conductive and high Li+ diffusion components coexist, is effective for assembling all-solid-state cells with improved rate capability.
Conclusions
A Fe- and Li-containing polysulfide positive electrode material (Li8FeS5–Li2FeS2 composite) was successfully prepared, and it showed a high specific capacity (>500 mA h g−1) with improved rate capability in all-solid-state cells. XRD results showed that this composite consisted of low-crystallinity Li2S and FeS, whereas high-resolution TEM analysis indicated the coexistence of small crystallites of Li2S, FeS, and Li2FeS2. This microstructure, in which highly conductive and high Li+ diffusion components coexist, resulted in higher electrical conductivity and improved rate capability in all-solid-state cells.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is based on the results obtained from a project, SOLiD-EV (JPNP18003), subsidized by the New Energy and Industrial Technology Development Organization (NEDO).References
- X. Ji and L. F. Nazar, J. Mater. Chem., 2010, 20, 9821–9826 RSC.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J.-M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed.
- Y.-X. Yu, J. Phys. Chem. C, 2019, 123, 205–213 CrossRef CAS.
- J.-H. Li, J. Wu and Y.-X. Yu, J. Mater. Chem. A, 2021, 9, 10186 RSC.
- P. Das and P. Sarkar, Phys. Chem. Chem. Phys., 2023, 25, 30536 RSC.
- K. A. J. Dilshad and M. K. Rabinal, Phys. Chem. Chem. Phys., 2023, 25, 11566 RSC.
- M. N. Obrovac and J. R. Dahn, Electrochem. Solid-State Lett., 2002, 5, A70–A73 CrossRef CAS.
- A. Hayashi, R. Ohtsubo, T. Ohtomo, F. Mizuno and M. Tatsumisago, J. Power Sources, 2008, 183, 422–426 CrossRef CAS.
- T. Shigedomi, Y. Fujita, T. Kishi, K. Motohashi, H. Tsukasaki, H. Nakajima, S. Mori, M. Tatsumisago, A. Sakuda and A. Hayashi, Chem. Mater., 2022, 34, 9745–9752 CrossRef CAS.
- M. Nagao, A. Hayashi and M. Tatsumisago, J. Mater. Chem., 2012, 22, 10015–10020 RSC.
- T. Takeuchi, H. Kageyama, K. Nakanishi, M. Tabuchi, H. Sakaebe, T. Ohta, H. Senoh, T. Sakai and K. Tatsumi, J. Electrochem. Soc., 2010, 157, A1196–A1201 CrossRef CAS.
- K. Han, J. Shen, C. M. Hayner, H. Ye, M. C. Kung and H. H. Kung, J. Power Sources, 2014, 251, 331–337 CrossRef CAS.
- T. Takeuchi, H. Kageyama, K. Nakanishi, M. Ogawa, T. Ohta, A. Sakuda, H. Sakaebe, H. Kobayashi and Z. Ogumi, J. Electrochem. Soc., 2015, 162, A1745–A1750 CrossRef CAS.
- A. Sakuda, T. Takeuchi, M. Shikano, H. Sakaebe and H. Kobayashi, Front. Energy Res., 2016, 4, 1–7 Search PubMed.
- A. Sakuda, T. Takeuchi, K. Okamura, H. Kobayashi, H. Sakaebe, K. Tatsumi and Z. Ogumi, Sci. Rep., 2014, 4, 4883 CrossRef CAS PubMed.
- Y. Kawasaki, H. Tsukasaki, T. Ayama, S. Mori, M. Deguchi, M. Tatsumisago, A. Sakuda and A. Hayashi, ACS Appl. Energy Mater., 2021, 4, 20–24 CrossRef CAS.
- M. Otoyama, T. Takeuchi, N. Taguchi, K. Kuratani and H. Sakaebe, ECS Adv., 2023, 2, 010501 CrossRef.
- C. D. Wei, H. T. Xue, X. D. Zhao and F. L. Tang, Phys. Chem. Chem. Phys., 2023, 25, 8515–8523 RSC.
- F. Izumi and T. Ikeda, Mater. Sci. Forum, 2000, 321, 198–203 Search PubMed.
- K. Nakanishi, S. Yagi and T. Ohta, AIP Conf. Proc., 2010, 1234, 931 CrossRef CAS.
- M. Kiguchi, T. Yokoyama, D. Matsumura, H. Kondoh, T. Ohta and Y. Kitajima, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 16205–16210 CrossRef CAS.
- R. D. Shannon, Structure and Bonding in Crystals, Academic Press. Inc., 1981, vol. ii, pp. 53–70 Search PubMed.
- H. Kobayashi, N. Takeshita, N. Mori, H. Takahashi and T. Kamimura, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 115203 CrossRef.
- S. Bodeur and J. M. Esteva, Chem. Phys., 1985, 100, 415–427 CrossRef CAS.
- ICSD (Inorganic Crystal Structure Database) code 68380 (Li2FeS2), 168079 (FeS), and 409397 (Li2S).
Footnote |
| † Electronic supplementary information (ESI) available: XAFS spectra. See DOI: https://doi.org/10.1039/d3ra08641k |
| This journal is © The Royal Society of Chemistry 2024 |