Open Access Article
Open Access ArticleAdsorption mechanism of Ca2+, Mg2+, Fe3+, and Al3+ ions in phosphoric acid–nitric acid solution on 001 × 7 and S957 resins
Yingyuan Hua,
Wenlong Zhanga,
Meizhao Xuea,
Rui Lv *b,
Caimei Fanb and
Ao Li*bc
*b,
Caimei Fanb and
Ao Li*bc
aCollege of Traditional Chinese Medicine and Food Engineering, Shanxi University of Chinese Medicine, Jinzhong, 030619, China
bCollege of Safety and Emergency Management and Engineering, Taiyuan University of Technology, Taiyuan, 030024, China. E-mail: lvrui5276@163.com; everliao007@qq.com
cJinneng Holding Group, Datong 037000, Shanxi, China
First published on 28th February 2024
Abstract
Selective removal of Ca2+ and Mg2+ ions using the 001 × 7 resin and Fe3+ and Al3+ ions using the S957 resin is able to achieve the deep purification of the phosphoric acid–nitric acid solution, but the adsorption behaviors of Fe3+ and Al3+ ions are seriously suppressed by phosphoric acid. In order to understand the interaction mechanism of separation processes and the influence of phosphoric acid, we first studied the bonding form of Ca2+, Mg2+, Fe3+, and Al3+ ions on 001 × 7 and S957 resins using FT-IR and XPS techniques; subsequently, quantum chemistry computation was carried out to further explore the bonding mechanism between the functional groups on resins and metal ions. FT-IR and XPS results reveal that for the adsorption process on the 001 × 7 resin, hydroxyls from sulfonic acid groups combine with Ca2+ and Mg2+ ions. Whereas Fe3+ and Al3+ ions are adsorbed on the S957 resin through an exchange reaction with hydroxyls on the phosphonic acid group but not on the sulfonic acid group. Quantum chemistry computation results reveal that the phosphonic acid group has a larger binding energy with Fe3+ and Al3+ ions. Thus, the S957 resin still presents great adsorption performance for Fe3+ and Al3+ ions despite the influence of dihydrogen phosphate ions in the phosphoric acid–nitric acid solution.
1 Introduction
Phosphoric acid–nitric acid solution is the neutralization mother liquor obtained using the nitric acid resolve phosphate ore, which can be used to produce nitrogen and phosphorus compound fertilizers.1,2 In addition to phosphoric acid (4.4 mol L−1) and nitric acid (1.1 mol L−1), this solution contains many Ca2+ ions (1.9 mol L−1) and a few Mg2+, Fe3+, and Al3+ ions (0.14–0.24 mol L−1). During the neutralization process, the presence of these metal ions leads to the formation of precipitates in the phosphoric acid–nitric acid solution, which makes the solution easily viscous and water-soluble phosphorus degenerate.3–5 In order to alleviate such problems, a precipitation method is usually used to remove most of the Ca2+ ions from phosphoric acid–nitric acid solution in industry.6 But limited by the solubility of metal ions, a small amount of Ca2+ ions still remain in the solution,7 and Mg2+, Fe3+, and Al3+ ions also are not removed. Therefore, the in-depth removal of these small four metal ions from the mother liquor is essential for practical production.Synthetic resins have been proven to effectively remove Ca2+, Mg2+, Fe3+, and Al3+ ions from phosphoric acid–nitric acid solution. Ca2+ and Mg2+ ions can be selectively removed by the 001 × 7 resin (containing a sulfonic acid group),8 and Fe3+ and Al3+ ions are selectively removed by the S957 resin (containing a sulfonic acid group and phosphonic acid group).9 Metal ions adsorbed on the 001 × 7 resin and S957 resin can be completely eluted with nitric acid and hydrochloric acid as regeneration solutions, respectively, achieving the recycling utilization of resins. Nevertheless, very little attention has been paid to the interaction mechanism of separation processes and the adsorption mechanism of the four metal ions from phosphoric acid–nitric acid solution using different resins.
Furthermore, it should be noted that the 001 × 7 resin presents great adsorption capacity for Fe3+ and Al3+ ions in pure water or nitric acid solution, while no adsorption capacity is observed in phosphoric acid solution.10 Adsorption capacity of S957 resin for Fe3+ and Al3+ ions also decrease significantly with the concentration of phosphoric acid increasing. Consequently, phosphoric acid has a particular influence on the adsorption process of Fe3+ and Al3+ ions on resins. Much work has been performed so far on the adsorption process of metal ions on resins and the influence of the coexisting component.11,12 It is believed that the dissociated phosphoric acid anion can combine with metal ions to form the phosphate chelate and suppress its adsorption behavior on the resin. However, there are relatively few studies devoted to proving this influencing mechanism from a microscopic view. Further effort on the adsorption mechanism is required to understand the effect of phosphoric acid.
