Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Application of natural zeolite adsorption in cooperation with photosynthesis for the post-treatment of microbial fuel cells†
Que Nguyen Hoa,
Taira Hidakab,
Mukhlis A. Rahmanc and
Naoko Yoshida *a
*a
aDepartment of Civil Engineering, Nagoya Institute of Technology, Nagoya, Japan. E-mail: yoshida.naoko@nitech.ac.jp
bDepartment of Environmental Engineering, Graduate School of Engineering, Kyoto University, Kyoto University Katsura, Nishikyo, Kyoto 615-8540, Japan
cAdvanced Membrane Technology Research Centre (AMTEC), School of Chemical and Energy Engineering (FCEE), Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor, Malaysia
First published on 22nd August 2024
Abstract
Microbial fuel cells (MFCs) are a promising technology that directly converts organic matter (OM) in wastewater into electricity while simultaneously degrading contaminants. However, MFCs are insufficient for the removal of nitrogenous compounds. Therefore, the post-treatment of MFCs is essential. This study was the first to use natural zeolite adsorption integrated with photosynthesis (ZP) for post-treating MFCs. In this system, no external energy was required; instead, natural light was used to promote the growth of photosynthetic microorganisms, thereby enhancing contaminants removal through the photosynthesis process. To assess the effectiveness of the method, comparisons were conducted under two conditions: dark (no photosynthesis) and light (with photosynthesis). In darkness, extending hydraulic retention time (HRT) enhanced COD and BOD removal by 19.8% and 28.9%, respectively. When exposed to natural light, improvements were even more notable, with COD and BOD removal reaching 32% and 40%, respectively. In both conditions, the method effectively removed NH4+, achieving 60% efficiency in darkness and 84.5% in light. This study showed that the adsorption capacity of the zeolite reached saturation when the cumulative liquid volume per unit weight of the zeolite exceeded 0.2 L g−1. The key functional photosynthetic microbes were investigated using 16S rRNA and 18S rRNA. This revealed the presence of microorganisms such as Chlorobium, Acidovorax, Novosphingobium, and Scenedesmus, which likely play a role in enhancing the efficiency of photosynthesis in removing contaminants. The study findings indicated that the integration of MFCs-ZP represents an eco-friendly approach capable of resource recovery from wastewater while also meeting discharge standards.
1. Introduction
Wastewater treatment (WWT) is essential to preserve the environment by mitigating water pollution. However, WWT is a process that requires a significant amount of energy. For instance, earlier research indicated that the electricity consumption for WWT accounted for 4% in the USA, 0.7% in China, and over 1% in Europe.1 Consequently, enhancing the energy efficiency of wastewater treatment plants is pertinent when considering both economic and environmental aspects.2 Hence, current innovations in WWT technologies are not only aimed at improving contaminant removal efficiency but also at reducing energy consumption. Among these technologies, microbial fuel cells (MFCs) have emerged as environmentally friendly electrical devices and have quickly evolved into sustainable systems. This is attributed to their capacity to simultaneously treat wastewater and generate energy.3 In MFCs, the oxidation of organic matter (OM) in wastewater produces electrons and protons in the anode compartment via exoelectrogens. Protons and electrons are transferred to the cathode chamber by the membrane and electrodes, respectively. These processes lead to the production of both clean water and energy.4 However, in certain cases, the application of MFCs technology alone does not meet the nitrogen effluent quality requirements5,6 because of the lack of oxidants, such as oxygen for nitrification and NO2− for the anammox process, in the anolyte in MFCs. In certain MFCs, ammonia removal is observed, but primarily in specific types that apply a gas diffusion membrane (GDM) or cation exchange membrane (CEM) to separate the anolyte and air. In both cases, the major mechanism involves the vaporization of the ammonia generated through the reduction of ammonium on the cathode.7–9 MFCs with GDM facilitate oxygen diffusion to the anolytes and enhance nitrification.10,11 However, MFCs equipped with anion exchange membranes (AEM) were superior in electricity generation owing to the migration of pH imbalances, as opposed to CEM and GDM. These facts highlight the trade-off between ammonia removal and electricity.The problem with ammonia removal is that the nitrification and anammox processes require oxidants. The dissolved oxygen (DO) concentration must exceed 0.5 mg L−1, the minimum concentration required for nitrite oxidation during nitrification.12 Intermittent aeration in MFCs partially reduces ammonia, enabling an increase in ammonia oxidizers and anammox despite the low removal efficiency.13 The exceptionally high anode potential can enable the anammox process in MFCs, although powerful oxidants, such as potassium persulfate, must be provided in the cathode.14 Alternative approaches include adsorption and indirect oxygen supplementation through photosynthesis. Two representative processes, adsorption15,16 and photosynthesis17 have been developed because of their ease of operation, cost-effectiveness, and high nitrogen removal efficiencies.
Adsorption is a mass transfer process in which substances accumulate at the interface of two phases, liquid–liquid or liquid–solid, and the adsorbing material is called an adsorbent.18 Hence, the effectiveness of adsorption in wastewater treatment relies on the characteristics of the adsorbent, such as negative electric charge, high surface area, and micro-/mesoporous properties, for the efficient removal of contaminants.19 Natural zeolites stand out because of their various advantages, particularly their roles as ion exchangers, catalysts, and adsorbents.20 Natural zeolites have a porous structure that can accommodate a wide variety of cations such as Na+, K+, Ca2+, Mg2+, and others. These positive ions are loosely bound and can be easily exchanged for others in the contact solution.21 This makes it an attractive approach for pollutant removal.22–24 Furthermore, zeolites are natural hydrated aluminosilicate materials with a high affinity for NH4+,25 potential for removal NH4+ from wastewater. Additionally, natural zeolites have a lower cost per gram of nitrogen removed compared to other polymeric cation exchange resins.26 For example, previous studies have indicated that zeolite eliminates approximately 90% NH4+ in industrial wastewater,27 as well as 82.97% of ammonia in synthetic wastewater.23 Additionally, Han et al. demonstrated that a constructed wetland incorporating zeolite as a substrate could significantly remove NH4+ and total nitrogen (TN) with percentage of 72.99%, and 70.71%, respectively, from swine wastewater.28
Oxygenic photosynthetic microorganisms generate oxygen via photosynthesis and have great potential for treating various nitrogen-contaminated wastewaters.17,29 These microorganisms can metabolize substrates such as nitrate and ammonia in the presence of light or oxygen.29 To date, numerous studies have employed photosynthetic microorganisms for removal nitrogen from various wastewaters: 83.2% of ammonia removal efficiency in chicken manure wastewater,30 90–94% of total nitrogen removal efficiency in poultry processing wastewater,31 72–98% of ammonia removal efficiency in synthetic wastewater.29
Therefore, zeolite adsorption and photosynthetic microorganisms are advantageous for nitrogen removal during wastewater treatment. However, earlier research primarily focused on eliminating nitrogen and OM from raw wastewater, as specifically mentioned in studies.32–34 This type of wastewater contains high concentrations of contaminants, which can positively affect the removal efficiency. Additionally, Meng Wang and co-authors employed natural zeolite and the photosynthetic microorganism Chlorella (comprising over 95% of the total cells) together to treat high-strength ammonium wastewater (1180 mg L−1 NH4+). In this study, the authors utilized artificial LED lights to promote algae growth.26 In our work, we utilized natural zeolites in cooperation with photosynthesis for nitrogen removal from wastewater as a secondary process, incorporated into MFCs. This involved lower concentrations of contaminants (i.e., OM and NH4+). We used natural light to promote the growth of photosynthetic microorganisms in the wastewater itself. To the best of our knowledge, there is limited research on this approach. The findings of this study show the significant potential for offering environmentally friendly solutions to effectively eliminate contaminants from wastewater, particularly nitrogen, when combined with MFCs. In this study, sewage was treated with MFCs, and the MFCs effluent was pumped into a reactor containing zeolite under both dark and light conditions to assess the effectiveness of photosynthetic microorganisms.
2. Material and methods
2.1. MFCs set-up experiment
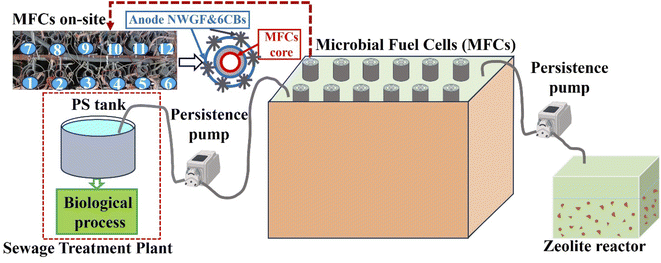
Fig. 1 shows a schematic diagram of zeolite adsorption for treatment of MFCs effluent. In this study, 12 units of MFCs reactors were used, each with a tubular structure with a diameter of 5 cm and a length of 100 cm. It features an air core and is equipped with a carbon-based cathode, an anion exchange membrane, a nonwoven graphite fabric anode, and five cylindrical anodes (4 cm in diameter and 100 cm in length). These anodes were constructed by bundling carbon fibers (T300B-3k-40B, Toray, Tokyo, Japan) with a stainless-steel wire and positioned around the core of the MFCs. The MFCs system was installed at the Ueda Water Treatment Center in Nagoya, Japan, which collects sewage from Nagoya City. Table 1 shows the quality of MFCs effluence, post treatment of the effluent involved zeolite adsorption and microbial photosynthetic as zeolite reactor was illuminated under natural daylight, which will be discussed in more detail in the following section. Natural zeolite, purchased from Shin Tohoku Chemical Industries (Miyagi, Japan), was used as the model adsorbent particle in the experiments. The particle size of natural zeolites has been reported to be 4–8 mm.| Parameters (mg L−1) | Conditions | ||
|---|---|---|---|
| Dark 1 | Dark 2 | Light | |
| Chemical oxygen demand (COD) | 53.4 | 69.9 | 62.0 |
| Biological oxygen demand (BOD) | 14.8 | 20.4 | 18.0 |
| NH4+ | 29.6 | 34.7 | |
| PO4+ | 6.1 | 6.3 | |
2.2. Zeolite adsorption and microbial photosynthesis
2.3. Measurement of NH4+ and PO43−
NH4+ concentration was assessed using an ion meter (TiN-9001, Toko Kagaku Kenkyusho, Tokyo, Japan). Standard solutions of NH4+–N at concentrations of 10 mg L−1 and 100 mg L−1 were prepared using ammonium chloride. To these solutions, 10 mL L−1 of NaOH solution (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v= 1
v= 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added to adjust the pH to 12 for both the standard solutions and samples. The NH4+ concentration was determined using a calibration curve. The concentration of PO43− was measured using a digital tester (HI 717, Hanna Instruments, Chiba City, Japan).
1) was added to adjust the pH to 12 for both the standard solutions and samples. The NH4+ concentration was determined using a calibration curve. The concentration of PO43− was measured using a digital tester (HI 717, Hanna Instruments, Chiba City, Japan).
2.4. Measurement of COD and BOD
In this study, the COD and BOD were measured to evaluate the degradation of organic matter (OM). These samples were periodically collected from the influent and effluents of the reactor under both dark and light conditions. The analyses were performed by Toa Environmental Services Co. in Nagoya, Japan. For the analysis of COD and BOD, 15 mL and 200 mL samples were collected, respectively. Both types of samples were stored at −20 °C prior to analysis.2.5. Microbial analysis
Biomass samples were collected from various locations within the reactor to study the microbial community. These locations included areas adhered to the walls of the zeolite reactor (Zeo-S), those attached to plates submerged in the reactor (Zeo-P), those affixed to the zeolite material (Zeo-Z), and those influencing the zeolite reactor (referred to as Zeo-in). These biomass samples were carefully preserved in 500 mL sterilized polyethylene containers and immediately transported to the laboratory. Upon arrival, they were stored at a temperature of 4 °C.Microorganisms were identified using 16S and 18S rRNA sequencing analysis with a high-throughput Illumina sequencing technique (Illumina MiSeq, Illumina Inc.). Polymerase chain reaction (PCR) amplification was performed using specific primer sets (Table 1) to amplify the 16S rRNA gene (V3–V4 region) and the 18S rRNA gene (V7–V8 region). Each amplification reaction was performed in triplicate, and the primer sequences used in this study are listed in Table 2. Furthermore, the microbial community was analyzed using Qiime2. In addition to Qiime2, the SILVA reference database was used to align sequences, taxonomic classification, and phylogenetic analysis of both 16S rRNA and 18S rRNA sequences. The gene sequence data were deposited in the DNA Data Bank of Japan under accession number DRA018819.
| Target genes | Primer | Sequence 5′ |
|---|---|---|
| 16S rRNA (V3–V4) | 341F | TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG |
| 805R | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC | |
| 18S rRNA (V7–V8) | 1183F | TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGAATTTGACTCAACACGGG |
| 1631R | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGTACAAAGGGCAGGGACG |
3. Results and discussion
3.1. Organic matter removal
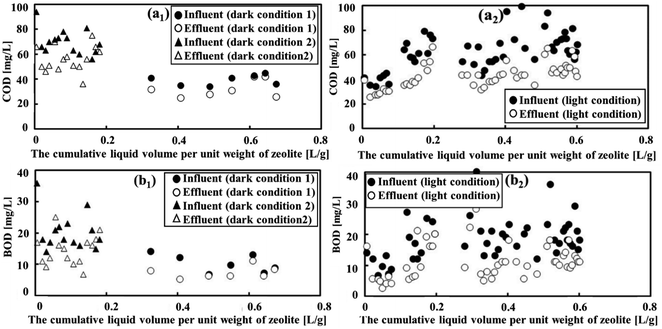
The assessment of treatment efficiency in a wastewater treatment plant (WWTP) is based on the effective removal of biodegradable and nonbiodegradable organic compounds, with COD and BOD serving as important parameters. In dark condition 1, low degradation of COD and BOD was observed, with average COD degradation from 53.4 mg L−1 to 15.9 mg L−1 and BOD degradation from 14.8 mg L−1 to 13.6 mg L−1 (Fig. 2(a1) and (b1)), corresponding to removal efficiencies of 15.9% and 8.14%, respectively. However, degradation increased when transitioning to condition 2, with the average COD and BOD decreasing from 69.9 mg L−1 to 56.1 mg L−1 and 20.3 mg L−1 to 14.5 mg L−1, respectively (as seen in Fig. 2(a1) and (b1)), resulting in a higher percentage removal of both parameters. Notably, the BOD removal efficiency increased to 28.9%, whereas the COD removal efficiency showed a slight increase to 19.8%.In darkness, the OM is considerably reduced owing to adsorption on the surface of the zeolites, as discussed in previous studies.35,36 The enhanced removal efficiencies of COD and BOD under dark condition 2 can be attributed to several factors. First, a decrease in the flow rate, leading to an extended HRT, increases the adsorption capacity of natural zeolite for OM. This finding aligns with prior research.37 Second, the high concentrations of BOD and COD had a substantial impact on the adsorption capacity of zeolite. This resulted in a noticeable improvement in the BOD and COD removal efficiencies from wastewater, as illustrated in Fig. 2(a1) and (b1).
Conversely, when subjected to light conditions, there was a substantial increase average degradation of both COD (from 62.4 mg L−1 to 42.6 mg L−1) and BOD (from 18.3 mg L−1 to 11.0 mg L−1) (refer to Fig. 2(a2) and (b2)), indicating removal efficiencies reaching 31.7% and 40.0%, respectively. These findings can be attributed to the increase in the number of photosynthetic microorganisms in the reactor under natural light illumination. The presence of light promoted the growth of both bacteria and microalgae,38 which engage in metabolic processes that enhance the degradation of OM. In the reactor, microalgae absorb CO2 and organic pollutants through photosynthesis, releasing O2 and producing OM. Simultaneously, bacteria consume oxygen and OM through respiration, creating a cycle of CO2 and O2 between the algae and bacteria. This collaboration between algae and bacteria enhances the efficiency of wastewater treatment, particularly in removing OM.39 The specific characteristics of these microorganisms are discussed in the following sections.
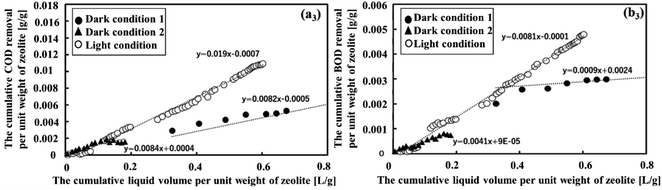
Fig. 3 clearly illustrates the variation in COD and BOD removal efficiencies across diverse conditions. The slopes for COD and BOD were 0.019 and 0.081, respectively, and it was evident that light conditions were optimal for effectively removing both COD and BOD following MFCs treatment. This was due to the presence of both zeolite-adsorbing and OM degradation bacteria under these conditions. Furthermore, it is noteworthy that under dark condition 2, the patterns of removal of COD and BOD exhibited remarkable similarity. They increased rapidly as the cumulative liquid volume per unit weight of zeolite increased from 0.005 to 0.16 L g−1 before stabilizing. This suggests that the zeolite adsorption could reach its saturation point, and further increasing the wastewater feed did not significantly affect its adsorption capacity. This observation was consistent with the findings of a study by Huang et al.40 Under dark condition 1, where the slopes were modest at 0.0082 and 0.0009 for COD and BOD, respectively, the removal efficiency was relatively low. This outcome was in line with the notion that a high flow rate, leading to reduced HRT, does not allow sufficient time for the zeolite to adsorb OM. Thus, dark condition 2 performed better than dark condition 1 in terms of COD and BOD removal. As a result, dark condition 2 was chosen to assess the removal of NH4+ and PO43− and to make a comparison with light conditions.
3.2. Removal of NH4+ and PO43− ions
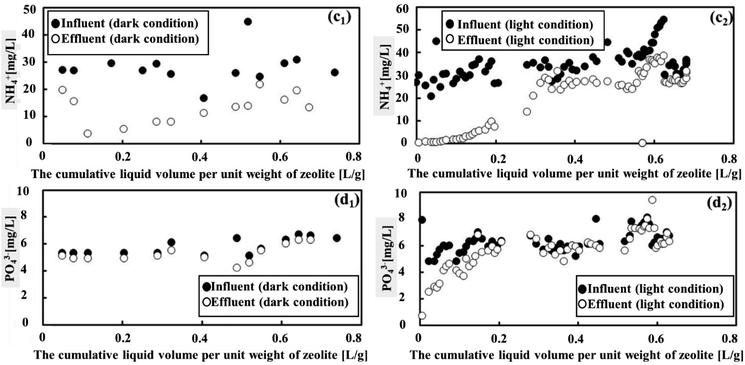
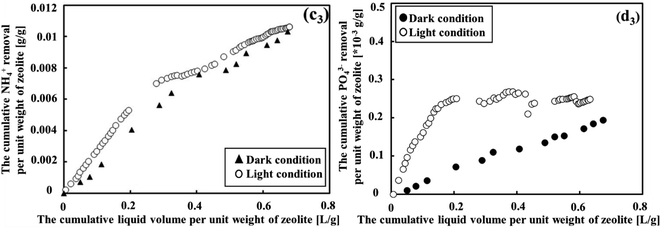
Under both light and dark conditions, zeolite adsorption and photosynthesis proved effective in removing NH4+ but were less efficient in removing PO43−, as shown in Fig. 4. When the cumulative liquid volume per unit weight of zeolite remained below 0.2 L g−1, it was the ideal condition for NH4+ removal. Within this range, NH4+ degradation displayed a high rate, with effluent concentrations of approximately 3.1 mg L−1 and 10.4 mg L−1 (as seen in Fig. 4(c1) and (c2)), corresponding to average removal efficiencies of 84.5% and 60% under light and dark conditions, respectively. The results demonstrated that the treatment system effectively removed NH4+ from sewage, showing performance comparable to the reported systems (see Table S1 in the ESI†). Thus, considering the NH4+ parameter, natural zeolite in cooperation with photosynthesis was more efficient compared to MFCs, which did not effectively remove this parameter from sewage, as shown in Fig. S1 (ESI†). Beyond this point, however, the removal efficiency began to decline. Considering the removal efficiencies of COD and BOD along with this observation, it can be concluded that the zeolite adsorption capacities reached saturation when the cumulative liquid volume per unit weight of zeolite exceeded 0.2 L g−1. In contrast, the degradation of PO43− was low in both conditions, showing average degradation declined from 5.8 mg L−1 to 5.4 mg L−1 and 6.3 mg L−1 to 5.7 mg L−1 for dark and light conditions, respectively. This was equivalent to the removal efficiency of PO43− of 5.54% in dark conditions and 14.9% in light conditions.In summary, the presence of photosynthetic microorganisms under light conditions noticeably enhanced the contaminant removal efficiency such as COD, BOD, and NH4+. The variation in the contaminant removal performance between dark and light conditions highlighted the significance of photosynthesis as an environmentally friendly approach for enhancing the removal of pollutants from MFCs-treated wastewater.
Fig. 5 presents the contrast in the removal efficiency between NH4+ and PO43−. Notably, cumulative NH4+ removal exhibited significant growth under both light and dark conditions. Initially, the light condition performed better than the dark condition; however, this difference diminished over time. Generally, the percentage of NH4+ removed from wastewater was relatively consistent across different conditions, with values of 43.8% under light conditions and 37.6% under dark conditions. To determine if there was a difference in efficiency between the two conditions, the Mann–Whitney U test was applied. The statistical test revealed a p-value of 0.2233, indicating that the NH4+ removal efficiency was not significantly different between dark and light conditions. In contrast, the cumulative PO43− removal per unit weight of the zeolite showed a slight increase. However, under light conditions, there was a more pronounced difference in cumulative PO43− removal compared to dark conditions. The Mann–Whitney U test revealed a p-value of 0.000174, indicating that the PO43− removal efficiency was significantly different between dark and light conditions. This difference could be attributed to the influence of photosynthetic microorganism activity under light condition. Therefore, in order to improve the removal of both NH4+ and PO43−, it was considered essential to optimize the photosynthesis process.
3.3. Microbial community diversity analysis
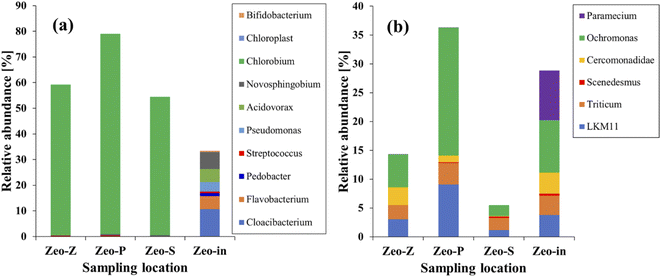
The previous section highlighted that the efficiency of contaminant removal increased under light conditions, which could be attributed to the increased activity of microorganisms. Analysis of 16S rRNA and 18S rRNA revealed the presence of more than 10 and 6 genera, respectively, in all samples (Fig. 6). Under 16S rRNA analysis, all genera were present in the Zeo-in samples. Notably, Chlorobium (53.92–78.02%), Novosphingobium (0.11–0.45%), Streptococcus (0.07–0.33%), and Cloacibacterium (0.08–0.13%) were identified as core genera, collectively accounting for nearly 78.9% of the total sequences. Whereas almost all the sequences detected in the Geo-in samples were no longer present because Chlorobium had become the predominant genus. This shift can be attributed to the fact that Chlorobium is a photosynthetic bacterium,41 that outcompetes non-photosynthetic microorganisms (NPM) for nutrients and OM in wastewater. This competitive advantage resulted in a decrease in the NPM population. Furthermore, previous studies have demonstrated Chlorobium's ability to remove COD from wastewater.17,42 Chlorobium has also been shown to oxidize reduced-sulfur compounds.43 When sulfur compounds are introduced into sewage, they contribute to the overall organic load by increasing both BOD and COD in wastewater. Consequently, the reduction in sulfur compounds led to a decrease in both BOD and COD in the sewage, a trend that aligns well with the findings presented in Fig. 2(a2) and (b2).Although dominant microorganisms such as Chlorobium and Streptococcus did not exhibit the capability to directly remove PO43− and NH4+, the concentrations of these substances still decreased. It was hypothesized that the microorganisms responsible for PO43− and NH4+ removal might still be present in the reactor, even if their sequences could not be detected. Notable examples of such microorganisms include Acidovorax, a genus within the family Comamonadaceae, which can accumulate phosphate in wastewater.44,45 Novosphingobium is renowned for its ability to break down a diverse range of aromatic hydrocarbons and plays a crucial role in the biogeochemical cycles of carbon, nitrogen, and chlorine in its surrounding environments. It exhibits the capability to convert nitrate into nitrite.46
Furthermore, the 18S rRNA analysis revealed the presence of Scenedesmus, a microorganism known for its capacity to eliminate nitrogen and phosphorus from wastewater47 (as shown in Fig. 6b). In wastewater, Scenedesmus can remove NH4+ in a two-way direct utilization by itself and remove nitrogen in the form of ammonia with air employed for the aeration of the medium. In addition, it can take up phosphorus from wastewater, leading to a reduction in the concentration of PO43− in the effluent.48 Normally, microalgae such as Scenedesmus can store phosphorus within biomass as polyphosphates. This storage actively participates in cell metabolism or is reserved when the external PO43− concentration becomes limited.49 Hence, the removal efficiency of COD, BOD, PO43−, and NH4+ increased under light conditions, which was clearly observed through the microorganism's activities.
3.4. Implication
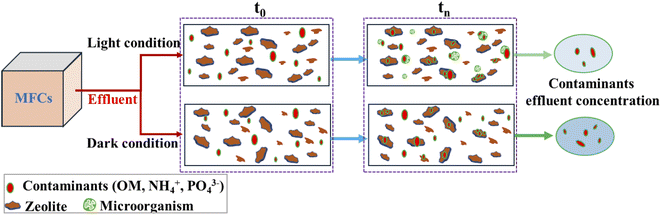
A conceptual model was employed to understand the kinetics of contaminant removal during the post-treatment of effluent from MFCs under both dark and light conditions using zeolite in collaboration with photosynthesis, as illustrated in Fig. 7. Regardless of the conditions, natural zeolites can remove contaminants through adsorption or ion exchange. Specifically, OM can be adsorbed onto zeolite surfaces via an electrostatic adsorption mechanism.34 However, this approach appeared to be ineffective, resulting in a low OM removal efficiency under dark conditions, as depicted in Fig. 2(a1) and (b1). This is because natural zeolites demonstrate limited adsorption of organics in aqueous solutions owing to their hydrophilic surfaces.34 Modifying the surface with surfactants can alter its functionality by introducing hydrophobic groups, thereby enhancing the adsorption capacities for various organics.34 When the zeolite was exposed to natural light, as proposed in this work, it exhibited enhanced efficiency in removing OM through photosynthesis, as depicted in Fig. 2(a2), (b2) and 3. In this study, due to the lack of nitrifying microbes detected in the microbial analysis of the reactor, the primary mechanism for NH4+ removal from the wastewater was hypothesized to be ion exchange. In this process, NH4+ is taken up by the zeolite through exchange with Na+, Ca2+, and K+ ions. Normally, Ca serves as the secondary primary cation in the crystal framework of natural zeolites, constituting 2.09% of the total atomic weight. Ca can be readily replaced by NH4+ in the solution.33 Previous research indicates that the adsorption capacity of natural zeolites for NH4+ ranges from 2.7 to 30.6 mg g−1.34 It has been reported that Ca2+ is released during the exchange process with NH4+, and in the presence of PO43− in the solution, it precipitates as Ca3(PO4)2 on the zeolite surface. This process contributes to the removal of phosphorous from wastewater, as described by reactions (1) and (2).32,33 However, this study revealed a low efficiency in removing PO43−, which is potentially influenced by competitive ion exchange between Ca2+ and NH4+ with another zeolite cation, leading to a reduction in the concentration of Ca2+ in the solution. Additionally, other factors, such as pH, can affect both the release of Ca2+ and the formation of Ca3(PO4)2 in solution.32,33 Hence, additional research is needed to enhance the removal of phosphate from wastewater using natural zeolites in collaboration with photosynthesis, with a significant concern regarding pH conditions.| Zeolite–Ca2+ + 2NH4+ → zeolite–2NH4+ + Ca2+ | (1) |
| Ca2+ + PO43− → Ca3(PO4)2↓ | (2) |
Therefore, the primary processes for removing contaminants from wastewater under dark conditions are adsorption and ion exchange. Additionally, under these conditions, a prolonged HRTs enhanced the interaction time between OM and zeolite, facilitating the removal of OM through adsorption. This was demonstrated under dark condition 2 (Fig. 2(a1) and (b1)). In contrast, under light conditions, the presence of microorganisms such as Chlorobium, Acidovorax, Novosphingobium, and Scenedesmus contributed to the removal of contaminants, as described in the previous section. Hence, utilizing zeolites in conjunction with photosynthesis represents a novel approach that offers advantages, such as cost-effectiveness and environmental friendliness. Furthermore, this approach addresses the limitations of MFCs in terms of nitrogen removal. In summary, this technique holds promise not only for MFCs but also for the secondary stages of other methods aimed at enhancing nitrogen and OM removal in effluents.
4. Conclusion
The findings revealed that factors such as flow rate and HRT influence contaminant concentrations, and the activity of photosynthetic microorganisms impacted the removal efficiency of COD, BOD, PO43−, and NH4+. The key findings are as follows.(1) Under dark conditions, a decrease in the flow rate combined with an increase in HRT resulted in improved removal efficiency for COD and BOD. This suggests that the contact time between OM and zeolite played a significant role in zeolite's adsorption of OM, leading to increased removal of OM from sewage.
(2) Under light conditions, the activities of microorganisms, such as Chlorobium, Acidovorax, Novosphingobium, and Scenedesmus appeared to contribute to a reduction in the concentrations of COD, BOD, PO43− and NH4+. Although the removal efficiency for PO43− was relatively low, the heightened activity of microorganisms under light condition created a substantial difference in PO43− removal between light and dark conditions.
These findings improve our understanding of the effectiveness of zeolite in conjunction with photosynthesis in eliminating OM and nutrients from MFCs effluents. Combining these methods is an ideal approach for reducing the carbon footprint of wastewater treatment systems. However, this system was unable to remove PO43− from sewage. Therefore, further research is needed to enhance the system's efficiency to effectively address all contaminants.
Data availability
Data for this article are available at https://figshare.com/account/home at https://doi.org/10.6084/m9.figshare.26113792.Author contributions
Q. N. H.: methodology, formal analysis, data curation, writing-original draft, software, visualization, writing-review & editing. T. H.: writing-review & editing. M. A. R.: writing-review & editing. N. Y.: conceptualization, methodology, writing-review & editing, supervision, funding acquisition, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was funded by MEXT/JSPS KAKENHI grant (grant number 22H01625) and the Science and Technology Research Partnership for Sustainable Development (SATREPS), a collaboration between the Japan Science and Technology Agency (JST) and the Japan International Cooperation Agency (JICA). Additional support was provided by the Kurita Water and Environment Foundation, NIPPON KOEI Co., Ltd, NIPPON KOEI Urban Space Co., Ltd, and TOYOBO Co., Ltd, all based in Japan. We extend our gratitude to Toshiyuki Yagi, Hideaki Hashimoto, and Kyo Ikeru, along with the staff of the Nagoya City Waterworks and Sewerage Bureau, for their technical assistance during the construction and analysis of the reactor. Their contributions have been crucial to the success of this research.References
- A. Maziotis, R. Sala-Garrido, M. Mocholi-Arce and M. Molinos-Senante, A comprehensive assessment of energy efficiency of wastewater treatment plants: an efficiency analysis tree approach, Sci. Total Environ., 2023, 885, 163539 CrossRef CAS PubMed.
- M. Molinos-Senante and A. Maziotis, Evaluation of energy efficiency of wastewater treatment plants: the influence of the technology and aging factors, Appl. Energy, 2022, 310, 118535 CrossRef.
- J. V. Boas, V. B. Oliveira, M. Simões and A. M. Pinto, Review on microbial fuel cells applications, developments and costs, J. Environ. Manage., 2022, 307, 114525 CrossRef CAS PubMed.
- G. Palanisamy, H.-Y. Jung, T. Sadhasivam, M. D. Kurkuri, S. C. Kim and S.-H. Roh, A comprehensive review on microbial fuel cell technologies: processes, utilization, and advanced developments in electrodes and membranes, J. Cleaner Prod., 2019, 221, 598–621 CrossRef CAS.
- Y. Park, S. Park, J. Yu, C. I. Torres, B. E. Rittmann and T. Lee, Complete nitrogen removal by simultaneous nitrification and denitrification in flat-panel air-cathode microbial fuel cells treating domestic wastewater, Chem. Eng. J., 2017, 316, 673–679 CrossRef CAS.
- Q. N. Ho, K. Mitsuoka and N. Yoshida, Microbial fuel cell in long-term operation and providing electricity for intermittent aeration to remove contaminants from sewage, Environ. Res., 2024, 259, 119503 CrossRef CAS PubMed.
- J. Desloover, A. Abate Woldeyohannis, W. Verstraete, N. Boon and K. Rabaey, Electrochemical resource recovery from digestate to prevent ammonia toxicity during anaerobic digestion, Environ. Sci. Technol., 2012, 46, 12209–12216 CrossRef CAS PubMed.
- A. Sotres, M. Cerrillo, M. Viñas and A. Bonmatí, Nitrogen removal in a two-chambered microbial fuel cell: establishment of a nitrifying–denitrifying microbial community on an intermittent aerated cathode, Chem. Eng. J., 2016, 284, 905–916 CrossRef CAS.
- R. A. Rozendal, T. H. J. A. Sleutels, H. V. Hamelers and C. J. Buisman, Effect of the type of ion exchange membrane on performance, ion transport, and pH in biocatalyzed electrolysis of wastewater, Water Sci. Technol., 2008, 57, 1757–1762 CrossRef CAS PubMed.
- H. Hiegemann, T. Littfinski, S. Krimmler, M. Lübken, D. Klein and K.-G. Schmelz, et al., Performance and inorganic fouling of a submergible 255 L prototype microbial fuel cell module during continuous long-term operation with real municipal wastewater under practical conditions, Bioresour. Technol., 2019, 294, 122227 CrossRef CAS PubMed.
- Y. Feng, W. He, J. Liu, X. Wang, Y. Qu and N. Ren, A horizontal plug flow and stackable pilot microbial fuel cell for municipal wastewater treatment, Bioresour. Technol., 2014, 156, 132–138 CrossRef CAS PubMed.
- K. Hanaki, C. Wantawin and S. Ohgaki, Nitrification at low levels of dissolved oxygen with and without organic loading in a suspended-growth reactor, Water Res., 1990, 24, 297–302 CrossRef CAS.
- A. Shimidzu, F. Tanaka, T. Matsumura, M. Sakoda, K. Iida and N. Yoshida, Optimizing low-voltage boosting for an air-cathode microbial fuel cell with an anion exchange membrane in a 246 L wastewater treatment reactor, Environ. Sci.: Water Res. Technol., 2024, 10, 296–303 RSC.
- S. Okabe, Bioelectrochemical anoxic ammonium nitrogen removal by an MFC driven single chamber microbial electrolysis cell, Chemosphere, 2021, 274, 129715 CrossRef PubMed.
- M. A. Al-Ghouti, M. A. Khraisheh, M. N. Ahmad and S. Allen, Adsorption behaviour of methylene blue onto Jordanian diatomite: a kinetic study, J. Hazard. Mater., 2009, 165, 589–598 CrossRef CAS PubMed.
- R. Rashid, I. Shafiq, P. Akhter, M. J. Iqbal and M. Hussain, A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method, Environ. Sci. Pollut. Res., 2021, 28, 9050–9066 CrossRef CAS PubMed.
- J. Chen, J. Wei, C. Ma, Z. Yang, Z. Li and X. Yang, et al., Photosynthetic bacteria-based technology is a potential alternative to meet sustainable wastewater treatment requirement?, Environ. Int., 2020, 137, 105417 CrossRef CAS PubMed.
- S. De Gisi, G. Lofrano, M. Grassi and M. Notarnicola, Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review, Sustainable Mater. Technol., 2016, 9, 10–40 CrossRef CAS.
- C. Lazaratou, D. Vayenas and D. Papoulis, The role of clays, clay minerals and clay-based materials for nitrate removal from water systems: a review, Appl. Clay Sci., 2020, 185, 105377 CrossRef CAS.
- E. Djubaedah, A. Wulandari and K. Krisnandi, Surface Area Modification of Natural Zeolite through NaCl Counterbalanced Treatment to Apply in Adsorption Heat Storage System, Evergreen: Jt. J. Novel Carbon Resour. Sci. Green Asia Strategy, 2020, 7, 26–31 CrossRef CAS.
- S. Montalvo, C. Huiliñir, R. Borja, E. Sánchez and C. Herrmann, Application of zeolites for biological treatment processes of solid wastes and wastewaters–a review, Bioresour. Technol., 2020, 301, 122808 CrossRef CAS PubMed.
- M. Rožić, Š. Cerjan-Stefanović, S. Kurajica, V. Vančina and E. Hodžić, Ammoniacal nitrogen removal from water by treatment with clays and zeolites, Water Res., 2000, 34, 3675–3681 CrossRef.
- M. R. Adam, M. H. D. Othman, S. K. Hubadillah, M. H. Abd Aziz and M. R. Jamalludin, Application of natural zeolite clinoptilolite for the removal of ammonia in wastewater, Mater. Today: Proc., 2023 Search PubMed , in press, corrected proof.
- C. J. Castro, H.-Y. Shyu, B. Hoque and D. H. Yeh, Evaluating the use of chemically modified clinoptilolite zeolite for the simultaneous recovery of ammonium and phosphate from blackwater, Environ. Sci.: Water Res. Technol., 2023, 9, 818–832 RSC.
- A. Malovanyy, H. Sakalova, Y. Yatchyshyn, E. Plaza and M. Malovanyy, Concentration of ammonium from municipal wastewater using ion exchange process, Desalination, 2013, 329, 93–102 CrossRef CAS.
- M. Wang, K. A. Payne, S. Tong and S. J. Ergas, Hybrid algal photosynthesis and ion exchange (HAPIX) process for high ammonium strength wastewater treatment, Water Res., 2018, 142, 65–74 CrossRef CAS PubMed.
- Y.-C. Chung, D.-H. Son and D.-H. Ahn, Nitrogen and organics removal from industrial wastewater using natural zeolite media, Water Sci. Technol., 2000, 42, 127–134 CrossRef CAS.
- Z. Han, J. Dong, Z. Shen, R. Mou, Y. Zhou and X. Chen, et al., Nitrogen removal of anaerobically digested swine wastewater by pilot-scale tidal flow constructed wetland based on in-situ biological regeneration of zeolite, Chemosphere, 2019, 217, 364–373 CrossRef CAS.
- A. Yang, G. Zhang, F. Meng, R. Zhi, P. Zhang and Y. Zhu, Nitrogen metabolism in photosynthetic bacteria wastewater treatment: a novel nitrogen transformation pathway, Bioresour. Technol., 2019, 294, 122162 CrossRef CAS PubMed.
- Q. Zhou, G. Zhang, X. Zheng and G. Liu, Biological treatment of high NH4+-N wastewater using an ammonia-tolerant photosynthetic bacteria strain (ISASWR2014), Chin. J. Chem. Eng., 2015, 23, 1712–1715 CrossRef CAS.
- T. Hülsen, K. Hsieh, S. Tait, E. M. Barry, D. Puyol and D. J. Batstone, White and infrared light continuous photobioreactors for resource recovery from poultry processing wastewater–a comparison, Water Res., 2018, 144, 665–676 CrossRef PubMed.
- N. Karapınar, Application of natural zeolite for phosphorus and ammonium removal from aqueous solutions, J. Hazard. Mater., 2009, 170, 1186–1191 CrossRef PubMed.
- L. Lin, C. Wan, D.-J. Lee, Z. Lei and X. Liu, Ammonium assists orthophosphate removal from high-strength wastewaters by natural zeolite, Sep. Purif. Technol., 2014, 133, 351–356 CrossRef CAS.
- S. Wang and Y. Peng, Natural zeolites as effective adsorbents in water and wastewater treatment, Chem. Eng. J., 2010, 156, 11–24 CrossRef CAS.
- L. Senila, A. Hoaghia, A. Moldovan, I. A. Török, D. Kovacs and D. Simedru, et al., The potential application of natural clinoptilolite-rich zeolite as support for bacterial community formation for wastewater treatment, Materials, 2022, 15, 3685 CrossRef CAS.
- D. Kallo, Applications of natural zeolites in water and wastewater treatment, Rev. Mineral. Geochem., 2001, 45, 519–550 CrossRef CAS.
- I. Vera-Puerto, M. Saravia, J. Olave, C. Arias, E. Alarcon and H. Valdes, Potential application of chilean natural zeolite as a support medium in treatment wetlands for removing ammonium and phosphate from wastewater, Water, 2020, 12, 1156 CrossRef CAS.
- Q. Wang, E. Childree, J. Box, M. López-Vela, D. Sprague and J. Cherones, et al., Microalgae can promote nitrification in poultry-processing wastewater in the presence and absence of antimicrobial agents, ACS ES&T Eng., 2023, 3, 568–579 Search PubMed.
- J.-L. Zhou, L. Yang, K.-X. Huang, D.-Z. Chen and F. Gao, Mechanisms and application of microalgae on removing emerging contaminants from wastewater: a review, Bioresour. Technol., 2022, 364, 128049 CrossRef CAS PubMed.
- Y. Huang, C. Song, L. Li and Y. Zhou, The mechanism and performance of zeolites for ammonia removal in the zeolite packed electrolysis reactor, Electrochemistry, 2014, 82, 557–560 CrossRef CAS.
- J. A. Eisen, K. E. Nelson, I. T. Paulsen, J. F. Heidelberg, M. Wu and R. J. Dodson, et al., The complete genome sequence of Chlorobium tepidum TLS, a photosynthetic, anaerobic, green-sulfur bacterium, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 9509–9514 CrossRef CAS.
- L. Azevedo, I. Castro, C. Leal, J. Araújo and C. Chernicharo, Performance and bacterial diversity of bioreactors used for simultaneous removal of sulfide, solids and organic matter from UASB reactor effluents, Water Sci. Technol., 2018, 78, 1312–1323 CrossRef CAS.
- Anoxygenic photosynthetic bacteria, ed. R. E. Blankenship, T. M. Michael and E. B. Carl, Kluwer Academic Publishers, Amsterdam, 1995, pp. 847–870 Search PubMed.
- H. Ge, D. J. Batstone and J. Keller, Biological phosphorus removal from abattoir wastewater at very short sludge ages mediated by novel PAO clade Comamonadaceae, Water Res., 2015, 69, 173–182 CrossRef CAS.
- I. Kamika, M. Coetzee, B. B. Mamba, T. Msagati and M. N. Momba, The impact of microbial ecology and chemical profile on the enhanced biological phosphorus removal (EBPR) process: a case study of Northern Wastewater Treatment Works, Johannesburg, Int. J. Environ. Res. Public Health, 2014, 11, 2876–2898 CrossRef.
- S. Feng, S. Xie, X. Zhang, Z. Yang, W. Ding and X. Liao, et al., Ammonium removal pathways and microbial community in GAC-sand dual media filter in drinking water treatment, J. Environ. Sci., 2012, 24, 1587–1593 CrossRef CAS.
- E. Zhang, B. Wang, Q. Wang, S. Zhang and B. Zhao, Ammonia–nitrogen and orthophosphate removal by immobilized Scenedesmus sp. isolated from municipal wastewater for potential use in tertiary treatment, Bioresour. Technol., 2008, 99, 3787–3793 CrossRef CAS.
- N. Tam and Y. Wong, Wastewater nutrient removal by Chlorella pyrenoidosa and Scenedesmus sp, Environ. Pollut., 1989, 58, 19–34 CrossRef CAS PubMed.
- O. J. H. Moreno, G. Pinto, A. Pollio, L. Frunzo, P. N. L. Lens and G. Esposito, Start-up of a nutrient removal system using Scenedesmus vacuolatus and Chlorella vulgaris biofilms, Bioresources and Bioprocessing, 2019, 6, 1–16 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04672b |
| This journal is © The Royal Society of Chemistry 2024 |