Open Access Article
Open Access ArticleLuminescence turn-on sensor for the selective detection of trace water and methanol based on a Zn(II) coordination polymer with 2,5-dihydroxyterephthalate†
Jitti
Suebphanpho
and
Jaursup
Boonmak
 *
*
Materials Chemistry Research Center, Department of Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand. E-mail: jaursup@kku.ac.th
First published on 25th March 2024
Abstract
A highly selective detection of trace water in organic solvents is urgently required for the chemical industry. In this work, the simple sonochemical method was used for producing a luminescent sensor, [Zn(H2dhtp)(2,2′-bpy)(H2O)]n (Zn-CP) (H2dhtp2− = 2,5-dihydroxyterephthalate and 2,2′-bpy = 2,2′-bipyridine). Zn-CP exhibits reversible thermally-induced and methanol-mediated structural transformation. Importantly, Zn-CP has exceptional water sensing performance in both dry methanol and dry ethanol, with high selectivity, wide linear ranges, and a low limit of detection (LOD) of 0.08% (v/v). Upon the incremental addition of water, the luminescent intensities enhanced and shifted, along with the emission color changing from green to greenish yellow. In addition, Zn-CP can detect methanol selectively through turn-on luminescence intensity with LODs of 0.28, 0.52, and 0.35% (v/v) in dry ethanol, dry n-propanol, and dry n-butanol, respectively. The excited-state proton transfer of linker H2dhtp2− via enol–keto tautomerism and collaboration with structural transformation could be attributed to the sensing mechanism.
1 Introduction
Over the past few decades, research in the field of coordination polymers (CPs) has received a great deal of attention because of their broad potential applications, such as heterogeneous catalysis, gas storage and separation, sensing, and magnetism.1–3 The building blocks of CPs are constructed by metal ions and organic ligands, and their physical properties can be directly tuned by the selection of metal ions and organic ligands on a coordination assembly.4–6 The reversible structural transformation with chromotropism of CPs has been attracting great attention due to their vital importance for application in thermo-sensors, chemical sensors, optical sensors, color indicators, and molecular switches.7–10 There are three main categories of structural changes: solid-state, solution-based, and solvent-mediated.11–13 The solvent-mediated structural transformation in CPs is rarely reported, owing to the dissolve-recrystallize process of crystallization, which commonly induces significant changes in their structures and properties.14,15Nowadays, water is considered a contaminant and impurity in dry organic products, food inspection, environmental monitoring, and pharmaceutical manufacturing.16–20 Trace water influences not only the yield of chemicals and drugs but also their activity and application.21 Up until now, the traditional Karl Fischer titration method and gas chromatography have been used for monitoring water, but these methods have certain limitations, involving the requirements of specialized instruments, well-trained personnel, and time-consuming processes.22,23 Therefore, the development of sensors for detecting water in organic products with simple operation, fast response, and low detection limits is highly desired.23,24 On the other hand, another important analyte is methanol, which is easily mixed into other alcohol solvents, especially ethanol, due to its analogous physical and chemical properties.25,26 Ethanol is an essential ingredient that is widely used in a variety of industries, including the food and chemical industries. When methanol is mixed with ethanol, the cost of producing ethanol is reduced. Furthermore, methanol is an alcohol that is toxic to mammals; consuming the compound will result in headaches, vomiting, blindness, or worse, representing one of the world's major risks to food safety.27,28 Therefore, the detection of methanol in alcohol solvents is of great significance and required.
It is well known that the excited-state intramolecular proton transfer (ESIPT) process of the organic ligand can greatly improve the sensitivity and selectivity of CPs sensing probes. Upon being stimulated by light, the ESIPT process can generate rapid intramolecular proton transfer between the hydrogen bond donors (–OH and –NH2) and hydrogen bond acceptors (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and –C
N– and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the molecular structure via tautomerism from the excited enol form to keto form.29–31 From this viewpoint, 2,5-dihydroxyterephthalate (H2dhtp2−) was selected to construct CPs for the sensing probe. The H2dhtp2− ligand consists of two carboxylate groups for constructing stable CPs and realizing a high-efficiency ESIPT process that originates from weak intramolecular H-bonds between carboxylate and hydroxyl groups.32 When adding the target molecule, it might interact with the –OH groups of H2dhtp2− and affect the enol–keto tautomerism during the ESIPT process, which suggests that luminescence emission peak position and intensity change after detection.22,33 Out of the above-mentioned considerations, we successfully prepared [Zn(H2dhtp)(2,2′-bpy)(H2O)]n (Zn-CP) (H2dhtp2− = 2,5-dihydroxyterephthalate and 2,2′-bpy = 2,2′-bipyridine) by the simple sonochemical method. Zn-CP presents a zigzag chain coordination polymer with an abundance of free hydroxy groups. The 1D zigzag chain structure of Zn-CP changes to 2D-layer Zn-CP-II by heating or soaking in methanol with chromotropism through the dehydration process. The luminescence properties of Zn-CP and Zn-CP-II in solid-state and suspension were studied. Furthermore, Zn-CP and Zn-CP-II can be applied for low water detection in dry methanol and dry ethanol with wide water detection ranges through the ESIPT mechanism, which produces enhanced and shifted emission spectra. In addition, by turning on luminescence intensity with low detection limits, Zn-CP is able to detect and discriminate methanol from a range of aliphatic alcohols.