Recent research efforts by the characterization of resin to explore the removal mechanism of metal ions from phosphoric acid have attracted much attention.13,14 Based on modern spectroscopic characterizations, such as Fourier transform infrared spectra (FT-IR) and X-ray photoelectron spectra (XPS), variation in the chemical components, elemental valence states and functional groups of resins (fresh, adsorbed metal ions, and regenerated) can be obtained and used to infer the bonding forms of the metal ion on the resin. Besides, density functional theory (DFT) calculation is a powerful theoretical tool that can provide effective evidence for the interaction mechanism of the microstructures.15,16 Thus, further quantum chemical calculation about the binding energies of metal ions with functional groups on the resins and phosphate anion in phosphoric acid solution will supply the theoretical support for the adsorption mechanism.
Therefore, in this study, we firstly focused on the adsorption mechanism for removal of Ca2+, Mg2+, Fe3+, and Al3+ ions from the phosphoric acid–nitric acid solution by 001 × 7 and S957 resins, using BET, FT-IR and XPS characterization techniques to investigate and compare the microstructure of resins before and after metal ions adsorption, and after metal ions elution. Furthermore, using chemical structures of 001 × 7 resin, S957 resin and dihydrogen phosphate ion as molecular models, quantum chemistry calculations about the binding energies of metal ions with the functional group on the resin and phosphate anions in the phosphoric acid solution were carried out to explore the bonding mechanism between the functional groups and metal ions and provide a theoretical basis for the adsorption mechanism of Ca2+, Mg2+, Fe3+, and Al3+ ions from phosphoric acid–nitric acid solution on 001 × 7 and S957 resins.
2 Experimental section
2.1. Materials and chemicals
Strong acid cation resin 001 × 7 and chelating resin S957 were obtained from Fuchen Chemical Reagent Factory and Purolite (China) Co., Ltd., respectively, and their characteristics were listed in Table 1. Analytical grade Ca(NO3)2·4H2O, Mg(NO3)2·6H2O, Fe(NO3)3·9H2O, Al(NO3)3·9H2O, and 65 wt% HNO3, 85 wt% H3PO4, 36 wt% HCl were used to prepare the synthetic solutions and the regeneration solutions, respectively.| Characteristics | 001 × 7 resin | S957 resin |
|---|---|---|
| Polymer structure | Gel-type polystyrene crosslinked with divinylbenzene | Macroporous polystyrene crosslinked with divinylbenzene |
| Functional groups | Sulfonic acid | Phosphonic acid and sulfonic acid |
| Ionic form | Na+ | H+ |
| Total exchange capacity | ≥1.8 equiv. L−1 | 18 g L−1 (iron capacity) |
| Specific gravity | 1.23–1.28 | 1.12 |
| Moisture holding capacity | 45–55% | 55–70% |
| Particle size | 0.3–1.2 mm | 0.3–0.8 mm |
| Operating temperature | 100 °C | 90 °C |
| pH range | 1–14 | 1–14 |
2.2. Dynamic adsorption and elution
Dynamic adsorption and elution processes were carried out in a glass column (internal diameter: 2 cm, length: 25 cm). The flow rate was controlled at 1 mL min−1 using a peristaltic pump (SHENCHEN, Lab 2015). The operations were performed as follows:(a) Synthetic resin (001 × 7 or S957) was firstly loaded into the column, then the deionized water was fed from the top of the column to rinse the resin. The resin in the column was collected as the sample of the resin before the adsorption of metal ions.
(b) Phosphoric acid–nitric acid solutions (4.4 mol L−1 phosphoric acid and 1.1 mol L−1 nitric acid) containing 0.25 mol L−1 Ca2+ and Mg2+ ions, or 0.15 mol L−1 Fe3+ and Al3+ ions were prepared according to the typical compositions of the neutralization mother liquor obtained from the Chemical Group Co. Ltd of China.10 The prepared solution passed through the column in the upflow mode. After the end of the adsorption process, the deionized water was fed from the top of the column to rinse the phosphoric acid–nitric acid solution retained. The resin in the column was collected as the sample of the resin after the adsorption of metal ions.
(c) The regeneration solution (7 mol L−1 nitric acid solution or 8 mol L−1 hydrochloric acid solution) was flown through the column upward. After the end of the elution process, the deionized water was fed from the top of the column to rinse the regeneration solution retained. Resin in the column was collected as the sample of the resin after the elution of metal ions.
2.3. Characterization
FT-IR spectra were recorded on a Shimadzu-8400 spectrometer in the range of 400–4000 cm−1 using KBr pellets. XPS spectra measurements were performed on a Thermo ESCALAB 250 with an Al Kα beam spot source (hν = 1486.6 eV, 150 W, 500 μm). The shift in the binding energy caused by the relative surface charges was referenced to the C 1s peak (284.8 eV) of the surface adventitious carbon. The surface area, pore volume, and pore size of the resin were measured by nitrogen adsorption–desorption isotherms using a Micromeritics ASAP-2460 instrument.2.4. Quantum chemistry computation
DFT calculations were run using the Gaussian 16 program package to comprehend the interaction mechanism of the metal ions with the resins. All the molecular structures in this study were built using Chem3D. The geometry optimizations and energy calculations were performed at the 6-31G(d) level of theory using the B3LYP method. The binding energies were calculated as follows:15–17| ΔE = E(adsorbent-metal ion complex) − E(adsorbent) − E(metal ion) | (1) |
3 Results and discussion
3.1. Adsorption mechanism on 001 × 7 resin
Fig. 1 presents the FT-IR spectra of 001 × 7 resins before and after the adsorption of Ca2+, Mg2+ ions, and after the elution of Ca2+, Mg2+ ions. The peaks around 3363–3523 cm−1 are assigned to the O–H stretching vibration of water absorbed and the sulfonic acid group.18–20 The peaks at 1181 and 1036 cm−1 are ascribed to S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretching vibration and symmetric stretching vibration of the sulfonic acid group, respectively.21,22 It can be observed from Fig. 1 that after the adsorption of Ca2+ and Mg2+ ions, the intensity of the characteristic peaks of O–H weakens, while the characteristic peaks of S
O asymmetric stretching vibration and symmetric stretching vibration of the sulfonic acid group, respectively.21,22 It can be observed from Fig. 1 that after the adsorption of Ca2+ and Mg2+ ions, the intensity of the characteristic peaks of O–H weakens, while the characteristic peaks of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are unchanged. After the elution of metal ions through nitric acid, the characteristic peaks of O–H recover and are almost the same as those before the adsorption of metal ions. These results reveal that during the adsorption process of Ca2+ and Mg2+ ions on 001 × 7 resin, the hydroxyl group on the sulfonic acid group combines with metal ions; meanwhile, the H+ ion is released, and after the regeneration with nitric acid, the hydroxyl is restored through the exchange reaction between the H+ ion at a higher concentration and the metal ion.
O are unchanged. After the elution of metal ions through nitric acid, the characteristic peaks of O–H recover and are almost the same as those before the adsorption of metal ions. These results reveal that during the adsorption process of Ca2+ and Mg2+ ions on 001 × 7 resin, the hydroxyl group on the sulfonic acid group combines with metal ions; meanwhile, the H+ ion is released, and after the regeneration with nitric acid, the hydroxyl is restored through the exchange reaction between the H+ ion at a higher concentration and the metal ion.
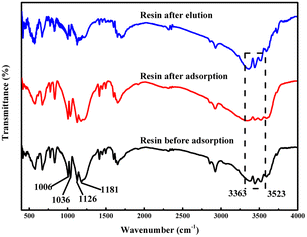 | ||
| Fig. 1 FT-IR spectra of 001 × 7 resins before and after the adsorption of Ca2+ and Mg2+ ions, and after the elution of Ca2+ and Mg2+ ions. | ||
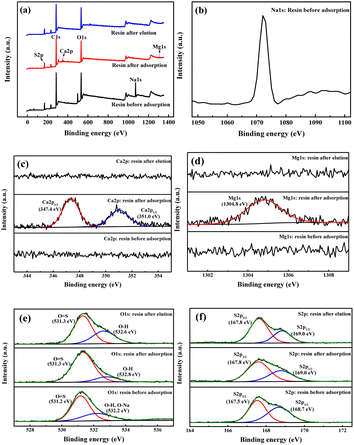
XPS spectra of 001 × 7 resins are shown in Fig. 2. The peaks centered at the binding energy of 347.4 eV and 351.0 eV correspond to Ca 2p3/2 and Ca 2p1/2, respectively.23,24 The peak of Mg 1s centers at the binding energy of 1304.8 eV.25,26 The spectrum of O 1s can be divided into two peaks centered at 531.3 eV and 532.8 eV, which are associated with O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and O–H, respectively.27 The spectrum of S 2p can be split into two peaks S 2p3/2 (167.8 eV) and S 2p1/2 (169.0 eV), which are assigned to S–O and S
S and O–H, respectively.27 The spectrum of S 2p can be split into two peaks S 2p3/2 (167.8 eV) and S 2p1/2 (169.0 eV), which are assigned to S–O and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.28
O, respectively.28
As shown in Fig. 2(a) and (b), due to the presence of the Na-type sulfonic acid group, XPS spectra of the fresh 001 × 7 resin exhibit Na 1s peak.29–31 After the adsorption of Ca2+ and Mg2+ ions, Na 1s peak disappears, while Ca 2p3/2, Ca 2p1/2 peaks and the Mg 1s peak appear. As a result of the exchange reaction between Ca2+ and Mg2+ ions and the H+ ion on the hydroxyl of the sulfonic acid group, the peak at the binding energy of 532.8 eV weakens, and the swell in the spectrum of S 2p for S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O almost disappears. After the of elution metal ions by nitric acid, Na 1s peak, Ca 2p3/2, Ca 2p1/2 peaks, and the Mg 1s peak disappear, which reveals that the Ca2+ and Mg2+ ions adsorbed on 001 × 7 resin are completely eluted and all of the sulfonic acid groups are H-type. In addition, the peak at the binding energy of 532.8 eV for O–H is enhanced, and the swell in the spectrum of S 2p for S
O almost disappears. After the of elution metal ions by nitric acid, Na 1s peak, Ca 2p3/2, Ca 2p1/2 peaks, and the Mg 1s peak disappear, which reveals that the Ca2+ and Mg2+ ions adsorbed on 001 × 7 resin are completely eluted and all of the sulfonic acid groups are H-type. In addition, the peak at the binding energy of 532.8 eV for O–H is enhanced, and the swell in the spectrum of S 2p for S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is the same as in the spectrum before adsorption.
O is the same as in the spectrum before adsorption.
3.2. Adsorption mechanism on S957 resin
Fig. 3 presents FT-IR spectra of S957 resin before and after the adsorption of Fe3+ and Al3+ ions and after the elution of Fe3+ and Al3+ ions. Apparently, after the adsorption of Fe3+ and Al3+ ions, the characteristic peak at 1001 cm−1 shifts to 1019 cm−1, and the peak intensity weakens, while the characteristic peak at 1081 cm−1 shifts to 1079 cm−1 and the peak intensity strengthens. However, because the characteristic peaks of the phosphonic acid group and sulfonic acid group are centered at 900–1200 cm−1,32–34 it is difficult to understand the bonding mechanism of Fe3+ and Al3+ ions with functional groups on S957 resin from its FT-IR spectra. Nevertheless, after the metal ions are eluted by hydrochloric acid, the characteristic peaks at 1001 cm−1 and 1081 cm−1 are recovered, and are almost the same as those before metal ion adsorption. This reveals that Fe3+ and Al3+ ions adsorbed on S957 resin are eluted successfully and functional groups are recovered.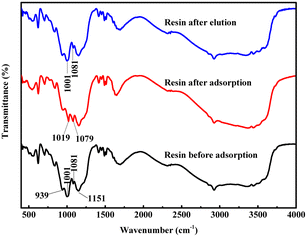 | ||
| Fig. 3 FT-IR spectra of S957 resin before and after the adsorption of Fe3+ and Al3+ ions, and after the elution of Fe3+, and Al3+ ions. | ||
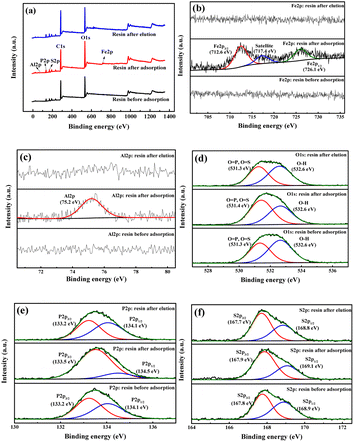
XPS spectra of S957 resin are shown in Fig. 4. The peaks centered at the binding energy of 712.6 eV and 726.1 eV correspond to Fe 2p3/2 and Fe 2p1/2, respectively, while the satellite peak of Fe 2p3/2 indicates binding energy of 717.4 eV.35–37 The peak of Al 2p centers at the binding energy of 75.2 eV.38–40 The spectrum of O 1s can be divided into two peaks centered at 531.3 eV (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P and O
P and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) and 532.8 eV (O–H).41 The spectrum of P 2p can be divided into two peaks centered at 133.2 eV (P 2p3/2) and 134.1 eV (P 2p1/2), which are associated with P
S) and 532.8 eV (O–H).41 The spectrum of P 2p can be divided into two peaks centered at 133.2 eV (P 2p3/2) and 134.1 eV (P 2p1/2), which are associated with P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and P–O, respectively.42,43 The spectrum of S 2p can be split into two peaks at 167.8 eV (S 2p3/2) and 168.9 eV (S 2p1/2).
O and P–O, respectively.42,43 The spectrum of S 2p can be split into two peaks at 167.8 eV (S 2p3/2) and 168.9 eV (S 2p1/2).
It can be observed from the XPS spectra of S957 resin that after the adsorption of Fe3+ and Al3+ ions, peaks of Fe 2p3/2, Fe 2p1/2, and Al 2p appear, and the peaks of O–H at the binding energy of 532.6 eV and P–O at the binding energy of 134.5 eV weaken, while the peaks of S 2p are almost unchanged. These results indicate that Fe3+ and Al3+ ions are adsorbed on S957 resin through the exchange reaction with H+ ions from the phosphonic acid group but not from the sulfonic acid group. After the metal ion elution, peaks for Fe 2p3/2, Fe 2p1/2 and Al 2p disappeared, and the peaks of O–H at the binding energy of 532.6 eV and P–O at the binding energy of 134.5 eV are enhanced, and are the same in comparison to the spectra before adsorption.
3.3. Bonding mechanism between functional groups on the resin and metal ions
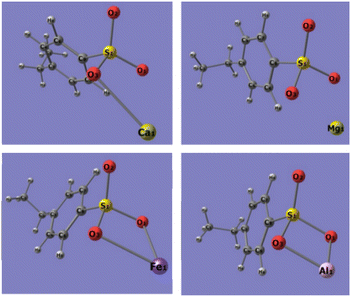
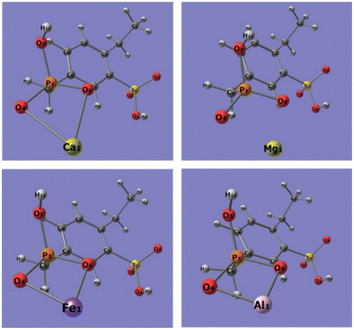
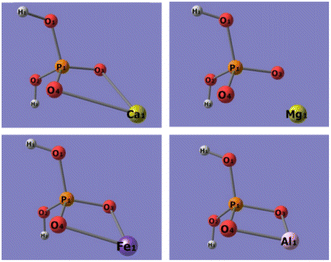
Quantum chemistry computation about the binding energies of different adsorbents (sulfonic acid group, phosphonic acid group, and dihydrogen phosphate ion) with Ca2+, Mg2+, Fe3+, and Al3+ ions was carried out to explore the bonding mechanism of the metal ions in phosphoric acid solution with functional groups on the resins. Based on the FT-IR and XPS spectra of 001 × 7 resins and S957 resins, adsorbent of the sulfonic acid group is built by structural units of 001 × 7 resin, in which sulfonic acid group loses the movable hydrogen ion, while adsorbent of the phosphonic acid group is built by the structural unit of S957 resin, in which phosphonic acid group loses a movable hydrogen ion and sulfonic acid group does not. Conformations of different adsorbents are shown in Fig. 5.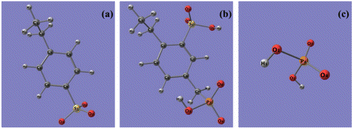 | ||
| Fig. 5 Conformations of different adsorbents, (a) sulfonic acid group, (b) phosphonic acid group, and (c) dihydrogen phosphate ion. | ||
Optimized conformations of the adsorbent-metal ion complexes are presented in Fig. 6–8. The binding energies of adsorbent-metal ion complexes are listed in Table 2. It can be observed that the differences in the binding energies of the sulfonic acid group-metal ion complexes and dihydrogen phosphate ion-metal ion complexes are 19.1662, 34.1315, 73.2515, and 98.7188 kJ mol−1 (Ca2+, Mg2+, Fe3+, and Al3+ ions, respectively). This indicates that dihydrogen phosphate ion has stronger binding energy to metal ions, and the differences in the binding energies of the two adsorbents with Ca2+ and Mg2+ ions are smaller, but the differences with Fe3+ and Al3+ ions are larger. Accordingly, under the influence of dihydrogen phosphate ion in the phosphoric acid solution, 001 × 7 resin containing only the sulfonic acid groups presents the adsorption ability to Ca2+ and Mg2+ ions while worse adsorption performance is seen for Fe3+ and Al3+ ions. Due to the weaker binding ability of the sulfonic acid group with Fe3+ and Al3+ ions, when Fe3+ and Al3+ ions in the phosphoric acid solution are adsorbed on the S957 resin, this group does not combine with Fe3+ and Al3+ ions.
| E(metal ion)/hartree | E(adsorbent)/hartree | E(adsorbent-metal ion complex)/hartree | ΔE/hartree | ΔE/kJ mol−1 | |
|---|---|---|---|---|---|
| −677.5218 (Ca2+) | –SO3− | −934.4345 | −1611.9707 | −0.0144 | −37.8072 |
| –PO3H− | −1541.5626 | −2219.1049 | −0.0205 | −53.8227 | |
| H2PO3− | −643.8381 | −1321.3816 | −0.0217 | −56.9733 | |
| −199.8449 (Mg2+) | –SO3− | −934.4345 | −1134.3397 | −0.0603 | −158.318 |
| –PO3H− | −1541.5626 | −1741.4824 | −0.0749 | −196.65 | |
| H2PO3− | −643.8381 | −843.7563 | −0.0733 | −192.449 | |
| −1263.2078 (Fe3+) | –SO3− | −934.4345 | −2197.6961 | −0.0538 | −141.252 |
| –PO3H− | −1541.5626 | −2804.8583 | −0.0879 | −230.781 | |
| H2PO3− | −643.8381 | −1907.1276 | −0.0817 | −214.503 | |
| −241.4268 (Al3+) | –SO3− | −934.4345 | −1176.2687 | −0.4074 | −1069.63 |
| –PO3H− | −1541.5626 | −1783.4511 | −0.4617 | −1212.19 | |
| H2PO3− | −643.8381 | −885.7099 | −0.4450 | −1168.35 | |
On the other hand, the differences in the binding energies of phosphonic acid group-metal ion complexes and dihydrogen phosphate ion-metal ion complexes are 3.1506, −4.2008, −16.2781, and −43.8459 kJ mol−1 (Ca2+, Mg2+, Fe3+, and Al3+ ions, respectively). That is, compared with the phosphonic acid group, the dihydrogen phosphate ion has stronger binding energy to the Ca2+ ion, while weaker binding energies for Mg2+, Fe3+, and Al3+ ions. Therefore, S957 resin containing the phosphonic acid group can hold great adsorption ability towards Fe3+ and Al3+ ions in the phosphoric acid solution, due to their higher binding performance with the phosphonic acid group while lower binding performance with the dihydrogen phosphate ion.
Thus, it can be concluded that due to the strong binding ability of the dihydrogen phosphate ions with Fe3+ and Al3+ ions, their adsorption behavior on the resin will be seriously affected by the coexisting phosphoric acid. Based on the order of the binding energy between the two metal ions and the adsorbent molecule: phosphonic acid group > dihydrogen phosphate ions > sulfonic acid group, the adsorption ability of 001 × 7 resin only containing sulfonic group to Fe3+ and Al3+ ions is very poor under the influence of dihydrogen phosphate ions dissociating from phosphoric acid, while S957 resin containing sulfonic acid group/phosphonic acid group has good adsorption capacity. Therefore, the coexisting components possessing strong binding ability with metal ions have a significant impact on their adsorption behaviors on the resins. Only the resin containing a strong binding ability functional group has the adsorption performance. Therefore, in order to further improve the separation efficiency of Fe3+ and Al3+ ions in the phosphoric acid solution, an important modification method is immobilizing a more efficient functional group on the resin, such as increasing the ratio of the phosphonic acid group and sulfonic acid group. The relevant modification experiments for the resins with different amounts of the phosphonic acid group and sulfonic acid group and their adsorption ability for Fe3+ and Al3+ ions from phosphoric acid solution are being studied in our laboratory.
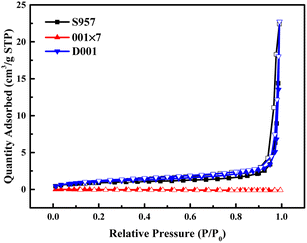
3.4. Influence of resin's porous structure
In order to further understand the adsorption mechanism, the adsorption processes of Ca2+, Mg2+, Fe3+, and Al3+ ions on D001 resin were carried out to explore the influence of the resin porous structure. According to resin characteristics supplied by the manufacturer, the D001 resin contains the same functional group as the 001 × 7 resin, but its polymer structure is macroporous polystyrene crosslinked with divinylbenzene, rather than the gel-type. As shown in the nitrogen adsorption–desorption isotherms of different resins (Fig. 9), the curves of D001 resin and S957 resin have smaller variations at P/P0 < 0.1, while upward tendency in 0.1 < P/P0 < 0.8. This indicates that the two resins contain mesoporous structures but do not contain microporous structures. Specific surface area, pore volume, and average pore size of D001 resin and S957 resin are 3.67 and 3.44 m2 g−1, 0.04 and 0.03 cm3 g−1, 36.35 and 48.80 nm, respectively. On the other hand, the amount of N2 adsorbed on the 001 × 7 resin at 0 < P/P0 < 1.0 is almost 0 cm3 g−1. This phenomenon is because the gel-type 001 × 7 resin only generates a microporous structure after absorbing water and swelling, but this kind of porous structure is very small, and the specific surface area and pore structure parameters of the 001 × 7 resin could not be obtained from the test results.Batch adsorption experiments indicate that the equilibrium adsorption capacities of D001 resin to Ca2+, Mg2+, Fe3+, and Al3+ ions in the phosphoric acid–nitric acid solution are 0.97, 0.71, 0.02, and 0.18 mmol g−1, respectively, which are almost the same with those of 001 × 7 resin (0.96, 0.74, 0.02, and 0.20 mmol g−1, respectively). Accordingly, it can be concluded that D001 resin and 001 × 7 resin have the same adsorption performances as the four metal ions in the phosphoric acid–nitric acid solution, thus the key factors in the adsorption ability of the resin are not the porous structure parameters but the functional groups on it.
