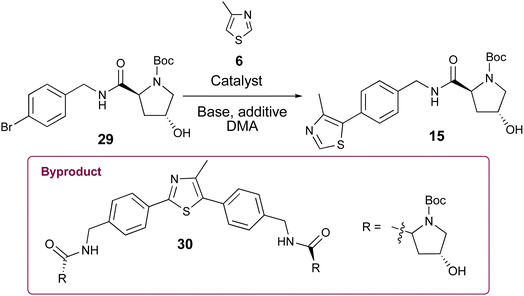
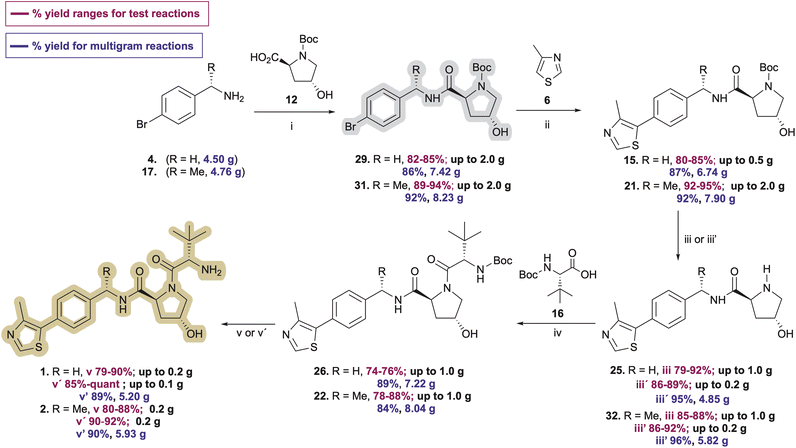
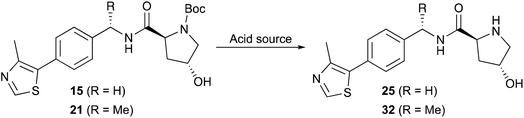
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Preparation of von Hippel-Lindau (VHL) E3 ubiquitin ligase ligands exploiting constitutive hydroxyproline for benzylic amine protection†
Diana M. Soto-Martínez a,
Garrett D. Clements
a,
Garrett D. Clements a,
John E. Díaza,
Joy Becher
a,
John E. Díaza,
Joy Becher a,
Robert C. Reynoldsb,
Christina Ochsenbauerb and
Timothy S. Snowden
a,
Robert C. Reynoldsb,
Christina Ochsenbauerb and
Timothy S. Snowden *ac
*ac
aDepartment of Chemistry and Biochemistry, The University of Alabama, 250 Hackberry Lane, Tuscaloosa, AL 35487, USA. E-mail: snowden@ua.edu
bDepartment of Medicine, Division of Hematology and Oncology, University of Alabama at Birmingham, Birmingham, AL 35294, USA
cCenter for Convergent Bioscience and Medicine, The University of Alabama, 720 2nd St., Tuscaloosa, AL 35401, USA
First published on 28th May 2024
Abstract
The von Hippel-Lindau (VHL) protein serves as the substrate recognition subunit of the multi-subunit Cullin-2 RING E3 ubiquitin ligase (CRL2VHL), which regulates intracellular concentrations of hypoxia inducible factors (HIFs) through a ubiquitin proteasome system (UPS) cascade. Strategic recruitment of CRL2VHL by bi- or trifunctional targeted protein degraders (e.g., PROTACs®) offers the prospect of promoting aberrant polyubiquitination and ensuing proteasomal degradation of disease-related proteins. Non-peptidic, L-hydroxyproline-bearing VHL ligands such as VH032 (1) and its chiral benzylic amine analog Me-VH032 (2), are functional components of targeted protein degraders commonly employed for this purpose. Herein, we compare two approaches for the preparation of 1 and 2 primarily highlighting performance differences between Pd(OAc)2 and Pd-PEPPSI-IPr for the key C–H arylation of 4-methylthiazole. Results from this comparison prompted the development of a unified, five-step route for the preparation of either VH032 (1) or Me-VH032 (2) in multigram quantities, resulting in yields of 56% and 61% for 1 and 2, respectively. Application of N-Boc-L-4-hydroxyproline rather than N-tert-butoxycarbonyl to shield the benzylic amine during the coupling step enhances step economy. Additionally, we identified previously undisclosed minor byproducts generated during arylation steps along with observations from amine deprotection and amidation reaction steps that may prove helpful not only for the preparation of 1 and 2, but for other VHL recruiting ligands, as well.
Introduction
The von Hippel-Lindau (VHL) tumor suppressor protein has multiple cellular roles, including serving as the substrate recognition subunit of the Cullin-2 RING E3 ubiquitin ligase CRL2VHL polyprotein. In this capacity, CRL2VHL is commonly recruited by targeted protein degraders (i.e., TPDs or PROTACs®1) and exploited for aberrant polyubiquitination of disease-related proteins, ideally resulting in the 26S proteasomal degradation of the target.1–3Established VHL recruiting ligands and ligands targeting the substrate recognition receptor CRBN of the CRL4 E3 ubiquitin ligase predominate as functional segments of reported TPDs. CRBN ligands offer enhanced bioavailability,4 ease of preparation, and affordability; however, they are generally inferior to VHL ligands in terms of thermal and chemical stability5–8 and target selectivity.9 Because CRL2VHL and CRL4CRBN have different capacities to form stable ternary complexes with TPDs and the targeted proteins, and their recruiting ligands impart distinct physicochemical properties to potential degraders, it is beneficial to evaluate members of both ligand classes in the early stages of TPD discovery to increase the probability of hit identification.10
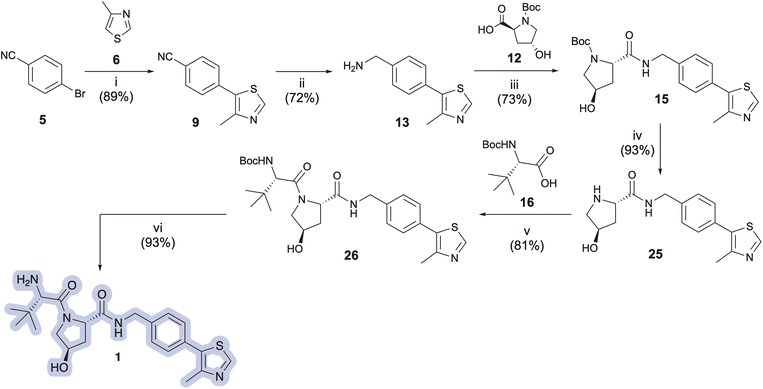
VH032 (1)11 and its chiral benzylic amine congener Me-VH032 (2)12 are among the most employed VHL ligands in TPDs. Reported syntheses of VH032 have relied upon C–H arylation of 4-methylthiazole 6 with Boc-protected benzylic amine 7, benzonitrile reduction of 9, or Suzuki–Miyaura cross coupling between 8 and 11 to access key intermediate 13.11,13–17 VH032 is subsequently assembled through sequential or convergent amidation and amine deprotection steps (Scheme 1A). Li and co-workers reported the highest yielding preparation of VH032, generating a 42.5 g batch in seven steps in 65% overall yield without chromatography.18 Researchers at Bio-Techne subsequently scaled up a comparable synthesis of 1 involving 7 to successfully prepare over 200 g batches.19 The routes originally reported by researchers at Arvinas and Yale and by the Ciulli group involving benzonitrile 5 remain popular, with multigram preparations of 1 completed in 25–65% overall yields in six steps from inexpensive commercial materials.11,14 Reported preparative methods for Me-VH032 (2) are more limited. Both researchers at Arvinas and the Wang group accessed 19 via C–H arylation of 6 using Boc-protected chiral benzylic amine 18, followed by amine deprotection and standard amide coupling procedures to form 2 (Scheme 1B).12,16,20
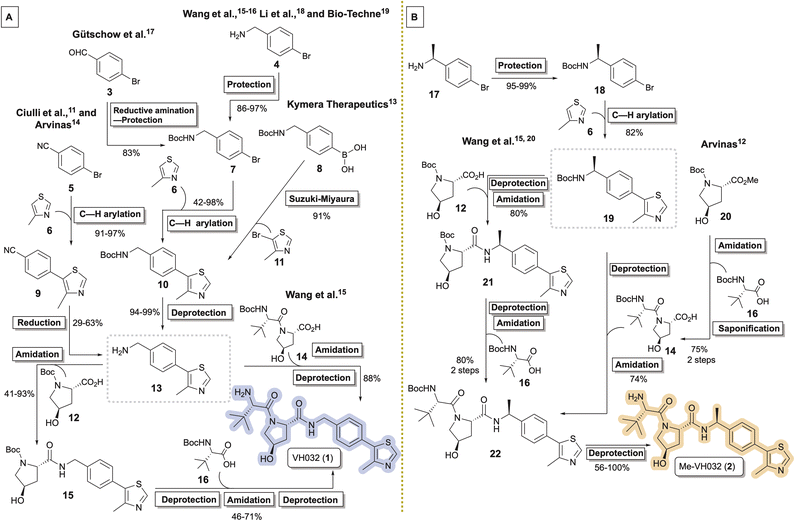 | ||
| Scheme 1 Synthetic routes (A) converging to key intermediate 13 en route to VH032 (1) and (B) diverging from 19 en route to Me-VH032 (2). | ||
Herein, we report a unified five-step strategy to prepare popular VHL recruiting ligand VH032 (1) and its higher affinity congener Me-VH032 (2) in multigram quantities. In addition, previously unreported observations related to minor byproduct formation during Pd-catalyzed 4-methylthiazole (6) arylation steps and during the sequential amine deprotection and amidation steps may prove helpful in the synthesis of these and other VHL recruiting ligands.
Results and discussion
Initial route
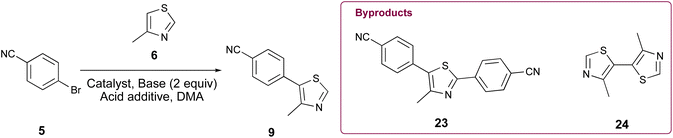
Initially, our goal was to improve the benzonitrile reduction step associated with popular synthetic routes for the multigram preparation of VH032 (1), since that step is yield limiting in published protocols involving synthetic intermediate 9 (Scheme 1A).11,14 We explored the preparation of requisite substrate 9 by initially comparing the C–H arylation conditions involving 2.0 equiv. 4-methylthiazole (6), 1.0 equiv. 4-bromobenzonitrile (5), 2.3 equiv. KOAc, and either 0.1 mol% or 3 mol% of Pd(OAc)2 in anhydrous DMA, as previously reported.11,14 The desired product 9 was obtained in excellent yields in both cases (Table 1, entries 1 and 2); however, both reactions generated two byproducts identified as 4,4′-(4-methylthiazole-2,5-diyl)dibenzonitrile (23) as a yellow solid (see page S11, Fig. 1S†) and 4,4′-dimethyl-5,5′-bithiazole (24) as a bright yellow solid (see page S12, Fig. 2S†), which were isolated in 5–8% combined yields (Fig. 1A). An attempt replacing KOAc with NaOAc under otherwise identical conditions to those in entry 2 offered comparable results (entry 3). Additional experiments using previously successful 3 mol% Pd(OAc)2 but only 1.3 equiv. of 6 at 100 °C or at 160 °C produced inferior results due to poor conversion of 9 in the first case and generation of multiple byproducts in the second (Table 1, entries 4 and 5).| Entry | Catalyst | Pd (mol%) | 6 (equiv.) | Base | Additive | [ ] (M) | T (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|
| a Scale = 250 mg of starting material 5 with purification by flash column chromatography.b Scale = 5.15 g of starting material 5 with purification by trituration using ice and H2O. | |||||||||
| 1 | Pd(OAc)2 | 0.1 | 2.0 | KOAc | 1.0 | 150 | 12 | 94 | |
| 2 | Pd(OAc)2 | 3 | 2.0 | KOAc | 0.5 | 150 | 5 | 88 | |
| 3 | Pd(OAc)2 | 3 | 2.0 | NaOAc | 0.5 | 150 | 3 | 86 | |
| 4 | Pd(OAc)2 | 3 | 1.3 | NaOAc | 0.5 | 160 | 4.5 | 47 | |
| 5 | Pd(OAc)2 | 3 | 1.3 | NaOAc | 0.5 | 100 | 5 | 30 | |
| 6 | Pd-PEPPSI-IPr | 0.5 | 2.0 | K2CO3 | AcOH | 0.25 | 125 | 3 | 70 |
| 7 | Pd-PEPPSI-IPr | 0.5 | 2.0 | K2CO3 | PivOH | 0.25 | 100 | 3 | 86 |
| 8 | Pd-PEPPSI-IPr | 0.5 | 2.0 | K2CO3 | PivOH | 0.25 | 125 | 2 | 93a |
| 9 | Pd-PEPPSI-IPr | 0.5 | 2.0 | K2CO3 | PivOH | 0.25 | 125 | 2 | 89b |
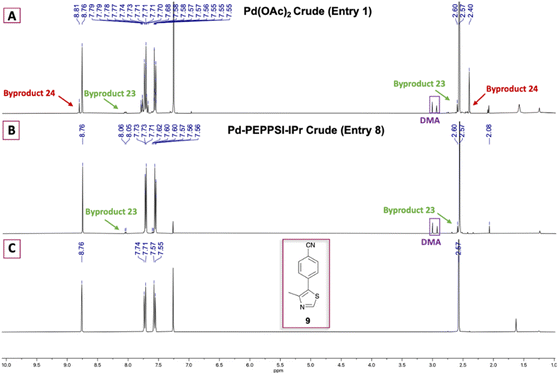 | ||
| Fig. 1 1H NMR comparison of crude 9 formed using (A) 0.1 mol% Pd(OAc)2 and (B) 0.5 mol% Pd-PEPPSI-IPr. (C) 1H NMR spectrum of purified 9. | ||
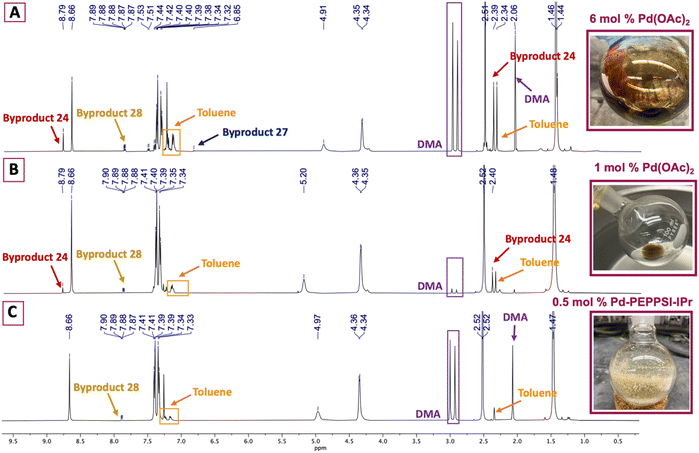
Various palladium pyridine-enhanced precatalyst preparation stabilization and initiation (PEPPSI™) precatalysts have been demonstrated to afford C–H arylation products, including 9, in high yields.21–23 However, to our knowledge, these precatalysts have not been reported for the preparation of VHL ligands. Treatment of 5 and 6 with Organ's commercial Pd-PEPPSI-IPr24,25 at varied reaction temperatures and reaction times (Table 1, entries 6–9) furnished 9 in yields comparable or superior to those involving Pd(OAc)2 with the benefit of slightly reduced formation of bis-arylated 23 and no evidence of 24 (Fig. 1B). In addition, crude 9 produced by Pd-PEPPSI-IPr-catalyzed C–H arylation can be triturated using crushed ice/cold H2O to afford pure 9 as a pearl-colored powder in 89% yield demonstrated on a 5.15 g scale with no evidence of residual 23 or 24 (Table 1, entry 10; Fig. 1C)—a technique that failed when 9 was prepared using catalytic Pd(OAc)2 due to co-precipitation of minor byproduct 24. This trituration process may be appealing for the preparation of 9 in applications wherein flash chromatography is undesirable.
With benzonitrile 9 in hand, we compared the reductants LiAlH4, NaBH4 with NiCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 or CoCl2,27 and LiBH4 plus trimethylsilyl chloride28 to generate key benzylic amine intermediate 13 with an interest in increasing the isolated yield while also considering scalability. Unable to improve upon previously reported results involving LiAlH4,14 we explored Singaram's (iBu)2AlBH4 reduction of 4-(4-methylthiazol-5-yl)benzonitrile (9).29 Our best results involved slight modifications to the originally reported conditions, largely to help manage B/Al-methanamine complex isolation from the associated solvogel produced upon quenching with methanol. Upon treating benzonitrile 9 for 2 hours with 1.1 equiv. of freshly prepared or briefly aged (iBu)2AlBH4, followed by biphasic extraction from introduced aqueous Rochelle's salt, the B/Al-methanamine adduct was obtained as a tacky, yellow solid. Subsequently heating the metalloid-complexed amine at reflux in 6 M HCl for 3 hours reproducibly afforded the desired methanamine 13 in 69–74% yields, which was modestly superior to reductions of 9 using LiAlH4 and appreciably better than those involving the other evaluated reductants.
26 or CoCl2,27 and LiBH4 plus trimethylsilyl chloride28 to generate key benzylic amine intermediate 13 with an interest in increasing the isolated yield while also considering scalability. Unable to improve upon previously reported results involving LiAlH4,14 we explored Singaram's (iBu)2AlBH4 reduction of 4-(4-methylthiazol-5-yl)benzonitrile (9).29 Our best results involved slight modifications to the originally reported conditions, largely to help manage B/Al-methanamine complex isolation from the associated solvogel produced upon quenching with methanol. Upon treating benzonitrile 9 for 2 hours with 1.1 equiv. of freshly prepared or briefly aged (iBu)2AlBH4, followed by biphasic extraction from introduced aqueous Rochelle's salt, the B/Al-methanamine adduct was obtained as a tacky, yellow solid. Subsequently heating the metalloid-complexed amine at reflux in 6 M HCl for 3 hours reproducibly afforded the desired methanamine 13 in 69–74% yields, which was modestly superior to reductions of 9 using LiAlH4 and appreciably better than those involving the other evaluated reductants.
Key intermediate 13 was next treated with N-protected (2S,4R)-4-hydroxyproline (12, Boc-L-Hyp), hexafluorophosphate azabenzotriazole tetramethyl uronium (HATU), and N,N-diisopropylethylamine (DIPEA) to provide N-Boc-protected pyrrolidine carboxylate intermediate 15 in 73% yield (Scheme 2). Amine deprotection using a CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TFA solution (1
TFA solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), followed by immediate freebasing and biphasic extraction from pH 12.5–13 aqueous medium afforded free amine 25 in 93% yield. The amidation strategy described for introduction of 12 to 13 was used to install Boc-L-t-leucine (16, Boc-L-Tle) onto 25, thereby furnishing penultimate VH032 product 26 in 81% yield. VH032 (1) was obtained using the amine deprotection/free basing strategy above in 93% yield, resulting in a 6-step preparation of 1 in 35% overall yield as free-based VH032 (1). The route offers comparable or superior step economy but lower overall yield compared to the best reported preparations of 1; however, identified improvements in select steps could be generally advantageous for the synthesis of other VHL ligands or unrelated target structures derived from 9 or 13. In addition, information gained from this initial approach inspired improvements for a more effective and expeditious preparative route to VH032 (1) and its analog Me-VH032 (2), vide infra.
1 v/v), followed by immediate freebasing and biphasic extraction from pH 12.5–13 aqueous medium afforded free amine 25 in 93% yield. The amidation strategy described for introduction of 12 to 13 was used to install Boc-L-t-leucine (16, Boc-L-Tle) onto 25, thereby furnishing penultimate VH032 product 26 in 81% yield. VH032 (1) was obtained using the amine deprotection/free basing strategy above in 93% yield, resulting in a 6-step preparation of 1 in 35% overall yield as free-based VH032 (1). The route offers comparable or superior step economy but lower overall yield compared to the best reported preparations of 1; however, identified improvements in select steps could be generally advantageous for the synthesis of other VHL ligands or unrelated target structures derived from 9 or 13. In addition, information gained from this initial approach inspired improvements for a more effective and expeditious preparative route to VH032 (1) and its analog Me-VH032 (2), vide infra.
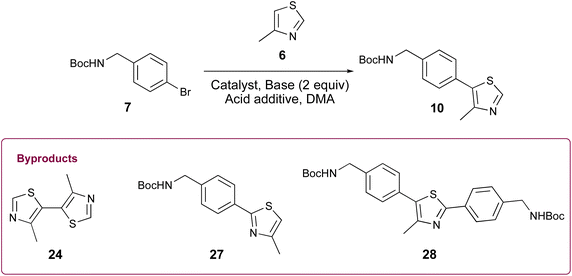
Appealing procedures featuring 4-methylthiazolylation of N-Boc protected benzylic amine derivatives (i.e., 7, 8, and 18, Scheme 1) as starting materials or early intermediates in the preparation of 1 and 2 have been reported.11,12 The advantage of these approaches for the preparation of 1 is obviation of the problematic benzonitrile reduction step required for transformation of 9 to 13 and employment of lower cost reactants than those required for Suzuki–Miyaura cross-coupling reactions.13 Given the comparative results between cat. Pd(OAc)2 and 0.5 mol% Pd-PEPPSI-IPr in the successful C–H arylation of 4-methylthiazole (6) using 5, we were interested to learn if the latter might offer some advantage in the C–H arylation of 6 using popular tert-butyl(4-bromobenzyl)carbamate 7. We initially evaluated conditions involving 6 mol% Pd(OAc)2, 2.3 equiv. methylthiazole (6), and 2.3 equiv. KOAc at 130 °C in anhydrous DMA (Table 2, entry 1),18 which furnished a viscous black oil following workup. The crude material was analyzed by 1H NMR spectroscopy, revealing signals for byproducts characterized as tert-butyl (4-(4-methylthiazol-5-yl)benzyl)carbamate (27), di-tert-butyl (((4-methylthiazole-2,5-diyl)bis(4,1-phenylene))bis(methylene))dicarbamate (28), and 4,4′-dimethyl-5,5′-bithiazole (24) along with desired product 10 (Fig. 2A). N-protected methylthiazole product 10 was isolated in 65% yield after flash chromatography and recrystallization using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 CHCl3/hexanes – the latter required to remove residual, bright yellow 24 (see page S12, Fig. 2S†) that was not fully removed by chromatography. Other isolated materials included 8% of unreacted 7 and minor byproducts that could not be isolated and characterized. A recently reported method for the synthesis of 10 from 7 involving 1 mol% Pd(OAc)2, 2.0 equiv. methylthiazole (6), 2.0 equiv. KOAc in anhydrous DMA at 95 °C performed appreciably better, producing desired product 10 in 85% yield after workup and flash chromatography (Table 2, entry 2).19 The crude material was analyzed by 1H NMR spectroscopy (Fig. 2B), reflecting formation of 24 and 28 as minor byproducts with no evidence of 27 or other substantial impurities. Meanwhile, an attempt replacing Pd(OAc)2 with Pd-PEPPSI-IPr under the optimal conditions established for 4-methythiazolylation of benzonitrile 3 (Table 2, entry 3) produced superior results, with limited formation of byproducts 27 and 28 and no evidence of 24 in the 1H NMR spectrum of the crude product (Fig. 2C). Consequently, product 10 was isolated in 91% yield as an off-white solid after flash chromatography.
4 CHCl3/hexanes – the latter required to remove residual, bright yellow 24 (see page S12, Fig. 2S†) that was not fully removed by chromatography. Other isolated materials included 8% of unreacted 7 and minor byproducts that could not be isolated and characterized. A recently reported method for the synthesis of 10 from 7 involving 1 mol% Pd(OAc)2, 2.0 equiv. methylthiazole (6), 2.0 equiv. KOAc in anhydrous DMA at 95 °C performed appreciably better, producing desired product 10 in 85% yield after workup and flash chromatography (Table 2, entry 2).19 The crude material was analyzed by 1H NMR spectroscopy (Fig. 2B), reflecting formation of 24 and 28 as minor byproducts with no evidence of 27 or other substantial impurities. Meanwhile, an attempt replacing Pd(OAc)2 with Pd-PEPPSI-IPr under the optimal conditions established for 4-methythiazolylation of benzonitrile 3 (Table 2, entry 3) produced superior results, with limited formation of byproducts 27 and 28 and no evidence of 24 in the 1H NMR spectrum of the crude product (Fig. 2C). Consequently, product 10 was isolated in 91% yield as an off-white solid after flash chromatography.
Route 2
Since the carbamate's role in 7 and 18 (Scheme 1) is to protect the Pd catalyst from amine coordination and associated side reactions that would complicate the C–H arylation step, we considered whether N-Boc-protected (2S,4R)-4-hydroxyproline (12, Boc-L-Hyp) might serve the same purpose with the hydroxyproline simultaneously acting as a constitutive moiety in the desired VHL ligand products, thereby eliminating an amine deprotection step. Researchers at GlaxoSmithKline reported this reaction in a 2014 patent using 2 mol% Pd(OAc)2 in N-methylpyrrolidinone (NMP) at 120 °C, obtaining 15 in 59% yield on an 8.0 g scale.30 No use of this approach or optimization is evidently reported beyond the one sentence reaction description in the patent. However, encouraged by the C–H arylation results highlighted in Tables 1 and 2, we compared the ability of Pd PEPPSI-IPr pre-catalyst to produce 15 from 29 relative to Pd(OAc)2 under various conditions, with the prospect of obviating the requirement for nitrile reduction or benzylic amine protection/deprotection steps en route to 15 or 21.Initially, we conducted the amidation of commercial Boc-L-Hyp (12) with 4-bromobenzylamine (4) using HATU (1.2 equiv.) and DIPEA (3.0 equiv.) in anhydrous acetonitrile at room temperature for 13 h, which afforded desired product 29 (Table 3) in 92–98% yields after column chromatography. However, the subsequent 4-methylthiazole C–H arylation step with 29 underwent incomplete conversion, likely due to partial deactivation of the catalyst by a small amount of residual tetramethylguanidinium or tetramethylurea byproduct that was not completely removed from 29 through either biphasic extraction or subsequent flash chromatography. With the idea of avoiding formation of potentially irremovable guanidinium or urea impurities, we switched to amidation of 4 with 12 using 1.3 equivalents of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl), 1.3 equivalents of hydroxybenzotriazole (HOBt) hydrate, and 2.3 equivalents of DIPEA in dimethylformamide (DMF). Under these conditions, pure 29 was obtained in 45–50% yields along with 15–20% yields of a byproduct (33, see pages S8–S9†) resulting from esterification of the secondary alcohol in 29 with 12. Comparable results were obtained from multiple amidation attempts involving varied reaction times and temperatures. However, replacing DMF with the mixed solvent system CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (5
DMF (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at −10 °C reported by Joullie and co-workers31,32 furnished 29 in 86% yield with no evidence of ester byproduct formation by 1H or 13C NMR spectroscopy in this or any subsequent amidation conducted on reaction scales ranging from 150 mg to >7 g of prepared 29.
1 v/v) at −10 °C reported by Joullie and co-workers31,32 furnished 29 in 86% yield with no evidence of ester byproduct formation by 1H or 13C NMR spectroscopy in this or any subsequent amidation conducted on reaction scales ranging from 150 mg to >7 g of prepared 29.
| Entry | Catalyst | Base | Additive | Pd (mol%) | [ ] (M) | T (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| a Reaction conducted in N-methylpyrrolidinone (NMP).b Scale = 7.4 g of 29. | ||||||||
| 1 | Pd(OAc)2 | KOAc | 2 | 0.4 | 120 | 18 | 58a | |
| 2 | Pd(OAc)2 | KOAc | 0.1 | 1.0 | 150 | 1.5 | 53 | |
| 3 | Pd(OAc)2 | KOAc | 3 | 0.5 | 150 | 2 | 53 | |
| 4 | Pd(OAc)2 | KOAc | 1 | 0.8 | 130 | 4 | 75 | |
| 5 | Pd-PEPPSI-IPr | K2CO3 | PivOH | 0.25 | 0.25 | 125 | 19 | 21 |
| 6 | Pd-PEPPSI-IPr | K2CO3 | PivOH | 0.5 | 0.25 | 100 | 21 | 37 |
| 7 | Pd-PEPPSI-IPr | K2CO3 | PivOH | 0.5 | 0.25 | 125 | 2 | 85 |
| 8 | Pd-PEPPSI-IPr | K2CO3 | PivOH | 0.5 | 0.25 | 125 | 2 | 88b |
| 9 | Pd-PEPPSI-IPr | K2CO3 | PivOH | 0.5 | 0.25 | 140 | 3 | 74 |
| 10 | Pd-PEPPSI-IPr | K2CO3 | PivOH | 1 | 0.25 | 100 | 19 | 73 |
| 11 | Pd-PEPPSI-IPr | K2CO3 | PivOH | 1 | 0.25 | 125 | 2.5 | 70 |
| 12 | Pd(OAc)2 | KOAc | 0.5 | 0.25 | 125 | 1.5 | 80 | |
With ample 29 in hand, we explored its utility in the C–H arylation of 4-methylthiazole (6). Invariably, 4-methylthiazole coupling reactions involving catalytic Pd(OAc)2 proved inferior with 29 relative to those explored with 5 or reported by others with 7. The conditions reported by the GSK researchers worked as described in our hands, affording pure 15 in 58% isolated yield on a 250 mg scale (Table 3, entry 1). Treating 29 with 1.0 mol% Pd(OAc)2 and 2.0 equiv. KOAc in anhydrous DMA at 130 °C provided the best initial results for the conversion of 29 to 15 (Table 3, entry 4), offering marked improvement over reactions conducted at different temperatures and catalyst concentrations (e.g., entries 2–3) in which byproducts including 24 and 30 were observed shortly after reaction commencement. 4-Methylthiazole coupling reactions with 29 involving 0.5% Pd-PEPPSI-IPr were generally more successful. Much like reaction attempts involving Pd(OAc)2 as summarized in Tables 1 and 2, we found the mol% of Pd-PEPPSI-IPr employed and reaction temperature significantly affected reaction outcomes in the generation of desired 15. Attempts at 100 °C or involving 0.25 mol% precatalyst proved sluggish with poor conversion (Table 3, entries 5 and 6), while those at 140 °C or involving 1 mol% Pd-PEPPSI-IPr showed high conversion but increased byproduct formation (Table 3, entries 9–11). The optimal conditions involved mixing 29 with pivalic acid (0.3 equiv.), K2CO3 (2 equiv.), and 0.5 mol% Pd-PEPPSI-IPr in DMA at 125 °C for 2 h (Table 3, entry 7). Under these conditions, yields of 15 invariably fall within the 85–88% range independent of the reaction scale evaluated (compare entry 7, conducted on a 250 mg scale with entry 8, conducted on a 7.4 g scale) or the batch produced. In addition, byproduct 30 formation was minimal, thereby facilitating rapid product purification relative to most reactions involving Pd(OAc)2. As a direct comparison of catalysts, we repeated the C–H arylation of 29 using Pd(OAc)2 in place of Pd-PEPPSI-IPr under the optimized conditions established with the latter and noted a slightly improved result relative to entry 4 (Table 3, entry 12), albeit still inferior to reactions completed using Pd-PEPPSI-IPr.
Pleased with the arylation results involving 6 and 29, we adapted the strategy to synthesize the key intermediate for the preparation of Me-VH032 (2). Commercial (S)-1-(4-bromophenyl)ethanamine (17) was amidated with Boc-L-Hyp (12) as described for 29, resulting in reproducibly high yields of 31 (averaging 90% in seven attempts) with no evidence of competing Boc-L-Hyp ester formation (Scheme 3). Treatment of resultant 31 with 6 (2.0 equiv.), pivalic acid (0.3 equiv.), K2CO3 (2 equiv.), and 0.5 mol% Pd-PEPPSI-IPr in DMA at 125 °C for 2 h cleanly furnished 21 in 95% yield, which is superior to reported C–H arylation of N-Boc-protected substrate 18 (Scheme 1).
Two common methods were evaluated to deprotect the prepared hydroxyproline intermediates 15 and 21. Initially, a TFA/CH2Cl2 solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 0.1 M) was used to remove the Boc group and obtain the ammonium trifluoroacetate salt, which was subjected directly to amidation with N-Boc-L-tert-leucine (Boc-L-Tle) 16 using 1.3 equivalents of both EDC·HCl and HOBt monohydrate and 3.5 equivalents of DIPEA. Alternatively, a 4 M HCl solution in methanol was used (0.5 M), and the crude product was triturated using anhydrous MTBE and dried under vacuum to afford the desired product 25 as its presumed bis-hydrochloride salt (Table 4), although the isolated solvent-free product masses contradict the assumed product molecular weight. The ammonium salt was subsequently treated with Boc-L-Tle (16), 1.3 equivalents each of EDC·HCl and HOBt monohydrate, and 3.5 equivalents of DIPEA. Amidation yields involving ammonium trifluoroacetate or chloride salts from 25 and 32 with Boc-L-Tle varied widely in our hands, ranging from ∼35 to 75% in multiple attempts. Better amidation results were observed using ammonium chloride salts, possibly because the associated trituration step of the deprotected amines resulted in reduced residual chloride and HCl compared with residual trifluoroacetate and TFA from its corresponding simple evaporation from deprotected 15 and 21. Such counterions are rarely quantified in published preparations of 25 or 32, and the extent of both counterion inclusion and product hydration likely varies from batch to batch and by method and length of product storage.
1 v/v, 0.1 M) was used to remove the Boc group and obtain the ammonium trifluoroacetate salt, which was subjected directly to amidation with N-Boc-L-tert-leucine (Boc-L-Tle) 16 using 1.3 equivalents of both EDC·HCl and HOBt monohydrate and 3.5 equivalents of DIPEA. Alternatively, a 4 M HCl solution in methanol was used (0.5 M), and the crude product was triturated using anhydrous MTBE and dried under vacuum to afford the desired product 25 as its presumed bis-hydrochloride salt (Table 4), although the isolated solvent-free product masses contradict the assumed product molecular weight. The ammonium salt was subsequently treated with Boc-L-Tle (16), 1.3 equivalents each of EDC·HCl and HOBt monohydrate, and 3.5 equivalents of DIPEA. Amidation yields involving ammonium trifluoroacetate or chloride salts from 25 and 32 with Boc-L-Tle varied widely in our hands, ranging from ∼35 to 75% in multiple attempts. Better amidation results were observed using ammonium chloride salts, possibly because the associated trituration step of the deprotected amines resulted in reduced residual chloride and HCl compared with residual trifluoroacetate and TFA from its corresponding simple evaporation from deprotected 15 and 21. Such counterions are rarely quantified in published preparations of 25 or 32, and the extent of both counterion inclusion and product hydration likely varies from batch to batch and by method and length of product storage.
| Entry | R | Acid source | [ ] (M) | Time (h) | Yield (%) ammonium salt | Yield (%) free-based |
|---|---|---|---|---|---|---|
| a % yield is calculated based on the mass of assumed bis-HCl salt, although the amount of residual HCl and water of hydration associated with the product was not determined.b Scale: 250 mg of 15 or 21.c Scale: 6.74 g of 15.d Scale: 6.66 g of 21. | ||||||
| 1 | H | HCl | 0.75 | 6 | 89a | — |
| 2 | H | HCl | 0.75 | 1 | 92a | 90b |
| 3 | H | HCl | 0.5 | 1.2 | 95a | 89b |
| 4 | H | HCl | 0.5 | 2 | 125a | 95c |
| 5 | Me | HCl | 0.5 | 1.5 | 102a | 92b |
| 6 | Me | HCl | 0.5 | 2 | 117a | 96d |
| 7 | Me | HCl | 0.3 | 1.2 | 92a | 83b |
| 8 | H | TFA | 0.1 | 1 | — | 92b |
| 9 | Me | TFA | 0.1 | 1 | — | 88b |
| 10 | Me | TFA | 0.25 | 1.1 | — | 75b |
Dissatisfied with the capriciousness of our amide coupling attempts using ammonium salts, and with particular concern regarding possible material loss during larger scale preparations of 1 and 2, we compared amine deprotection procedures involving freebasing the isolated crude conjugate acids of 15 and 21 by liquid–liquid extraction. Free-basing involved dissolving the dry trifluoroacetate salts in a mixture of dichloromethane-deionized water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) with constant stirring, followed by dropwise addition of concentrated aqueous NaOH solution to achieve pH = 12.5–13.0. Liquid–liquid extractions were conducted until product was absent from the DCM phase by TLC (typically, 8–11 small volume extractions). Free-based compounds 25 and 32, isolated upon treatment of 15 and 21 with TFA
1 v/v) with constant stirring, followed by dropwise addition of concentrated aqueous NaOH solution to achieve pH = 12.5–13.0. Liquid–liquid extractions were conducted until product was absent from the DCM phase by TLC (typically, 8–11 small volume extractions). Free-based compounds 25 and 32, isolated upon treatment of 15 and 21 with TFA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHCl2, were obtained in 92% and 88% yields, respectively (Table 4, entries 8 and 9; Scheme 3, iii). Similarly, triturated hydrochloride salts were free-based by dissolving the solid in a mixture of DCM–MeOH–dH2O (4
CHCl2, were obtained in 92% and 88% yields, respectively (Table 4, entries 8 and 9; Scheme 3, iii). Similarly, triturated hydrochloride salts were free-based by dissolving the solid in a mixture of DCM–MeOH–dH2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v)—with MeOH required to promote dissolution using a limited solvent volume—followed by dropwise addition of concentrated aqueous NaOH solution to achieve pH = 12.5–13.0. Liquid–liquid extraction as described above yielded free-based products 25 and 32 in 89% and 92% yields from 15 and 21, respectively (Table 4, entries 3 and 5; Scheme 3, iii′). No purification was necessary after either procedure.
1 v/v)—with MeOH required to promote dissolution using a limited solvent volume—followed by dropwise addition of concentrated aqueous NaOH solution to achieve pH = 12.5–13.0. Liquid–liquid extraction as described above yielded free-based products 25 and 32 in 89% and 92% yields from 15 and 21, respectively (Table 4, entries 3 and 5; Scheme 3, iii′). No purification was necessary after either procedure.
After successfully producing free amines 25 and 32, the compounds were treated with N-Boc-tert-leucine (16) using 1.3 equivalents each of EDC·HCl and HOBt monohydrate and 2.3 equivalents of DIPEA in DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (5
DMF (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at −10 °C to afford 26 and 22 in 76% and 88% yields on up to 1 g preparations (Scheme 3, iv), respectively. N-Deprotection of the N-tert-butoxycarbamoyl tert-leucine in 26 and 22 using TFA in DCM, followed by solvent evaporation and biphasic extraction at pH 12.5–13.0 produced free-based target ligands 1 and 2 in 90% and 88% yields, respectively. Comparatively, treatment of 26 and 22 with 4 M HCl in MeOH followed by trituration of the hydrochloride salt of 1 with Et2O33 and that of 2 with MTBE prior to the freebasing workup afforded 1 and 2 in 89% and 92% yields, respectively (Scheme 3). As a result, the overall yields for the five-step synthesis of neutral VHL ligands 1 and 2 on ∼200 mg scales were 46% for VH032 (1) and 62% for Me-VH032 (2).
1 v/v) at −10 °C to afford 26 and 22 in 76% and 88% yields on up to 1 g preparations (Scheme 3, iv), respectively. N-Deprotection of the N-tert-butoxycarbamoyl tert-leucine in 26 and 22 using TFA in DCM, followed by solvent evaporation and biphasic extraction at pH 12.5–13.0 produced free-based target ligands 1 and 2 in 90% and 88% yields, respectively. Comparatively, treatment of 26 and 22 with 4 M HCl in MeOH followed by trituration of the hydrochloride salt of 1 with Et2O33 and that of 2 with MTBE prior to the freebasing workup afforded 1 and 2 in 89% and 92% yields, respectively (Scheme 3). As a result, the overall yields for the five-step synthesis of neutral VHL ligands 1 and 2 on ∼200 mg scales were 46% for VH032 (1) and 62% for Me-VH032 (2).
Encouraged by these findings, preparations of VH032 (1) and methyl-VH032 (2) were scaled starting with 5.15 g of Boc-L-Hyp (12) as the limiting reagent. The yields of each step from the multigram-scale preparations were consistent with those from the corresponding smaller scale reactions conducted during reaction and procedural optimizations—apart from the deprotections of 15 and 21 using HCl in MeOH and the amidation of 16 with 25 to afford 26, which proved more effective on a multigram scale (Scheme 3). Quantitative NMR analyses of both final free-based amine products indicated purities of 97% for 1 and 98% for 2 from their multigram scale preparations. In total, these unified approaches for the preparation of VH032 (1) and Me-VH032 (2) resulted in 56% and 61% overall yields, respectively—the highest yet reported for 2—thereby producing >5 g of product in each case in five steps from commercial materials.
Conclusions
We compared approaches to prepare the popular VHL recruiting ligands VH032 (1) and Me-VH032 (2), thereby finding commercial Pd-PEPPSI-IPr pre-catalyst to be particularly effective for the requisite C–H arylation of 4-methylthiazole (6), even when the N-protected 4-hydroxyproline segment of 1 and 2, rather than a conventional amine protecting group, serves as the benzylic amine blocking moiety. Subsequent optimizations in amine deprotection and ensuing amidation steps produced multigram quantities of the high purity VHL ligands. The final route, which is common to formation of either 1 or 2, features unprecedented step economy (i.e., five steps from inexpensive commercial materials) and comparable or improved overall yields of 1 and 2 relative to other scalable approaches. In addition, minor byproducts formed during arylations under various conditions are reported for the first time, and more extensive characterization of key intermediates is offered to assist those generating 1, 2 or other VHL ligands featuring (2S,4R)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (i.e., 25 or 32) segments.Experimental
All reactants, reagents, and solvents were purchased from Sigma-Aldrich, Ambeed, or VWR suppliers. Reactions were monitored by thin-layer chromatography (TLC) on pre-coated 0.25 mm silica glass plates (60F254) purchased from Silicycle and visualized using UV light (254 nm or 365 nm), an I2 chamber, and/or either ninhydrin or KMnO4 stain with mild charring. Flash chromatography was performed using silica gel (60 Å, 230–400 mesh) from Silicycle pre-dried in a 150 °C oven for at least 24 h with a manual column or a Teledyne ISCO Combiflash Rf 200i. 1H and 13C NMR spectra were recorded on a Bruker NEO-500 spectrometer with a cryoprobe. All reported 1H and 13C chemical shifts (δH, δC) are referenced to the residual 1H signal of deuterated solvents (CDCl3: 1H = 7.26 ppm, 13C = 77.16 ppm; (CD3)2CO: 1H = 2.05 ppm, 13C = 29.84 ppm; CD3OD: 1H = 3.31 ppm, 13C = 49.00 ppm).34 Mass spectra were recorded using a Waters Xevo G2-XS QToF with ACUITU UPLC M-Class equipped with ESI and a high-performance orthogonal-acceleration Time of Flight (oaToF) mass analyzer (MS2). Melting points were determined with a MelTemp 1001D capillary melting point apparatus and were uncorrected. 1,2,4,5-Tetrachloro-3-nitrobenzene (99.85% pure) from Sigma Aldrich was used as the internal standard for quantitative NMR studies. Specific rotations were determined using an AUTOPOL IV automatic polarimeter. FTIR spectra were obtained with a JASCO FT/IR-4100 spectrometer.General procedure I: amidation of Boc-L-Hyp (12) and Boc-L-Tle (16) to form 22, 26, 29, 31
To a flame-dried round-bottom flask equipped with a magnetic stir bar and septum were added 12 or 16 (1.0 equiv.), EDC·HCl (1.3 equiv.), and HOBt monohydrate (1.3 equiv.). Amine (1.1 equiv.) dissolved in dry CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (5
DMF (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) was added to the reaction flask to create a 0.25 M solution of 12 or 16. The resulting white suspension was cooled to −10 °C with stirring for 5 minutes, then DIPEA (2.3 equiv.) was added dropwise. The reaction mixture was stirred at −10 °C for 1 h then the temperature was increased to 4 °C followed by gradual warming to room temperature until full conversion was evident by TLC (generally 15–20 h). The reaction was quenched by adding deionized water, and the aqueous phase was extracted successively with CH2Cl2 then EtOAc. The combined organic phases were washed with an aqueous solution of NaHCO3 to remove much of the remaining HOBt, then dried over anhydrous sodium sulfate, filtered, and concentrated by rotary evaporation followed by azeotropic distillation with toluene by rotary evaporation (55–60 °C water bath) to remove residual DMF. The crude product was purified by flash column chromatography using oven-dried silica and 4–5% MeOH in DCM as the eluent to afford the desired amide product 22, 26, 29, or 31.
1 v/v) was added to the reaction flask to create a 0.25 M solution of 12 or 16. The resulting white suspension was cooled to −10 °C with stirring for 5 minutes, then DIPEA (2.3 equiv.) was added dropwise. The reaction mixture was stirred at −10 °C for 1 h then the temperature was increased to 4 °C followed by gradual warming to room temperature until full conversion was evident by TLC (generally 15–20 h). The reaction was quenched by adding deionized water, and the aqueous phase was extracted successively with CH2Cl2 then EtOAc. The combined organic phases were washed with an aqueous solution of NaHCO3 to remove much of the remaining HOBt, then dried over anhydrous sodium sulfate, filtered, and concentrated by rotary evaporation followed by azeotropic distillation with toluene by rotary evaporation (55–60 °C water bath) to remove residual DMF. The crude product was purified by flash column chromatography using oven-dried silica and 4–5% MeOH in DCM as the eluent to afford the desired amide product 22, 26, 29, or 31.
General procedure II: C–H arylation using 0.5 mol% Pd-PEPPSI-IPr to form 15 and 21
To a two-neck round-bottom flask equipped with a magnetic stir bar and condenser were added bromoaryl derivatives 29 or 31 (1.0 equiv.), anhydrous potassium carbonate (2.0 equiv.), Pd-PEPPSI-IPr (0.5 mol%), pivalic acid (0.3 equiv.), 4-methylthiazole 6 (2.0 equiv.) in anhydrous DMA (0.25 M). The charged flask was placed into an oil bath that had been pre-equilibrated to 125 °C, and the reaction was heated for 2 h at that temperature. The mixture was cooled to room temperature, quenched with deionized water (equal to the DMA volume used in the reaction), then the aqueous phase was extracted with EtOAc (5 × ∼5 mL mmol−1). The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated by rotary evaporation to remove EtOAc. Residual DMA was removed by azeotropic distillation by rotary evaporation between 55 and 60 °C using toluene. The crude product was purified by flash column chromatography using oven-dried silica and 5% MeOH in DCM to afford the desired product 15 or 21.General procedure III: amine deprotection to form 1, 2, 25, 32
The Boc-protected amine 15, 21, 22, or 26 (1.0 equiv.) was stirred in a solution of 4 M HCl in methanol (2 mL mmol−1) at 4 °C for 2 h in a one-neck round-bottom flask equipped with a magnetic stir bar. The volatile components were removed by rotary evaporation at 33 °C, and the resulting oil was dried overnight under vacuum. The hydrochloride salt was stirred in cold MTBE (for compounds 25, 32 and 2) or diethyl ether (for 1) for 1–2 h at 4 °C then the solid was collected by filtration and rinsed with cold MTBE or Et2O, as appropriate, to obtain an off-white amorphous solid. The solid was dissolved in a mixture of MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM
DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :vH2O (3
:vH2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4:1 v/v, 8 mL mmol−1) at 4 °C, then a concentrated NaOH solution was slowly added with rapid mixing until the pH reached 12.5 to 13.0. The aqueous phase was extracted using small volumes of DCM until no product was evident in the extracting solvent by TLC (typically 8–11 times). The combined extracts were dried over anhydrous sodium sulfate, filtered, and concentrated by rotary evaporation at 33 °C to afford the desired product as analytically pure, free-based amine.
4:1 v/v, 8 mL mmol−1) at 4 °C, then a concentrated NaOH solution was slowly added with rapid mixing until the pH reached 12.5 to 13.0. The aqueous phase was extracted using small volumes of DCM until no product was evident in the extracting solvent by TLC (typically 8–11 times). The combined extracts were dried over anhydrous sodium sulfate, filtered, and concentrated by rotary evaporation at 33 °C to afford the desired product as analytically pure, free-based amine.
m.p.: 93–95 °C. Rf: 0.10 (5% MeOH/CH2Cl2).
FT-IR (KBr, plate) νmax (cm−1): 3360, 3298, 3072, 2954, 2869, 1668, 1624, 1553, 1439, 1416, 1221, 1199, 1080, 962, 848, 751.
1H NMR (500 MHz, CDCl3, OH signal not evident in 1H NMR spectrum) δ (ppm): 8.65 (s, 1H, CH thiazole), 7.65 (t, J = 6.1 Hz, 1H, NHC = O), 7.31 (d, J = 8.5 Hz, 2H, Ar–H), 7.29 (d, J = 8.5 Hz, 2H, Ar–H), 4.71 (t, J = 8.1 Hz, 1H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N Hyp), 4.45–4.41 (m, 2H, ((CH2)CHOH) Ar–CH2–N), 4.27 (dd, J = 15.2, 5.7 Hz, 1H, Ar–CH2–N), 3.71 (d, J = 11.3 Hz, 1H, CH2 Hyp), 3.58 (dd, J = 11.0, 3.8 Hz, 1H, CH2 Hyp), 3.29 (s, 1H, O
C–CH–N Hyp), 4.45–4.41 (m, 2H, ((CH2)CHOH) Ar–CH2–N), 4.27 (dd, J = 15.2, 5.7 Hz, 1H, Ar–CH2–N), 3.71 (d, J = 11.3 Hz, 1H, CH2 Hyp), 3.58 (dd, J = 11.0, 3.8 Hz, 1H, CH2 Hyp), 3.29 (s, 1H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N Boc-L-Tle), 2.79 (s br, 2H, NH2), 2.48 (s, 3H, CH3 thiazole), 2.29 (ddd, J = 13.3, 8.6, 4.4 Hz, 1H, CH2 Hyp), 2.12 (ddt, J = 13.3, 7.7, 1.8 Hz, 1H, CH2 Hyp), 0.91 (s, 9H, C(CH3)3).
C–CH–N Boc-L-Tle), 2.79 (s br, 2H, NH2), 2.48 (s, 3H, CH3 thiazole), 2.29 (ddd, J = 13.3, 8.6, 4.4 Hz, 1H, CH2 Hyp), 2.12 (ddt, J = 13.3, 7.7, 1.8 Hz, 1H, CH2 Hyp), 0.91 (s, 9H, C(CH3)3).
13C NMR (126 MHz, CDCl3) δ (ppm): 173.90 (CONH Hyp), 171.70 (CONH Boc-L-Tle), 150.38 (CH thiazole), 148.49 (C thiazole), 138.46 (C–Ar), 131.71 and 130.83 (C–Ar, C thiazole), 129.48 and 127.92 (CH–Ar), 70.07 (CH Hyp), 60.54 (CH Boc-L-Tle), 58.97 (CH Hyp), 56.89 (CH2 Hyp), 43.05 (Ar–CH2–N), 37.16 (CH2 Hyp), 35.73 (C t-Bu Boc-L-Tle), 26.15 (CH3 t-Bu Boc-L-Tle), 16.15 (CH3 thiazole).
HRMS QToF-ESI: calculated for C22H31N4O3S [M + H+] m/z 431.2117; found m/z 431.2117.
| [α]D20 = +34.3 (c = 0.035 in MeOH). |
m.p.: 180–181 °C. Rf: 0.10 (5% MeOH/CH2Cl2).
FT-IR (KBr plate), νmax (cm−1): 3364, 3282, 3066, 2955, 2869, 1672, 1631, 1539, 1448, 1416, 1222, 1085, 968, 851, 835, 755.
1H NMR (500 MHz, CDCl3,; OH signal not evident in 1H NMR spectrum) δ (ppm): 8.60 (s, 1H, CH thiazole), 7.89 (d, J = 7.6 Hz, 1H, NHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 7.31 (s, 4H, Ar–H), 5.00 (p, J = 7.1 Hz, 1H, Ar–CH–N), 4.70 (dd, J = 9.5, 7.6 Hz, 1H, O
O), 7.31 (s, 4H, Ar–H), 5.00 (p, J = 7.1 Hz, 1H, Ar–CH–N), 4.70 (dd, J = 9.5, 7.6 Hz, 1H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N Hyp), 4.38 (s br, 1H, (CH2)CH–OH), 3.65 (d, J = 11.0 Hz, 1H, CH2, CH2 Hyp), 3.54 (dd, J = 10.9, 4.1 Hz, 1H, CH2 Hyp), 3.29 (s, 1H, O
C–CH–N Hyp), 4.38 (s br, 1H, (CH2)CH–OH), 3.65 (d, J = 11.0 Hz, 1H, CH2, CH2 Hyp), 3.54 (dd, J = 10.9, 4.1 Hz, 1H, CH2 Hyp), 3.29 (s, 1H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N Boc-L-Tle), 2.44 (s, 3H, CH3 thiazole), 2.25 (ddd, J = 12.8, 7.8, 4.8 Hz, 1H, CH2 Hyp), 2.02 (m, 1H, CH2 Hyp), 1.42 (d, J = 7.0 Hz, 3H, CH3), 0.98 (s, 9H, C(CH3)3).
C–CH–N Boc-L-Tle), 2.44 (s, 3H, CH3 thiazole), 2.25 (ddd, J = 12.8, 7.8, 4.8 Hz, 1H, CH2 Hyp), 2.02 (m, 1H, CH2 Hyp), 1.42 (d, J = 7.0 Hz, 3H, CH3), 0.98 (s, 9H, C(CH3)3).
13C NMR (126 MHz, CDCl3), δ (ppm): 173.97 (CONH Hyp), 170.51 (CONH Boc-L-Tle), 150.39 (CH thiazole), 148.49 (C thiazole), 143.78 (C–Ar), 131.76 (C thiazole), 130.77 (C–Ar), 129.59 and 126.43 (CH–Ar), 69.97 (CH Hyp), 60.53 (CH Boc-L-Tle), 58.76 (CH Hyp), 56.77 (CH2 Hyp), 48.96 (Ar–CH–N), 36.74 (CH2 Hyp), 35.77 (C t-Bu Boc-L-Tle), 26.24 (CH3 t-Bu Boc-L-Tle), 22.45 (CH3), 16.18 (CH3 thiazole).
HRMS QToF-ESI: calculated for C23H33N4O3S [M + H+] m/z 445.2273; found m/z 445.2276.
| [α]D20 = −128.0 (c = 0.025 in MeOH). |
m.p.: 78–80 °C. Rf: 0.45 (5% MeOH/CH2Cl2).
FT-IR (KBr plate) νmax (cm−1): 3305, 3076, 2978, 2930, 1672, 1546, 1409, 1162, 858, 755.
1H NMR (500 MHz, CD3OD) δ (ppm): 8.874 and 8.865 (each s, 1H, CH, CH thiazole major and minor rotamer17), 7.43 and 7.42 (each s, 4H, the major and minor rotamer,17 Ar–H), 4.63–4.20 (m, 4H, Ar–CH2–N, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N, (CH2)CH–OH), 3.64–3.54 (m, 1H, CH2 Hyp), 3.54–3.43 (m, 1H, CH2 Hyp), 2.47 (s, 3H, CH3 thiazole), 2.34–2.20 (m, 1H, CH2 Hyp), 2.03 (ddd, J = 13.1, 8.6, 4.5 Hz, 1H, CH2 Hyp), 1.47 and 1.33 (s each, 9H, OC(CH3)3, the major and minor rotamer17).
C–CH–N, (CH2)CH–OH), 3.64–3.54 (m, 1H, CH2 Hyp), 3.54–3.43 (m, 1H, CH2 Hyp), 2.47 (s, 3H, CH3 thiazole), 2.34–2.20 (m, 1H, CH2 Hyp), 2.03 (ddd, J = 13.1, 8.6, 4.5 Hz, 1H, CH2 Hyp), 1.47 and 1.33 (s each, 9H, OC(CH3)3, the major and minor rotamer17).
13C NMR (126 MHz, CD3OD, major rotamer17) δ (ppm): 175.56 (CONH), 156.17 (NCO), 152.92 (CH thiazole), 149.11 (C thiazole), 140.31 (C–Ar), 133.25 and 131.86 (C–Ar and C Thiazole), 130.51 and 129.69 (CH–Ar), 81.58 (C N-Boc), 70.05 (CH Hyp), 60.81 (CH Hyp), 56.00 (CH2 Hyp), 43.78 (Ar–CH2–N), 40.86 (CH2 Hyp), 28.54 (CH3 t-Bu N-Boc), 15.79 (CH3 thiazole).
HRMS QToF-ESI: calculated for C21H28N3O4S [M + H+] m/z 418.1801; found m/z 418.1808.
m.p: 89–91 °C. Rf: 0.40 (5% MeOH/CH2Cl2).
FT-IR (KBr plate) νmax (cm−1): 3409, 3072, 2977, 2932, 1664, 1542, 1413, 1162, 1092, 858, 829, 751.
1H NMR (500 MHz, CD3OD; the major and minor rotamer; NH and OH signals not evident in 1H NMR spectrum) δ (ppm): 8.88 (s, 1H, CH thiazole), 7.48–7.40 (s, 4H, Ar–H), 5.20–5.01 (m, 1H, Ar–CH–N), 4.48–4.21 (m, 2H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N, (CH2)CH–OH), 3.65–3.54 (m, 1H, CH2 Hyp), 3.54–3.42 (m, 1H, CH2 Hyp), 2.49 (s, 3H, CH3 thiazole), 2.29–2.19 (m, 1H, CH2 Hyp), 1.95 (ddd, J = 13.1, 8.7, 4.5 Hz, 1H, CH2 Hyp), 1.53 (d, J = 7.1 Hz, 3H, CH3), 1.49 and 1.43 (s each, 9H, OC(CH3)3, minor and major rotamer).
C–CH–N, (CH2)CH–OH), 3.65–3.54 (m, 1H, CH2 Hyp), 3.54–3.42 (m, 1H, CH2 Hyp), 2.49 (s, 3H, CH3 thiazole), 2.29–2.19 (m, 1H, CH2 Hyp), 1.95 (ddd, J = 13.1, 8.7, 4.5 Hz, 1H, CH2 Hyp), 1.53 (d, J = 7.1 Hz, 3H, CH3), 1.49 and 1.43 (s each, 9H, OC(CH3)3, minor and major rotamer).
13C NMR (126 MHz, CD3OD, major rotamer) δ (ppm): 174.63 (CONH), 156.17 (NCO), 152.86 (CH thiazole), 149.06 (C thiazole), 145.50 (C–Ar), 133.28 and 131.61 (C–Ar or C thiazole), 130.50 and 127.73 (CH–Ar), 81.54 (C N-Boc), 70.04 (CH Hyp), 60.53 (CH Hyp), 56.06 (CH2 Hyp), 49.86 (Ar–CH–N), 40.70 (CH2 Hyp), 28.64 (CH3 t-Bu N-Boc), 22.21 (CH3), 15.81 (CH3 thiazole).
HRMS QToF-ESI: calculated for C22H30N3O4S [M + H+] m/z 432.1957; found m/z 432.1956.
m.p.: 224–226 °C. Rf: 0.35 (5% MeOH/CH2Cl2).
FT-IR (KBr plate) νmax (cm−1): 3287, 3061, 2973, 2939, 2874, 1683, 1624, 1542, 1501, 1453, 1368, 1169, 837, 755.
1H NMR (500 MHz, CDCl3, the major and minor rotamer; OH signal not evident in 1H NMR spectrum) δ (ppm): 8.67 (s, 1H, CH thiazole), 7.65 (d, J = 7.9 Hz, 1H, NHC = O), 7.38 (d, J = 8.3 Hz, 2H, Ar–H), 7.36 (d, J = 8.3 Hz, 2H, Ar–H), 5.28 (d, J = 9.2 Hz, 1H, NH), 5.07 (p, J = 7.1 Hz, 1H, Ar–CH–N), 4.72 (t, J = 7.9 Hz, 1H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N Hyp), 4.48 (s br, 1H, ((CH2)CH–OH)), 4.22 (d, J = 9.3 Hz, 1H, O
C–CH–N Hyp), 4.48 (s br, 1H, ((CH2)CH–OH)), 4.22 (d, J = 9.3 Hz, 1H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N Boc-L-Tle), 4.00 (d, J = 11.4 Hz, 1H, CH2 Hyp), 3.59 (dd, J = 11.4, 3.7 Hz, 1H, CH2 Hyp), 3.44 (s, 1H, OH Hyp), 2.51 (s, 3H, CH3 thiazole), 2.43 (ddd, J = 12.8, 7.8, 4.6 Hz, 1H, CH2 Hyp), 2.05 (dd, J = 13.5, 8.1 Hz, 1H, CH2 Hyp), 1.46 (d, J = 6.9 Hz, 3H, CH2 Hyp), 1.40 (s, 9H, major rotamer, OC(CH3)3), 1.01 (s, 9H, C(CH3)3 Boc-L-Tle).
C–CH–N Boc-L-Tle), 4.00 (d, J = 11.4 Hz, 1H, CH2 Hyp), 3.59 (dd, J = 11.4, 3.7 Hz, 1H, CH2 Hyp), 3.44 (s, 1H, OH Hyp), 2.51 (s, 3H, CH3 thiazole), 2.43 (ddd, J = 12.8, 7.8, 4.6 Hz, 1H, CH2 Hyp), 2.05 (dd, J = 13.5, 8.1 Hz, 1H, CH2 Hyp), 1.46 (d, J = 6.9 Hz, 3H, CH2 Hyp), 1.40 (s, 9H, major rotamer, OC(CH3)3), 1.01 (s, 9H, C(CH3)3 Boc-L-Tle).
13C NMR (126 MHz, CDCl3, the major rotamer) δ (ppm): 173.0 (CONH Hyp), 169.7 (CONH Boc-L-Tle), 156.6 (NCO), 150.6, (CH thiazole) 148.5 (C thiazole), 143.3 (C–Ar), 131.8 and 130.9 (C–Ar or C thiazole), 129.7 and 126.6 (CH–Ar), 80.6 (C N-Boc), 70.2 (CH Hyp), 59.1 (CH Boc-L-Tle), 58.4 (CH Hyp), 56.6 (CH2 Hyp), 49.0 (Ar–CH–N), 35.4 (CH2 Hyp), 35.0 (C t-Bu Boc-L-Tle) 28.4 (CH3 N-Boc), 26.6 (CH3 t-Bu Boc-L-Tle), 22.4 (CH3), 16.1 (CH3 thiazole).
HRMS QToF-ESI: calculated for C28H41N4O5S [M + H+] m/z 545.2798; found m/z 545.2794.
m.p.: 108–110 °C. Rf: 0.10 (5% MeOH/CH2Cl2).
FT-IR (KBr plate) νmax (cm−1): 3312, 3077, 2925, 2862, 1653, 1520, 1417, 852, 807.
1H NMR (500 MHz, CD3OD) δ (ppm): 8.86 (s, 1H, CH thiazole), 7.42 (d, J = 8.0 Hz, 2H, Ar–H), 7.37 (d, J = 8.0 Hz, 2H, Ar–H), 4.43 (s, 2H, Ar–CH2–N), 4.36 (m, 1H, (CH2)CHOH), 3.94 (t, J = 8.2 Hz, 1H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N), 3.01 (dd, J = 11.9, 3.9 Hz, 1H, CH2 Hyp), 2.90 (d, J = 12.0 Hz, 1H, CH2 Hyp), 2.46 (s, 3H, CH3), 2.18 (dd, J = 13.3, 8.0 Hz, 1H, CH2 Hyp), 1.86 (ddd, J = 13.5, 8.6, 5.0 Hz, 1H, CH2 Hyp).
C–CH–N), 3.01 (dd, J = 11.9, 3.9 Hz, 1H, CH2 Hyp), 2.90 (d, J = 12.0 Hz, 1H, CH2 Hyp), 2.46 (s, 3H, CH3), 2.18 (dd, J = 13.3, 8.0 Hz, 1H, CH2 Hyp), 1.86 (ddd, J = 13.5, 8.6, 5.0 Hz, 1H, CH2 Hyp).
13C NMR (126 MHz, CD3OD) δ (ppm): 177.13 (CONH), 152.83 (CH thiazole), 149.10 (C thiazole), 140.34 (C–Ar), 133.26 and 131.68 (C–Ar or C thiazole), 130.46 and 128.97 (CH–Ar), 73.52 (CH Hyp), 60.78 (CH Hyp), 56.05 (CH2 Hyp), 43.37 (Ar–CH2–N), 41.01 (CH2 Hyp), 15.82 (CH3 thiazole).
HRMS QToF-ESI: calculated for C16H19N3O2S [M + H+] m/z 318.1276; found m/z 318.1287.
m.p.: 161–163 °C. Rf: 0.45 (5% MeOH/CH2Cl2).
FT-IR (KBr plate) νmax (cm−1): 3445, 3423, 3312, 3078, 2970, 2873, 1686, 1631, 1551, 1504, 1440, 1368, 1231, 1166, 763.
1H NMR (500 MHz, CDCl3, the major and minor rotamer;17 OH signal not evident in 1H NMR spectrum) δ (ppm): 8.67 (s, 1H, CH thiazole), 7.48 (t, J = 6.0 Hz, 1H, NHC = O), 7.33 (d, J = 8.3 Hz, 2H, Ar–H), 7.30 (d, J = 8.3 Hz, 2H, Ar–H), 5.23 (d, J = 9.1 Hz, 1H, NHC = O), 4.72 (t, J = 7.8 Hz, 1H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N Hyp), 4.53 (dd, J = 15.0, 6.5 Hz, 1H, Ar–CH2–N), 4.51 (m, s br, 1H, (CH2)CH–OH), 4.28 (dd, J = 15.0, 5.2 Hz, 1H, Ar–CH2–N), 4.17 (d, J = 9.2 Hz, 1H, O
C–CH–N Hyp), 4.53 (dd, J = 15.0, 6.5 Hz, 1H, Ar–CH2–N), 4.51 (m, s br, 1H, (CH2)CH–OH), 4.28 (dd, J = 15.0, 5.2 Hz, 1H, Ar–CH2–N), 4.17 (d, J = 9.2 Hz, 1H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N Boc-L-Tle), 3.98 (d, J = 11.2 Hz, 1H, 3.5 Hz, 1H, CH2 Hyp), 3.61 (dd, J = 11.3, 3.8 Hz, 1H, 3.5 Hz, 1H, CH2 Hyp), 2.49 (s, 3H, CH3 thiazole overlapping signal of H from CH2), 2.47–2.44 (m, overlapping signal of CH3, 1H, CH2 Hyp), 2.11–2.04 (m, 1H, CH2 Hyp), 1.39 (s, 9H, major and minor rotamer,17 OC(CH3)3), 0.91 (s, 9H, major rotamer,17 C(CH3)3 Boc-L-Tle).
C–CH–N Boc-L-Tle), 3.98 (d, J = 11.2 Hz, 1H, 3.5 Hz, 1H, CH2 Hyp), 3.61 (dd, J = 11.3, 3.8 Hz, 1H, 3.5 Hz, 1H, CH2 Hyp), 2.49 (s, 3H, CH3 thiazole overlapping signal of H from CH2), 2.47–2.44 (m, overlapping signal of CH3, 1H, CH2 Hyp), 2.11–2.04 (m, 1H, CH2 Hyp), 1.39 (s, 9H, major and minor rotamer,17 OC(CH3)3), 0.91 (s, 9H, major rotamer,17 C(CH3)3 Boc-L-Tle).
13C NMR (126 MHz, CDCl3, the major rotamer17) δ (ppm): 172.56 (CONH Hyp), 170.93 (CONH Boc-L-Tle), 156.636 (NCO), 150.48 (CH thiazole), 148.47 (C thiazole), 138.23 (C–Ar), 131.74 and 130.74 (C–Ar or C thiazole), 129.56 and 128.11 (CH–Ar), 80.39 (C N-Boc), 70.15 (CH Hyp), 58.97 (CH Boc-L-Tle), 58.59 (CH Hyp), 56.59 (CH2 Hyp), 43.28 (Ar–CH2–N), 36.11 (CH2 Hyp), 35.16 (C t-Bu Boc-L-Tle), 28.42 (CH3 N-Boc), 26.41 (CH3 t-Bu Boc-L-Tle), 16.10 (CH3 thiazole).
HRMS QToF-ESI: calculated for C27H39N4O5S [M + H+] m/z 531.2641; found m/z 531.2648.
m.p.:127–129 °C. Rf: 0.50 (5% MeOH/CH2Cl2).
FT-IR (KBr plate) νmax (cm−1): 3313, 2975, 2927, 1713, 1672, 1540, 1417, 1368, 1221, 759.
1H NMR (500 MHz, CD3OD, NH and OH signals not evident in 1H NMR spectrum) δ (ppm): 7.46 and 7.44 (d, J = 8.3 Hz, 2H, the major and minor rotamer,35 Ar–H), 7.24 (d, J = 8.3 Hz, 2H, and Ar–H), 4.58–4.12 (m, 4H, Ar–CH2–N, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N, (CH2)CH–OH), 3.61–3.52 (m, 1H, CH2 Hyp), 3.52–3.43 (m, 1H, CH2 Hyp), 2.23 (m, 1H, CH2 Hyp), 2.00 (ddd, J = 13.1, 8.6, 4.5 Hz, 1H, CH2 Hyp), 1.47–1.33, (s each, 9H, OC(CH3)3, minor and major rotamer34).
C–CH–N, (CH2)CH–OH), 3.61–3.52 (m, 1H, CH2 Hyp), 3.52–3.43 (m, 1H, CH2 Hyp), 2.23 (m, 1H, CH2 Hyp), 2.00 (ddd, J = 13.1, 8.6, 4.5 Hz, 1H, CH2 Hyp), 1.47–1.33, (s each, 9H, OC(CH3)3, minor and major rotamer34).
13C NMR (126 MHz, CD3OD, major rotamer34) δ (ppm): 175.53 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ONH), 156.14 (C
ONH), 156.14 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 139.29 (C–Ar), 132.60 and 130.99 (CH–Ar), 122.02 (C–Br), 81.58 (C N-Boc), 70.02 (CH Hyp), 60.74 (CH Hyp), 55.97 (CH2 Hyp), 43.48 (Ar–CH2–N), 40.80 (CH2 Hyp), 28.51 (CH3 t-Bu N-Boc).
O), 139.29 (C–Ar), 132.60 and 130.99 (CH–Ar), 122.02 (C–Br), 81.58 (C N-Boc), 70.02 (CH Hyp), 60.74 (CH Hyp), 55.97 (CH2 Hyp), 43.48 (Ar–CH2–N), 40.80 (CH2 Hyp), 28.51 (CH3 t-Bu N-Boc).
HRMS QToF-ESI: calculated for C17H23N2O4NaBr [M + Na+] m/z 421.0739; found m/z 421.0731.
m.p.: 153–155 °C. Rf: 0.45 (5% MeOH/CH2Cl2).
FT-IR (KBr plate) νmax (cm−1): 3309, 2977, 2935, 1740, 1660, 1548, 1414, 1366, 1228, 770.
1H NMR (500 MHz, CD3OD, the major and minor rotamer; NH and OH signals not evident in NMR spectra) δ (ppm): 7.48–7.44 (m, 2H, and Ar–H), 7.28 and 7.24 (d each, J = 8.3 Hz, 2H, major and minor rotamer, Ar–H), 4.98 (q, J = 7.0 Hz, 1H, Ar–CH–N), 4.39–4.27 (m, 2H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N, (CH2)CHOH), 3.59–3.51 (m, 1H, CH2 Hyp), 3.48–3.36 (m, 1H, CH2 Hyp), 2.30–2.11 (m, 1H, CH2 Hyp), 1.89 (ddd, J = 13.1, 8.8, 4.5 Hz, 1H, CH2 Hyp), 1.45 (d, J = 7.1 Hz, 3H, CH3), 1.48–1.36 (s each, 9H, OC(CH3)3, major and minor rotamer).
C–CH–N, (CH2)CHOH), 3.59–3.51 (m, 1H, CH2 Hyp), 3.48–3.36 (m, 1H, CH2 Hyp), 2.30–2.11 (m, 1H, CH2 Hyp), 1.89 (ddd, J = 13.1, 8.8, 4.5 Hz, 1H, CH2 Hyp), 1.45 (d, J = 7.1 Hz, 3H, CH3), 1.48–1.36 (s each, 9H, OC(CH3)3, major and minor rotamer).
13C NMR (126 MHz, CD3OD, major rotamer) δ (ppm): 174.58 (CONH), 156.12 (NCO), 144.51 (C–Ar), 132.58 and 129.10 (CH–Ar), 121.68 (C–Ar), 81.53 (C N-Boc), 70.01 (CH Hyp), 60.43 (CH Hyp), 56.02 (CH2 Hyp), 49.61 (Ar–CH2–N), 40.62 (CH2 Hyp), 28.62 (CH3 t-Bu N-Boc), 22.10 (CH3).
HRMS Q-tof-ESI: calculated for C18H25N2O4NaBr [M + Na+] m/z 435.0895; found m/z 435.0898.
m.p.: 112–113 °C. Rf: 0.10 (5% MeOH/CH2Cl2).
FT-IR (KBr plate) νmax (cm−1): 3299, 3058, 2980, 2928, 1642, 1542, 961, 840, 763, 737.
1H NMR (500 MHz, CD3OD, NH and OH signals not evident in NMR spectra) δ (ppm): 8.86 (s, 1H, CH thiazole), 7.43 (m, 4H, Ar–H), 5.04 (q, J = 7.0 Hz, 1H, Ar–CH–N), 4.37–4.35 (m, 1H, (CH2)CH–OH), 3.91 (t, J = 8.3 Hz, 1H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH–N), 3.06 (dd, J = 12.0, 4.2 Hz, 1H, CH2 Hyp), 2.89 (dt, J = 11.9, 1.8 Hz, 1H, CH2 Hyp), 2.47 (s, 3H, CH3 thiazole), 2.15 (ddt, J = 13.4, 7.8, 1.8 Hz, 1H, CH2 Hyp), 1.82 (ddd, J = 13.7, 8.7, 5.1 Hz, 1H, CH2 Hyp), 1.49 (d, J = 7.1 Hz, 3H, CH3).
C–CH–N), 3.06 (dd, J = 12.0, 4.2 Hz, 1H, CH2 Hyp), 2.89 (dt, J = 11.9, 1.8 Hz, 1H, CH2 Hyp), 2.47 (s, 3H, CH3 thiazole), 2.15 (ddt, J = 13.4, 7.8, 1.8 Hz, 1H, CH2 Hyp), 1.82 (ddd, J = 13.7, 8.7, 5.1 Hz, 1H, CH2 Hyp), 1.49 (d, J = 7.1 Hz, 3H, CH3).
13C NMR (126 MHz, CD3OD): δ (ppm) 174.38 (CONH), 151.48 (CH thiazole), 147.72 (C thiazole), 143.98 (C–Ar), 131.91 and 130.24 (C–Ar C thiazole), 129.14 and 126.27 (CH–Ar), 72.08 (CH Hyp), 59.27 (CH Hyp), 54.64 (CH2 Hyp), 48.33 (Ar–CH–N), 39.61 (CH2 Hyp), 21.00 (CH3), 14.46 (CH3 thiazole).
HRMS QToF-ESI: calculated for C17H22N3O2S [M + H+] m/z 332.1433; found m/z 332.1423.
Quantitative NMR product purity analysis. Compounds 1 and 2 were dissolved in anhydrous CDCl3 to make 0.041 M solutions. The quantitative NMR standard 1,2,4,5-tetrachloro-3-nitrobenzene (99.85% pure) was used as the internal standard in a ratio of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (m/m). 500 MHz 1H NMR spectra were obtained for each mixture with a relaxation delay of 16 s at 298 K for 1 and 313 K for 2. The purity of each compound was calculated by applying the following equation:
1 (m/m). 500 MHz 1H NMR spectra were obtained for each mixture with a relaxation delay of 16 s at 298 K for 1 and 313 K for 2. The purity of each compound was calculated by applying the following equation: 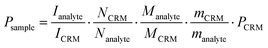 , where PSample = purity of the sample as mass fraction; PCRM = purity of the Certificated Reference Material (CMR) as mass fraction; IAnalyte = integration of the analyte signal; ICRM = integration of the CRM signal; NAnalyte = number of analyte protons; NCRM = number of CRM protons; MAnalyte = molecular weight of the analyte; MCRM = molecular weight of the CRM; mSample = mass of sample analysed; mCRM = mass of CRM analysed.
, where PSample = purity of the sample as mass fraction; PCRM = purity of the Certificated Reference Material (CMR) as mass fraction; IAnalyte = integration of the analyte signal; ICRM = integration of the CRM signal; NAnalyte = number of analyte protons; NCRM = number of CRM protons; MAnalyte = molecular weight of the analyte; MCRM = molecular weight of the CRM; mSample = mass of sample analysed; mCRM = mass of CRM analysed.
Author contributions
DMSM, GDC, JED, and JB conducted the synthesis experiments and completed compound characterization. DMSM and JED conducted quantitative NMR studies and authored the ESI.† DMSM and TSS wrote the original manuscript. JED and GDC reviewed and edited the manuscript. TSS conceptualized and supervised all studies. TSS, RCR, and CO acquired financial support. All authors have given approval of the final version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers: R21Al157362 and R21Al170213. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.Notes and references
- M. Bekes, D. R. Langley and C. M. Crews, Nat. Rev. Drug Discov., 2022, 21, 181–200, DOI:10.1038/s41573-021-00371-6.
- C. J. Diehl and A. Ciulli, Chem. Soc. Rev., 2022, 51, 8216–8257, 10.1039/d2cs00387b.
- N. Setia, H. T. A. Almuqdadi and M. Abid, Eur. J. Med. Chem., 2024, 265, 116041, DOI:10.1016/j.ejmech.2023.116041.
- K. R. Hornberger and E. M. V. Araujo, J. Med. Chem., 2023, 66, 8281–8287, DOI:10.1021/acs.jmedchem.3c00740.
- D. Mi, Y. Li, H. Gu, Y. Li and Y. Chen, Eur. J. Med. Chem., 2023, 256, 115444, DOI:10.1016/j.ejmech.2023.115444.
- A. Bricelj, Y. L. Dora Ng, D. Ferber, R. Kuchta, S. Muller, M. Monschke, K. G. Wagner, J. Kronke, I. Sosic, M. Gutschow and C. Steinebach, ACS Med. Chem. Lett., 2021, 12, 1733–1738, DOI:10.1021/acsmedchemlett.1c00368.
- D. K. Brownsey, B. C. Rowley, E. Gorobets, B. S. Gelfand and D. J. Derksen, Chem. Sci., 2021, 12, 4519–4525, 10.1039/d0sc05442a.
- P. Lu, L. Wang, M. Song and T. J. Hang, J. Pharm. Biomed. Anal., 2015, 114, 159–167, DOI:10.1016/j.jpba.2015.05.018.
- D. P. Bondeson, B. E. Smith, G. M. Burslem, A. D. Buhimschi, J. Hines, S. Jaime-Figueroa, J. Wang, B. D. Hamman, A. Ishchenko and C. M. Crews, Cell Chem. Biol., 2018, 25, 78.e75–87.e75, DOI:10.1016/j.chembiol.2017.09.010.
- H. Jiang, H. Xiong, S. X. Gu and M. Wang, Front. Chem., 2023, 11, 1098331, DOI:10.3389/fchem.2023.1098331.
- C. Galdeano, M. S. Gadd, P. Soares, S. Scaffidi, I. Van Molle, I. Birced, S. Hewitt, D. M. Dias and A. Ciulli, J. Med. Chem., 2014, 57, 8657–8663, DOI:10.1021/jm5011258.
- K. Raina, J. Lu, Y. Qian, M. Altieri, D. Gordon, A. M. Rossi, J. Wang, X. Chen, H. Dong, K. Siu, J. D. Winkler, A. P. Crew, C. M. Crews and K. G. Coleman, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 7124–7129, DOI:10.1073/pnas.1521738113.
- N. Ji, N. Mainolfi and M. Weiss, US Pat., WO2020/010210A1, 2020, 2020 Search PubMed.
- A. P. Crew, K. Raina, H. Dong, Y. Qian, J. Wang, D. Vigil, Y. V. Serebrenik, B. D. Hamman, A. Morgan, C. Ferraro, K. Siu, T. K. Neklesa, J. D. Winkler, K. G. Coleman and C. M. Crews, J. Med. Chem., 2018, 61, 583–598, DOI:10.1021/acs.jmedchem.7b00635.
- X. Han, C. Wang, C. Qin, W. Xiang, E. Fernandez-Salas, C. Y. Yang, M. Wang, L. Zhao, T. Xu, K. Chinnaswamy, J. Delproposto, J. Stuckey and S. Wang, J. Med. Chem., 2019, 62, 941–964, DOI:10.1021/acs.jmedchem.8b01631.
- J. Hu, B. Hu, M. Wang, F. Xu, B. Miao, C. Y. Yang, M. Wang, Z. Liu, D. F. Hayes, K. Chinnaswamy, J. Delproposto, J. Stuckey and S. Wang, J. Med. Chem., 2019, 62, 1420–1442, DOI:10.1021/acs.jmedchem.8b01572.
- M. Gütschow, C. Steinebach, S. A. Voell, L. P. Vu, A. Bricelj, I. Sosič and G. Schnakenburg, Synthesis, 2020, 52, 2521–2527, DOI:10.1055/s-0040-1707400.
- W. Yan, B. S. Pan, J. Shao, H. K. Lin and H. Y. Li, ACS Omega, 2022, 7, 26015–26020, DOI:10.1021/acsomega.2c00245.
- M. S. Cooper, M. C. Norley, S. Armitage, J. O. Cresser-Brown, A. K. Edmonds, S. Goggins, J. P. Hopewell, B. Karadogan, K. A. Knights, T. J. Nash, C. S. Oakes, W. J. O'Neill, S. J. Pridmore, H. J. Maple and G. P. Marsh, Org. Biomol. Chem., 2023, 21, 8344–8352, 10.1039/d3ob00983a.
- M. Wang, J. Lu, M. Wang, C. Y. Yang and S. Wang, J. Med. Chem., 2020, 63, 7510–7528, DOI:10.1021/acs.jmedchem.0c00471.
- L. Q. Hu, R. L. Deng, Y. F. Li, C. J. Zeng, D. S. Shen and F. S. Liu, Organometallics, 2018, 37, 214–226, DOI:10.1021/acs.organomet.7b00784.
- B. B. Ma, X. B. Lan, D. S. Shen, F. S. Liu and C. Xu, J. Organomet. Chem., 2019, 897, 13–22, DOI:10.1016/j.jorganchem.2019.06.016.
- D. Bensalah, L. Mansour, M. Sauthier, N. Gurbuz, I. Ozdemir, L. Beji, R. Gatri and N. Hamdi, RSC Adv., 2023, 13, 31386–31410, 10.1039/d3ra06334h.
- M. G. Organ, S. Avola, I. Dubovyk, N. Hadei, E. A. B. Kantchev, C. J. O'Brien and C. Valente, Chem.–Eur. J., 2006, 12, 4749–4755, DOI:10.1002/chem.200600206.
- C. J. O'Brien, E. A. B. Kantchev, C. Valente, N. Hadei, G. A. Chass, A. Lough, A. C. Hopkinson and M. G. Organ, Chem.–Eur. J., 2006, 12, 4743–4748, DOI:10.1002/chem.200600251.
- S. Caddick, D. B. Judd, A. K. d. K. Lewis, M. T. Reich and M. R. V. Williams, Tetrahedron, 2003, 59, 5417–5423, DOI:10.1016/s0040-4020(03)00858-5.
- S. W. Heinzman and B. Ganem, J. Am. Chem. Soc., 1982, 104, 6801–6802, DOI:10.1021/ja00388a063.
- A. Giannis and K. Sandhoff, Angew Chem. Int. Ed. Engl., 2003, 28, 218–220, DOI:10.1002/anie.198902181.
- G. Amberchan, R. A. Snelling, E. Moya, M. Landi, K. Lutz, R. Gatihi and B. Singaram, J. Org. Chem., 2021, 86, 6207–6227, DOI:10.1021/acs.joc.0c03062.
- S. A. Campos, J. D. Harling, A. H. Miah, I. E. David Smith, US Pat., WO2014/108452A1, 2014 Search PubMed.
- D. L. Boger, S. Miyazaki, S. H. Kim, J. H. Wu, S. L. Castle, O. Loiseleur and Q. Jin, J. Am. Chem. Soc., 1999, 121, 10004–10011, DOI:10.1021/ja992577q.
- B. Cao, H. Park and M. M. Joullie, J. Am. Chem. Soc., 2002, 124, 520–521, DOI:10.1021/ja017277z.
- Trituration using cold MTBE prompted some product loss due to partial dissolution of the ammonium chloride salt of 1. No dissolution of the hydrochloride salt of 2 was observed using MTBE.
- N. R. Babij, E. O. McCusker, G. T. Whiteker, B. Canturk, N. Choy, L. C. Creemer, C. V. De Amicis, N. M. Hewlett, P. L. Johnson, J. A. Knobelsdorf, F. Z. Li, B. A. Lorsbach, B. M. Nugent, S. J. Ryan, M. R. Smith and Q. Yang, Org. Process Res. Dev., 2016, 20, 661–667, DOI:10.1021/acs.oprd.5b00417.
- H. Ding, A. Shigenaga, K. Sato, K. Morishita and A. Otaka, Org. Lett., 2011, 13, 5588–5591, DOI:10.1021/ol202316v.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra, additional experimental procedures, and photographs and characterization data of isolated impurities from C–H arylation reactions. See DOI: https://doi.org/10.1039/d4ra01974a |
| This journal is © The Royal Society of Chemistry 2024 |