O) of the molecular structure via tautomerism from the excited enol form to keto form.29–31 From this viewpoint, 2,5-dihydroxyterephthalate (H2dhtp2−) was selected to construct CPs for the sensing probe. The H2dhtp2− ligand consists of two carboxylate groups for constructing stable CPs and realizing a high-efficiency ESIPT process that originates from weak intramolecular H-bonds between carboxylate and hydroxyl groups.32 When adding the target molecule, it might interact with the –OH groups of H2dhtp2− and affect the enol–keto tautomerism during the ESIPT process, which suggests that luminescence emission peak position and intensity change after detection.22,33 Out of the above-mentioned considerations, we successfully prepared [Zn(H2dhtp)(2,2′-bpy)(H2O)]n (Zn-CP) (H2dhtp2− = 2,5-dihydroxyterephthalate and 2,2′-bpy = 2,2′-bipyridine) by the simple sonochemical method. Zn-CP presents a zigzag chain coordination polymer with an abundance of free hydroxy groups. The 1D zigzag chain structure of Zn-CP changes to 2D-layer Zn-CP-II by heating or soaking in methanol with chromotropism through the dehydration process. The luminescence properties of Zn-CP and Zn-CP-II in solid-state and suspension were studied. Furthermore, Zn-CP and Zn-CP-II can be applied for low water detection in dry methanol and dry ethanol with wide water detection ranges through the ESIPT mechanism, which produces enhanced and shifted emission spectra. In addition, by turning on luminescence intensity with low detection limits, Zn-CP is able to detect and discriminate methanol from a range of aliphatic alcohols.
2 Experimental section
2.1 Materials and methods
All chemicals and reagents were commercially available and used without further purification. FT-IR spectra were recorded over the range 4000–600 cm−1, using a Bruker Tensor 27 Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) spectrophotometer. Powder X-ray diffraction (PXRD) patterns were obtained from 5° to 50° at a speed of 0.5 s per step on EMPYREAN PANALYTICAL using monochromatic CuKα radiation at room temperature. Thermogravimetric analyses (TGA) were measured using a TG-DTA 2010S MAC thermal analyzer under an N2 atmosphere at a heating rate of 10 °C min−1 and a temperature range of 35–700 °C. The sonicator used in this study was an Elmasonic S30H (maximum 280 W at 50 Hz). The luminescence spectra were obtained by using a Shimadzu RF-6000 spectrofluorometer with a continuous Xe lamp at room temperature.2.2 Preparation of [Zn(H2dhtp)(2,2′-bpy)(H2O)]n (Zn-CP)
The synthesis of Zn-CP in this work was developed using the simple sonochemical method rather than the previously reported solvothermal reaction at 60 °C for 4 days.34 Sodium acetate was used as a modulator in order to control the particle size of the product. Zn(NO3)2·6H2O 0.2 mmol (56 mg), H4dhtp 0.2 mmol (40 mg), 2,2′-bpy 0.2 mmol (31 mg), sodium acetate 0.2 mmol (54 mg), and NaOH 0.4 mmol (16 mg) were dissolved in 10 mL of a mixed solvent of water/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The mixture was sonicated for 30 min at room temperature. After the sonication process, the yellow powder of Zn-CP was obtained. Then, the product was centrifuged, and washed several times with water, and dried overnight at 70 °C. Yield: (78% based on Zn(II) salt). FT-IR (ATR, cm−1) 3194(m), 1597(s), 1490(m), 1475(m), 1428(s), 1321(s), 1238(s), 1062(w), 1021(w), 804(m), 754(s).
1 v/v). The mixture was sonicated for 30 min at room temperature. After the sonication process, the yellow powder of Zn-CP was obtained. Then, the product was centrifuged, and washed several times with water, and dried overnight at 70 °C. Yield: (78% based on Zn(II) salt). FT-IR (ATR, cm−1) 3194(m), 1597(s), 1490(m), 1475(m), 1428(s), 1321(s), 1238(s), 1062(w), 1021(w), 804(m), 754(s).
2.3 Preparation of [Zn(H2dhtp)(2,2′-bpy)]n (Zn-CP-II)
The yellow powder of Zn-CP was heated at 140 °C for 3 hours, and then the white powder of Zn-CP-II was obtained. FT-IR (ATR, cm−1) 3184(m), 1598(s), 1472(m), 1440(s), 1380(m), 1361(m), 1109(m), 1024(m), 866(m), 789(s), 760(s).2.4 Water sensing experiment
To carry out the luminescent detection for water, 10 mg of the ground Zn-CP or Zn-CP-II was added to 100 mL of dry methanol or dry ethanol. After that, the mixture solution was sonicated at room temperature for 30 min, and a suspension of Zn-CP or Zn-CP-II in a methanolic or ethanolic solution was obtained. Then, 4 mL of the Zn-CP or Zn-CP-II suspension was pipetted into a 5 mL volumetric flask. After that, a different water level from 0.5 to 20% (v/v) was added. The volume of the suspension containing water was adjusted to 5 mL with dry methanol or dry ethanol, giving the different concentrations of water in the Zn-CP or Zn-CP-II luminescent probe. After that, this suspension was transferred to a quartz cuvette, followed by monitoring emission intensity upon excitation at 360 nm.2.5 Methanol sensing experiment
10 mg of Zn-CP was added to 100 mL of dry ethanol, then it was sonicated for 30 min. After that, the suspension of Zn-CP in an ethanolic solution was obtained. The experiment was performed by the incremental addition of methanol to 5 mL of ethanolic suspension of Zn-CP in 10 mL of a volumetric flask to make the final content of methanol in the range of 5–50% (v/v). After that, the luminescence intensity was measured by exciting at 360 nm. Furthermore, the methanol sensing of Zn-CP in dry n-propanol or dry n-butanol can be carried out in the same way, but instead of dry ethanol to dry n-propanol or dry n-butanol.3 Results and discussion
3.1 Structural characterization of Zn-CP and Zn-CP-II
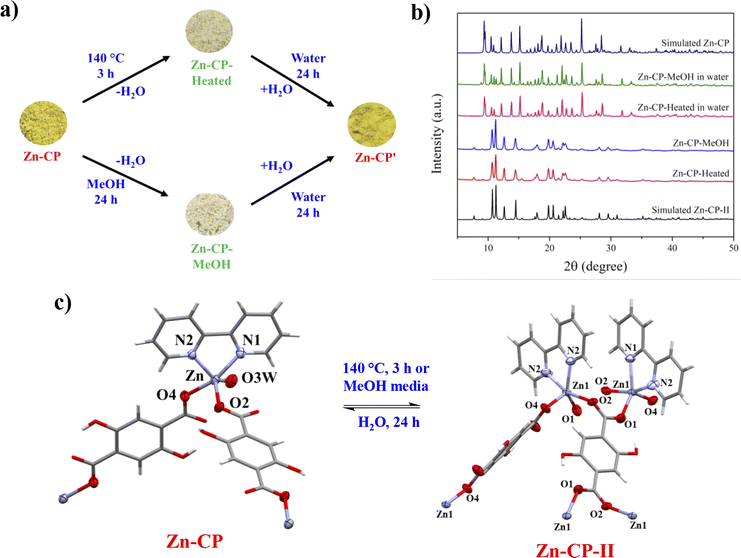
A Zn-CP was prepared by the simple sonochemical method instead of the solvothermal method previously reported.34 The sonochemical reaction serves to facilitate fast preparation and reduce energy consumption such as pressure or high-temperature heating. The powder X-ray diffraction (PXRD) pattern of Zn-CP matches well with the reported structural phase (Fig. S1†), suggesting that they are isostructural structures.34 The structure of Zn-CP presents a 3D supramolecular framework. Each Zn(II) center is five-coordinated and consists of two oxygen atoms from different H2dhtp2− ligands, two nitrogen atoms from a chelating 2,2′-bpy ligand, and one oxygen atom from a water molecule (Fig. S2†). Monodentate H2dhtp2− ligands bridge two Zn(II) to form a 1D zigzag chain. For the packing structure of Zn-CP, each 1D zigzag chain is linked together through π–π interaction between the aromatic ring of H2dhtp2− and the pyridine ring of 2,2′-bpy along the c-axis, generating a 2D-layer structure with free hydroxy group of H2dhtp2−, which is the active site for the ESIPT process (Fig. S3†). The adjacent 2D layers of Zn-CP are assembled to the 3D supramolecular framework by hydrogen bonding along the b-axis between the hydrogen atom of the coordinated water molecule and the uncoordinated oxygen atom of the carboxyl group in different units (Fig. S4†). The TGA analysis of Zn-CP displays a weight loss of 4.01% at temperatures around 100 °C that indicates the release of a coordinated water molecule (anal. Calc., 4.13%). In addition, the Zn-CP shows high thermal stability up to 300 °C, and then the organic ligands were decomposed (Fig. S8†).Zn-CP-II was prepared by heating Zn-CP at 140 °C for 3 h. The PXRD pattern of as-synthesized Zn-CP-II is well matched with that of the simulated pattern of reported single crystal data,34 confirming the identical crystalline phase purity (Fig. S1†).The crystal structure of Zn-CP-II displays a 3D supramolecular framework. The Zn(II) center is five-coordinated and surrounded by three oxygen atoms of carboxylate groups from different H2dhtp2− ligands and two nitrogen atoms from a chelating 2,2′-bpy ligand (Fig. S5†). The carboxylate groups of H2dhtp2− ligands show the bidentate and monodentate bridging modes for connecting with four and two Zn(II) centers, respectively, which generate the 2D layer of Zn-CP-II along the bc plane (Fig. S6†). For the packing motif of Zn-CP-II, each 2D layer is connected through intramolecular hydrogen bonds between different hydroxyl groups of different H2dhtp2− ligands and hydrogen bonds between hydroxyl groups and carboxylate groups of H2dhtp2− ligands. Moreover, each 2D-layer is packed by intramolecular π–π interactions between pyridyl rings of different 2,2′-bpy, as shown in Fig. S6.† Furthermore, the adjacent 2D layers of Zn-CP-II are assembled to the 3D supramolecular framework through interlayer C–H⋯π interactions among the hydrogen atoms from the adjacent 2,2′-bpy units and benzene rings of H2dhtp2− ligands (Fig. S7†).
3.2 Reversible thermal- and methanol-induced structural transformation in Zn-CP
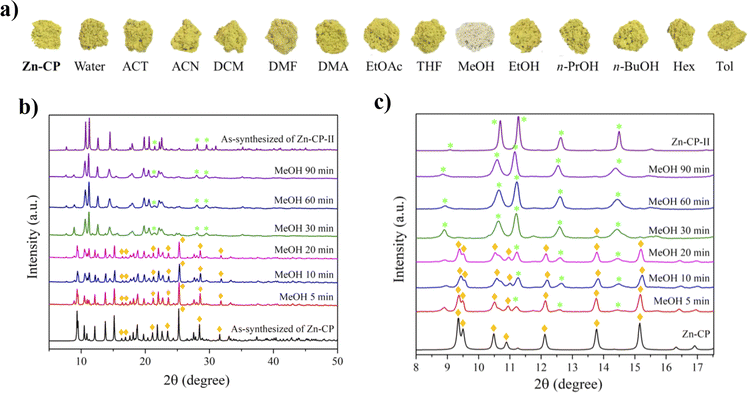
According to the TGA results, upon heating Zn-CP at 140 °C for 3 h, the coordinated water molecule in Zn-CP was removed to be an activated form (denoted as Zn-CP-Heated), and the color was also changed from yellow to white (Fig. 1a). The reversible structural transformation of Zn-CP relating to its dehydration and rehydration processes was monitored by FTIR and PXRD techniques. The FTIR spectrum of Zn-CP-Heated shows that the ν(OH)water at 3031 cm−1 clearly disappears compared with the original Zn-CP (Fig. S9†). In addition, the PXRD patterns of Zn-CP-Heated are different from Zn-CP, indicating the structural transformation after the dehydration process. Also, the PXRD pattern of Zn-CP-Heated matched the reported structure of [Zn(H2dhtp)(2,2′-bpy)] (Zn-CP-II).34To examine the solvent-mediated structural transformation, 50 mg of the ground Zn-CP was soaked in 14 different solvents at room temperature. Interestingly, the color of Zn-CP was changed from yellow to white only in the methanol media, as shown in Fig. 1a (denoted as Zn-CP-MeOH). In contrast, Zn-CP shows no significant color change in other solvents after soaking for 24 h (Fig. 2a). The PXRD pattern of Zn-CP-MeOH shows the same diffraction pattern as the simulated Zn-CP-II, confirming that the structural changed from the 1D chain of Zn-CP to the 2D layer of Zn-CP-II. Time-dependent PXRD patterns of Zn-CP in methanol were also investigated. When Zn-CP is soaked in methanol for 5–20 min, the majority of the products are the same as Zn-CP, which is greater than Zn-CP-II. Nevertheless, some diffraction peaks of Zn-CP-II at 2θ = 11.2°, 12.6°, and 14.5° were obtained, which indicate the mixed phases between Zn-CP and Zn-CP-II. After soaking in methanol for more than 30 min, some characteristic diffraction peaks of Zn-CP at 2θ = 9.3°, 9.5°, 10.9°, 12.1°, 13.8°, 15.1°, 25.2°, 28.5°, and 31.6° disappeared. Also, only the appearance of diffraction peaks of Zn-CP-II is obtained after soaking in methanol for 60 min (Fig. 2b and c), indicating that the structure completely changed from Zn-CP to Zn-CP-II. It is worth mentioning that Zn-CP does not exhibit a change in color or structural transformation upon contact with other common organic solvents (Fig. 2a), which corresponds to the PXRD patterns (Fig. S10†). Therefore, the structural transformation with chromotropism is selective toward methanol over other solvents. The structural transformation mechanism could be determined by the size35 and hydrogen bonding ability of methanol, which are confirmed by Kamlet–Taft parameters36 (Table S1†). Methanol has a tiny size and high hydrogen bond donor (α) and acceptor (β) parameters; thus, it is easily accessible to the lattice structure of Zn-CP. Also, the coordinated water molecule of Zn-CP has the highest hydrogen bond donor parameter, which generates strong hydrogen bonds between them.37 Due to the strong hydrogen bonding, the coordinated water molecule can be removed from the coordination sphere, which indicates the methanol-mediated structural transformation. Furthermore, Zn-CP-Heated and Zn-CP-MeOH can be restored to the original crystalline phase of Zn-CP after being soaked in water at room temperature for 24 h. The rehydrated crystalline solid after soaking in water for 24 h shows the same PXRD patterns as the original phase of Zn-CP (Fig. 1b). Also, the color was returned to that of the original one, as shown in Fig. 1a. These results confirm the reversible thermal-induced and MeOH-mediated structural phase transformation with chromotropism in Zn-CP. It is worth mentioning that Zn-CP-II (both Zn-CP-Heated and Zn-CP-MeOH) cannot return to Zn-CP after exposure to air for a month, implying the high stability of Zn-CP-II in air.
3.3 Luminescence properties of Zn-CP and Zn-CP-II
The solid-state luminescence properties of H4dhtp, 2,2′-bpy, Zn-CP, and Zn-CP-II were investigated (Fig. 3). Upon excitation at 360 nm, the luminescence intensity of free H4dhtp and 2,2′-bpy ligands is observed at 477 and 526 nm, respectively, showing the blue and white colors under UV light that originate from the π* to n or π* to π electronic transition.35,37 In contrast, Zn-CP and Zn-CP-II show the quenching luminescent emission in solid-state when compared with the free ligands, and other CPs containing H4dhtp ligand normally show strong emission in solid-state, as shown in Table S2.† The quenching phenomenon in Zn-CP and Zn-CP-II is unique because the H4dhtp ligand is a strong luminescent organic chromophore that can be changed from enol to keto via excited-state intramolecular proton transfer (ESIPT) between hydrogen atoms from the hydroxy group to the carboxylate group after being excited by UV-light. The weak luminescent intensity of Zn-CP could be attributed to (i) the loss of energy from the vibration of the phenolic ligand in the excited state,38,39 (ii) the molecular aggregation and characteristics of molecular stacking. Generally, J-aggregation (slip-stacking/head-to-tail interaction) enhances the fluorescence emission over H-aggregation (face-to-face interaction) for ESIPT.40–42 The packing structure of Zn-CP and Zn-CP-II displayed intermolecular face-to-face interaction between rings of ligands in 1D chains and 2D layers (Fig. S3 and S6†), which originated the aggregation-caused quenching (ACQ) effect. (iii) The ligand-to-ligand charge transfer (LLCT) in Zn-CP. The previous study used DFT to calculate the molecular orbital simulation of [Zn(bpy)(dhtp)0.5]n.43 The composition of this compound is the same as that of Zn-CP and Zn-CP-II, but with different packing structures and coordination modes of dhtp4−. The HOMO is provided by the C and O atoms of the dhtp4− when emitting light energy, it transfers to the 2,2′-bpy on the LUMO, which is the LLCT process causing the quenching luminescence intensity.43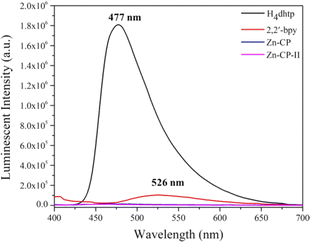 | ||
| Fig. 3 Solid-state PL spectra of free H4dhtp, 2,2′-bpy, Zn-CP, and Zn-CP-II upon excitation at 360 nm. | ||
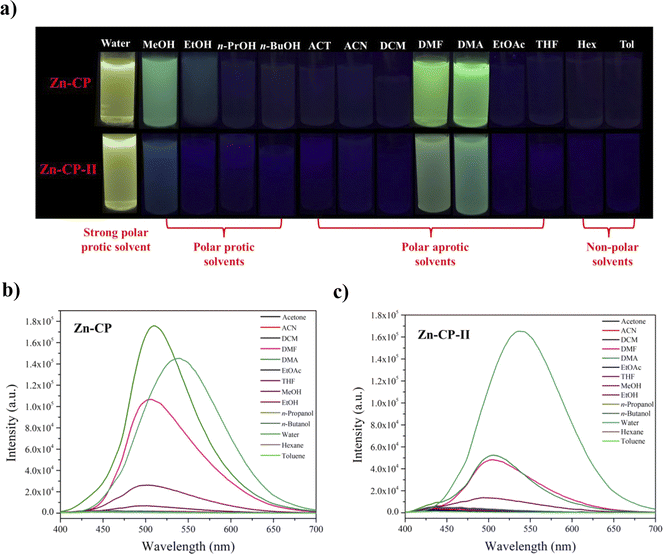
The suspension of Zn-CP and Zn-CP-II showed strong emission in water, methanol, DMF, and DMA, as shown in Fig. 4. In strong polar protic solvents like water, Zn-CP and Zn-CP-II showed strong luminescence intensity at 537 nm with yellow emission. This phenomenon could be attributed to the intermolecular proton transfer process between water and proton in the hydroxyl group of H2dhtp2−. Other polar protic solvents such as MeOH, EtOH, n-propanol (n-PrOH), and n-butanol (n-BuOH), polar aprotic solvents such as acetone (ACT), acetonitrile (ACN), dichloromethane (DCM), dimethyl formamide (DMF), dimethylacetamide (DMA), ethyl acetate (EtOAc), tetrahydrofuran (THF), and non-polar solvents such as hexane (Hex) and toluene (Tol) show the green luminescence color at 503, 502, 507, and 510 nm in MeOH, EtOH, DMF, and DMA, respectively, depending on solvent polarity.44,45 Zn-CP and Zn-CP-II contain hydroxyl groups in H2dhtp2− thus methanol or ethanol can easily interact with hydroxyl groups due to their small size with high hydrogen bond donor and acceptor parameters. DMF and DMA suspensions display green emission due to their higher polarizability (Table S1†). Moreover, the high polarity of water can be more stabilized in the excited state than the ground state due to solvent relaxation, resulting in a decreased energy band gap and a red-shitted emission when compared to other solvents (Fig. 4b and c).
3.4 Water sensing of Zn-CP and Zn-CP-II
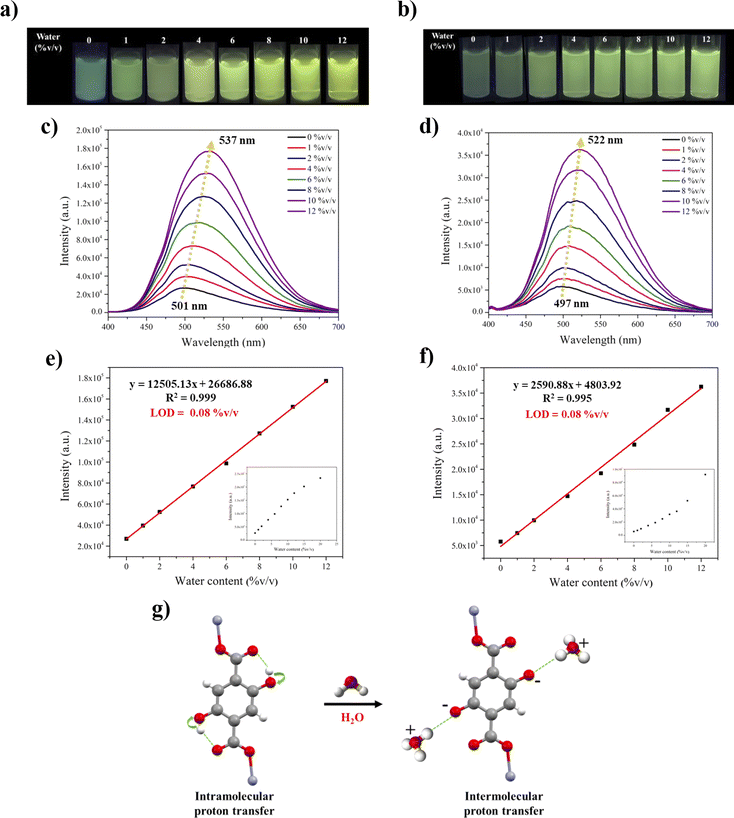
Based on the above results, the emission spectra of Zn-CP and Zn-CP-II in water suspensions show significantly red-shifted emission when compared to other solvents (Fig. 4b), so the utilization of Zn-CP and Zn-CP-II for trace amounts of water in dry methanol or dry ethanol was investigated. As shown in Fig. 5, the luminescence intensity upon adding water to the Zn-CP suspension was dramatically increased and red-shifted from 501 to 537 nm (≈35 nm) and 497 to 522 nm (≈25 nm) in dry methanol and dry ethanol, respectively (Fig. 5c and d). The luminescence color under the irradiation of a 365 nm UV lamp is switched from green to green-yellow (Fig. 5a and b), corresponding with the CIE diagram (Fig. S11†). The correlations between luminescence intensity and water contents are fitted, exhibiting good linear ranges at low water content 0–12% v/v with R2 values of 0.999 and 0.995 in dry methanol and dry ethanol, respectively. The limit of detection (LOD) for a trace of water is determined by LOD = 3σ/S (σ is the standard deviation of the luminescence test for 10 blank solutions, and S is the slope of the linear equation curve); the LODs of water in both dry methanol and dry ethanol were calculated to be as low as 0.08% v/v (Fig. 5e and f). Moreover, the luminescence response of Zn-CP-II toward water in dry methanol and dry ethanol was examined under the same conditions as in Zn-CP (Fig. S14†). It was revealed that the luminescence intensity of Zn-CP-II was gradually raised and red-shifted from 502 to 536 nm (≈35 nm) and 497 to 511 nm (≈14 nm) with increasing water contents in dry methanol and dry ethanol, respectively. The luminescence color has a striking transition from blue to green-yellow and blue to green in dry methanol and dry ethanol, respectively, which is beneficial for visual detection by the naked eye under a 365 nm UV lamp and confirmed by CIE diagrams (Fig. S15†). A good linear relationship between luminescence intensity and water contents was obtained in 0–20 and 1–10% v/v with R2 = 0.999 in both dry methanol and dry ethanol. The LODs of water in dry methanol and dry ethanol in Zn-CP-II suspension were calculated to be 0.05 and 0.04% v/v, respectively. The LOD values for water in dry methanol and dry ethanol of Zn-CP and Zn-CP-II are comparable to other CPs, as shown in Table S3,† and lower than those found in AEHC (hydrated ethyl alcohol fuel: 0.8 in China, 1.0% in the US, and 4.9% in Brazil).46 The water sensing mechanism for Zn-CP could be explained as follows: (i) when water was added to a dry methanol or dry ethanol suspension of Zn-CP, the suspension became more polar, increasing the luminescence intensity; (ii) water molecules may acquire a proton from the hydroxy groups of the H2dhtp2− ligand, generating H3O+ along with the intermolecular proton transfer process (Fig. 5g), resulting in the red-shifted emission.21,22,47 Additionally, the stability of Zn-CP after water sensing can be confirmed by FTIR and PXRD techniques. The FTIR spectra and PXRD patterns of Zn-CP display identical patterns to those of the pristine Zn-CP (Fig. S12 and S13†), implying that the structure did not change from Zn-CP to Zn-CP-II during the water sensing process. In the case of Zn-CP-II, the FTIR spectra show some characteristic peaks of Zn-CP after water sensing (Fig. S16†) and the PXRD pattern of Zn-CP-II after water sensing in dry methanol or dry ethanol shows mixed crystalline phases between Zn-CP and Zn-CP-II (Fig. S17†), implying the structural transformation process from Zn-CP-II to Zn-CP. The water molecules can be coordinated with Zn(II) centers, which corresponds to a change from a 2D-layer of Zn-CP-II to a 1D-zigzag chain of Zn-CP. The water sensing mechanism of Zn-CP-II can be explained based on the following considerations: (i) at low water content, the majority phase is Zn-CP-II, which is confirmed by its characteristic emission color (blue-green emission color, Fig. S14a†); (ii) at high water content, the bright-green emission color was obtained, confirming the majority phase of Zn-CP. Moreover, the luminescence intensity increased, and red-shifted emission could be caused by an intermolecular proton transfer between the hydroxy and water molecules. From these results, Zn-CP and Zn-CP-II show not only facile water detection without a complicated method but also are very cost-effective and environmentally friendly.3.5 MeOH sensing of Zn-CP
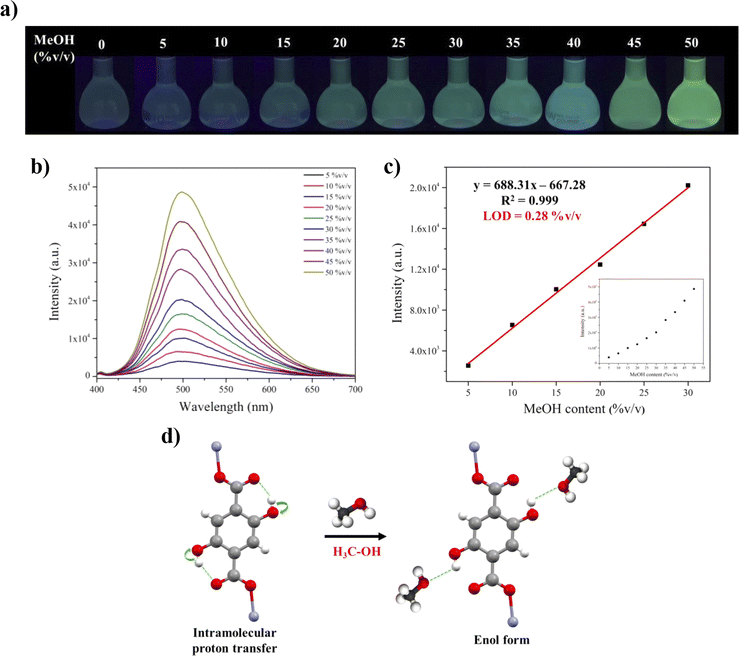
When comparing the luminescence intensity at the maximum peak of the Zn-CP probe in four different alcohols (Fig. 4b), methanol has the highest luminescence intensity (Table S4†). Thus, we further explored the performance of the Zn-CP probe in detecting methanol in various dry alcohol suspensions. Upon adding different methanol levels (0–50% v/v) to dry ethanol, the luminescence intensity gradually increased (Fig. 6b). The luminescence color under the irradiation of a 365 nm UV lamp switches from blue to green, which can be easily observed. The correlation between the luminescence intensity and methanol contents (5–30% v/v) is fitted. The linear relationship is Y = 688.31X − 667.28, with the calibration curve R2 = 0.999, implying a good linear relationship between the luminescence intensity and methanol content. The result shows that methanol in dry ethanol can be detected using the Zn-CP probe with a LOD value of 0.28% v/v (Fig. 6c). According to the excellent luminescence sensing performance of Zn-CP for detecting methanol in dry ethanol, its luminescence sensing behavior in other alcohols, such as dry n-propanol and dry n-butanol, was further investigated. The results of luminescence sensing are shown in Fig. S18 and 19.† The luminescence intensity increased with the increase in methanol content, showing a turn-on luminescence response (Fig. S18b and S19b†). The luminescence intensity and methanol content showed a good linear relationship with R2 values of 0.996 and 0.998 and low LOD values of 0.52 and 0.35% v/v in dry n-propanol and dry n-butanol, respectively. The linear ranges and LODs are summarized in Table S5,† which are comparable with the reported luminescence methanol sensors. These results prove that the Zn-CP probe can detect methanol in three common dry alcohol solvents with good linear ranges and low LODs. The sensing mechanism can be explained by the ESIPT process. Methanol is a small molecule with high polarizability when compared with ethanol, n-propanol, and n-butanol. Upon adding the methanol to the dry alcohol solvents, the methanol can easily interact with the hydroxyl group of H2dhtp2− in Zn-CP, implying that it reduces the O–H vibration energy and increases luminescence intensity with green emission.35 This phenomenon effectively interrupts excited-state intramolecular proton transfer from the hydroxyl group and carboxylate group, showing the enol form in H2dhtp2− ligand,22 as shown in Fig. 6d. Moreover, the PXRD pattern of Zn-CP after methanol sensing in dry ethanol shows mixed-phases of diffraction peaks between Zn-CP and Zn-CP-II, implying that the structural transformation occurred after adding methanol to dry ethanol. In contrast, the PXRD patterns of Zn-CP after methanol sensing in dry n-propanol and dry n-butanol were well fitted with the PXRD pattern of as-synthesized Zn-CP, indicating that the framework of Zn-CP was still maintained after methanol sensing in dry n-propanol and dry n-butanol (Fig. S20†).4 Conclusions
The simple synthetic method for Zn-CP can be developed using the sonochemical method. Zn-CP exhibits a reversible structural transformation to Zn-CP-II, with color change induced by thermal and methanol-mediated dehydration and rehydration processes. The luminescence properties of Zn-CP and Zn-CP-II show weak emission intensities in solid-state but strong emission intensities in suspension with high polar solvents. Zn-CP and Zn-CP-II contain an ESIPT-sensitive H4dhtp linker, which not only enhances the interaction between solvent molecules and the frameworks but also facilitates electron transfer via uncoordinated hydroxyl groups in the frameworks. These probes can be used to detect trace amounts of water in dry methanol and dry ethanol. The results show high selectivity, wide linear ranges with good linear relationships, and low LODs. In addition, they can be used for methanol detection in various dry alcohol suspensions, which turn-on luminescence intensity. This work has established a promising model for detecting trace water and methanol in dry organic solvents.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Funding for this work was provided by National Research Council of Thailand (NRCT) with Grant No. N41A640144, Material Chemistry Research Center, Research and Graduate Studies, Khon Kaen University. J. S. was financially supported by the Development and Promotion of Science and Technology Talents Project (DPST).References
- W. L. Leong and J. J. Vittal, Chem. Rev., 2011, 111, 688–764 CrossRef CAS PubMed.
- X. Zhang, W. Wang, Z. Hu, G. Wang and K. Uvdal, Coord. Chem. Rev., 2015, 284, 206–235 CrossRef CAS.
- W. Xu, K. B. Thapa, Q. Ju, Z. Fang and W. Huang, Coord. Chem. Rev., 2018, 373, 199–232 CrossRef CAS.
- N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- A. V. Desai, S. Sharma, S. Let and S. K. Ghosh, Coord. Chem. Rev., 2019, 395, 146–192 CrossRef CAS.
- A. Halder, B. Bhattacharya, F. Haque and D. Ghoshal, Cryst. Growth Des., 2017, 17, 6613–6624 CrossRef CAS.
- M. Arıcı, O. Z. Yeşilel and M. Taş, Dalton Trans., 2015, 44, 1627–1635 RSC.
- J. Suebphanpho, S. Wannapaiboon, S. Youngme and J. Boonmak, Cryst. Growth Des., 2020, 20, 7439–7449 CrossRef CAS.
- F. Klongdee, S. Leelasubcharoen, S. Youngme and J. Boonmak, RSC Adv., 2021, 11, 12218–12226 RSC.
- S. Tunsrichon, S. Youngme and J. Boonmak, Inorg. Chem., 2021, 60, 18242–18250 CrossRef CAS PubMed.
- S. M. Mobin, A. K. Srivastava, P. Mathur and G. K. Lahiri, Dalton Trans., 2010, 39, 1447–1449 RSC.
- W.-W. Dong, J. Zhao and L. Xu, Cryst. Growth Des., 2008, 8, 2882–2886 CrossRef CAS.
- X. Cui, A. N. Khlobystov, X. Chen, D. H. Marsh, A. J. Blake, W. Lewis, N. R. Champness, C. J. Roberts and M. Schröder, Chem.–Eur. J., 2009, 15, 8861–8873 CrossRef CAS PubMed.
- V. A. Friese and D. G. Kurth, Coord. Chem. Rev., 2008, 252, 199–211 CrossRef CAS.
- J. Liu, Y.-X. Tan and J. Zhang, Cryst. Growth Des., 2012, 12, 5164–5168 CrossRef CAS.
- J. Li, P. Du, J. Chen, S. Huo, Z. Han, Y. Deng, Y. Chen and X. Lu, Anal. Chem., 2020, 92, 8974–8982 CrossRef CAS PubMed.
- S.-Y. Zhu and B. Yan, Ind. Eng. Chem. Res., 2018, 57, 16564–16571 CrossRef CAS.
- S. K. Sachan and G. Anantharaman, Inorg. Chem., 2022, 61, 18340–18345 CrossRef CAS PubMed.
- Y. Zhou, D. Zhang, W. Xing, J. Cuan, Y. Hu, Y. Cao and N. Gan, Anal. Chem., 2019, 91, 4845–4851 CrossRef CAS PubMed.
- H.-Q. Yin, J.-C. Yang and X.-B. Yin, Anal. Chem., 2017, 89, 13434–13440 CrossRef CAS PubMed.
- S.-Y. Li, X. Yan, J. Lei, W.-J. Ji, S.-C. Fan, P. Zhang and Q.-G. Zhai, ACS Appl. Mater. Interfaces, 2022, 14, 55997–56006 CrossRef CAS PubMed.
- J. Othong, J. Boonmak, F. Kielar and S. Youngme, ACS Appl. Mater. Interfaces, 2020, 12, 41776–41784 CrossRef CAS PubMed.
- T. Wiwasuku, J. Suebphanpho, S. Ittisanronnachai, V. Promarak, J. Boonmak and S. Youngme, RSC Adv., 2023, 13, 18138–18144 RSC.
- B. Bhattacharya, A. Halder, L. Paul, S. Chakrabarti and D. Ghoshal, Chem.–Eur. J., 2016, 22, 14998–15005 CrossRef CAS PubMed.
- R. R. Fonseca, R. D. Gaspar, I. M. Raimundo Jr and P. P. Luz, J. Rare Earths, 2019, 37, 225–231 CrossRef CAS.
- M. Latha, R. Aruna-Devi, N. Bogireddy, S. E. Rios, W. Mochan, J. Castrellon-Uribe and V. Agarwal, RSC Adv., 2020, 10, 22522–22532 RSC.
- Z. Jin, H. He, H. Zhao, T. Borjigin, F. Sun, D. Zhang and G. Zhu, Dalton trans., 2013, 42, 13335–13338 RSC.
- T. Wiwasuku, J. Othong, J. Boonmak, V. Ervithayasuporn and S. Youngme, Dalton trans., 2020, 49, 10240–10249 RSC.
- A. C. Sedgwick, L. Wu, H.-H. Han, S. D. Bull, X.-P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Chem. Soc. Rev., 2018, 47, 8842–8880 RSC.
- A. D. Pournara, A. Douvali, S. Diamantis, G. S. Papaefstathiou, A. G. Hatzidimitriou, S. Kaziannis, C. Kosmidis, T. Lazarides and M. J. Manos, Polyhedron, 2018, 151, 401–406 CrossRef CAS.
- V. Trannoy, N. Guillou, C. Livage, C. Roch-Marchal, M. Haouas, A. Léaustic, C. Allain, G. Clavier, P. Yu and T. Devic, Inorg. Chem., 2019, 58, 6918–6926 CrossRef CAS PubMed.
- K. Jayaramulu, P. Kanoo, S. J. George and T. K. Maji, Chem. Commun., 2010, 46, 7906–7908 RSC.
- A. Halder, A. Maiti, S. Dinda, B. Bhattacharya and D. Ghoshal, Cryst. Growth Des., 2021, 21, 6110–6118 CrossRef CAS.
- L. Zhang, J. Chen, Y. Ling and Z. Chen, Chem. Lett., 2014, 43, 997–998 CrossRef CAS.
- R. Díaz-Torres and S. Alvarez, Dalton trans., 2011, 40, 10742–10750 RSC.
- M. J. Kamlet, J. L. M. Abboud, M. H. Abraham and R. Taft, J. Org. Chem., 1983, 48, 2877–2887 CrossRef CAS.
- G. Mehlana, G. Ramon and S. A. Bourne, CrystEngComm, 2013, 15, 9521–9529 RSC.
- J. Heine and K. Müller-Buschbaum, Chem. Soc. Rev., 2013, 42, 9232–9242 RSC.
- N. Barman, D. Singha and K. Sahu, J. Phys. Chem. A, 2013, 117, 3945–3953 CrossRef CAS PubMed.
- P. Alam, C. Climent, P. Alemany and I. R. Laskar, J. Photochem. Photobiol., C, 2019, 41, 100317 CrossRef CAS.
- Z. He, C. Ke and B. Z. Tang, Acs Omega, 2018, 3, 3267–3277 CrossRef CAS PubMed.
- V. S. Padalkar and S. Seki, Chem. Soc. Rev., 2016, 45, 169–202 RSC.
- L. Dun, B. Zhang, J. Wang, H. Wang, X. Chen and C. Li, Cryst., 2020, 10, 1105 CrossRef CAS.
- X. Liu, Y. Wang, Y. Wang, Y. Tao, X. Fei, J. Tian and Y. Hou, Photochem. Photobiol., 2021, 20, 1183–1194 CrossRef CAS PubMed.
- X. Meng, L. Song, H. Han, J. Zhao and D. Zheng, Spectrochim. Acta, Part A, 2022, 265, 120383 CrossRef CAS PubMed.
- H. Li, W. Han, R. Lv, A. Zhai, X.-L. Li, W. Gu and X. Liu, Anal. Chem., 2019, 91, 2148–2154 CrossRef CAS PubMed.
- P. Huang, Y. Liu, A. Karmakar, Q. Yang, J. Li, F.-Y. Wu and K.-Y. Deng, Dalton trans., 2021, 50, 6901–6912 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Figures for crystal structures, FT-IR spectra, PXRD patterns of Zn-CP and Zn-CP-II, TG curve of Zn-CP, PL spectra and CIE diagrams and other data. See DOI: https://doi.org/10.1039/d4ra00500g |
| This journal is © The Royal Society of Chemistry 2024 |