Open Access Article
Open Access ArticleEvaluation of sustained drug release performance and osteoinduction of magnetron-sputtered tantalum-coated titanium dioxide nanotubes
Jing Zhana,
Li Lia,
Lili Yao b,
Zheng Caoa,
Weiwei Louc,
Jianying Zhangd,
Jinsong Liu*b and
Litao Yao
b,
Zheng Caoa,
Weiwei Louc,
Jianying Zhangd,
Jinsong Liu*b and
Litao Yao *a
*a
aDepartment of Dentistry, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, 3# Qingchun East Road, Shangcheng District, Hangzhou 310058, Zhejiang, China. E-mail: 3320080@zju.edu.cn
bSchool and Hospital of Stomatology, Wenzhou Medical University, 268# Xueyuan West Road, Lucheng District, Wenzhou, Zhejiang, China. E-mail: jinsong0719@wmu.edu.cn
cDepartment of Stomatology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, China
dInternational Healthcare Center, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310058, China
First published on 23rd January 2024
Abstract
Modifying the drug-release capacity of titanium implants is essential for maintaining their long-term functioning. Titanium dioxide nanotube (TNT) arrays, owing to their drug release capacity, are commonly used in the biomaterial sphere. Their unique half open structure and arrangement in rows increase the drug release capacity. However, their rapid drug release ability not only reduces drug efficiency but also produces excessive local and systemic deposition of antibiotics. In this study, we designed a tantalum-coated TNT system for drug-release optimization. A decreased nanotube size caused by the tantalum nanocoating was observed through SEM and analyzed (TNT: 110 nm, TNT-Ta1: 80 nm, TNT-Ta3: 40 nm, TNT-Ta5: 20 nm, TNT-Ta7: <5 nm). XPS analysis revealed the distribution of the chemical components, especially that of the tantalum element. In vitro experiments showed that the tantalum nanocoating enhanced cell proliferation; in particular, TNT-Ta5 possessed the best cell viability (about 1.18 of TNT groups at 7d). It also showed that the tantalum nanocoating had a positive effect on osteogenesis (especially TNT-Ta5 and TNT-Ta7). Additionally, hydrophilic/hydrophobic drug (vancomycin/raloxifene) release results indicated that the TNT-Ta5 group possessed the most desirable sustained release capacity. Moreover, in this drug release system, the hydrophobic drug showed more sustained release capacity than the hydrophilic drug (vancomycin: sustained release for more than 48 h, raloxifene: sustained release for more than 168 h). More importantly, TNT-Ta5 is proved to be an appropriate drug release system, which possesses cytocompatibility, osteogenic capacity, and sustained drug release capacity.
1. Introduction
Titanium implants are widely applied in implantology owing to their excellent advantages such as good corrosion resistance, plasticity, and biocompatibility.1–4 However, the antibacterial scarcity, poor osteo-induction ability, and potential allergic reaction of titanium implants may cause postoperative complications, thus, leading to premature implant loss.3,5–7 Moreover, titanium-based implants can be easily modified by physical, chemical, and biological techniques.8–10 In addition, with these surface modifications, both surface energy and osteogenesis could be promoted.11 Among these techniques, the combination of drugs and implants is an effective method to enhance the pro-osteogenic and antibacterial activities.12–14 Titanium dioxide nanotube arrays (TNTs) have been developed as an excellent drug container, which have the advantages of neat arrangement, controllable dimeter, high surface-to-volume ratio, and excellent biocompatibility.15 Previous studies have proposed to prepare TNTs on medical implants as a drug release platform for clinical application.16 Moreover, TNTs with drug loading properties, biocompatibility, and pro-osteogenic promotion ability have attracted much interest,17,18 whereas rapid drug release caused by the large and straight diameter of TNTs would compromise their application.17,19 Therefore, effective techniques are urgently needed to control drug release from TNTs, promoting their biological properties and avoiding excessive local drug concentration.Numerous methods have been developed to optimize the drug release property of TNTs.20–22 For instance, a layer-by-layer (LBL) self-assembly method was applied to slow down the drug release rate.23 However, the LBL formed by electrostatic interactions between polycations and polyanions was easy to be detached.23 Moreover, a super-hydrophobic titanium dioxide nanotube (S-TNT) array drug delivery system was investigated to control drug release, while the osteogenic activity was still inadequate.24 Compared to polymer coating techniques, functional metal/metal oxide coating methods possess advantages of simplicity, anti-corrosion properties, and high stability.25–27 Therefore, controlling drug release from TNTs via metal/metal oxide coating is an effective method to achieve sustained drug release.
Based on the purpose of fabricating a drug release system by metal/metal oxide coating, the question still remained whether the narrowed outer diameter of nanotube could decrease the drug release rate and prolong the drug release duration. A previous study had covered the titanium dioxide nanotube array with ZnO and Sr to narrow the outer diameter of TNTs and enhance the osteogenic property, whereas the absence of drug release test failed to prove the function of drug release property.15 Our previous study coated Ga2O3 nanocoating on TNTs by magnetron sputtering to endow the titanium implant with antibacterial and osteogenic property, proving that Ga2O3-coated TNTs was a promising drug release system.28 However, Ga2O3 nano-coating was strictly dose-dependent and thus too thin to retard the drug releasing rate. Compared with Ga2O3, tantalum possessed excellent mechanical property and pro-osteogenic activity, which was conducive to osteointegration.29 Moreover, the ideal porosity, modulus of elasticity, and coefficient of friction made it easy to reduce the stress shielding effectively.30,31 Therefore, we deposited a tantalum nanocoating on TNTs in this study to narrow the orifice diameter of the nano-tube by magnetron sputtering to fabricate a tantalum modified drug release system. Afterward, the hydrophilic/hydrophobic drug (vancomycin/raloxifene) was loaded as the model drug to evaluate the drug release property, and in vitro experiments were applied to evaluate the osteo-inductive property. We speculated that the covering of a suitable tantalum nanocoating could form a desirable drug delivery system, which could realize controllable drug release and osteogenic differentiation promotion. Two issues were intended to be solved in this study as follows: (1) selecting the objective group with sustained drug release property and excellent osteogenic ability. (2) Evaluating the biocompatibility, pro-osteogenic property, and drug release performance of the tantalum-modified drug release system by in vitro experiment.