4 Conclusions
We studied the adsorption mechanism of Ca2+, Mg2+, Fe3+, and Al3+ ions on 001 × 7 and S957 resins using FT-IR and XPS characterization techniques and quantum chemistry computation between the functional groups and metal ions. Results reveal that the adsorption mechanism of the 001 × 7 resin is the ion exchange reaction between the hydroxyl on the sulfonic acid group and Ca2+, Mg2+ ions. The adsorption mechanism of the S957 resin involves the ion exchange reaction between hydroxyl on the phosphonic acid group and Fe3+, Al3+ ions, while the sulfonic acid group and porous structural parameters have little influence. Quantum chemistry computation results reveal that the strong binding ability of dihydrogen phosphate ions to Fe3+ and Al3+ ions makes their adsorption behavior on the resin affected seriously by the coexisting phosphoric acid. As a consequence, only the resin containing a strong binding ability functional group, such as the phosphonic acid group, has the adsorption performance for Fe3+ and Al3+ ions from the phosphoric acid solution.Author contributions
Yingyuan Hu: conceptualization, methodology, writing – original draft, writing – review and edition. Wenlong Zhang: methodology, supervision. Meizhao Xue: investigation, data curation. Rui Lv: methodology, data curation, resources, supervision. Caimei Fan: methodology, supervision. Ao Li: investigation, data curation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was sponsored by the Natural Science Foundation for Young Scientists of Shanxi Province (No. 202103021223111, No. 202203021212347), Doctoral Research Start-up Fund (No. 2020BK08) and Scientific and Technological Innovation Capacity Cultivation Program (2023PY-TH-04) of Shanxi University of Chinese Medicine, Jinneng Coal Industry Group Co., Ltd. and Taiyuan University of Technology Postdoctoral project (No. 275226).Notes and references
- X. L. Yin, S. W. Tang, T. Zhang, Y. He and L. Lv, Ind. Eng. Chem. Res., 2023, 62, 6311–6321 CAS.
- D. H. Xu, B. H. Zhong, X. L. Wang, X. Li, Y. J. Zhong, Z. J. Yan, J. X. Yang, X. B. Li, Y. M. Wang and X. H. Zhou, Chinese J. Chem. Eng., 2022, 41, 170–175 CrossRef CAS.
- G. R. Campbell, Y. K. Leong, C. C. Berndt and J. L. Liow, Chem. Eng. Sci., 2006, 61, 5856–5866 CrossRef CAS.
- J. Ando and J. R. Lehr, J. Agric. Food. Chem., 1968, 16, 391–398 CrossRef CAS.
- J. C. Brosheer and F. A. Lenfesty, J. Agric. Food. Chem., 1958, 6, 827–833 CrossRef CAS.
- H. J. Jing, X. X. Zheng, C. M. Fan, J. Q. Feng, Y. F. Wang and G. Y. Ding, Adv. Mater. Res., 2011, 146, 1066–1069 Search PubMed.
- Y. Zhang and M. Muhammed, J. Chem. Eng. Data, 1989, 34(1), 121–124 CrossRef CAS.
- R. Lv, Q. Xi, T. Li, R. Li, X. C. Zhang, J. X. Liu, C. M. Fan, J. Q. Feng, L. Y. Zhang and Z. H. Wang, Chinese J. Chem. Eng., 2019, 27, 2930–2936 CrossRef.
- R. Lv, Y. Y. Hu, Z. H. Jia, R. Li, X. C. Zhang, J. X. Liu, C. M. Fan, J. Q. Feng, L. Y. Zhang and Z. H. Wang, Ind. Eng. Chem. Res., 2019, 58, 21641–21648 CrossRef CAS.
- R. Lv, Y. Y. Hu, Z. H. Jia, R. Li, X. C. Zhang, J. X. Liu, C. M. Fan, J. Q. Feng, L. Y. Zhang and Z. H. Wang, Hydrometallurgy, 2019, 188, 194–200 CrossRef CAS.
- D. S. Eldridge, R. J. Crawford and I. H. Harding, J. Colloid Interface Sci., 2015, 454, 20–26 CrossRef CAS PubMed.
- N. Kabay, M. Demircioğlu, H. Ekinci, M. Yüksel, M. Sağlam, M. Akçay and M. Streat, Ind. Eng. Chem. Res., 1998, 37, 2541–2547 CrossRef CAS.
- X. K. Leng, Y. J. Zhong, D. H. Xu, X. L. Wang and L. Yang, Chinese J. Chem. Eng., 2019, 27, 1050–1057 CrossRef CAS.
- C. L. He, M. W. Qi, Y. Liu, Z. Z. Liu, Y. Z. Wei, T. Fujita, G. F. Wang, S. J. Ma, W. C. Yang and J. Y. Gan, Hydrometallurgy, 2024, 224, 106230 CrossRef CAS.
- R. Q. Ma, T. Wang, T. B. Huang, W. L. Sun, S. Qiao and W. Liu, J. Mol. Liq., 2020, 312, 113432 CrossRef CAS.
- Y. Y. Luo, C. Liu, W. H. Xu and M. G. Shan, Sep. Purif. Technol., 2024, 333, 125858 CrossRef CAS.
- D. Tang, Y. L. Zhao, G. J. Xu, T. Yang and D. D. Han, J. Clean. Prod., 2023, 415, 137651 CrossRef CAS.
- F. S. Liu, X. L. Ma, H. Li, Y. Y. Wang, P. Cui, M. Guo, H. L. Yaxin, W. P. Lu, S. J. Zhou and M. Z. Yu, Fuel, 2020, 266, 117149 CrossRef CAS.
- P. B. Zhang, H. Wu, M. M. Fan, W. J. Sun, P. P. Jiang and Y. M. Dong, Fuel, 2019, 235, 426–432 CrossRef CAS.
- M. Goswami and P. Phukan, J. Environ. Chem. Eng., 2017, 5, 3508–3517 CrossRef CAS.
- Z. Z. Lin, M. T. Luo, Y. D. Zhang, X. X. Wu, Y. H. Fu, F. M. Zhang and W. D. Zhu, Appl. Catal. Gen., 2018, 563, 54–63 CrossRef CAS.
- T. Wang, C. H. Zhao, J. Xu and D. J. Sun, Colloid. Surface., 2013, 417, 256–263 CrossRef CAS.
- Y. F. Chen and X. K. Tang, Appl. Surf. Sci., 2020, 512, 145663 CrossRef CAS.
- Y. F. Zhang, Q. S. Wang, S. N. Gao, H. M. Jiang and C. G. Meng, Micropor. Mesopor. Mat., 2018, 266, 14–23 CrossRef CAS.
- M. Santamaria, F. D. Quarto, S. Zanna and P. Marcus, Electrochim. Acta, 2008, 53, 1314–1324 CrossRef.
- S. Ardizzone, C. L. Bianchi, M. Fadoni and B. Vercelli, Appl. Surf. Sci., 1997, 119, 253–259 CrossRef CAS.
- M. M. Nasef and H. Saidi, Appl. Surf. Sci., 2006, 252, 3073–3084 CrossRef CAS.
- Y. Jin, J. Shi, F. M. Zhang, Y. J. Zhong and W. D. Zhu, J. Mol. Catal. Chem., 2014, 383–384, 167–171 CrossRef CAS.
- Z. W. Zhao, Y. X. Song, X. B. Min, Y. J. Liang, L. Y. Chai and M. Q. Shi, J. Non-Cryst. Solids, 2016, 452, 238–244 CrossRef CAS.
- S. Y. Park, C. H. Champness and I. Shih, J. Electron. Spectrosc., 2015, 205, 23–28 CrossRef CAS.
- L. Silipigni, L. Schirò, L. M. Scolaro, G. D. Luca and G. Salvato, Appl. Surf. Sci., 2011, 257, 10888–10892 CrossRef CAS.
- L. L. Luo, F. S. Li, H. C. Zhang, Y. Z. Duan and W. C. Wang, Sep. Purif. Technol., 2023, 323, 124485 CrossRef.
- D. Kołodyńska, D. Fila and Z. Hubicki, Chem. Eng. Process., 2020, 147, 107767 CrossRef.
- L. Zhang, H. Y. Jin, C. H. Shen and S. J. Gao, Chem. Phys., 2020, 532, 110594 CrossRef CAS.
- H. Wang, F. X. Yin, G. R. Li, B. H. Chen and Z. Q. Wang, Int. J. Hydrogen Energy, 2014, 39, 16179–16186 CrossRef CAS.
- J. Gurgul, K. Łątka, I. Hnat, J. Rynkowski and S. Dzwigaj, Micropor. Mesopor. Mat., 2013, 168, 1–6 CrossRef CAS.
- T. Yamashita and P. Hayes, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
- K. I. Maslakov, Y. A. Teterin, S. V. Stefanovsky, S. N. Kalmykov, A. Y. Teterin, K. E. Ivanov and S. S. Danilov, J. Non-Cryst. Solids, 2018, 482, 23–29 CrossRef CAS.
- L. Wang, T. Shinohara and B. P. Zhang, Appl. Surf. Sci., 2010, 256, 5807–5812 CrossRef CAS.
- S. Attavar, M. Diwekar, M. R. Linford, M. A. Davis and S. Blair, Appl. Surf. Sci., 2010, 256, 7146–7150 CrossRef CAS.
- B. Zhang, Y. Cao, S. T. Li, Z. Jiang, G. W. He and H. Wu, J. Membr. Sci., 2016, 518, 243–253 CrossRef CAS.
- P. Piotrowski, A. Krogul-Sobczak and A. Kaim, J. Environ. Chem. Eng., 2019, 7, 103147 CrossRef CAS.
- J. Bassil, S. Roualdès, V. Flaud and J. Durand, J. Membr. Sci., 2014, 461, 1–9 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |