Open Access Article
Open Access ArticleNovel mono substituted pyridoimidazoisoquinoliniums via a silver-catalyzed intramolecular cyclization and their applications in cellular imaging†
Masato Kawakubo,
Yoshikazu Inoh,
Yuki Murata ,
Mio Matsumura
,
Mio Matsumura *,
Tadahide Furuno
*,
Tadahide Furuno * and
Shuji Yasuike
* and
Shuji Yasuike
School of Pharmaceutical Sciences, Aichi Gakuin University, 1-100 Kusumoto-cho, Chikusa-ku, Nagoya 464-8650, Japan. E-mail: m-matsu@dpc.agu.ac.jp; furuno@dpc.agu.ac.jp
First published on 22nd March 2024
Abstract
Cationic heterocycles, an important class of organic compounds soluble in polar solvents, have been gaining attention in the construction of fluorescent probes. This paper reports the quick synthesis of novel pyrido[1′,2′;2,3]imidazo[5,1-a]isoquinoliniums starting from 2-(2-ethynylphenyl)imidazo[1,2-a]pyridines at room temperature via intramolecular cyclization by employing a catalytic amount of silver trifluoromethanesulfonate in addition to lithium trifluoromethanesulfonate and silica gel as the counter anion source and additive, respectively. The designed pyridoimidazoisoquinoliniums consisted of an imidazo[1,2-a]pyridine fused isoquinolinium. The X-ray diffraction results revealed that pyrido[1′,2′;2,3]imidazo[5,1-a]isoquinolinium trifluoromethanesulfonate contained considerable planar parent skeletons and interacted by π–π stacking with neighbouring molecules. Furthermore, in a methanol solution the designed 6-phenyl derivative exhibited strong fluorescence in the 420–450 nm region in addition to strong mitochondrial specificity in a cell staining assay.
Introduction
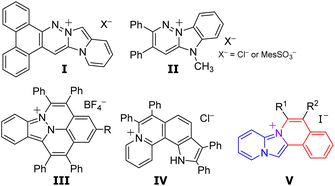
Fluorescence bioimaging has been gaining significant attention, as a powerful tool for the visualization of specific organelles in live cells, since it enables the monitoring of biological activities and consequently helps in disease diagnosis.1–3 Organic fluorescent dyes have been used to enhance the contrast for the imaging of biological specimens.4–10 Consequently, several studies have investigated the design of organelle-targeted probes. Charged polycyclic heteroarenes, characterized by both fluorescence properties stemming from their π-conjugated skeletons and high solubilities attributed to their ionic structures, have been gaining significant attention. However, the majority of charged polycyclic heteroarenes with three or more condensed rings reported so far possess a benzene-fused quinolinium, quinolizinium, or cinnolinium skeleton, indicating a notable lack of structural diversity. Only a few studies have reported the application of cationic fluorophores fused with two or more heteroaromatic compounds in intercellular imaging (Fig. 1).Vaquero et al. reported the synthesis and properties of fluorescent DNA probes consisting of benzocinnolinium fused imidazo[1,5-a]pyridine (I)11,12 and pyridazinium fused benzimidazole (II).13 Maheswari et al. reported the application of a synthesised azafluoranthenium including cinnolinium and indazole (III) in mitochondrial visualization in living cells.14 You et al. reported that aza[4]helicenes, pyrrolo[3,2-k]phenanthridiziniums exhibited fluorescence in the acidic environment of lysosomes.15 Our group has recently reported the synthesis of pyrido[1′,2′;2,3]imidazo[5,1-a]isoquinoliniums consisting of imidazo[1,2-a]pyridine and isoquinolium.16 The designed isoquinoliums were successfully employed in the intracellular imaging of the endoplasmic reticulum in particular.17 However, only a few approaches have been proposed for the synthesis of pyrido[1′,2′;2,3]imidazo[5,1-a]isoquinoliniums.16–19 Furthermore, the properties of their derivatives and applications are still ambiguous.
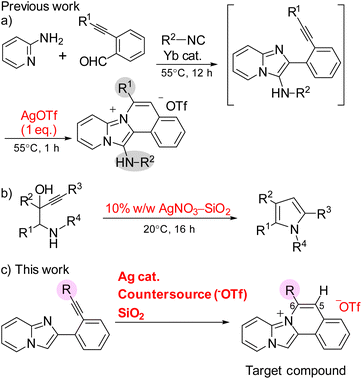
The intermolecular nucleophilic cyclization of alkynes with a nitrogen-functional side chain mediated by silver complexes is a facile and powerful method for the synthesis of key heterocycles.20–23 Zhou et al. conducted the synthesis of 13-aminopyrido[1′,2′;2,3]imidazo[5,1-a]isoquinoliniums by the Ag-mediated three-component domino reaction of 2-alkynylbenzaldehydes, 2-aminopyridines, and isocyanides at 55 °C (Scheme 1a).18 However, the cyclization reaction required stoichiometric amounts of silver(I) trifluoromethanesulfonate (AgOTf) because the trifluoromethanesulfonate also acted as a counter anion for the products, limiting the application of their synthetic method. Knight and coworkers discovered that various 3-alkynyl-hydroxyalkanamine derivatives can undergo nucleophilic cyclizations to provide the corresponding pyrroles using catalytic amount of 10% w/w AgNO3–SiO2 (Scheme 1b).24 This 5-endo-dig cyclization was promoted by a silver catalyst supported on a silica gel and required 16 h under mild conditions. Consequently, this study reported the rapid synthesis of pyrido[1′,2′;2,3]imidazo[5,1-a]isoquinoliniums at room temperature via the silver-catalysed nucleophilic cyclization of 2-ethynylphenylimidazopyridine with lithium trifluoromethanesulfonate (LiOTf) and silica gel as the counter anion source and additive, respectively (Scheme 1c). The obtained compounds were characterized by different spectroscopic techniques and their application in cell imaging was also investigated.
 | ||
| Scheme 1 Ag-mediated intermolecular nucleophilic cyclization of alkynes with nitrogen-functional side chain. | ||
Results and discussion
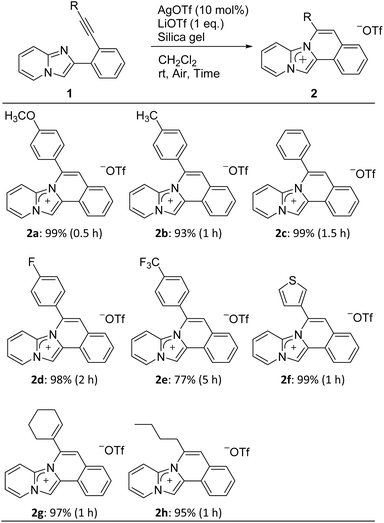
The cyclization precursors, 2-(2-ethynylphenyl)imidazo[1,2-a]pyridines 1, were easily synthesised by the Sonogashira cross-coupling reaction between 2-(2-iodophenyl)imidazo[1,2-a]pyridine and various terminal acetylenes.16 These precursors were then treated with AgOTf (10 mol%) as the Lewis acid, LiOTf (1 equiv.) as the counter anion source, and silica gel (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w vs. AgOTf) as the additive under aerobic conditions in CH2Cl2 at room temperature (Table 1). All reactions followed the 6-endo-diagonal type to give the desired ring-closure products 2a–h in good to excellent yields. Precursors 1a–d bearing electron donors or halide groups on the terminal benzene ring provided the corresponding products 2a–d in a short reaction time and high yields. On the other hand, 2e was obtained in a lower yield and required longer reaction times due to the stronger electron-withdrawing trifluoromethyl group of 1e. Furthermore, ethynylimidazopyridines 1f–h with a heterocyclic thiophene, vinyl, or alkyl group, respectively, afforded the respective products 2f–h within one hour. All obtained products were easily purified by silica gel column chromatography. Additionally, they completely soluble in polar solvent such as CH3OH and DMSO, showed less solubility in CH2Cl2, and was insoluble in water. The addition of silica gel was essential and promoted the cyclization reaction while the yield was less than 9% yield without silica gel.24,25 Nevertheless, the reason behind effect of silica gel is still not clear.
1 w/w vs. AgOTf) as the additive under aerobic conditions in CH2Cl2 at room temperature (Table 1). All reactions followed the 6-endo-diagonal type to give the desired ring-closure products 2a–h in good to excellent yields. Precursors 1a–d bearing electron donors or halide groups on the terminal benzene ring provided the corresponding products 2a–d in a short reaction time and high yields. On the other hand, 2e was obtained in a lower yield and required longer reaction times due to the stronger electron-withdrawing trifluoromethyl group of 1e. Furthermore, ethynylimidazopyridines 1f–h with a heterocyclic thiophene, vinyl, or alkyl group, respectively, afforded the respective products 2f–h within one hour. All obtained products were easily purified by silica gel column chromatography. Additionally, they completely soluble in polar solvent such as CH3OH and DMSO, showed less solubility in CH2Cl2, and was insoluble in water. The addition of silica gel was essential and promoted the cyclization reaction while the yield was less than 9% yield without silica gel.24,25 Nevertheless, the reason behind effect of silica gel is still not clear.
| a Reaction conditions: 1 (0.5 mmol), LiOTf (0.5 mmol), AgOTf (0.05 mmol), silica gel (1.27 g), CH2Cl2 (4 mL). |
|---|
 |
The molecular structures of the cyclized products 2 were characterised by NMR, HRMS, IR, UV/Vis, and fluorescent spectroscopy. The 1H NMR results revealed that the imidazole ring had a lower electron density than that of bicyclic imidazo[1,2-a]pyridine. The chemical shifts of the CH signals in the imidazole ring (13-position of the pyridoimidazoisoquinolinium skeleton and 3-position of the imdazopyridine skeleton) shifted downfield by 0.64–0.90 ppm after cyclization.
The single-crystal X-ray diffraction results of 2b (Fig. 2), and the values of the selected geometrical parameters (Table 2) revealed that the tetracyclic parent skeleton were virtually coplanar (mean deviation 0.069 Å) to each other. The four imidazole C–N bonds were almost identical in length and similar to the partial double bond lengths in five-membered heterocycles.26 These results indicated that the imidazole moiety of the product retained the resonance characteristics of the imidazolium cation. Furthermore, the oxygen atom (O1) in trifluoromethanesulfonate, used as a counter anion, possessed a negative charge and thus interacted with the cationic 2b. Consequently, O− coordinated with the cationic carbon (C1) at a distance of 3.104(2) Å, where the interatomic distance was shorter than the sum of the van der Waals radii (3.25 Å).27 A side-view of the crystal packing structure revealed the formation of π–π stacking between the two tetracyclic planes with distances of 3.399 and 3.490 Å (Fig. 2b).
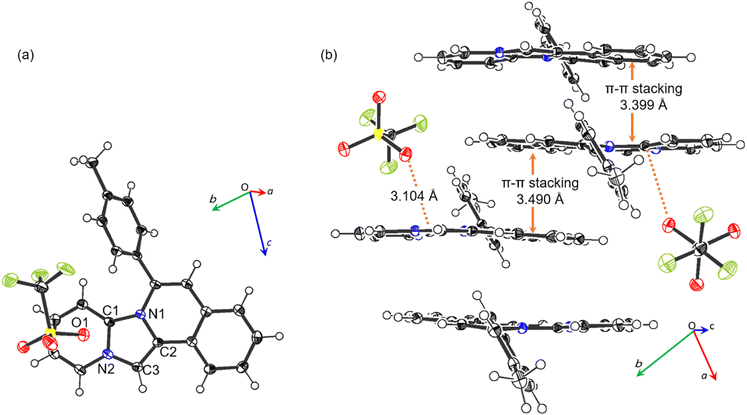 | ||
| Fig. 2 Crystal structure of 2b. (a) Top view and (b) packing structure from side view, with 50% provability thermal ellipsoids. | ||
| C1–N1 | 1.373(2) | C3–N2 | 1.379(2) |
| C1–N2 | 1.372(2) | C1⋯O1 | 3.104(2) |
| C2–N1 | 1.406(2) |
The optical properties of 2 were characterized by UV absorption and fluorescence spectroscopy in CH3OH, and the corresponding data are shown in Fig. 3 and Table 3. These compounds exhibited trimodal absorption bands in the range of 340–375 nm. Furthermore, the absorption maxima (λabs) of these compounds were not affected by the substituents at the 6-position on the tetracyclic skeleton, while the emission maxima (λem) increasingly redshifted as the electron-donating nature of the substituent increased from 421 to 447 nm for 2a–e, respectively. Thienyl, vinyl, and alkyl substituted compounds 2f–h exhibited emissions at 435, 438, and 419 nm, respectively. All compound has high fluorescence intensities with quantum yields (ΦF) in the range of 39–63%. Additionally, these compounds exhibited very little solvent dependence in CH2Cl2 as nonpolar solvent (λabs = 341, λem = 425 nm for 2c) and HEPES buffer (λabs = 340, λem = 426 nm) (Fig. S1†).
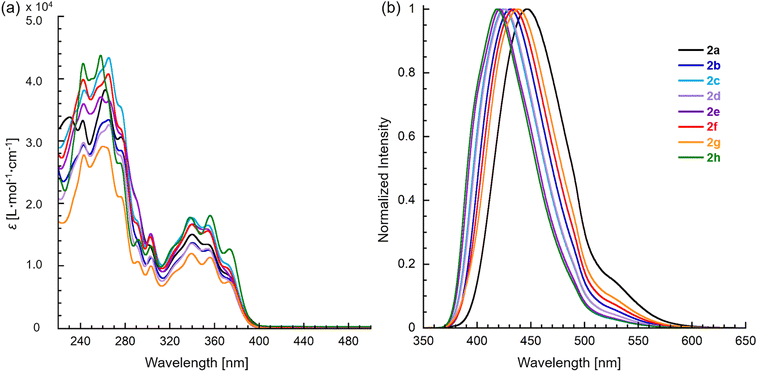 | ||
| Fig. 3 (a) Absorption and (b) fluorescence spectra of pyridoimidazoisoquinoliniums 2a–h in CH3OH. The excitation wavelength of fluorescence are at 340 nm. | ||
| Compd | R | λabs (nm) | λemb (nm) | ΦFb (%) | |
|---|---|---|---|---|---|
| a In CH3OH.b Excitation at 340 nm, and quantum yield using anthracene as standard. | |||||
| 2a | 4-CH3OC6H4 | 340 | 354 | 447 | 42 |
| 2b | 4-CH3C6H4 | 341 | 355 | 432 | 49 |
| 2c | C6H5 | 340 | 354 | 426 | 62 |
| 2d | 4-FC6H4 | 340 | 354 | 427 | 49 |
| 2e | 4-CF3C6H4 | 341 | 353 | 421 | 63 |
| 2f | 3-Thienyl | 340 | 354 | 435 | 39 |
| 2g | 1-Cyclohexenyl | 340 | 356 | 438 | 43 |
| 2h | n-Butyl | 338 | 356 | 419 | 63 |
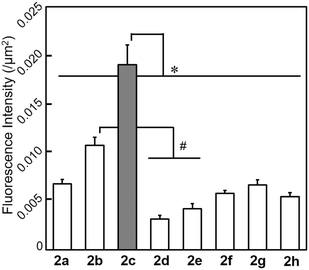
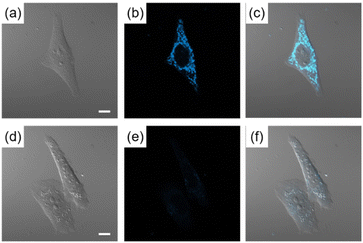
The possibility of employing the obtained compounds in cellular imaging was then investigated. Consequently, the live human cervical cancer (HeLa) cells were incubated for 30 min with each derivative (2 μM). The standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay showed that none of the compounds 2a–h affected cell viability under imaging conditions (Fig. S2†). The confocal laser scanning microscopic (CLSM) images revealed that the tetracyclic compounds were selectively localized in the cytoplasm without being transported into the nucleus (Fig. S3†). Additionally, autofluorescence without staining with the compounds was confirmed to be negligible. The intracellular fluorescence intensity of 2c was the strongest, while those of the other compounds were not correlated with the fluorescent quantum yields in CH3OH (Fig. 4). The subcellular localization of compound 2c was investigated by colocalization microscopy techniques. The HeLa cells were treated with 2 μM of 2c and commercially available organelle trackers i.e., MitoTracker Green, ER Tracker Green, and LysoTracker Green (Fig. 5 and S4†). Compound 2c was localized mitochondrial specificity, unlike our previously reported pyrideimidazoisoquinolinium with 5,6-disubstitutions.17 Therefore we further investigated the propulsion of localization and how it is affected upon disrupting the mitochondrial membrane potential (MMP). The intracellular fluorescence intensity of 2c significantly decreased upon a treatment with a membrane depolarising agent, carbonyl cyanide m-chlorophenyl hydrazone (CCCP), which reduced the MMP (Fig. 6).28,29 These results indicated that the specific localization of 2c on the mitochondrial depended on MMP.
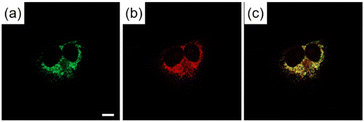 | ||
| Fig. 5 CLSM images of HeLa cells incubated for 30 min with (a) MitoTracker Green (200 nM) for 30 min and (b) 2c (2 μM). (c) Is the mergence image of (a) and (b). Scale bar: 10 μm. | ||
Conclusion
This study reported the synthesis of novel charged heteroarenes consisting of imidazo[1,2-a]pyridine-fused isoquinolines from 2-(2-ethynylphenyl)imidazo[1,2-a]pyridines by intramolecular cyclization. The reaction proceeded with the catalytic activity of AgOTf, with LiOTf as the counter anion source and silica gel as the additive at room temperature for a short reaction times. The X-ray diffraction results revealed that pyrido[1′,2′;2,3]imidazo[5,1-a]isoquinolinium trifluoromethanesulfonate 2b exhibited considerable planar parent tetracyclic skeletons and formed π–π stacking with neighbouring molecules. In a methanol solution, the newly synthesised pyridoimidazoisoquinolinium exhibited strong fluorescence. Finally, the possible application of the obtained pyridoimidazoisoquinoliniums in cell imaging was assessed. The results revealed that the 6-phenyl derivative 2c showed strong localized mitochondrial specificity. These result are extremely important basic knowledge for the development of cell staining agents using ionic compounds consisting of imidazopyridine skeleton. Further investigations on the wide scope of cyclization by Ag catalysts with silica gel to afford cationic heteroacenes and elucidating the intracellular-imaging mechanism are currently underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by a research grant from Institute of Pharmaceutical Life Sciences, Aichi Gakuin University and Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan (M. K.).Notes and references
- J. Zhang, X. Chai, X.-P. He, H.-J. Kim, J. Yoon and H. Tian, Chem. Soc. Rev., 2019, 48, 683–722 RSC.
- M. Gao, F. Yu, C. Lv, J. Choo and L. Chen, Chem. Soc. Rev., 2017, 46, 2237–2271 RSC.
- H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke and Y. Urano, Chem. Rev., 2010, 110, 2620–2640 CrossRef CAS PubMed.
- X. Tian, L. C. Murfin, L. Wu, S. E. Lewis and T. D. James, Chem. Sci., 2021, 12, 3406–3426 RSC.
- J. V. Jun, D. M. Chenoweth and E. J. Petersson, Org. Biomol. Chem., 2020, 18, 5747–5763 RSC.
- E. Kozma and P. Kele, Org. Biomol. Chem., 2019, 17, 215–233 RSC.
- L. Wang, M. S. Frei, A. Salim and K. Johnsson, J. Am. Chem. Soc., 2019, 141, 2770–2781 CrossRef CAS PubMed.
- J. Zhou and H. Ma, Chem. Sci., 2016, 7, 6309–6315 RSC.
- Y. Tang, D. Lee, J. Wang, G. Li, J. Yu, W. Lin and J. Yoon, Chem. Soc. Rev., 2015, 44, 5003–5015 RSC.
- M. Baker, Nature, 2010, 466, 1137–1140 CrossRef CAS PubMed.
- P. Bosch, V. García, B. S. Bilen, D. Sucunza, A. Domingo, F. Mendicuti and J. J. Vaquero, Dyes Pigm., 2017, 138, 135–146 CrossRef CAS.
- P. Bosch, D. Sucunza, F. Mendicuti, A. Domingo and J. J. Vaquero, Org. Chem. Front., 2018, 5, 1916–1927 RSC.
- P. Bosch, G. Marcelo, A. Matamoros-Recio, D. Sucunza, F. Mendicuti, A. Domingo and J. J. Vaquero, Dyes Pigm., 2021, 192, 109443 CrossRef CAS.
- S. Mayakrishnan, M. Tamizmani, C. Balachandran, S. Aoki and N. U. Maheswari, Org. Biomol. Chem., 2021, 19, 5413–5425 RSC.
- L. Yan, W. Ma, J. Lan, H. Cheng, Z. Bin, D. Wu and J. You, Chem. Sci., 2021, 12, 2419–2426 RSC.
- M. Kawakubo, Y. Inaguma, Y. Murata, M. Matsumura and S. Yasuike, Tetrahedron Lett., 2022, 105, 154054 CrossRef CAS.
- M. Kawakubo, Y. Inoh, Y. Inaguma, R. Kokubugata, Y. Murata, M. Matsumura, T. Furuno and S. Yasuike, ChemistrySelect, 2023, 8, e202303017 CrossRef CAS.
- H. Zhou, W. Wang, O. Khorev, Y. Zhang, Z. Miao, T. Meng and J. Shen, Eur. J. Org Chem., 2012, 2012, 5585–5594 CrossRef CAS.
- Z. Qi, S. Yu and X. Li, J. Org. Chem., 2015, 80, 3471–3479 CrossRef CAS PubMed.
- C. R. Nathaniel, M. Neetha and G. Anilkumar, Appl. Organomet. Chem., 2021, 35, e6141 CrossRef CAS.
- A. K. Clarke, H. E. Ho, J. A. Rossi-Ashton, R. J. K. Taylor and W. P. Unsworth, Chem.–Asian J., 2019, 14, 1900–1911 CrossRef CAS PubMed.
- G. Fang and X. Bi, Chem. Soc. Rev., 2015, 44, 8124–8173 RSC.
- M. Harmata, in Silver in Organic Chemistry, ed. P. Belmont, John Wiley & Sons Inc, Hoboken, 2010, vol. 5, pp. 143–162 Search PubMed.
- C. M. Sharland, J. Singkhonrat, M. NajeebUllah, S. J. Hayes, D. W. Knight and D. G. Dunford, Tetrahedron Lett., 2011, 52, 2320–2323 CrossRef CAS.
- M. Kawakubo, H. Ohta, Y. Murata, M. Matsumura and S. Yasuike, unpublished data.
- F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1–S19 RSC.
- J. Emsley, in The Elements, Clarendon Press, Oxford, 1998 Search PubMed.
- B. S. Padman, M. Bach, G. Lucarelli, M. Prescott and G. Ramm, Autophagy, 2013, 9, 1862–1875 CrossRef CAS PubMed.
- F. Sivandzade, A. Bhalerao and L. Cucullo, Bio-Protoc., 2019, 9, e3128 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2324904. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ra01210k |
| This journal is © The Royal Society of Chemistry 2024 |