2. Materials and methods
2.1 Materials
Titanium foils (thickness: 0.2 mm, length: 10 mm, width: 10 mm) were purchased from Northwest Institute (Xi'an, China) and washed with acetone, ethanol, and distilled water for 10 min before the experiment. Ammonium fluoride and glycerin were purchased from Aladdin Biotech (Shanghai, China). The hydrophilic drug (vancomycin) and hydrophobic drug (raloxifene) were purchased from Sigma Chemical. FITC-labeled phalloidin, MTT solution, dihydrochloride (DAPI), bicinchoninic acid kit (BCA), alkaline phosphatase (ALP), and alizarin red staining kit were purchased from Beyotime Biotechnology Co. (Shanghai, China). Tantalum target materials were supplied by ZhongNuo Advanced Materials Technology Co. (Beijing, China).2.2 Sample preparation
The anodization procedure was conducted according to our previous study. In short, the electrolyte containing 50 wt% deionized water, 10 wt% ammonium fluoride, and 50 wt% glycerin was prepared. The pretreated pure titanium sheet (anode) and the platinum foils (cathode) were kept a distance of 10 mm and immersed in the electrolyte for anodization. The anodization parameters were set as follows: the direct current (DC) potential of 30 V for 1.5 h at 30 °C. After reaction, the anodized samples were taken out and ultrasonically cleaned with acetone, ethanol, and deionized water for 5 min. Finally, these samples were dried and stored in a vacuum device.2.3 Magnetron sputtering of tantalum
The tantalum nanocoating was deposited onto TNTs via DC magnetron sputtering machine (VTC-600-2HD, Kejing Auto-Instrument Co., Shenyang, China). The prepared TNTs were placed on the rotating stage and 99.9% high purity tantalum target was used for magnetron sputtering. The specific parameters of magnetron sputtering were as follows: the power, voltage, and gas pressure were kept the same, while the sputtering durations was set as 1 min, 3 min, 5 min, and 7 min and denoted as TNT-Ta1, TNT-Ta3, TNT-Ta5, and TNT-Ta7, respectively. The specific parameters of magnetron sputtering are illustrated in Table 1. Therefore, the groups involved in this study were Ti, TNT, TNT-Ta1, TNT-Ta3, TNT-Ta5, and TNT-Ta7.| Variable factors | Ta1 | Ta3 | Ta5 | Ta7 |
|---|---|---|---|---|
| Power (watt) | 100 | 100 | 100 | 100 |
| Sputtering rate (nm s−1) | 1 | 1 | 1 | 1 |
| Base pressure (Pa) | 5 × 10−4 | 5 × 10−4 | 5 × 10−4 | 5 × 10−4 |
| Working pressure (Pa) | 0.5 | 0.5 | 0.5 | 0.5 |
| Time (min) | 1 | 3 | 5 | 7 |
2.4 Surface characterization
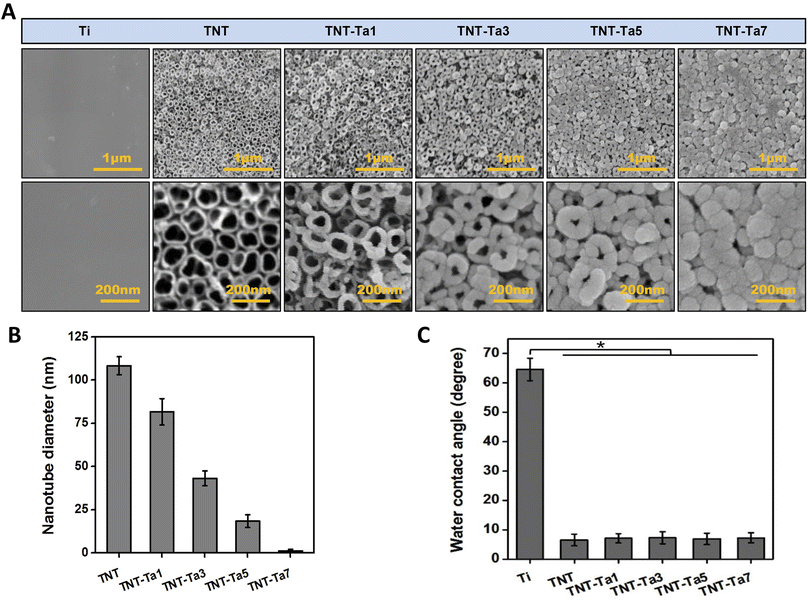
Field emission scanning electron microscopy was used to detect the surface structure of different samples (FESEM, Nova NanoSEM200, FEI Co., USA), and the diameter of TNTs was analyzed by IPwin60. The chemical composition of Ti, TNT, TNT-Ta1, TNT-Ta3, TNT-Ta5, and TNT-Ta7 was detected via X-ray photoelectron spectroscopy (XPS, K-alpha, Thermo, USA), and the amount of Ta element was measured by XPS quantitative analysis. The hydrophilicity of the surface was detected by a water contact angle system (Model 200, Future Scientific, China). The crystal structure was accessed by X-ray diffraction (XRD, Philips X'Pert Pro). Four samples in each group were applied for water contact angle measurement. In short, 5 μL distilled water was dropped on the sample surface for 15 s at room temperature before imaging. Finally, water contact angle analysis (WCA, SL200B, Solon) was performed to evaluate the hydrophilic property. The average roughness of different substrates (6 samples/group) was detected in the examination range (10 μm × 10 μm) by atomic force microscopy (AFM, Veeco Co, USA). In this way, roughness characterization including 3D images, mean roughness (Sa), and RMS roughness (Sq) of different substrates was acquired. Among them, Sa reflected the arithmetic average deviations of the profile height from the mean surface, and Sq indicated the root mean square average of the profile height deviations from the mean surface.2.5 Drug release test
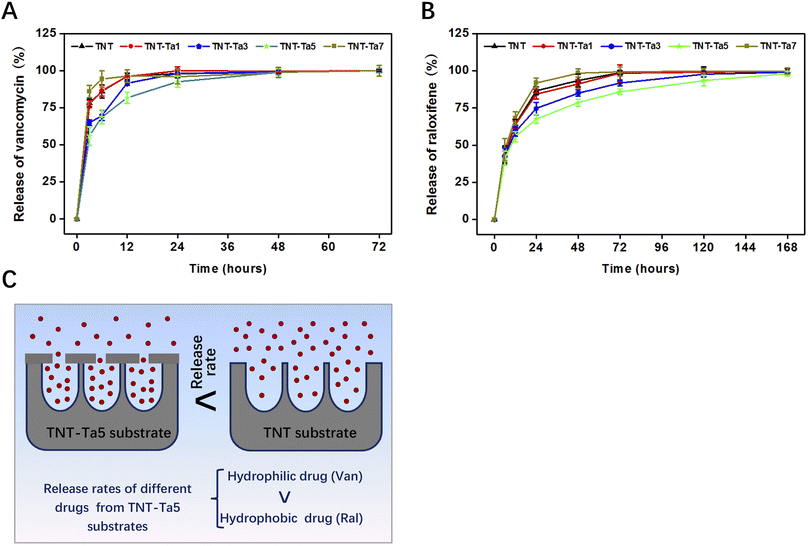
Vancomycin solution (1 mg mL−1, 10 mL) and raloxifene solution (1 mg mL−1, 10 mL) were prepared at room temperature. The drug-loaded samples were prepared by immersing in two solutions with 2 h of shaking. Therefore, the drug would be fully loaded into the nano-tube arrays of TNTs in this way. Afterward, each group of samples was put into 10 mL deionized water for 1 min to remove the residual solution, which was dried with nitrogen. Next, the samples were immersed in PBS solution (pH 7.4) at 37 °C for solution collection.For the vancomycin-loaded samples, the immersion solution (0.5 mL) was collected and replaced with fresh PBS at the immersing duration of 12, 24, 36, 48, 60, and 72 h.
As for the raloxifene-loaded samples, the immersion solution (0.5 mL) was collected and replaced with fresh PBS at the incubation time points of 24, 48, 72, 96, 120, 144, and 168 h. The amount of vancomycin/raloxifene released into PBS was measured via a Nanodrop 2000 instrument at a wavelength of 280 nm.
2.6 In vitro cell evaluation
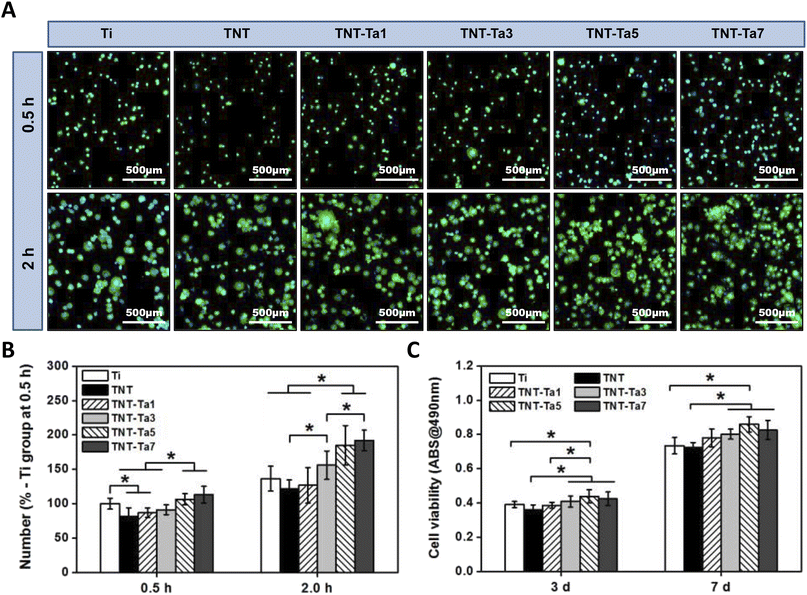
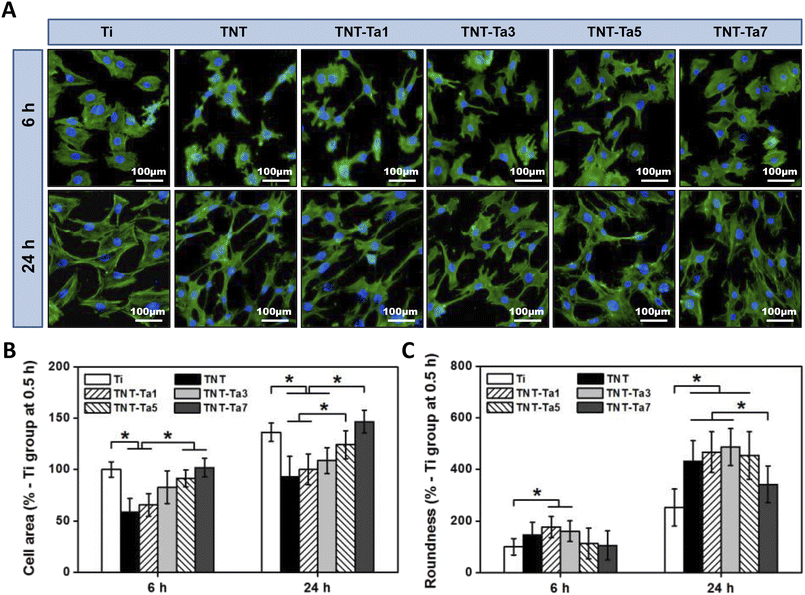
As for early adhesion, we used sterilized PBS to remove the impurities after 0.5 and 2 h of cell incubation (seeding density: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per cm2). Next, 0.2% TritonX-100 was used to punch after cell fixation with 4% paraformaldehyde for 1 h. Afterward, the cytoskeleton was stained with FITC-labeled phalloidin for 30 min, and the nucleus was stained with DAPI for 15 min. The treated specimens were then washed thrice with PBS and observed using a fluorescence microscope from five directions. The experiment was repeated three times.
000 per cm2). Next, 0.2% TritonX-100 was used to punch after cell fixation with 4% paraformaldehyde for 1 h. Afterward, the cytoskeleton was stained with FITC-labeled phalloidin for 30 min, and the nucleus was stained with DAPI for 15 min. The treated specimens were then washed thrice with PBS and observed using a fluorescence microscope from five directions. The experiment was repeated three times.
| Target genes | Primers |
|---|---|
| Runx2 | Forward 5′-CCACAAGGACAGAGTCAGATTACA-3′ |
| Reverse 5′-TGGCTCAGATAGGAGGGGTA-3′ | |
| ALP | Forward 5′-GGCCAGCTACACCACAACA-3′ |
| Reverse 5′-CTGAGCGTTGGTGTTATATGTCTT-3′ | |
| COL-1 | Forward 5′-CCTGAGCCAGCAGATTGA-3′ |
| Reverse 5′-TCCGCTCTTCCAGTCAG-3′ | |
| OPN | Forward 5′-CCCGGTGAAAGTGACTGATT-3′ |
| Reverse 5′-TTCTTCAGAGGACACAGCATTC-3′ | |
| OCN | Forward 5′-AGACTCCGGCGCTACCTT-3′ |
| Reverse 5′-CTCGTCACAAGCAGGGTTAAG-3′ | |
| GAPDH | Forward 5′-GGCATTGCTCTCAATGACAA-3′ |
| Reverse 5′-TGTGAGGGAGATGCTCAGTG -3′ |
2.7 Statistical analysis
All data in this study were expressed as means ± standard deviation (SD). The statistical analysis was performed through Student's t-test and one-way analysis of variance (ANOVA) using Origin Pro at 95% and 99% confidence levels.3. Results and discussion
3.1 Surface characterization
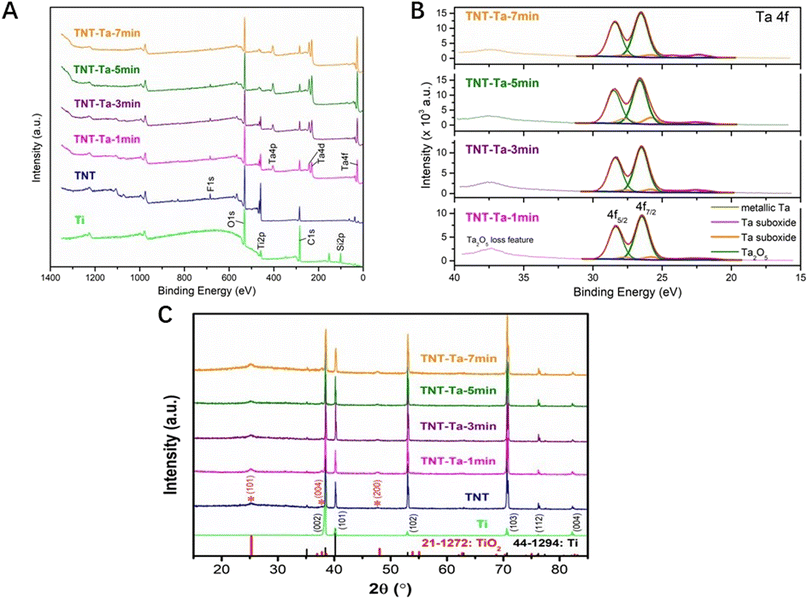
The surface structure of different specimens was characterized by FE-SEM (Fig. 1A). The Ti samples showed smooth surface with no obvious impurities, while an ordered TiO2 nanotubes possessed an average outer diameter of 110 nm (Fig. 1A and C). After tantalum nanocoating deposition, the diameter of TiO2 nanotubes was decreased with the extension of sputtering duration (TNT-Ta1: 80 nm; TNT-Ta3: 40 nm; TNT-Ta5: 20 nm; TNT-Ta7 <5 nm). Moreover, FE-SEM showed that the deposited tantalum nanocoating was dense and uniformly distributed over the TNTs. The procedure of magnetron sputtering had advantages of uniform distribution, dense and controllable layer, low temperature requirement, and high bind strength.33 Zeliang Ding et al. prepared multilayer Cu-mTa2O5 composite coatings on Ti6Al4V alloy for biomedical applications by magnetron sputtering, realizing an enhanced anticorrosion performance and superior antibacterial effects.34 As for surface topography, previous studies proved that TNTs with large diameter (110–150 nm) were beneficial for osteogenic differentiation, while a small diameter (15–70 nm) was conducive to the cell proliferation of MC3T3-E1.35 Among them, TNTs with diameter of 15 nm possessed the highest cell biocompatibility.17 Moreover, a previous study fabricated TNTs with a diameter of 25 nm and 80 nm on the titanium surface to evaluate the biological property of mesenchymal stem cells (BMSCs). The results indicated that TNTs with 25 nm size had significantly higher osteogenic gene related expression than those with 80 nm size.36 However, previous studies showed that TiO2 with pore diameter of 70 nm exhibited promising osteo-induction property, which was mainly attributed to the favorable hydrophilicity, protein adsorption ability, and pro-osteogenic functions caused by the “capillary” of nanotube topography and hydroxyl groups.37,38 Nevertheless, the structure with small nanotube diameter was conducive to chondrogenic differentiation. The surface structure with small nanotube diameter was found to impede the endochondral ossification.39 Therefore, TNTs with different sizes of nanotube diameter possessed different biological functions under different conditions. Though the most suitable nanotube diameter of TNTs has not been standardized yet, the biological response of different substrates was the joint effect of tantalum and surface physical properties. In this study, the tantalum nanocoating was applied to adjust the diameter of the nanotube to optimize the drug release property. TNTs with decreased nanotube diameter (TNT-Ta1: 80 nm; TNT-Ta3: 40 nm; TNT-Ta5: 20 nm; TNT-Ta7 <5 nm) were successfully fabricated by magnetron sputtering parameters given in Table 1.In order to detect the surface elemental and binding compositions, XPS analysis was performed, as shown in Fig. 3A and C. The electronic orbitals of Ta 4p, Ta 4d, and Ta 4f were observed in tantalum-modified samples corresponding to the binding energies of 405, 230, and 25 eV. Meanwhile, compared with TNT, the binding energies of Ti 2p were decreased in the tantalum-modified samples, indicating the success of tantalum deposition and that the tantalum nanocoating was thicker with the increase of the sputtering duration. The detailed scan data in Fig. 3C showed that tantalum element was composed of Ta2O5 (+5), metallic Ta, and suboxides with low valence (+1 to +4). Moreover, Ta2O5 (+5) was prominent in all tantalum-modified samples, which was mainly attributed to the oxidation.40
In addition, the hydrophilic property and crystalline phase structure also affect the surface physical property.41 Fig. 3C showed that the TNT surface possessed high hydrophilic property (5 ± 2.33°) compared with pure Ti (65 ± 2.67°), which was consistent with our previous studies.28 The contact angle decreased 92% compared to that of pure Ti. This reason was complex, which might be related to the reaction products containing hydroxide compounds on the TNT surfaces, the introduction of oxygen elements, the nanotube arrays that provided space for liquid penetration, and the alteration of surface roughness.42,43 According to previous studies, the nanotube arrays that provide the liquid to penetrate might be the prominent reason for the decrease of the contact angle.40 The TNTs substrates in our study showed porous structure with large diameter (Fig. 1A and C), containing more water molecules and reducing the water contact angle. Notwithstanding, the nanotube arrays with larger diameter could provide more penetration space and thus create a more hydrophilic surface. Consistent with a previous study that the nanocoating significantly increased the water contact angle of TNTs,28 the water contact angle of TNT-Ta1, TNT-Ta3, TNT-Ta5, and TNT-Ta7 was almost the same as that of TNTs (TNT-Ta1: 5.6 ± 2.38°, TNT-Ta3: 6.8 ± 5.28°, TNT-Ta5: 5.9 ± 6.78°, TNT-Ta7: 6.6 ± 7.83°). However, the reasons are not yet fully elucidated and we will be able to clarify it in our further experiment. The increase in the surface roughness and the alternation of surface topography might influence the result.44,45
The XRD patterns of the produced specimens were presented in Fig. 3C. It exhibited the characteristic peaks of Ti (JCPDS: 44-12-94), which was consistent with our previous study.28 The main diffraction peaks of XRD were the characteristic peaks of titanium: 35.03°, 38.37°, 40.17°, 53.02°, 63.94°, 70.65°, 76.25°, and 77.33° (card number JCPDS: 44-12-94). On the other hand, the TiO2 diffraction peaks (card number JCPDS: 21-1272) 25.2°, 37.8°, and 48° emerged after anodization, which proved the composition of TNTs. From these results, no obvious Ta signal can be detected by XRD evaluation, indicating that the deposition of the tantalum nanocoating could not change the crystalline phase structure. The reason may be attributed to the thinness of the tantalum coating, indicating that the magnetron sputtering technique was insufficient to produce the Ta crystal phase.46 The surface roughness was compared in Fig. 2. Merely depending on the Sa value to compare the surface roughness was inadequate, which reflected the arithmetic average deviations of the profile height from the mean surface. Therefore, the parameter Sq was taken to indicate the root mean square average of the profile height deviations from the mean surface, which could react to the maximum heights and depths of the profile sensitively.47,48 Fig. 2 showed that pure Ti possessed minimum roughness (Sa: 34 nm, Sq: 44.8 nm), while the anodization treatment increased the surface roughness significantly (Sa: 69.8 nm, Sq: 87.5 nm). Moreover, the deposition of the tantalum nanocoating also remarkably increased the surface roughness, and TNT-Ta7 (Sa: 199.6 nm, Sq: 221 nm) was the roughest substrate. The Sa/Sq values of all the substrates were ranked in the order Ti < TNT < TNT-Ta1 < TNT-Ta3 < TNT-Ta5 < TNT-Ta7, indicating that the surface roughness increased on prolonging the sputtering duration.
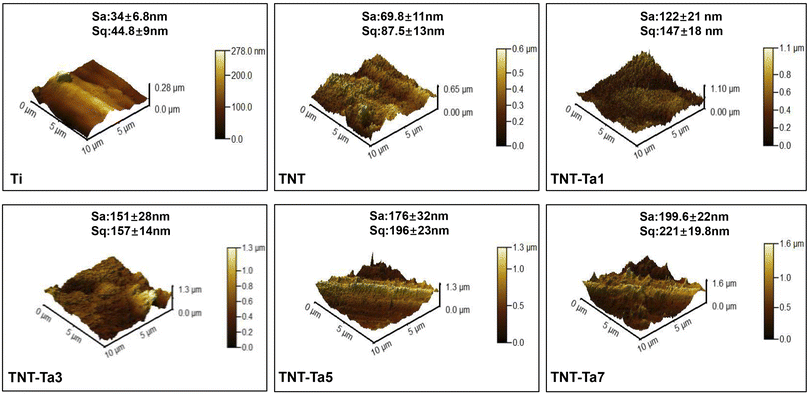 | ||
| Fig. 2 AFM images showing the average roughness (Sa) and root mean square deviation of the surface (Sq) of different substrates (n = 6). | ||
3.2 In vitro cell experiment
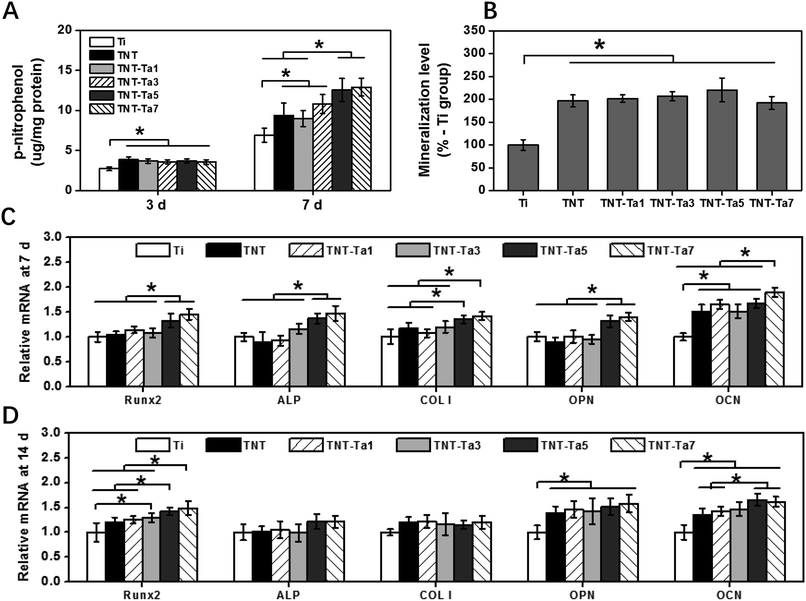
In order to further access the osteogenic property of different specimens, alizarin red staining was evaluated to access the mineralization level. As shown in Fig. 6B, pro-osteoblasts cultured on TNT (196 ± 13%) and TNT-Ta specimens showed significantly enhanced (P < 0.05) mineralization compared with Ti (100 ± 11%), which might explain that tantalum nanocoating played a pivotal role in the mature stage of osteogenic differentiation.57 Among the TNT-Ta specimens, the TNT-Ta5 group showed the highest mineralization level (220 ± 26%), indicating the osteogenic promotion property of suitable tantalum nanocoating and corresponding to previous studies.40,58 On the one hand, tantalum promoted the proliferation and adhesion of pro-osteoblasts and enhanced the mineralization level by the Wnt/β-catenin and TGF-β/smad signal pathway.59 On the other hand, tantalum could activate the MAPK/ERK signaling pathway and thus promote osteogenesis-related gene expression.45 Except for the tantalum function, the decreased nanotube diameter of TNTs also plays an important role in osteogenic promotion. A previous study proved the TNTs with smaller diameter (<30 nm) showed enhanced ALP activity and ECM mineralization, while TNTs with large diameter exhibited a negative effect on cell growth.17 Additionally, previous studies also proved that TiO2 with pore diameter of 20 nm promoted osteogenic induction, while osteoblastic maturation promotion was observed on surfaces with a pore diameter of 50 nm.37 As a result, the surface structure properties, including surface roughness and topography, could not only influence the physical properties, such as wettability and energy, but also the biological behavior. For instance, a previous study had proved that highly rough pure titanium coating promoted the osteogenic differentiation.60 It also indicated that surfaces with a higher roughness were beneficial for the expression of osteogenesis-related genes, while surfaces with lower roughness of 15 nm were conducive to chondrogenic gene expression.61 Therefore, the biological response of objective surfaces was affected by the combination of surface properties including wettability, surface roughness, and surface topography.
In order to evaluate the osteogenic property at a genetic level, the RT-qPCR experiment was conducted. After 7 days of cell culture, the expression of Runx2, ALP, OPN, OCN, and COL-1 on the TNT-Tpa5 and TNT-Ta7 group was statistically higher than those on the control group (P < 0.05). Unlike 7 d of cell culture, the RT-qPCR results at 14 d showed that both the TNT-Ta5 and TNT-Ta7 group promoted the expression of Runx2, OPN, and OCN significantly (P < 0.05) when compared with Ti and TNT. Among these osteogenesis-related genes, Runx2 was regarded as an important transcription factor of osteogenic induction and bone to implant osteointegration.62 Meanwhile, the TNT-Ta5 group (1.33 ± 0.13/1.41 ± 0.075) showed significantly higher expression level of Runx2 than Ti (1.00 ± 0.09/1.00 ± 0.18), TNT (1.03 ± 0.07/1.19 ± 0.10), and TNT-Ta1 (1.14 ± 0.07/1.24 ± 0.07) both after 7/14 days of incubation. These results indicated that the tantalum nanocoating plays a pivotal role in osteogenesis-related gene expression (Runx2, ALP, OPN, OCN, and COL-1) and thus promoted the osteogenic property. It was corresponding to a previous study that porous tantalum scaffold could enhance the expression of osteogenesis-related gene (ALP, OSX, COL-1, OSN, and OCN) expression by the activation of the MAPK/ERK signaling pathway.63 Furthermore, the TNT-Ta5 group was regarded as an ideal group with the best cytocompatibility and excellent osteogenic ability, indicating that the tantalum nanocoating with an appropriate thickness on TNTs could provide conducive cell condition.64 Moreover, the phenomenon that ALP gene expression was decreased after 14 days of cell culture was consistent with a previous study, which was mainly attributed to the transfer to mineralization.15 It indicated that the tantalum nanocoating promoted the gene expression of Runx2, ALP, OPN, OCN, and COL-1 at the early stage and thus enhanced the ALP activity and osteogenic induction. Afterward, the gene expression of Runx2, OPN, and OCN was further promoted at a later stage to further enhance the mineralization and osteogenic property.
4. Conclusion
In this study, a new tantalum nanocoating modified drug release system was established by the combination of anodization and magnetron sputtering. This tantalum modified drug delivery system was expected to enhance the biological function and sustained drug release ability. In view of the relevant experimental results and analysis, the following conclusions could be summarized.• The surface characterization results showed that the tantalum-modified drug delivery system was successfully prepared and tantalum element was present as Ta2O5.
• The in vitro cell experiment indicated that TNT-Ta5 and TNT-Ta7 possessed superior pro-osteogenic induction property, which upregulated OCN, Runx2, and OPN gene expression and thus enhanced the osteogenic differentiation.
• The drug release test indicated that the TNT-Ta5 group had the best sustained drug release property, especially for the hydrophobic drug (168 h).
• The suitable tantalum nano-coating on TNTs (TNT-Ta5) was confirmed as a promising strategy for implant design.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work is supported by the National Natural Science Foundation of China (82071170), Traditional Chinese Medicine Science and Technology Program of Zhejiang Province (2023ZR109) and Wenzhou Public Welfare Science and Technology Project (Y2020209).References
- J. Liang, S. Xu, M. Shen, B. Cheng, Y. Li, X. Liu, D. Qin, A. Bellare and L. Kong, Osteogenic activity of titanium surfaces with hierarchical micro-/nano-structures obtained by hydrofluoric acid treatment, Int. J. Nanomed., 2017, 12, 1317 CrossRef CAS PubMed.
- H. Wang, Q. Xu, H. Hu, C. Shi, Z. Lin, H. Jiang, H. Dong and J. Guo, The fabrication and function of strontium-modified hierarchical micro/nano titanium implant, Int. J. Nanomed., 2020, 8983–8998 CrossRef CAS PubMed.
- P. Jiang, Y. Zhang, R. Hu, B. Shi, L. Zhang, Q. Huang, Y. Yang, P. Tang and C. Lin, Advanced surface engineering of titanium materials for biomedical applications: From static modification to dynamic responsive regulation, Bioact. Mater., 2023, 27, 15–57 CAS.
- S. Kligman, Z. Ren, C.-H. Chung, M. A. Perillo, Y.-C. Chang, H. Koo, Z. Zheng and C. Li, The impact of dental implant surface modifications on osseointegration and biofilm formation, J. Clin. Med., 2021, 10(8), 1641 CrossRef CAS PubMed.
- K. A. Kravanja and M. Finšgar, A review of techniques for the application of bioactive coatings on metal-based implants to achieve controlled release of active ingredients, Mater. Des., 2022, 110653 CrossRef CAS.
- X. He, F.-X. Reichl, S. Milz, B. Michalke, X. Wu, C. M. Sprecher, Y. Yang, M. Gahlert, S. Röhling and H. Kniha, Titanium and zirconium release from titanium-and zirconia implants in mini pig maxillae and their toxicity in vitro, Dent. Mater., 2020, 36(3), 402–412 CrossRef CAS PubMed.
- A. Rezvan, E. Sharifikolouei, A. Lassnig, V. Soprunyuk, C. Gammer, F. Spieckermann, W. Schranz, Z. Najmi, A. Cochis and A. C. Scalia, Antibacterial activity, cytocompatibility, and thermomechanical stability of Ti40Zr10Cu36Pd14 bulk metallic glass, Mater. Today Bio, 2022, 16, 100378 CrossRef CAS PubMed.
- J. C. Souza, M. B. Sordi, M. Kanazawa, S. Ravindran, B. Henriques, F. S. Silva, C. Aparicio and L. F. Cooper, Nano-scale modification of titanium implant surfaces to enhance osseointegration, Acta Biomater., 2019, 94, 112–131 CrossRef CAS PubMed.
- L. Sun, X. Chen, K. Ma, R. Chen, Y. Mao, R. Chao, H. Wang, B. Yu, J. Wang and S. Zhang, Novel Titanium Implant: a Three-dimensional Multifunction Architecture with Charge-trapping and Piezoelectric Self-stimulation, Adv. Healthcare Mater., 2023, 2202620 CrossRef CAS PubMed.
- C. Tang, J. Deng, R. Xu, J. Li, C. Yin, Y. Yang, Y. Zhou and F. Deng, Micro/nano-modified Titanium Surfaces Accelerate Osseointegration via Rab7-dependent Mitophagy, Biomater. Sci., 2023, 11(2), 666–677 RSC.
- J. Tang, L. Chen, D. Yan, Z. Shen, B. Wang, S. Weng, Z. Wu, Z. Xie, J. Shao and L. Yang, Surface functionalization with proanthocyanidins provides an anti-oxidant defense mechanism that improves the long-term stability and osteogenesis of titanium implants, Int. J. Nanomed., 2020, 1643–1659 CrossRef CAS PubMed.
- N. Sarkar and S. Bose, Controlled delivery of curcumin and vitamin K2 from hydroxyapatite-coated titanium implant for enhanced in vitro chemoprevention, osteogenesis, and in vivo osseointegration, ACS Appl. Mater. Interfaces, 2020, 12(12), 13644–13656 CrossRef CAS PubMed.
- D. Aggarwal, V. Kumar and S. Sharma, Drug-loaded biomaterials for orthopedic applications: A review, J. Controlled Release, 2022, 344, 113–133 CrossRef CAS PubMed.
- S. Dai, L. Jiang, L. Liu, J. Chen, Y. Liao, S. He, J. Cui, X. Liu, A. Zhao and P. Yang, Photofunctionalized and drug-loaded TiO2 nanotubes with improved vascular biocompatibility as a potential material for polymer-free drug-eluting stents, ACS Biomater. Sci. Eng., 2020, 6(4), 2038–2049 CrossRef CAS PubMed.
- K. Zhang, Y. Zhu, X. Liu, Z. Cui, K. W. Yeung, H. Pan and S. Wu, Sr/ZnO doped titania nanotube array: an effective surface system with excellent osteoinductivity and self-antibacterial activity, Mater. Des., 2017, 130, 403–412 CrossRef CAS.
- L. Yuan, X. Xu, X. Song, L. Hong, Z. Zhang, J. Ma and X. Wang, Effect of bone-shaped nanotube-hydrogel drug delivery system for enhanced osseointegration, Biomater. Adv., 2022, 137, 212853 CrossRef CAS PubMed.
- K. Wang, H. Jin, Q. Song, J. Huo, J. Zhang and P. Li, Titanium dioxide nanotubes as drug carriers for infection control and osteogenesis of bone implants, Drug Delivery Transl. Res., 2021, 11(4), 1456–1474 CrossRef CAS PubMed.
- Y. Liu, C. Xie, F. Zhang and X. Xiao, pH-responsive TiO2 nanotube drug delivery system based on iron coordination, J. Nanomater., 2019, 1–7 Search PubMed.
- T. Guo, K. Gulati, H. Arora, P. Han, B. Fournier and S. Ivanovski, Orchestrating soft tissue integration at the transmucosal region of titanium implants, Acta Biomater., 2021, 124, 33–49 CrossRef CAS PubMed.
- F. Wu, J. Xu, R. Yan, B. Hu, G. Li, M. Jin, X. Jiang, J. Li, P. Tang and J. Zhu, In vitro and in vivo evaluation of antibacterial activity of polyhexamethylene guanidine (PHMG)-loaded TiO2 nanotubes, Biomed. Mater., 2020, 15(4), 045016 CrossRef CAS PubMed.
- J. Wu, J. Huang, J. Yun, J. Yang, J. Yang, A. Fok and Y. Wang, Enzyme-directed biomineralization coating on TiO2 nanotubes and its positive effect on osteogenesis, ACS Biomater. Sci. Eng., 2019, 5(6), 2769–2777 CrossRef CAS PubMed.
- B. Tao, Y. Deng, L. Song, W. Ma, Y. Qian, C. Lin, Z. Yuan, L. Lu, M. Chen and X. Yang, BMP2-loaded titania nanotubes coating with pH-responsive multilayers for bacterial infections inhibition and osteogenic activity improvement, Colloids Surf., B, 2019, 177, 242–252 CrossRef CAS PubMed.
- X. Ma, D. Zhao, Y. Xiang, Y. Hua, W. Zhao, Y. Cui and Z. Zhang, Layer-by-layer self-assembly and clinical application in orthopedics, J. Mater. Sci. Technol., 2022, 147, 241–268 CrossRef.
- J. Zhou, M. A. Frank, Y. Yang, A. R. Boccaccini and S. Virtanen, A novel local drug delivery system: Superhydrophobic titanium oxide nanotube arrays serve as the drug reservoir and ultrasonication functions as the drug release trigger, Mater. Sci. Eng. C, 2018, 82, 277–283 CrossRef CAS PubMed.
- M. Mesgari, A. H. Aalami and A. Sahebkar, Antimicrobial activities of chitosan/titanium dioxide composites as a biological nanolayer for food preservation: A review, Int. J. Biol. Macromol., 2021, 176, 530–539 CrossRef CAS PubMed.
- C. Mutalik, G. Okoro, D. I. Krisnawati, A. Jazidie, E. Q. Rahmawati, D. Rahayu, W.-T. Hsu and T.-R. Kuo, Copper sulfide with morphology-dependent photodynamic and photothermal antibacterial activities, J. Colloid Interface Sci., 2022, 607, 1825–1835 CrossRef CAS PubMed.
- T. Zhang, Y. Liu, F. Zhang and X. Xiao, Polylysine-modified titania nanotube arrays for local drug delivery, Micro Nano Lett., 2018, 13(1), 93–95 CrossRef CAS.
- L. Yao, A. M. Al-Bishari, J. Shen, Z. Wang, T. Liu, L. Sheng, G. Wu, L. Lu, L. Xu and J. Liu, Osseointegration and anti-infection of dental implant under osteoporotic conditions promoted by gallium oxide nano-layer coated titanium dioxide nanotube arrays, Ceram. Int., 2023, 49(14), 22961–22969 CrossRef CAS.
- H. Gao, J. Yang, X. Jin, X. Qu, F. Zhang, D. Zhang, H. Chen, H. Wei, S. Zhang and W. Jia, Porous tantalum scaffolds: Fabrication, structure, properties, and orthopedic applications, Mater. Des., 2021, 210, 110095 CrossRef CAS.
- X. Hu, S. Mei, F. Wang, J. Qian, D. Xie, J. Zhao, L. Yang, Z. Wu and J. Wei, Implantable PEKK/tantalum microparticles composite with improved surface performances for regulating cell behaviors, promoting bone formation and osseointegration, Bioact. Mater., 2021, 6(4), 928–940 CAS.
- K.-H. Cheon, C. Park, M.-H. Kang, I.-G. Kang, M.-K. Lee, H. Lee, H.-E. Kim, H.-D. Jung and T.-S. Jang, Construction of tantalum/poly (ether imide) coatings on magnesium implants with both corrosion protection and osseointegration properties, Bioact. Mater., 2021, 6(4), 1189–1200 CAS.
- L. Li, L. Yao, L. Wu, Q. Ma, S. Lin, X. Shen, C. Huang and L. Sheng, The characteristic and osteogenic effect of a nanoporous coating of zirconia implant, Ceram. Int., 2022, 48(17), 24260–24267 CrossRef CAS.
- J. Wu, K. Ueda and T. Narushima, Fabrication of Ag and Ta co-doped amorphous calcium phosphate coating films by radiofrequency magnetron sputtering and their antibacterial activity, Mater. Sci. Eng. C, 2020, 109, 110599 CrossRef CAS PubMed.
- Z. Ding, Y. Wang, Q. Zhou, Z. Ding, Y. Wu, Y. Zhu, W. Shi and Q. He, The preparation and properties of multilayer Cu-MTa2O5 composite coatings on Ti6Al4V for biomedical applications, Nanomaterials, 2019, 9(10), 1498 CrossRef CAS PubMed.
- L. Xing, H. Zheng, Y. Cao and S. Che, Coordination polymer coated mesoporous silica nanoparticles for pH-responsive drug release, Adv. Mater., 2012, 24(48), 6433–6437 CrossRef CAS PubMed.
- L. Zhao, L. Liu, Z. Wu, Y. Zhang and P. K. Chu, Effects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiation, Biomaterials, 2012, 33(9), 2629–2641 CrossRef CAS PubMed.
- J. S. Khaw, C. R. Bowen and S. H. Cartmell, Effect of TiO2 nanotube pore diameter on human mesenchymal stem cells and human osteoblasts, Nanomaterials, 2020, 10(11), 2117 CrossRef CAS PubMed.
- Y. Li, S. Wang, Y. Dong, P. Mu, Y. Yang, X. Liu, C. Lin and Q. Huang, Effect of size and crystalline phase of TiO2 nanotubes on cell behaviors: a high throughput study using gradient TiO2 nanotubes, Bioact. Mater., 2020, 5(4), 1062–1070 Search PubMed.
- M. J. Gupte, W. B. Swanson, J. Hu, X. Jin, H. Ma, Z. Zhang, Z. Liu, K. Feng, G. Feng and G. Xiao, Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization, Acta Biomater., 2018, 82, 1–11 CrossRef CAS PubMed.
- Z. Cao, L. Li, L. Yang, L. Yao, H. Wang, X. Yu, X. Shen, L. Yao and G. Wu, Osteoinduction evaluation of fluorinated hydroxyapatite and tantalum composite coatings on magnesium alloys, Front. Chem., 2021, 9, 727356 CrossRef CAS PubMed.
- M. F. Kunrath, A. L. Vargas, P. Sesterheim, E. R. Teixeira and R. Hubler, Extension of hydrophilicity stability by reactive plasma treatment and wet storage on TiO2 nanotube surfaces for biomedical implant applications, J. R. Soc., Interface, 2020, 17(170), 20200650 CrossRef CAS PubMed.
- C. Pan, T. Liu, Y. Yang, T. Liu, Z. Gong, Y. Wei, L. Quan, Z. Yang and S. Liu, Incorporation of Sr2+ and Ag nanoparticles into TiO2 nanotubes to synergistically enhance osteogenic and antibacterial activities for bone repair, Mater. Des., 2020, 196, 109086 CrossRef CAS.
- X. Yang, Q. Wang, Y. Zhang, H. He, S. Xiong, P. Chen, C. Li, L. Wang, G. Lu and Y. Xu, A dual-functional PEEK implant coating for anti-bacterial and accelerated osseointegration, Colloids Surf., B, 2023, 224, 113196 CrossRef CAS PubMed.
- S. S. Nisar, S. Arun and H. C. Choe, Plasma electrolytic oxidation coatings on femtosecond laser-treated Ti-6Al-4V alloy for bio-implant use arrays, Surf. Coat. Technol., 2023, 464, 129553 CrossRef CAS.
- X. Dou, X. Wei, G. Liu, S. Wang, Y. Lv, J. Li, Z. Ma, G. Zheng, Y. Wang and M. Hu, Effect of porous tantalum on promoting the osteogenic differentiation of bone marrow mesenchymal stem cells in vitro through the MAPK/ERK signal pathway, J. Orthop. Translat., 2019, 19, 81–93 CrossRef PubMed.
- L. Li, L. Yao, H. Wang, X. Shen, W. Lou, C. Huang and G. Wu, Magnetron sputtering of strontium nanolayer on zirconia implant to enhance osteogenesis, Mater. Sci. Eng. C, 2021, 127, 112191 CrossRef CAS PubMed.
- S. Wu, X. Shen, M. Chen, K. H. R. Yie, Z. Zhou, M. A. Al-Baadani, K. Fang, A. M. Al-Bishari, Z. Deng, J. Liu and L. Yao, Multifunctional TaCu-nanotubes coated titanium for enhanced bacteriostatic, angiogenic and osteogenic properties, Mater. Sci. Eng. C, 2021, 120, 111777 CrossRef CAS PubMed.
- G. Jin, H. Qin, H. Cao, S. Qian, Y. Zhao, X. Peng, X. Zhang, X. Liu and P. K. Chu, Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium, Biomaterials, 2014, 35(27), 7699–7713 CrossRef CAS PubMed.
- T. Lu, J. Wen, S. Qian, H. Cao, C. Ning, X. Pan, X. Jiang, X. Liu and P. K. Chu, Enhanced osteointegration on tantalum-implanted polyetheretherketone surface with bone-like elastic modulus, Biomaterials, 2015, 51, 173–183 CrossRef CAS PubMed.
- W.-T. Hsieh, Y.-S. Liu, Y.-h. Lee, M. G. Rimando, K.-h. Lin and O. K. Lee, Matrix dimensionality and stiffness cooperatively regulate osteogenesis of mesenchymal stromal cells, Acta Biomater., 2016, 32, 210–222 CrossRef CAS PubMed.
- X. Shen, K. Fang, K. H. R. Yie, Z. Zhou, Y. Shen, S. Wu, Y. Zhu, Z. Deng, P. Ma and J. Ma, High proportion strontium-doped micro-arc oxidation coatings enhance early osseointegration of titanium in osteoporosis by anti-oxidative stress pathway, Bioact. Mater., 2022, 10, 405–419 CAS.
- S. Bencharit, T. Morelli, S. Barros, J. T. Seagroves, S. Kim, N. Yu, K. Byrd, C. Brenes and S. Offenbacher, Comparing initial wound healing and osteogenesis of porous tantalum trabecular metal and titanium alloy materials, J. Oral. Implantol., 2019, 45(3), 173–180 CrossRef PubMed.
- L. Wu, Y. Dong, L. Yao, C. Liu, A. M. Al-Bishari, K. H. R. Yie, H. Zhang, J. Liu and G. Wu, Nanoporous tantalum coated zirconia implant improves osseointegration, Ceram. Int., 2020, 46(11), 17437–17448 CrossRef CAS.
- T. Wang, Z. Weng, X. Liu, K. W. Yeung, H. Pan and S. Wu, Controlled release and biocompatibility of polymer/titania nanotube array system on titanium implants, Bioact. Mater., 2017, 2(1), 44–50 Search PubMed.
- M. Deng, S. G. Kumbar, L. S. Nair, A. L. Weikel, H. R. Allcock and C. T. Laurencin, Biomimetic structures: biological implications of dipeptide-substituted polyphosphazene–polyester blend nanofiber matrices for load-bearing bone regeneration, Adv. Funct. Mater., 2011, 21(14), 2641–2651 CrossRef CAS.
- Z. Wang, B. Li, Q. Cai, X. Li, Z. Yin, B. Li, Z. Li and W. Meng, Advances and Prospects in Antibacterial-Osteogenic Multifunctional Dental Implant Surface, Front. bioeng. biotechnol., 2022, 10, 921338 CrossRef PubMed.
- Y. Zhang, R. Luo, J. Tan, J. Wang, X. Lu, S. Qu, J. Weng and B. Feng, Osteoblast behaviors on titania nanotube and mesopore layers, Regener. Biomater., 2017, 4(2), 81–87 CAS.
- X. Wang, B. Ning and X. Pei, Tantalum and its derivatives in orthopedic and dental implants: Osteogenesis and antibacterial properties, Colloids Surf., B, 2021, 208, 112055 CrossRef CAS PubMed.
- H. Qian, T. Lei, Z. Ye, Y. Hu and P. Lei, From the performance to the essence: the biological mechanisms of how tantalum contributes to osteogenesis, Biomed Res. Int., 2020, 2020, 5162524 Search PubMed.
- A. Martín Vilardell, N. Cinca i Luis, N. Garcia Giralt, S. Dosta Parras, I. García Cano, X. Nogués Solán and J. M. Guilemany, Osteoblastic cell response on high-rough titanium coatings by cold spray, J Mater Sci Mater Med., 2018, 29, 2 CrossRef PubMed.
- C. C. Wang, Y. Hsu, F. Su, S. Lu and T.-M. Lee, Effects of passivation treatments on titanium alloy with nanometric scale roughness and induced changes in fibroblast initial adhesion evaluated by a cytodetacher, J Biomed Mater Res A, 2009, 88(2), 370–383 CrossRef PubMed.
- X. Zhang, M. Yang, L. Lin, P. Chen, K. Ma, C. Zhou and Y. Ao, Runx2 overexpression enhances osteoblastic differentiation and mineralization in adipose-derived stem cells in vitro and in vivo, Calcif. Tissue Int., 2006, 79, 169–178 CrossRef CAS PubMed.
- C. Luo, C. Wang, X. Wu, X. Xie, C. Wang, C. Zhao, C. Zou, F. Lv, W. Huang and J. Liao, Influence of porous tantalum scaffold pore size on osteogenesis and osteointegration: a comprehensive study based on 3D-printing technology, Mater. Sci. Eng. C, 2021, 129, 112382 CrossRef CAS PubMed.
- M. Zhang, Y. Xue, X. Huang, D. Ma, J. Gao, S. Yu, L. Zhu and Y. Wu, Cytocompatibility and osteogenic activity of Ta-Ti-O nanotubes anodically grown on Ti6Al4V alloy, Appl. Surf. Sci., 2023, 614, 156165 CrossRef CAS.
- E. D. Freitas, R. A. Bataglioli, J. Oshodi and M. M. Beppu, Antimicrobial peptides and their potential application in antiviral coating agents, Colloids Surf., B, 2022, 112693 CrossRef CAS PubMed.
- K. D. Jandt and D. C. Watts, Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials, Dent. Mater., 2020, 36(11), 1365–1378 CrossRef CAS PubMed.
- M. F. Kunrath and M. M. Campos, Metallic-nanoparticle release systems for biomedical implant surfaces: Effectiveness and safety, Nanotoxicology, 2021, 15(6), 721–739 CrossRef CAS PubMed.
- N. Poth, V. Seiffart, G. Gross, H. Menzel and W. Dempwolf, Biodegradable chitosan nanoparticle coatings on titanium for the delivery of BMP-2, Biomolecules, 2015, 5(1), 3–19 CrossRef CAS PubMed.
- D. Ionita, D. Bajenaru-Georgescu, G. Totea, A. Mazare, P. Schmuki and I. Demetrescu, Activity of vancomycin release from bioinspired coatings of hydroxyapatite or TiO2 nanotubes, Int. J. Pharm., 2017, 517(1–2), 296–302 CrossRef CAS PubMed.
- M. F. Kunrath, F. A. Shah and C. Dahlin, Bench-to-bedside: Feasibility of nano-engineered and drug-delivery biomaterials for bone-anchored implants and periodontal applications, Mater. Today Bio, 2023, 18, 100540 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |