 Open Access Article
Open Access ArticleThe synergistic effect of adsorption and Fenton oxidation for organic pollutants in water remediation: an overview
Junzhe Song†
ab,
Linan Zhu†c,
Sheng Yu*c,
Guobiao Li b and
Dong Wang
b and
Dong Wang *ab
*ab
aKey Laboratory of Green Process and Engineering, National Engineering Research Center of Green Recycling for Strategic Metal Resources, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: wangdong@ipe.ac.cn
bGanjiang Innovation Academy, Chinese Academy of Sciences, Ganzhou, 341007, China
cSchool of Mechanical and Materials Engineering, Washington State University, Pullman, WA 99164, USA. E-mail: shengyuacdemic@163.com
First published on 22nd October 2024
Abstract
Water pollution from industrial sources presents a significant environmental challenge due to the presence of recalcitrant organic contaminants. These pollutants threaten human health and necessitate effective remediation strategies. This article reviewed the synergistic application of adsorption and Fenton oxidation for water treatment. Adsorption, a common technique, concentrates pollutants onto a solid surface, but offers limited degradation. Fenton oxidation, an advanced oxidation process (AOP), utilizes hydroxyl radicals for efficient organic compound breakdown. When adsorption and Fenton oxidation combine, adsorption pre-concentrates pollutants, boosting Fenton oxidation effectiveness. This review delves into the mechanisms and advantages of this integrated approach, highlighting its potential for enhanced removal of organic contaminants. The discussion encompasses the mechanisms of Fenton oxidation and the synergistic effects it has with adsorption. Additionally, various support materials employed in this combined process are explored, including carbon-based supports (activated carbon, graphene, carbon nanotubes and biochar), metal–organic frameworks (MOFs), and clays. Finally, the applicability of this approach to diverse wastewater streams, such as medical and industrial wastewater, is addressed. The review contains 105 references and summarizes the key findings and future perspectives for this promising water remediation technology.
1 Introduction
Nowadays, with the fast growth of industries including the chemical industry, agriculture and animal husbandry, food processing and manufacturing industry, textile waste treatment and pharmaceutical manufacturing, the large number of organic contaminants in wastewater that are related to these industries have caused severe environmental problems.1–3 Indeed, most of these organic pollutants are not only toxic but also difficult to be degraded in natural conditions. Thus, these organic pollutants could always penetrate the barrier of the human body through breathing or the food chain, resulting in health problems such as cardiovascular system disease, nervous system disease, immune system diseases, and respiratory system diseases. Therefore, the removal of toxic organic pollutants is still a major challenge in upcoming years.4–6 In the recent decade, various methods have been developed for the removal of toxic organic pollutants, and adsorption stands out among them because of its ease of use, low cost, speed of pollution removal, and minimal sludge creation.7,8 Adsorption is basically a separation-based technique (a phase-changing technology) that just redirects the pollutants from water to another phase, producing sorbents that are contaminated and need to be disposed of in the side stream or subjected to additional treatment to prevent the possibility of secondary contamination.Particularly, the advanced oxidation processes (AOPs) constitute a promising technique for the treatment of toxic organic pollutants due to its highly oxidizing ability. Indeed, AOPs is a technology to effectively oxidize toxic and non-degradable compounds based on intermediary of hydroxyl and other radicals. AOPs are very commonly used to treat pharmaceutical wastewater within last decade. AOPs contains different approaches such as Fenton, ozonation, sonolysis, photocatalysis and wet air oxidation. The Fenton reaction is a kind of advanced oxidation process (AOPs). It combines H2O2 with Fe2+, which has a strong oxidation ability in acidic solutions and can effectively degrade organic pollutants in the wastewater field.9 Compared with other AOP, the Fenton reaction has the following advantages, making it one of the most promising strategies:10
(i) Easy to operate: the Fenton reaction is simple to operate and does not require complex equipment or conditions. This makes it more convenient for practical applications.
(ii) Fast and non-polluting: the Fenton reaction has a fast reaction rate and does not produce harmful by-products. This helps efficiently treat organic pollutants in water bodies.
(iii) High degradation efficiency: the Fenton reaction can effectively degrade organic pollutants, including refractory compounds. Its strong oxidation ability makes it perform well in water purification.
Therefore, Fenton reaction has broad potential in the removal of environmental pollutants, for example, it can be used to treat the removal of pollutants in soil: Fenton reaction can effectively degrade organic pollutants by generating hydroxyl radicals (˙OH). Secondly, Fenton reaction can also convert organic dyes and other pollutants in industrial waste into harmless products. In addition, the Fenton reaction can also degrade antibiotic residues in wastewater: by reacting with hydrogen peroxide, the hydroxyl radicals generated by the Fenton reaction can effectively decompose antibiotic molecules.
As Fenton reaction is of great significance in pollutant treatment, and recent studies have shown that the synergistic effect of adsorption and Fenton reaction can greatly improve the efficiency of pollutant interpretation. However, there have been no review articles on the synergistic effects of adsorption and Fenton reaction in recent years. Therefore, as a review paper, this article reviews the recent research on the synergistic effect of adsorption and Fenton reaction in the field of water pollutant treatment. It is of great importance for researchers in related fields and practitioners in the field of wastewater treatment.
Even though adsorption is an effective method of treating waste water, it could be limited by adsorbent material which would cause more cost and the properties of adsorbents would also affect the whole process. Combining different water treatment techniques would help enhance the treatment performance and lower the cost and treatment time. Due to the significant chemical input, Fenton oxidation when used alone can be costly and damaging to the environment.11 Dual-functional materials are being researched more and more as heterogeneous catalysts and adsorbents to address these issues.7,8,11 These substances have the capacity to simultaneously remove contaminants through Fenton oxidation (degradation-based removal) and adsorption (separation-based removal). These composites' dual activity allows for the coupling of Fenton oxidation and adsorption for enhanced treatment effectiveness while lowering the costs and restrictions of separate procedures (via material renewal and more pollutant removal). Additional light input (photo-Fenton), which has also been investigated, has the potential to improve heterogeneous Fenton processes even more. By utilizing the same components, these integrated treatments can be applied both in situ (without separating the adsorbent) and ex situ (after separating the adsorbent). This can be done by combining or impregnating multiple materials to create a hybrid material that has the dual functionality, or by employing a single material that serves both purposes.
Also, the limitations of both processes can be addressed by the integration. Adsorption requires huge amount of absorbent and regeneration of spent absorbents. AOPs are designed to degrade adsorbed organic pollutants which also regenerating adsorbent. Thus, AOPs can occur with adsorption simultaneously which can ensure continuous supply of adsorbent.
It should be noted that in addition to toxic organic pollutants, heavy metals such as Cr, Hg, Cu, Pb, etc.12 are also typical pollutants in water. However, due to limited space and the existing recent review article in this field, this paper only introduced the combination of Fenton oxidation process and adsorption in removal of non-biodegradable organic compounds, and different materials could be used in the support of combination of adsorption and Fenton process, such as carbon-based material, graphitic carbon nitride and MOFs are discussed. Furthermore, the applications are involved such as medical wastewater, industrial wastewater and other types of wastewaters.
2 Mechanism of Fenton oxidation and the synergistic effects of adsorption
For the mechanism of the Fenton reaction, researchers have put forward many theories, but there are still some disputes. However, the strong oxidation of ·OH in the Fenton reaction process is supported by most scholars. Fenton reaction is mainly formed by two reactions. Firstly, Fe2+ and H2O2 react to produce ·OH, which degrades organic pollutants, as shown in eqn (1)–(4). Secondly, Fe3+ generated by oxidation will undergo a series of reactions with H2O2 and can be reduced to Fe2+ as shown in eqn (5) and (6). Specific reactions are as follows:13| Fe2+ + H2O2 → Fe3+ + HO− + ·OH | (1) |
| ·OH + RH → R· + H2O | (2) |
| R· + Fe3+ → R+ + Fe2+ | (3) |
| ·OH + Fe2+ → ·OH + Fe3+ | (4) |
| Fe3+ + H2O2 → Fe⋯OOH2+ + H+ | (5) |
| Fe⋯OOH2+ → Fe2+ + HO· | (6) |
Due to the limitations of Fenton process, Fenton could be modified with different conditions such as photo-Fenton, electro-Fenton and photo-electro-Fenton. The combination of photo/electro with tradition Fenton greatly improved the reaction efficiency, and their pathways of promotion are illustrated in the following.
2.1 Photo-Fenton
Since the 1990s, photo heterogeneous Fenton reactions have been used for the degradation of structured compounds.14 The photo Fenton reaction can be briefly summarized as follows. Iron acts as a catalyst and cycles through oxidation and reduction, and Fe2+ reacts with H2O2 in a very rapid reaction to produce ·OH, which is oxidized to Fe3+. The Fe3+ can absorb light in the range of ultraviolet light–visible light while producing ·OH, which is reduced again to Fe2+, as shown in the reaction formula (7). The iron reduction can also occur in the dark, as shown in eqn (8). Since eqn (7) is much faster than eqn (8), light can increase the reaction rate. Thus, the process depends largely on the concentration of iron and the wavelength of the light.15,16| Fe3+ + H2O + hν → Fe2+ + ·OH + H+ | (7) |
| Fe3+ + H2O2 → Fe2+ + HO2· + H+ | (8) |
2.2 Electro-Fenton
Electrochemical advanced oxidation processes (EAOPs) are considered to be one of the most promising advanced oxidation technologies, among which the high efficiency and cleanliness of the electric-Fenton process have been extensively studied.17 The reaction mechanism can be interpreted as that H2O is oxidized at the anode to produce ·OH (see eqn (9)), and O2 is reduced at the cathode to produce H2O2 (see eqn (10)). At the same time, Fe2+ consumed in the Fenton reaction can be reduced to Fe2+ at the cathode through Fe3+ (see eqn (11)).18| H2O → ·OH + H+ + e− | (9) |
| O2 + 2H+ + 2e− → H2O2 | (10) |
| Fe3+ + e− → Fe2+ | (11) |
A large amount of iron(II) salt is required in traditional Fenton compared to electro-Fenton. Due to the regeneration of Fe2+ in electro-Fenton, the electro-Fenton process overcomes the shortcomings of the classical Fenton process, including avoiding the use of large amounts of Fe2+ and the formation of large amounts of ferric hydroxide sludge.19
2.3 Photo-electro-Fenton
Photo-electro-Fenton technology irradiates artificial UV-visible light or natural sunlight into the electric Fenton system based on the electric Fenton technology. Compared with the electric Fenton technology, the photoelectric Fenton technology can improve the mineralization efficiency of organic pollutants and has increasingly prominent advantages in the treatment of organic wastewater. Moreover, the solar light resource is cheap and renewable, so they can greatly save the treatment cost of organic pollutants.Photo-electro-Fenton (PEF) process is based on photochemical/photocatalytic actions assisted by UV irradiation. The intensity and wavelength of incident light affects the destruction rate of organic pollutants. The photo-electro-Fenton contains simultaneous support of electrogenerated H2O2, Fe2+ and UV illumination of the solution to destruct organic pollutants. In PEF process, photoreduction of Fe(OH)2+ and the predominant Fe3+ species in acid medium and/or the photolysis of complexes of Fe(III) with generated carboxylic acids would increase the amount of hydroxyl radical produced. The UV irradiation enhance the process of electro-Fenton which helps produce Fe2+ according to eqn (9)–(11). The synergistic effect of photo and electro Fenton provides better efficiency of decomposing organic pollutants.
It is known that cathodic two-electron reduction:
| Fe(OH)2+ + hν → Fe2+ + ·OH | (12) |
| Fe(OOCR)2+ + hν → Fe2+ + CO2 + ·R | (13) |
| H2O2 + hν → 2·OH | (14) |
However, the application of artificial ultraviolet light for the homogeneous photo-Fenton reaction also has disadvantages. In addition to the system's restriction on the acidity and alkalinity of solution, the use of the artificial ultraviolet light requires an external power supply and the electrical cost is relatively high, which hinders the application of homogeneous photo-Fenton technology in the industrial processing.20 The solar photovoltaic Fenton technology overcomes this shortcoming well. Solar Fenton technology refers to the use of sunlight irradiation solution (usually λ > 300 nm), greatly saving the application cost. In addition, this solar-assisted electro-Fenton technology has better degradation and mineralization effects on organic pollutants than the original photo-Fenton technology, because the combination of powerful ultraviolet input from sunlight and photolysis with λ > 400 nm enhances decarboxylation of Fe3+ carboxylate species.21 This solar-assisted electro Fenton technology also has fatal shortcomings, because the daily sunshine duration varies in different regions and climates, and the intensity of solar sunlight is greatly affected by the weather and the cycle of four seasons, so the practical application of this method is also limited.
2.4 Synergistic effect of Fenton and adsorption
Generally, Fenton reactions can be classified into homogeneous and heterogeneous Fenton reactions. Homogeneous Fenton reactions include classical, modified Fenton reactions (sono-Fenton, photo-Fenton, electro-Fenton, sono-electro-Fenton, photo-electro-Fenton, sono-photo-Fenton, and solar photoelectron-Fenton), Heterogeneous Fenton reactions use recyclable solid catalysts instead of Fe2+ which helps overcome the disadvantages of homogeneous Fenton such as narrowed pH range.5 The homogeneous system can only produce a good reaction in pH = 2.5–3.5. When pH > 4, a large amount of Fe(OH), commonly known as iron mud, will be produced.22 A relatively narrow pH range not only limits the application of the Fenton reaction but also produces a large amount of iron mud which forms secondary pollution and increases the treatment cost.4 Therefore, the homogeneous Fenton reaction has not been applied to industrial wastewater treatment on a large scale due to the cost and reaction conditions. On the contrary, the heterogeneous Fenton catalyst provides an advantage of long lifetime which effectively reduces the concentration of iron bivalent. Iron ions are slowly leached, so the working pH range will be expanded, which will not cause secondary pollution to the environment.Besides, various functional materials or support can also provide more active sites for the Fenton catalyst, thus showing higher catalytic activity and accelerating Fe(III)/Fe(II) redox cycle. While this can be ascribed to the synergistic effect of between Fenton and adsorption. The synergistic effect come from the effective adsorption of pollutants onto heterogeneous Fenton catalysis via ion exchange, electrostatic interactions, hydrophobic attractions, π–π stacking, hydrogen bonding, and/or van der Waals forces interactions, as well as the acceleration of electron transfer during the Fenton reaction, thus enhancing the whole process efficiency.
The synergistic effect of Fenton and adsorption can be studied through its improvement and limitations. Some research vividly shows that the combination of Fenton and adsorption can improve the removal percentage. In Gvoic's research, the decolorization percentage for homogeneous and heterogeneous Fenton process was 79% and 54% respectively with 59% and 33% COD removal. With the addition of adsorption, a huge enhance of the process is discovered. The toxicity reduction reached 95% and decolorization achieved 90%. Thus, it would be extremely beneficial to combine Fenton and adsorption (Fig. 1).23
 | ||
| Fig. 1 Proposed adsorption mechanism of yellow dye degradation products on biochar (this figure has been reproduced with permission from Gvoic et al.23 from Springer, copyright 2024). | ||
In the future, the performance and life of new Fenton catalysts and catalyst support will be the focus of the research. The new Fenton technology is expected to be more efficient and environmentally friendly in the application of large-scale industrial wastewater.
3 Supports used in the combination of adsorption and Fenton oxidation process
The integration of adsorption with the Fenton oxidation paradigm is chiefly evident in the heterogeneous Fenton process.24 The foundational premise of this combined methodology lies in the adsorptive capture of pollutants onto specific substrates, facilitated by a spectrum of mechanisms such as ion exchange, electrostatic interactions, hydrophobic attractions, π–π stacking, hydrogen bonding, and van der Waals forces interactions.25 Substrates that undergo functionalization to increase their adsorptive sites can foster accelerated electron transfer during the Fenton reaction, culminating in an enhanced process efficiency.26 This synergistic mechanism obviates the need for excessive dosages and mitigates the production of iron-rich sludge, a residual byproduct typically linked with the traditional homogeneous Fenton process.27 Furthermore, this approach adeptly navigates the limitations inherent to the conventional homogeneous Fenton process, particularly those related to aquatic environmental parameters such as pH.28 Owing to the advantages conferred by these combined interactions, support materials have emerged as a focal point of scholarly exploration. A comprehensive analysis of contemporary research indicates that the primary substrates utilized as supports include carbon-based materials,29 MOFs,30 and minerals/clay.31 Ensuing discussions will delve into these material classifications and synthesize the avant-garde design philosophies that characterize them.3.1 Carbon based materials
Activated carbon (AC) is widely used for pollutant adsorption due to its economic efficiency, large specific surface area per unit volume, significant surface reactivity, and strong affinity for various types of dissolved organic compounds.35 It can also serve as a substrate for simultaneous adsorption and Fenton oxidation processes. In this context, activated carbon assumes a dual function: it serves as an adsorbent and plays a crucial role in facilitating the Fenton reaction, which is typically conducted in sequential stages.29 By acting as a support matrix for essential Fenton reaction elements such as iron, activated carbon increases the reaction's effectiveness while preserving its inherent adsorption capabilities. AC is used extensively across various Fenton reactions, including traditional Fenton processes, electro-Fenton, photo-Fenton, and sono-Fenton methodologies. This versatility underscores its adaptability and effectiveness in various advanced oxidation processes. The Fenton reaction involves the interaction between hydrogen peroxide (H2O2) and ferrous ions (Fe2+) to generate hydroxyl radicals (˙OH) for the degradation of organic pollutants. This reaction results in the oxidation of Fe2+ to ferric ions (Fe3+), which are subsequently reduced back to Fe2+, thus perpetuating the cycle.12 Contemporary research has focused on integrating the Fenton oxidation process with activated carbon (AC) adsorption for the remediation of various pollutants. For instance, Cui J. et al. concentrated on the removal of residual organic compounds in manganese (Mn) electrochemical solutions.36 Their findings revealed that employing a dose of 3.75 g L−1 of AC at a temperature of 70 °C for 120 minutes significantly enhanced the Chemical Oxygen Demand (COD) removal rate to 93.1% during the activated carbon stage (see Fig. 2).
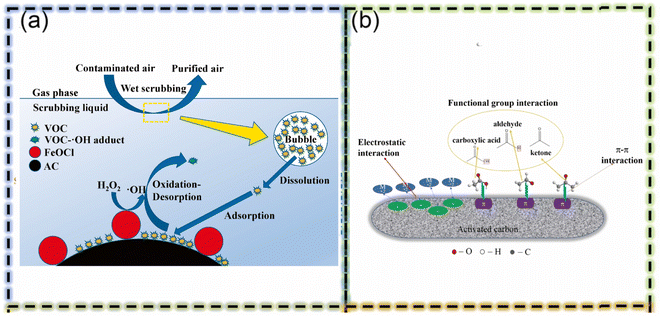 | ||
| Fig. 2 (a) Schematic illustration of DCE removal by wet scrubbing using FeOCl/AC.36 (b) The possible mechanism of Mn2+ adsorption over AC36 (this figure has been reproduced with permission from Najm et al.35 from Elsevier, copyright 2020). | ||
Indeed, several studies explored modifying activated carbon (AC) with magnetic properties for improved water treatment. Duan introduced an innovative method for producing magnetic activated carbon (MAC) by immersing porous activated carbon in iron oxide, followed by the co-precipitation of ferrous and ferric iron onto the carbon's surface.29 This modification led to the degradation of over 90% of a 4-chlorophenol solution within an hour, with a consistent removal efficiency above 80% over five regeneration cycles. This enhancement indicates a significant improvement in AC's adsorption capacity for iron following magnetic modification, thereby maintaining high efficiency over multiple cycles. Amir' group developed a nanocomposite, Fe3O4 magnetic activated carbon (Fe3O4@AC), using a co-precipitation method. Characterized by its pseudo-spherical shape, strong magnetic field, and large surface area, Fe3O4@AC demonstrated high reusability and chemical stability. It showed efficient oxidation performance over a wide pH range.37
In addition, activated carbon can be used in traditional Fenton reactions in combination with a fluidized bed reactor. Bello et al. explored the effectiveness of granular activated carbon (GAC) in a fluidized bed Fenton process for removing COD and color from wastewater.28 In conjunction with a fluidized bed reactor, GAC expanded the surface area, promoting iron oxide crystallization and enhanced pollutant adsorption. When compared to the SiO2 fluidized carrier, the GAC process exhibited a 20% improvement in removal efficiency and was efficient in removing COD within a pH range of 3 to 7, potentially obviating the need for conventional acidification and neutralization stages. Lyu et al. designed an integrated system that combined adsorption with Fenton oxidation in a fluidized bed reactor for dye removal.38 Activated carbon not only facilitated dye adsorption but also served as a site for iron precipitation and crystallization, acting as a heterogeneous catalyst. Fe3+ produced during the Fenton reaction precipitated on the activated carbon surface as FeO(OH) or Fe(OH)3, which could be recovered and reused as a catalyst. The remaining Fe3+ was adsorbed on the activated carbon as organic iron complexes, minimizing iron hydroxide sludge production in the reactor. The above studies illustrate the key role of activated carbon as an adsorbent and carrier in enhancing Fenton reaction efficiency. Its synergistic effect in the Fenton process helps improve the overall efficiency of the reaction. Thus, the optimization of the Fenton reaction can be achieved by combining it with other catalytic methods. One particularly straightforward and promising approach is to combine the Fenton reaction with electrical energy, often called the electro-Fenton process. The Fenton reaction's core is the electron transfer cycle, which is driven by the redox cycling of iron ions between the Fe2+ and Fe3+ states, facilitated by hydrogen peroxide. In the electro-Fenton process, introducing an external electric field can directly affect the electron transfer cycle, and the rate of electron gain and loss can be controlled through it. This new approach conforms to the basic principles of redox chemistry and provides a promising way to optimize the degradation of organic pollutants in various environmental remediation applications. The integration of activated carbon with the electro-Fenton reaction has led to significant advancements in the field, as evidenced by recent works. Researchers are developing improved Electro-Fenton (EF) technologies for wastewater treatment. One key advancement is the ACSS cathode, a cost-effective design that combines activated carbon (for H2O2 generation and adsorption) with stainless steel mesh (for current distribution). This approach proved efficient in dye removal over multiple cycles.39 Another promising direction is integrating EF with adsorption, as demonstrated by Godínez et al.'s reactor design. This setup combines porous carbon electrodes, granular activated carbon, and Fe(II) ions for enhanced H2O2, hydroxyl radical generation, and organic pollutant removal (Fig. 3).40
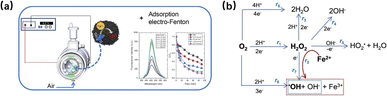 | ||
| Fig. 3 (a) Side view of the electrochemical reactor air vent valve, (b) equations describing the chemical and electrochemical reactivity of H2O2.40 | ||
This innovative setup utilized a cation-exchange resin to supply Fe(II) ions to an activated carbon adsorption bed positioned between two porous carbon electrodes. This configuration synergistically combined activated carbon adsorption with the electro-Fenton process, leading to effective H2O2 and hydroxyl radical (OH) generation and the removal of model dyes from both synthetic and real aqueous solutions. The system demonstrated that the introduction of pure oxygen could enhance OH formation. Furthermore, the compaction level of activated carbon within the reactor significantly affected its adsorption and electrochemical performance, with denser packing leading to increased surface area and electrical conductivity. Song et al. developed an innovative flow-through heterogeneous electro-Fenton (FHEF) reactor to improve mass transfer for the efficient and stable degradation of trimethoprim (TMP).41
These studies illustrated above collectively highlight the versatility and efficacy of activated carbon in enhancing electro-Fenton processes, offering promising avenues for the efficient removal of pollutants and advancing environmental remediation technologies. The exploration of combining activated carbon with optical Fenton processes, such as photo-Fenton, represents another promising direction for enhancing reaction efficiency in environmental remediation. Given its ability to utilize natural sunlight as an energy source, photo-Fenton offers an economical and environmentally friendly alternative to the electro-Fenton process. The work of Babaei et al. serves as a notable example in this context.42 They employed a direct chemical co-precipitation method to embed magnetic nanoparticles (MNPs) onto powdered activated carbon, creating magnetically recyclable composites (MNPs@C) specifically designed for the eradication of Direct Red 16 (DR16) in water-based systems. These MNPs@C composites, characterized by a mesoporous structure and a zero-point charge (pHzpc) of approximately 6.2, demonstrated a catalytic efficiency of 47% during the UV-Fenton process. This efficiency indicates that MNPs@C effectively collaborates with UV radiation and hydrogen peroxide (H2O2) to enhance catalytic activity for DR16 degradation. In a related study, Jiang Z.-Y. and his team developed composites of activated carbon fiber and cobalt ferrite (ACF/CoFe2O4) using a hydrothermal method.43 The CoFe2O4 nanoparticles were uniformly deposited onto activated carbon fibers, leading to a synergistic interaction between the components. This integration resulted in a reduction of the bandgap of CoFe2O4 nanoparticles from 1.82 eV to 1.62 eV, enhancing the photocatalytic activity. The effective collaboration of CoFe2O4 and ACF within the composite boosted the catalytic potential of the photo-Fenton agents. The observed changes in the valence states of Co and Fe in the composite suggest that both Co3+/Co2+ and Fe3+/Fe2+ redox cycles contribute to the Fenton-like reactions. This composite was found to be efficient in producing hydroxyl radicals in the photo-Fenton system, thereby facilitating the oxidative degradation of atrazine (ATZ), a commonly used herbicide.
This review will now focus on studying graphene and carbon nanotubes in the context of ordinary Fenton reactions, following an exploration of activated carbon materials. Like activated carbon, these advanced materials are of significant interest for their potential to enhance the efficiency of Fenton-based processes.
As illustrated in Table 1, several graphene and carbon nanotube based supports are investigated in recent years.
| Pollutants | Supports | Ref. |
|---|---|---|
| Methylene blue | CNF-GO-Fe aerogel | 47 |
| Sulfadiazine | 3D-GN@nZVI | 45 |
| Methylene blue | Fe3O4/RGO-2 | 48 |
| Mitoxantrone | rGO/Fe NPs | 49 |
| Phenol | GO@Fe3O4 | 32 |
| αE2 | FeNPs/rGO | 50 |
| Tetracycline | Fe3O4/3D-PU-G | 51 |
| Sulfadiazine | Pt1-FeOX/G | 52 |
| Phenol | OCNT-Fe | 53 |
| Acyclovir | MMT/rGO/Fe3O4 | 54 |
| Rhodamine B | GF@CuS–Fe3O4 | 55 |
Sajab et al. created a highly effective porous cellulose nano-fibrils (CNF) aerogels amalgamated with graphene oxide-iron (III) nano-composites (CNF-GO-Fe aerogel) for removing methylene blue from water.47 It combines superior adsorption with Fenton oxidation (adding iron significantly boosts efficiency), achieving double the removal rate compared to adsorption alone. This innovative material shows promise for industrial wastewater treatment due to its reusability. Another recent work introduced a promising method for cleaning water by combining adsorption with a built-in Fenton-like reaction.45 Their design uses iron nanoparticles trapped within a 3D graphene network (3D-GN@nZVI). This material effectively removes sulfadiazine (SDZ) from water through a combination of adsorption and degradation, since the 3D-GN matrix's exemplary electronic conductivity precipitated the formation of multiple micro-electrolytic cells encircling the nZVI particles. This research shows promise for future water treatment technologies prehensive adsorption and reaction with SDZ and its derivatives.
Zhang et al. synthesized colloidal Fe3O4 nanoparticles through a hydrothermal process and subsequently immobilized them on a three-dimensional graphene aerogel framework.48 This process of adherence, driven by electrostatic interactions, was facilitate entirely through colloidal coagulation effects (CCE), obviating the need for auxiliary bonding agents. The Fe3O4/RGO-2 composite exhibited an impressive adsorption capacity, with its adsorption isotherms and kinetics analysis for Methylene Blue (MB) revealing a maximum adsorption capacity of 163.83 mg g−1. The catalytic activity in the heterogeneous Fenton reaction was predominantly surface-centric, with minimal iron leaching, attributable to the synergistic interplay between zero-dimensional Fe3O4 nanoparticles and graphene nano-sheets. This amalgamation rendered Fe3O4/RGO-2 a highly efficacious adsorbent and heterogeneous Fenton catalyst. The composite's enhanced performance under alkaline conditions, leading to complete MB degradation within 60 minutes, is particularly noteworthy. Besides, a highly effective system with reduced graphene oxide and iron nanoparticles (rGO/Fe NPs) for removing mitoxantrone (MTX) from water, was also developed.49 This combination of reduced graphene oxide and iron nanoparticles achieves a remarkable 99.8% MTX removal rate at neutral pH. The secret lies in its ability to generate hydroxyl radicals, which effectively target MTX over other contaminants. Additionally, Wang et al. addressed the challenge of phenol contamination by synthesizing Fe3O4 nanocrystals onto graphene oxide (GO) nanosheets, thereby creating a GO@Fe3O4 composite.32 This novel composite emerged as a potent catalyst in phenol degradation, with a key mechanism involving the adsorption of phenol molecules at the Fe3O4-GO interface through coordination and hydrogen bonds, pivotal for facilitating non-radical degradation pathways. In the Fenton reaction, the presence of H2O2 significantly enhanced the GO@Fe3O4 composite's catalytic efficiency, underscoring its potential in diverse environmental remediation applications. Another interesting work explored a method for removing αE2 from water using FeNPs/rGO.50 Their findings suggest that π–π bonds form between αE2 and rGO, while the oxidation process alters the FeNPs, promoting the generation of hydroxyl radicals. This dual mechanism-adsorption and Fenton-like oxidation-significantly improves αE2 removal efficiency. In 2023, Hong et al. addressed the challenges associated with traditional Fenton-like catalysts like Fe3O4, particularly its limited operational pH range, metal leaching, and suboptimal catalytic efficiency.51 The proposed Fe3O4/three-dimensional (3D) graphene composite, Fe3O4/3D-PU-G (H), synthesized through a solvothermal process followed by mild post-heating, exhibited an exceptional performance. This was largely attributed to the formation of a stable, compact 3D porous conductive network, enhancing electron transfer between Fe species and H2O2 and thereby facilitating the generation of hydroxyl radicals (˙OH). This advancement in the Fe3O4/3D-PU-G composite technology marks a pivotal step in environmental remediation, enabling rapid degradation of tetracycline without the need for external energy inputs.
Graphene and carbon nanotubes not only play the traditional role of adsorbents and carriers in the field of Fenton reactions. These nanomaterials manifest exceptional adaptability and efficiency when integrated into specialized Fenton methodologies, namely electro-Fenton and photo-Fenton reactions. The electro-Fenton process harnesses electrical energy to amplify the production of hydroxyl radicals, while the photo-Fenton reaction exploits light, often of solar origin, to activate the catalyst. The unique electrical and optical properties inherent to graphene and carbon nanotubes significantly augment the efficacy of these processes. Their integration not only enhances the degradation efficiency of organic pollutants but also paves the way for more energy-efficient and environmentally sustainable remediation techniques. As a result, these nanomaterials represent a seminal advancement in the field of environmental remediation, particularly in scenarios where conventional Fenton reactions may encounter limitations.
For instance, Song et al. demonstrated this by creating Pt-FeOX single atoms on graphene (Pt1-FeOX/G).52 This catalyst efficiently generates H2O2, crucial for the degradation process. The study highlights the role of material structure in performance (Fig. 4). Notably, using single atoms reduces material consumption and enhances efficiency. Additionally, the excellent conductivity of graphene and carbon nanotubes benefits the electro-Fenton process. Similarly, iron-doped oxidized carbon nanotubes (OCNT-Fe) as a catalyst for electro-Fenton applications also developed.53 Oxygen groups on the OCNT surface aid in Fe(II) regeneration, improving efficiency. Notably, using a Fe catalyst avoids the need for dissolved iron (Fe2+), expanding the operational pH range and reducing sludge formation compared to traditional Fenton processes. This method generates hydroxyl radicals (˙OH) via a continuous oxygen reduction reaction (ORR), forming H2O2 as an intermediate that decomposes into ˙OH. The process relies solely on environmentally friendly O2 and H2O, eliminating the need for additional H2O2. Also, the combination of iron oxide nanoparticles with reduced graphene oxide and montmorillonite (MMT/rGO/Fe3O4) could for the catalyst for 3D electro-Fenton systems.54 This approach leverages the strengths of each material for efficient acyclovir (ACV) degradation. Since it produces more hydroxyl radicals and facilitates Fe(II) regeneration compared to traditional Fenton methods. However, limitations like corrosion and stability require further solution. A recent work reported by Matos and colleagues, synthesized nanocomposites comprising graphene sheets (GF) combined with CuS, Fe3O4, and CuS–Fe3O4 (GF@CuS–Fe3O4).55 These nanocomposites, specifically GF@Fe3O4, GF@CuS, and GF@CuS–Fe3O4, were fabricated through the amalgamation of two or three different components, each possessing complementary effects, aimed at enhancing the efficiency and stability of the photocatalyst. Within this ensemble, Fe3O4 assumes the role of a Fenton-type catalyst, CuS functions as a visible light photocatalyst, and GF serves as a charge carrier. Fe3O4 and/or CuS nanoparticles were directly anchored to GF without the need for additional chemical treatments. The adsorption and photocatalytic properties of these GF-based nanocomposites were meticulously investigated for the removal of rhodamine B (RhB) contaminants at room temperature. This research comprehensively demonstrates the synergistic effect arising from the combination of graphene and carbon nanotubes in the photo-Fenton reaction, ultimately leading to a marked improvement in catalytic efficiency.
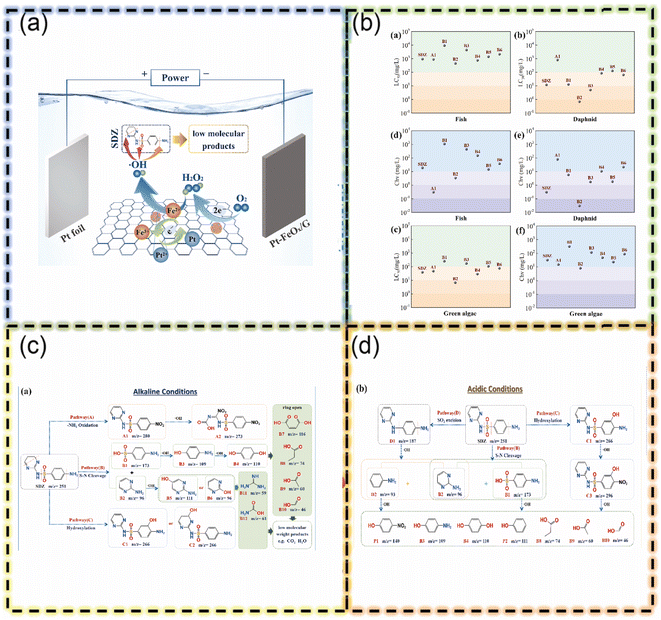 | ||
| Fig. 4 (a) Schematic diagram of SDZ degradation in the heterogeneous EF system with Pt1-FeOX/G cathode. (b) Acute toxicity (a–c) and chronic toxicity (d–f) of SDZ and its generated products established via ECOSAR in conjunction with trophic aquatic organisms (fish, daphnids, and green algae) (for interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article). Proposed degradation pathways for SDZ in the heterogeneous EF system with Pt1-FeOX/G cathode under alkaline condition (c) and acidic condition (d) (this figure has been reproduced with permission from Song et al.52 from Elsevier, copyright 2024). | ||
Recent work of g-C3N4 based support material in Fenton reactions actively participates through several mechanisms: g-C3N4's unique structure makes it effective in electro-Fenton and photo-Fenton processes as well. Its rich nitrogen atoms readily capture transition metals for catalysis. Also, the double C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in g-C3N4 improve current efficiency compared to graphene under electrical stimulation. This allows for in situ H2O2 generation at the cathode, crucial for electro-Fenton degradation. For instance the g-C3N4 could form Fe–N coordination complex: Wang et al. showed g-C3N4's π bonds form a complex with Fe(III) ions, creating oxygen vacancy (OV) oxides.33 These oxides enhance electron transfer between Fe and nearby H2O2, leading to O2 evolution. Further research explored co-doping g-C3N4 with metals like Na and Fe can improve catalytic activity56,57 by (i) increased active sites: co-doping creates more sites for Fe and enhances electron transfer; (ii) metal synergy: copper, for example, accelerates the rate-limiting step and boosts H2O˙ generation.58–60
C bonds in g-C3N4 improve current efficiency compared to graphene under electrical stimulation. This allows for in situ H2O2 generation at the cathode, crucial for electro-Fenton degradation. For instance the g-C3N4 could form Fe–N coordination complex: Wang et al. showed g-C3N4's π bonds form a complex with Fe(III) ions, creating oxygen vacancy (OV) oxides.33 These oxides enhance electron transfer between Fe and nearby H2O2, leading to O2 evolution. Further research explored co-doping g-C3N4 with metals like Na and Fe can improve catalytic activity56,57 by (i) increased active sites: co-doping creates more sites for Fe and enhances electron transfer; (ii) metal synergy: copper, for example, accelerates the rate-limiting step and boosts H2O˙ generation.58–60
Biochar derived from plant biomass is gaining traction in Fenton reactions due to its ability to combine adsorption and catalysis. Alizadeh et al. created a biochar-based nanocomposite (MC@nano-Fe3O4) by incorporating magnetic nanoparticles into microbial cellulose.34 This material effectively removes tetracycline (TC) through adsorption onto its high surface area and subsequent degradation via electron transfer between metal ions in the biochar, mimicking a Fenton-like process. The composite's impressive performance and reusability make it a promising candidate for water treatment. In a study of similar import, Wang et al. exemplify this through the use of corncob biochar as a precursor to synthesize a cost-effective magnetic iron–copper bimetallic nanomaterial (MBC), targeted for the removal of ciprofloxacin (CIP) from aqueous solutions.66 The innovative synthesis of MBC involves a combination of coprecipitation and pyrolysis techniques. Crucially, the presence of H2O2 in the aqueous solution activates MBC, resulting in a significant enhancement of its adsorption capacity for CIP in the MBC/H2O2 system during the Fenton reaction. This leads to a comprehensive interaction between CIP and the magnetic iron–copper bimetallic components on the MBC material through direct adsorption. Subsequently, the degradation of CIP is achieved via electron transfer and redox cycles among the metal ions. This process epitomizes a typical synergistic effect, where catalytic degradation is seamlessly coupled with adsorption, thereby illustrating the potential of biochar-based materials in advanced environmental remediation techniques (Fig. 5).
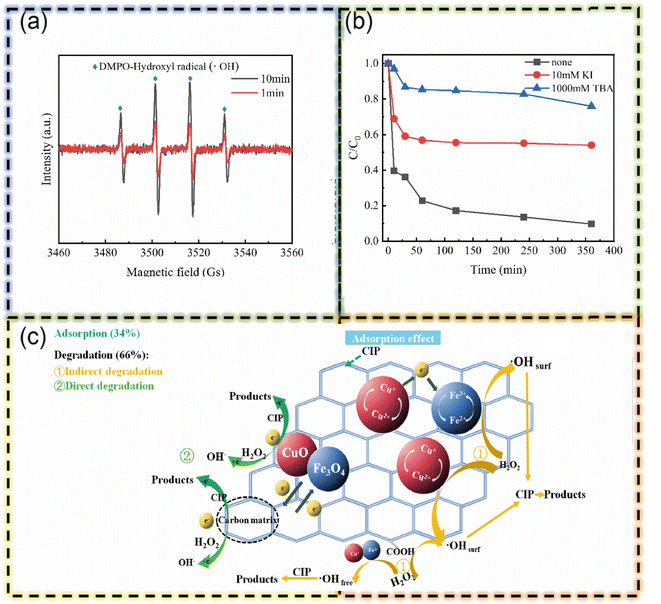 | ||
| Fig. 5 (a) Comparison of EPR spectra at 1 min and 10 min in the MBC/H2O2. (b) Effect of TBA or KI scavengers on the removal of CIP in the MBC/H2O2. (c) Mechanisms of CIP removal in the MBC/H2O2 (this figure has been reproduced with permission from Wang H. et al.66 from Elsevier, copyright 2020). | ||
Inspired by algae-based biochar, Yu et al. created biochar/iron oxide composites from Aegagropila linnaei algae (AL) for BPA removal.67 The cost-effective AL is activated with KOH to form a biochar with a high surface area, then functionalized with ferrous sulfate to introduce Fe3O4 nanoparticles. This composite effectively removes BPA through a combination of adsorption and Fenton-like reactions triggered by the iron oxide. This approach highlights the potential of biochar/iron oxide composites for efficient BPA removal.
While biochar is derived from a variety of sources, its modification bestows it with advantageous electrochemical properties, notably improved conductivity and reduced impedance, which are essential for its integration into the electro-Fenton process For instance, Deng et al. created a nitrogen-doped biochar cathode (B@Ni-F) from waste giant trees.68 This cathode effectively degrades sulfadiazine (SMR) by combining adsorption and Fenton-like reactions. This research highlights the potential of biochar for sustainable wastewater treatment (Fig. 6).
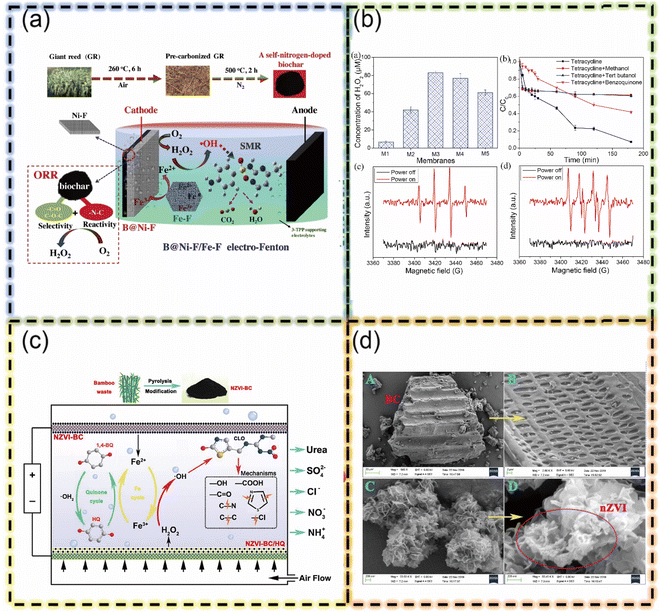 | ||
| Fig. 6 (a) B@Ni-F cathode in electro-Fenton for sulfamerazine degradation (b) concentration of electro-generated H2O2 by EF catalytic membranes (c) the ecotoxicity evaluation of degradation products indicated that CLO degradation in 3D–ICE–EF system (d) FE-SEM characterizations of BC and NZVI–BC (this figure has been reproduced with permission from Deng F. et al.68 from Elsevier, copyright 2019). | ||
Biochar derived from waste materials like bamboo and agricultural residue shows promise for wastewater treatment via electro-Fenton processes.69,70 These biochar electrodes effectively remove pollutants through a combination of adsorption and Fenton-like reactions. For example, bamboo waste as a source of biochar with composed of zero-valent iron nanoparticle hybrids (NZVIeBC) could efficiently removes clothianidin (CLO) by first adsorbing it and then degrading it through an electro-Fenton reaction with zero-valent iron nanoparticles.69 Meanwhile, utilizing Fe/N co-doped biochar (Fe-g-C3N4/biochar) membrane degrades tetracyclines while minimizing membrane fouling.70 Its high activity stems from abundant functional groups and nitrogen species, promoting efficient hydroxyl radical generation. These studies highlight biochar's potential for sustainable and adaptable electro-Fenton treatment of organic pollutants in wastewater.
While biochar-enhanced electro-Fenton processes effectively treat pollutants, integrating biochar with renewable energy sources like light offers a more promising path towards even more sustainable water treatment. Exploring biochar in photo-Fenton remediation could unlock new avenues for degradation using clean and abundant solar energy. This approach aligns with sustainability goals and potentially offers greater efficiency and lower environmental impact than traditional methods.
Other biochar supports such as starch and red mud also plays importance roles in Fenton reaction. Cai et al. designed a novel starch-derived carbon (SC)-modified and copper-doped (Fe, Cu)S/CuFe2O4 (CFS/CFO@SC) composite for efficient 17α-ethinylestradiol (EE2) removal. The unique structure of CFS/CFO@SC facilitates high EE2 adsorption and promotes efficient charge transfer for photogenerated electron–hole separation. This synergistic effect, combining adsorption and photo-Fenton mechanisms, enables efficient EE2 degradation. Their work highlights the potential of integrating adsorption and photocatalysis for organic pollutant treatment.71 Lin et al. leverage biochar's adsorption and photocatalysis for efficient dye removal.73 Their iron-containing red mud biochar (RMBC) effectively adsorbs Acid Orange 7 (AO7) dye. Upon light irradiation, Fe in RMBC undergoes redox cycling, generating hydroxyl radicals (˙OH) that degrade AO7 via photo-Fenton reactions. This study highlights the potential of biochar for integrating adsorption and photocatalysis in wastewater treatment. The integration of biochar with various Fenton reactions, employing a synergistic approach of initial adsorption followed by Fenton processing, is pivotal in enhancing the overall reaction efficiency. This principle holds across different applications of biochar, whether in traditional Fenton, electro-Fenton, or photo-Fenton reactions. A critical aspect of this synergy is the enhancement of the material's adsorption capacity. The ability of biochar to effectively adsorb pollutants sets the stage for the subsequent Fenton reaction. Equally crucial is the promotion of the redox cycle of Fe2+/Fe3+ within the system, which is the core of the Fenton process, generating reactive species that are essential for pollutant degradation.
Carbon materials exhibit high adsorption capacity, effectively removing pollutants from water, and can anchor Fenton catalytic ions, such as iron, thereby enhancing Fenton reaction efficiency. This dual functionality allows carbon materials to serve both as exceptional adsorbents and optimal matrices for integrating catalysts, including iron ions. Additionally, their inherent electrical conductivity facilitates the integration with electrical and photonic energies, thereby improving electron mobility and the overall efficiency of Fenton processes.
3.2 MOFs
In developing new materials for adsorption and Fenton catalysis, four factors are crucial: a strong intrinsic adsorption capacity for initial pollutant capture, the ability to bind or support catalytic metals like iron, high electrical conductivity for efficient electron transfer, and an abundance of functional groups to chelate metals, thereby enhancing catalytic activity. These functional groups not only stabilize metal ions but also position them optimally for enhanced catalytic activity, leading to the formation of robust and active catalytic sites. Such considerations ensure efficient pollutant degradation and robust Fenton catalysis, making carbon materials highly valuable in environmental decontamination efforts. Drawing from the aforementioned insights, it is apparent that Metal–Organic Frameworks (MOFs) thereby eliciting considerable interest within the scientific fraternity. Several types of MOFs have been investigated in Fenton reactions, and the relevant literature in this review are listed in the followed table. (See Table 2).| MOF-based adsorbent/catalyst | Target pollutants | Operational conditions | Mechanism | Ref. | |
|---|---|---|---|---|---|
| Adsorption | Fenton | ||||
| NH2-MIL-88B | Pefloxacin | pH: 6, C0: 0.5 g L−1 and T: 30 °C, adsorbent/catalyst dosage: 0.5 g L−1, H2O2: 8 mM | π–π stacking/electrostatic/functional group interaction | ˙OH | 74 |
| MFC@Mn–NH2-UiO-66@IL | Methylene blue | pH: 6, C0: 50 mg L−1, T: 25 °C, adsorbent/catalyst dosage: 0.5 g L−1, time: 250 min, H2O2: 30 mM | π–π stacking/electrostatic/hydrogen bonds interaction | ˙OH | 75 |
| Fe/Ni@ZIF-8 | Ofloxacin | pH: 6.32, C0: 10 mg L−1, T: 25 °C, adsorbent/catalyst dosage: 0.5 g L−1, time: 210 min, H2O2: 10 mM | π–π stacking/electrostatic interaction | ˙OH | 76 |
| MnxCo3−x@C | Ciprofloxacin | pH: 3, C0: 20 mg L−1, T: 25 °C, I: 20 mA, time: 60 min, Fe2+: 0.02 mm, Na2SO4: 0.05 M | π–π stacking/electrostatic/hydrogen bonds interaction | Electron transfer/HO2˙/O2˙−/˙OH | 77 |
| xFe-N/C | Carbamazepine | pH: 7, C0: 5 mg L−1, T: 25 °C, I: 15 mA, catalyst dosage: 50 mg L−1, time: 100 min | π–π stacking/electrostatic/hydrogen bonds interaction | Electron transfer 1O2/O2˙−/˙OH | 78 |
| FeNi1/15-BDC | Methylene blue/methyl orange | pH (methylene blue): 6.5, pH (methyl orange): 6.9, C0: 40 mg L−1, T: 30 °C, adsorbent/catalyst dosage: 0.1 g L−1, time: 40 min, H2O2: 10 mM | π–π stacking/electrostatic/interaction | ˙OH/1O2/h+ | 44 |
| Co0.8Fe0.2N-NC | Methylene blue/methyl orange | pH (methylene blue): natural pH, pH (methyl orange): natural pH, C0: 20 mg L−1, T: 25 °C, adsorbent/catalyst dosage: 0.1 g L−1, time: 40 min, H2O2: 0.75 mL, 300 W fluorescent lamp | π–π stacking/electrostatic/hydrogen bonds interaction | ˙OH/˙O2−/h+ | 17 |
| MOFs (2Fe/Co)/CNF | Methylene blue/erythromycin | pH (methylene blue): 3, pH (erythromycin): 3, C0 (methylene blue): 50 mg L−1, C0 (erythromycin): 20 mg L−1, T: 25 °C, adsorbent/catalyst dosage: 0.1 g L−1, time: 30 min, 500 W Xe lamp | π–π stacking/electrostatic/interaction | ˙OH | 79 |
At their core, MOFs consist of two fundamental components: metallic ions or their clusters, and organic segments, often referred to as linkers. These organic segments predominantly manifest as bi-, tri-, or tetradentate ligands.80,81 A notable aspect of MOF synthesis is the utilization of homogeneous ligands, albeit with varying metallic constituents.82 Such versatility facilitates the precise modulation of MOF pore dimensions, extending from microporous to mesoporous frameworks, through the strategic selection of diverse metallic elements.83 This attribute of tunability bestows upon MOFs the capacity to meticulously customize their pore size and architecture. Their adaptable pore interfaces confer a distinct advantage to MOFs, particularly in applications centered around adsorption, enabling the selective entrapment of various guest molecules distinguished by their unique functional groups. Recent studies highlight MOFs' ability to adsorb and neutralize environmental pollutants, emphasizing their extensive porosity. Iron-based MOFs, for example, have intrinsic Fenton catalytic capabilities, eliminating the need for additional metals. Organic ligands enhance catalytic sites by chelating supported metals, opening new research avenues in doping MOFs with diverse elements for efficient catalysts. These properties make MOFs promising for adsorption and Fenton reactions.84 In the realm of environmental remediation, Fe-based Metal–Organic Frameworks (MOFs) have emerged as promising catalysts due to the widely recognized efficacy of iron as a catalyst in the Fenton reaction. In their notable study, Ma H et al. explored the use of functional NH2-MIL-88B MOFs, which exhibit both adsorption and Fenton oxidation capabilities, for the degradation of pefloxacin in aqueous solutions.74 This integrated approach marries the benefits of adsorption with those of advanced oxidation processes. The intrinsically strong adsorption capacity of the MOF material plays a crucial role in this methodology. It facilitates the mass transfer of organic pollutants to the surface of the material designed for advanced oxidation. Once on the surface, the advanced oxidation process, leveraging the Fenton reaction, effectively degrades the adsorbed organic pollutants. The synergy between adsorption and Fenton oxidation in the Fe-based MOF (NH2-MIL-88B) framework leads to the successful removal of organic pollutants, either through adsorption or through Fenton-based oxidative degradation. This study by Ma H. et al. underscores the potential of MOFs, particularly Fe-based variants, in environmental cleanup strategies. The dual functionality of these MOFs, enabling both adsorption and catalytic degradation, presents a compelling case for their application in the efficient and effective removal of contaminants from aqueous environments.
Metal–organic frameworks (MOFs) inherently contain Fenton catalytic metals, providing them with natural Fenton catalytic capabilities. The functional groups on MOFs facilitate efficient loading of catalytic metals, increasing active sites and enhancing effectiveness. Interaction mechanisms such as electrostatic interactions, hydrogen bonding, and π–π stacking significantly boost the adsorption efficiency for organic pollutants. These interactions between MOF functional groups and pollutants lead to more effective capture and degradation of contaminants. Simple modifications can enhance both adsorption capacity and Fenton catalytic efficiency in MOFs, creating a synergistic effect. This synergy results in a combined impact that exceeds the sum of individual processes, making the dual enhancement more effective than separate applications of adsorption and Fenton catalysis.
In their groundbreaking research, Lu R. et al. utilized UiO-66 series metal–organic frameworks (MOFs) as the foundational substrate, leveraging their renowned chemical and hydrothermal stability. These MOFs underwent a sophisticated functionalization process with Mn(II)-doped ionic liquids (ILs), culminating in the formation of Fe3O4@Zr-MOFs featuring core–shell structures. This innovative approach was adeptly applied to the removal of methylene blue (MB) from wastewater.75 The enhanced adsorptive capacity of this MOF material in aqueous environments can be attributed not merely to its layered and porous structure, which provides a high surface area and porosity, but also to the electrostatic interactions between the MOF's functional groups and MB. The adsorption capacity is further augmented by hydrogen bonding and π–π stacking interactions. Additionally, the Fe3O4 supported by the MOF plays a pivotal role in catalyzing the Fenton reaction. The incorporation of zirconium (Zr) within the MOF framework is instrumental in facilitating electron transfer between Fe and Zr, leading to the creation of dual active sites for Fenton-like reactions. This synergy results in the rapid decomposition of H2O2 in the Zr-MOF and Fe3O4 systems, producing hydroxyl radicals for the oxidative breakdown of MB. This study exemplifies a prototypical synergistic interaction between adsorption and Fenton reactions.
The MOF material, characterized by its multi-layered porous structure and the presence of specialized functional groups, exhibits a remarkable pollutant adsorption capacity. This is further enhanced by electrostatic interactions, hydrogen bonding, and π–π stacking, surpassing the adsorption efficiencies of previously discussed carbonaceous materials.
Moreover, the inclusion of Zr in the MOF establishes dual active centers with Fe3O4, significantly enhancing the Fenton reaction. This research underscores the inherent Fenton catalytic potential of the metals contained within the MOF material. Diverging from the carbon-based materials earlier mentioned, MOFs serve not only as supports for adsorbents and catalysts but also as active catalysts in their own right. Furthermore, MOFs can be utilized as catalyst supports to develop multiple catalytic cores, thus enhancing catalytic efficiency. The dual functionality of MOFs as both adsorbents and catalysts, coupled with their ability to house multiple catalytic sites, markedly improves the synergistic efficacy of adsorption and Fenton reactions. In pursuit of augmenting Fenton efficiency, the approach of bimetal doping emerges as a promising strategy. In this context, Zhang T. et al. conducted a groundbreaking study by doping Ni and Fe bimetals into a Zn-containing Zeolitic imidazolate frameworks (ZIFs) material, ZIF-8. This innovative endeavor led to the creation of the Fe/Ni@ZIF-8 composite material, which demonstrated remarkable efficacy in the oxygen Fenton oxidation of ofloxacin (OFX).76 The mechanism underlying the removal of OFX via Fe/Ni@ZIF-8 involves a two-pronged approach: initially, OFX is adsorbed onto the Fe/Ni@ZIF-8 composite through a combination of π–π bond intercalation and electrostatic interactions. Following this adsorption, the adsorbed OFX undergoes oxidative degradation, mediated by hydroxyl radicals (˙OH) generated through the Fenton reaction, catalyzed by the Fe/Ni bimetallic nanoparticles supported on ZIF-8 in the presence of H2O2. This process of OFX removal using the Fe/Ni@ZIF-8 composite exemplifies a typical outcome of the synergistic effect combining ZIF-8 adsorption and Fe/Ni Fenton-like oxidation. The study not only highlights the enhanced catalytic efficiency achieved through bimetal doping in MOFs but also underscores the potential of these composite materials in advanced oxidation processes (Fig. 7).
 | ||
| Fig. 7 Reusability of Fe/Ni@ZIF-8 in adsorption and Fenton-like oxidation (a); practical removal efficiency of OFX from three wastewater by Fe/Ni@ZIF-8 under non-Fenton (0–120 min) and Fenton-like (>120 min) conditions (b) (this figure has been reproduced with permission from Zhang T. et al.76 from Elsevier, copyright 2022). | ||
Metal–organic frameworks (MOFs) excel in integrating adsorption and Fenton reactions. Their large surface area, porous structure, and functionalizable groups enhance adsorption efficiency through mechanisms like electrostatic interactions, hydrogen bonding, and π–π stacking. MOFs with Fenton-active metals like iron possess inherent catalytic abilities. Their functional groups chelate metal ions, enabling efficient loading of Fenton-active metals and creating multiple active centers that boost Fenton efficiency. MOFs demonstrate superior Fenton activity compared to other carbon materials. This synergy of strong adsorption and robust Fenton catalysis makes MOFs highly effective for pollutant removal, simplifying and enhancing environmental cleanup. Furthermore, MOFs are compatible with other catalytic modalities, notably electro-Fenton catalysis. Their conductive framework, porous structure, and high surface area make them suitable as electrode materials or supports for electrocatalysts, facilitating electron transfer processes crucial in electrocatalysis. In electro-Fenton systems, MOFs enhance the electrochemical generation of Fenton reagents, boosting hydroxyl radical production for efficient pollutant degradation. In the context of advanced Fenton processes, the electro-Fenton system presents a significant advancement, particularly in terms of operational efficiency and cost-effectiveness. A key feature of this system is its ability to generate hydrogen peroxide (H2O2) in situ via the two-electron oxygen reduction reaction (ORR) on the cathode. This capability circumvents the need for continuous addition of H2O2 to the reaction mixture, thereby reducing operational expenses.19 An additional noteworthy feature of metal–organic frameworks (MOFs) that further augments their utility in Fenton processes is their excellent electrical conductivity. When MOFs are subjected to an electric field, the conductivity of the material facilitates an accelerated movement of electrons within the framework. Metal–organic frameworks (MOFs) enhance Fenton processes due to their excellent electrical conductivity, which accelerates electron movement under an electric field. This increased electron mobility boosts the generation of reactive species like hydroxyl radicals (˙OH), which are central to the Fenton reaction, thereby improving pollutant degradation rates. This is particularly beneficial in electro-Fenton systems, where MOFs' rapid electron transfer processes synergize with electrochemical aspects, enhancing overall efficiency. In a notable study, Huang S. et al. developed Mn/Co MOF derivatives (MnxCo3−x@C-GF) for ciprofloxacin (CIP) treatment. By adjusting the Mn/Co ratio in the MOF-74 precursor, they achieved a hierarchical porous structure, which enhanced adsorption and electrochemical activity. This structure facilitated rapid CIP adsorption and faster electron transfer, improving Mn2+/3+/4+ and Co3+/2+ Fenton-like reactions, generating more active radicals (˙OH) and thus enhancing degradation performance. This study highlights MOFs' potential in electro-Fenton processes, creating efficient, cost-effective, and environmentally sustainable pollutant degradation solutions.77 Similarly, Guo J. et al. encapsulated an iron precursor in a ZIF-8 cage, forming a single-atom Fe-N/C structure after calcination. This MOF efficiently adsorbs carbamazepine (CBZ) and rapidly degrades it via hydroxyl radicals (˙OH) and oxygen (O2) generated from H2O2 reacting with single-atom iron sites. This study demonstrates the use of MOFs for single-atom catalysts, showcasing the synergistic effects of adsorption and electro-Fenton processes. The material effectively activates H2O2 over a wide pH range, offering new insights into MOFs' potential for environmental remediation and advanced pollutant degradation. Both studies exemplify the innovative use of MOFs in enhancing electro-Fenton processes and creating multifunctional catalysts for environmental cleanup (Fig. 8).78
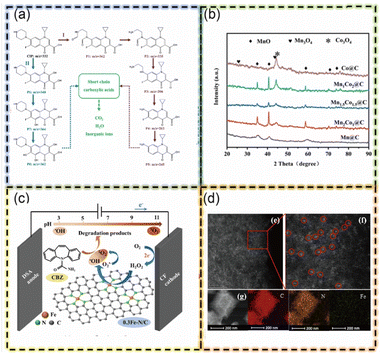 | ||
| Fig. 8 (a) Proposed pathways for degradation of CIP. (b) XRD patterns of MnxCo3−x@C (this figure has been reproduced with permission from Huang S. et al.77 from Elsevier, copyright 2022). (c) Schematic diagram of possible CBZ degradation mechanism by 0.3Fe-N/C/hetero-EF. (d) HAADF-STEM and corresponding element maps of 0.3Fe-N/C (this figure has been reproduced with permission from Guo J. et al.78 from Elsevier, copyright 2022). | ||
Beyond their significant role in electro-Fenton reactions, Metal–Organic Frameworks (MOFs) have also garnered widespread interest in the realm of photo-Fenton processes. In photo-Fenton reactions, MOFs can act as both catalysts and supports for catalysts, leveraging light energy to enhance the generation of hydroxyl radicals (˙OH) through the Fenton reaction. The versatility of MOFs allows for the tuning of their optical properties, making them responsive to different wavelengths of light. This adaptability is crucial in photo-Fenton processes, as it enables the activation of the Fenton reaction under light irradiation, potentially expanding the range of operational conditions and enhancing the efficiency of pollutant degradation. Moreover, the structural diversity and modifiability of MOFs offer opportunities for designing materials that can effectively harness solar energy, thereby contributing to the development of more sustainable and energy-efficient photo-Fenton systems.
In a notable advancement in pollutant remediation, Wu Q. et al. engineered an iron–nickel bimetallic organic framework (FeNiX-BDC) targeting the adsorption and photo-Fenton degradation of methylene blue (MB) and methyl orange (MO). The specific ratio of Ni and Fe optimized the MOF's surface area, pore volume, and surface charge, enhancing its adsorption capacity and photocatalytic properties. This synergy significantly improved the removal rate of these pollutants, demonstrating FeNiX-BDC's potential in environmental cleanup.30 Aligning with evolving research trends, Miao S. et al. synthesized magnetic MOF-derived nitrogen-doped cobalt–iron bimetallic carbon composite nanomaterials (Co0.8Fe0.2N-NC). Created through self-assembly and annealing, this material features nitrogen doping into a Co–Fe MOF structure, enhancing adsorption and Fenton activity. The interplay between cobalt–iron centers and H2O2 amplified active species generation, expediting MB degradation. Co0.8Fe0.2N-NC demonstrated superior performance, being 2.9 times more effective than traditional Fenton systems and 25.2 times more than conventional photocatalytic systems. This study highlights significant advances in pollutant degradation and the stability of nitrogen-doped bimetallic MOFs.44 (see Fig. 9). In 2023, Li B. et al. innovatively prepared an electrospun cathode (MOFs (2Fe/Co)/CNF) with photocatalytic and electrocatalytic functionalities. Utilizing graphene templates, carbonization, and in situ growth, this material effectively degraded dyes like methylene blue and antibiotics like erythromycin. The flexible electrospun cathode's unique heterostructure, facilitated by carbon nanofibers (CNF), promoted efficient electron transport and suppressed electron–hole recombination, enhancing catalytic performance. This system, combining adsorption and photo-electro-Fenton processes, represents a significant leap in pollutant degradation technologies' efficiency and effectiveness.79 The array of research discussed above clearly illustrates the extensive utilization of metal–organic frameworks (MOFs) in systems that harness both adsorption and Fenton synergistic processes. MOFs inherently possess a strong adsorption force, and when they contain Fenton catalytic metals, they also function as effective catalysts. This dual functionality is a cornerstone of their utility in environmental remediation. A key aspect of MOFs is the array of functional groups present on their surface, which not only contribute to the structural advantages of the material in adsorption but also facilitate various interactions. These interactions include electrostatic attractions, hydrogen bonding, and π–π stacking, all of which collectively enhance the adsorptive force of the MOFs. Furthermore, these functional groups can chelate with Fenton metals, thereby improving the efficiency of Fenton-based reactions. In addition to these properties, the high conductivity and photosensitivity of MOF materials expand their applicability. These characteristics enable MOFs to participate not only in adsorption and traditional Fenton processes but also in more complex systems involving electro-Fenton and photo-Fenton reactions. The culmination of these capabilities is the ability of MOFs to engage in adsorption and photo-electro-Fenton processes, thereby offering a multifaceted approach to the synergistic degradation of organic pollutants. This versatility positions MOFs as a pivotal material in the field of environmental science, particularly in the efficient and sustainable removal of a wide range of contaminants.
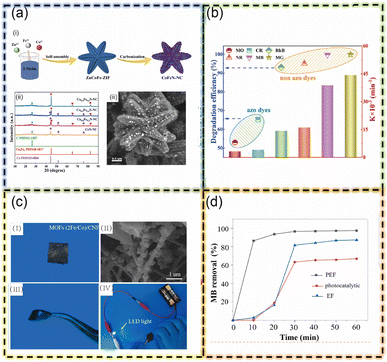 | ||
| Fig. 9 (a) (i) Schematic diagram of CoxFe1−xN-NC prepared by self-assembly and annealing. (ii) XRD patterns of CoxFe1−xN-NC materials with various molar ratios of Co and Fe after carbonization. (iii) SEM, (b) Relationship between degradation efficiency and degradation rate constant of six organic dyes (T = 30 ± 1 °C; Co0.8Fe0.2N-NC = 0.1 g L−1; C0 = 40 mg L−1; natural pH; H2O2 = 0.75 mL; 300 W fluorescent lamp). (c) (I) MOFs (2Fe/Co)/CNF, (II) SEM diagrams, (III) the flexibility, (IV) the conductivity (this figure has been reproduced with permission from Miao S. et al.44 from Elsevier, copyright 2022). (d) The MB removal efficiency with different treatments (with PEF, EF and photocatalyst, respectively). | ||
3.3 Minerals/clay
In addressing the challenges of organic pollutant treatment, especially in the context of adsorption and Fenton reactions, the practical limitations of metal–organic frameworks (MOFs), such as high material costs and difficulties in mass production, necessitate the exploration of alternative materials. Consequently, attention has shifted towards mineral/clay matrix materials, which are increasingly recognized for their potential in large-scale industrial applications due to their cost-effectiveness and scalability. Mineral/clay matrices, characterized by their inherent porous structure and expansive specific surface area, demonstrate exceptional adsorption capabilities. These properties render them comparable to carbonaceous matrices and MOFs in terms of effectiveness as adsorbents. The primary mechanisms underpinning the adsorption capabilities of these materials include capillary action, surface tension dynamics, hydrogen bond formation, and van der Waals forces. Notable among the spectrum of mineral/clay matrices are substances such as kaolin, diatomaceous earth, sepiolite, bentonite, perlite, SiO2, attapulgite, vermiculite, fly ash, and opal. Montmorillonite, in particular, stands out for its immense specific surface area and substantial cation exchange capacity.85 The inherent qualities of mineral/clay matrices, including their abundance, economic viability, chemical stability, and thermal consistency, position them as ideal candidates for adsorbents and precursor materials. Moreover, the ease of modification of these materials facilitates the loading of metal substances with Fenton catalytic effects, thereby enabling their application in Fenton reactions. This capacity for modification enhances the suitability of mineral/clay matrix materials for adsorption and Fenton synergy. Consequently, mineral/clay matrix materials emerge as exceptionally viable materials for the treatment of organic pollutants, particularly in contexts where large-scale production and cost-effectiveness are paramount (Fig. 10).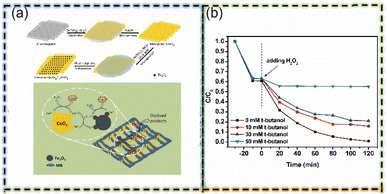 | ||
Fig. 10 (a) Schematic diagram for the preparation of mesoporous Fe3O4/CeO2 and MB removal mechanism by mesoporous Fe3O4/CeO2 composite. (b) Effect of t-butanol on removal of MB in Fe3O4/CeO2 (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce = 1 Ce = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) catalyzed system (this figure has been reproduced with permission from Li et al.85 from Elsevier, copyright 2017). 1) catalyzed system (this figure has been reproduced with permission from Li et al.85 from Elsevier, copyright 2017). | ||
It is note that the clay-based supports are also efficient in heterogeneous Fenton catalyst function for PFAS decomposition. Indeed, the per- and polyfluoroalkyl substances (PFAS) are manufactured chemicals that have been widely used in industry, and continuously exposed in water in the form of perfluorooctanoic acid (PFOA). It is reported that an iron–clay(montmorillonite)-cyclodextrin(β-CD)-DFB (decafluorobiphenyl) with the iron-clay segment containing a heterogeneous Fenton catalyst function, could adsorbed and oxidized more than 65% of long-chain PFAS within 10 min.86
In the realm of environmental remediation, similar to other materials previously discussed, mineral/clay matrix materials have been explored for their potential in synergistic applications combining adsorption with various Fenton processes. This exploration encompasses integrating these materials in traditional Fenton reactions, electro-Fenton systems, and photo-Fenton processes.
In each of these scenarios, the inherent properties of mineral/clay materials, such as their wide specific surface area, porosity, and chemical stability, contribute significantly to the overall efficiency of the combined processes. The versatility of these materials, coupled with their cost-effectiveness and abundance, makes them a promising area of research in the development of advanced environmental remediation technologies. Their integration into various Fenton-based systems exemplifies a sustainable approach to addressing the complex challenge of organic pollutant degradation.
3.4 The art of material design
To maximize the combined benefits of adsorption and Fenton oxidation for water purification, it is imperative to enhance the adsorption capacity and improve the Fenton reaction's efficiency.Firstly, the selected substrate must exhibit robust adsorption characteristics, which are primarily driven by factors such as an extensive specific surface area and a porous structure. Leading strategies to boost adsorption capacity emphasize the use of materials with inherent porous structures, particularly activated carbon. Upon establishing the fundamental properties of the adsorption medium, enhancing its adsorption capacity may require introducing additional components. For example, materials rich in π bonds can leverage the π–π bond stacking mechanism to fortify their adsorption capabilities. Prominent materials in this context include graphene, carbon nanotubes, and g-C3N4. The choice of these materials is not merely based on their intrinsic π bonds; their ability to host hydrophilic functional groups is also crucial.82 These groups, naturally efficient at adsorption, further heighten the overall adsorptive potential of the substrate. Moreover, electrostatic interactions present a vital tool for enhancing adsorption. The synergistic combination of electrostatic interactions, π–π stacking, and hydrogen bonding underpins many Metal–Organic Frameworks (MOFs), signaling a renewed perspective in materials science.
Secondly, to achieving the Fenton reaction's efficiency, the increasing active sites and enhancing electron transfer in the architectures of supports are called for.
However, within applied research, particularly in environmental engineering and conservation, the dual concerns of cost-effectiveness and sustainability frequently emerge. This shows that utilizing cost-effective mineral or clay-based materials provides practical solutions. When these substrates undergo specific scientific alterations, they significantly improve their inherent capacities, facilitating a robust synergy between adsorption processes and Fenton oxidation in multifaceted aquatic environments. This methodology elevates contaminant removal efficiency and promotes prudent resource allocation. From an environmental and sustainable materials science perspective, biochar stands out. As a carbon-dense, renewable material derived from biomass, biochar exhibits a range of physicochemical attributes, making it apt for water treatment applications and a substrate of considerable research relevance. Its untapped potential presents opportunities for innovative strides in sustainable water treatment techniques.
4 Application
The synergistic prowess of combining adsorption with Fenton oxidation, showcasing its efficacy in mitigating water pollution. Indeed, these applications span several domains, including medical wastewater treatment,91 industrial wastewater treatment,32 and urban wastewater treatment.92 This broad array of applications not only demonstrates the versatility of these methods but also highlights their potential to contribute significantly to environmental conservation efforts.4.1 Medical wastewater
The domain of medical wastewater represents one of the most critically affected areas in terms of organic pollutant contamination. The effective degradation of organic pollutants in medical wastewater emerges as a paramount application field. Medical wastewater is predominantly characterized by trace concentrations of soluble drugs91 and antibiotics.25 These contaminants, due to their minuscule concentrations, often elude the efficacy of traditional water treatment methods like precipitation and neutralization, which are typically insufficient in such scenarios.25 Considering these challenges, the synergistic employment of adsorption and Fenton oxidation has emerged as a highly effective strategy for treating such contaminated water systems.In practical scenarios, especially for large-scale treatment of diverse organic pollutants in medical waste liquids, the selection of Fenton catalytic materials predominantly focuses on carbonaceous and mineral materials.93 As a typical carbonaceous material, biochar materials are of particular interest due to their high specific surface area, akin to other adsorbents, and their effective absorption of harmful substances found in medical waste liquids. The production of biochar involves the pyrolysis of biomass materials under low-oxygen conditions, a process that transforms waste materials into beneficial products, thereby reducing waste accumulation and environmental pollution.62 Biochar's relatively low production and application costs render it a better way for real water treatment than other advanced materials like graphene, carbon nanotubes, and Metal–Organic Frameworks (MOFs).25,91–94
For instance, Zhou and colleagues have innovatively developed biochar-based adsorptive catalysts derived from waste leather (WLBC) by chemically modifying waste leather. This modification is designed for the dual purpose of adsorption and Fenton co-processing of sulfamethoxazole (SMX), an antibiotic frequently present in large-scale medical waste liquids.25 The chemically modified WLBC demonstrates exceptional efficiency in adsorbing trace quantities of SMX, utilizing mechanisms such as electrostatic attraction, hydrophobic interaction, and π–π interactions. Additionally, the surface functional groups of WLBC are adept at chelating chromium ions, thereby facilitating their incorporation into the WLBC. In an environment containing hydrogen peroxide (H2O2), these chromium ions catalyze the Cr(VI)/Cr(III) redox cycle, resulting in the prolific generation of hydroxyl radicals (˙OH). These radicals play a crucial role in the Fenton degradation of SMX that is absorbed on the WLBC. This study proposes a feasible strategy for the large-scale treatment of antibiotic-laden medical waste. Particularly laudable is the utilization of waste leather as the raw material, effectively embodying a waste-to-treatment approach for the control of organic pollutants. This innovative method not only addresses the pressing issue of waste management but also significantly contributes to the sustainable handling of environmental contaminants.
Similarly employing biochar materials, the research team led by Della-Flora A. has demonstrated the effective use of avocado seed activated carbon (ASAC) in medical wastewater treatment.91 In 2020, the team led by Della-Flora A. implemented a novel approach combining adsorption with solar-driven Fenton reactions (SPF) using avocado seed activated carbon (ASAC) to address the presence of the anticancer drug flutamide (FLUT) in medical waste. This method involved the use of ASAC as an adsorbent in conjunction with SPF to effectively treat FLUT. The primary step in this process is the adsorption of FLUT onto the ASAC surface, facilitated primarily by π–π interactions. Additionally, the surface activated ASAC, characterized by its abundance of functional groups, allows for the even distribution of iron ions on its surface. In the presence of the Fenton reagent hydrogen peroxide (H2O2), the iron laden ASAC catalyzes the generation of hydroxyl radicals (˙HO) through the Fe2+/Fe3+ redox cycle, enhanced by sunlight. These hydroxyl radicals are instrumental in the rapid Fenton degradation of FLUT absorbed on the ASAC surface. This process exemplifies the effective synergy.
4.2 Industrial wastewater treatment
Industrial wastewater represents a significant challenge in the realm of organic pollution, characterized by a diverse array of organic contaminants. This complexity is due to the variety of industries contributing to wastewater, each with its specific type of organic pollutants. The predominant contaminants encapsulate Chemical Oxygen Demand (COD), Total Organic Carbon (TOC), salicylic acid (SA), phenol, and related compounds. The primary culprits, as identified, are wastewater from textiles, refineries, dairies, and pharmaceutical sectors.32 Additionally, the industrial environment's complexity and the diversity of wastewater characteristics, such as varying pH values, necessitate a broad range of materials for effective treatment using adsorption and Fenton synergies between adsorption and photo-Fenton mechanisms. Following this, in 2021, the same team progressed to degrading a mixture of nine drugs, including fluconazole, using the identical material (ASAC) in combination with Solar Fenton (SPF).91 The mechanisms of adsorption and Fenton reactions in this instance were analogous to those previously described. The integration of adsorption and photo-Fenton processes resulted in remarkably high degradation rates for the target pharmaceuticals. Specifically, fluconazole experienced a degradation rate of up to 80%, while the remaining eight drugs in the mixture were degraded by up to 99%. This research not only presents an advanced methodology for wastewater treatment but also underscores the efficacy of utilizing natural materials like avocado seed carbon in environmental remediation, offering sustainable and efficient solutions for managing pharmaceutical pollutants (Fig. 11). | ||
| Fig. 11 Scanning electron microscopy of ASAC and proposed adsorption mechanisms and interaction of FLUT and TPs present after solar photo-Fenton treatment in a competitive and of HWW matrix (this figure has been reproduced with permission from Della-Flora A. et al.91 from Elsevier, copyright 2021). | ||
In the treatment of industrial wastewater, the materials employed to exploit the synergy between adsorption and Fenton processes are diverse.95–103 These include: (i) activated carbon: widely used due to its high surface area and porous structure, making it effective for absorbing a wide range of organic pollutants; (ii) graphene and carbon nanotubes: known for their exceptional adsorption capacities and ability to enhance catalytic processes, these materials are effective in removing complex organic compounds; (iii) graphitic carbon nitride (g-C3N4): this material is notable for its stability and photocatalytic properties, making it suitable for photo-Fenton applications; (iv) biochar: a sustainable option, biochar is effective in absorbing various organic pollutants and is often used in conjunction with Fenton reactions; (v) Metal–Organic Frameworks (MOFs): MOFs are known for their high porosity and customizable structure, which can be tailored for specific pollutants; (vi) minerals/clay materials: these materials are valued for their natural abundance, adsorption capabilities, and stability, making them suitable for a range of applications. When treating industrial wastewater, the combination of adsorption with various Fenton processes is applied, including traditional Fenton, electro-Fenton, and photo-Fenton. Each combination offers unique advantages and is selected based on the specific requirements of the wastewater being treated.
4.3 Urban wastewater treatment
The final realm of application for the synergy of adsorption and Fenton processes holds significant relevance to everyday life, particularly in urban sewage treatment. Given the unique characteristics of urban sewage, the selection of materials for practical applications is crucial. Predominantly, activated carbon, biochar, and minerals/clay are employed extensively in actual urban sewage treatment due to their efficacy and safety.92,104–108For instance, impregnating granular activated carbon (GAC) with Fe ions has been proved to be an efficient method for the degradation of the antibiotics for urban wastewater.92 Meanwhile the biochar also utilized for organic pollutant degradation.107 Particularly, the innovative work by Zeng et al., explored the direct application of iron-containing minerals, specifically pyrite (FeS2), goethite (α-FeOOH), and magnetite (Fe3O4), in the restoration of groundwater.108 These minerals, characterized by their affordability and abundant availability, are intrinsically valuable in wastewater treatment due to their inherent composition. Each of these minerals naturally contains Fe ions, which serve as Fenton catalysts. Additionally, the ore materials themselves exhibit notable adsorption properties. Their utility is further enhanced by their electrical conductivity and magnetic properties, which allow them to function dually as both adsorbents and catalysts, that is particularly advantageous in the treatment process.
5 Conclusion and perspectives
This review provides an overview of the application of the combined action of adsorption and Fenton oxidation in water remediation. In conclusion, the combined action of adsorption and Fenton oxidation is an effective water remediation technology that can effectively remove organic pollutants from water. The mechanisms of this combined action mainly include: the enrichment effect of the adsorbent on pollutants, the catalytic effect of the adsorbent on the Fenton oxidation reaction, and the protective effect of the adsorbent on hydroxyl radicals. This combined action has been used to remove a variety of organic pollutants from water, including aromatics, phenols, and pesticides. However, there are still rooms for future improvement for Fenton oxidations:(i) New adsorbent/catalyst composite materials to improve the efficiency and stability of the combined action are required for future study. The adsorbent not only adsorbs pollutants but also catalyzes the Fenton oxidation process. This dual function accelerates the degradation of organic compounds. Therefore, the investigating the optimal conditions for this catalytic effect such as pH, temperature, and adsorbent dosage remains an important avenue for research.
(ii) The application effect of the combined action in complex water bodies are still need to be investigated. It is needed to conduct a more in-depth study on the application effects of combined action in complex water bodies. This includes considering the interactions between different components of wastewater, environmental factors, and possible synergistic effects. Research in this field will help optimize the treatment strategies for complex water bodies based on the Fenton reaction.
(iii) Develop economic and large-scale application technologies of the combined action are also called for. For instance, the utilization of mineral or clay-based materials or other cost-effective materials in Fenton reaction may provide practical solutions.
In conclusion the combined action of adsorption and Fenton oxidation holds promise for sustainable water remediation. Addressing the above outlined research gaps will contribute to its future success.
Data availability
No new data were collected or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 52374419), Double Thousand Plan of Jiangxi Province (jxsq2020105012, jxsq2020101043).Notes and references
- Y. Li, Y. Liu, L. Feng and L. Zhang, A review: Manganese-driven bioprocess for simultaneous removal of nitrogen and organic contaminants from polluted waters, Chemosphere, 2023, 314, 137655 CrossRef CAS PubMed.
- F. C. R. Costa, C. R. dos Santos and M. C. S. Amaral, Trace organic contaminants removal by membrane distillation: A review on mechanisms, performance, applications, and challenges, Chem. Eng. J., 2023, 464, 142461 CrossRef CAS.
- S. R. Kafle, et al., Advancement of membrane separation technology for organic pollutant removal, Water Sci. Technol., 2024, wst2024117, DOI:10.2166/wst.2024.117.
- W. Luo, et al., Efficient Removal of Organic Pollutants with Magnetic Nanoscale BiFeO3 as a Reusable Heterogeneous Fenton-Like Catalyst, Environ. Sci. Technol., 2010, 44, 1786–1791 CrossRef CAS PubMed.
- B. Jain, A. K. Singh, H. Kim, E. Lichtfouse and V. K. Sharma, Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes, Environ. Chem. Lett., 2018, 16, 947–967 CrossRef CAS.
- S. S. A. Shah, et al., Removal of antibiotics from wastewater via metal-organic frameworks (MOFs) as adsorbents, Toxin Rev., 2024, 1–23 Search PubMed.
- M. Usman, et al., Sorption of nalidixic acid onto micrometric and nanometric magnetites: Experimental study and modeling, Appl. Surf. Sci., 2014, 299, 136–145 CrossRef CAS.
- R. Rashid, I. Shafiq, P. Akhter, M. J. Iqbal and M. Hussain, A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method, Environ. Sci. Pollut. Res., 2021, 28, 9050–9066 CrossRef CAS PubMed.
- M. T. Samadi, et al., The utility of ultraviolet beam in advanced oxidation-reduction processes: a review on the mechanism of processes and possible production free radicals, Environ. Sci. Pollut. Res., 2024, 31, 6628–6648 CrossRef CAS PubMed.
- G. Khajouei, H. O. Finklea and L.-S. Lin, UV/chlorine advanced oxidation processes for degradation of contaminants in water and wastewater: A comprehensive review, J. Environ. Chem. Eng., 2022, 10, 107508 CrossRef CAS.
- N. A. Khan, A. H. Khan, P. Tiwari, M. Zubair and M. Naushad, New insights into the integrated application of Fenton-based oxidation processes for the treatment of pharmaceutical wastewater, J. Water Process Eng., 2021, 44, 102440 CrossRef.
- Y. Zhu, W. Fan, W. Feng, Y. Wang, S. Liu, Z. Dong and X. Li, A critical review on metal complexes removal from water using methods based on Fenton-like reactions: Analysis and comparison of methods and mechanisms, J. Hazard. Mater., 2021, 414, 125517 CrossRef CAS PubMed.
- M. Bayat, M. Sohrabi and S. J. Royaee, Degradation of phenol by heterogeneous Fenton reaction using Fe/clinoptilolite, J. Ind. Eng. Chem., 2012, 18, 957–962 CrossRef CAS.
- J. J. Pignatello, Dark and photoassisted iron(3+)-catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide, Environ. Sci. Technol., 1992, 26, 944–951 CrossRef CAS.
- H. Kusic, N. Koprivanac, S. Horvat, S. Bakija and A. L. Bozic, Modeling dye degradation kinetic using dark- and photo-Fenton type processes, Chem. Eng. J., 2009, 155, 144–154 CrossRef CAS.
- S. Malato Rodríguez, et al., Engineering of solar photocatalytic collectors, Sol. Energy, 2004, 77, 513–524 CrossRef.
- E. Brillas, I. Sirés and M. A. Oturan, Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton's Reaction Chemistry, Chem. Rev., 2009, 109, 6570–6631 CrossRef CAS PubMed.
- M. Panizza, et al., Complete mineralization of the antibiotic amoxicillin by electro-Fenton with a BDD anode, J. Appl. Electrochem., 2014, 44, 1327–1335 CrossRef CAS.
- P. V. Nidheesh and R. Gandhimathi, Trends in electro-Fenton process for water and wastewater treatment: An overview, Desalination, 2012, 299, 1–15 CrossRef CAS.
- E. Brillas, A review on the degradation of organic pollutants in waters by UV photoelectro-Fenton and solar photoelectro-Fenton, J. Braz. Chem. Soc., 2014, 25, 393–417 CAS.
- E. Brillas, A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies, Chemosphere, 2020, 250, 126198 CrossRef CAS PubMed.
- P. V. Nidheesh, R. Gandhimathi and S. T. Ramesh, Degradation of dyes from aqueous solution by Fenton processes: a review, Environ. Sci. Pollut. Res., 2013, 20, 2099–2132 CrossRef CAS PubMed.
- V. Gvoic, et al., Synergistic effect of Fenton oxidation and adsorption process in treatment of azo printing dye: DSD optimization and reaction mechanism interpretation, Environ. Technol., 2024, 45, 1781–1800 CrossRef CAS PubMed.
- R. Lin, et al., Synergistic effects of oxidation, coagulation and adsorption in the integrated Fenton-based process for wastewater treatment: A review, J. Environ. Manage., 2022, 306, 114460 CrossRef CAS PubMed.
- C. Zhou, et al., Coupling adsorption and in situ Fenton-like oxidation by waste leather-derived materials in continuous flow mode towards sustainable removal of trace antibiotics, Chem. Eng. J., 2021, 420, 130370 CrossRef CAS.
- C. Liu, et al., Dual reaction centers promote adsorption-photo Fenton synergistic efficient removal of tetracycline by reduced graphene oxide/CuFe2O4-oxygen vacancies, Appl. Surf. Sci., 2022, 606, 154890 CrossRef CAS.
- A.-Y. Zhang, et al., Heterogeneous Fenton decontamination of organoarsenicals and simultaneous adsorption of released arsenic with reduced secondary pollution, Chem. Eng. J., 2018, 344, 1–11 CrossRef CAS.
- M. M. Bello, A. A. A. Raman and A. Asghar, Activated carbon as carrier in fluidized bed reactor for Fenton oxidation of recalcitrant dye: Oxidation-adsorption synergy and surface interaction, J. Water Process Eng., 2020, 33, 101001 CrossRef.
- Z. Duan, et al., Magnetic Fe3O4/activated carbon for combined adsorption and Fenton oxidation of 4-chlorophenol, Carbon, 2020, 167, 351–363 CrossRef CAS.
- Q. Wu, M. S. Siddique and W. Yu, Iron–nickel bimetallic metal-organic frameworks as bifunctional Fenton-like catalysts for enhanced adsorption and degradation of organic contaminants under visible light: Kinetics and mechanistic studies, J. Hazard. Mater., 2021, 401, 123261 CrossRef CAS PubMed.
- X. Meng, et al., Co-adsorption of As(III) and phenanthrene by schwertmannite and Fenton-like regeneration of spent schwertmannite to realize phenanthrene degradation and As(III) oxidation, Environ. Res., 2021, 195, 110855 CrossRef CAS PubMed.
- X. Wang, et al., Adsorption Dependent Fenton-like Catalysis on Graphene Oxide-Fe3O4 Composite: Electron Transfer Mediated by Target-catalyst Interactions, Surf. Interfaces, 2022, 29, 101749 CrossRef CAS.
- Y. Wang, et al., Enhanced Fenton-like efficiency by the synergistic effect of oxygen vacancies and organics adsorption on FexOy-d-g-C3N4 with Fe–N complexation, J. Hazard. Mater., 2021, 408, 124818 CrossRef CAS PubMed.
- Z. Alizadeh and A. Rezaee, Tetracycline removal using microbial cellulose@nano-Fe3O4 by adsorption and heterogeneous Fenton-like processes, J. Mol. Liq., 2022, 366, 120199 CrossRef CAS.
- I. N. Najm, V. L. Snoeyink, B. W. Lykins and J. Q. Adams, Using Powdered Activated Carbon: A Critical Review, J.–Am. Water Works Assoc., 1991, 83, 65–76 CrossRef CAS.
- J. Cui, et al., Residual organics removal from manganese electrochemical solution using combined Fenton oxidation process with adsorption over activated carbon, Environ. Sci. Pollut. Res., 2020, 27, 44240–44248 CrossRef CAS PubMed.
- N. Sharifi, A. Nasiri, S. Silva Martínez and H. Amiri, Synthesis of Fe3O4@activated carbon to treat metronidazole effluents by adsorption and heterogeneous Fenton with effluent bioassay, J. Photochem. Photobiol., C, 2022, 427, 113845 CrossRef CAS.
- C. Lyu, D. Zhou and J. Wang, Removal of multi-dye wastewater by the novel integrated adsorption and Fenton oxidation process in a fluidized bed reactor, Environ. Sci. Pollut. Res., 2016, 23, 20893–20903 CrossRef PubMed.
- W. Zhou, et al., Activated carbon as effective cathode material in iron-free Electro-Fenton process: Integrated H2O2 electrogeneration, activation, and pollutants adsorption, Electrochim. Acta, 2019, 296, 317–326 CrossRef CAS PubMed.
- J. D. García-Espinoza, I. Robles, A. Durán-Moreno and L. A. Godínez, Study of simultaneous electro-Fenton and adsorption processes in a reactor containing porous carbon electrodes and particulate activated carbon, J. Electroanal. Chem., 2021, 895, 115476 CrossRef.
- Y. Song, A. Wang, S. Ren, Y. Zhang and Z. Zhang, Flow-through heterogeneous electro-Fenton system using a bifunctional FeOCl/carbon cloth/activated carbon fiber cathode for efficient degradation of trimethoprim at neutral pH, Environ. Res., 2023, 222, 115303 CrossRef CAS PubMed.
- A. A. Babaei, et al., Comparative treatment of textile wastewater by adsorption, Fenton, UV-Fenton and US-Fenton using magnetic nanoparticles-functionalized carbon (MNPs@C), J. Ind. Eng. Chem., 2017, 56, 163–174 CrossRef CAS.
- Z.-Y. Jiang, et al., Hydrothermal deposition of CoFe2O4 nanoparticles on activated carbon fibers promotes atrazine removal via physical adsorption and photo-Fenton degradation, J. Environ. Chem. Eng., 2021, 9, 105940 CrossRef CAS.
- S. Miao, et al., Metal-organic frameworks-derived CoFeN-NC materials with the enhanced catalytic activity and selectivity for the degradation of organic dyes via adsorption and heterogeneous photo-Fenton, Appl. Surf. Sci., 2022, 601, 154028 CrossRef CAS.
- Y. Yang, et al., Adsorption and degradation of sulfadiazine over nanoscale zero-valent iron encapsulated in three-dimensional graphene network through oxygen-driven heterogeneous Fenton-like reactions, Appl. Catal., B, 2019, 259, 118057 CrossRef CAS.
- Md. N. Pervez, et al., A bifunctional-FeOOH@GCA nanocomposite for enhanced adsorption of arsenic and photo Fenton-like catalytic conversion of As(III), Environ. Technol. Innovation, 2021, 22, 101437 CrossRef CAS.
- M. S. Sajab, et al., Bifunctional graphene oxide–cellulose nanofibril aerogel loaded with Fe(III) for the removal of cationic dye via simultaneous adsorption and Fenton oxidation, RSC Adv., 2016, 6, 19819–19825 RSC.
- F. Zhang, X. Xue, X. Huang and H. Yang, Adsorption and heterogeneous Fenton catalytic performance for magnetic Fe3O4/reduced graphene oxide aerogel, J. Mater. Sci., 2020, 55, 15695–15708 CrossRef CAS.
- J. Wu, M. Lin, X. Weng, G. Owens and Z. Chen, Pre-adsorption and Fenton-like oxidation of mitoxantrone using hybrid green synthesized rGO/Fe nanoparticles, Chem. Eng. J., 2021, 408, 127273 CrossRef CAS.
- L. Liu, J. Lin, G. Owens and Z. Chen, New insights on removal mechanism of 17α-estradiol based on adsorption and Fenton-like oxidation by FeNPs/rGO, Sep. Purif. Technol., 2022, 283, 120222 CrossRef CAS.
- C. Hong, et al., Hierarchical porous structure of urushiol mediated Fe3O4/three-dimensional graphene composites towards enhanced Fenton degradation of tetracycline, Chem. Eng. Sci., 2023, 281, 119111 CrossRef CAS.
- X. Song, et al., Accelerated removal of sulfadiazine by heterogeneous electro-Fenton system with Pt-FeOX/graphene single-atom alloy cathodes, J. Environ. Manage., 2024, 349, 119541 CrossRef CAS PubMed.
- Y. Lu, X. Qin, K. Wang, S. Chen and X. Quan, Accelerated Fe(II) regeneration on Fe-doped oxidized carbon nanotube enabling highly-efficient electro-Fenton process for pollutants removal, Sep. Purif. Technol., 2023, 320, 124196 CrossRef CAS.
- N. Cai, et al., Three-dimensional heterogeneous electro-Fenton system with reduced graphene oxide based particle electrode for Acyclovir removal, Chin. Chem. Lett., 2024, 35, 108514 CrossRef CAS PubMed.
- R. Matos, et al., Design and photo-Fenton performance of Graphene/CuS/Fe3O4 tertiary nanocomposites for Rhodamine B degradation, Catal. Today, 2023, 418, 114132 CrossRef CAS.
- X. Wang, M. He and Z. Nan, Effects of adsorption capacity and activity site on Fenton-like catalytic performance for Na and Fe co-doped g-C3N4, Sep. Purif. Technol., 2021, 256, 117765 CrossRef CAS.
- Y. Ren, C. Liu, N. Li, B. Lai and J. Li, A bimetallic co-doped iron and copper in polymeric graphitic carbon nitride to enhance catalytic activity and reusability of Fenton-like oxidation, Sep. Purif. Technol., 2023, 316, 123782 CrossRef CAS.
- D. Chen, et al., Fabrication of a novel gas diffusion electrode (g-C3N4/CB/PTFE-GF) for electro-Fenton treatment of amaranth dye, J. Water Process Eng., 2023, 56, 104357 CrossRef.
- G. Pan and Z. Sun, Cu-doped g-C3N4 catalyst with stable Cu0 and Cu+ for enhanced amoxicillin degradation by heterogeneous electro-Fenton process at neutral pH, Chemosphere, 2021, 283, 131257 CrossRef CAS PubMed.
- M. Liu, et al., Novel Cu-Fe bi-metal oxide quantum dots coupled g-C3N4 nanosheets with H2O2 adsorption-activation trade-off for efficient photo-Fenton catalysis, Appl. Catal., B, 2022, 301, 120765 CrossRef CAS.
- V. Gargiulo, et al., Assessing the Potential of Biochars Prepared by Steam-Assisted Slow Pyrolysis for CO2 Adsorption and Separation, Energy Fuels, 2018, 32, 10218–10227 CrossRef CAS PubMed.
- C. Zhang, L. Liu, M. Zhao, H. Rong and Y. Xu, The environmental characteristics and applications of biochar, Environ. Sci. Pollut. Res., 2018, 25, 21525–21534 CrossRef CAS PubMed.
- P. D. Dissanayake, et al., Sustainable gasification biochar as a high efficiency adsorbent for CO2 capture: A facile method to designer biochar fabrication, Renewable Sustainable Energy Rev., 2020, 124, 109785 CrossRef CAS.
- G. Liu, et al., Biochar/layered double hydroxides composites as catalysts for treatment of organic wastewater by advanced oxidation processes: A review, Environ. Res., 2023, 234, 116534 CrossRef CAS PubMed.
- G. Song, et al., Tailoring biochar for persulfate-based environmental catalysis: Impact of biomass feedstocks, J. Hazard. Mater., 2022, 424, 127663 CrossRef CAS PubMed.
- H. Wang, H. Wang, H. Zhao and Q. Yan, Adsorption and Fenton-like removal of chelated nickel from Zn-Ni alloy electroplating wastewater using activated biochar composite derived from Taihu blue algae, Chem. Eng. J., 2020, 379, 122372 CrossRef CAS.
- C. Yu, et al., Development of a novel biochar/iron oxide composite from green algae for bisphenol-A removal: Adsorption and Fenton-like reaction, Environ. Technol. Innovation, 2022, 28, 102647 CrossRef CAS.
- F. Deng, et al., A biochar modified nickel-foam cathode with iron-foam catalyst in electro-Fenton for sulfamerazine degradation, Appl. Catal., B, 2019, 256, 117796 CrossRef CAS.
- C. Zhang, et al., Heterogeneous electro–Fenton using three–dimension NZVI–BC electrodes for degradation of neonicotinoid wastewater, Water Res., 2020, 182, 115975 CrossRef CAS PubMed.
- Z. Yin, et al., Novel Fe/N co-doping biochar based electro-Fenton catalytic membrane enabling enhanced tetracycline removal and self-cleaning performance, J. Cleaner Prod., 2023, 402, 136731 CrossRef CAS.
- X. Cai, et al., Construction of a C-decorated and Cu-doped (Fe,Cu)S/CuFe2O4 solid solution for photo-Fenton degradation of hydrophobic organic contaminant: Enhanced electron transfer and adsorption capacity, Chemosphere, 2022, 296, 134005 CrossRef CAS PubMed.
- Z. Xiang, et al., Incorporating Se vacancies into FeSe2/Fe2O3@C to enhance H2O2 adsorption for efficient photo-Fenton removal of As(III), Appl. Surf. Sci., 2022, 606, 154879 CrossRef CAS.
- K. Lin, et al., Heterogeneous photo-Fenton degradation of acid orange 7 activated by red mud biochar under visible light irradiation, Environ. Pollut., 2023, 327, 121454 CrossRef CAS PubMed.
- H. Ma, B. Yu, Q. Wang, G. Owens and Z. Chen, Enhanced removal of pefloxacin from aqueous solution by adsorption and Fenton-like oxidation using NH2-MIL-88B, J. Colloid Interface Sci., 2021, 583, 279–287 CrossRef CAS PubMed.
- R. Lu, et al., IL-functionalized Mn(II)-doped core–shell Fe3O4@Zr-MOF nanomaterials for the removal of MB from wastewater based on dual adsorption/Fenton catalysis, New J. Chem., 2022, 46, 8534–8544 RSC.
- T. Zhang, P. Wu, G. Owens and Z. Chen, Adsorption and Fenton-like oxidation of ofloxacin in wastewater using hybrid MOF bimetallic Fe/Ni nanoparticles, Chemosphere, 2022, 307, 135936 CrossRef CAS PubMed.
- S. Huang, et al., In-situ fabrication from MOFs derived MnxCo3-x@C modified graphite felt cathode for efficient electro-Fenton degradation of ciprofloxacin, Appl. Surf. Sci., 2022, 586, 152804 CrossRef CAS.
- J. Guo, et al., Single iron atoms embedded in MOF-derived nitrogen-doped carbon as an efficient heterogeneous electro-Fenton catalyst for degradation of carbamazepine over a wide pH, Sep. Purif. Technol., 2022, 302, 122141 CrossRef CAS.
- B. Li, et al., Enhanced photo-electro-Fenton degradation performance using graphene structure-oriented MOFs (2Fe/Co)/CNF cathode membrane, J. Cleaner Prod., 2023, 401, 136782 CrossRef CAS.
- L. Ma, C. Abney and W. Lin, Enantioselective catalysis with homochiral metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1248–1256 RSC.
- S. Yu, et al., Computational modeling guided design of metal–organic frameworks for photocatalysis – a mini review, Catal. Sci. Technol., 2023, 13, 6583–6603 RSC.
- H. Furukawa, et al., Ultrahigh Porosity in Metal-Organic Frameworks, Science, 2010, 329, 424–428 CrossRef CAS PubMed.
- S. H. Jhung, N. A. Khan and Z. Hasan, Analogous porous metal–organic frameworks: synthesis, stability and application in adsorption, CrystEngComm, 2012, 14, 7099–7109 RSC.
- H. Chen, et al., Intramolecular modulation of iron-based metal organic framework with energy level adjusting for efficient photocatalytic activity, Appl. Catal., B, 2022, 302, 120823 CrossRef CAS.
- K. Li, Y. Zhao, C. Song and X. Guo, Magnetic ordered mesoporous Fe3O4/CeO2 composites with synergy of adsorption and Fenton catalysis, Appl. Surf. Sci., 2017, 425, 526–534 CrossRef CAS.
- S. Kundu and A. Radian, Surface confinement of per-fluoroalkyl substances on an iron-decorated clay-cyclodextrin composite enables rapid oxidation by hydroxyl radicals, Chem. Eng. J., 2022, 431, 134187 CrossRef CAS.
- L. Liu, et al., Iron-included mesoporous silica thin slice as adsorbent and Fenton-like catalyst for the adsorption and degradation of dye in wastewater, Ceram. Int., 2019, 45, 15475–15481 CrossRef CAS.
- J. Zhang, H. Pang, S. Gray, S. Ma, Z. Xie and L. Gao, PFAS removal from wastewater by in-situ formed ferric nanoparticles: Solid phase loading and removal efficiency, J. Environ. Chem. Eng., 2021, 9, 105452 CrossRef CAS.
- Y. Chai, et al., Noble metal particles confined in zeolites: synthesis, characterization, and applications, Adv. Sci., 2019, 6, 1900299 CrossRef PubMed.
- Q. Zhu, et al., Ethylene glycol assisted synthesis of hierarchical Fe-ZSM-5 nanorods assembled microsphere for adsorption Fenton degradation of chlorobenzene, J. Hazard. Mater., 2020, 385, 121581 CrossRef CAS PubMed.
- A. Della-Flora, M. L. Wilde, D. Lima, E. C. Lima and C. Sirtori, Combination of tertiary solar photo-Fenton and adsorption processes in the treatment of hospital wastewater: The removal of pharmaceuticals and their transformation products, J. Environ. Chem. Eng., 2021, 9, 105666 CrossRef CAS.
- S. G. Michael, et al., Solar photo-Fenton oxidation followed by adsorption on activated carbon for the minimisation of antibiotic resistance determinants and toxicity present in urban wastewater, Appl. Catal., B, 2019, 244, 871–880 CrossRef CAS.
- P. Lv, C. Liu and Z. Rao, Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications, Renewable Sustainable Energy Rev., 2017, 68, 707–726 CrossRef CAS.
- T. T. N. Phan, H. L. Nguyen, V. T. Le, C. N. Phan and T. H. Pham, Mesoporous LaFeO3: Synergistic Effect of Adsorption and Visible Light Photo-Fenton Processes for Phenol Removal from Refinery Wastewater, J. Chem., 2021, 2021, e5841066 Search PubMed.
- Y.-P. Chen, L.-M. Yang, J. Paul Chen and Y.-M. Zheng, Electrospun spongy zero-valent iron as excellent electro-Fenton catalyst for enhanced sulfathiazole removal by a combination of adsorption and electro-catalytic oxidation, J. Hazard. Mater., 2019, 371, 576–585 CrossRef CAS PubMed.
- F. Yu, Y. Zhang, Y. Zhang, Y. Gao and Y. Pan, Promotion of the degradation perfluorooctanoic acid by electro-Fenton under the bifunctional electrodes: Focusing active reaction region by Fe/N co-doped graphene modified cathode, Chem. Eng. J., 2023, 457, 141320 CrossRef CAS.
- M. Tamang and K. K. Paul, Application of Activated Carbon-supported Heterogeneous Fenton Catalyst for the Treatment of Real Coking Wastewater: Characterization and Optimization of Treatment Parameters, Water, Air, Soil Pollut., 2023, 234, 77 CrossRef CAS.
- S. V. Gupta and Md. Ahmaruzzaman, Designing of a magnetically recoverable (Ce+3, +4/Fe+2. Fe+3) oxide@ 2D g-C3N4 surface for enhanced photo-Fenton catalytic degradation of Bismarck-Brown R and Congo red dyes: kinetics, optical properties,and mechanistic pathway studies, Int. J. Environ. Anal. Chem., 2023, 1–32 CrossRef.
- M. Bayrakdar, S. Atalay and G. Ersöz, Efficient treatment for textile wastewater through sequential photo Fenton-like oxidation and adsorption processes for reuse in irrigation, Ceram. Int., 2021, 47, 9679–9690 CrossRef CAS.
- K. He, et al., Enhanced oxidation and recovery of phosphorous from hypophosphite wastewater: Key role of heterogeneous E-Fenton system with MOFs derived hierarchical Mn-Fe@PC modified cathode, Surf. Interfaces, 2023, 39, 102957 CrossRef CAS.
- Y. Zhang, C. Liu, B. Xu, F. Qi and W. Chu, Degradation of benzotriazole by a novel Fenton-like reaction with mesoporous Cu/MnO2: Combination of adsorption and catalysis oxidation, Appl. Catal., B, 2016, 199, 447–457 CrossRef CAS.
- A. Kuleyin, et al., Combining Electro-Fenton and adsorption processes for reclamation of textile industry wastewater and modeling by Artificial neural Networks, J. Electroanal. Chem., 2022, 921, 116652 CrossRef CAS.
- C. Zang, K. Yu, S. Hu and F. Chen, Adsorption-depended Fenton-like reaction kinetics in CeO2-H2O2 system for salicylic acid degradation, Colloids Surf., A, 2018, 553, 456–463 CrossRef CAS.
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, Carbon nanodots: synthesis, properties and applications, J. Mater. Chem., 2012, 22, 24230–24253 RSC.
- Y. Yang, et al., Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review, Water Res., 2020, 184, 116200 CrossRef CAS PubMed.
- S. Li, et al., S-scheme MIL-101(Fe) octahedrons modified Bi2WO6 microspheres for photocatalytic decontamination of Cr(VI) and tetracycline hydrochloride: Synergistic insights, reaction pathways, and toxicity analysis, Chem. Eng. J., 2023, 455, 140943 CrossRef CAS.
- M. Lai, J. Li, H. Li, Y. Gui and J. Lü, Adsorption-reduction of Fe(III) by different biochars and their co-activation of H2O2 for oxidation of refractory pollutants, Catal. Commun., 2023, 176, 106626 CrossRef CAS.
- G. Zeng, et al., Natural iron minerals in an electrocatalytic oxidation system and in situ pollutant removal in groundwater: Applications, mechanisms, and challenges, Sci. Total Environ., 2023, 871, 161826 CrossRef CAS PubMed.
Footnote |
| † These two authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |
